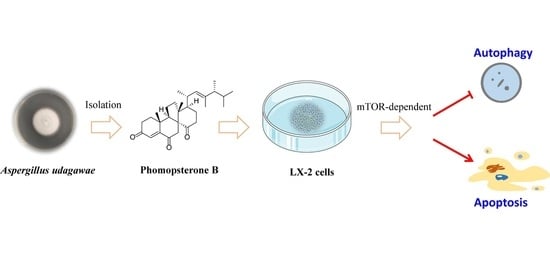
Phomopsterone B Alleviates Liver Fibrosis through mTOR-Mediated Autophagy and Apoptosis Pathway
Abstract
1. Introduction
2. Results
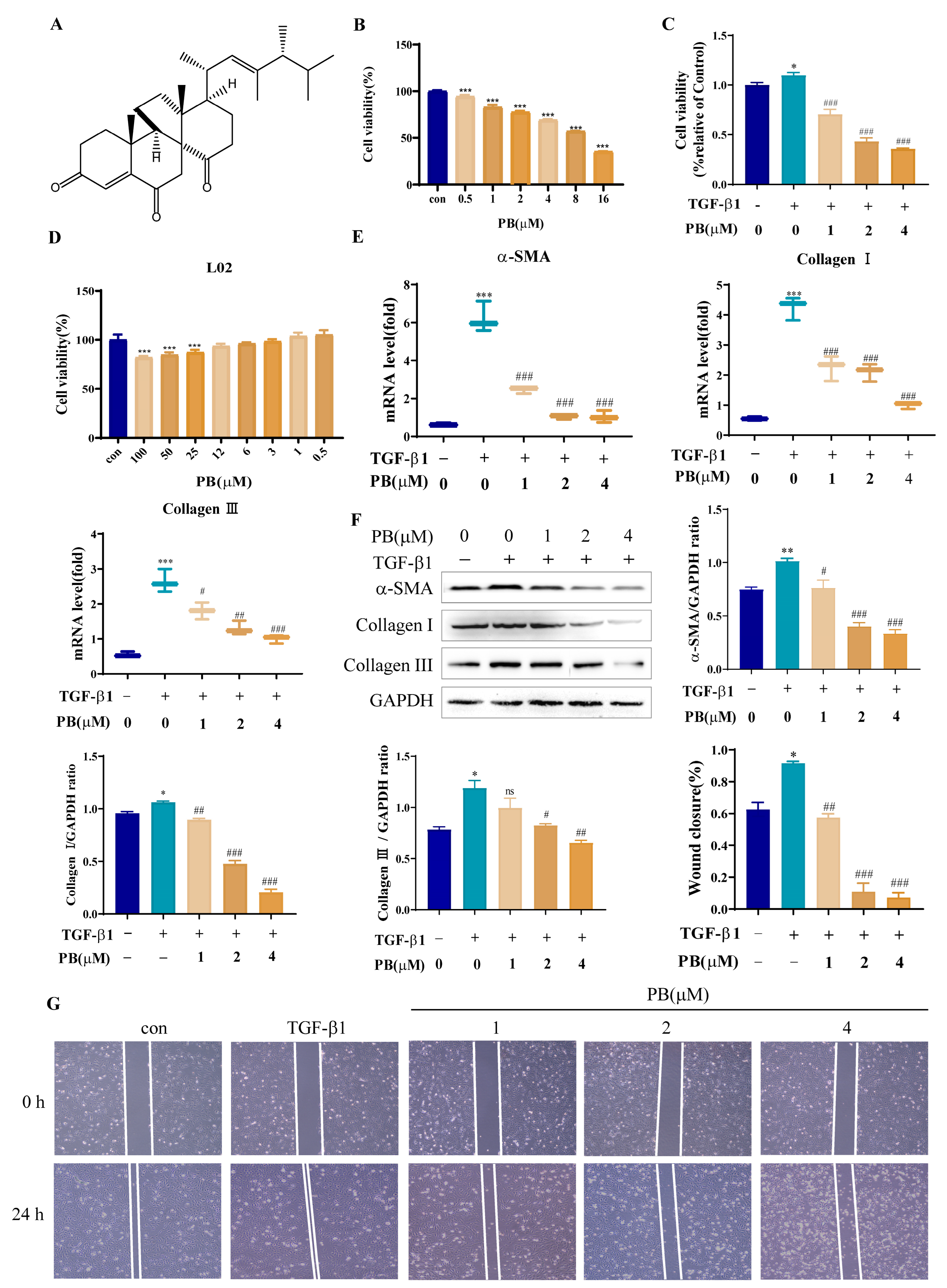
2.1. PB Prevents the Proliferation, Activation, and Migration of LX-2 Cells
2.2. Molecular Docking
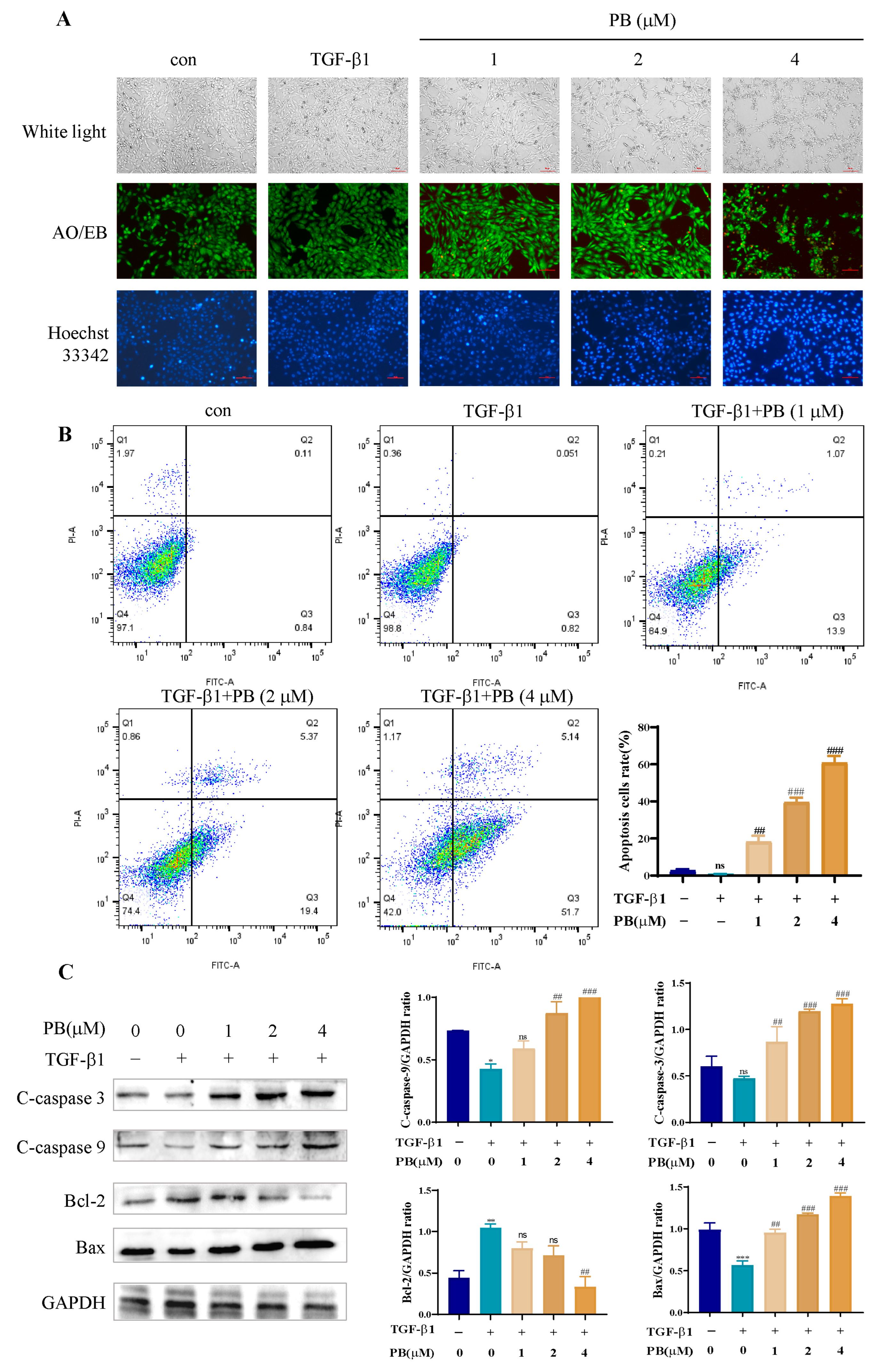
2.3. PB-Induced LX-2 Cell Apoptosis
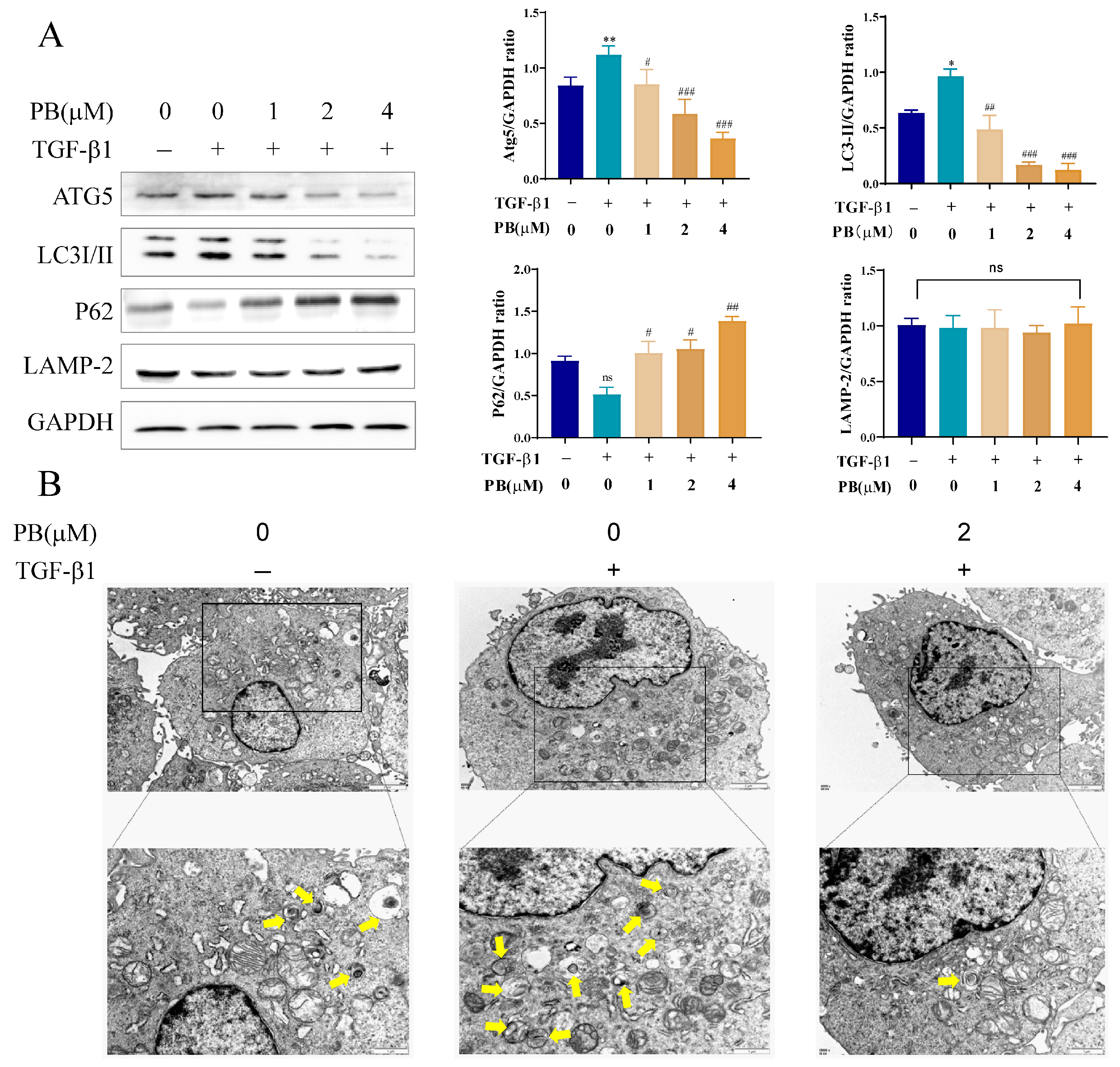
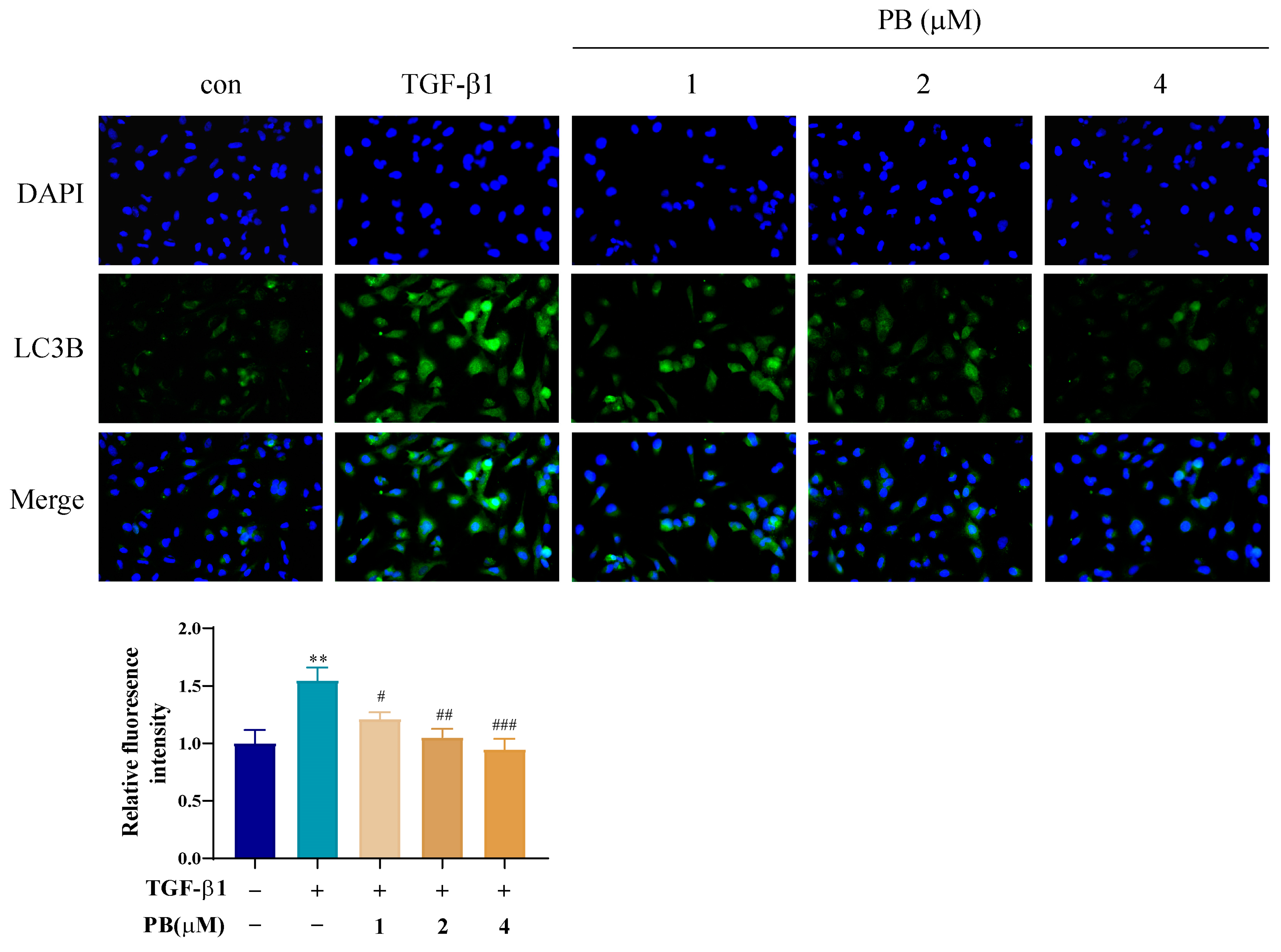
2.4. PB Suppressed Autophagy in Activated LX-2 Cells
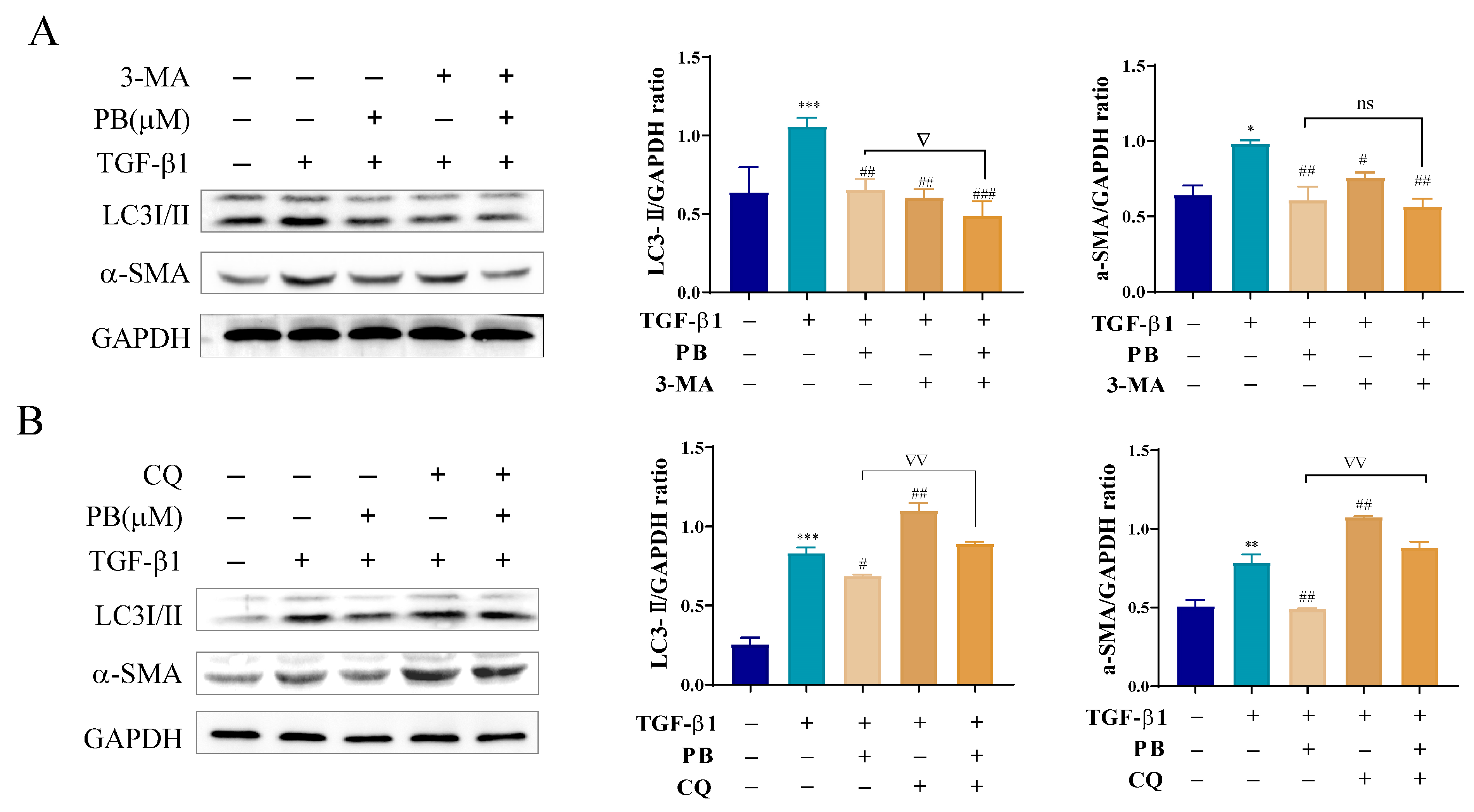
2.5. Inhibition of Autophagy Promotes Anti-Fibrosis Effects of PB In Vitro
2.6. PB Regulates Autophagy and Apoptosis through mTOR-Dependent Mechanism in LX-2 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Viability Assay
4.3. Cell Wound Healing Assay
4.4. Cell Fluorescence Assay
4.5. Immunofluorescent Staining
4.6. Flow Cytometric Analysis
4.7. Quantitative Real-Time PCR
4.8. Western Blot Analysis
4.9. Molecular Docking
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastro. Hepat. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Hernandez-Gea, V.; Friedman, S.L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol.-Mech. 2011, 6, 425–456. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wang, J.; Wang, J.; Zhou, Q.; He, Q.; Weng, Q. Intercellular crosstalk of hepatic stellate cells in liver fibrosis: New insights into therapy. Pharmacol. Res. 2020, 155, 104720. [Google Scholar] [CrossRef] [PubMed]
- Krizhanovsky, V.; Yon, M.; Dickins, R.A.; Hearn, S.; Simon, J.; Miething, C.; Yee, H.; Zender, L.; Lowe, S.W. Senescence of activated stellate cells limits liver fibrosis. Cell 2008, 134, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Thornberry, N.A. Caspases: Key mediators of apoptosis. Chem. Biol. 1998, 5, R97–R103. [Google Scholar] [CrossRef]
- Moghtaderi, H.; Sepehri, H.; Delphi, L.; Attari, F. Gallic acid and curcumin induce cytotoxicity and apoptosis in human breast cancer cell MDA-MB-231. BioImpacts 2018, 8, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Du, X.S.; Li, H.D.; Yang, X.J.; Li, J.J.; Xu, J.J.; Chen, Y.; Xu, Q.Q.; Yang, L.; He, C.S.; Huang, C.; et al. Wogonin attenuates liver fibrosis via regulating hepatic stellate cell activation and apoptosis. Int. Immunopharmacol. 2019, 75, 105671. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yang, X.; He, Y.; Zhao, Y.; Chen, Y.; Yang, Y.; Xiao, P. Mechanism of the anti-liver fibrosis effect of Periplaneta americana extracts that promote apoptosis of HSC-T6 cells through the Bcl-2/Bax signaling pathway. J. Asia-Pac. Entomol. 2023, 26, 102094. [Google Scholar] [CrossRef]
- Gao, J.; Wei, B.; de Assuncao, T.M.; Liu, Z.; Hu, X.; Ibrahim, S.; Cooper, S.A.; Gao, S.; Shah, V.H.; Kostallari, E. Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J. Hepatol. 2020, 73, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Allaire, M.; Rautou, P.E.; Codogno, P.; Lotersztajn, S. Autophagy in liver diseases: Time for translation? J. Hepatol. 2019, 70, 985–998. [Google Scholar] [CrossRef]
- Thoen, L.F.; Guimarães, E.L.; Dollé, L.; Mannaerts, I.; Najimi, M.; Sokal, E.; van Grunsven, L.A. A role for autophagy during hepatic stellate cell activation. J. Hepatol. 2011, 55, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Ichimiya, T.; Yamakawa, T.; Hirano, T.; Yokoyama, Y.; Hayashi, Y.; Hirayama, D.; Wagatsuma, K.; Itoi, T.; Nakase, H. Autophagy and autophagy-related diseases: A review. Int. J. Mol. Sci. 2020, 21, 8974. [Google Scholar] [CrossRef]
- Lucantoni, F.; Martnez-Cerezuela, A.; Gruevska, A.; Moragrega, Á.B.; Víctor, M.; Víctor, V.M.; Juan, V.; Esplugues, J.V.; Blas-García, A.; Apostolova, N. Understanding the implication of autophagy in the activation of hepatic stellate cells in liver fibrosis: Are we there yet? J. Pathol. 2021, 254, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Tan, L.; Hu, M. The role of autophagy in hepatic fibrosis. Am. J. Transl. Res. 2021, 13, 5747. [Google Scholar] [PubMed]
- Li, X.; Sun, R.; Liu, R. Natural products in licorice for the therapy of liver diseases: Progress and future opportunities. Pharmacol. Res. 2019, 144, 210–226. [Google Scholar] [CrossRef]
- Pan, X.; Ma, X.; Jiang, Y.; Wen, J.; Yang, L.; Chen, D.; Cao, X.; Peng, C. A Comprehensive Review of Natural Products against Liver Fibrosis: Flavonoids, Quinones, Lignans, Phenols, and Acids. Evid.-Based Compl. Alt. Med. 2020, 2020, 7171498. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chai, C.; He, Y.; Li, F.; Hao, X.; Cao, F.; Gu, L.; Liu, J.; Hu, Z.; Zhang, Y. Periconiastone A, an antibacterial ergosterol with a pentacyclo [8.7. 0.01, 5.02, 14.010, 15] heptadecane system from Periconia sp. TJ403-rc01. Org. Lett. 2019, 21, 8469–8472. [Google Scholar] [CrossRef]
- Deng, S.; Xu, H.; Jiang, H.; Ma, Z. Formal total synthesis of dankasterone B. Org. Chem. Front. 2022, 9, 3961–3965. [Google Scholar] [CrossRef]
- Chen, P.; Wang, C.; Yang, R.; Xu, H.; Wu, J.; Jiang, H.; Chen, K.; Ma, Z. Asymmetric total synthesis of dankasterones A and B and periconiastone a through radical cyclization. Angew. Chem. Int. Ed. 2021, 60, 5512–5518. [Google Scholar] [CrossRef]
- Duecker, F.L.; Heinze, R.C.; Heretsch, P. Synthesis of swinhoeisterol A, dankasterone A and B, and periconiastone A by radical framework reconstruction. J. Am. Chem. Soc. 2020, 142, 104–108. [Google Scholar] [CrossRef]
- Amagata, T.; Tanaka, M.; Yamada, T.; Doi, M.; Minoura, K.; Ohishi, H.; Yamori, T.; Numata, A. Variation in cytostatic constituents of a sponge-derived Gymnascella dankaliensis by manipulating the carbon source. J. Nat. Prod. 2007, 70, 1731–1740. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, Y.; Xie, S.; Sun, W.; Guo, Y.; Li, X.N.; Liu, J.; Li, H.; Wang, J.; Luo, Z.; et al. Phomopsterones A and B, two functionalized ergostane-type steroids from the endophytic fungus Phomopsis sp. TJ507A. Org. Lett. 2017, 19, 258–261. [Google Scholar] [CrossRef]
- Pagadala, N.S.; Syed, K.; Tuszynski, J. Software for molecular docking: A review. Biophys. Rev. 2017, 9, 91–102. [Google Scholar] [CrossRef]
- Kaur, A.; Sharma, S. Mammalian target of rapamycin (mTOR) as a potential therapeutic target in various diseases. Inflammopharmacology 2017, 25, 293–312. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yuan, W.G.; He, P.; Lei, J.H.; Wang, C.X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Delive. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Strasser, A.; Cory, S.; Adams, J.M. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011, 30, 3667–3683. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Bio. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Moscat, J.; Karin, M.; Diaz-Meco, M.T. p62 in cancer: Signaling adaptor beyond autophagy. Cell 2016, 167, 606–609. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, H.; Yuan, Y.; Gao, J.; Shen, Z.; Cheng, Y.; Shen, Y.; Wang, R.R.; Wang, X.; Hu, W.W.; et al. Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy 2013, 9, 1321–1333. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, B.; Xie, J.; Jiang, X.; Xiao, B.; Hu, X.; Xiang, J. Aspirin attenuates liver fibrosis by suppressing TGF-β1/Smad signaling. Mol. Med. Rep. 2022, 25, 181. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, S.M.; Hur, W.; Kang, B.-Y.; Lee, H.L.; Nam, H.; Yoo, S.H.; Sung, P.S.; Kwon, J.H.; Jang, J.W.; et al. Tenofovir disoproxil fumarate directly ameliorates liver fibrosis by inducing hepatic stellate cell apoptosis via downregulation of PI3K/Akt/mTOR signaling pathway. PLoS ONE 2021, 16, e0261067. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Wang, D.; Xu, Y.; Dong, R.; Yang, Y.; Lv, Q.; Chen, X.; Zhang, Z. The upstream pathway of mTOR-mediated autophagy in liver diseases. Cells 2019, 8, 1597. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Gestwicki, J.E.; Murphy, L.O.; Klionsky, D.J. Potential therapeutic applications of autophagy. Nat. Rev. Drug Discov. 2007, 6, 304–312. [Google Scholar] [CrossRef]
- Kumara, H.K.; Suhas, R.; Suyoga Vardhan, D.M.; Shobha, M.; Channe Gowda, D. A correlation study of biological activity and molecular docking of Asp and Glu linked bishydrazones of quinazolinones. RSC Adv. 2018, 8, 10644–10653. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Cui, H.; Liu, G.; Zhao, X.; Li, W.; Piao, G. How can synergism of traditional medicines benefit from network pharmacology? Molecules 2017, 22, 1135. [Google Scholar] [CrossRef]
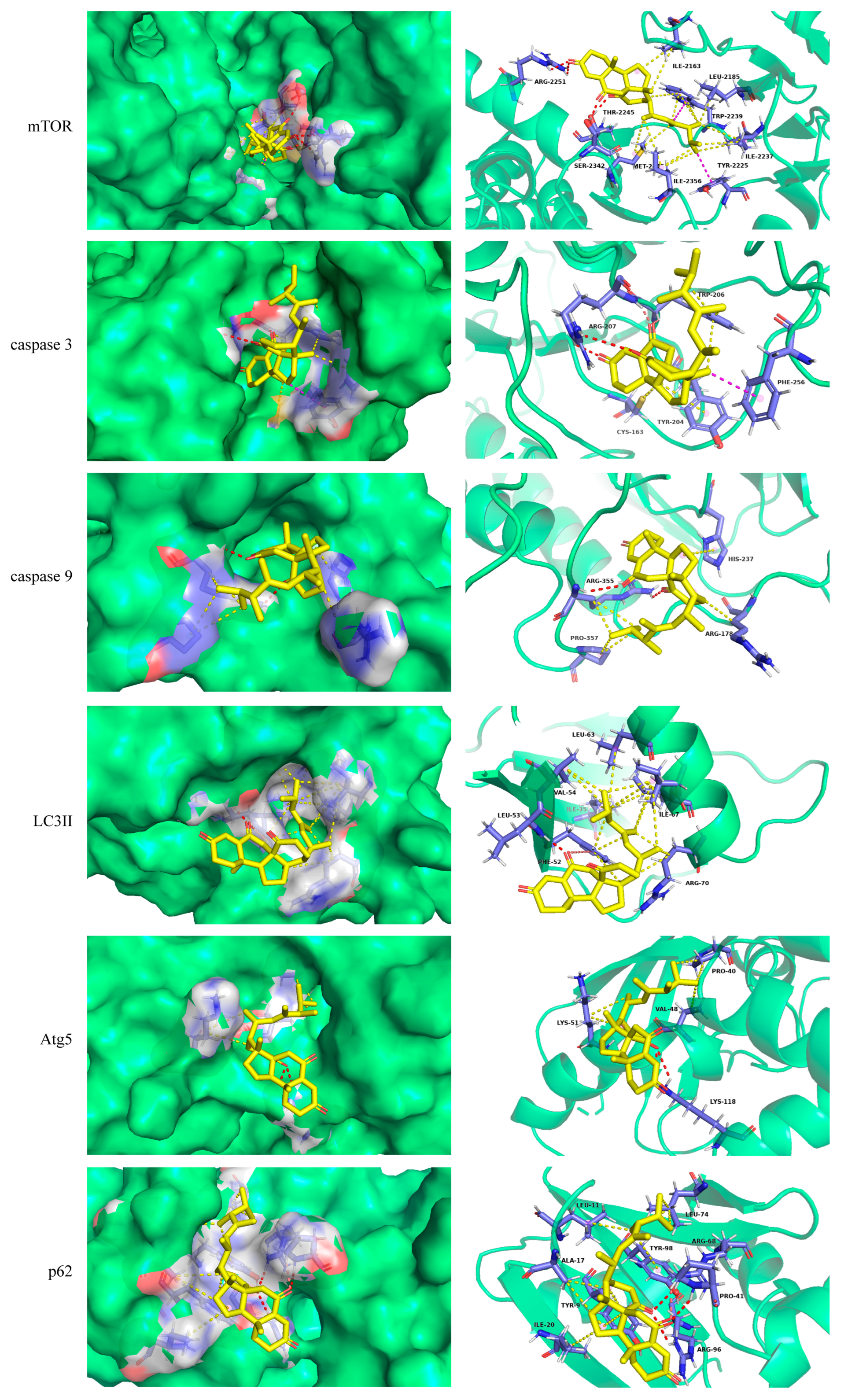


| Protein | Binding Energy kcal/mol | Hydrogen Bond Interaction | Distance Å |
|---|---|---|---|
| mTOR | −9.4 | ARG-2251 | 2.2, 2.7 |
| SER-2342 | 2.0 | ||
| THR-2245 | 2.0 | ||
| caspase 3 | −8.1 | ARG-207 | 1.9, 2.4, 3.4 |
| TRP206 | 2.8 | ||
| caspase 9 | −7.3 | ARG-355 | 2.3, 3.0 |
| Atg5 | −6.9 | LYS118 | 2.8, 3.1 |
| LC3II | −6.9 | LEU-53 | 1.8 |
| PHE-52 | 2.9 | ||
| p62 | −7.8 | PRO41 | 2.5 |
| ARG68 | 2.8 | ||
| ARG96 | 3.0 |
| Gene | Forward | Reverse |
| α-SMA | TCATGGTCGGTATGGGTCAG | CGTTGTAGAAGGTGTGGTGC |
| Collagen I | TGGAGAGGAAGGAAAGCGAG | ACCAGCTTCACCAGGAGATC |
| Collagen III | AAAAGGGGAGCTGGCTACTT | GAATTTCTGGGTTGGGGCAG |
| GAPDH | TCAAGAAGGTGGTGAAGCAGG | TCAAAGGTGGAGGAGTGGGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, M.-L.; Zhang, L.-J.; Luo, Y.; Xu, S.-Y.; Long, X.-M.; Ao, J.-L.; Liao, S.-G.; Zhu, Q.-F.; He, X.; Xu, G.-B. Phomopsterone B Alleviates Liver Fibrosis through mTOR-Mediated Autophagy and Apoptosis Pathway. Molecules 2024, 29, 417. https://doi.org/10.3390/molecules29020417
Peng M-L, Zhang L-J, Luo Y, Xu S-Y, Long X-M, Ao J-L, Liao S-G, Zhu Q-F, He X, Xu G-B. Phomopsterone B Alleviates Liver Fibrosis through mTOR-Mediated Autophagy and Apoptosis Pathway. Molecules. 2024; 29(2):417. https://doi.org/10.3390/molecules29020417
Chicago/Turabian StylePeng, Mei-Lin, Li-Jie Zhang, Yan Luo, Shi-Ying Xu, Xing-Mei Long, Jun-Li Ao, Shang-Gao Liao, Qin-Feng Zhu, Xun He, and Guo-Bo Xu. 2024. "Phomopsterone B Alleviates Liver Fibrosis through mTOR-Mediated Autophagy and Apoptosis Pathway" Molecules 29, no. 2: 417. https://doi.org/10.3390/molecules29020417
APA StylePeng, M.-L., Zhang, L.-J., Luo, Y., Xu, S.-Y., Long, X.-M., Ao, J.-L., Liao, S.-G., Zhu, Q.-F., He, X., & Xu, G.-B. (2024). Phomopsterone B Alleviates Liver Fibrosis through mTOR-Mediated Autophagy and Apoptosis Pathway. Molecules, 29(2), 417. https://doi.org/10.3390/molecules29020417