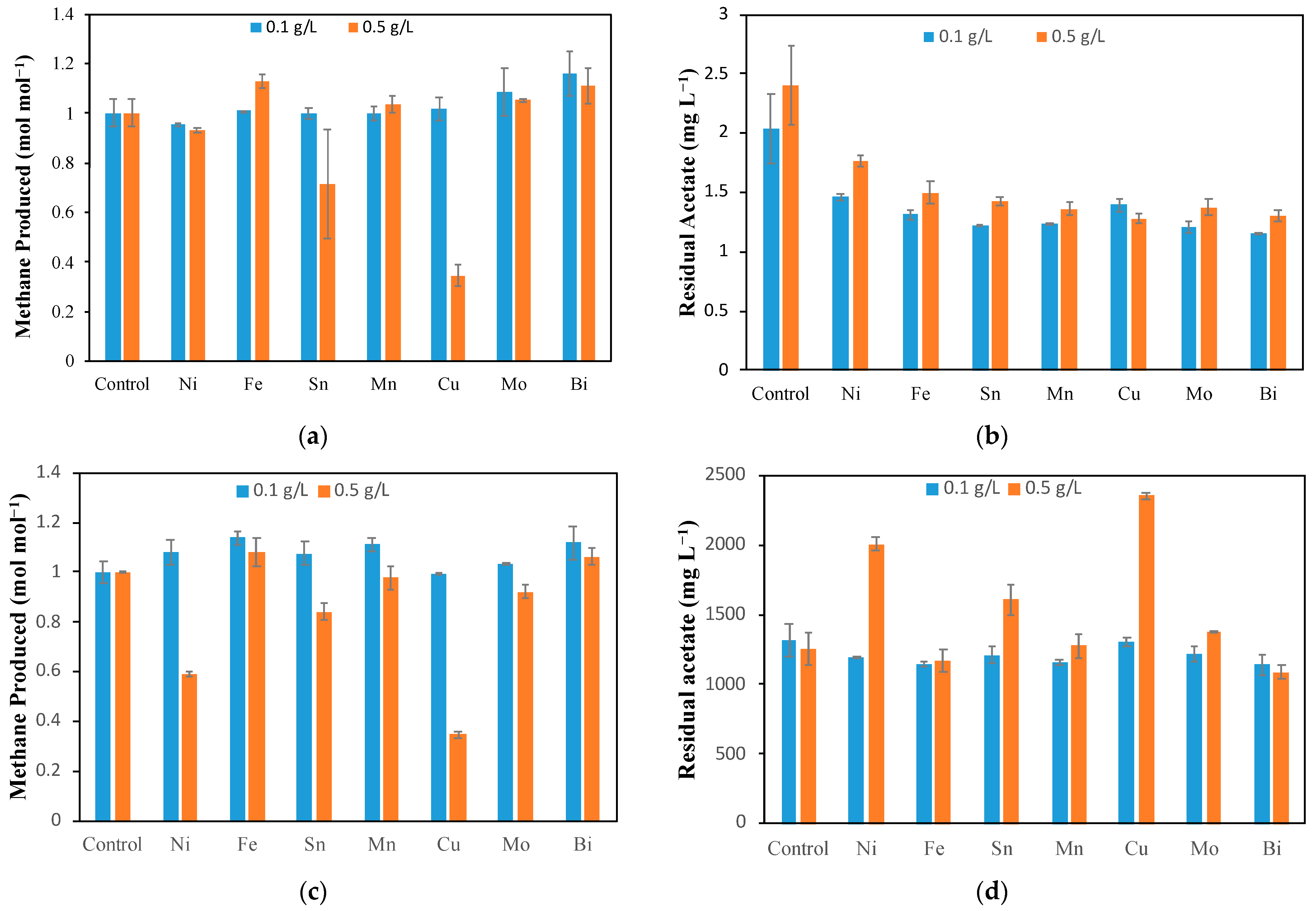2.1. Batch H2 and Acetate Consumption Tests
While transition metal ions can be hypothesized to improve bioelectrochemical CO2 reduction, some metal ions can inhibit microbial activity, i.e., metal catalysts need to be biocompatible. Thus, a series of batch tests were carried out to evaluate the impact of metal ions towards two key microbial populations responsible for CH4 production and expected to be present in the cathodic biofilm in the MESC: hydrogenotrophic and acetoclastic archaea. Notably, acetogenic bacteria capable of producing VFAs from CO2 were also expected to be present in the biofilm. The batch tests were carried out using dissolved metal cations and molybdate at relatively high concentrations and under non-bioelectrochemical conditions (no applied current). In the subsequent MESC experiments, two transition metal ions that showed a positive impact on CH4 production were added to the MESC cathodic liquid.
The microbial formation of CH
4 from CO
2 and H
2 can be achieved through two competing pathways. While detailed knowledge of bioelectrochemical reactions leading to the conversion of CO
2 to CH
4 and acetate is lacking, research agrees that the main reactions that occur during CO
2 bio-electrochemical conversion can be classified as based on direct and indirect electron transfer. Direct electron transfer (DET) refers to the process by which electroactive microorganisms accept electrons directly from the surface of the cathode. The DET pathway is associated with the electron transfer occurring in the outer membrane of the microbes through cytochromes or membrane-bound redox proteins that are in contact with the biocathode [
15,
20]. The following reduction reaction, known as direct autotrophic methanogenesis, illustrates direct CH
4 formation at the cathode surface [
21,
22]:
Indirect electron transfer corresponds to the mechanism by which CO
2 conversion products are produced via intermediates, namely hydrogen (H
2) or acetate, which act as electron donors (
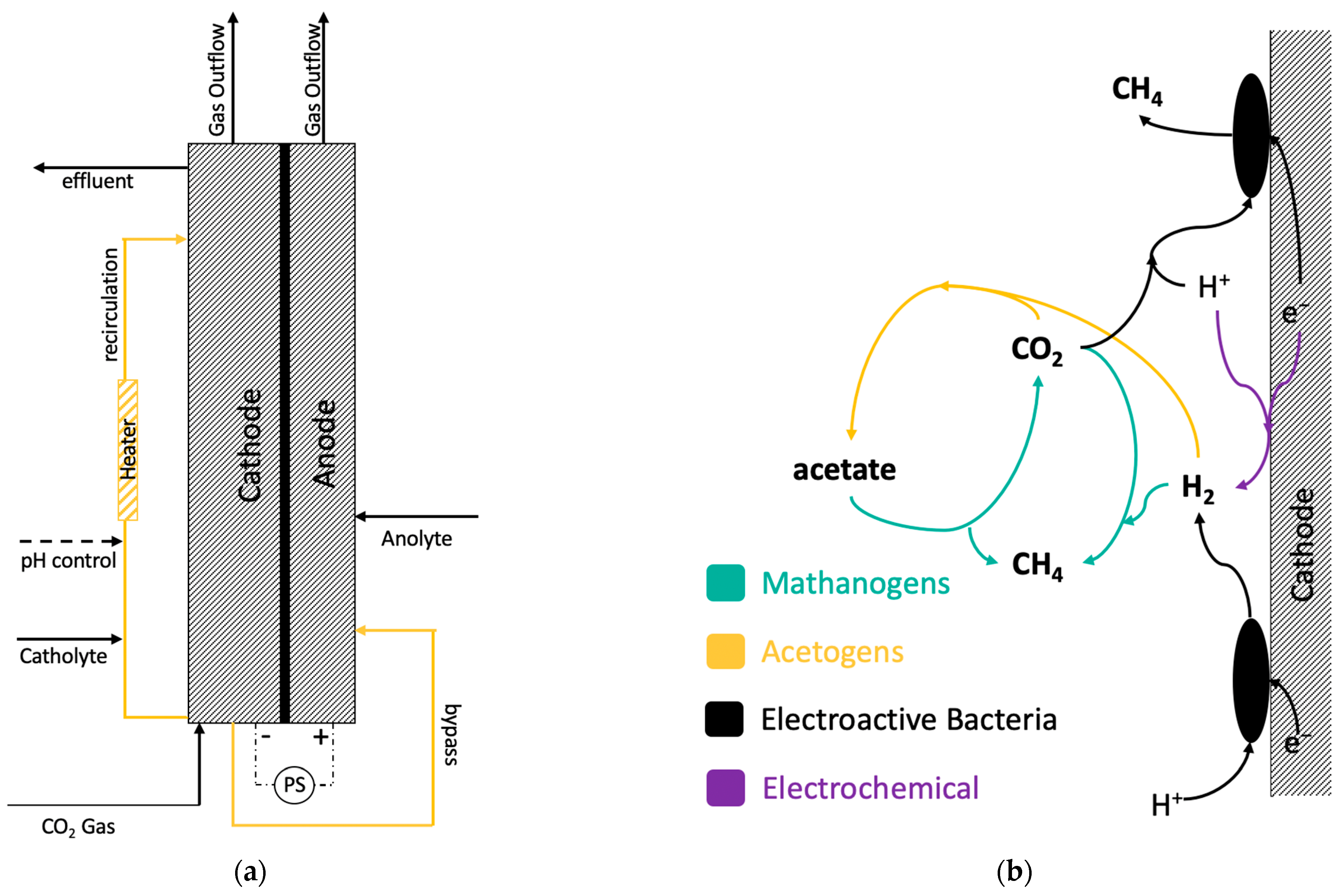
Figure 1b). H
2 can be produced electrochemically on the surface of the cathode via water electrolysis or biotically by some types of electroactive microorganisms in the following reaction occurring in a slightly alkaline environment [
23]:
Then, hydrogenotrophic methanogens in the biofilm reduce CO
2 to CH
4 via the following bioreaction [
21,
24]:
Acetate can also be produced directly at the cathode by electroactive microorganisms or indirectly (with H
2 as an intermediate) by acetogenic microorganisms via the reduction of CO
2 as follows [
25,
26,
27]:
Finally, acetoclastic methanogens present in the biofilm are able to use the acetate produced to form CH
4 as follows:
The schematic of these bioelectrochemical transformations is represented in
Figure 1b. Accordingly, batch CO
2/H
2 consumption tests were conducted to evaluate the impact of transition metals on all three groups of microorganisms.
As mentioned above, in the batch H
2/CO
2 consumption tests, dissolved metal salts were added to test bottles to study the impact of these metal ions on the microbial conversion of CO
2 to CH
4 and VFAs. Two concentrations of metal ions were used in these tests: 0.1 g L
−1 and 0.5 g L
−1. Notably, in addition to Ni
2+, Fe
2+, Cu
2+, Sn
2+, Mn
2+, and Bi
3+ cations, the molybdate (as MoO
42−) anion was also tested.
Figure 1a shows the normalized CH
4 production (with respect to control), while
Figure 1b shows the acetate concentration in the bottles at the completion of the test. Concentrations of other VFAs such as propionate, butyrate, and valerate were below the corresponding detection limits (1 mg L
−1) and are not shown in the graphs.
For most of metal ions at a concentration of 0.1 g L
−1, it is evident that there was an insignificant variation in CH
4 production in the presence of dissolved metal ions and very low residual acetate concentrations, with a slight improvement in CH
4 production seen in bottles containing Bi
3+ (16%), MoO
42− (8.5%), and Fe
2+ (1.1%), whereas for 0.5 g L
−1, the results varied significantly. At the higher concentration of metal ions, inhibition was observed for Sn and Cu ions, with CH
4 production declining by 15% and 70%, respectively (relative to control). This is validated by various studies that show that Cu is especially toxic to methanogens [
28,
29,
30]. However, at lower Sn and Cu concentrations of 0.1 g L
−1, there was no negative effect on CH
4 production.
Other metals showed little to no significant variation in CH
4 production at both ion concentrations. However, even though the presence of metal ion salts in the bottle tests showed no significant improvement in CH
4 production, previous studies [
16,
31] indicated that the use of these metals in an MESC may improve CH
4 and/or acetate production, as mentioned in the introduction. Such improvement can be either achieved through the improved electrochemical production of H
2 or by enhancing the DET pathway of CH
4 production through electromethanogenesis [
32]. It is important to note that the impact of metal ions in the bottle tests provides some insight on the toxicity of the metal ions. However, any improved conversion may not translate to the same effects in an MESC. In an MESC, there is an electric current that reduces the metal ions to zero-charge metals that deposit on the cathode surface (electrodeposition). Hence, the effects observed in an MESC to be discussed later in the text are due to a combination of influence of metal ions and metal deposited on the surface of the cathode.
Furthermore, the analysis of acetate concentrations at the end of each test showed some differences (
Figure 1b). Although all acetate concentrations at the end of each test were always low (below 3 mg L
−1) and close to the analytical threshold of 1 mg L
−1, for all bottles with metal ions, residual acetate concentrations were lower as compared to the control, with a slight deviation among tests containing different metal ions. It is important to note that acetate is simultaneously produced by acetogens and utilized by acetoclastic methanogens as described by Equations (4) and (5). Furthermore, the difference in the remaining acetate concentration is less than 1 mg L
−1 between control and metal ion bottles (
Figure 1b). This amount is very low compared to what is seen in the anaerobic reactors producing biogas. Notably, the low residual concentration of acetate and other VFAs in the CO
2/H
2 bottle tests could be indicative of a low acetogenic activity (i.e., insignificant acetate production) as well as high rate of acetate conversion to CH
4 by acetoclastic methanogens. Considering that the anaerobic sludge used for inoculating the bottles originated from an anaerobic digester that treats agricultural wastes and contained a substantial population of acetoclastic methanogens, it was important to also estimate the impact of metal ions on acetoclastic methanogenesis.
An analysis of H
2, CO
2, and CH
4 headspace concentrations in the test bottles demonstrated a close-to-stoichiometric CH
4 production (
Table 1). Based on Equation (3), every mole of CH
4 produced needed 4 moles of H
2 to be used. Indeed, the H
2-to-CH
4 ratio was close to 4 for all the tests performed, with the exception of bottles with Cu (at metal ion concentration of 0.5 mg L
−1), where the value was higher. It can be hypothesized that because of Cu toxicity, products other than CH
4 and VFAs (e.g., formate) were formed in this case or the biotransformation pathways were affected due to Cu toxicity.
The second part of activity tests was dedicated to evaluating the impact of transition metal ions on methanogenic acetoclastic activity, resulting in the conversion of acetate to CH
4 and CO
2 (Equation (5)). The normalized CH
4 production (with respect to control) observed in these tests is shown in
Figure 1c. It is evident that at the low metal ion concentration (0.1 g L
−1), there was little to no effect on the CH
4 produced for all metal ions; however, at the concentration of 0.5 g L
−1, CH
4 production inhibition was clearly observed for Ni, Cu, and Sn. Ni inhibition led to about a 40% lower CH
4 production relative to the control, whereas it was about 60% and 20% for Cu and Sn, respectively. When these results were compared with the H
2 activity test (
Figure 1a), it can be concluded that dissolved Cu inhibited both acetoclastic and hydrogenotrophic methanogenic activities. Fe and Bi consistently showed a slight improvement in CH
4 production at both concentrations, while Mo and Mn showed an improvement only at 0.1 g L
−1.
Figure 1d shows the residual acetate in the bottles after the end of the experiment. At 0.1 g L
−1, comparable amounts of acetate were used for all the metal ions and control. However, at 0.5 g L
−1, we see more residual acetate for Ni, Sn, and Cu.
Table S1 (in Supplementary Materials) shows the amount of acetate used, the amount of CH
4 produced, and the ratio of CH
4 produced to acetate used. This ratio was about one for all the bottles at both concentrations, which is consistent with the expected reaction stoichiometry (Equation (5)), indicating that although Cu, Ni, and Sn reduced the rate of acetate conversion to CH
4, the conversion stoichiometry of this microbial biotransformation was not affected. This also suggests that in the biotransformation of acetate, only CH
4 is predominantly formed even though there were other microbial species present other than acetoclastic methanogen.
From these results, it can be concluded that the presence of transition metal ions had little to no effect on the observed stoichiometry of CH4 production from acetate. At the same time, acetate consumption was significantly slower in the presence of 0.5 g L−1 of Ni and Cu, i.e., these metals significantly suppressed acetoclastic methanogenic activity. Although there was a 20% inhibition of acetate consumption in the presence of Sn at a concentration of 0.5 g L−1, this value was lower than both Cu and Ni inhibition. Furthermore, inhibition was not observed at the lower Sn concentration of 0.1 g L−1.
Overall, the batch tests helped to exclude Ni and Cu from further testing in the MESC experiments. Notably, Ni is a well-known HER catalyst [
33], while several recent works demonstrated promising catalytic properties of Cu for the direct electrochemical reduction of CO
2 [
7,
34,
35]. While the utilization of these transition metals at an MESC cathode would support the electrocatalytic conversion of CO
2, which could be complementary to bioelectrochemical conversion, the high toxicity of these metals towards methanogenic populations would limit microbial activity. Our previous studies with an MESC cathode enhanced by alloy electrodeposition (Ni-Fe and Ni-Fe-Mn alloys) [
17,
36] showed an improved production of CH
4 and acetate when using a Ni-based alloy. It can be suggested that the impact of electrodeposited (solid) Ni on CH
4 production differs from that of Ni cations (Ni
2+).
Of the remaining three transition metals tested in the batch tests described above, Bi and Sn were of interest due to their known catalytic properties in the electrochemical conversion of CO
2 [
37,
38]. Bi performed well and seemed to have a positive contribution to CH
4 production in both H
2/CO
2 and acetate consumption tests. Also, Sn showed no inhibition of CH
4 production at 0.1 g L
−1. Moreover, previous research conducted by Qui et al. [
18] suggests that Sn facilitated acetate production via the production of formate, which effectively acts as an electron donor. Based on these considerations, it was decided to test the impact of Bi and Sn on CO
2 conversion in an MESC.
2.2. MESC Operation with Bismuth Ion Injection
Following batch tests, the impact of Bi and Sn ions on the bioelectrochemical conversion of CO2 to CH4 was studied in MESCs with a continuous supply of CO2. These cells were inoculated with the same anaerobic sludge as used for batch activity tests, after homogenizing it. Before inoculation, each MESC was operated for 2–5 days under abiotic conditions in order to electrochemically characterize the system and determine the amount of H2 gas produced electrochemically.
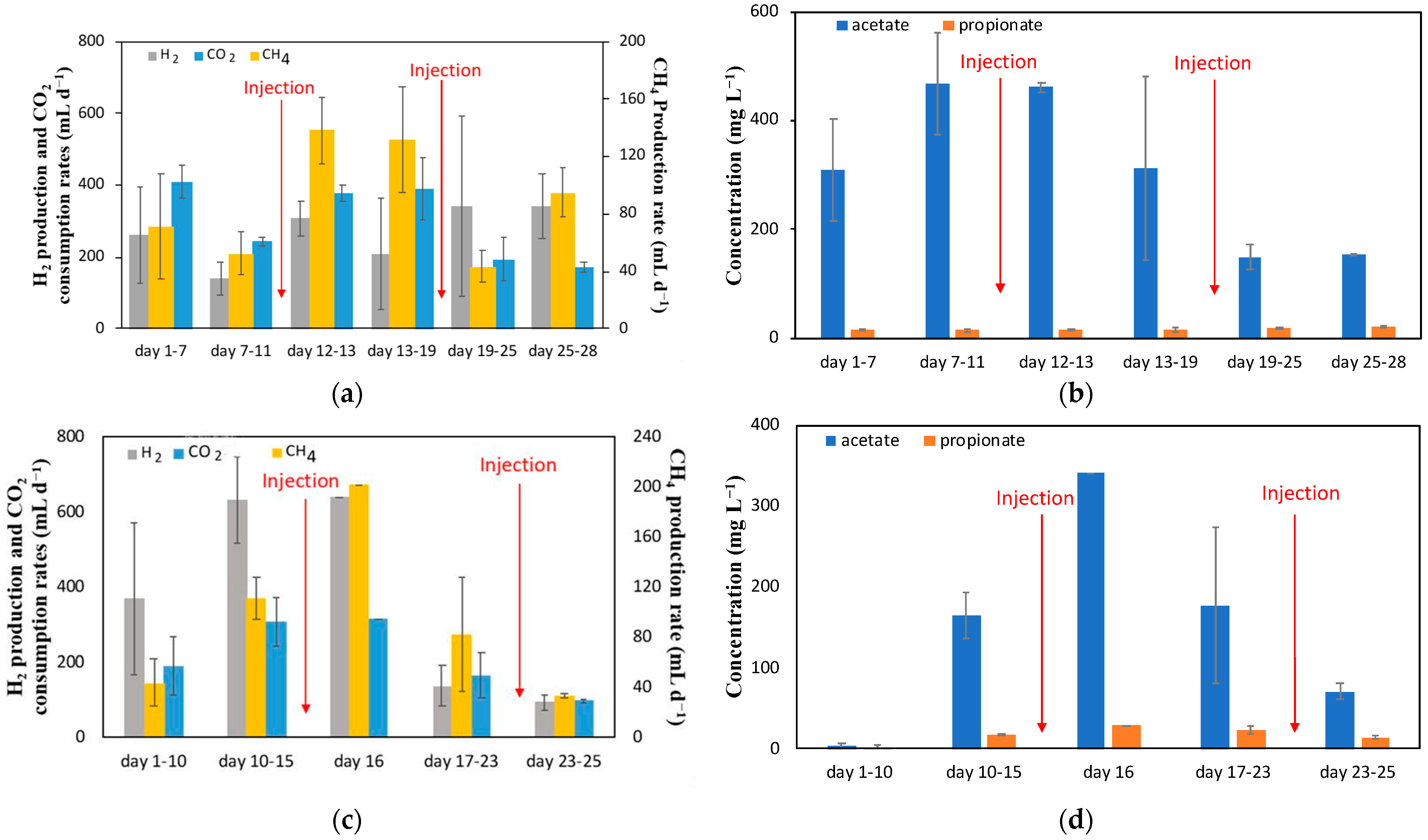
The results of the Bi injection experiment are summarized in
Figure 2a,b, which shows average volumetric rates of product (CH
4, H
2, acetate, and propionate) formation as well as CO
2 consumption. The MESC operation was started at a constant current of 50 mA. Although not shown, at this current and under abiotic conditions, a H
2 production of around 500 mL d
−1 was observed, corresponding to an energy consumption of 7 Wh/L
H2.
Starting from inoculation with anaerobic sludge, the Bi MESC was given 11 days for the biofilm to grow and for CH
4 production to reach a steady state before introducing the Bi solution. The CH
4 formation rate before injections was 53 ± 15 mL d
−1. Bi solution was injected on day 12 to obtain an initial concentration of Bi ions of 0.1 g L
−1. Following this injection, the CH
4 production rate increased almost immediately to 138 ± 23 mL d
−1. Limitations in the supply of CO
2, and hence the availability of dissolved CO
2/carbonate, indicated by the low cathode off-gas concentration of CO
2 (2.4%), was subsequently observed. In order to avoid a CO
2-limited production of CH
4, its inflow was increased by 50% to 720 mL d
−1 on day 16, leading to a CO
2 off-gas concentration of 62%. It is important to note that the increase in CO
2 supply was simultaneous with changes in current. Not only could this have affected the microbial population, but an increase in current could cause an increase in H
2 supplied electrochemically. With an increased CO
2 supply and simultaneous increase in current, there was an immediate increase in H
2 consumed, shown in
Figure 2a. This indicates that H
2 was used by either hydrogenotrophic methanogens or acetogens. To avoid H
2 limitation, the current setpoint was gradually increased from 50 mA to 80 mA, starting on day 15 and ending on day 18.
As expected, the increase in current resulted in an increase in H
2 gas produced. However, the current increase seems to have negatively affected the methanogenic activity, as seen by the increased standard deviation (higher fluctuations) in CH
4 production witnessed between days 13 and 19 (
Figure 2a). It can be suggested that the increased partial pressure of H
2 negatively affected acetoclastic methanogens, decreasing the acetate conversion to CH
4. Furthermore, the increased production of H
2 could disrupt the biofilm formed on the cathode (carbon felt) surface. Notably, acetoclastic methanogens have a slow growth rate [
39] and perform better in biofilm-based systems. A higher growth rate of acetogenic bacteria is expected to promote the proliferation of this microbial population in the biofilm as well as in the cathodic liquid at high dissolved H
2 concentrations.
Acetate production was observed throughout the entire test with a much lower concentration of propionate also detected (
Figure 2b), while the production of other VFAs was negligible. At the MESC startup, some abiotic (electrochemical) acetate production was observed with a steady-state acetate concentration of 35 mg L
−1 (not shown in the figure). After inoculation, the acetate concentration increased due to acetogenic activity, reaching a steady-state value of about 468 ± 94 mg L
−1 by day 11 (
Figure 2b). Immediately after the first injection of Bi ions, the acetate concentration was maintained at around 461 ± 8 mg L
−1, before falling to a final measured concentration of 150 mg L
−1 on day 19.
These changes in acetate concentration over time can be either attributed to a short-term increase in activity of acetoclastic methanogens, which convert acetate to CH4, as shown in the batch activity tests, or to a decrease in acetogenic activity following Bi injection. Once the MESC current was increased to 80 mA, acetate concentration decreased likely due to a combination of lower acetogenic activity and the competition for H2 between acetogenic bacteria and hydrogenotrophic methanogens as well as the possible adverse impact of the increased partial pressure of H2, which could also result in a lower acetate concentration. Furthermore, the formation of products other than CH4 and short chain fatty acids cannot be excluded.
The second Bi injection was carried out on day 18. There was a notable decrease in product formation observed immediately after this injection, with CH
4 production reaching 74 ± 11 mL d
−1 initially (
Figure 2a, days 19–25) before plateauing at 95 ± 17 mL d
−1 (
Figure 2a, days 25–28). Furthermore, the acetate concentration declined towards the end of the test, plateauing at a final concentration of 155 ± 1 mg L
−1 (
Figure 2b).
Before the first Bi injection, the Coulombic efficiency (calculated based on H
2, CH
4, and acetate production) was at 92 ± 20%. After the first injection, the Coulombic efficiency was fluctuating, exceeding 100% initially due to the transition to a new steady state, before converging to about 90%. After the second injection, the Coulombic efficiency stabilized at about 60%. The decrease in Coulombic efficiency, which evidences a decrease in the MESC performance after the second Bi injection, supports the observed reduction in CH
4 and acetate production rates. This is suggestive of the reduced uptake of electrons by the microbial community particularly for the bioconversion of CO
2 to CH
4 and acetate as the Coulombic efficiency determination is based on H
2 and these two major products of CO
2 conversion. Thus, a decrease in Coulombic efficiency values suggests the formation of products that are not measured. In particular, the formation of ethanol, medium chain fatty acids such as caproate, and alcohols such as butanol and hexanol was previously observed [
40,
41].
2.3. MESC Operation with Tin Ion Injection
Based on the results of the Bi injection experiment, the MESC with Sn injections was maintained at 80 mA and 720 mL d
−1 of CO
2 from the beginning of the experiment. Inoculation was carried out on day zero. Immediately after inoculation, an increase in CH
4 production was observed, whereas an increase in acetate concentration was observed only after a few days, indicating the development of the biofilm, which can be seen in
Figure 2c and
Figure 2d, respectively. The steady-state CH
4 production before Sn
2+ injection was about 111 ± 17 mL d
−1, a value almost double that observed with the Bi ion injection experiment. This is expected as starting at a higher current resulted in the availability of more H
2 gas for product formation.
The first injection was performed on day 15 while the second was performed on day 23. The first injection led to a short-term increase in CH
4 production (day 16,
Figure 2c), which eventually decreased to a much lower level. The first day after injection, 202 mL d
−1 of CH
4 was observed, a value about 82% higher than the steady-state production before injection. This value eventually decreased to about 35 mL d
−1 before the second injection, shown in
Figure 2c.
After the second injection, there was no change in CH
4 production (days 23–25 in
Figure 2c). This lack of response of CH
4 production could be due to the depletion of H
2 gas in the system, which could be caused by the lack of cathode surface area available for the abiotic electrochemical production of H
2 by water splitting. Another possibility is that all the H
2 produced is being consumed to form products that are not measured, as mentioned above.
This hypothesis is supported by Coulombic efficiency estimations showing significantly lower values towards the end of this test. In fact, before any Sn injection, the Coulombic efficiency was about 90% (comparable to that of Bi). After the first injection, the value was stable at close to 100%, indicating that all products were accounted for. After the second injection, the Coulombic efficiency declined to about 22 ± 4%. This trend was the same as that observed with the Bi injections, indicating that the second metal ion injection caused product formation that was not measured.
From
Figure 2d, with the first injection, it is evident that acetate levels increased to 341 mg L
−1 before following a decreasing trend, indicating that the injection of Sn caused a temporary increase in acetogenic activity. Acetate levels eventually decreased to 177 ± 96 mg L
−1 between days 17 and 23. Unlike the first injection, there was no short-term increase in acetate concentration after the second injection; however, there was a decrease to 71 ± 10 mg L
−1.
Looking at the results of both metal ion injections, it seems that the injection of metal ions only caused a short-term improvement in CH
4 production. This aligns with what was seen in the H
2/CO
2 activity tests (
Figure 1a,b), in which CO
2 and H
2 consumption was observed within 24 h. The injection of the metal salts ultimately leads to electrodeposition of the metals on the surface of the cathode, which is negatively charged. Both Sn and Bi are known to help facilitate H
2 evolution [
42,
43].
Faraday’s equation indicated that metal ions are immediately deposited on the surface of the cathode. In fact, for 0.1 g L−1, it would take Sn only 7 min to completely deposit, whereas it would take only 6 min for Bi, assuming that all current is used for the electrodeposition. Although the actual rate of electrodeposition was slower due to bioelectrochemical and electrochemical reactions occurring simultaneously, the expected concentration of metal ions is expected to diminish quickly, which agrees well with the observed short-term impact of each injection on microbial activity. Therefore, it would be of interest to try including these salts in the nutrient solution (catholyte) or to carry out the electrodeposition of metals before the start-up of MESC operation.
Considering that the electrodeposition of transition metals such as Bi and Sn is expected to improve the electrochemical properties of the carbon felt cathode, in the following tests, the carbon felt cathode was first modified by electrodeposition of the two metals on its surface prior to the CO2 conversion experiments in the MESC, and their performance on the CO2 conversion to CH4 and VFAs was evaluated subsequently.
2.4. MESC Operation with Sn- and Bi-Modified Carbon Felt Cathode
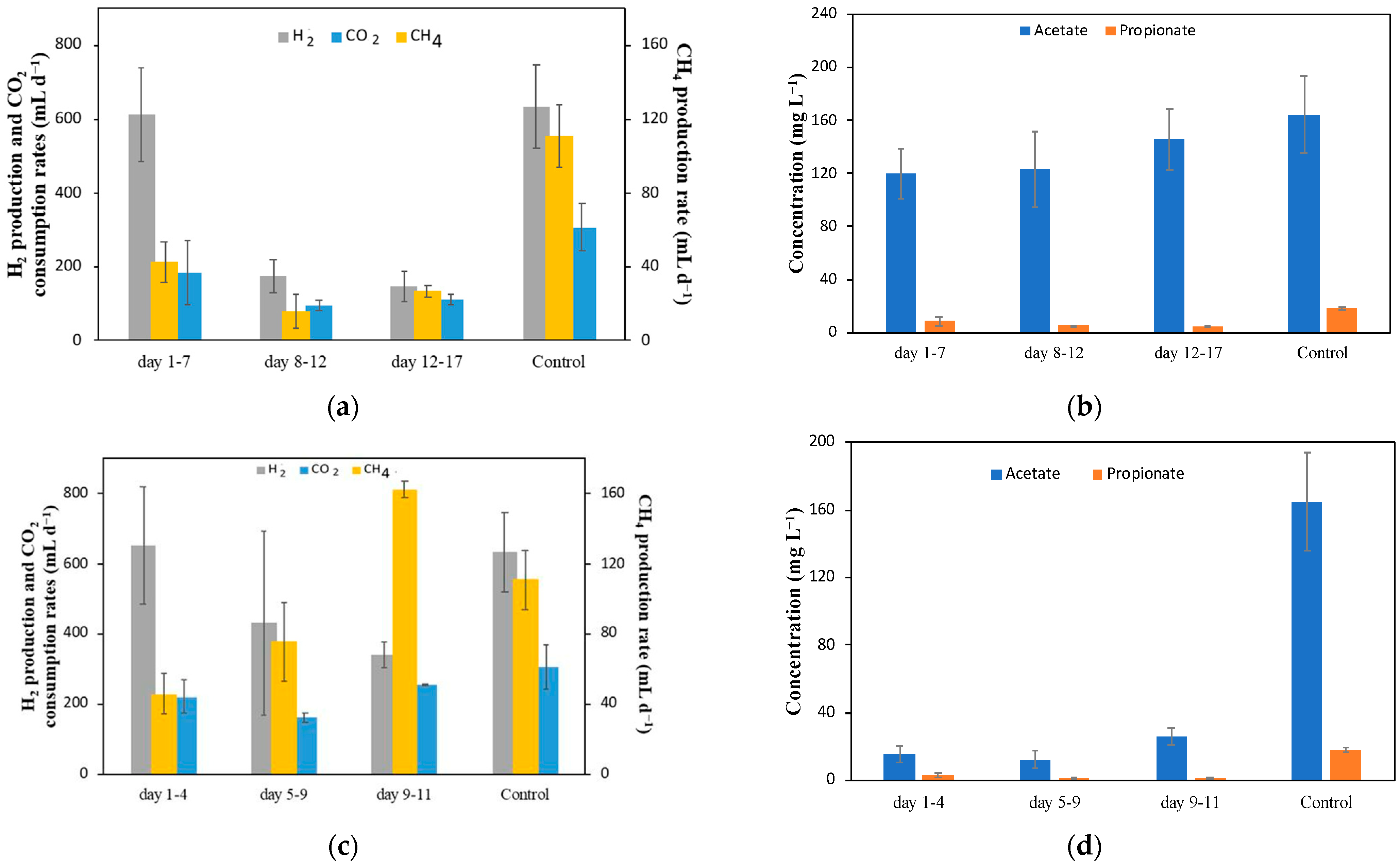
The next part of this research led to the investigation of whether the same two metals (Sn and Bi) would lead to a long-term amelioration of CH
4 production if they were electrochemically deposited on the surface of the carbon felt before inoculation. It is important to note that the control data that will be used as the basis for comparison in the experiments described further in the text, are the values collected before the injection of metal ions in the previous experiment performed under the same operating conditions (720 mL d
−1 CO
2 and 80 mA) (
Figure 2c); here, the CH
4 production averaged at 111 ± 17 mL d
−1 and that of acetate averaged at 165 ± 29 mg L
−1 (also shown in
Figure 3), corresponding to specific volumetric production rates of CH
4 and acetate of 555 ± 85 mL (L
c d)
−1 and 66 ± 12 mg (L
c d)
−1, respectively.
Figure 3 shows the results for the MESC with a Bi-modified carbon felt electrode. The Bi MESC was inoculated on day zero. The electrodeposition of Bi on the carbon felt electrode showed a negative effect in comparison to the control, with a lower level of CH
4 production as in the Bi injection test, albeit with some fluctuations, as seen in
Figure 4a. CH
4 production in the first few days was high but, eventually, this level lowered by about half (
Figure 3a). This could be attributed to the decrease in H
2 gas available for CH
4 production. This, in turn, could be due to the biofilm growth which decreases the available surface for the electrochemical formation of H
2, as discussed previously. However, it could also more likely be because other microbial species have developed within the biofilm, resulting in more competition for H
2 to be used for the formation of other products. In fact, Coulombic efficiency values in this experiment started at values near 100%; however, this decreased with time, reaching 28.5 ± 6% on average. Again, as with previous experiments, we can hypothesize the production of components that were not measured.
At steady-state, the CH
4 production rate eventually leveled to about 27 ± 3 mL d
−1, which is about 24% of the CH
4 production observed in the previous MESC tests prior to the Sn injections (
Figure 2c). The acetate concentration, on the other hand, was 145 ± 23 mg L
−1, at a level comparable to the control. This indicates that the electrodeposition of Bi adversely affected the conversion rate of CO
2 to CH
4, or this suggests that Bi
3+ enhanced the activity of acetogenic and methanogenic populations, while, on the other hand, Bi metal inhibits methanogenesis (contrary to the observation of the batch activity tests) and has a negligible impact on acetogenesis.
SEM/EDX results (shown later in the manuscript) confirmed the successful electrodeposition of Bi, although it was present at less than 0.05 wt%. The lack of improvement in long-term CH4 production may be in part due to the low amount of Bi deposited at the cathode surface.
The abiotic electrodeposition (pre-deposition) of Sn on the carbon felt cathode was carried out under the same conditions as that of Bi (current of 320 mA for 24 h).
Figure 3c,d show the gas flow rates and VFA concentrations, respectively, for the MESC experiment on a Sn-modified carbon felt electrode. After inoculation, a consistent increase in CH
4 production was observed. In fact, compared to the Bi-modified cathode test (
Figure 3a,b), the Sn-modified cathode yielded more CH
4 with time, reaching the highest value of 163 ± 5 mL d
−1 (815 mL (L
c d)
−1), as can be seen in
Figure 3c. Although this CH
4 production was lower (by 19%) than the maximum achieved in the Sn injection experiment (
Figure 2c day 16), the increased production in this MESC was stable over a few days, indicating improvement and stability over a longer period. Furthermore, this showed about a 47% improvement compared to the control experiments, which indicates that Sn, when electrodeposited on carbon felt prior to inoculation as a metal, improved CH
4 production.
Compared to CH
4, the H
2 production rate behaved in the inverse way. This is to be expected since CO
2 and H
2 are used for the production of CH
4 and acetate.
Figure 3d shows the average acetate concentration within the MESC. It is important to note that although there was no significant change with time, the acetate concentration was low, reaching a maximum value of 31 mg L
−1. It is therefore evident that the presence of metallic Sn on the carbon felt surface promotes CH
4 production by activating the pathway of acetoclastic methanogens (Equation (5)). This aligns with the results seen with the Sn salt injections (
Figure 2c).
Coulombic efficiency values were more consistent in this experiment between the beginning and end with an average value of 99.7 ± 26%. This indicates that in this experiment, almost all products (CH4 and acetate) were accounted for.
SEM/EDX results (shown in
Figure 4) confirmed the electrodeposition of Sn on the carbon felt surface. There was about 1.52 wt% of Sn deposited on the surface. Compared to the Bi-modified carbon felt electrode, the higher CH
4 production seen in
Figure 3c is attributed to the increase in metal presence, which can improve electron transfer. As described above, direct CH
4 production occurs by direct electron transfer between the cathode and electroactive microorganisms (Equation (1)). The presence of metallic Sn facilitates electron transfer as compared to the electron transfer when just carbon felt is used. As such, the production of CH
4 without intermediates is facilitated. This process requires less energy and thus improves the efficiency of CO
2 conversion.
Table 2 below summarizes the results obtained by the experiments employing Sn and Bi both as ions and metals on the cathode. The data show that metal salt injections into an MESC caused a temporary increase in CH
4 production. Sn and Bi ion injections seem to temporarily improve methanogenic activity, which is consistent to what was seen in the batch activity tests. In all experiments, Sn had more promising results than Bi with higher increases in CH
4 production, indicating that it is the superior catalyst to Bi for enhancing the bioelectrochemical conversion of CO
2. The Sn-modified carbon felt electrode especially gave a stable, high CH
4 production, reaching about 163 ± 5 mL d
−1 relative to the control. Although this was lower than the CH
4 production rate observed after the first injection of Sn ions in the MESC experiment (
Table 2), when compared to the average of the control values, this shows a clear increase in CH
4 production.
Interestingly, the results observed in the MESC experiments did not entirely reflect the results obtained in the activity tests. The activity tests showed that Sn reduced the methanogenic activity at a high concentration (0.5 g L−1), whereas the presence of Bi improved CH4 production both at low and high concentrations. In the MESC experiments, we observed a positive improvement in CH4 production when using Sn, both with injected ions and with Sn electrodeposition on the carbon felt cathode before inoculation, whereas Bi had an effect only with ion injections. This observation highlights the difference between the impact of transition metal ions on the microbial (methanogenic and acetogenic) activities and on the catalyst–biofilm interactions in an MESC. While metal ions can disrupt microbial metabolic activity, Sn electrodeposition improved H2 production, either through an improved HER or by improving the direct electron transfer to electroactive methanogens and acetogens.
2.5. Cathode Characterization
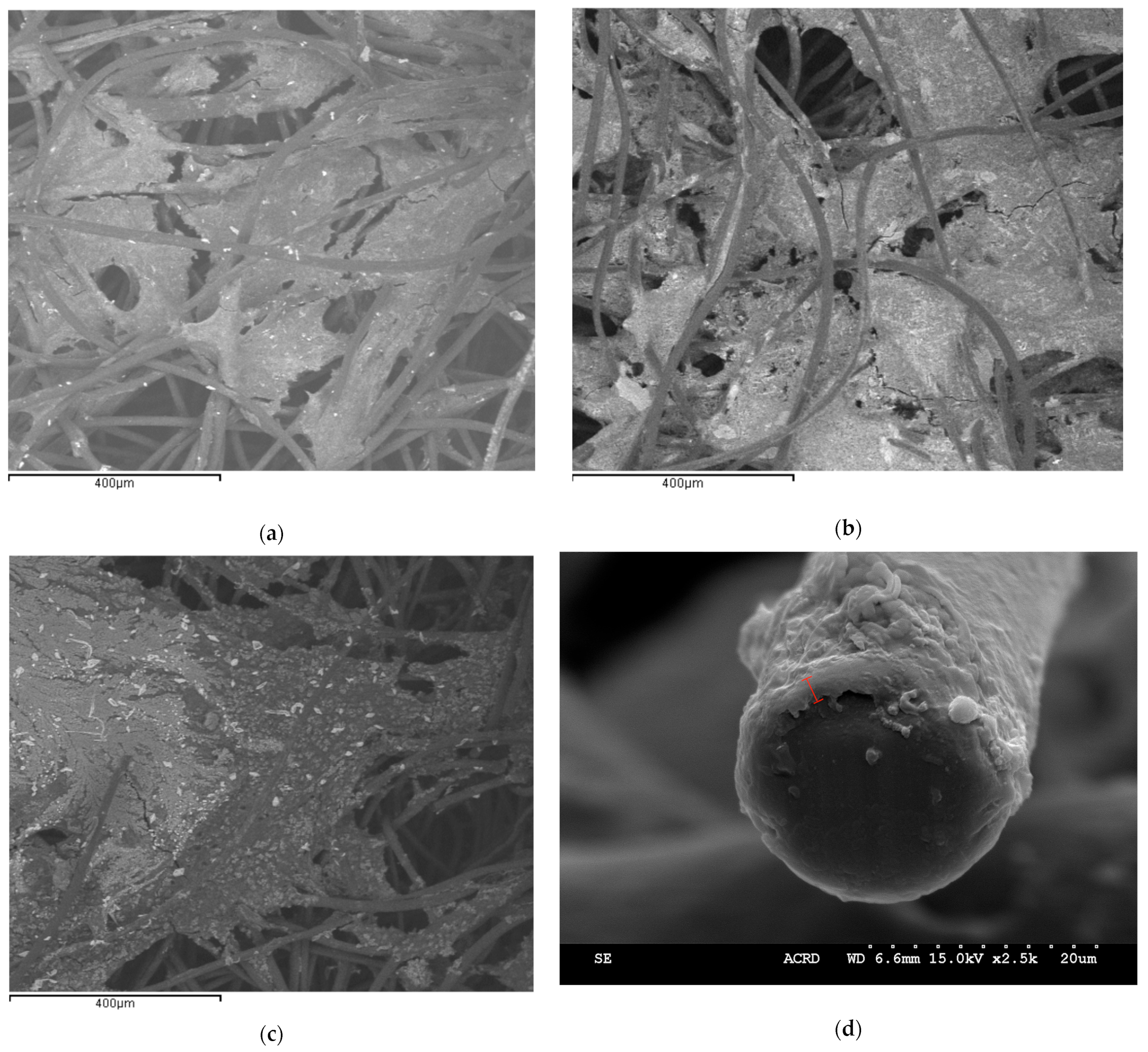
Following the end of each of these experiments, pieces of the cathode were sent for SEM imaging.
Figure 4 depicts sample SEM images showing the presence of a biofilm on the Sn- and Bi-modified carbon felt cathode. As can be seen in
Figure 4a–c, the biofilm blocked some of the pores of the carbon felt, thus limiting CO
2, carbonate, and proton transport into the 3D carbon felt cathode, potentially limiting the rate of CO
2 conversion. Similarly, the diffusion-limited transport of CO
2 conversion products and H
2 could be detrimental to the overall rate of CO
2 conversion, as the accumulation of products and high local pH inside the carbon felt limited bioelectrochemical reactions. This indicates that this could have been a contributing factor to the limited CO
2 conversion since blocked pores mean that CO
2/CO
32− does not have access to the microorganisms grown within the carbon felt pores.
Figure 4d shows a tip of the carbon felt strand of the Bi-modified experiment with grown biofilm. The biofilm thickness could be estimated to be about 24 μm.
The SEM/EDX results confirmed the presence of both Sn and Bi molecules albeit at very low amounts. In the Bi injection experiment, Bi was present at a maximum of 0.55 wt.%, whereas in the Sn injection experiment, there was a maximum of 0.47 wt.% of Sn detected. In the Bi predeposition experiment (Bi-modified carbon felt cathode) (
Figure 4c,d), there was less than 0.05 wt.% of Bi on the carbon felt surface, while in the Sn predeposition experiment (
Figure 4a,b), Sn was detected at 1.52 wt.%. It is important to note that of all the SEM images, for both injection and electrodeposition, Sn was more consistently measured, whereas in some Bi images, Bi was sometimes not detected on certain parts of the carbon felt surface.
Overall, Sn was present on the surface at a higher amount than Bi, which is to be expected since metallic Sn formation from its salt has a more negative standard electrode potential than that of metallic Bi formation from its salt. Furthermore, Sn salt is more soluble than the Bi salt used for the test. This was the case for both metal injection and predeposition experiments. Importantly, following each injection for metal ion injection experiments, the concentration of Bi and Sn ions declined over time due to the recirculation and washout nature of the MESC.