Three-Dimensional Hydrogen-Bonded Porous Metal-Organic Framework for Natural Gas Separation with High Selectivity
Abstract
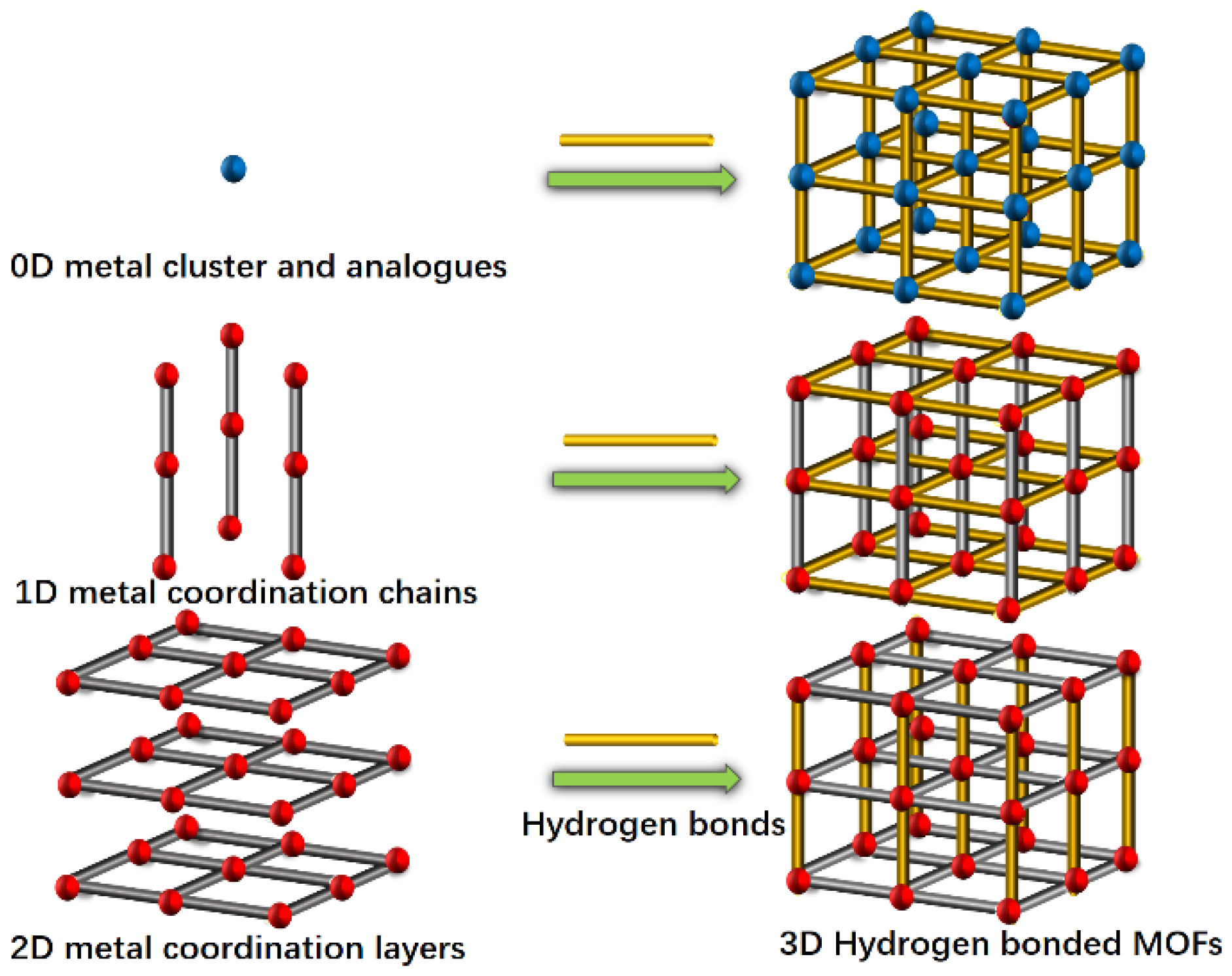
1. Introduction
2. Results and Discussion
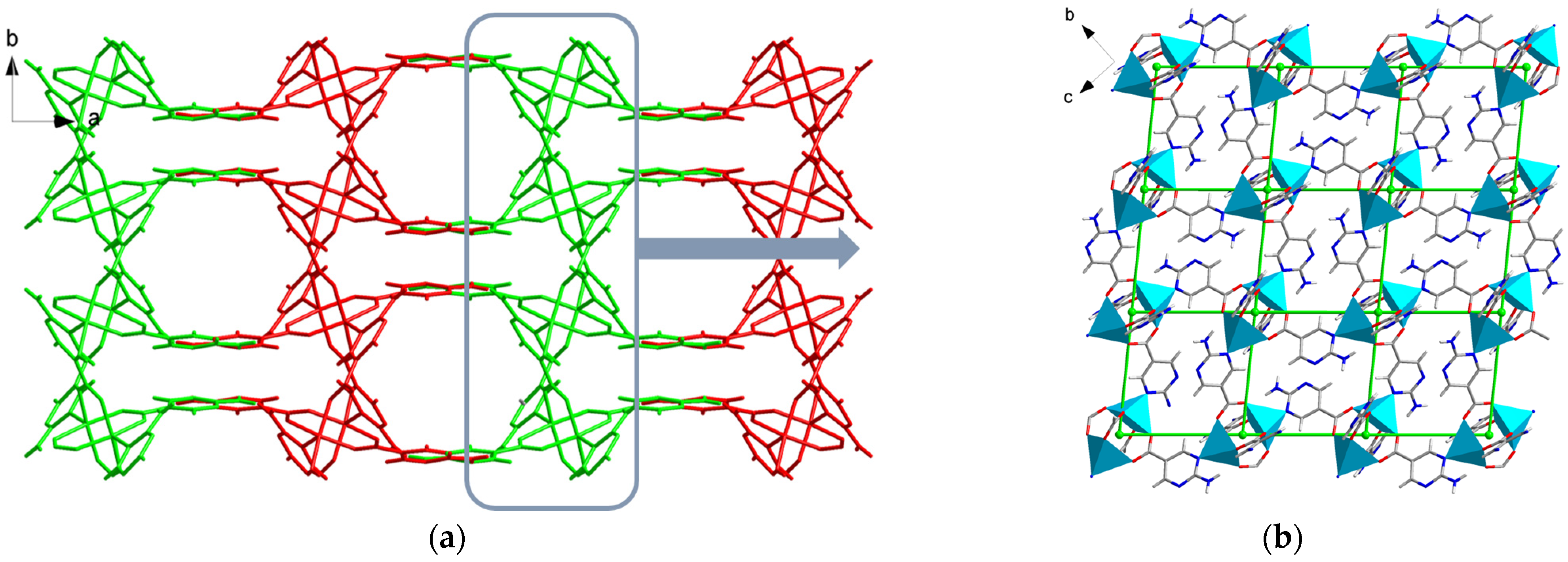
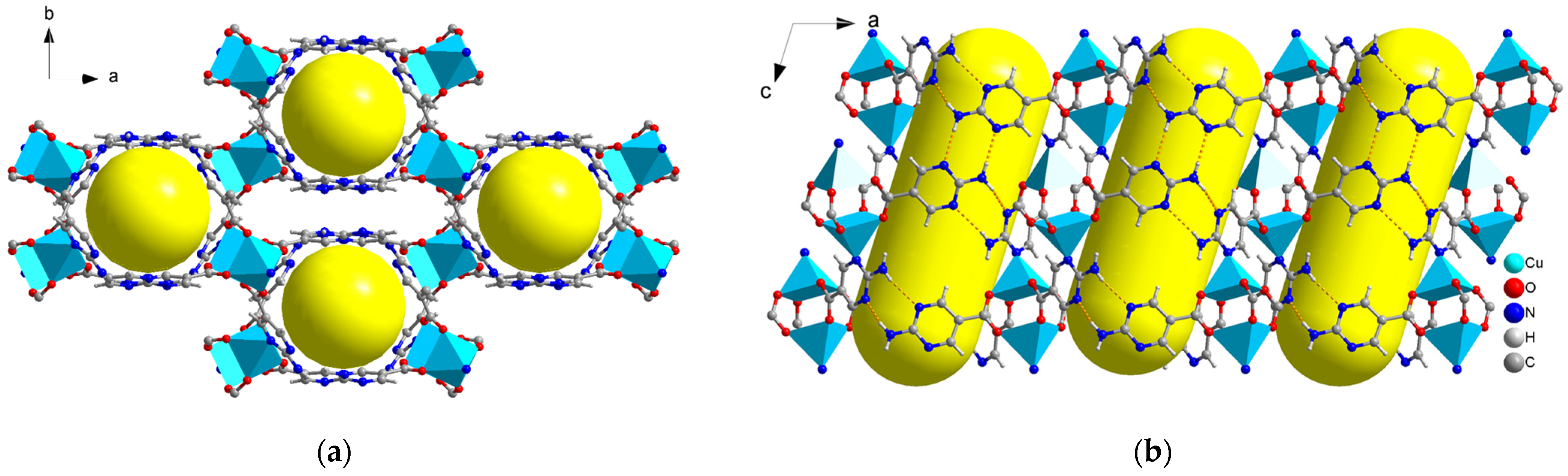
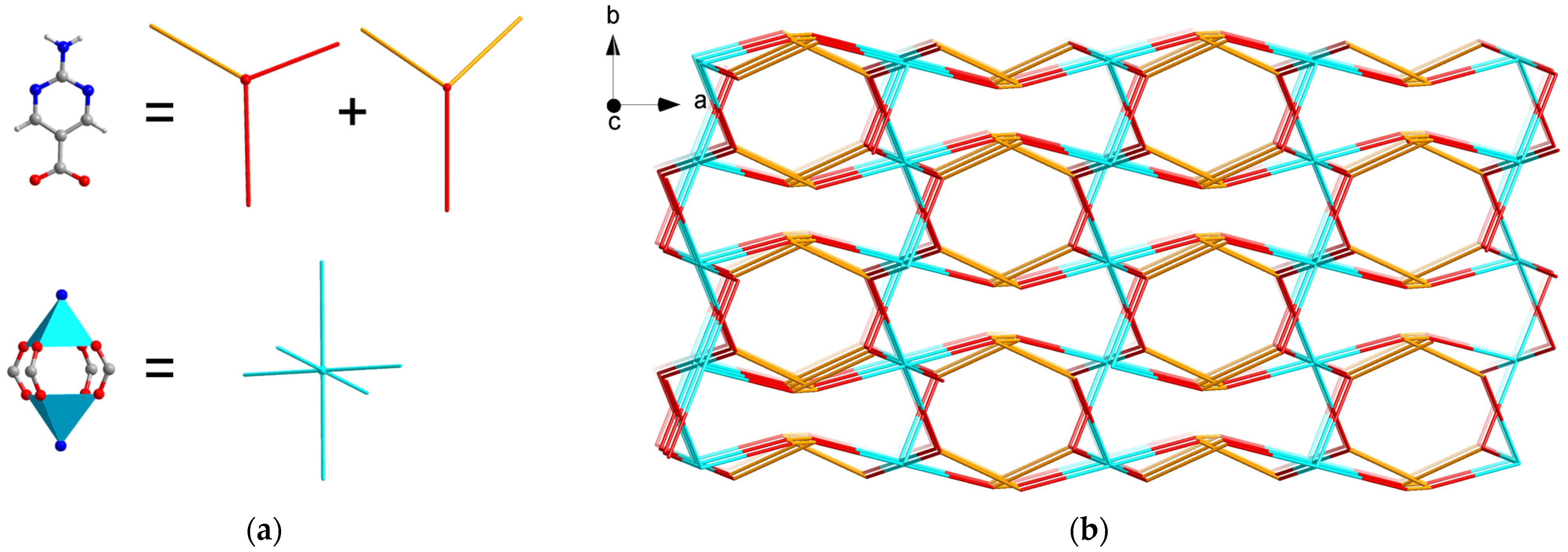
2.1. Structure Description
2.2. Characterization of TJU-Dan-5
2.3. Gas Adsorption of H2 and N2
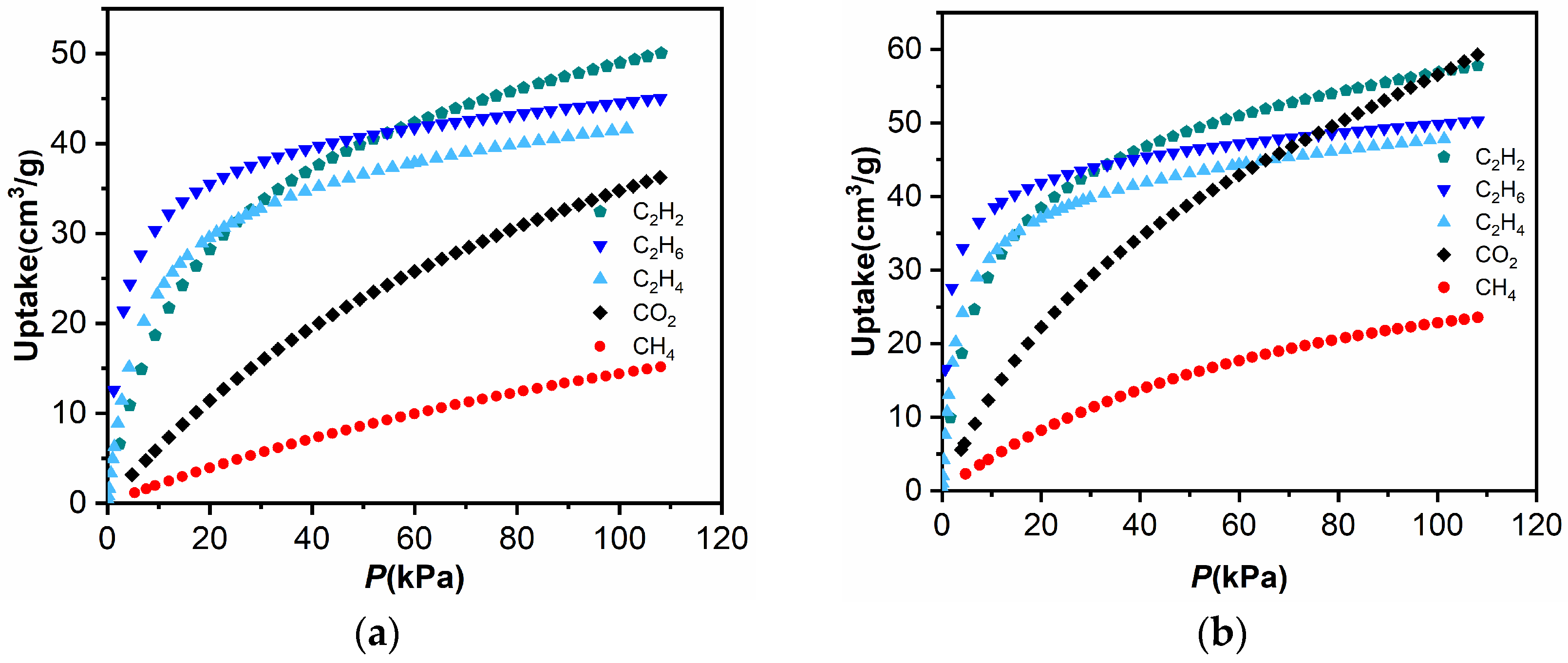
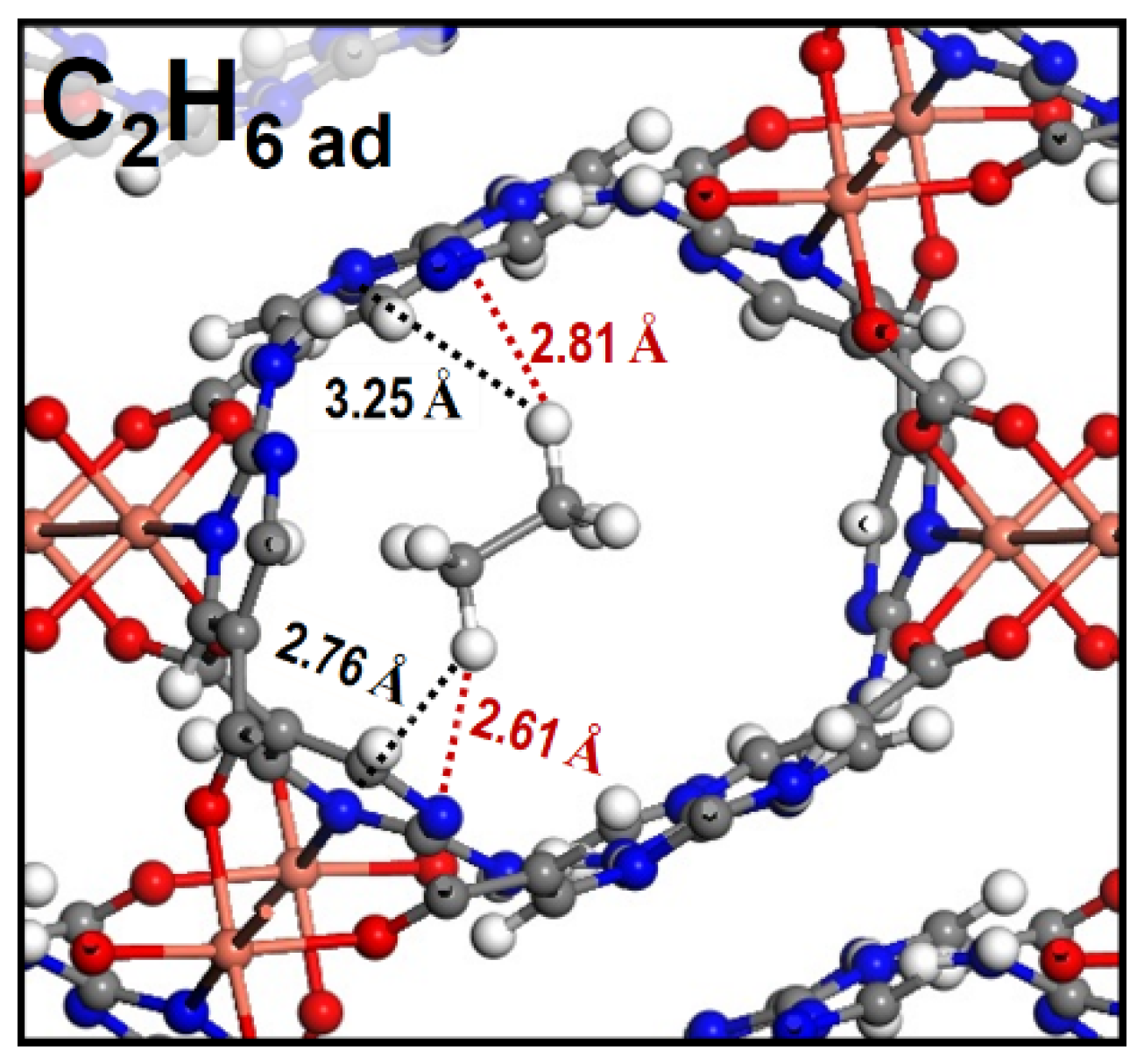
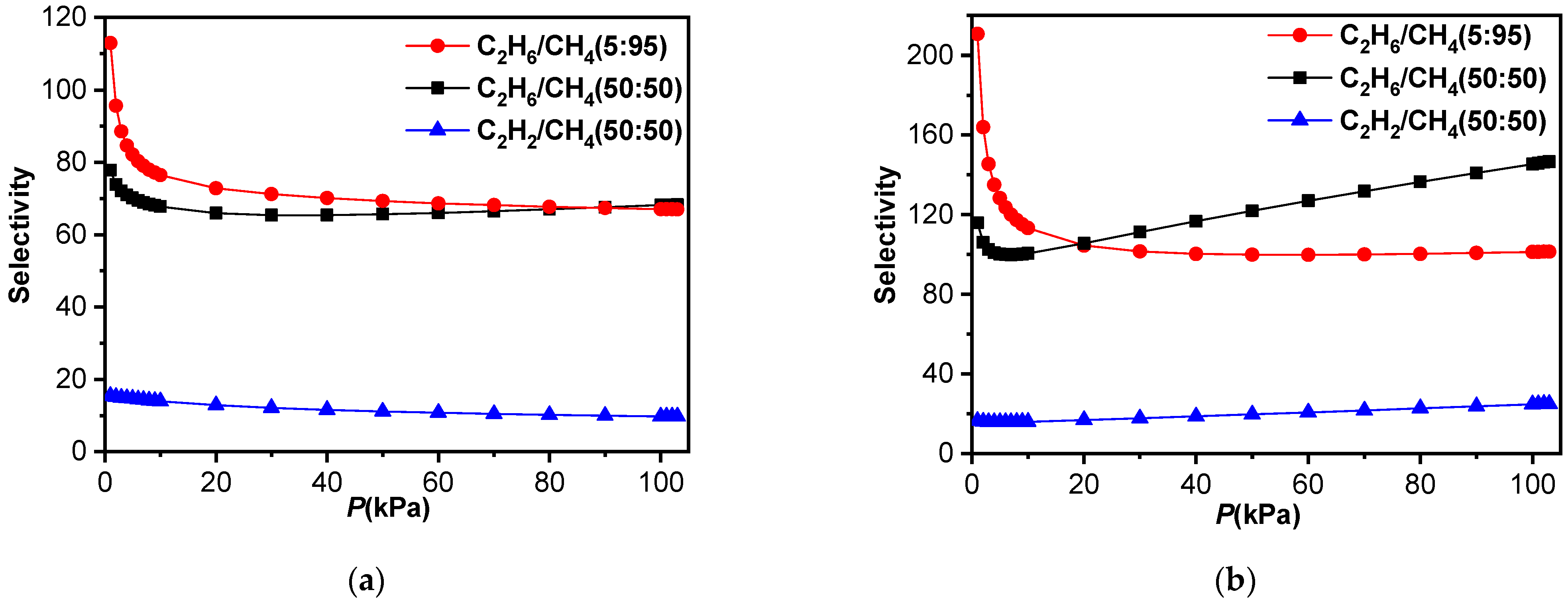
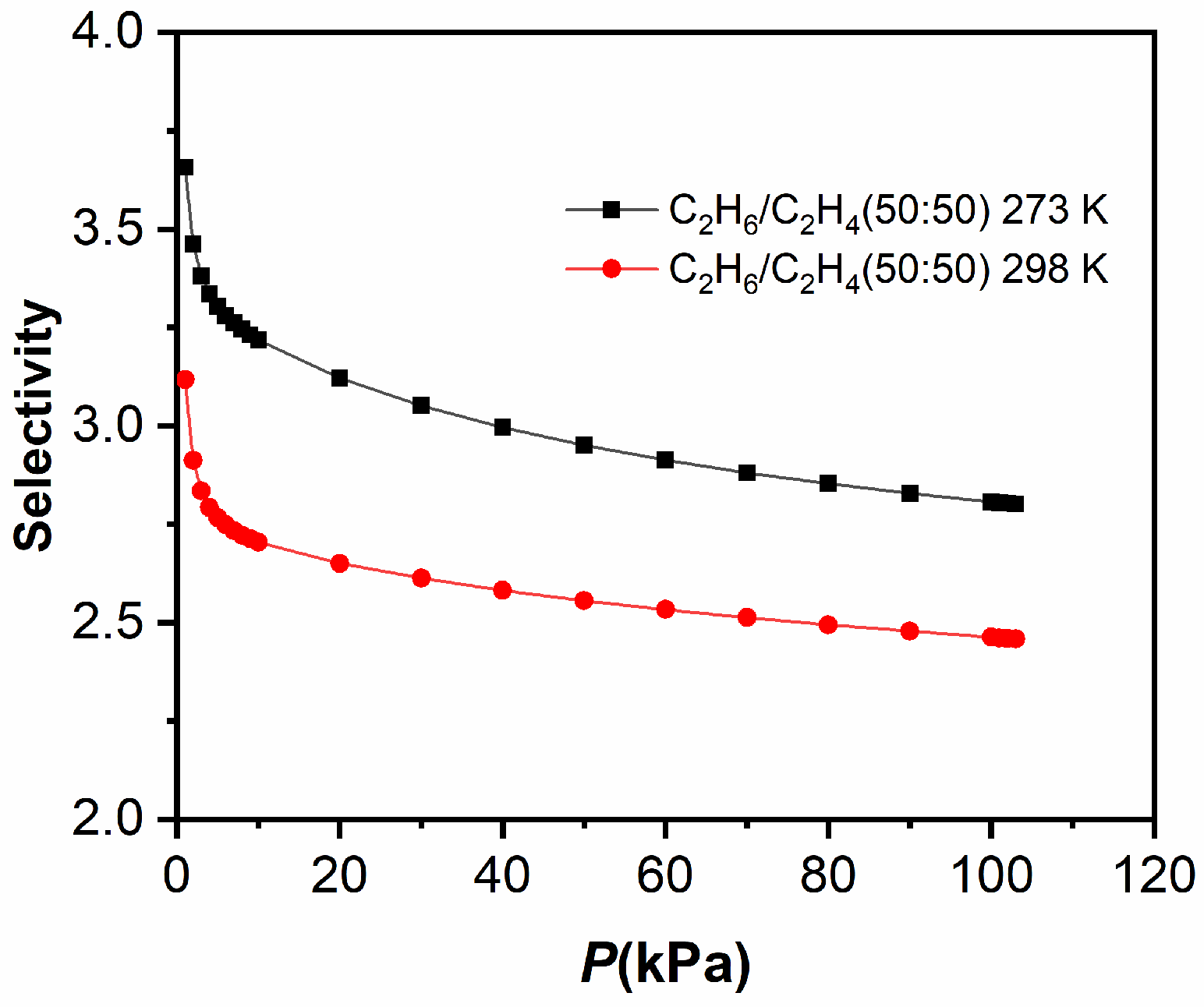
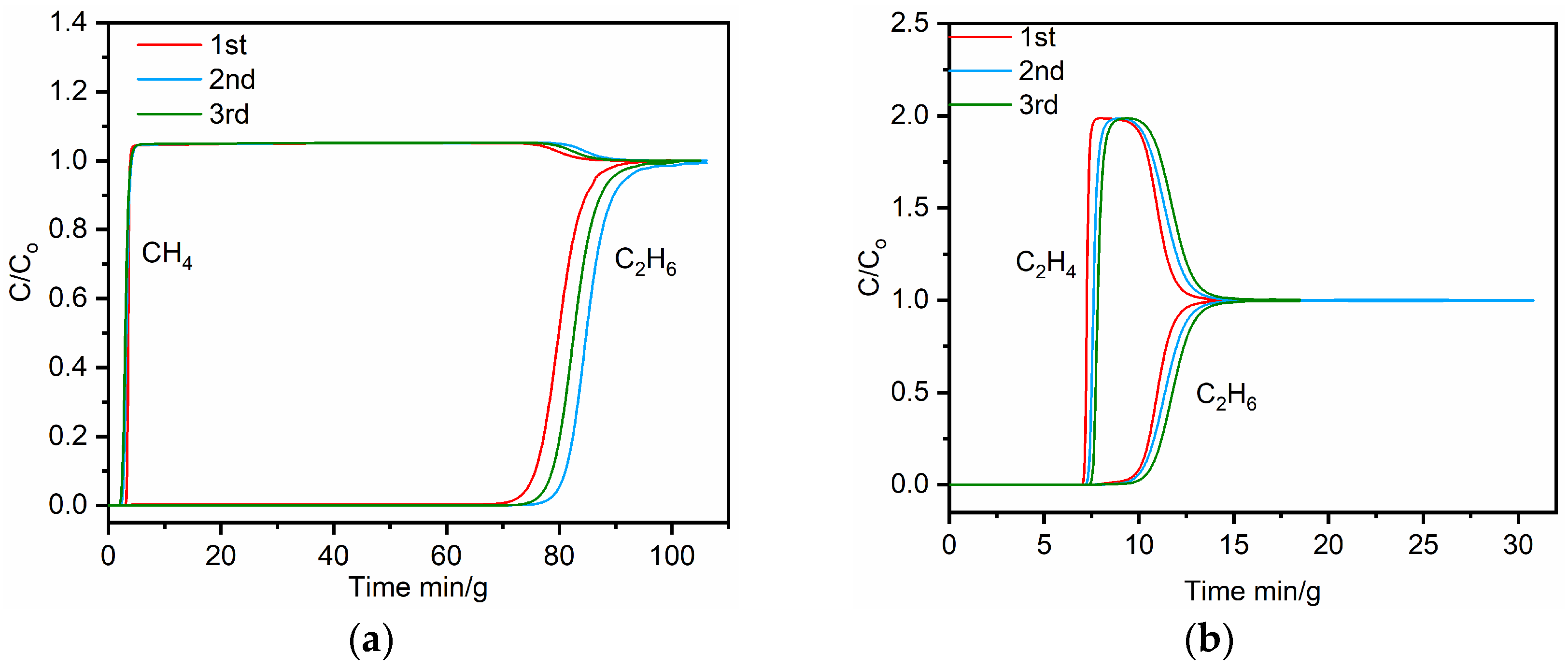
2.4. Separations of C2H6/CH4 and C2H6/C2H4
3. Materials and Methods
3.1. General Naterials and Methods
3.2. Synthesis of TJU-Dan-5
3.3. X-ray Crystallography
3.4. Gas Adsorption Measurements
3.5. Breakthrough Measurements
3.6. Density Functional Theory Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane storage in metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.Y. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef]
- Della Rocca, J.; Liu, D.; Lin, W. Nanoscale Metal-organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, W.; Li, W.; Liu, L.; Zhao, D.; Liu, X.; Hu, T.; Fan, L. Heterometallic ZnHoMOF as a dual-responsive luminescence sensor for efficient detection of hippuric acid biomarker and nitrofuran antibiotics. Molecules 2023, 28, 6274. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, W.; Liu, X.; Zhao, D.; Liu, L.; Yin, J.; Li, X.; Zhang, G.; Fan, L. Two chemorobust cobalt(II) organic frameworks as high sensitivity and selectivity sensors for efficient detection of 3-nitrotyrosine biomarker in serum. Cryst. Growth Des. 2023, 23, 7716–7724. [Google Scholar] [CrossRef]
- Xiao, X.; Zou, L.; Pang, H.; Xu, Q. Synthesis of micro/nanoscaled metal-organic frameworks and their direct electrochemical applications. Chem. Soc. Rev. 2020, 49, 301–331. [Google Scholar] [CrossRef]
- Kurmoo, M. Magnetic metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1353–1379. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S.-I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Lu, W.; Wei, Z.; Gu, Z.Y.; Liu, T.F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle Iii, T.; et al. Tuning the structure and function of metal–organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.T.; Wu, L.Z. Hydrogen Bonded Supramolecular Structures; Springer: Berlin/Heidelberg, Germany, 2015; p. 87. [Google Scholar]
- Brunet, P.; Simard, M.; Wuest, J.D. Molecular Tectonics. Porous hydrogen-bonded networks with unprecedented structural integrity. J. Am. Chem. Soc. 1997, 119, 2737–2738. [Google Scholar] [CrossRef]
- Lin, R.B.; He, Y.; Li, P.; Wang, H.; Zhou, W.; Chen, B. Multifunctional porous hydrogen-bonded organic framework materials. Chem. Soc. Rev. 2019, 48, 1362–1389. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Gipson, J.; Beobide, G.; Castillo, O.; Fröba, M.; Hoffmann, F.; Luque, A.; Pérez-Yáñez, S.; Román, P. Paddle-wheel shaped copper(II)-adenine discrete entities as supramolecular building blocks to afford porous supramolecular metal-organic frameworks (SMOFs). Cryst. Growth Des. 2014, 14, 4019–4029. [Google Scholar] [CrossRef]
- Thomas-Gipson, J.; Pérez-Aguirre, R.; Beobide, G.; Castillo, O.; Luque, A.; Pérez-Yáñez, S.; Román, P. Unravelling the growth of supramolecular metal-organic frameworks based on metal-nucleobase entities. Cryst. Growth Des. 2015, 15, 975–983. [Google Scholar] [CrossRef]
- Beobide, G.; Castillo, O.; Cepeda, J.; Luque, A.; Pérez-Yáñez, S.; Román, P.; Thomas-Gipson, J. Metal-carboxylato-nucleobase systems: From supramolecular assemblies to 3D porous materials. Coord. Chem. Rev. 2013, 257, 2716–2736. [Google Scholar] [CrossRef]
- An, J.; Fiorella, R.P.; Geib, S.J.; Rosi, N.L. Synthesis, structure, assembly, and modulation of the CO2 adsorption properties of a zinc-adeninate macrocycle. J. Am. Chem. Soc. 2009, 131, 8401–8403. [Google Scholar] [CrossRef]
- Nugent, P.S.; Rhodus, V.L.; Pham, T.; Forrest, K.; Wojtas, L.; Space, B.; Zaworotko, M.J. A robust molecular porous material with high CO2 uptake and selectivity. J. Am. Chem. Soc. 2013, 135, 10950–10953. [Google Scholar] [CrossRef]
- Sava, D.F.; Kravtsov, V.C.; Eckert, J.; Eubank, J.F.; Nouar, F.; Eddaoudi, M. Exceptional stability and high hydrogen uptake in hydrogen-bonded metal-organic cubes possessing ACO and AST zeolite-like topologies. J. Am. Chem. Soc. 2009, 131, 10394–10396. [Google Scholar] [CrossRef]
- Alkordi, M.H.; Brant, J.A.; Wojtas, L.; Kravtsov, V.C.; Cairns, A.J.; Eddaoudi, M. Zeolite-like metal-organic frameworks (ZMOFs) based on the directed assembly of finite metal-organic cubes (MOCs). J. Am. Chem. Soc. 2009, 131, 17753–17755. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, T.; Li, G.; Wojtas, L.; Huo, Q.; Eddaoudi, M.; Liu, Y. From metal-organic squares to porous zeolite-like supramolecular assemblies. J. Am. Chem. Soc. 2010, 132, 18038–18041. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kan, L.; Li, J.; Liu, Y.; Eddaoudi, M. Quest for zeolite-like supramolecular assemblies: Self-assembly of metal-organic squares via directed hydrogen bonding. Angew. Chem. Int. Ed. 2020, 59, 19659–19662. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Yáñez, S.; Beobide, G.; Castillo, O.; Cepeda, J.; Luque, A.; Román, P. Structural diversity in a copper(II)/isophthalato/9-methyladenine system. From one- to three-dimensional metal-biomolecule frameworks. Cryst. Growth Des. 2013, 13, 3057–3067. [Google Scholar] [CrossRef]
- Duan, H.; Dan, W.Y.; Fang, X.D. Zinc-coordinated MOFs complexes regulated by hydrogen bonds: Synthesis, structure and luminescence study toward broadband white-light emission. J. Solid State Chem. 2018, 260, 159–164. [Google Scholar] [CrossRef]
- Manna, B.; Mukherjee, S.; Desai, A.V.; Sharma, S.; Krishna, R.; Ghosh, S.K. A π-electron deficient diaminotriazine functionalized MOF for selective sorption of benzene over cyclohexane. Chem. Commun. 2015, 51, 15386–15389. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, B.; Yu, X.; Zou, X.; Liu, X.; Zhang, L.; Liu, Y. A stable Y(III)-based amide-functionalized metal-organic framework for propane/methane separation and knoevenagel condensation. Inorg. Chem. 2022, 61, 3708–3715. [Google Scholar] [CrossRef]
- Liao, P.Q.; Zhang, W.X.; Zhang, J.P.; Chen, X.M. Efficient purification of ethene by an ethane-trapping metal-organic framework. Nat. Commun. 2015, 6, 8697. [Google Scholar] [CrossRef]
- Liao, P.Q.; Huang, N.Y.; Zhang, W.X.; Zhang, J.P.; Chen, X.M. Controlling guest conformation for efficient purification of butadiene. Science 2017, 356, 1193–1196. [Google Scholar] [CrossRef]
- Bao, Z.; Xie, D.; Chang, G.; Wu, H.; Li, L.; Zhou, W.; Wang, H.; Zhang, Z.; Xing, H.; Yang, Q.; et al. Fine Tuning and specific binding sites with a porous hydrogen-bonded metal-complex framework for gas selective separations. J. Am. Chem. Soc. 2018, 140, 4596–4603. [Google Scholar] [CrossRef]
- Li, J.; Dai, C.; Cao, Y.; Sun, X.; Li, G.; Huo, Q.; Liu, Y. Lewis basic site (LBS)-functionalized zeolite-like supramolecular assemblies (ZSAs) with high CO2 uptake performance and highly selective CO2/CH4 separation. J. Mater. Chem. A 2017, 5, 21429–21434. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, X.; Kang, Z.; Liu, X.; Sun, D. Isoreticular chemistry within metal-organic frameworks for gas storage and separation. Coord. Chem. Rev. 2021, 443, 213968. [Google Scholar] [CrossRef]
- ZareKarizi, F.; Joharian, M.; Morsali, A. Pillar-layered MOFs: Functionality, interpenetration, flexibility and applications. J. Mater. Chem. A 2018, 6, 19288–19329. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Kyprianidou, E.J.; Papaefstathiou, G.S.; Manos, M.J.; Tasiopoulos, A.J. A flexible Cd2+ metal organic framework with a unique (3,3,6)-connected topology, unprecedented secondary building units and single crystal to single crystal solvent exchange properties. CrystEngComm 2012, 14, 8368–8373. [Google Scholar] [CrossRef]
- Sun, L.; Xing, H.; Xu, J.; Liang, Z.; Yu, J.; Xu, R. A novel (3,3,6)-connected luminescent metal–organic framework for sensing of nitroaromatic explosives. Dalton Trans. 2013, 42, 5508–5513. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.W. Hydrogen storage in metal-organic frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Liang, J.; Huang, Y.; Li, H.; Lin, Z.; Cao, R. Water-stable anionic metal-organic framework for highly selective separation of methane from natural gas and pyrolysis gas. ACS Appl. Mater. Interfaces 2016, 8, 9777–9781. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Z.; Xiang, S.; Fronczek, F.R.; Krishna, R.; Chen, B. A microporous metal-organic framework for highly selective separation of acetylene, ethylene, and ethane from methane at room temperature. Chem. Eur. J. 2012, 18, 613–619. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, Q.; Ding, M.; Gao, Y.; Xue, D.; Bai, J. Modifying a partial corn-sql layer-based (3,3,3,3,4,4)-c topological MOF by substitution of OH− with Cl− and its highly selective adsorption of C2 hydrocarbons over CH4. Dalton Trans. 2021, 50, 4840–4847. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.V.; Le, H.T.N.; Ha, H.Q.; Duong, X.N.T.; Nguyen, L.H.T.; Doan, T.L.H.; Nguyen, H.L.; Truong, T. A five coordination Cu(II) cluster-based MOF and its application in the synthesis of pharmaceuticals via sp3 C–H/N–H oxidative coupling. Catal. Sci. Technol. 2017, 7, 3453–3458. [Google Scholar] [CrossRef]
- Plonka, A.M.; Chen, X.; Wang, H.; Krishna, R.; Dong, X.; Banerjee, D.; Woerner, W.R.; Han, Y.; Li, J.; Parise, J.B. Light hydrocarbon adsorption mechanisms in two calcium-based microporous metal organic frameworks. Chem. Mat. 2016, 28, 1636–1646. [Google Scholar] [CrossRef]
- Yoon, T.-U.; Baek, S.B.; Kim, D.; Kim, E.-J.; Lee, W.-G.; Singh, B.K.; Lah, M.S.; Bae, Y.-S.; Kim, K.S. Efficient separation of C2 hydrocarbons in a permanently porous hydrogen-bonded organic framework. Chem. Commun. 2018, 54, 9360–9363. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yan, L.; Niu, W.; Feng, Y.; Fu, Q.; Zhang, S.; Zhang, Y.; Li, L.; Gu, X.; Dai, P.; et al. Adsorption in reversed order of C2 hydrocarbons on an ultramicroporous fluorinated metal-organic framework. Angew. Chem. Int. Ed. 2022, 61, e202204046. [Google Scholar]
- Lin, R.-B.; Wu, H.; Li, L.; Tang, X.-L.; Li, Z.; Gao, J.; Cui, H.; Zhou, W.; Chen, B. Boosting ethane/ethylene separation within isoreticular ultramicroporous metal-organic frameworks. J. Am. Chem. Soc. 2018, 140, 12940–12946. [Google Scholar] [CrossRef]
- Li, L.; Lin, R.-B.; Krishna, R.; Li, H.; Xiang, S.; Wu, H.; Li, J.; Zhou, W.; Chen, B. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites. Science 2018, 362, 443–446. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Z.; Xiang, S.; Fronczek, F.R.; Krishna, R.; Chen, B. A robust doubly interpenetrated metal-organic framework constructed from a novel aromatic tricarboxylate for highly selective separation of small hydrocarbons. Chem. Commun. 2012, 48, 6493–6495. [Google Scholar] [CrossRef]
- Duan, J.; Yan, R.; Qin, L.; Wang, Y.; Wen, L.; Cheng, S.; Xu, H.; Feng, P. Highly selective gaseous and liquid-phase separation over a novel cobalt(II) metal-organic framework. ACS Appl. Mater. Interfaces 2018, 10, 23009–23017. [Google Scholar] [CrossRef]
- Liu, K.; Ma, D.; Li, B.; Li, Y.; Yao, K.; Zhang, Z.; Han, Y.; Shi, Z. High storage capacity and separation selectivity for C2 hydrocarbons over methane in the metal-organic framework Cu–TDPAT. J. Mater. Chem. A 2014, 2, 15823–15828. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, X.; Yue, L.; Fan, L.; He, Y. A microporous MOF constructed by cross-linking helical chains for efficient purification of natural gas and ethylene. Inorg. Chem. 2021, 60, 14969–14977. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Lü, J.; Li, H.-F.; Liu, T.-F.; Cao, R. Robust Microporous Porphyrin-Based Hydrogen-Bonded Organic Framework for Highly Selective Separation of C2 Hydrocarbons versus Methane. Cryst. Growth Des. 2019, 19, 4157–4161. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.-L.; Lv, J.; Ma, L.; Lin, R.-B.; Cui, H.; Zhang, J.; Zhang, Z.; Xiang, S.; Chen, B. A novel mesoporous hydrogen-bonded organic framework with high porosity and stability. Chem. Commun. 2020, 56, 66–69. [Google Scholar] [CrossRef]
- Sikdar, N.; Bonakala, S.; Haldar, R.; Balasubramanian, S.; Maji, T.K. Dynamic entangled porous framework for hydrocarbon (C2–C3) storage, CO2 capture, and separation. Chem. Eur. J. 2016, 22, 6059–6070. [Google Scholar] [CrossRef]
- Bruker. SAINT, V8.34A; Bruker AXS Inc.: Madison, WI, USA, 2013. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Kratzert, D. FinalCif, V104. Available online: https://dkratzert.de/finalcif.html (accessed on 20 April 2020).
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Zhang, X.J.; Shang, C.; Liu, Z.P. Double-ended surface walking method for pathway building and transition state location of complex reactions. J. Chem. Theory Comput. 2013, 9, 5745–5753. [Google Scholar] [CrossRef]
- Wei, G.F.; Liu, Z.P. Restructuring and hydrogen evolution on Pt nanoparticle. Chem. Sci. 2015, 6, 1485–1490. [Google Scholar] [CrossRef]

| MOF | BET | C2H6/CH4 (50:50) | C2H6/CH4 (5:95) | C2H2/CH4 (50:50) | T (K) | Gas Uptake C2H6 & CH4 | Refs. |
|---|---|---|---|---|---|---|---|
| TJU-Dan-5 * | 748 | 146.5 | 101.2 | 25.0 | 273 | 50.3/23.6 | This work |
| 68 | 67 | 15.4 | 298 | 45.03/15.2 | This work | ||
| UTSA-35 * | 742.7 | 15 | / | 19 | 296 | 51/9 | [50] |
| [Co2(5,4-PMIA)2(TPOM)0.5] | 920 | 34.7 | / | 32.0 | 300 | 81.9/16.9 | [51] |
| FJI-C4 * | 690 | 39.7 | / | 51 | 298 | 66.3/18.4 | [41] |
| UTSA-33 * | 660 | 20 | / | 17.1 | 296 | 59/12 | [42] |
| Cu-TDPAT | 1938 | 16.4 | / | 154.3 | 273 | 217.7/51.6 | [52] |
| 12.1 | / | 127.1 | 298 | 154.4/28.3 | [52] | ||
| JLU-MOF112 * | 1553 | 12 | 24 | / | 298 | 107.7/10.2 | [29] |
| SNNU-Bai67 | 989.5 | 38.3 | / | 50.5 | 298 | 91.2/15.8 | [43] |
| VNU-18 | 900 | 27.3 | / | 41.5 | 298 | 72.8/18.5 | [44] |
| SBMOF-2 | 195 | 26 | / | 18 | 298 | 59.8/16.2 | [45] |
| ZJNU-119 * | 950 | 20.9 | / | 62.9 | 298 | 89.2/37.5 | [53] |
| HOF-BTBa | 955 | 17.7 | / | 12.5 | 273 | 95.4/13.44 | [46] |
| 13.7 | 9.3 | 295 | 69.2/10.08 | [46] | |||
| PFC-5 * | 256 | 19 | / | 15 | 298 | 25.9/8.0 | [54] |
| HOF-14 | 2573 | 6.3 | 3.7 | 298 | 44.2/7.8 | [55] | |
| MAF-49 | 170 | / | / | 316 | 36/22 | [30] | |
| [Zn2(bdc)2(bpndi)] | 565 | 175 | / | 496 | 298 | 44/8 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dan, W.; Wei, G.; Fang, X. Three-Dimensional Hydrogen-Bonded Porous Metal-Organic Framework for Natural Gas Separation with High Selectivity. Molecules 2024, 29, 424. https://doi.org/10.3390/molecules29020424
Dan W, Wei G, Fang X. Three-Dimensional Hydrogen-Bonded Porous Metal-Organic Framework for Natural Gas Separation with High Selectivity. Molecules. 2024; 29(2):424. https://doi.org/10.3390/molecules29020424
Chicago/Turabian StyleDan, Wenyan, Guangfeng Wei, and Xiangdong Fang. 2024. "Three-Dimensional Hydrogen-Bonded Porous Metal-Organic Framework for Natural Gas Separation with High Selectivity" Molecules 29, no. 2: 424. https://doi.org/10.3390/molecules29020424
APA StyleDan, W., Wei, G., & Fang, X. (2024). Three-Dimensional Hydrogen-Bonded Porous Metal-Organic Framework for Natural Gas Separation with High Selectivity. Molecules, 29(2), 424. https://doi.org/10.3390/molecules29020424







