Polymorphism and Red Photoluminescence Emission from 5s2 Electron Pairs of Sb(III) in a New One-Dimensional Organic–Inorganic Hybrid Based on Methylhydrazine: MHy2SbI5
Abstract
1. Introduction
2. Results
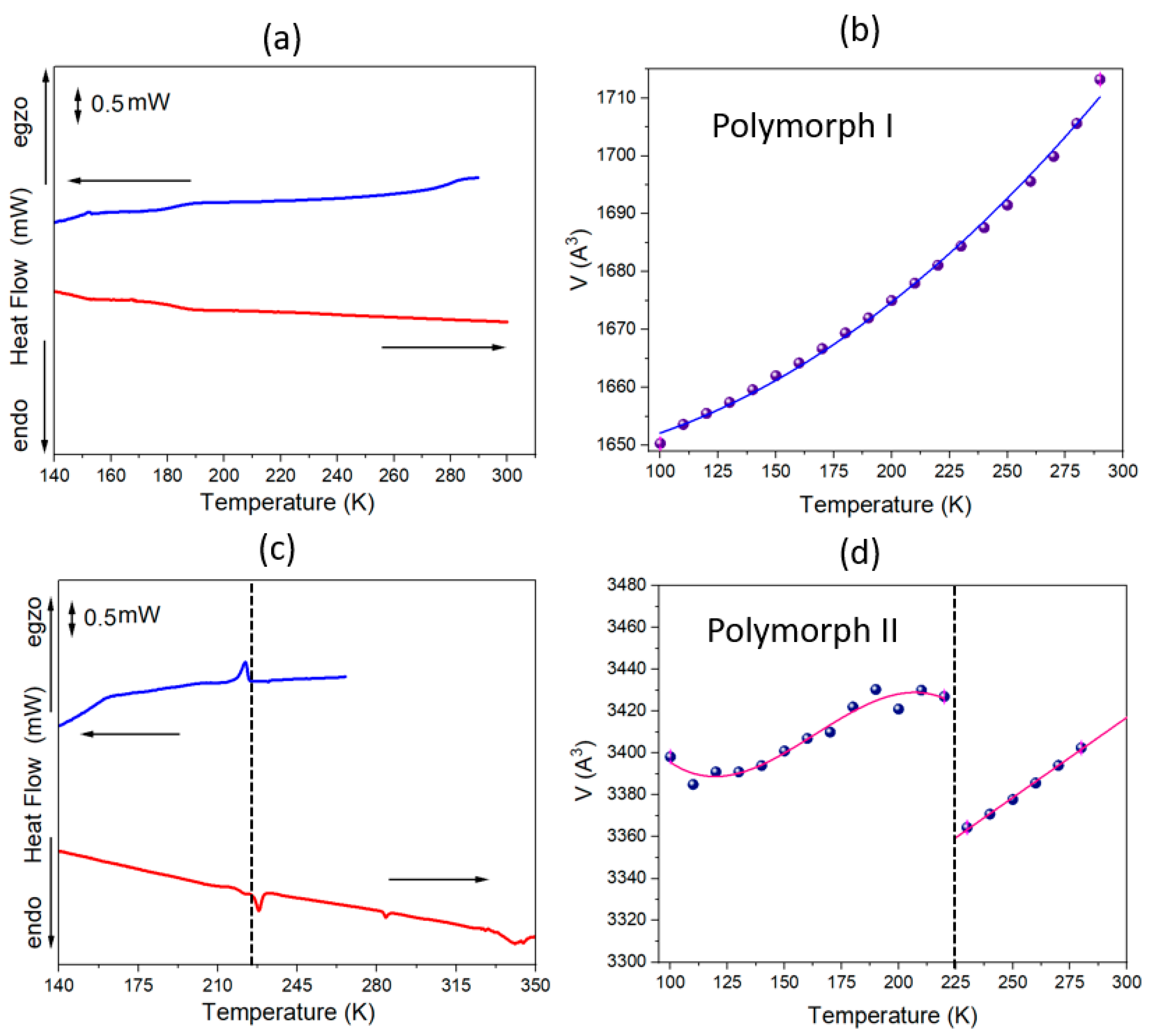
2.1. Thermal Properties of MHy2SbI5
2.2. Crystal Structure of MHy2SbI5
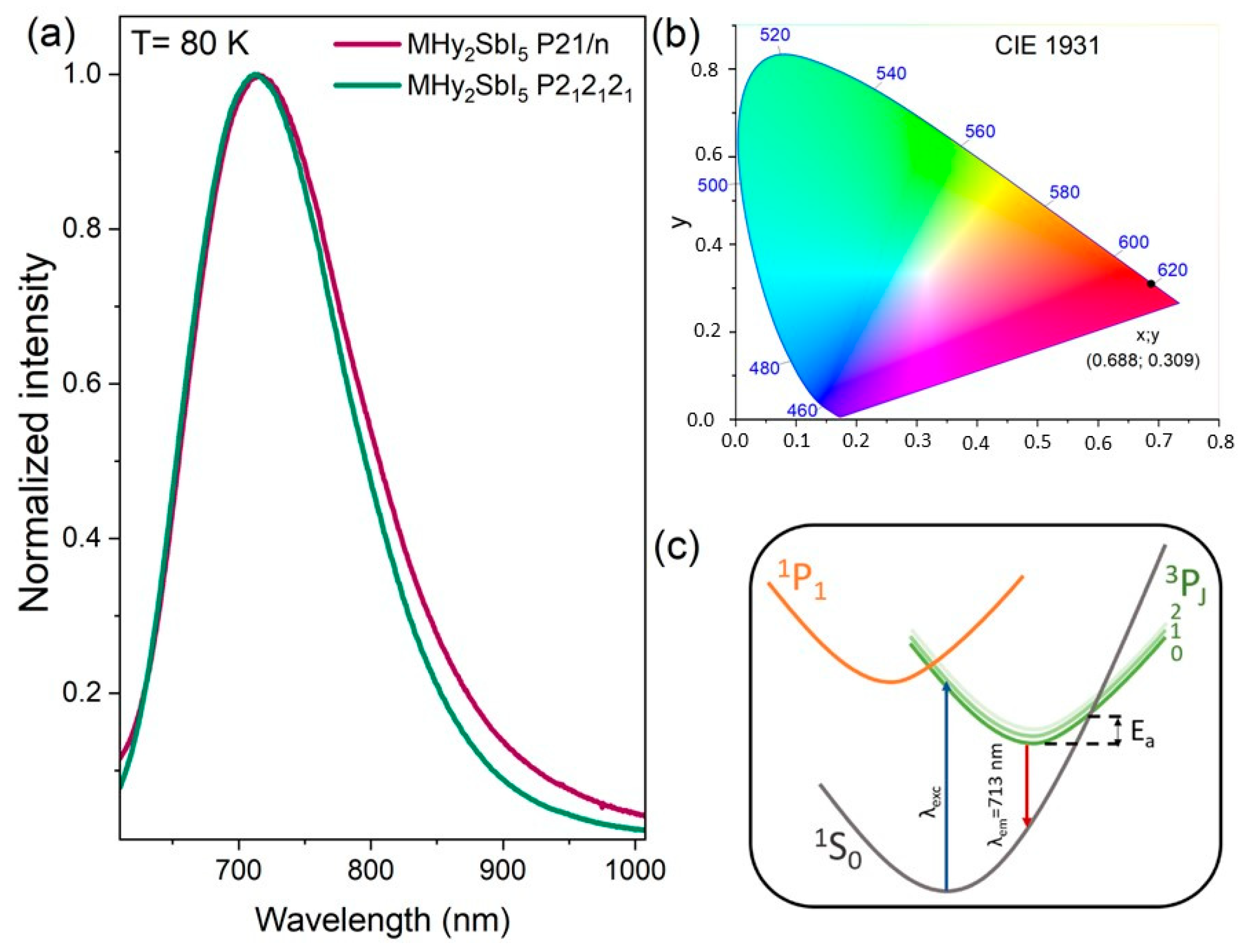
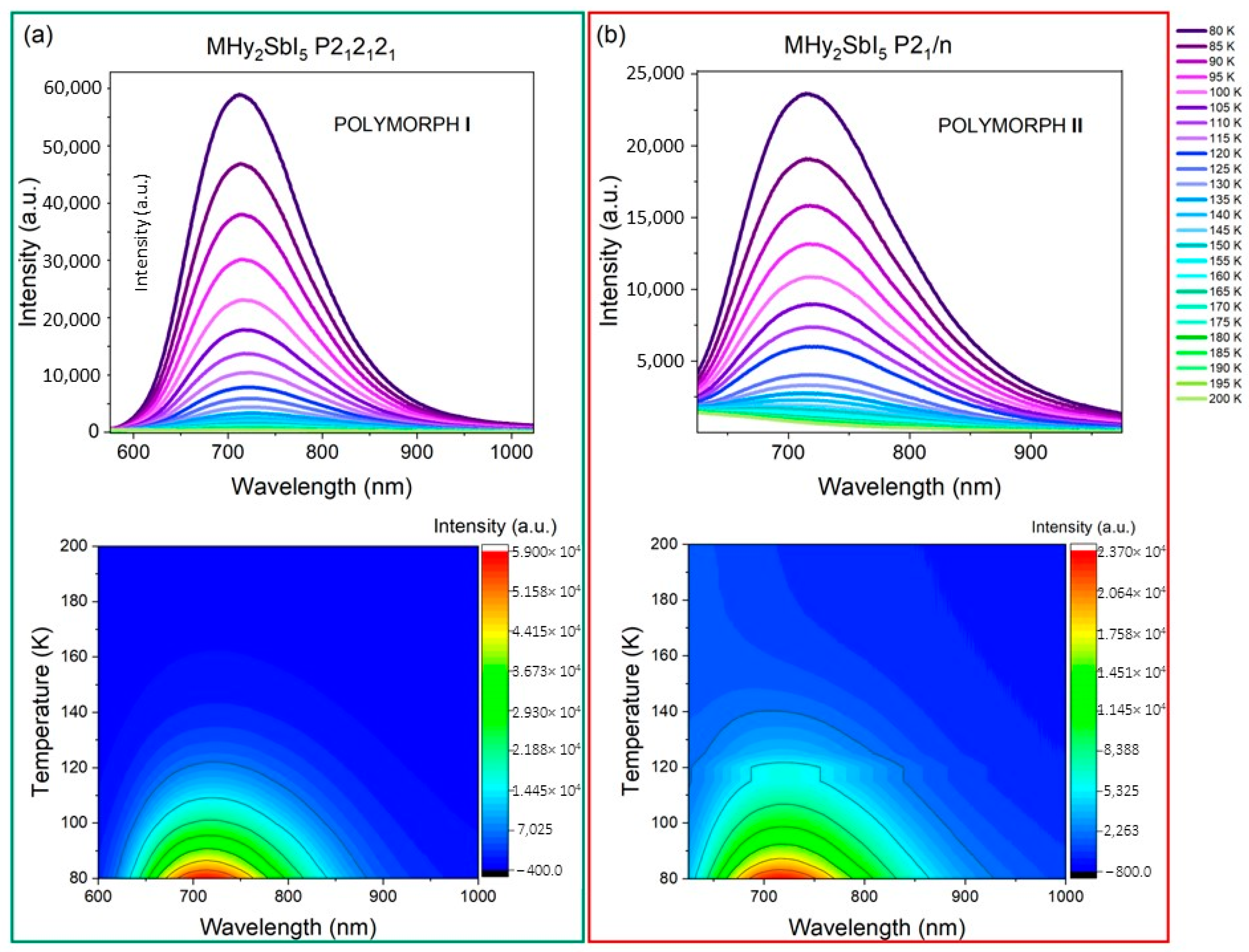
2.3. Luminescence Properties
3. Conclusions
4. Experimental Details
4.1. Synthesis
4.2. Single-Crystal X-Ray Diffraction
4.3. Thermal Analysis
4.4. Electrical Measurements
4.5. Optical Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient Planar Heterojunction Perovskite Solar Cells by Vapour Deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Gwan Park, T.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Su Moon, C.; Joong Jeon, N.; Correa-Baena, J.-P.; et al. Efficient Perovskite Solar Cells via Improved Carrier Management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-Halide Anion Engineering for α-FAPbI 3 Perovskite Solar Cells. Nature 2021, 592, 381. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Chen, M.; Zong, Y.; Huang, J.; Pang, S.; Padture, N.P. Doping and Alloying for Improved Perovskite Solar Cells. J. Mater. Chem. A 2016, 4, 17623. [Google Scholar] [CrossRef]
- Ono, L.K.; Juarez-Perez, E.J.; Qi, Y. Progress on Perovskite Materials and Solar Cells with Mixed Cations and Halide Anions. ACS Appl. Mater. Interfaces 2017, 9, 30197–30246. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Liao, Y.; Wei, Q.; Wang, Z.; Xiang, B.; Ke, Y.; Liu, W.; Ning, Z. Highly Stable Hybrid Perovskite Light-Emitting Diodes Based on Dion-Jacobson Structure. Sci. Adv. 2019, 5, 8072–8088. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, X.; Yin, W.; Wang, H.; Hu, Y.; Zhang, Q.; Shi, Z.; Colvin, V.L.; Yu, W.W.; Zhang, Y. Ruddlesden–Popper Perovskites: Synthesis and Optical Properties for Optoelectronic Applications. Adv. Sci. 2019, 6, 1900941. [Google Scholar] [CrossRef]
- Cortecchia, D.; Yin, J.; Petrozza, A.; Soci, C. White Light Emission in Low-Dimensional Perovskites. J. Mater. Chem. C 2019, 7, 4956. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cho, H.; Lee, T.W. Metal Halide Perovskite Light Emitters. Proc. Natl. Acad. Sci. USA 2016, 113, 11694–11702. [Google Scholar] [CrossRef]
- Wang, Y.; Song, L.; Chen, Y.; Huang, W. Emerging New-Generation Photodetectors Based on Low-Dimensional Halide Perovskites. ACS Photonics 2020, 7, 10–28. [Google Scholar] [CrossRef]
- Lun, M.-M.; Zhou, F.-L.; Fu, D.-W.; Ye, Q. Multi-Functional Hybrid Perovskites with Triple-Channel Switches and Optical Properties. J. Mater. Chem. C 2022, 10, 11371–11378. [Google Scholar] [CrossRef]
- Li, H.-J.; Liu, Y.-L.; Chen, X.-G.; Gao, J.-X.; Wang, Z.-X.; Liao, W.-Q. High-Temperature Dielectric Switching and Photoluminescence in a Corrugated Lead Bromide Layer Hybrid Perovskite Semiconductor. Inorg. Chem. 2019, 58, 10357–10363. [Google Scholar] [CrossRef]
- Chen, W.; Bhaumik, S.; Veldhuis, S.A.; Xing, G.; Xu, Q.; Grätzel, M.; Mhaisalkar, S.; Mathews, N.; Sum, T.C. Giant Five-Photon Absorption from Multidimensional Core-Shell Halide Perovskite Colloidal Nanocrystals. Nat. Commun. 2017, 8, 15198. [Google Scholar] [CrossRef] [PubMed]
- Mączka, M.; Ptak, M.; Gągor, A.; Stefańska, D.; Zarȩba, J.K.; Sieradzki, A. Methylhydrazinium Lead Bromide: Noncentrosymmetric Three-Dimensional Perovskite with Exceptionally Large Framework Distortion and Green Photoluminescence. Chem. Mater. 2020, 32, 1667–1673. [Google Scholar] [CrossRef]
- Mączka, M.; Gagor, A.; Zaręba, J.K.; Stefanska, D.; Drozd, M.; Balciunas, S.; Šimėnas, M.; Banys, J.; Sieradzki, A. Three-Dimensional Perovskite Methylhydrazinium Lead Chloride with Two Polar Phases and Unusual Second-Harmonic Generation Bistability above Room Temperature. Chem. Mater. 2020, 32, 4072–4082. [Google Scholar] [CrossRef]
- Mączka, M.; Zarȩba, J.K.; Gągor, A.; Stefańska, D.; Ptak, M.; Roleder, K.; Kajewski, D.; Soszyński, A.; Fedoruk, K.; Sieradzki, A. 2PbBr4, a Ferroelectric Hybrid Organic-Inorganic Perovskite with Multiple Nonlinear Optical Outputs. Chem. Mater. 2021, 33, 2331–2342. [Google Scholar] [CrossRef]
- Mączka, M.; Ptak, M.; Gągor, A.; Stefańska, D.; Sieradzki, A. Layered Lead Iodide of [Methylhydrazinium]2PbI4 with a Reduced Band Gap: Thermochromic Luminescence and Switchable Dielectric Properties Triggered by Structural Phase Transitions. Chem. Mater. 2019, 31, 8563–8575. [Google Scholar] [CrossRef]
- Li, R.; Wang, Z.; Zhu, T.; Ye, H.; Wu, J.; Liu, X.; Luo, J. Stereochemically Active Lone Pair Induced Polar Tri-Layered Perovskite for Record-Performance Polarized Photodetection. Angew. Chem. Int. Ed. 2023, 62, e202308445. [Google Scholar] [CrossRef]
- Shi, Z.; Guo, J.; Chen, Y.; Li, Q.; Pan, Y.; Zhang, H.; Xia, Y.; Huang, W.; Shi, Z.; Guo, J.; et al. Lead-Free Organic-Inorganic Hybrid Perovskites for Photovoltaic Applications: Recent Advances and Perspectives. Adv. Mater. 2017, 29, 1605005. [Google Scholar] [CrossRef]
- Chatterjee, S.; Pal, A.J. Tin(IV) Substitution in (CH3NH3)3Sb2I9: Toward Low-Band-Gap Defect-Ordered Hybrid Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 35194–35205. [Google Scholar] [CrossRef]
- Lu, L.; Pan, X.; Jonhua, L.; Sun, Z. Recent Advances and Optoelectronic ApplicationsofLead-Free HalideDoublePerovskites. Chem. Eur. J. 2020, 26, 16975–16984. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xia, Z.; Chen, D.; Dai, F.; Hao, S.; Zhou, G.; Liu, Q.; Wolverton, C. Materials for Optical, Magnetic and Electronic Devices Crystal Structure and Luminescence Properties of Lead-Free Metal Halides (C6H5CH2NH3)3MBr. J. Mater. Chem. C 2020, 8, 7322–7329. [Google Scholar] [CrossRef]
- Yang, W.; Chu, K.B.; Zhang, L.; Ding, X.; Sun, J.; Liu, J.Z.; Deng, J. Lead-free molecular ferroelectric [N,N-dimethylimidazole]3Bi2I9 with narrow bandgap. Mater. Des. 2020, 193, 108868. [Google Scholar] [CrossRef]
- Zhang, W.; Tao, K.; Ji, C.; Sun, Z.; Han, S.; Zhang, J.; Wu, Z.; Luo, J. (C6H13N)2BiI5: A One-Dimensional Lead-Free Perovskite-Derivative Photoconductive Light Absorber. Inorg. Chem. 2018, 57, 4239–4243. [Google Scholar] [CrossRef]
- Skorokhod, A.; Mercier, N.; Allain, M.; Manceau, M.; Katan, C.; Kepenekian, M. From Zero- to One-Dimensional, Opportunities and Caveats of Hybrid Iodobismuthates for Optoelectronic Applications. Inorg. Chem. 2021, 60, 17123–17131. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Leblanc, N.; Mercier, N.; Auban-Senzier, P.; Pasquier, C. Thermally Induced Bi(III) Lone Pair Stereoactivity: Ferroelectric Phase Transition and Semiconducting Properties of (MV)BiBr5(MV= Methylviologen). Chem. Mater. 2009, 21, 4099–4101. [Google Scholar] [CrossRef]
- Ju, D.; Jiang, X.; Xiao, H.; Chen, X.; Hua, X.; Tao, X. Narrow band gap and high mobility of lead-free perovskite single crystal Sn-doped MA3Sb2I9. J. Mater. Chem. A 2018, 6, 20753. [Google Scholar] [CrossRef]
- Zaleski, J.; Pietraszko, A. Structure at 200 and 298 K and X-ray Investigations of the Phase Transition at 242 K of [NH2(CH3)2]3Sb2Cl9 (DMACA). Acta Crystallogr. B 1996, 52, 287–295. [Google Scholar] [CrossRef]
- Matuszewski, J.; Sobczyk, L. Ferroelectricity and Phase Transitions in Tris (Dimethylammonium) Nonabromodiantimonate (III). Ferroelectrics 1987, 74, 339–345. [Google Scholar] [CrossRef]
- Louvain, N.; Mercier, N.; Boucher, F. α-to β-(Dmes)BiI5 (Dmes) Dimethyl(2-Ethylammonium)Sulfonium Dication): Umbrella Reversal of Sulfonium in the Solid State and Short I⋯I Interchain ContactssCrystal Structures, Optical Properties, and Theoretical Investigations of 1D Iodobismuthates. Inorg. Chem 2009, 48, 879–888. [Google Scholar] [CrossRef]
- Li, Y.; Yang, T.; Liu, X.; Han, S.; Wang, J.; Ma, Y.; Guo, W.; Luo, J.; Sun, Z. A Chiral Lead-Free Photoactive Hybrid Material with a Narrow Bandgap. Inorg. Chem. Front. 2020, 7, 2770. [Google Scholar] [CrossRef]
- Wenhua Bi, B.; Louvain, N.; Mercier, N.; Luc, J.; Rau, I.; Kajzar, F.; Sahraoui, B.; Mercier, N.; Bi, W.; Louvain, N.; et al. A Switchable NLO Organic-Inorganic Compound Based on Conformationally Chiral Disulfide Molecules and Bi(III)I5 Iodobismuthate Networks. Adv. Mater. 2008, 20, 1013–1017. [Google Scholar] [CrossRef]
- Tao, K.; Li, Y.; Ji, C.; Liu, X.; Wu, Z.; Han, S.; Sun, Z.; Luo, J. A Lead-Free Hybrid Iodide with Quantitative Response to X-ray Radiation. Chem. Mater. 2019, 31, 5927–5932. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Guo, C.-X.; Ni, C.-Y.; Ren, Z.-G.; Li, H.-X.; Lang, J.-P. Iodine-Induced Solvothermal Formation of Viologen Iodobismuthates. Eur. J. Inorg. Chem 2010, 33, 5326–5333. [Google Scholar] [CrossRef]
- Li, X.; Traoré, B.; Kepenekian, M.; Li, L.; Stoumpos, C.C.; Guo, P.; Even, J.; Katan, C.; Kanatzidis, M.G. Bismuth/Silver-Based Two-Dimensional Iodide Double and One-Dimensional Bi Perovskites: Interplay between Structural and Electronic Dimensions. Chem. Mater. 2021, 33, 6206–6216. [Google Scholar] [CrossRef]
- Gągor, A.; Banach, G.; Wȩcławik, M.; Piecha-Bisiorek, A.; Jakubas, R. The Lone-Pair-Electron-Driven Phase Transition and Order-Disorder Processes in Thermochromic (2-MIm)SbI4 Organic-Inorganic Hybrid. Dalton Trans. 2017, 46, 16605–16614. [Google Scholar] [CrossRef]
- Gagor, A.; Wecławik, M.; Bondzior, B.; Jakubas, R. Periodic and Incommensurately Modulated Phases in a (2-Methylimidazolium)Tetraiodobismuthate(III) Thermochromic Organic-Inorganic Hybrid. CrystEngComm 2015, 17, 3286–3296. [Google Scholar] [CrossRef]
- Mccall, K.M.; Morad, V.; Benin, B.M.; Kovalenko, M.V. Efficient Lone-Pair-Driven Luminescence: Structure−Property Relationships in Emissive 5s2 Metal Halides. ACS Mater. Lett. 2020, 2, 1218–1232. [Google Scholar] [CrossRef]
- Wang, Z.-P.; Wang, J.-Y.; Li, J.-R.; Feng, M.-L.; Zou, G.-D.; Huang, X.-Y. [Bmim]2SbCl5: A Main Group Metal-Containing Ionic Liquid Exhibiting Tunable Photoluminescence and White-Light Emission. Chem. Commun. 2015, 51, 3094–3097. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Liang, P.; Zhou, T.; Wang, L.; Xie, R.-J. Dual-Band Luminescent Lead-Free Antimony Chloride Halides with Near-Unity Photoluminescence Quantum Efficiency. Chem. Mater. 2019, 31, 9363–9371. [Google Scholar] [CrossRef]
- Zhou, C.; Worku, M.; Neu, J.; Lin, H.; Tian, Y.; Lee, S.; Zhou, Y.; Han, D.; Chen, S.; Hao, A.; et al. Facile Preparation of Light Emitting Organic Metal Halide Crystals with Near-Unity Quantum Efficiency. Chem. Mater. 2018, 30, 2374–2378. [Google Scholar] [CrossRef]
- Benin, B.M.; McCall, K.M.; Wörle, M.; Morad, V.; Aebli, M.; Yakunin, S.; Shynkarenko, Y.; Kovalenko, M.V. The Rb7Bi3−3xSb3xCl16 Family: A Fully Inorganic Solid Solution with Room-Temperature Luminescent Members. Angew. Chem. 2020, 132, 14598–14605. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Tao, L.; Shen, N.; Hu, B.; Gong, L.; Li, J.; Chen, X.; Huang, X.; Wang, Z.; et al. Hybrid Chloroantimonates(III): Thermally Induced Triple-Mode Reversible LuminescentS Witching and Laser-Printable Rewritable LuminescentP Aper. Angew. Chem. 2019, 131, 10079–10083. [Google Scholar] [CrossRef]
- Morad, V.; Shynkarenko, Y.; Yakunin, S.; Brumberg, A.; Schaller, R.D.; Kovalenko, M. V Disphenoidal Zero-Dimensional Lead, Tin, and Germanium Halides: Highly Emissive Singlet and Triplet Self-Trapped Excitons and X-ray Scintillation. J. Am. Chem. Soc. 2019, 141, 9764–9768. [Google Scholar] [CrossRef] [PubMed]
- Mccall, K.M.; Stoumpos, C.C.; Kostina, S.S.; Kanatzidis, M.G.; Wessels, B.W. Strong Electron−Phonon Coupling and Self-Trapped Excitons in the Defect Halide Perovskites A3M2I9 (A = Cs, Rb; M = Bi, Sb). Chem. Mater. 2017, 29, 4129–4145. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhou, C.; Tian, Y.; Shu, Y.; Messier, J.; Wang, J.C.; Van De Burgt, L.J.; Kountouriotis, K.; Xin, Y.; Holt, E.; et al. One-Dimensional Organic Lead Halide Perovskites with Efficient Bluish White-Light Emission. Nat. Commun. 2017, 8, 14051. [Google Scholar] [CrossRef]
- Liu, K.; Deng, C.; Li, C.; Zhang, X.; Cao, J.; Yao, J.; Zhao, J.; Jiang, X.; Lin, Z.; Liu, Q. Hybrid Metal-Halide Infrared Nonlinear Optical Crystals of (TMEDA)MI5 (M = Sb, Bi) with High Stability. Adv. Opt. Mater. 2021, 9, 24. [Google Scholar] [CrossRef]
- Li, P.-F.; Tang, Y.-Y.; Liao, W.-Q.; Ye, H.-Y.; Zhang, Y.; Fu, D.-W.; You, Y.-M.; Xiong, R.-G. A Semiconducting Molecular Ferroelectric with a Bandgap Much Lower than That of BiFeO3. NPG Asia Mater. 2016, 9, e342. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Wei, Z.; Li, P.F.; Tang, Y.-Y.; Liao, W.Q.; Cai, H.; Xiong, R.-G. Thin Films The Narrowest Band Gap Ever ObservedinMolecular Ferroelectrics: Hexane-1,6-Diammonium Pentaiodobismuth(III). Angew. Chem. Int. Ed. 2018, 57, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-X.; Hua, X.-N.; Chen, X.-G.; Mei, G.-Q.; Liao, W.-Q. [C6N2H18][SbI5]: A Lead-Free Hybrid Halide Semiconductor with Exceptional Dielectric Relaxation. Inorg. Chem. 2019, 58, 4343. [Google Scholar] [CrossRef]
- Skorokhod, A.; Hleli, F.; Hajlaoui, F.; Karoui, K.; Allain, M.; Zouari, N.; Mercier, N. Layered Arrangement of 1D Wavy Chains in the Lead-Free Hybrid Perovskite (PyrCO2H)2BiI5: Structural Investigations and Properties. Eur. J. Inorg. Chem. 2021, 2021, 1452–1458. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, P.; You, X.; Wei, Z. A Hybrid Organic-Inorganic Bismuth Iodine Material Showing High Phase Transition Point and Low Bandgap. Eur. J. Inorg. Chem. 2022, 18, e202200172. [Google Scholar] [CrossRef]
- Mao, C.-Y.; Liao, W.-Q.; Wang, Z.-X.; Li, P.-F.; Lv, X.-H.; Ye, H.-Y.; Zhang, Y. Structural Characterization, Phase Transition and Switchable Dielectric Behaviors in a New Zigzag Chain Organic–Inorganic Hybrid Compound: [C3H7NH3]2SbI5. Dalton Trans. 2016, 45, 5229–5233. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.M.F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar]
- Kubelka, P.; Munk, F. A Contribution to the Optics of Pigments. Z. Technol. Phys. 1931, 12, 593–599. [Google Scholar]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties And Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-Gap Energy Estimation from Diffuse Reflectance Measurements on Sol-Gel and Commercial TiO2: A Comparative Study. J. Sol. Sci. Technol. 2012, 16, 1–7. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV−Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Li, C.; Luo, C.; Zhou, W.; Zhang, R.; Zheng, D.; Han, P. Highly Efficient Emission in Lead-Free Inorganic Vacancy-Ordered Sb-Bi-Alloyed Halide Quadruple Perovskites. Chem. Phys. Lett. 2022, 795, 139536. [Google Scholar] [CrossRef]
- Mcclure, E.T.; Ball, M.R.; Windl, W.; Woodward, P.M. Cs2AgBiX6 (X = Br, Cl): New Visible Light Absorbing, Lead-Free Halide Perovskite Semiconductors. Chem. Mater. 2016, 28, 1348–1354. [Google Scholar] [CrossRef]
- Saparov, B.; Hong, F.; Sun, J.P.; Duan, H.S.; Meng, W.; Cameron, S.; Hill, I.G.; Yan, Y.; Mitzi, D.B. Thin-Film Preparation and Characterization of Cs3Sb2I9: A Lead-Free Layered Perovskite Semiconductor. Chem. Mater. 2015, 27, 5622–5632. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]

| Crystal Data | Polymorph I | Polymorph II | ||
|---|---|---|---|---|
| Chemical Formula | (CN2H7)2[SbI5] | |||
| Molecular weight (Formula mass/g·mol−1) Crystal system Space group | 836.31 | 836.31 | 836.31 | 836.31 |
| Orthorhombic | Orthorhombic | Monoclinic | Orthorhombic | |
| P212121 | P212121 | P21/n | P212121 | |
| Temperature (K) | 100 | 295 | 290 | 365 |
| a [Å] | 8.901 (3) | 8.835 (3) | 8.648 (3) | 8.737 (3) |
| b [Å] | 10.291 (4) | 10.550 (4) | 37.271 (9) | 10.689 (4) |
| c [Å] | 18.004 (5) | 18.395 (5) | 10.668 (4) | 18.754 (5) |
| V [Å3] | 1649.2 (10) | 1714.6 (10) | 3437.7 (19) | 1751.4 (10) |
| β [◦] | 91.32 (3) | |||
| Z | 4 | 4 | 8 | 4 |
| Data collection | ||||
| No. of measured, independent, observed [I > 2σ(I)] reflections | 12,731, 3129, 3018 | 15,315, 3244, 2913 | 57,907, 8809, 5784 | 15,653, 3325, 1711 |
| Rint | 0.027 | 0.025 | 0.035 | 0.057 |
| Refinement | ||||
| R[F2 > 2σ(F2)], | 0.032 | 0.028 | 0.035 | 0.048 |
| wR(F2), S | 0.079, 1.08 | 0.064, 1.05 | 0.087, 1.05 | 0.157, 1.02 |
| Δρmax, Δρmin (e Å−3) | 2.39, −1.52 | 0.82, −0.77 | 0.99, −0.86 | 0.92, −0.85 |
| Twin refinement | Refined as an inversion twin | |||
| Absolute structure parameter | 0.38 (14) | 0.43 (13) | 0.34 (12) | |
| Polymorph I | Polymorph II | |||
|---|---|---|---|---|
| Temperature | 100 K | 295 K | 290 K | 365 K |
| Average bond length (Å) | 3.024 | 3.039 | 3.041 | 3.050 |
| Polyhedral volume (Å3) | 36.81 | 37.39 | 37.42 | 37.75 |
| Distortion index (bond length) | 0.041 | 0.045 | 0.047 | 0.052 |
| Bond angle variance (deg.2) | 4.38 | 2.78 | 6.61 | 6.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rowińska, M.; Stefańska, D.; Bednarchuk, T.J.; Zaręba, J.K.; Jakubas, R.; Gągor, A. Polymorphism and Red Photoluminescence Emission from 5s2 Electron Pairs of Sb(III) in a New One-Dimensional Organic–Inorganic Hybrid Based on Methylhydrazine: MHy2SbI5. Molecules 2024, 29, 455. https://doi.org/10.3390/molecules29020455
Rowińska M, Stefańska D, Bednarchuk TJ, Zaręba JK, Jakubas R, Gągor A. Polymorphism and Red Photoluminescence Emission from 5s2 Electron Pairs of Sb(III) in a New One-Dimensional Organic–Inorganic Hybrid Based on Methylhydrazine: MHy2SbI5. Molecules. 2024; 29(2):455. https://doi.org/10.3390/molecules29020455
Chicago/Turabian StyleRowińska, Magdalena, Dagmara Stefańska, Tamara J. Bednarchuk, Jan K. Zaręba, Ryszard Jakubas, and Anna Gągor. 2024. "Polymorphism and Red Photoluminescence Emission from 5s2 Electron Pairs of Sb(III) in a New One-Dimensional Organic–Inorganic Hybrid Based on Methylhydrazine: MHy2SbI5" Molecules 29, no. 2: 455. https://doi.org/10.3390/molecules29020455
APA StyleRowińska, M., Stefańska, D., Bednarchuk, T. J., Zaręba, J. K., Jakubas, R., & Gągor, A. (2024). Polymorphism and Red Photoluminescence Emission from 5s2 Electron Pairs of Sb(III) in a New One-Dimensional Organic–Inorganic Hybrid Based on Methylhydrazine: MHy2SbI5. Molecules, 29(2), 455. https://doi.org/10.3390/molecules29020455







