Abstract
The present study evaluated the antioxidant and antidiabetic properties of Medicago sativa and Solidago virgaurea extracts enriched in polyphenolic compounds. The extracts were obtained by accelerated solvent extraction (ASE) and laser irradiation. Then, microfiltration was used for purification, followed by nanofiltration used to concentrate the two extracts. The obtained extracts were analyzed to determine their antioxidant activity using DPPH radical scavenging and reducing power methods. The antidiabetic properties have been investigated in vitro on a murine insulinoma cell line (β-TC-6) by the inhibition of α-amylase and α-glucosidase. M. sativa obtained by laser irradiation and concentrated by nanofiltration showed the highest DPPH• scavenging (EC50 = 105.2 ± 1.1 µg/mL) and reducing power activities (EC50 = 40.98 ± 0.2 µg/mL). M. sativa extracts had higher inhibition on α-amylase (IC50 = 23.9 ± 1.2 µg/mL for concentrated extract obtained after ASE, and 26.8 ± 1.1), while S. virgaurea had the highest α-glucosidase inhibition (9.3 ± 0.9 µg/mL for concentrated extract obtained after ASE, and 8.6 ± 0.7 µg/mL for concentrated extract obtained after laser extraction). The obtained results after evaluating in vitro the antidiabetic activity showed that the treatment with M. sativa and S. virgaurea polyphenolic-rich extracts stimulated the insulin secretion of β-TC-6 cells, both under normal conditions and under hyperglycemic conditions as well. This paper argues that M. sativa and S. virgaurea polyphenolic-rich extracts could be excellent natural sources with promising antidiabetic potential.
1. Introduction
Type 2 diabetes mellitus (DM) is a major public health problem due to its continuously increasing prevalence, chronic complications, and because it is a major risk factor for cardiovascular diseases with economic and social implications. Prior studies have shown that synthetic antidiabetic drugs used for treating type 2 diabetes are associated with adverse side effects. For instance, thiazolidinedione causes heart failure, and sulfonylureas causes hypoglycemia and weight gain [1]. The interest in natural biologically active compounds comes from the recognition that they have very few side effects compared to synthetic compounds and that research in recent decades demonstrated the importance of the synergistic effect of bioactive compounds in a natural mixture. The high cost of synthetic antidiabetic drugs and adverse side effects make it necessary to seek a green and cheap alternative solution to managing this disease.
Natural products have recently gained great interest for diabetic treatment, including traditional herbal remedies, plant extracts, and their chemical components [2]. Polyphenols (phenolic acids, flavones, and isoflavones) are compounds of widespread interest in the nutritional and medicinal fields. These natural compounds are considered essential components due to their antioxidative, anti-inflammatory, antimicrobial, anti-mutagenic, estrogenic, and anticancer properties, combined with their ability to modulate critical cellular enzyme functions [3,4,5].
The interest in natural biologically active compounds comes from the recognition that they have very few side effects compared to synthetic compounds, and from recently conducted research, which demonstrated the importance of the synergistic effect of bioactive compounds in a natural mixture. Some studies reported that there is a relationship between the antidiabetic and antioxidant properties of medicinal plants [6]. A therapeutic alternative for diabetes treatment consists of the reduction in post-prandial hyperglycemia with natural products. This is obtained by delaying glucose absorption via α-glucosidase and α-amylase inhibiting, enzymes involved in carbohydrate hydrolysis in the digestive tract [7]. Many studies have highlighted that bioactive compounds with antioxidant activity, especially polyphenols, have the ability to inhibit these enzymes and could be beneficial in the management of diabetes mellitus [2,8].
Studies showed that a good choice of extraction technique, solvents, and extraction conditions might support the content of targeted bioactive compounds in the final extract, leading to increased efficacy of the final product. Although intensive investigations have been conducted in recent decades, researchers are still seeking stable plant sources of natural antioxidants and highly efficient extraction technologies. Nowadays, there is more interest in green and sustainable extraction methods [9,10,11,12]. Green methods offer the advantages of a shorter extraction time, higher selectivity, and lower organic solvent expense. Lately, ultrasound-assisted and accelerated solvent extraction (ASE), using organic solvents at high pressure and temperature, has been intensively studied [13,14,15,16]. Recently, a new method called laser irradiation showed great value in extractive technology. Yet there is only one study related to this method [17]. Laser irradiation is used to intensify the exposed environment’s heating and increase the reaction speed of the extraction process, resulting in an increased amount of biologically active substances extracted from plants (e.g., anthocyanins, polysaccharides, proteins, polyphenolics, minerals, etc.) [18,19].
Medicago sativa (lucerne; Fabaceae family) contains significant quantities of polyphenols, especially isoflavones known as phytoestrogens [20,21]. M. sativa is one of the most prevalent forage crops, but it also has a long tradition of use in folk medicine for central nervous and digestive system disorders and for curing other ailments, including cancer [22,23,24,25]. However, only a few research studies have been directed toward the antidiabetic potential of M. sativa [26,27,28].
Solidago virgaurea (goldenrod; Asteraceae family) is a medicinal plant used in popular medicine to treat numerous diseases, especially as a urological agent in kidney and bladder inflammation [29,30]. A recent study showed the antidiabetic potentials of this plant [31]. According to the literature, its pharmacodynamic activity is attributed to the presence of biologically active compounds, especially flavonoids, considered to be the most essential [20,32,33].
Since flavonoids are thermolabile compounds, high consideration was paid to the extraction and concentration of these compounds in the present study. In this context, we compared the antioxidant and antidiabetic activities of the two medicinal plant extracts enriched in polyphenols, using accelerated solvent extraction and laser irradiation extraction, coupled with concentration by nanofiltration. These obtained final products are meant to be used in antidiabetic management from safe and natural resources. The in vitro antidiabetic study was carried out by inhibiting amylase and glucosidase, as well as on a murine insulinoma cell line (β-TC-6).
2. Results and Discussion
2.1. Chemical Characterization
The first objective of the study was to obtain the polyphenolic-rich extracts of Medicago sativa and Solidago virgaurea using two green extraction methods: accelerated solvent extraction (ASE) and laser irradiation extraction (LE), followed by nanofiltration, used to concentrate the extracts. The previous results of the authors demonstrated the efficiency of the nanofiltration process in the concentration of polyphenolic compounds (phenolic acids, flavonoids, and isoflavonoids) [34].
Accelerated solvent extraction (ASE) involves the use of solvents at high temperatures and pressures. High temperatures accelerate the kinetics of the extraction process, while increased pressure keeps the solvent below its boiling point, thus obtaining fast and safe extractions. Some parameters such as solvent, temperature, the number of extraction cycles, and the static time are very important for the efficient extraction of polyphenolic compounds. However, due to the particularities of the compounds of interest and the fact that, at high temperatures, their structure could be damaged, leading to loss of their activity, the extraction was carried out in 3 extraction cycles performed at 60 °C [35]. Another study showed that the optimal ASE parameters were 50% ethanol, 150 °C, two extraction cycles, and 10 min static time [36].
The HPLC-MS method has been used for the phenolic acid, flavonoid, and isoflavonoid profile characterization of plant extract samples. The target bioactive compounds are presented in Table 1.

Table 1.
Contents of target compounds in the extracts.
The data obtained showed that laser irradiation is an efficient extraction method for some flavonoids and isoflavonoids (e.g., quercetin 3-D glucoside, quercitrin, naringenin, and vitexin), but both methods ensure efficient extraction of the desired compounds. Hence, this extraction method enhancing these valuable compounds is of particular interest, especially because it has been poorly studied thus far. Laser irradiation is a very new method of selective extraction, which demonstrated the efficiency in the extraction of polyphenols from plants at 552 nm, 660 nm, and 785 nm [17]. This method was effective in the case of flavonoid and isoflavonoid compound extraction from our studied plants, leading to a significant amount of extract (depending on the capacity of the extractor) obtained within less time.
However, this is the first study that uses this combination of wavelengths in laser extraction and that demonstrates the efficiency in the extraction, especially of flavonoids and isoflavonoids.
At the same time, with ASE, higher values were obtained for other compounds from the class of flavonoids and isoflavonoids. The comparison of the total flavonoid and isoflavonoid compound values in the samples indicates close values for the extracts obtained by both methods. Using a 50% (v/v) hydroalcoholic solution represents a reduction in the cost of the extraction process versus using pure solvents while maintaining a high extraction yield of the targeted compounds. This could be explained due to the different solubility of the extracted polyphenolic compounds, some of which have a higher solubility in water (phenolic acids), while others are extracted with a higher yield in ethanol.
Several studies indicated that M. sativa is a rich source of phytoestrogens. HPLC-MS analysis showed that the highest rutin content was the dominant flavonoid in all of the plant extracts. Rutin, quercetin, kaempferol, naringenin, formononetin, and genistein were also reported in other studies [18,20]. Tucak et al. found that genistein represents 30.33% of total phytoestrogens, followed by kaempferol, with 26.84% in the total amount of phytoestrogens [37]. However, biochanin A and vitexin were detected only in M. sativa seeds and sprouts, not in the aerial part [38]. Among the isoflavones, vitexin is found in the largest amount. The determined values of the phytoestrogens investigated in this research differed from the results obtained by the above-mentioned studies, which are most likely related to the type of cultivar, stages of maturity, extraction method, and other factors. Chlorogenic acid was the main phenolic acid from all extracts.
The HPLC–MS analysis of S. virgaurea extracts showed a significant content of rutin, quercetin 3-β-D-glucoside, and vitexin. Daidzein was not detected in S. virgaurea extracts. Our data confirmed the results of previously published studies reporting significant amounts of rutin in S. virgaurea species [32]. To the best of our knowledge, formononetin, biochanin A, and vitexin were not reported previously in goldenrod hydroalcoholic extracts.
2.2. Total Antioxidant Activity
Polyphenolic compounds are a group of natural compounds with a biologically active potential; hence, they have an antioxidant effect. The results for the total flavonoid content and antioxidant activity (DPPH and Fe(III) reducing power methods) in the various extracts compared with ascorbic acid (vitamin C) as standard, known for its antioxidant properties, are displayed in Table 2.

Table 2.
Total flavonoid content and antioxidant activity of analyzed extracts.
The DPPH scavenging assay is a frequently utilized method to evaluate antioxidant activity. The EC50 values related to the DPPH radical scavenging activity for all extracts were higher than vitamin C, showing a moderate antioxidant activity, with M. sativa obtained by laser irradiation and concentrated by nanofiltration being the most active extract (EC50 = 105.2 ± 1.1 µg/mL). The free radical inhibition results for M. sativa are in accord with the previous study, which showed strong antioxidant activity of extracts obtained from M. sativa (IC50 of the extract = 245.18 ± 48.41 μg/mL) [39].
Comparing the obtained results, we can observe that although the extracts of S. virgaurea have a much higher content of flavonoids, they have a lower antioxidant activity than M. sativa. This result can be explained by a higher content of other compounds, with the antioxidant activity present in the extracts of M. sativa, such as phenolic acids or other isoflavone compounds, not quantified in the studied extracts. Our results about the antioxidant activity of S. virgaurea extracts confirm the results of the other research, but it must be taken into account that the antioxidant activity is dependent on the solvent and the extraction method applied [40].
The reducing power showed significant differences between the examined extracts compared to ascorbic acid as a standard, due to the highest EC50 value obtained. M. sativa polyphenolic-rich extracts had the highest reducing power activity. The reducing power of the extracts can be determined by the hydrogen donation ability, which stabilizes the molecules by acceptance of hydrogen ions in the extracts. The reducing power results revealed that all tested extracts had good abilities to donate electrons, which were involved in the antioxidant activity.
The DPPH assay can be applied to both lipophilic and hydrophilic compounds, while the reducing power assay was more sensitive to hydrophilic compounds [41].
The free radical scavenging activity and the reducing power depend on the number of hydroxyl groups attached to a benzene ring, as such groups could donate hydrogen to stabilize free radicals. Thus, the lack of a positive correlation between the reducing power and the scavenging DPPH free radical activity may only result from different reaction mechanisms and steric accessibilities of the reagents and each type of antioxidant in the two assays.
2.3. Antidiabetic Activity
- α-amylase and α-glucosidase inhibition
One of the alternative approaches regarding the prevention/modulation of postprandial hyperglycemia consists of using natural therapeutic inhibitors of α-amylase and α-glucosidase, as they are key enzymes in starch digestion. Our results for these enzymes’ inhibition by the tested polyphenolic-rich extracts are presented in Table 3.

Table 3.
α-amylase and α-glucosidase enzyme inhibition of analyzed extracts.
M. sativa extracts had higher inhibitory activity on α-amylase (IC50 = 23.9 ± 1.2 µg/mL for concentrated extract obtained after ASE, and 26.8 ± 1.1 µg/mL for concentrated extract obtained after LE), while S. virgaurea had the highest α-glucosidase inhibition compared with acarbose, used as standard. S. virgaurea extracts showed the best α-glucosidase inhibition (IC50 of 9.3 ± 0.9 µg/mL for concentrated extract obtained after ASE, and 8.6 ± 0.7 µg/mL for concentrated extract obtained after LE), almost 7 times lower than acarbose (IC50 of 66.5 ± 4.2 µg/mL).
The inhibitory activities of the rutin, the main compound identified in extracts, were higher than those of the acarbose. Thus, rutin can be considered one of the compounds responsible for the activity of the extracts. The milder inhibition of α-amylase than α-glucosidase of all studied extracts could eliminate the major drawback of current drugs with side effects [42].
Flavonoids, such as rutin, quercitrin, and isoquercitrin (quercetin 3-β-D-glucoside), but also isoflavones (daidzein, genistein, vitexin), have been previously reported to have a hypoglycemic effect and stronger inhibitory effect on α-glucosidase. [43,44,45]. In recent years, several studies have reported the inhibitory activity of phenolic acids on α-amylase and α-glucosidase. Latest studies showed that the interaction between polyphenolic compounds and α-amylase/α-glucosidase consists of the hydrogen bond between the amino acid residues of the enzymes and the hydroxyl groups in the polyphenolic compounds. These interactions disrupt the protein structure of the enzymes, leading to a decrease in enzyme activity [46].
The polyphenols’ inhibitory activity on α-amylase and α-glucosidase has been related to the number of hydroxyl groups, the number of double bonds on aromatic rings A and B, as well as the heterocyclic ring C [47]. Thus, the phenolic acids with more hydroxyl groups (chlorogenic, caffeic, and gallic acids) presented a higher inhibition effect against both enzymes [48].
This study suggests that combinations of polyphenolic compounds from the studied plants have a synergic effect on α-amylase and α-glucosidase inhibition.
Recent in vivo studies showed the anti-hyperglycemic effect of S. virgaurea, but the antidiabetic activity of S. virgaurea has rarely been studied [49]. However, the α-amylase and α-glucosidase inhibition by S. virgaurea extract was not found in the literature.
- Effect of extracts on insulin secretion by β-TC6 cell lines
In this study, we investigated the complementary in vitro antidiabetic activity of M. sativa and S. virgaurea polyphenolic-rich extracts, at the tested concentrations (10–250 μg/mL), on a murine insulinoma cell line (β-TC-6).
The obtained results showed that higher insulin concentrations were obtained after treatment with all the tested extracts compared to the control, both in normal conditions (5.6 mM) and in hyperglycemic conditions (16.7 mM) (Figure 1).
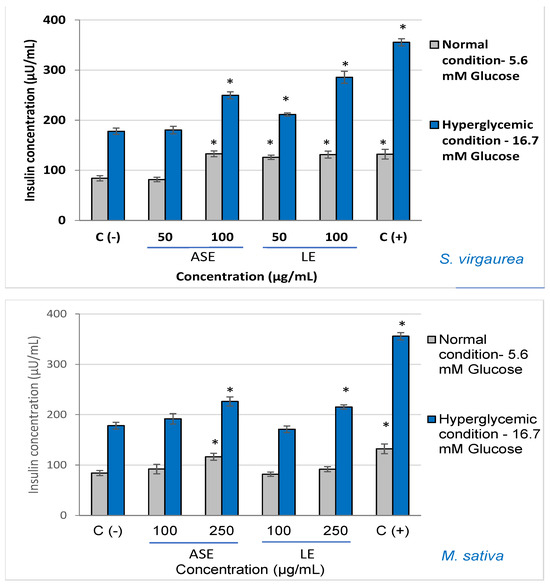
Figure 1.
Effect of polyphenolic-rich extracts on insulin secretion, after treatment with different concentrations, in both normal and hyperglycemic conditions. C (−)—negative control, untreated cells. C (+)—positive control, cells treated with alanine. * p < 0.05, compared to the untreated cells.
Under normal glycemic conditions (5.6 mM), the highest concentration of the insulin was found in two specific cases of cell treatment with either 50 µg/mL (~134 µU/mL) S. virgaurea concentrated extract obtained by ASE extraction or cell treatment with 100 µg/mL (~132 µU/mL) S. virgaurea concentrated extract obtained by LE extraction. In the case of the control stimulated with 5.6 mM glucose and untreated, the secreted insulin concentration was ~84 μU/mL.
Likewise, similar results of the effectiveness of stimulating insulin secretion were also obtained in hyperglycemic conditions (16.7 mM). Thus, the best results were obtained after the treatment with S. virgaurea concentrated extract obtained after ASE extraction at 50 µg/mL (~249 µU/mL), S. virgaurea concentrated extract obtained after LE extraction at 100 µg/mL (~286 µU/mL), and M. sativa extracts 250 µg/mL; for the stimulated and untreated control, the secreted insulin concentration was ~178 μU/mL. Alanine was used as a positive control, demonstrating its ability to significantly stimulate insulin secretion.
Interestingly, even though M. sativa, a related and well-known phytotherapeutic plant, is traditionally used as an anti-diabetic agent, its α-glucosidase- and α-amylase-inhibitory properties were poorly investigated [50].
3. Materials and Methods
3.1. Materials
Flavonoid and isoflavonoid compounds, including rutin, quercitrin, quercetin 3-β-D-glucoside, quercetin, isorhamnetin, formononetin, genistein, naringenin, biochanin A, and vitexin, were purchased from Sigma–Aldrich (Schnelldorf, Germany), daidzein was obtained from Fluka (Buchs, Switzerland), luteolin and kaempferol were purchased from Carl Roth (Karlsruhe, Germany), 2,2-difenil-1-picrilhidrazil (DPPH), potassium ferricyanide, sodium carbonate (Na2CO3), dinitrosalicylic acid (DNS), α-amylase from hog pancreas, α-glucosidase from Saccharomyces cerevisiae, and 4-nitrophenyl α-d-glucopyranoside (NPG) were purchased from Sigma–Aldrich, and iron chloride was bought from Fluka. All other used reagents, methanol (Riedel-de Haen), and ethanol (Chemical Company) were of chromatographic or analytical purity; the ultra-pure water was obtained using the distillation apparatus from Evoqua Water Technologies (Pittsburgh, PA, USA).
The medicinal plants were collected from the Cluj county (Romania) natural site, and voucher specimens were stored in the Herbarium of Babes-Bolyai University from Cluj-Napoca (code: 868.786 for Solidago virgaurea L.; code: 622172 for Medicago sativa).
3.2. Extract Preparation
Two green extraction methods were used to study their influence on bioactive compound extraction: accelerated solvent extraction (ASE) and laser irradiation (LE).
3.2.1. ASE Extraction
Accelerate solvent extraction of dried and grounded M. sativa and S. virgaurea was realized by Dionex ASE 350 System (Thermo Fisher Scientific Inc., Waltham, MA USA). Each stainless steel cell (100 mL) equipped with a cellulose filter was filled with 15 g of dried and fine-milled plant and diatomaceous earth and the ASE conditions were set as solvent—ethanol/water (50/50, v/v), temperature—60 °C, static time—10 min, number of cycles—3. The extracts were collected in a 250 mL flask and stored at 4 °C before concentration. According to the ASE extract volume, the concentration of the extracts was 9% (w/v).
3.2.2. Laser Irradiation Extraction
LE extraction was performed in the same conditions with ASE; 9 g of the dry plant (aerial parts) was mixed with 100 mL of ethanol/water (50/50, v/v), and extracted for 30 min assisted with laser radiation at a combined 1270 and 1550 nm. The LE extraction used a steel extractor (made by the Apel Laser S.R.L) provided with a lid with two windows through which laser irradiation was carried out (Figure 2).

Figure 2.
Laser-assisted extraction installation.
Subsequently, all extracts were purified by microfiltration through a Millipore membrane (0.2 µm pores) and concentrated by nanofiltration through Sterlitech membranes NF90 with a cut-off of 150–300 Da using a KMS Laboratory Cell CF-1 module. The concentrated extracts were stored in a freezer at −20 °C for use in further analysis.
3.3. Analysis of Polyphenolic Compounds
3.3.1. Quantification of Total Polyphenols and Flavonoids
Polyphenolic compound content determination was performed using the Folin–Ciocalteu method [51]. A total of 2 mL extract and 2 mL Folin–Ciocalteu reagent were mixed and filtered; then, 0.5 mL of the filtrate was added to 9.5 mL sodium carbonate 20% and absorbance was read at 760 nm. A calibration curve of different concentrations of chlorogenic acid solutions (10 µg/mL–1000 µg/mL) was used for expressing the results as chlorogenic acid equivalent (CA), with the equation y = 0.0016x + 0.013 (R2 = 0.9945).
Total flavonoid content was quantified using the aluminum chloride colorimetric method [52]. A total of 2 mL of extract and 3 mL of methanol were mixed. After filtration, 1 mL filtrate was added to 1 mL sodium acetate solution, 0.6 mL of aluminum chloride solution, and 2.4 mL methanol. The absorbance was measured at 430 nm and the flavonoid content was calculated based on a rutin calibration curve (y = 0.0073x − 0.0357; R2 = 0.9959).
3.3.2. HPLC-MS Analysis
HPLC analysis was realized using an HPLC Shimadzu system consisting of a SIL-20AC autosampler, two LC-20AD pumps, a DGU-20A degasser, and a CTO-20A column oven with an LC Solution software var. 5.1. The HPLC was coupled to a mass spectrometer detector, LCMS-2010 with an ESI interface using negative ionization mode using the following parameters: detector voltage, 1.8 kV; interface voltage 4 kV; heat block temperature, 200 °C; CDL temperature, 200 °C; interface temperature, 250 °C; and nebulization gas (N2) flow rate, 1.5 L min−1. A previously developed HPLC-MS method [53] was used for the identification and quantification of polyphenol compounds, and analyses were performed on a Kromasil 100-5-C18 2.1 × 150 mm column and with an elution gradient of mobile phase (formic acid in water, pH = 3, solvent A and formic acid in MeCN, pH = 3, solvent B, 0–20 min 5–30% solvent B, 20.01–40 min 30% solvent B, 40.01–50 min 50% solvent B, 50.01–52 min 50–5% solvent B, 52.01–62 min 5% solvent B) and a gradient of flow rate (0.1 mL min−1 from 0–5, 15.01–35, and 60.01–62 min and 0.2 mL min−1 between 5.01–15 and 35.01–60 min). The selected ion monitoring (SIM) mode was used and the corresponding peaks of the compound fragment ions ([M-H]-: 163, 169, 179, 253, 267, 269, 271, 283, 285, 301, 315, 317 353, 431, 447, 463, and 609) were obtained for quantitative analysis.
3.4. Antioxidant Assays
3.4.1. DPPH Radical Scavenging
The DPPH assay was carried out as described by Bondet et al. [54] with slight modifications. A total of 100 µL extract with different concentrations was mixed with 1000 µL DPPH (2,2-diphenyl-1-picrylhydrazyl), 0.25 mM solution, and 1.9 mL methanol. The absorbance was measured at 517 nm, and the extracts’ scavenging activity was determined by the following formula:
where RSA = radical scavenging activity; Ac = control absorbance; and As = sample absorbance. Results were presented as inhibition, in EC50 (μg/mL).
RSA (%) = [(Ac − As)/Ac] × 100,
3.4.2. Fe (III) Reducing Power Assay
The reducing power assay is based on the reduction of iron (III) to iron (II) and was performed using Berker’s method [55]. The polyphenolic-rich extracts (0.1 mL with varying concentrations) were mixed with 2.5 mL sodium phosphate buffer (0.2 M) and 2.5 mL potassium ferricyanide (1%) and then were kept at 50 °C for 20 min. Thereafter, 2.5 mL of trichloroacetic acid (10%) was added. Finally, an aliquot of 2.5 mL mixture was combined with 2.5 mL water followed by 0.5 mL of iron chloride solution (0.1%) and UV absorbance was read at 700 nm. Results were presented as EC50 (μg/mL), the extract concentration giving an absorbance of 0.5 for reducing power, and was calculated from the graph of absorbance against extract concentration.
3.5. Antidiabetic Assay
3.5.1. α-Amylase- and α-Glucosidase-Inhibitory Activities
The ability of extracts to inhibit α-amylase and α-glycosidase enzymes was examined to establish the plant’s potential as an antidiabetic.
The α-amylase inhibition analysis was achieved according to our previous study [56]. Briefly, 100 μL of the extracts was added to 250 μL α-amylase from hog pancreas (EC 3.2.1.1) solution in phosphate buffer (pH 6.9) and was maintained at 37 °C for 20 min. Then, 250 μL starch solution was added and incubated at 37 °C for 30 min. Subsequently, 500 μL DNS was added, and the mixture was heated at 90 °C for 5 min. Absorbance measurements were performed at 540 nm.
The α-glucosidase-inhibitory activity was evaluated using a slightly modified method of Ranilla et al. [57]. Samples (60 μL) with different concentrations were incubated with 120 μL of α-glucosidase from Saccharomyces cerevisiae (EC 3.2.1.20) solution (0.5 U/mL) and 720 μL phosphate buffer (0.1 M, pH 6.9), at 37 °C, for 15 min. After that, 120 μL of NPG substrate solution was added, and the mixture was incubated at 37 °C, for 15 min. Then, 480 μL of 0.2 M Na2CO3 solution was added to this mixture to stop the reaction, and the absorbance was read at 405 nm. The results were calculated using the following formula:
Values were compared with the standard drug acarbose. IC50 values (concentration of the extract that inhibits 50% enzyme activity) were obtained from the linear regression analysis.
3.5.2. In Vitro Insulin Secretion Assay
In vitro cytocompatibility of extracts was evaluated on pancreatic βTC-6 cells in an experimental model of direct contact by MTT assay. The results obtained showed that extracts were biocompatible in the range of 10–100 µg/mL for S. virgaurea extracts and 10–250 µg/mL for M. sativa extracts. At higher concentrations, cellular viability decreased in a dose-dependent manner. Based on the results obtained by MTT assay, we selected the highest concentrations at which samples did not affect cell viability (>80%) and we tested them further to detect the extracts’ effect on insulin secretion in an in vitro model.
MTT assay was used for cytocompatibility testing according to the international standard ISO 10993-5/2009, as previously described [58].
In vitro evaluation of antidiabetic activity was conducted on a mice insulinoma cell line (βTC-6). βTC-6 cells were purchased from Cell Lines Service (CLS, Germany) and were grown in DMEM medium supplemented with 10% FBS (fetal bovine serum) and 1% PSN (penicillin−streptomycin–neomycin) antibiotic mixture at 37 °C and 5% CO2. βTC-6 cells were seeded in a 24-well plate at a density of 1 × 105 cells/mL. After 24 h of cultivation in standard conditions, βTC-6 cells were cultivated in normal (5.6 mM) and hyperglycemic (16.7 mM) conditions in the absence and presence of extracts for 1 h, at 37 °C. The culture medium was then collected, centrifuged for 10 min at 1500 rpm, and stored at −20 °C until insulin measurement. Insulin secretion was determined by Mouse Ins1/Insulin-1 ELISA Kit, according to the manufacturer’s recommendations (Sigma–Aldrich Chemie GmbH, Schnelldorf, Germany). L-alanine (10 mM) was used as the reference stimulant of insulin secretion from pancreatic beta cells. Alanine was used as a positive control because it has been noted to stimulate insulin secretion significantly. Studies have shown that alanine is consumed by b-cell lines and islet cells and increases insulin secretion, findings that are supported by observations from other β-cell lines, including murine and human [59].
3.6. Statistical Analysis
Three independent experiments were carried out and the obtained data were presented as mean ± standard deviation (SD) (n = 3). The sample pair of interest was analyzed using the paired Student’s t-test (Microsoft Excel 2018 software). Significant statistical differences were considered p < 0.05.
4. Conclusions
In this research, Medicago sativa and Solidago virgaurea extracts were obtained using accelerated solvent extraction and laser irradiation extraction, coupled with concentration by nanofiltration. The laser irradiation method at the combined wavelengths of 1270 and 1550 nm, reported for the first time in this paper, was efficient in the case of flavonoid and isoflavonoid compound extraction from M. sativa and S. virgaurea. M. sativa polyphenolic-rich extracts had the best values for antioxidant activity (DPPH and Fe(III) reducing power methods). The polyphenolic-rich extracts from both plants showed a significative inhibition on α-amylase and α-glucosidase, correlated with their total phenolic acid, flavonoid, and isoflavonoid high contents. Additionally, the obtained results showed that the studied extracts stimulate insulin secretion in vitro. S. virgaurea polyphenolic-rich extracts showed the strongest stimulatory effect on insulin secretion in the in vitro β-TC-6 pancreatic beta cell stimulation model. Our results revealed that the M. sativa and S. virgaurea enriched with polyphenols could be used as an alternative therapy in the management of diabetes.
Further studies should be designed to explore the mechanism of inhibition of digestive enzymes by the studied extracts.
Author Contributions
Conceptualization, G.P.; investigation, G.P., E.N., C.A., A.A. and A.-M.S.-G.; supervision, G.L.R.; writing—original draft preparation, G.P. and A.-M.S.-G.; writing—review and editing, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Research, Innovation and Digitization, CCCDI-UEFISCDI, project number PN-III-P2-2.1-PED-2021-1185, and was partially supported through the Competitiveness Operational Program, Axis 1, Action 1.2.3, SMIS no. 105535 (FITO-COMP), subsidiary 1869 and the Core-Program, project PN 7N/23-02-0101/2023.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank Apel Laser S.R.L. for providing the laser extractor.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Haq, F.; Siraj, A.; Ameer, M.; Hamid, T.; Rahman, M.; Khan, S.; Khan, S.; Masud, S. Comparative Review of Drugs Used in Diabetes Mellitus—New and Old. J. Diabetes Mellit. 2021, 11, 115–131. [Google Scholar] [CrossRef]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A.; et al. Antidiabetic phytochemicals from medicinal plants: Prospective candidates for new drug discovery and development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic Activity, Biological Effect and Bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Famuyiwa, S.O.; Sanusi, K.; Faloye, K.O.; Yilmaz, Y.; Ceylan, U. Antidiabetic and antioxidant activities: Is there any link between them? New J. Chem. 2019, 43, 13326–13329. [Google Scholar] [CrossRef]
- de Souza, P.M.; de Sales, P.M.; Simeoni, L.A.; Silva, E.C.; Silveira, D.; de Oliveira Magalhães, P. Inhibitory activity of α-amylase and α-glucosidase by plant extracts from the Brazilian cerrado. Planta Med. 2012, 78, 393–399. [Google Scholar] [CrossRef]
- Nicolle, E.; Souard, F.; Faure, P.; Boumendjel, A. Flavonoids as promising lead compounds in type 2 diabetes mellitus: Molecules of interest and structure-activity relationship. Curr. Med. Chem. 2011, 18, 2661–2672. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ravindran, R.; Walsh, O.; O’Doherty, J.; Jaiswal, A.K.; Tiwari, B.K.; Rajauria, G. Evaluation of Ultrasound, Microwave, Ultrasound–Microwave, Hydrothermal and High Pressure Assisted Extraction Technologies for the Recovery of Phytochemicals and Antioxidants from Brown Macroalgae. Mar. Drugs 2021, 19, 309. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Zabot, G.L.; Reyes, F.G.R.; Iglesias, A.H.; Martínez, J. Integration of pressurized liquids and ultrasound in the extraction of bioactive compounds from passion fruit rinds: Impact on phenolic yield, extraction kinetics and technical-economic evaluation. Innov. Food Sci. Emerg. Technol. 2021, 67, 102549. [Google Scholar] [CrossRef]
- Mihelčič, A.; Lisjak, K.; Vanzo, A. Accelerated solvent extraction of phenols from lyophilised ground grape skins and seeds. Beverages 2023, 9, 4. [Google Scholar] [CrossRef]
- Khongthaw, B.; Chauhan, P.K.; Dulta, K.; Kumar, V.; Ighalo, J.O. A comparison of conventional and novel phytonutrient extraction techniques from various sources and their potential applications. J. Food Meas. Charact. 2023, 17, 1317–1342. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Revilla-Ruiz, P.; Hernández-Méndez, J. Pressurized liquid extraction in the analysis of food and biological samples. J. Chromatogr. A 2005, 1089, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Borrull, F.; Pocurull, E.; Marcé, R.M. Pressurized liquid extraction: A useful technique to extract pharmaceuticals and personal-care products from sewage sludge. TrAC Trends Anal. Chem. 2010, 29, 752–764. [Google Scholar] [CrossRef]
- Perra, M.; Leyva-Jiménez, F.-J.; Manca, M.L.; Manconi, M.; Rajha, H.N.; Borrás-Linares, I.; Segura-Carretero, A.; Lozano-Sánchez, J. Application of pressurized liquid extraction to grape by-products as a circular economy model to provide phenolic compounds enriched ingredient. J. Clean. Prod. 2023, 402, 136712. [Google Scholar] [CrossRef]
- Pirvu, L.C.; Nita, S.; Rusu, N.; Bazdoaca, C.; Neagu, G.; Bubueanu, C.; Udrea, M.; Udrea, R.; Enache, A. Effects of laser irradiation at 488, 514, 532, 552, 660, and 785 nm on the aqueous extracts of Plantago lanceolata L.: A comparison on chemical content, antioxidant activity and caco-2 viability. Appl. Sci. 2022, 12, 5517. [Google Scholar] [CrossRef]
- Galanakis, C. Food Waste Recovery: Processing Technologies, Industrial Techniques, and Applications, 2nd ed.; Elsevier: Vienna, Austria, 2020. [Google Scholar]
- Panchev, I.N.; Kirtchev, N.A.; Dimitrov, D.D. Possibilities for application of laser ablation in food technologies. Innov. Food Sci. Emerg. Technol. 2011, 12, 369–374. [Google Scholar] [CrossRef]
- Wyse, J.M.; Latif, S.; Gurusinghe, S.; Berntsen, E.D.; Weston, L.A.; Stephen, C.P. Characterization of phytoestrogens in Medicago sativa L. and grazing beef cattle. Metabolites 2021, 11, 550. [Google Scholar] [CrossRef]
- Bajkacz, S.; Baranowska, I.; Buszewski, B.; Kowalski, B.; Ligor, M. Determination of flavonoids and phenolic acids in plant materials using SLE-SPE-UHPLC-MS/MS method. Food Anal. Method. 2018, 11, 3563–3575. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. Phytochemical and pharmacological potential of Medicago sativa: A review. Pharm. Biol. 2011, 49, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.I.; Mosbach, E.H.; Matoba, N.; Suh, S.O.; McSherry, C.K. The effect of alfalfa-corn diets on cholesterol metabolism and gallstones in prairie dogs. Lipids 1990, 25, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Gaweł, E.; Grzelak, M.; Janyszek, M. Lucerne (medicago sativa L.) in the human diet—Case reports and short reports. J. Herb. Med. 2017, 10, 8–16. [Google Scholar] [CrossRef]
- Dutu, L.E.; Istudor, V.; Loloiu, T.; Radulescu, V. Research on polyphenolic compounds from Medicago sativa L. Farmacia 2002, 50, 44–56. [Google Scholar]
- Mansourzadeh, S.; Esmaeili, F.; Shabani, L.; Gharibi, S. Trans-differentiation of mouse mesenchymal stem cells into pancreatic β-like cells by a traditional anti-diabetic medicinal herb Medicago sativa L. J. Tradit. Complement. Med. 2022, 12, 466–476. [Google Scholar] [CrossRef]
- Eruygur, N.; Dincel, B.; Kutuk Dincel, N.; Ucar, E. Comparative study of in vitro antioxidant, acetylcholinesterase and butyrylcholinesterase activity of alfalfa (Medicago sativa L.) collected during different growth stages. Open Chem. 2018, 16, 963–967. [Google Scholar] [CrossRef]
- Gray, A.M.; Flatt, P.R. Pancreatic and extra-pancreatic effects of the traditional anti-diabetic plant, Medicago sativa (lucerne). Br. J. Nutr. 1997, 78, 325–334. [Google Scholar] [CrossRef]
- Abdel Motaal, A.; Ezzat, S.M.; Tadros, M.G.; El-Askary, H.I. In vivo anti-inflammatory activity of caffeoylquinic acid derivatives from Solidago virgaurea in rats. Pharm. Biol. 2016, 54, 2864–2870. [Google Scholar] [CrossRef]
- Borchert, V.E.; Czyborra, P.; Fetscher, C.; Goepel, M.; Michel, M.C. Extracts from Rhois aromatica and Solidaginis virgaurea inhibit rat and human bladder contraction. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 281–286. [Google Scholar] [CrossRef]
- Zehra, S.A.; Bhattarai, P.; Zhang, J.; Liu, Y.; Parveen, Z.; Sajid, M.; Zhu, L. In vitro and In vivo Evaluation of the Antidiabetic Activity of Solidago virgaurea Extracts. Curr. Bioact. Compd. 2023, 19, 68–78. [Google Scholar]
- Jasicka-Misiak, I.; Makowicz, E.; Stanek, N. Chromatographic fingerprint, antioxidant activity, and colour characteristic of polish woundwort (Solidago virgaurea L.) honey and flower. Eur. Food Res. Technol. 2018, 244, 1169–1184. [Google Scholar] [CrossRef]
- Tămaş, M.; Vostinaru, O.; Soran, L.; Lung, I.; Opris, O.; Toiu, A.; Gavan, A.; Dinte, E.; Mogosan, C. Antihyperuricemic, anti-inflammatory and antihypertensive effect of a dry extract from Solidago virgaurea L. (Asteraceae). Sci. Pharm. 2021, 89, 27. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Tache, A.; Radu, G.L.; Parvulescu, V. Application of nanofiltration process for concentration of polyphenolic compounds from Geranium robertianum and Salvia officinalis extracts. Chem. Biochem. Eng. Q. 2011, 25, 49–56. [Google Scholar]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Tucak, M.; Čupić, T.; Horvat, D.; Popović, S.; Krizmanić, G.; Ravlić, M. Variation of phytoestrogen content and major agronomic traits in alfalfa (Medicago sativa L.) populations. Agronomy 2020, 10, 87. [Google Scholar] [CrossRef]
- Chiriac, E.R.; Chiţescu, C.L.; Sandru, C.; Geană, E.-I.; Lupoae, M.; Dobre, M.; Borda, D.; Gird, C.E.; Boscencu, R. Comparative study of the bioactive properties and elemental composition of red clover (Trifolium pratense) and alfalfa (Medicago sativa) sprouts during germination. Appl. Sci. 2020, 10, 7249. [Google Scholar] [CrossRef]
- Raeeszadeh, M.; Moradi, M.; Ayar, P.; Akbari, A. The Antioxidant Effect of Medicago sativa L. (Alfalfa) Ethanolic Extract against Mercury Chloride (HgCl2) Toxicity in Rat Liver and Kidney: An in Vitro and in Vivo Study. Evid.-Based Complement. Altern. Med. 2021, 2021, 8388002. [Google Scholar] [CrossRef]
- Demir, H.; Acik, L.; Bali, E.B.; Koç, L.Y.; Kaynak, G. Antioxidant and antimicrobial activities of Solidago virgaurea extracts. Afr. J. Biotechnol. 2009, 8, 274–279. [Google Scholar]
- Sahreen, S.; Khan, M.R.; Khan, R.A. Evaluation of antioxidant activities of various solvent extracts of Carissa opaca fruits. Food Chem. 2010, 122, 1205–1211. [Google Scholar] [CrossRef]
- Pinto, M.D.S.; Ranilla, L.G.; Apostolidis, E.; Lajolo, F.M.; Genovese, M.I.; Shetty, K. Evaluation of anti-hyperglycemia and anti-hypertension potential of native Peruvian fruits using in vitro models. J. Med. Food 2009, 12, 278–291. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.T.; Yin, Y.C.; Xing, S.; Li, W.N.; Fu, X.Q. Hypoglycemic effect and mechanism of isoquercitrin as an inhibitor of dipeptidyl peptidase-4 in type 2 diabetic mice. RSC Adv. 2018, 8, 14967–14974. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.C.; Peng, R.Y.; Chen, Y.T.; Xu, H.X.; Zhang, Q.F. In vitro and in vivo Inhibitory Activity of C-glycoside Flavonoid Extracts from Mung Bean Coat on Pancreatic Lipase and α-glucosidase. Plant Foods Hum. Nutr. 2023, 78, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Fang, H.; Zhang, H.; Ye, X. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef] [PubMed]
- Amaral, S.; Mira, L.; Nogueira, J.M.F.; Silva, A.P.; Florêncio, M.H. Plant extracts with anti-inflammatory properties—A new approach for characterization of their bioactive compounds and establishment of structure–antioxidant activity relationships. Bioorg. Med. Chem. 2009, 17, 1876–1883. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Fursenco, C.; Calalb, T.; Uncu, L.; Dinu, M.; Ancuceanu, R. Solidago virgaurea L.: A Review of its ethnomedicinal uses, phytochemistry, and pharmacological activities. Biomolecules 2020, 10, 1619. [Google Scholar] [CrossRef]
- Jakupović, L.; Kalvarešin, M.; Bukovina, K.; Poljak, V.; Vujić, L.; Zovko Končić, M. Optimization of Two Eco-Friendly Extractions of Black Medick (Medicago lupulina L.) Phenols and Their Antioxidant, Cosmeceutical, α-Glucosidase and α-Amylase Inhibitory Properties. Molecules 2021, 26, 1610. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Lin, J.-Y.; Tang, C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Cristea, V.; Deliu, C.; Oltean, B.; Butiuc-Keul, A.; Brummer, A.; Albu, C.; Radu, G.L. Soilless Cultures for Pharmaceutical Use and Biodiversity Conservation. Acta Hortic. 2009, 843, 157–164. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanism of antioxidant activity using the DPPH free radical method. Leb. Wiss Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Berker, K.; Guclu, K.; Tor, I.; Apak, R. Comparative evaluation of Fe (III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP) and ferricyanide reagents. Talanta 2007, 72, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Neagu, E.; Paun, G.; Albu, C.; Eremia, S.A.-M.V.; Radu, G.L. Artemisia abrotanum and Symphytum officinale Polyphenolic Compounds-Rich Extracts with Potential Application in Diabetes Management. Metabolites 2023, 13, 354. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Phenolic compounds antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Biores. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef]
- Iosageanu, A.; Ilie, D.; Craciunescu, O.; Seciu-Grama, A.-M.; Oancea, A.; Zarnescu, O.; Moraru, I.; Oancea, F. Effect of Fish Bone Bioactive Peptides on Oxidative, Inflammatory and Pigmentation Processes Triggered by UVB Irradiation in Skin Cells. Molecules 2021, 26, 2691. [Google Scholar] [CrossRef]
- Newsholme, P.; Cruzat, V.; Arfuso, F.; Keane, K. Nutrient regulation of insulin secretion and action. J. Endocrinol. 2014, 221, R105–R120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).