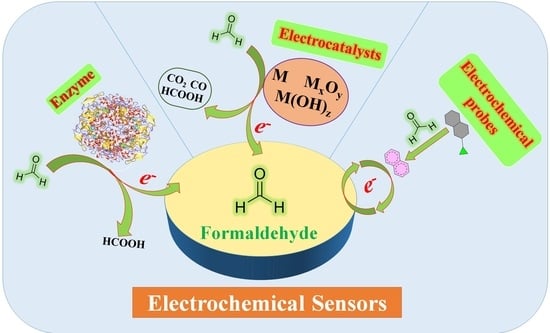
Recent Advances in Electrochemical Sensors for Formaldehyde
Abstract
1. Introduction
2. Sensing Mechanisms of Electrochemical Formaldehyde Sensors
3. Electrochemical Sensors for Formaldehyde
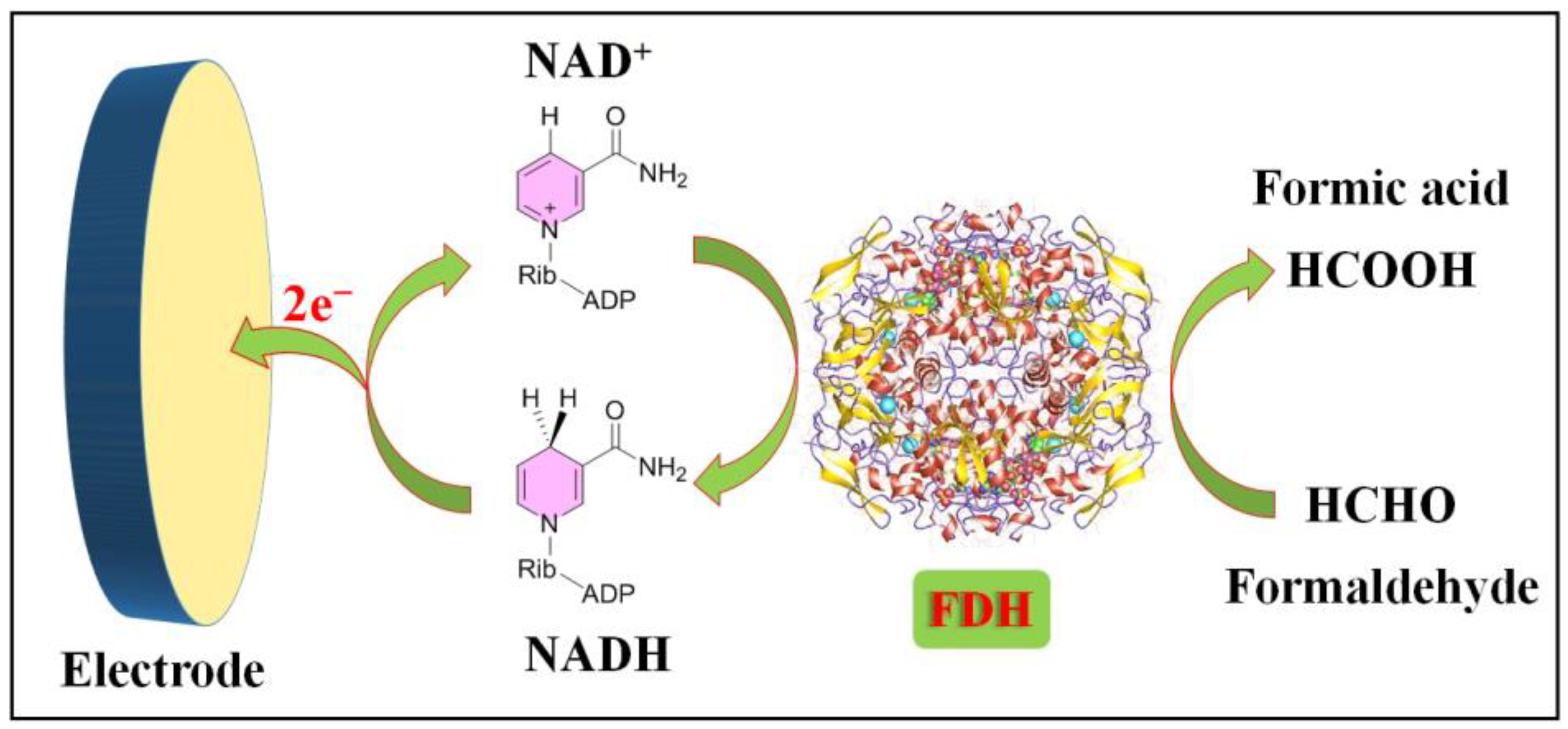
3.1. Electrochemical Sensors Rely on Bioenzymes
3.1.1. Formaldehyde-Dehydrogenase-Based Formaldehyde Sensors
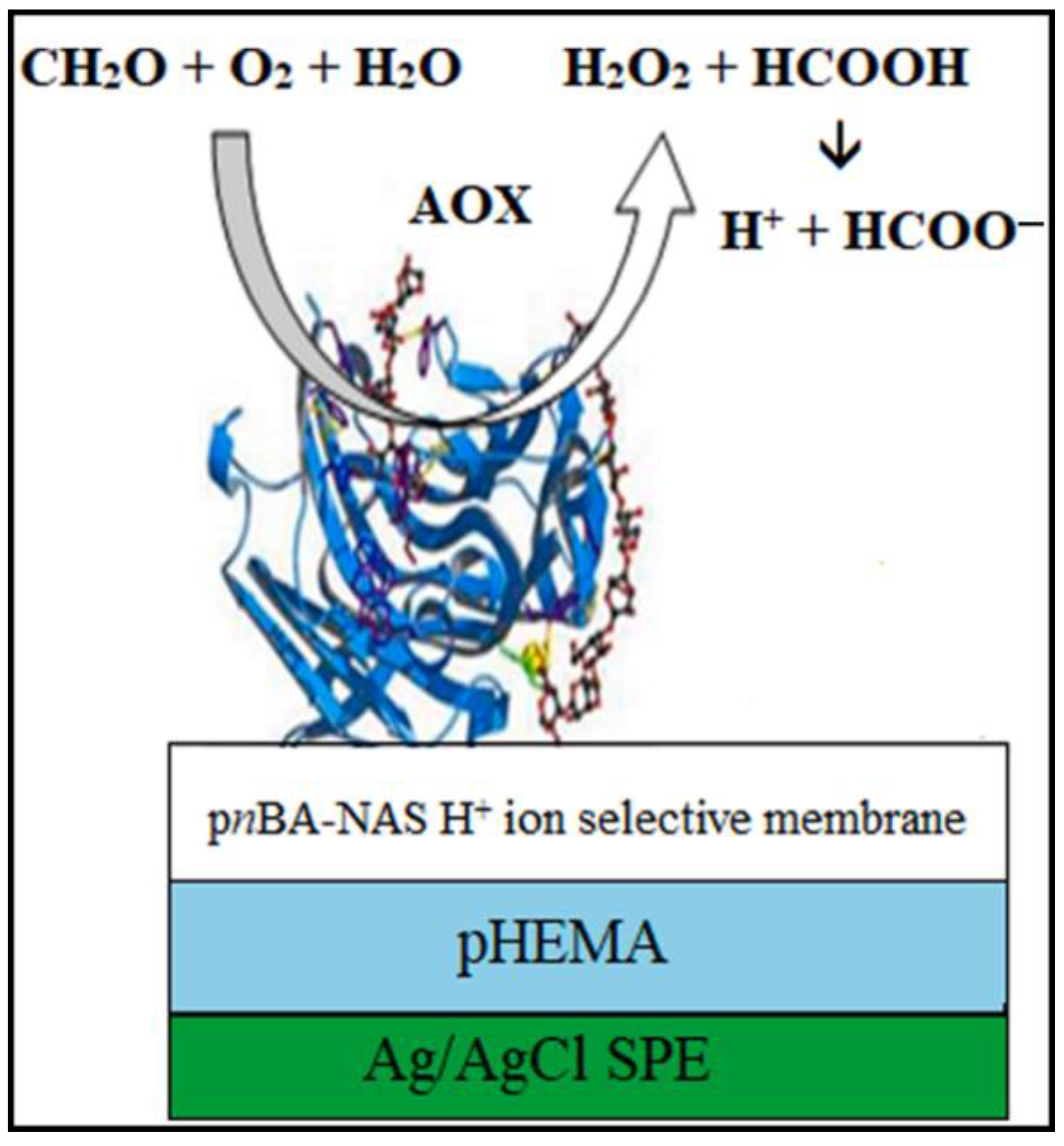
3.1.2. Alcohol-Oxidase-Based Formaldehyde Sensors
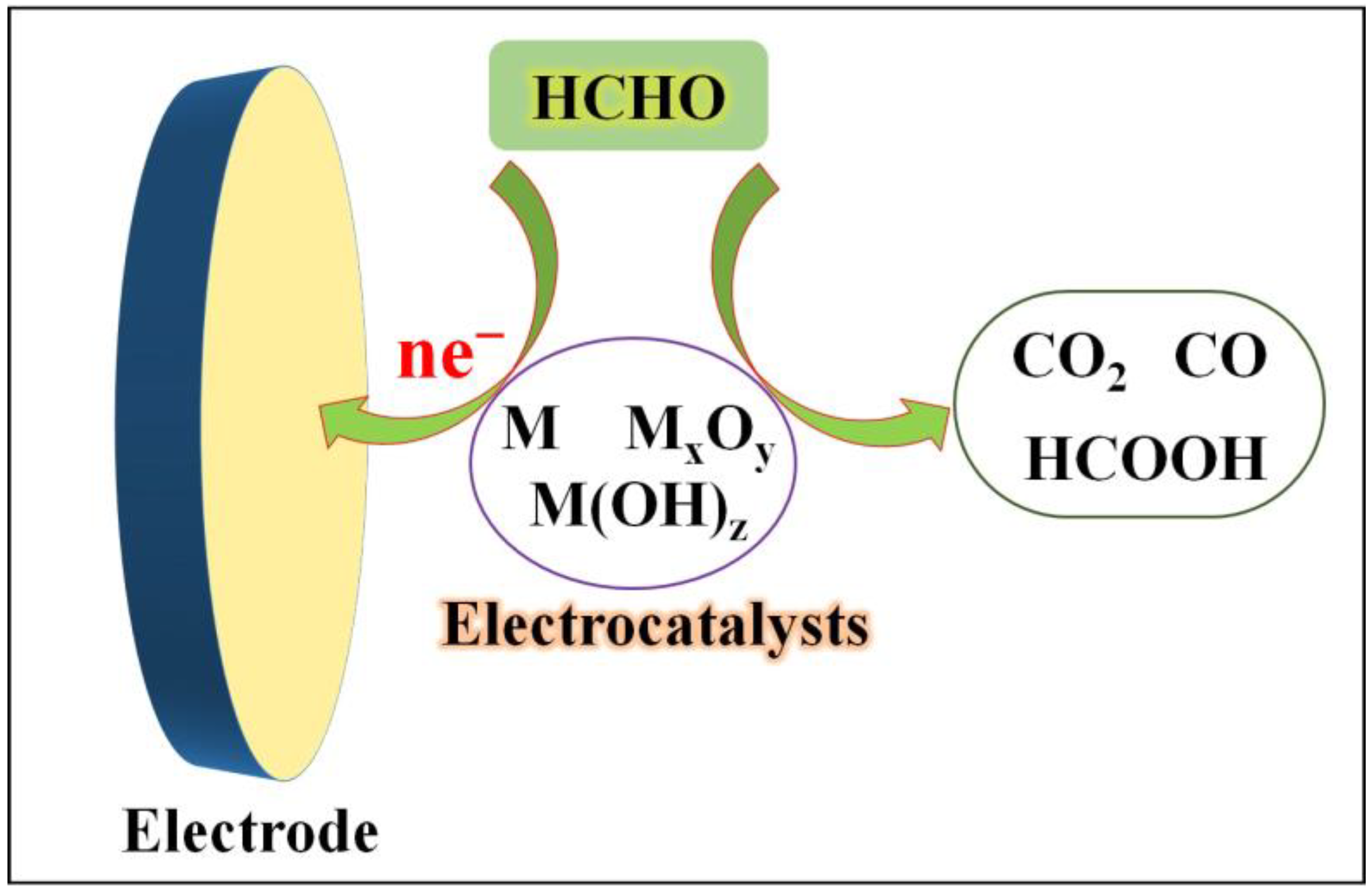
3.2. Electrochemical Sensors Rely on Electrocatalysts
3.2.1. Noble-Metal-Based Formaldehyde Sensors
- Au-based electrocatalysts
- Pt-based electrocatalysts
- Pd-based electrocatalysts
- Ag-based electrocatalysts
3.2.2. Bimetallic-Based Formaldehyde Sensors
3.2.3. Transition Metals and Their Oxide-Based Formaldehyde Sensors
- Nickel-based electrocatalysts
- Copper-based electrocatalysts
3.2.4. Organic-Polymer-Electrocatalysts-Based Formaldehyde Sensors
3.3. Electrochemical Sensors Rely on Derivative Reagents
4. Summaries
4.1. Conclusions
4.2. Challenges and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| ALDHs | Aldehyde dehydrogenases |
| AOX | Alcohol oxidase |
| BDMA | 1,4-Benzenedimethaneamine functionalized graphene |
| BP | Buckypaper |
| CFP | Carbon fiber paper |
| Ch | Choline |
| CHIT | Chitosan |
| CILE | Carbon ionic liquid electrode |
| CNF | Carbon nanofiber |
| CNTs | Carbon nanotubes |
| COD | Chemical oxygen demand |
| CPE | Carbon paste electrode |
| CV | Cyclic voltammetry |
| CYPs | Cytochrome P450 |
| DDL | 3,5-Diacetyl-1,4-dihydromethylpyridine |
| DET | Direct electron transfer |
| DFT | Density functional theory |
| EDTA | Ethylenediaminetetraacetic acid |
| EIS | Impedance spectroscopy |
| EQCM | Electrochemical quartz crystal microbalance |
| ESPB | Self-powered biosensor |
| FI | Flow injection |
| FTIR | Fourier-transform infrared spectroscopy |
| GCE | Glassy carbon electrode |
| GMA-co-MTM | Glycidyl methacrylate-co-3-methylthienyl methacrylate |
| HOPG | Highly oriented pyrolytic graphite |
| HPA | Hydrazinium polyacrylate |
| IDGE | Interdigitated gold electrodes |
| IL | Ionic liquid |
| ITO | Indium tin oxide |
| LDA | Linear discriminant analysis |
| LIG | Laser-induced graphene |
| LOD | Detection limit |
| MiPAN | Molecular imprinted polymer of acrylonitrile |
| MIPs | Molecularly imprinted polymers |
| MWCNTs | Multi-walled carbon nanotubes |
| NAD+ | Nicotinamide adenine dinucleotide |
| nBA-NAS | n-Butyl acrylate-N-acryloxysuccinimide |
| NCs | Nanoclusters |
| NF | Nickel foam |
| NFC | Near-field communication |
| NFs | Nanofibers |
| Ni/P-CPE | Nickel-doped P nanozeolite carbon paste electrode |
| NPs | Nanoparticles |
| NQS | 1,2-Naphthoquinone-4-sulfonic acid |
| NS | Nanosheets |
| NW | Nanowire |
| PAA | Polyacrylic acid |
| PAD | Amperometric detection |
| PAN | Polyaniline |
| PANI | Polyaniline |
| PB | Prussian blue |
| PBA | 1-Pyrenebutyric acid |
| pDA | Polydopamine |
| PDOS | Projected Density of States |
| PdPs-CMs | Palladium particles and carbon microspheres |
| pHEMA | Poly(2-hydroxyethyl methacrylate) |
| PMAH | Poly(methacryloyl hydrazide) |
| PMG | Poly-methylene green |
| POs-EA | Os(bpy)2-poly(vinylpyridine) |
| PPy | Polypyrrole |
| PS | Porous silicon |
| Pt NPs-SPUME | Pt nanoparticles-screen-printed carbon ultramicroelectrode |
| PVA | Poly(vinyl alcohol) |
| Px | p-Xylylenediamine |
| SBA-16 | Mesoporous silica Santa Barbara Amorphous No. 16 |
| SCE | Saturated calomel electrode |
| SOMC | Surface organometallic chemistry |
| SPCE | Screen-printed carbon electrode |
| SPCPtEs | Screen-printed platinized carbon electrodes |
| SPE | Screen-printed electrode |
| SSAO | Semicarbazide-sensitive amine oxidase |
| TNAs | TiO2 nanotube arrays |
| TOC | Total organic carbon |
| VOCs | Volatile organic compounds |
| WGE | Paraffin-impregnated graphite electrode |
References
- Peng, Z.; Jiang, X.; Si, C.; Joao Cárdenas-Oscanoa, A.; Huang, C. Advances of Modified Lignin as Substitute to Develop Lignin-Based Phenol-Formaldehyde Resin Adhesives. ChemSusChem 2023, 16, e202300174. [Google Scholar] [CrossRef] [PubMed]
- Horáková, P.; Kočí, K. Continuous-Flow Chemistry and Photochemistry for Manufacturing of Active Pharmaceutical Ingredients. Molecules 2022, 27, 8536. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.-Y.; Pascoe, M. Disinfectants and antiseptics: Mechanisms of action and resistance. Nat. Rev. Microbiol. 2024, 22, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Sarika, P.R.; Nancarrow, P.; Khansaheb, A.; Ibrahim, T. Bio-Based Alternatives to Phenol and Formaldehyde for the Production of Resins. Polymers 2020, 12, 2237. [Google Scholar] [CrossRef] [PubMed]
- Tuesuwan, B.; Vongsutilers, V. Nitrosamine Contamination in Pharmaceuticals: Threat, Impact, and Control. J. Pharm. Sci. 2021, 110, 3118–3128. [Google Scholar] [CrossRef] [PubMed]
- Fappiano, L.; Carriera, F.; Iannone, A.; Notardonato, I.; Avino, P. A Review on Recent Sensing Methods for Determining Formaldehyde in Agri-Food Chain: A Comparison with the Conventional Analytical Approaches. Foods 2022, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Klose, R.J.; Zhang, Y. Regulation of histone methylation by demethylimination and demethylation. Nat. Rev. Mol. Cell Biol. 2007, 8, 307–318. [Google Scholar] [CrossRef]
- Blande, J.D.; Holopainen, J.K.; Niinemets, Ü. Plant volatiles in polluted atmospheres: Stress responses and signal degradation. Plant Cell Environ. 2014, 37, 1892–1904. [Google Scholar] [CrossRef]
- Obata, T. Diabetes and semicarbazide-sensitive amine oxidase (SSAO) activity: A review. Life Sci. 2006, 79, 417–422. [Google Scholar] [CrossRef]
- Goyal, V.; Sarki, N.; Narani, A.; Naik, G.; Natte, K.; Jagadeesh, R.V. Recent advances in the catalytic N-methylation and N-trideuteromethylation reactions using methanol and deuterated methanol. Coord. Chem. Rev. 2023, 474, 214827. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Lin, Z.-J.; Li, C.-C.; Lin, X.; Shan, S.-K.; Guo, B.; Zheng, M.-H.; Li, F.; Yuan, L.-Q.; Li, Z.-h. Epigenetic regulation in metabolic diseases: Mechanisms and advances in clinical study. Signal Transduct. Target. Ther. 2023, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivera, J.C.; Sherman, M.W.; Wang, D.Y.S.; Chuvalo-Abraham, J.C.L.; Hildebrandt Ruiz, L.; Contreras, L.M. RNA oxidation in chromatin modification and DNA-damage response following exposure to formaldehyde. Sci. Rep. 2020, 10, 16545. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, L.; Barbosa, E.; Charao, M.F.; Brucker, N. Formaldehyde toxicity reports fromin vitroandin vivostudies: A review and updated data. Drug Chem. Toxicol. 2022, 45, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Zhang, X.J. Formaldehyde in biological systems: Involving sources, related diseases and reaction-based fluorescent detection. TrAC Trends Anal. Chem. 2023, 168, 117298. [Google Scholar] [CrossRef]
- Dighe, S.U.; Khan, S.; Soni, I.; Jain, P.; Shukla, S.; Yadav, R.; Sen, P.; Meeran, S.M.; Batra, S. Synthesis of β-Carboline-Based N-Heterocyclic Carbenes and Their Antiproliferative and Antimetastatic Activities against Human Breast Cancer Cells. J. Med. Chem. 2015, 58, 3485–3499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.Y.; He, X.Q.; Yang, P.Y.; Zong, T.Y.; Sun, P.; Sun, R.C.; Yu, T.; Jiang, Z.R. The cellular function and molecular mechanism of formaldehyde in cardiovascular disease and heart development. J. Cell. Mol. Med. 2021, 25, 5358–5371. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.S.; Kim, H.S.; Jung, J.H.; Lee, C.M.; Ahn, Y.S.; Seo, Y.R. Formaldehyde exposure and leukemia risk: A comprehensive review and network-based toxicogenomic approach. Genes Environ. 2021, 43, 13. [Google Scholar] [CrossRef] [PubMed]
- Möhner, M.; Liu, Y.M.; Marsh, G.M. New insights into the mortality risk from nasopharyngeal cancer in the national cancer institute formaldehyde worker cohort study. J. Occup. Med. Toxicol. 2019, 14, 4. [Google Scholar] [CrossRef]
- Kwak, K.; Paek, D.; Park, J.T. Occupational exposure to formaldehyde and risk of lung cancer: A systematic review and meta-analysis. Am. J. Ind. Med. 2020, 63, 312–327. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.J. Comparison of standard methods and gas chromatography method in determination of formaldehyde emission from MDF bonded with formaldehyde-based resins. Bioresour. Technol. 2005, 96, 1457–1464. [Google Scholar] [CrossRef]
- Ma, B.K.; Xu, F.J.; He, M.; Lin, Y.Q.; Hu, G.H.; Zhang, M.; Zhao, X.Y.; Liu, W.L. Detection of residual formaldehyde in N-butyl-2-cyanoacrylate by high-performance liquid chromatography with rhodamine B hydrazide. Microchem. J. 2020, 158, 105222. [Google Scholar] [CrossRef]
- Chen, S.L.; Cheng, C.L. Determination of gaseous formaldehyde by derivatization using magnetic multiwalled carbon nanotubes (MWCNTs) modified with 2,4-dinitrophenylhydrazine (DNPH) and high-performance liquid chromatography—Ultraviolet detection (HPLC-UV). Instrum. Sci. Technol. 2022, 50, 174–189. [Google Scholar] [CrossRef]
- Lian, X.Y.; Zhang, M.G.; Sun, X.T.; Song, C.F.; Yuan, H.F.; Guo, L.H.; Li, X.Y. Online real time determination of free formaldehyde content during polymerization process of phenolic resin by NIR spectra and a modeling-free method. Polym. Test. 2021, 93, 106584. [Google Scholar] [CrossRef]
- Nie, X.M.; Chen, Z.Y.; Tian, Y.P.; Chen, S.; Qu, L.L.; Fan, M.B. Rapid detection of trace formaldehyde in food based on surface-enhanced Raman scattering coupled with assembled purge trap. Food Chem. 2021, 340, 127930. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.G.; Lu, L.Q.; Lin, L.; Xu, A.W. A silver nanoparticle based surface enhanced resonance Raman scattering (SERRS) probe for the ultrasensitive and selective detection of formaldehyde. Nanoscale 2012, 4, 7358–7361. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Aquino, C.; Costero, A.M.; Gil, S.; Gaviña, P. A New Environmentally-Friendly Colorimetric Probe for Formaldehyde Gas Detection under Real Conditions. Molecules 2018, 23, 2646. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Musto, C.J.; Suslick, K.S. A Simple and Highly Sensitive Colorimetric Detection Method for Gaseous Formaldehyde. J. Am. Chem. Soc. 2010, 132, 4046–4047. [Google Scholar] [CrossRef]
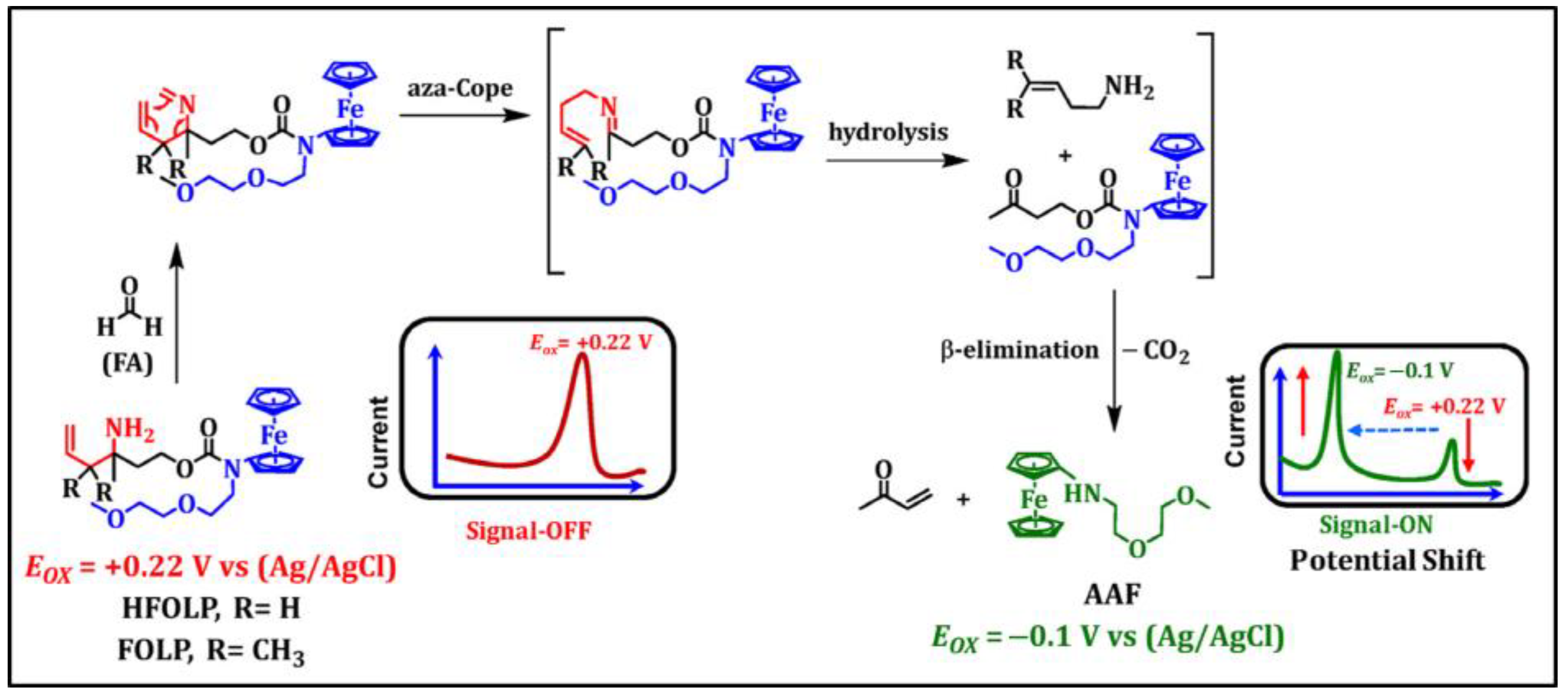
- Brewer, T.F.; Chang, C.J. An Aza-Cope Reactivity-Based Fluorescent Probe for Imaging Formaldehyde in Living Cells. J. Am. Chem. Soc. 2015, 137, 10886–10889. [Google Scholar] [CrossRef]
- Hao, Y.Q.; Zhang, Y.T.; Zhang, A.M.; Sun, Q.L.; Zhu, J.; Qu, P.; Chen, S.; Xu, M.T. A benzothiazole-based ratiometric fluorescent probe for detection of formaldehyde and its applications for bioimaging. Spectrochim. Acta Part A 2020, 229, 117988. [Google Scholar] [CrossRef]
- Tang, Y.H.; Kong, X.Q.; Xu, A.; Dong, B.L.; Lin, W.Y. Development of a Two-Photon Fluorescent Probe for Imaging of Endogenous Formaldehyde in Living Tissues. Angew. Chem. Int. Ed. 2016, 55, 3356–3359. [Google Scholar] [CrossRef]
- Liang, M.N.; Shao, C.Y.; Zhang, Q.; Zhang, C.; Wang, Y.X.; Zheng, X.Q.; Lu, S. High-Performance Formaldehyde Sensing Using Paper-Based Fluorescent Copper Nanoclusters. IEEE Sens. J. 2023, 23, 2076–2084. [Google Scholar] [CrossRef]
- Yuan, G.Q.; Ding, H.Y.; Peng, L.P.; Zhou, L.Y.; Lin, Q.L. A novel fluorescent probe for ratiometric detection of formaldehyde in real food samples, living tissues and zebrafish. Food Chem. 2020, 331, 127221. [Google Scholar] [CrossRef] [PubMed]
- Röth, D.; Molina-Franky, J.; Williams, J.C.; Kalkum, M. Mass Spectrometric Detection of Formaldehyde-Crosslinked PBMC Proteins in Cell-Free DNA Blood Collection Tubes. Molecules 2023, 28, 7880. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.Y.; Sun, J.N.; Wang, Y.C.; Ye, M.Y.; Cheng, H.Y. Rapid determination of aldehydes in food by high-throughput reactive paper spray ionization mass spectrometry. J. Food Compos. Anal. 2022, 114, 104814. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Yu, Y.; Zhang, C.B.; Song, N.N.; Guo, Z.J.; Liang, M.M. Highly Sensitive and Selective Detection of Formaldehyde via Bio-Electrocatalysis over Aldehyde Dehydrogenase. Anal. Chem. 2022, 94, 15827–15831. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Zhang, L.Y.; Cheng, B.; Fan, J.J.; Yu, J.G. A high-response formaldehyde sensor based on fibrous Ag-ZnO/In2O3 with multi-level heterojunctions. J. Hazard. Mater. 2021, 413, 125352. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Mi, Q.; Wang, D.Y.; Li, T.T. MXene/Co3O4 composite based formaldehyde sensor driven by ZnO/MXene nanowire arrays piezoelectric nanogenerator. Sens. Actuators B 2021, 339, 129923. [Google Scholar] [CrossRef]
- Sun, X.X.; Zhang, H.; Huang, L.; Hao, S.; Zhai, J.F.; Dong, S.J. A naked-eye readout self-powered electrochemical biosensor toward indoor formaldehyde: On-site detection and exposure risk warning. Biosens. Bioelectron. 2021, 177, 112975. [Google Scholar] [CrossRef]
- Sun, X.X.; Zhang, H.; Hao, S.; Zhai, J.F.; Dong, S.J. A Self-Powered Biosensor with a Flake Electrochromic Display for Electrochemical and Colorimetric Formaldehyde Detection. ACS Sens. 2019, 4, 2631–2637. [Google Scholar] [CrossRef]
- Liu, X.; Qi, X.; Gu, Y.; Huang, X.; Liang, P. Titanium mesh as the anode of electrochemically active biofilm sensor for improved sensitivity in water toxicity real-time early-warning. Biosens. Bioelectron. 2023, 241, 115692. [Google Scholar] [CrossRef]
- Li, T.; Liao, C.M.; An, J.K.; Zhou, L.A.; Tian, L.L.; Zhou, Q.X.; Li, N.; Wang, X. A highly sensitive bioelectrochemical toxicity sensor and its evaluation using immediate current attenuation. Sci. Total Environ. 2021, 766, 142646. [Google Scholar] [CrossRef] [PubMed]
- Ayad, M.M.; Abdelghafar, M.E.; Torad, N.L.; Yamauchi, Y.; Amer, W.A. Green synthesis of carbon quantum dots toward highly sensitive detection of formaldehyde vapors using QCM sensor. Chemosphere 2023, 312, 137031. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, E.; Zeinali, S. Formaldehyde detection using quartz crystal microbalance (QCM) nanosensor coated by nanoporous MIL-101(Cr) film. Microporous Mesoporous Mater. 2020, 300, 110065. [Google Scholar] [CrossRef]
- Chu, Z.X.; Song, Q.; Zhang, Y.Q.; Jiang, J. Recent progress in the development of organic small-molecule and functional material fluorescent probes for formaldehyde detection and imaging. Coord. Chem. Rev. 2023, 495, 215338. [Google Scholar] [CrossRef]
- Zheng, J.J.; Liu, W.C.; Lu, F.N.; Tang, Y.; Yuan, Z.Q. Recent Progress in Fluorescent Formaldehyde Detection Using Small Molecule Probes. J. Anal. Test. 2022, 6, 204–215. [Google Scholar] [CrossRef]
- Liu, X.; Li, N.; Li, M.; Chen, H.; Zhang, N.N.; Wang, Y.L.; Zheng, K.B. Recent progress in fluorescent probes for detection of carbonyl species: Formaldehyde, carbon monoxide and phosgene. Coord. Chem. Rev. 2020, 404, 213109. [Google Scholar] [CrossRef]
- Wang, L.Y.; Gao, J.K.; Xu, J.Q. QCM formaldehyde sensing materials: Design and sensing mechanism. Sens. Actuators B 2019, 293, 71–82. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Chen, J.H.; Hu, L.L.; Tan, Y.; Liu, S.H.; Yin, J. Recent advances in formaldehyde-responsive fluorescent probes. Chin. Chem. Lett. 2017, 28, 1935–1942. [Google Scholar] [CrossRef]
- Bruemmer, K.J.; Brewer, T.F.; Chang, C.J. Fluorescent probes for imaging formaldehyde in biological systems. Curr. Opin. Chem. Biol. 2017, 39, 17–23. [Google Scholar] [CrossRef]
- Min, Y.R.; Yuan, C.Y.; Fu, D.L.; Liu, J.Q. Formaldehyde Gas Sensors Fabricated with Polymer-Based Materials: A Review. Chemosensors 2023, 11, 134. [Google Scholar] [CrossRef]
- Lou, C.M.; Lei, G.L.; Liu, X.H.; Xie, J.Y.; Li, Z.S.; Zheng, W.; Goel, N.; Kumar, M.; Zhang, J. Design and optimization strategies of metal oxide semiconductor nanostructures for advanced formaldehyde sensors. Coord. Chem. Rev. 2022, 452, 214280. [Google Scholar] [CrossRef]
- Ishak, S.; Johari, S.; Ramli, M.M.; Darminto, D. Formaldehyde gas sensing using metal oxide semiconductor: A brief review. Sens. Rev. 2022, 42, 554–567. [Google Scholar] [CrossRef]
- Han, Z.J.; Qi, Y.; Yang, Z.Y.; Han, H.C.; Jiang, Y.Y.; Du, W.J.; Zhang, X.; Zhang, J.Z.; Dai, Z.F.; Wu, L.L.; et al. Recent advances and perspectives on constructing metal oxide semiconductor gas sensing materials for efficient formaldehyde detection. J. Mater. Chem. C 2020, 8, 13169–13188. [Google Scholar] [CrossRef]
- Kukkar, D.; Vellingiri, K.; Kaur, R.; Bhardwaj, S.K.; Deep, A.; Kim, K.H. Nanomaterials for sensing of formaldehyde in air: Principles, applications, and performance evaluation. Nano Res. 2019, 12, 225–246. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Chen, D.Z.; Yuan, Y.J. Thin-Film Sensors for Detection of Formaldehyde: A Review. IEEE Sens. J. 2015, 15, 6749–6760. [Google Scholar] [CrossRef]
- Dai, H.; Gong, L.S.; Xu, G.F.; Li, X.H.; Zhang, S.P.; Lin, Y.Y.; Zeng, B.S.; Yang, C.P.; Chen, G.N. An electrochemical impedimetric sensor based on biomimetic electrospun nanofibers for formaldehyde. Analyst 2015, 140, 582–589. [Google Scholar] [CrossRef] [PubMed]
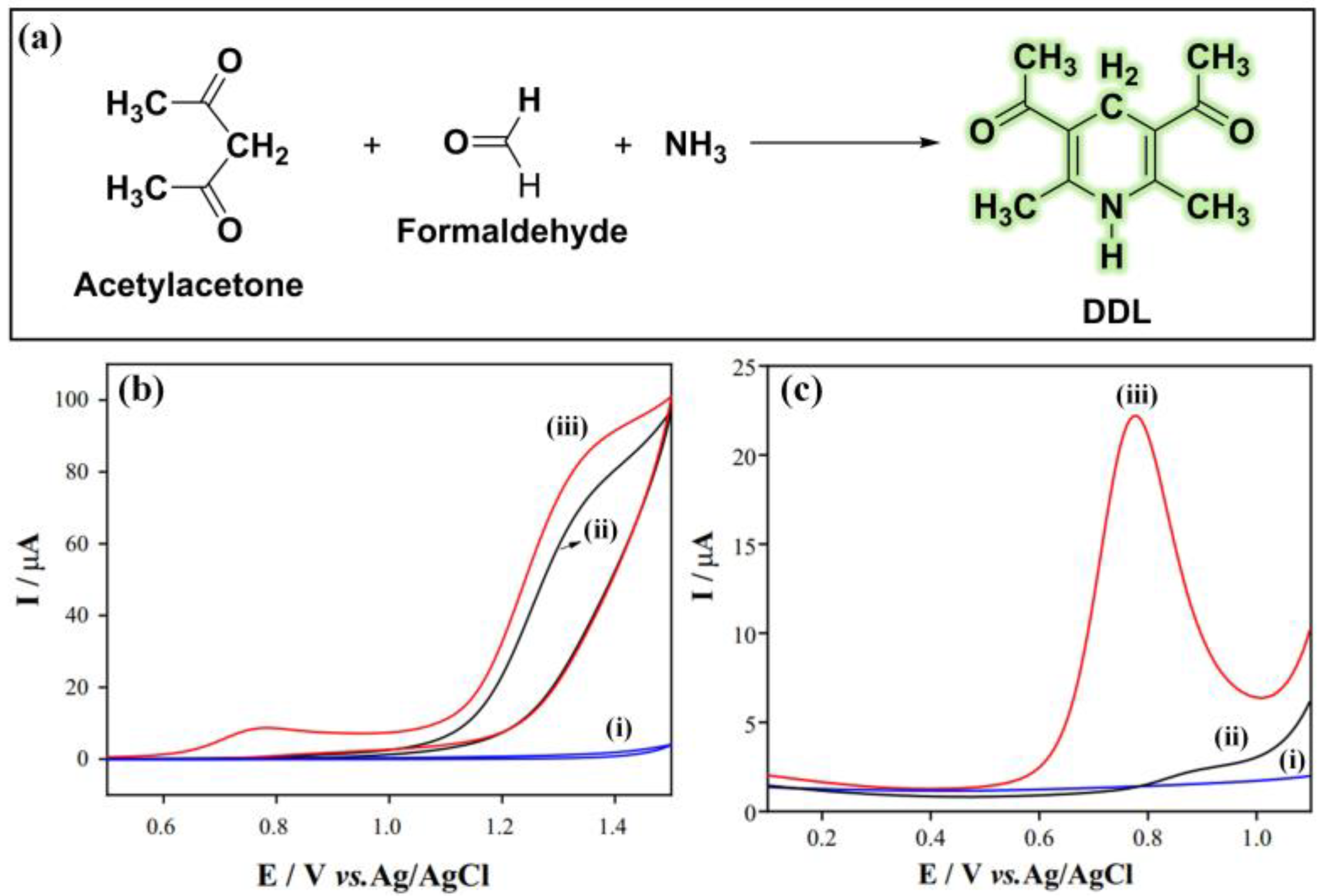
- Pinto, G.F.; Rocha, D.P.; Richter, E.M.; Muñoz, R.A.A.; Silva, S.G. Indirect determination of formaldehyde by square-wave voltammetry based on the electrochemical oxidation of 3,5-diacetyl-1,4-dihydrolutidine using an unmodified glassy-carbon electrode. Talanta 2019, 198, 237–241. [Google Scholar] [CrossRef]
- Kumaravel, S.; Wu, S.H.; Chen, G.Z.; Huang, S.T.; Lin, C.M.; Lee, Y.C.; Chen, C.H. Development of ratiometric electrochemical molecular switches to assay endogenous formaldehyde in live cells, whole blood and creatinine in saliva. Biosens. Bioelectron. 2021, 171, 112720. [Google Scholar] [CrossRef]
- Hammerle, M.; Hall, E.A.H.; Cade, N.; Hodgins, D. Electrochemical enzyme sensor for formaldehyde operating in the gas phase. Biosens. Bioelectron. 1996, 11, 239–246. [Google Scholar] [CrossRef]
- Herschkovitz, Y.; Eshkenazi, I.; Campbell, C.E.; Rishpon, J. An electrochemical biosensor for formaldehyde. J. Electroanal. Chem. 2000, 491, 182–187. [Google Scholar] [CrossRef]
- Vastarella, W.; Nicastri, R. Enzyme/semiconductor nanoclusters combined systems for novel amperometric biosensors. Talanta 2005, 66, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, O.; Shleev, S.; Gayda, G.; Demkiv, O.; Gonchar, M.; Gorton, L.; Csöregi, E.; Nistor, M. Bi-enzyme biosensor based on NAD+- and glutathione-dependent recombinant formaldehyde dehydrogenase and diaphorase for formaldehyde assay. Sens. Actuators B 2007, 125, 1–9. [Google Scholar] [CrossRef]
- Achmann, S.; Hämmerle, M.; Moos, R. Amperometric enzyme-based biosensor for direct detection of formaldehyde in the gas phase:: Dependence on electrolyte composition. Electroanalysis 2008, 20, 410–417. [Google Scholar] [CrossRef]
- Shimomura, T.; Itoh, T.; Sumiya, T.; Mizukami, F.; Ono, M. Electrochemical biosensor for the detection of formaldehyde based on enzyme immobilization in mesoporous silica materials. Sens. Actuators B 2008, 135, 268–275. [Google Scholar] [CrossRef]
- Korpan, Y.I.; Soldatkin, O.O.; Sosovska, O.F.; Klepach, H.M.; Csöregi, E.; Vocanson, F.; Jaffrezic-Renault, N.; Gonchar, M.V. Formaldehyde-sensitive conductometric sensors based on commercial and recombinant formaldehyde dehydrogenase. Microchim. Acta 2010, 170, 337–344. [Google Scholar] [CrossRef]
- Bareket, L.; Rephaeli, A.; Berkovitch, G.; Nudelman, A.; Rishpon, J. Carbon nanotubes based electrochemical biosensor for detection of formaldehyde released from a cancer cell line treated with formaldehyde-releasing anticancer prodrugs. Bioelectrochemistry 2010, 77, 94–99. [Google Scholar] [CrossRef]
- Marzuki, N.; Abu Bakar, F.; Salleh, A.; Heng, L.Y.; Yusof, N.A.; Siddiquee, S. Electrochemical Biosensor Immobilization of Formaldehyde Dehydrogenase with Nafion for Determination of Formaldehyde from Indian Mackerel (Rastrelliger kanagurta) Fish. Curr. Anal. Chem. 2012, 8, 534–542. [Google Scholar] [CrossRef]
- Ozoner, S.K.; Erhan, E.; Yilmaz, F.; Ergenekon, P.; Anil, I. Electrochemical biosensor for detection of formaldehyde in rain water. J. Chem. Technol. Biotechnol. 2013, 88, 727–732. [Google Scholar] [CrossRef]
- Nguyen-Boisse, T.T.; Saulnier, J.; Jaffrezic-Renault, N.; Lagarde, F. Miniaturised enzymatic conductometric biosensor with Nafion membrane for the direct determination of formaldehyde in water samples. Anal. Bioanal. Chem. 2014, 406, 1039–1048. [Google Scholar] [CrossRef]
- Premaratne, G.; Farias, S.; Krishnan, S. Pyrenyl carbon nanostructures for ultrasensitive measurements of formaldehyde in urine. Anal. Chim. Acta 2017, 970, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Prasad, S.; Krishnan, P.; Gajjala, S. A Novel Electrochemical Biosensor Based on Hematite (α-Fe2O3) Flowerlike Nanostructures for Sensitive Determination of Formaldehyde Adulteration in Fruit Juices. Food Bioprocess Technol. 2019, 12, 1659–1671. [Google Scholar] [CrossRef]
- Kundu, M.; Bhardwaj, H.; Pandey, M.K.; Krishnan, P.; Kotnala, R.K.; Sumana, G. Development of electrochemical biosensor based on CNT-Fe3O4 nanocomposite to determine formaldehyde adulteration in orange juice. J. Food Sci. Technol. 2019, 56, 1829–1840. [Google Scholar] [CrossRef]
- Kundu, M.; Rajesh; Krishnan, P.; Gajjala, S. Comparative Studies of Screen-Printed Electrode Based Electrochemical Biosensor with the Optical Biosensor for Formaldehyde Detection in Corn. Food Bioprocess Technol. 2021, 14, 726–738. [Google Scholar] [CrossRef]
- Teiserskyte, V.; Urbonavicius, J.; Ratautas, D. A direct electron transfer formaldehyde dehydrogenase biosensor for the determination of formaldehyde in river water. Talanta 2021, 234, 122657. [Google Scholar] [CrossRef] [PubMed]
- Gajjala, R.K.R.; Gade, P.S.; Bhatt, P.; Vishwakarma, N.; Singh, S. Enzyme decorated dendritic bimetallic nanocomposite biosensor for detection of HCHO. Talanta 2022, 238, 123054. [Google Scholar] [CrossRef] [PubMed]
- Dzyadevych, S.V.; Arkhypova, V.N.; Korpan, Y.I.; El’skaya, A.V.; Soldatkin, A.P.; Jaffrezic-Renault, N.; Martelet, C. Conductometric formaldehyde sensitive biosensor with specifically adapted analytical characteristics. Anal. Chim. Acta 2001, 445, 47–55. [Google Scholar] [CrossRef]
- Ling, Y.P.; Heng, L.Y. A Potentiometric Formaldehyde Biosensor Based on Immobilization of Alcohol Oxidase on Acryloxysuccinimide-modified Acrylic Microspheres. Sensors 2010, 10, 9963–9981. [Google Scholar] [CrossRef]
- Román, L.D.D.; Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Merino-Sánchez, C.; Merino-Amayuelas, M.P.; Arcos-Martínez, M.J. Fabrication and characterization of disposable sensors and biosensors for detection of formaldehyde. Talanta 2011, 86, 324–328. [Google Scholar] [CrossRef]
- Sigawi, S.; Smutok, O.; Demkiv, O.; Gayda, G.; Vus, B.; Nitzan, Y.; Gonchar, M.; Nisnevitch, M. Detection of Waterborne and Airborne Formaldehyde: From Amperometric Chemosensing to a Visual Biosensor Based on Alcohol Oxidase. Materials 2014, 7, 1055–1068. [Google Scholar] [CrossRef]
- Nurlely; Ahmad, M.; Heng, L.Y.; Tan, L.L. Potentiometric enzyme biosensor for rapid determination of formaldehyde based on succinimide-functionalized polyacrylate ion-selective membrane. Measurement 2021, 175, 109112. [Google Scholar] [CrossRef]
- Khlupova, M.; Kuznetsov, B.; Demkiv, O.; Gonchar, M.; Csöregi, E.; Shleev, S. Intact and permeabilized cells of the yeast Hansenula polymorpha as bioselective elements for amperometric assay of formaldehyde. Talanta 2007, 71, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Korpan, Y.I.; Gonchar, M.V.; Sibirny, A.A.; Martelet, C.; El’skaya, A.V.; Gibson, T.D.; Soldatkin, A.P. Development of highly selective and stable potentiometric sensors for formaldehyde determination. Biosens. Bioelectron. 2000, 15, 77–83. [Google Scholar] [CrossRef]
- Knake, R.; Jacquinot, P.; Hauser, P.C. Amperometric detection of gaseous formaldehyde in the ppb range. Electroanalysis 2001, 13, 631–634. [Google Scholar] [CrossRef]
- Ngamchana, S.; Surareungchai, W. Sub-millimolar determination of formalin by pulsed amperometric detection. Anal. Chim. Acta 2004, 510, 195–201. [Google Scholar] [CrossRef]
- Baez-Gaxiola, M.R.; Fernández-Sánchez, C.; Mendoza, E. Gold cluster based electrocatalytic sensors for the detection of formaldehyde. Anal. Methods 2015, 7, 538–542. [Google Scholar] [CrossRef]
- Nellaiappan, S.; Kumar, A.S.; Nisha, S.; Pillai, K.C. In-situ preparation of Au(111) oriented nanoparticles trapped carbon nanofiber-chitosan modified electrode for enhanced bifunctional electrocatalysis and sensing of formaldehyde and hydrogen peroxide in neutral pH solution. Electrochim. Acta 2017, 249, 227–240. [Google Scholar] [CrossRef]
- Xi, H.T.; Chen, X.G.; Cao, Y.; Xu, J.J.; Ye, C.Z.; Deng, D.W.; Zhang, J.S.; Huang, G.H. Electrochemical determination of formaldehyde via reduced AuNPs@PPy composites modified electrode. Microchem. J. 2020, 156, 104846. [Google Scholar] [CrossRef]
- Fu, D.L.; Chen, T.; Cheng, Y.J.; Li, A.H.; Liu, H.L.; Cheng, Z.F.; Li, P.F.; Liu, J.Q. A molecularly imprinted electrochemical sensing platform based on the signal amplification system fabricated with the theoretically optimized monomer for specific determination of formaldehyde. Sens. Actuators B 2021, 344, 130260. [Google Scholar] [CrossRef]
- Huang, X.Z.; Li, Y.N.; Witherspoon, E.; He, R.; Petruncio, G.; Paige, M.; Li, M.T.; Liu, T.C.; Amine, K.; Wang, Z.; et al. Species-Selective Detection of Volatile Organic Compounds by Ionic Liquid-Based Electrolyte Using Electrochemical Methods. ACS Sens. 2023, 8, 3389–3399. [Google Scholar] [CrossRef]
- Jin, G.P.; Li, J.; Peng, X. Preparation of platinum nanoparticles on polyaniline-coat multi-walled carbon nanotubes for adsorptive stripping voltammetric determination of formaldehyde in aqueous solution. J. Appl. Electrochem. 2009, 39, 1889–1895. [Google Scholar] [CrossRef]
- Chou, C.H.; Chang, J.L.; Zen, J.M. Effective analysis of gaseous formaldehyde based on a platinum-deposited screen-printed edge band ultramicroelectrode coated with Nafion as solid polymer electrolyte. Sens. Actuators B 2010, 147, 669–675. [Google Scholar] [CrossRef]
- He, J.H.; Zhang, S.T.; Cai, Y.H.; Song, Z.R. A Sensitive Electrochemical Sensor for the Detection of Formaldehyde Based on L-Alanine/Pt-Nanoparticles Modified Glassy Carbon Electrode. Asian J. Chem. 2013, 25, 10121–10126. [Google Scholar] [CrossRef]
- Chen, Y.A.; Liu, X.H.; Zhang, W.; Zhang, Y.; Li, L.J.; Cao, Z.Z.; Wang, H.; Jia, G.; Gao, Y.F.; Liu, J.R. Electrocatalytic oxidation of formaldehyde on direct electrodeposited graphene-platinum nanoparticles composites electrode. Anal. Methods 2013, 5, 3915–3919. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Z.; Zhi, Z.; Gao, W.; Sun, W.; Hua, Z.; Wu, Y. Practical and Efficient: A Pocket-Sized Device Enabling Detection of Formaldehyde Adulteration in Vegetables. ACS Omega 2022, 7, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.J.; Yi, Q.F. A novel nanoporous palladium catalyst for formaldehyde electro-oxidation in alkaline media. Rare Met. 2011, 30, 102–105. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Cai, Z.Q.; Chen, M.Q.; Cheng, F.L. A novel electrochemical sensor for formaldehyde based on palladium nanowire arrays electrode in alkaline media. Electrochim. Acta 2012, 68, 172–177. [Google Scholar] [CrossRef]
- Qiao, J.; Guo, Y.J.; Song, J.P.; Zhang, Y.C.; Sun, T.J.; Shuang, S.M.; Dong, C. Synthesis of a Palladium-Graphene Material and Its Application for Formaldehyde Determination. Anal. Lett. 2013, 46, 1454–1465. [Google Scholar] [CrossRef]
- Zhang, J.; Shangguan, L.Z.; Dong, C. Electrocatalytic oxidation of formaldehyde and formic acid at Pd nanoparticles modified glassy carbon electrode. Micro Nano Lett. 2013, 8, 704–708. [Google Scholar] [CrossRef]
- Ejaz, A.; Ahmed, M.S.; Jeon, S. Synergistic Effect of 1,4-Benzenedimethaneamine Assembled Graphene Supported Palladium for Formaldehyde Oxidation Reaction in Alkaline Media. J. Electrochem. Soc. 2016, 163, B163–B168. [Google Scholar] [CrossRef]
- Kongkaew, S.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. A preparation of homogeneous distribution of palladium nanoparticle on poly (acrylic acid)-functionalized graphene oxide modified electrode for formalin oxidation. Electrochim. Acta 2017, 247, 229–240. [Google Scholar] [CrossRef]
- Gor’kov, K.V.; Talagaeva, N.V.; Kleinikova, S.A.; Dremova, N.N.; Vorotyntsev, M.A.; Zolotukhina, E.V. Palladium-polypyrrole composites as prospective catalysts for formaldehyde electrooxidation in alkaline solutions. Electrochim. Acta 2020, 345, 136164. [Google Scholar] [CrossRef]
- Liu, X.G.; Chen, W.J.; Zhang, X. Highly Active Palladium-Decorated Reduced Graphene Oxides for Heterogeneous Catalysis and Electrocatalysis: Hydrogen Production from Formaldehyde and Electrochemical Formaldehyde Detection. Nanomaterials 2022, 12, 1890. [Google Scholar] [CrossRef] [PubMed]
- Torrarit, K.; Kongkaew, S.; Samoson, K.; Kanatharana, P.; Thavarungkul, P.; Chang, K.H.; Abdullah, A.F.L.; Limbut, W. Flow Injection Amperometric Measurement of Formalin in Seafood. ACS Omega 2022, 7, 17679–17691. [Google Scholar] [CrossRef] [PubMed]
- Soleh, A.; Saisahas, K.; Promsuwan, K.; Saichanapan, J.; Thavarungkul, P.; Kanatharana, P.; Meng, L.Y.; Mak, W.C.; Limbut, W. A wireless smartphone-based “tap-and-detect” formaldehyde sensor with disposable nano-palladium grafted laser-induced graphene (nanoPd@LIG) electrodes. Talanta 2023, 254, 124169. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.; Joo, Y.; Cho, J.C.; Choi, J.M.; Kim, J.Y.; Lee, S.; Jeon, S. Synthesis and catalytic activity of Ag nanoparticles dispersed on nitrogen-doped GOPx toward direct electrooxidation of formaldehyde. J. Electroanal. Chem. 2018, 813, 31–38. [Google Scholar] [CrossRef]
- Mu, Z.Y.; Zhou, X.L.; Fan, Z.; Fu, C.Q.; Dan, Z.H.; Chang, H. Fabrication of Nanoporous Silver and Its Superior Sensitivity to Formaldehyde. Rare Metal. Mat. Eng. 2019, 48, 3252–3257. [Google Scholar]
- Yang, Y.L.; Mu, Z.Y.; Fan, Z.; Dan, Z.H.; Wang, Y.; Chang, H. Nanoporous Silver via Electrochemical Dealloying and Its Superior Detection Sensitivity to Formaldehyde. Acta Metall. Sin. 2019, 55, 1302–1310. [Google Scholar]
- Sun, W.; Sun, G.Q.; Qin, B.; Xin, Q. A fuel-cell-type sensor for detection of formaldehyde in aqueous solution. Sens. Actuators B 2007, 128, 193–198. [Google Scholar] [CrossRef]
- Zhou, Z.L.; Kang, T.F.; Zhang, Y.; Cheng, S.Y. Electrochemical sensor for formaldehyde based on Pt-Pd nanoparticles and a Nafion-modified glassy carbon electrode. Microchim. Acta 2009, 164, 133–138. [Google Scholar] [CrossRef]
- Safavi, A.; Farjami, F. Electrochemical Design of Ultrathin Palladium Coated Gold Nanoparticles as Nanostructured Catalyst for Amperometric Detection of Formaldehyde. Electroanalysis 2011, 23, 1842–1848. [Google Scholar] [CrossRef]
- Safavi, A.; Momeni, S.; Tohidi, M. Silver-Palladium Nanoalloys Modified Carbon Ionic Liquid Electrode with Enhanced Electrocatalytic Activity Towards Formaldehyde Oxidation. Electroanalysis 2012, 24, 1981–1988. [Google Scholar] [CrossRef]
- Yi, Q.F.; Zuo, G.K.K. Nanoporous Pt Catalyst Modified by Sn Electrodeposition for Electrochemical Oxidation of Formaldehyde. Chin. J. Chem. 2012, 30, 151–156. [Google Scholar] [CrossRef]
- Pötzelberger, I.; Mardare, C.C.; Burgstaller, W.; Hassel, A.W. Maximum electrocatalytic oxidation performance for formaldehyde in a combinatorial copper-palladium thin film library. Appl. Catal. A-Gen. 2016, 525, 110–118. [Google Scholar] [CrossRef]
- Qiao, J.; Chang, J.J.; Wang, H.J.; Sun, T.J.; Dong, C. Determination of Formaldehyde with a Platinum-Palladium-Graphene Nanocomposite Glassy Carbon Electrode. Anal. Lett. 2017, 50, 80–90. [Google Scholar] [CrossRef]
- Kavian, S.; Azizi, S.N.; Ghasemi, S. Novel bimetallic nanoporous Pd-Cu-SBA-16/CPE as a highly sensitive sensor for determination of formaldehyde. J. Electroanal. Chem. 2017, 799, 308–314. [Google Scholar] [CrossRef]
- Nachaki, E.O.; Ndangili, P.M.; Naumih, N.M.; Masika, E. Nickel-Palladium-Based Electrochemical Sensor for Quantitative Detection of Formaldehyde. Chemistryselect 2018, 3, 384–392. [Google Scholar] [CrossRef]
- Li, S.; Cheng, Y.R.; Shi, J.C.; Wang, X.F.; Xu, P.C.; Ying, C.; Yuan, Z.; Sen, Z.; Li, X.X. Electrochemical Sensor with Bimetallic Pt-Ag Nanoparticle as Catalyst for the Measurement of Dissolved Formaldehyde. J. Electrochem. Soc. 2022, 169, 047507. [Google Scholar]
- Zhang, J.X.; Lv, F.; Li, Z.H.; Jiang, G.Y.; Tan, M.J.; Yuan, M.L.; Zhang, Q.H.; Cao, Y.P.; Zheng, H.Y.; Zhang, L.L.; et al. Cr-Doped Pd Metallene Endows a Practical Formaldehyde Sensor New Limit and High Selectivity. Adv. Mater. 2022, 34, 2105276. [Google Scholar] [CrossRef]
- Chen, P.Y.; Yang, H.H.; Zen, J.M.; Shih, Y. Sensitive and Simple Flow Injection Analysis of Formaldehyde Using an Activated Barrel Plating Nickel Electrode. J. AOAC Int. 2011, 94, 1585–1591. [Google Scholar] [CrossRef]
- Zhang, J.; Shangguan, L.Z.; Shuang, S.M.; Dong, C. Electrocatalytic oxidation of formaldehyde and methanol on Ni(OH)2/Ni electrode. Russ. J. Electrochem. 2013, 49, 888–894. [Google Scholar] [CrossRef]
- Azizi, S.N.; Ghasemi, S.; Amiripoura, F. Nickel/P nanozeolite modified electrode: A new sensor for the detection of formaldehyde. Sens. Actuators B 2016, 227, 1–10. [Google Scholar] [CrossRef]
- Wen, X.; Xi, J.J.; Long, M.; Tan, L.; Wang, J.J.; Yan, P.; Zhong, L.F.; Liu, Y.; Tang, A.D. Ni(OH)2/Ni based on TiO2 nanotube arrays binder-free electrochemical sensor for formaldehyde accelerated detection. J. Electroanal. Chem. 2017, 805, 68–74. [Google Scholar] [CrossRef]
- Hassaninejad-Darzi, S.K. Application of Synthesized NaA Nanozeolite as a Novel Supported Electrode for the Formaldehyde Electro-catalytic Oxidation. Fuel Cells 2018, 18, 82–95. [Google Scholar] [CrossRef]
- TrivediJ, D.; Crosse, J.; Tanti, J.; Cass, A.J.; Toghill, K.E. The electrochemical determination of formaldehyde in aqueous media using nickel modified electrodes. Sens. Actuators B 2018, 270, 298–303. [Google Scholar] [CrossRef]
- Daemi, S.; Moalem-Banhangi, M.; Ghasemi, S.; Ashkarran, A.A. An efficient platform for the electrooxidation of formaldehyde based on amorphous NiWO4 nanoparticles modified electrode for fuel cells. J. Electroanal. Chem. 2019, 848, 113270. [Google Scholar] [CrossRef]
- Trafela, S.; Zavasnik, J.; Sturm, S.; Rozman, K.Z. Formation of a Ni(OH)2/NiOOH active redox couple on nickel nanowires for formaldehyde detection in alkaline media. Electrochim. Acta 2019, 309, 346–353. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Rehman, A. Facile and scalable fabrication of nanostructured nickel thin film electrodes for electrochemical detection of formaldehyde. Anal. Methods 2020, 12, 4028–4036. [Google Scholar] [CrossRef]
- Zhou, F.L.; Wang, Q.; Huang, K.; Jiang, X.; Zou, Z.R.; Xiong, X.L. Flame synthesis of NiO nanoparticles on carbon cloth: An efficient non-enzymatic sensor for glucose and formaldehyde. Microchem. J. 2020, 159, 105505. [Google Scholar] [CrossRef]
- Li, S.J.; Li, M.; Yang, H.; Shao, J.Y.; Meng, Z.C. Facile Synthesis of Flower-like Ni(OH)2/rGO Nanocomposite as Sensitive Electrochemical Sensor for Formaldehyde Detection. Int. J. Electrochem. Sci. 2022, 17, 22079. [Google Scholar] [CrossRef]
- Zhou, J.X.; Zhao, L.; Wang, Q.; Zuo, L.X.; Zhao, A.; Yu, H.M.; Jiang, X.; Xiong, X.L. Plasma-induced chemical etching generating Ni3S2 for formaldehyde detection. Chin. Chem. Lett. 2022, 33, 3035–3038. [Google Scholar] [CrossRef]
- Zhuo, K.; Wang, J.H.; Hou, W.; Cheng, Y.Q.; Sang, S.B. SnO2 doped NiO heterostructure nanofibers prepared by electrostatic spinning: A novel sensor for catalytic oxidation of formaldehyde. Microchem. J. 2022, 180, 107579. [Google Scholar] [CrossRef]
- Chai, A.W.; Wang, C.C.; Chen, C.Y. Acicular Nickel on Carbon Nanofiber as Electrochemical Sensor Electrode for the Rapid Detection of Aqueous and Gaseous Formaldehyde at Room Temperature. Electroanalysis 2023, 35, 220036. [Google Scholar] [CrossRef]
- Trafela, S.; Krishnamurthy, A.; Soderznik, K.Z.; Kavcic, U.; Karlovits, I.; Klopcic, B.; Sturm, S.; Zuzek, K. IoT Electrochemical Sensor with Integrated Ni(OH)2-Ni Nanowires for Detecting Formaldehyde in Tap Water. Sensors 2023, 23, 4676. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Khalilzadeh, B.; Shadjou, N.; Karim-Nezhad, G.; Saghatforoush, L.; Kazeman, I.; Abnosi, M.H. A New Kinetic-Mechanistic Approach to Elucidate Formaldehyde Electrooxidation on Copper Electrode. Electroanalysis 2010, 22, 168–176. [Google Scholar] [CrossRef]
- Jing, Z.H.; Lin, X.Q. Electrocatalytic Oxidation of Formaldehyde on Copper Oxide Nano-crystalline Modified Glassy Carbon Electrode. Chin. J. Chem. 2010, 28, 2359–2363. [Google Scholar] [CrossRef]
- Pötzelberger, I.; Mardare, A.I.; Hassel, A.W. Copper-nickel combinatorial library screening for electrocatalytic formaldehyde oxidation. Phys. Status Solid A 2017, 214, 1600552. [Google Scholar] [CrossRef]
- Momeni, S.; Sedaghati, F. CuO/Cu2O nanoparticles: A simple and green synthesis, characterization and their electrocatalytic performance toward formaldehyde oxidation. Microchem. J. 2018, 143, 64–71. [Google Scholar] [CrossRef]
- Hajilari, F.; Farhadi, K.; Eskandari, H.; Allahnouri, F. Application of Cu/porous silicon nanocomposite screen printed sensor for the determination of formaldehyde. Electrochim. Acta 2020, 355, 136751. [Google Scholar] [CrossRef]
- Rahman, M.M. Efficient formaldehyde sensor development based on Cu-codoped ZnO nanomaterial by an electrochemical approach. Sens. Actuators B 2020, 305, 127541. [Google Scholar] [CrossRef]
- Zhang, S.L.; Wen, X.; Long, M.; Xi, J.J.; Hu, J.Q.; Tang, A.D. Fabrication ofCuO/Cu/TiO2 nanotube arrays modified electrode for detection of formaldehyde. J. Alloys Compd. 2020, 829, 154568. [Google Scholar] [CrossRef]
- Yang, L.; Yang, J.; Dong, Q.Y.; Zhou, F.L.; Wang, Q.; Wang, Z.P.; Huang, K.; Yu, H.M.; Xiong, X.L. One-step synthesis of CuO nanoparticles based on flame synthesis: As a highly effective non-enzymatic sensor for glucose, hydrogen peroxide and formaldehyde. J. Electroanal. Chem. 2021, 881, 114965. [Google Scholar] [CrossRef]
- Kumar, H.; Kumari, N.; Singh, D. Quantum dots decorated polyaniline plastic nanocomposites as a novel amperometric sensor for formaldehyde: Experimental and theoretical approach. Talanta Open 2022, 6, 100141. [Google Scholar] [CrossRef]
- Fukunaga, M.T.; Guimaraes, J.R.; Bertazzoli, R. Kinetics of the oxidation of formaldehyde in a flow electrochemical reactor with TiO2/RuO2 anode. Chem. Eng. J. 2008, 136, 236–241. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Sato, A.; Iwai, S.; Tomono, K.; Nakayama, M. A novel formaldehyde sensor based on the pseudocapacitive catalysis of birnessite. Electrochem. Commun. 2013, 29, 55–58. [Google Scholar] [CrossRef]
- Alolaywi, H.Y.; Duanghathaipornsuk, S.; Kim, S.S.; Li, C.H.; Jinschek, J.R.; Kim, D.S.; Alba-Rubio, A.C. Electrochemical MoOx/Carbon Nanocomposite-Based Gas Sensor for Formaldehyde Detection at Room Temperature. J. Electrochem. Soc. 2021, 168, 067525. [Google Scholar] [CrossRef]
- Dong, Q.Y.; Yang, L.; He, Z.Y.; Zhang, Y.; Tang, X.; Tang, J.Y.; Huang, K.; Zou, Z.R.; Xiong, X.L. Co-Based Transition Metal Hydroxide Nanosheet Arrays on Carbon Cloth for Sensing Glucose and Formaldehyde. ACS Appl. Nano Mater. 2021, 4, 5076–5083. [Google Scholar] [CrossRef]
- Ganie, A.S.; Bano, S.; Sultana, S.; Sabir, S.; Khan, M.Z. Ferrite Nanocomposite based Electrochemical Sensor: Characterization, Voltammetric and Amperometric Studies for Electrocatalytic Detection of Formaldehyde in aqueous Media. Electroanalysis 2021, 33, 233–248. [Google Scholar] [CrossRef]
- Nag, S.; Pradhan, S.; Naskar, H.; Roy, R.B.; Tudu, B.; Pramanik, P.; Bandyopadhyay, R. A Simple Nano Cerium Oxide Modified Graphite Electrode for Electrochemical Detection of Formaldehyde in Mushroom. IEEE Sens. J. 2021, 21, 12019–12026. [Google Scholar] [CrossRef]
- Padmalaya, G.; Vardhan, K.H.; Kumar, P.S.; Ali, M.A.; Chen, T.W. A disposable modified screen-printed electrode using egg white/ZnO rice structured composite as practical tool electrochemical sensor for formaldehyde detection and its comparative electrochemical study with Chitosan/ZnO nanocomposite. Chemosphere 2022, 288, 132560. [Google Scholar] [CrossRef]
- Wicaksono, W.P.; Fadilla, N.I.; Zamar, A.A.; Fadillah, G.; Anugrahwati, M.; Anas, A.K.; Kadja, G.T.M. Formaldehyde electrochemical sensor using graphite paste-modified green synthesized zinc oxide nanoparticles. Inorg. Chem. Commun. 2022, 143, 109729. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Lin, F.F.; Wei, T.; Fu, D.G.; Pei, L.Z. Synthesis and Electrochemical Performance of Polypyrrole/Graphene Nanocomposites for the Detection of Formaldehyde. Int. J. Electrochem. Sci. 2019, 14, 4371–4382. [Google Scholar] [CrossRef]
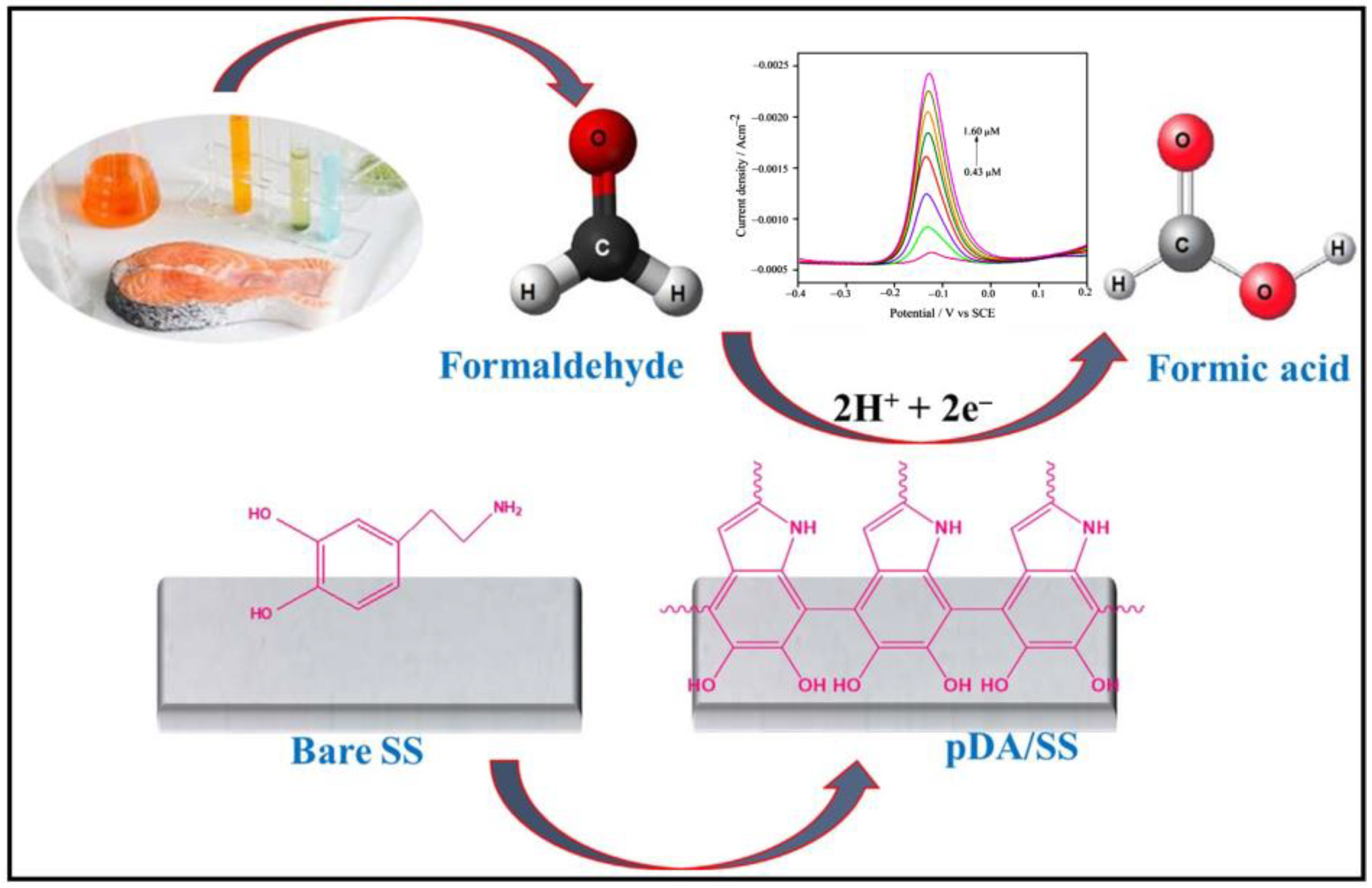
- Philip, A.S.; Rison, S.; Cherian, A.R.; Akshaya, K.B.; George, L.; Varghese, A. Electrochemical Sensing of Formaldehyde in Fish Samples Using a Polydopamine-Modified Stainless Steel Electrode. ECS J. Solid State Sci. Technol. 2021, 10, 067003. [Google Scholar] [CrossRef]
- Nag, S.; Pradhan, S.; Das, D.; Tudu, B.; Bandyopadhyay, R.; Roy, R.B. Fabrication of a Molecular Imprinted Polyacrylonitrile Engraved Graphite Electrode for Detection of Formalin in Food Extracts. IEEE Sens. J. 2022, 22, 42–49. [Google Scholar] [CrossRef]
- Wang, Y.F.; Liu, D.L.; Han, J.J.; Guo, A.R. Detection of formaldehyde by cyclic voltammetry using a PANI/GO composite film-modified electrode. Ionics 2022, 28, 2457–2468. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, L.; Huang, X.; Ju, W.; Liang, Y.; Tao, Z.; Yang, Y.; Zhu, B.; Wei, G. Efficient formaldehyde sensor based on PtPd nanoparticles-loaded nafion-modified electrodes. Nanotechnology 2024, 35, 025704. [Google Scholar] [CrossRef]
- Luo, M.C.; Zhao, Z.L.; Zhang, Y.L.; Sun, Y.J.; Xing, Y.; Lv, F.; Yang, Y.; Zhang, X.; Hwang, S.; Qin, Y.N.; et al. PdMo bimetallene for oxygen reduction catalysis. Nature 2019, 574, 81–85. [Google Scholar] [CrossRef]
- Dang, Q.; Lin, H.P.; Fan, Z.L.; Ma, L.; Shao, Q.; Ji, Y.J.; Zheng, F.F.; Geng, S.Z.; Yang, S.Z.; Kong, N.N.; et al. Iridium metallene oxide for acidic oxygen evolution catalysis. Nat. Commun. 2021, 12, 6007. [Google Scholar] [CrossRef]
- Ta, H.Q.; Mendes, R.G.; Liu, Y.; Yang, X.Q.; Luo, J.P.; Bachmatiuk, A.; Gemming, T.; Zeng, M.Q.; Fu, L.; Liu, L.J.; et al. In Situ Fabrication of Freestanding Single-Atom-Thick 2D Metal/Metallene and 2D Metal/ Metallene Oxide Membranes: Recent Developments. Adv. Sci. 2021, 8, 2100619. [Google Scholar] [CrossRef]
- Deng, K.; Zhou, T.Q.; Mao, Q.Q.; Wang, S.Q.; Wang, Z.Q.; Xu, Y.; Li, X.N.A.; Wang, H.J.; Wang, L. Surface Engineering of Defective and Porous Ir Metallene with Polyallylamine for Hydrogen Evolution Electrocatalysis. Adv. Mater. 2022, 34, 2110680. [Google Scholar] [CrossRef]
- Li, X.C.; Shen, P.; Luo, Y.J.; Li, Y.H.; Guo, Y.L.; Zhang, H.; Chu, K. PdFe Single-Atom Alloy Metallene for N2 Electroreduction. Angew. Chem. Int. Ed. 2022, 61, e202205923. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Guo, Y.N.; Sun, F.Y.; Wang, S.Y.; Kang, Y.Q.; Xu, X.T.; Zhao, J.J.; You, J.; Eguchi, M.; Yamauchi, Y.; et al. Nanoarchitectonics of Metallene Materials for Electrocatalysis. ACS Nano 2023, 17, 13017–13043. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ma, Z.Y.; Li, X.C.; Kang, J.L.; Ma, D.W.; Chu, K. Single-Atom Bi Alloyed Pd Metallene for Nitrate Electroreduction to Ammonia. Adv. Funct. Mater. 2023, 33, 2209890. [Google Scholar] [CrossRef]
- Li, X.T.; Shen, P.; Li, X.C.; Ma, D.W.; Chu, K. Sub-nm RuOX Clusters on Pd Metallene for Synergistically Enhanced Nitrate Electroreduction to Ammonia. ACS Nano 2023, 17, 1081–1090. [Google Scholar] [CrossRef]
- Liu, Y.D.; Dinh, K.N.; Dai, Z.F.; Yan, Q.Y. Metallenes: Recent Advances and Opportunities in Energy Storage and Conversion Applications. ACS Mater. Lett. 2020, 2, 1148–1172. [Google Scholar] [CrossRef]
- Xie, M.H.; Tang, S.S.; Zhang, B.W.; Yu, G.H. Metallene-related materials for electrocatalysis and energy conversion. Mater. Horiz. 2023, 10, 407–431. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Y.; Jiao, L.; Wang, H.J.; Wu, Z.C.; Wei, X.Q.; Wu, Y.; Wu, N.N.; Hu, L.Y.; Zhong, H.; et al. Atomically thin bismuthene nanosheets for sensitive electrochemical determination of heavy metal ions. Anal. Chim. Acta 2022, 1235, 340510. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.J.; Jiao, L.; Wu, N.N.; Xu, W.Q.; Wu, Z.C.; Wu, Y.; Hu, P.; Gu, W.L.; Zhu, C.Z. Defect engineering of PdMo metallene for sensitive electrochemical detection of dopamine. Chem. Eng. J. 2023, 466, 143075. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 Nanotubes: Recent Advances in Synthesis and Gas Sensing Properties. Sensors 2013, 13, 14813–14838. [Google Scholar] [CrossRef]
- Azer, B.B.; Gulsaran, A.; Pennings, J.R.; Saritas, R.; Kocer, S.; Bennett, J.L.; Abhang, Y.D.; Pope, M.A.; Abdel-Rahman, E.; Yavuz, M. A Review: TiO2 based photoelectrocatalytic chemical oxygen demand sensors and their usage in industrial applications. J. Electroanal. Chem. 2022, 918, 116466. [Google Scholar] [CrossRef]
- Hao, Y.Q.; Li, T.; Luo, L.J.; Fan, S.N.; Chen, S.; Zhang, Y.T.; Tang, Z.L.; Xu, M.T.; Zeng, R.J.; Chen, S. A reaction based dual-modal probe for fluorescent and photoelectrochemical determination of thiophenol. Sens. Actuators B 2022, 369, 132405. [Google Scholar] [CrossRef]
- Li, T.; Hao, Y.Q.; Dong, H.; Li, C.L.; Liu, J.X.; Zhang, Y.T.; Tang, Z.L.; Zeng, R.J.; Xu, M.T.; Chen, S. Target-Induced In Situ Formation of Organic Photosensitizer: A New Strategy for Photoelectrochemical Sensing. ACS Sens. 2022, 7, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Luo, Y.; Hao, Y.; Han, H.; Hu, X.; Yang, Y.; Long, X.; He, J.; Zhang, P.; Zeng, R.; et al. A responsive organic probe based photoelectrochemical sensor for hydrazine detection. Spectrochim. Acta Part A 2024, 305, 123463. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.; Li, H.H.; Deng, L.; Zhao, S.F.; Ouyang, J.; Wen, M.; Chen, W.S.; Zeng, K.; Wei, C.W.; Liu, Y.N. A Cascade Nanozyme with Amplified Sonodynamic Therapeutic Effects through Comodulation of Hypoxia and Immunosuppression against Cancer. ACS Nano 2022, 16, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.Q.; Gao, X.Y.; Wen, M.; Liu, Y.H.; Li, Y.Q.; Wei, C.A.W.; Wu, X.B.; Zou, Y.Y.; Li, J.H.; Li, X.L.; et al. Biocomputation with MnTiO3 Piezoelectric Enzymes for Programed Catalysis of Tumor Death. ACS Appl. Mater. Interfaces 2022, 14, 28199–28210. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.Y.; Li, L.B.; Tang, X.N.; Zhai, W.J.; Hong, Y.S.; Hu, T.; Yuan, K.; Chen, Y.W. Pyrolysis-free polymer-based oxygen electrocatalysts. Energy Environ. Sci. 2021, 14, 2789–2808. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Mohamad, A.A.; Nguyen, M.T.; Yonezawa, T.; Qin, J.; Thamyongkit, P.; Somwangthanaroj, A.; Kheawhom, S. Recent advances in oxygen electrocatalysts based on tunable structural polymers. Mater. Today Chem. 2022, 23, 100632. [Google Scholar] [CrossRef]
- Ma, J.; Wu, W.; Xiao, X.; Feng, Y.; Hao, Y.; Zhang, J.; Liu, C.; Zhang, P.; Chen, J.; Zeng, R.; et al. New insight into electropolymerization of melamine. II: Low onset potential deposition of polymelamine with trace active bromine. Electrochim. Acta 2022, 410, 139991. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Zuo, Y.; Chen, S.; Zhuo, Y.; Lu, M.; Shi, G.; Gu, H. In Vivo Monitoring of pH in Subacute PD Mouse Brains with a Ratiometric Electrochemical Microsensor Based on Poly(melamine) Films. ACS Sens. 2022, 7, 235–244. [Google Scholar] [CrossRef]
- Menart, E.; Jovanovski, V.; Hocevar, S.B. Novel hydrazinium polyacrylate-based electrochemical gas sensor for formaldehyde. Sens. Actuators B 2017, 238, 71–75. [Google Scholar] [CrossRef]
- Li, H.B.; Li, Y.L.; Li, M.Y.; Xu, L.Q.; Li, J. Facile and ultrasensitive electrochemical impedance sensing for formaldehyde based on silver ions doped in controllable and homogeneous silica microspheres. Sens. Actuators B 2019, 284, 657–662. [Google Scholar] [CrossRef]
- Dvořák, P.; Ramos, R.M.; Vyskočil, V.; Rodrigues, J.A. A new electroanalytical methodology for the determination of formaldehyde in wood-based products. Talanta 2020, 217, 121068. [Google Scholar] [CrossRef] [PubMed]
- Bruemmer, K.J.; Walvoord, R.R.; Brewer, T.F.; Burgos-Barragan, G.; Wit, N.; Pontel, L.B.; Patel, K.J.; Chang, C.J. Development of a General Aza-Cope Reaction Trigger Applied to Fluorescence Imaging of Formaldehyde in Living Cells. J. Am. Chem. Soc. 2017, 139, 5338–5350. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, Y.; Huang, M.; Wang, S.; Wang, J.; Liao, K.; Wu, X.; Zhou, Q.; Zhang, X.; Wu, Y.-D.; et al. Systematic investigation of the aza-Cope reaction for fluorescence imaging of formaldehyde in vitro and in vivo. Chem. Sci. 2021, 12, 13857–13869. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.; Zhang, W.; Liu, J.; Wang, Y.-L.; Du, Z.; Song, B.; Xu, Z.P.; Yuan, J. “Dual-Key-and-Lock” Ruthenium Complex Probe for Lysosomal Formaldehyde in Cancer Cells and Tumors. J. Am. Chem. Soc. 2019, 141, 8462–8472. [Google Scholar] [CrossRef]




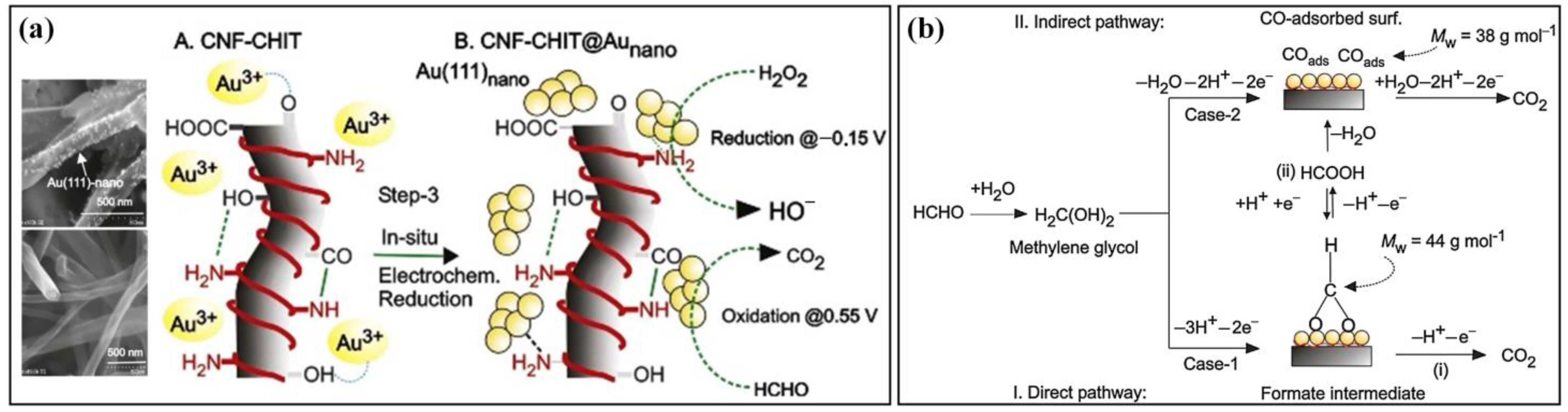
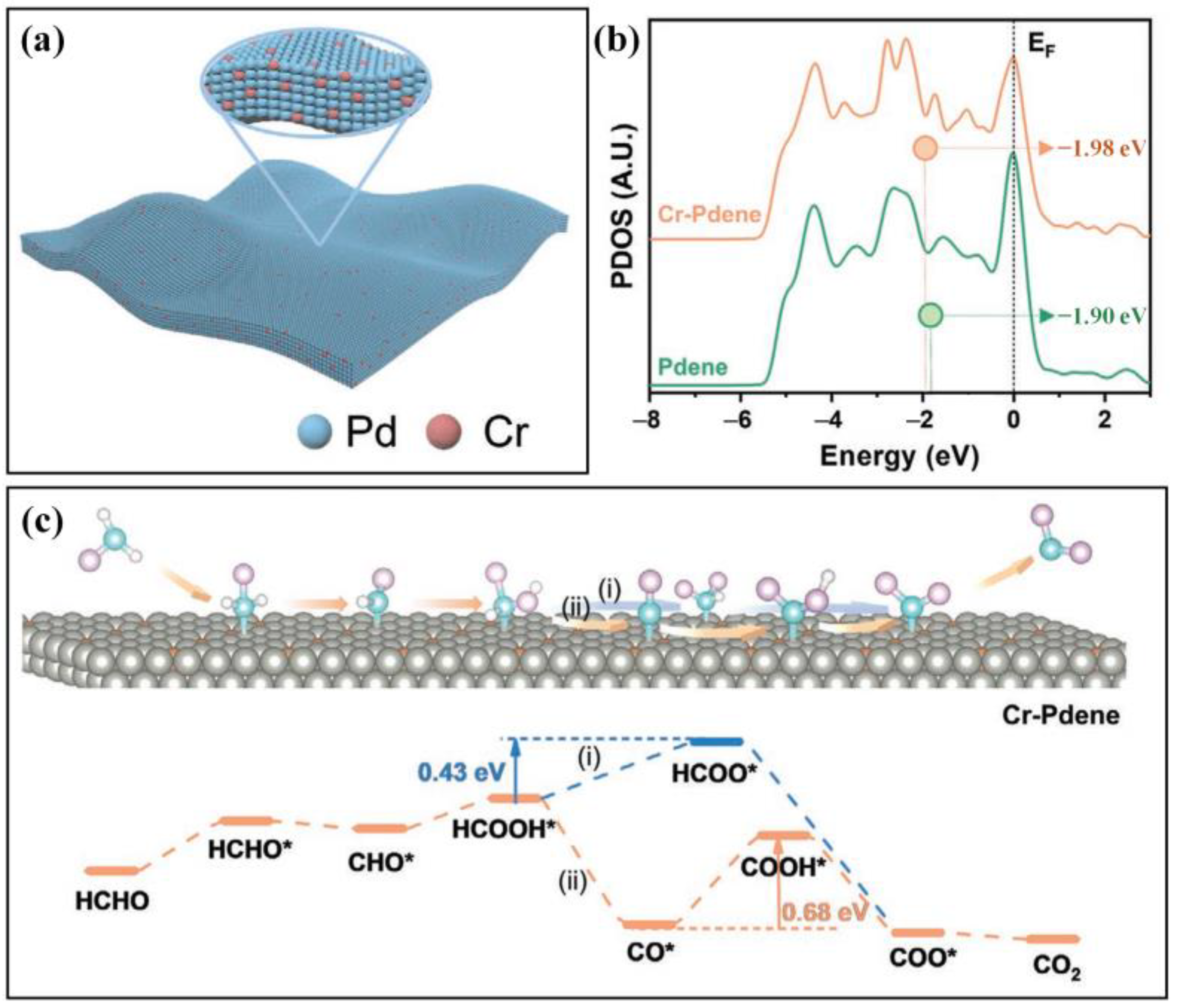
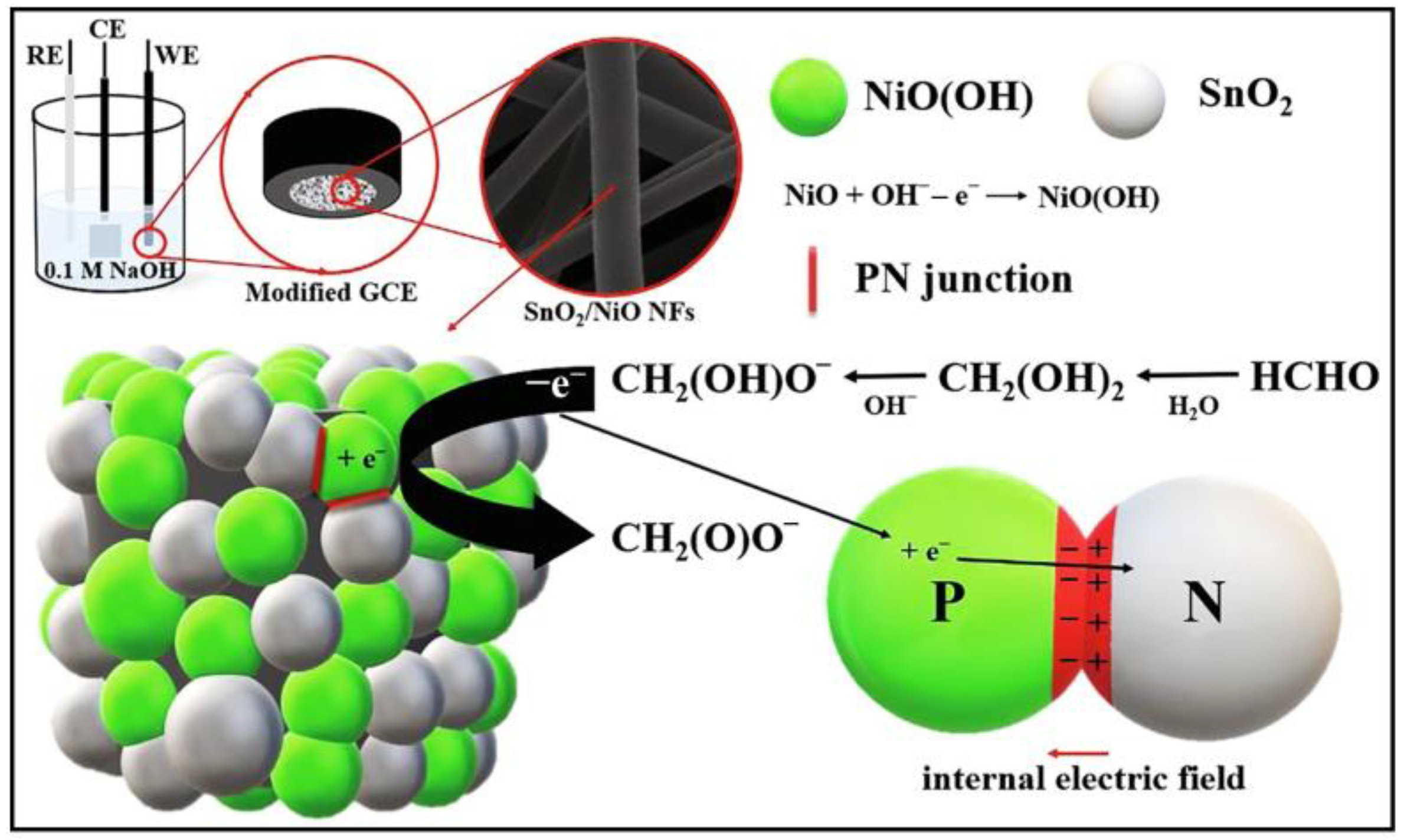

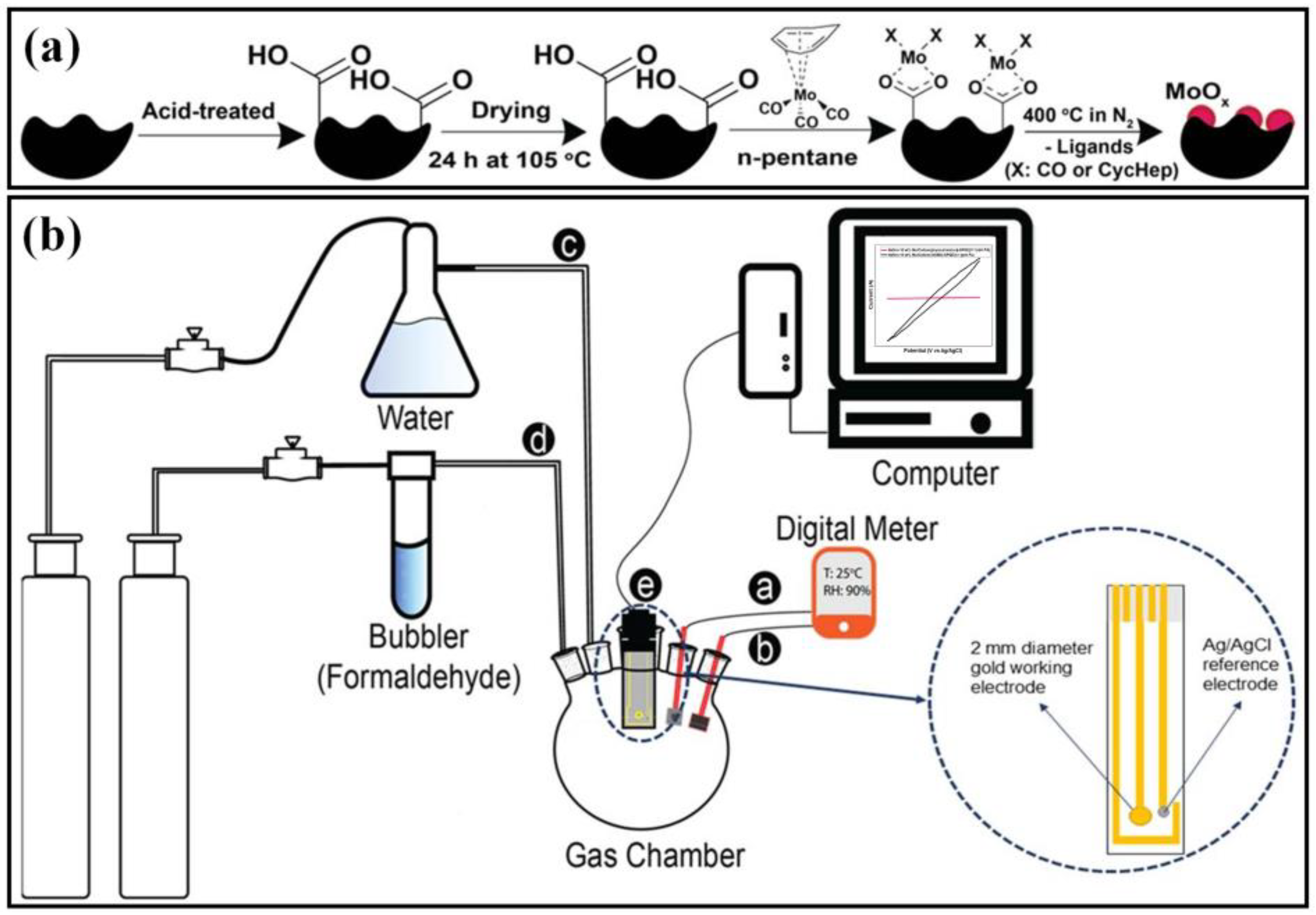

| Electrode Materials | Signal Mode | Dynamic Range | Detection Limit | Applications | Ref. |
|---|---|---|---|---|---|
| Pt/FDH | Amperometry | 0–6 vppm | 0.3 vppm | Gas sensing | [60] |
| SPEs/POs-EA/FDH | Amperometry | 0.030–1.5 mg∙mL−1 | 30 ng∙mL−1 | -- | [61] |
| Au/CdS/FDH@Nylon | Amperometry | 0.05–1 µg∙mL−1 | 41 ng∙mL−1 | -- | [62] |
| Graphite/PVI-Os/DPH/FDH | Amperometry | 0.05–0.5 mM | 32 µM | -- | [63] |
| PTFE/graphite/FDH | Amperometry | 0–15 ppm | 0.03 ppm | -- | [64] |
| GCE/FDH@FSM8.0/Nylon | Amperometry | 1.2–617 µM | 1.1 µM | -- | [65] |
| Au electrodes/Dextran@FDH | Conductometry | 10–200 mM | -- | -- | [66] |
| SPEs/CNTs | Amperometry | 0.1–100 μM | -- | -- | [67] |
| Au/Nafion@FDH | Amperometry | 0.1–10 ppm | 0.016 ppm | Fish, Mackerel | [68] |
| GCE/poly(GMA-co-MTM)@FDH/PPyfilm | Amperometry | 3.3–100 μM | 0.15 μM | Water | [69] |
| Interdigitated gold electrodes/Nafion@FDH-GA | Conductometry | 0–10 mM | 18 μM | Water | [70] |
| AuSPE/MWNT@PBA-FDH | Amperometry | 10 ppb to 10 ppm | 6 ppb | Urine | [71] |
| ITO/α-Fe2O3/FDH | Amperometry | 0.0–0.3 mg∙L−1 | 0.02 mg∙L−1 | Juice | [72] |
| ITO/CNT-Fe3O4/FDH | Amperometry | 0.05–0.50 mg∙L−1 | 0.05mg∙L−1 | Juice | [73] |
| Bioanode: BP/PMG/FDH Cathode: CFP/Au NPs/PB | Amperometry Colorimetry | 0.01–0.35 mM 0.01-0.045 mM | 0.006 mM | Water | [39] |
| SPE/FDH/BSA ITO/α-Fe2O3/FDH | Amperometry Colorimetry | 0.01–0.5 mg/L 0.01–0.5 mg/L | 0.03 mg/L 0.03 mg/L | Corn | [74] |
| Bioanode: FDH/PMG/BP Cathode: ITO/PB | Amperometry Colorimetry | 80 and 3000 ppb | Gas | [38] | |
| Au electrode/Au NPs/FDH | Amperometry | 0.25–2.0 mM | 0.05 mM | Water | [75] |
| SPE/ErGO/AuPd/Cys/FDH | Amperometry | 1–100 μM | 0.3 μM | Fish | [76] |
| Cu electrode/ALDHs | Amperometry | 10−15−10−5 M | 10−15 M | -- | [35] |
| Gold interdigitated electrode/DEAE-dextran@lactitol/BSA@AOX | Conductometry | 0.05−500 mM | 0.05 mM | -- | [77] |
| SPE(Ag/AgCl)/pH transducer/poly(nBA-NAS)-AOX | Potentiometry | 0.3–316.2 mM | 0.3 mM | Shrimp | [78] |
| SPCPtEs/BSA@AOX | Amperometry | 60-460 µM | 60 µM | Histogen | [79] |
| Carbon (or gold) electrode/AOX&HRP(aq.) | Amperometry | 0–5 mM | -- | Gas | [80] |
| SPE(Ag/AgCl)/pHEMA/poly(nBA-NAS)-AOX | potentiometry | 0.5–220.0 mM | 0.1 mM | Fish | [81] |
| Electrocatalysts | Electrode Materials | Electrolyte | Dynamic Range | Detection Limit | Applications | Ref. |
|---|---|---|---|---|---|---|
| Au | Nafion/Au | H2SO4 | 13 ppb–10 ppm | 13 ppb | [84] | |
| Au | Au disk electrode | NaOH | 0–10 mM | 0.0129 mM | [85] | |
| Au NCs | SPCE/Au NCs | NaOH | 1–10 mM | 0.93 mM | Water samples | [86] |
| Au NPs | GCE/CNF-CHIT@Aunano | Phosphate buffer | 0.1–10 mM | 3.54 μg∙L−1 | Hair dye | [87] |
| Au NPs | AuNPs/PPy/GCE | NaOH | 0.4–2.1 mM | 0.4 mM | Milk | [88] |
| Au NCs | PDA@Au NCs-MIPs/HOPG | H2SO4 | 0.2 μM–0.02 M | 0.1 μM | Octopuses | [89] |
| Au | Au electrode | IL/DMSO | 5.30–53.00 μM | 0.53μM | [90] | |
| Pt NPs | WGE/Ch/MWCNTs/PAN/Pt | H2SO4 | 10−9–10−3 M | 4.6 × 10−11 M | [91] | |
| Pt NPs | Pt NPs-SPUME | Nafion films | 0–5.1 ppm | 80 ppb | Gas | [92] |
| Pt NPs | GCE/Pt NPs/L-alanine | NaOH | 0.3–1050 µmol/L | 0.14 µM | Water | [93] |
| Pt | GCE/Graphene–Pt | H2SO4 | 0–2 mM | 0.04 mM | [94] | |
| Pt | SPE/Pt | NaOH | 100–1000 μmol/L | Vegetable | [95] | |
| Pd NPs | Ti/Pd NPs | NaOH | 0-20 mM | 38.6 µM | [96] | |
| Pd NWs | GCE/Pd NWs | NaOH | 2 µM to 1 mM | 0.5 µM | [97] | |
| Pd | GCE/Nafion-Graphene-Pd | NaOH | 7.75–62.0 µM | 3.15µM | [98] | |
| Pd NPs | GCE/Pd NPs | NaOH | 30 µM–14 mM | 10µM | [99] | |
| Pd NPs | GCE/GO-BDMA-Pd | KOH | 1 µM–18 mM | 0.35μM | Tomato sauce | [100] |
| Pd NPs | GCE/GO-PAA-Pd NPs | KOH | 50 µM–50 mM | 16 μM | Food | [101] |
| Pd | GCE/Ppy-Pd | NaOH | 0.001–0.1 mM | 0.9μM | [102] | |
| Pd | GCE/Nafion@rGO-Pd | NaOH | 2–20 mM | [103] | ||
| Pd NPs | GCE/CMs-Pd NPs | NaOH | 0.025–15.00 mM | 8 μM | Seafood | [104] |
| Pdnano | nanoPd@LIG | KOH | 0.01–4.0 mM | 6.4 μM | Seafood, etc. | [105] |
| Ag NPs | GCE/GOPx-Ag NPs | KOH | 1 μM–70 mM | 0.167 μM | [106] | |
| Ag Nanoporous | Cu/Ag | KOH | 10–100 mM | [107] | ||
| Ag Nanoporous | Cu/Ag | KOH | 10–100 mM | [108] | ||
| Pt-Ru | PtRu black anode | Nafion®-117 | 0.002–1.25 g·L−1 | -- | Gas | [109] |
| Pt-Pd | GCE/Nf/Pt–Pd | H2SO4 | 10 μM–1 mM | 3 μM | Water | [110] |
| Pd-Au | CILE/Au/Pd | NaOH | 0.015–1.4 mM 1.4-56.7 mM | 3 μM | [111] | |
| Ag-Pd | CILE/AgPd | NaOH | 10 µM–70 mM | 2 µM | Water | [112] |
| Sn-Pt | Ti/Sn/nanoPt | H2SO4 | 0.003–0.1 M | 0.506 mM | [113] | |
| Cu-Pd | Glass slides/Cu-Pd | NaOH | -- | -- | -- | [114] |
| Pd-Pt | GCE/Nafion-graphene-Pd-Pt | H2SO4 | 4.50 µM–0.180 mM | 2.85µM | Water | [115] |
| Pd-Cu | Pd-Cu-SBA-16/CPE | NaOH | 1.79–121.86 mM | 16 μM | -- | [116] |
| Ni-Pd | Ni-Pd/GCE | NaOH | 10 μM–1 mM | 5.4μM | Water | [117] |
| Pt–Ag | Pt–Ag/rGO/SPE | 1–100 μM | 1 μM | Juice, etc. | [118] | |
| Cr-Pdene | Cr-doped Pd metallene (Cr-Pdene)/GCE | 1–5μM | 1 μM | Gas, Food | [119] | |
| Ni | BPE-Ni | NaOH | 0.037–10 μg∙mL−1 | 0.23 μg∙L−1 | -- | [120] |
| Ni(OH)2 | Ni/Ni(OH)2 | NaOH | 70 µM–16 mM | 20 µM | -- | [121] |
| Ni(OH)2 | CPE/Ni/P(Ni-doped P nanozeolite) | NaOH | 0.02–11.5 mM | 5.8 μM | Water | [122] |
| Ni(OH)2 | TNAs/Ni/Ni(OH)2 | KOH | 1.3–13 mM | 33.4 μM | [123] | |
| Ni(OH)2 | CPE/NaA nanozeolite/Ni(OH)2 | NaOH | 6.0–231 μM | 2 μM | Water | [124] |
| Ni(OH)2 | GCE/Ni-Ni(OH)2 | KOH | 0.01–1 mM | 6 μM | Water | [125] |
| NiWO4 | CPE/NiWO4 | NaOH | 0.008–1 mM | 3.6 μM | Water | [126] |
| Ni(OH)2 | Ni-NWs/Ni(OH)2 | KOH | 20 μM–2 mM 2–20 mM | 0.8 μM | -- | [127] |
| Ni(OH)2 | FTO/Ni/Ni(OH)2 | KOH | 0–6.5 mM | 8.3 μM | Juice | [128] |
| NiO | CC (carbon cloth)/NiO | NaOH | 5 μM–3 mM | 7.45 nM | Water | [129] |
| Ni(OH)2 | GCE/rGO/Ni(OH)2 | KOH | 0.1–100 mM | 60 μM | Wastewater | [130] |
| Ni3S2 | Nickel foam (NF)/Ni3S2 | NaOH | 0.002–5.45 mM | 1.23 μM | Water | [131] |
| SnO2@NiO | GCE/SnO2@NiO | NaOH | 0.1–28 mM | 2.8 nM | -- | [132] |
| Ni/Ni(OH)2 | CNFs/Ni/Ni(OH)2 | NaOH | 0.05–91.5 mM, 0.5–10 ppm | 0.36 ppm | Gas | [133] |
| Ni(OH)2 | SPEs/Ni NWs/Ni(OH)2 | NaOH | 0.8 µM–10 mM | LOQ: 0.8 µM | Water | [134] |
| Cu | Copper Electrode | NaOH | 1 µM–1 mM | 0.019 mM | -- | [135] |
| CuO NPs | GCE/CuO NPs | NaOH | 1.0 µM–10.0 mM | 0.25 µM | -- | [136] |
| Cu–Ni | Cu–Ni | NaOH | 3–100 mM | -- | -- | [137] |
| Cu2O/CuO | CILE/Cu2O/CuO | NaOH | 0.1–110 mM | 10 μM | -- | [138] |
| Cu NPs | SPCE/PS/Cu NPs | NaOH | 0.4–4 mM 1–300 mM | 0.0124 μM | Water | [139] |
| CuO/ZnO | GCE/CZO(Cu-codoped ZnO) | Phosphate buffer | 2 nM–6 mM | 4.1 nM | Water | [140] |
| CuO-CuOOH | Ti/TiO2/Cu/CuO | NaOH | 65 μM–7.8 mM | 25.0 μM | -- | [141] |
| CuO | CC/CuO | NaOH | 20 μM–3 mM | 26 nM | Milk | [142] |
| CuO | CuO/polyaniline (PANI) | Phosphate buffer | -- | 1 μM | -- | [143] |
| TiO2/RuO2 | Ti/Ru0.3Ti0.7O2 | K2SO4 | -- | -- | -- | [144] |
| MnO2 | GCE/MnO2 | Na2SO4/H2SO4 | 0.02–0.2 mM 0.2–2 mM | 10.2 μM | -- | [145] |
| MoOx | SPGE/Carbon/MoOx-Nafion | Nafion | -- | 60 ppb | Gas | [146] |
| Co(OH)2 | CC/Co(OH)2 nanosheet arrays | NaOH | 4 µM–5.45 mM | 0.57 μM | Not specified | [147] |
| Ag2S@g-C3N4 | GCE/NiFe2O4/Ag2S@g-C3N4 | Phosphate buffer | 0.9–120 mM | 1.63 μM | -- | [148] |
| GP@CeO2 | GP@CeO2 | NaOH | 25–120 μM 120–1000 μM | 1 μM | Mushroom | [149] |
| ZnO | SPCE/Egg albumin@ZnO SPCE/Chitosan@ZnO | Phosphate buffer | 1–5 μM 1–9 μM | 6.2 nM | Urine | [150] |
| ZnO | GPE/ZnO NPs | Phosphate buffer | 0–100 mM | 18 μM | -- | [151] |
| Polypyrrole | GCE/Graphene@Polypyrrole | KCl | 0.001–2 mM | 0.028 μM | -- | [152] |
| pDA | Stainless Steel Electrode/pDA | H2SO4 | 0.43–1.60 μM | 0.14 μM | Fish | [153] |
| MiPAN@GP | GP@MiPAN | NaOH | 10 μM–1 mM | 0.63 μM | Mushroom, fish | [154] |
| PANI/GO | GE/PANI@GO | HClO4 | 0.1–20 μM | 0.0185 μM | -- | [155] |
| Derivative Reagents | Signal Mode | Dynamic Range | Detection Limit | Applications | Ref. |
|---|---|---|---|---|---|
 | EIS | 1 µM–10 mM | 0.8 μM | -- | [57] |
 | Amperometry | 4–16 ppm | sub-ppm | Gas | [180] |
 | EIS | 0.05–1 µg∙mL−1 | 41 ng∙mL−1 | Gas | [181] |
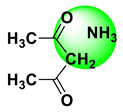 | Amperometry | 0.4–40.0 mg∙L−1 | 0.13 mg∙L−1 | Mushrooms | [58] |
| Amperometry | 15–500 μM | 0.57 mg∙kg−1 | Wood | [182] | |
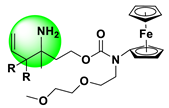 | Amperometry | 0.12–1000 μM | 48.2 nM | Blood, Cell | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Hao, Y.; Huang, L.; Luo, Y.; Chen, S.; Xu, M.; Chen, W. Recent Advances in Electrochemical Sensors for Formaldehyde. Molecules 2024, 29, 327. https://doi.org/10.3390/molecules29020327
Yang Y, Hao Y, Huang L, Luo Y, Chen S, Xu M, Chen W. Recent Advances in Electrochemical Sensors for Formaldehyde. Molecules. 2024; 29(2):327. https://doi.org/10.3390/molecules29020327
Chicago/Turabian StyleYang, Yufei, Yuanqiang Hao, Lijie Huang, Yuanjian Luo, Shu Chen, Maotian Xu, and Wansong Chen. 2024. "Recent Advances in Electrochemical Sensors for Formaldehyde" Molecules 29, no. 2: 327. https://doi.org/10.3390/molecules29020327
APA StyleYang, Y., Hao, Y., Huang, L., Luo, Y., Chen, S., Xu, M., & Chen, W. (2024). Recent Advances in Electrochemical Sensors for Formaldehyde. Molecules, 29(2), 327. https://doi.org/10.3390/molecules29020327