Synthesis and Structural and Optical Behavior of Dehydrohelicene-Containing Polycyclic Compounds
Abstract
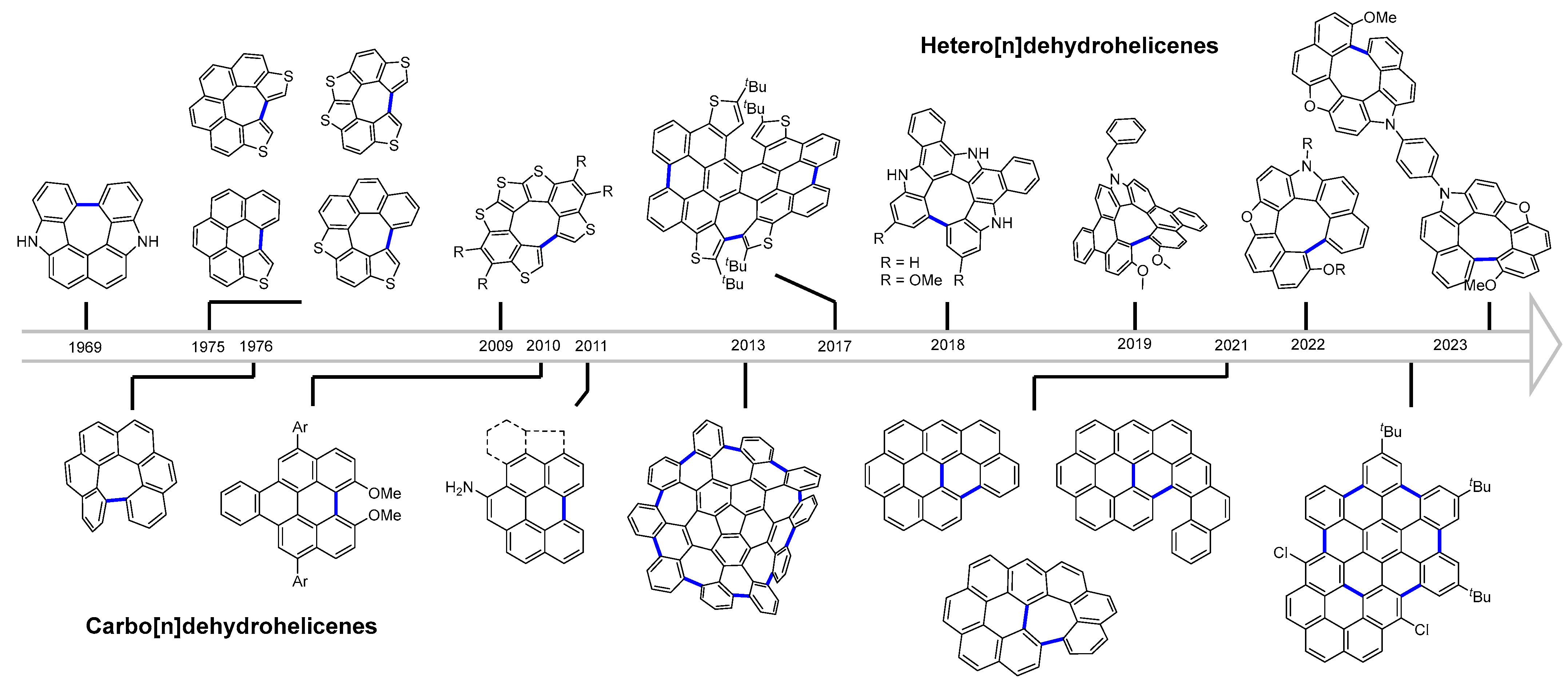
1. Introduction
2. Carbo[n]dehydrohelicenes
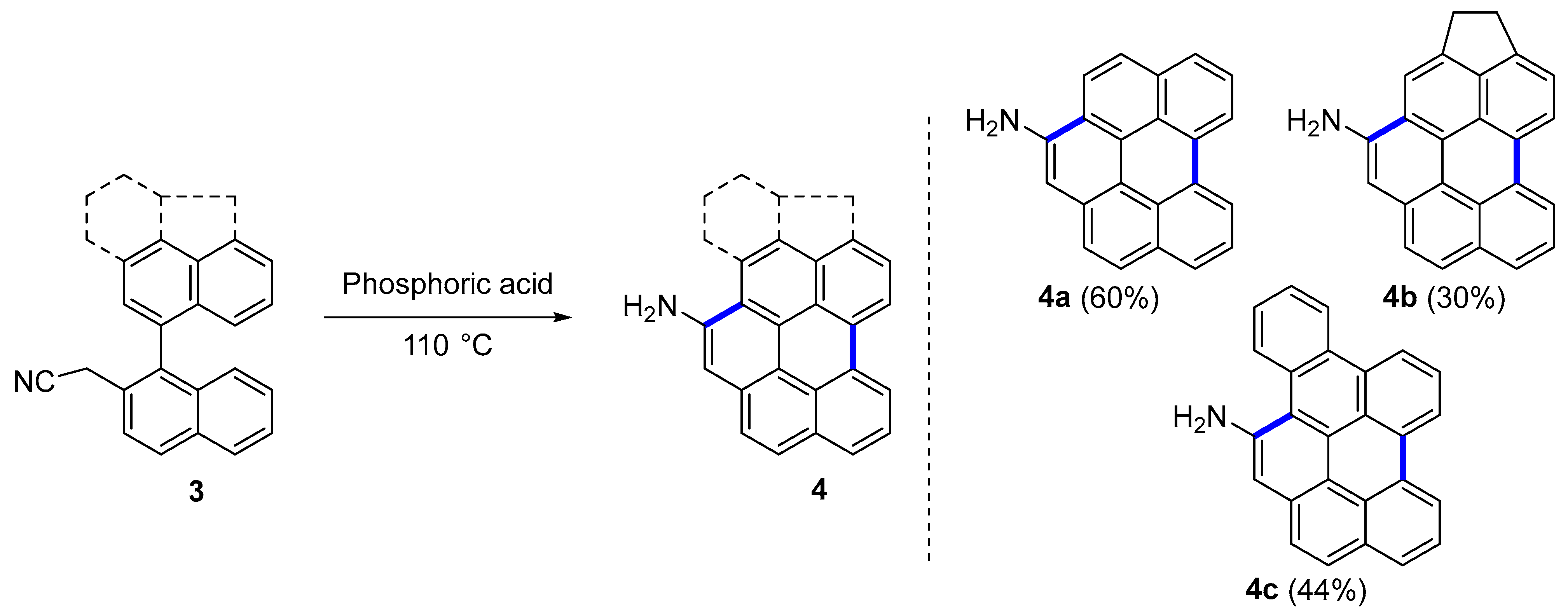
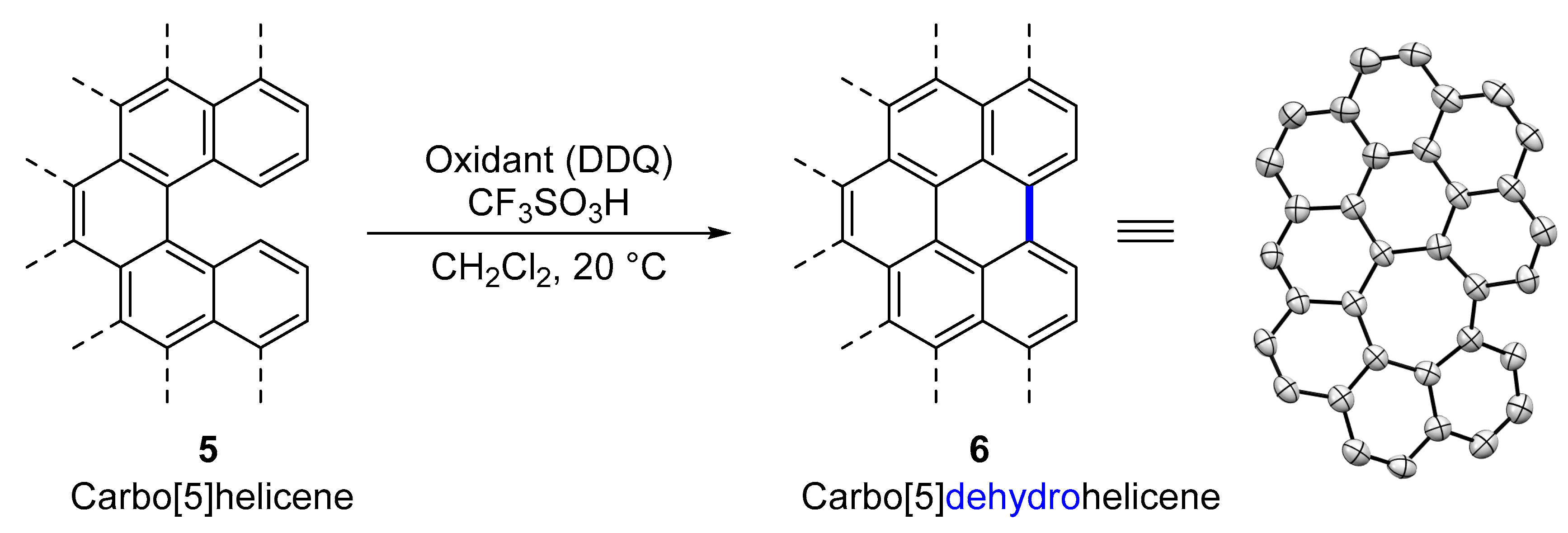
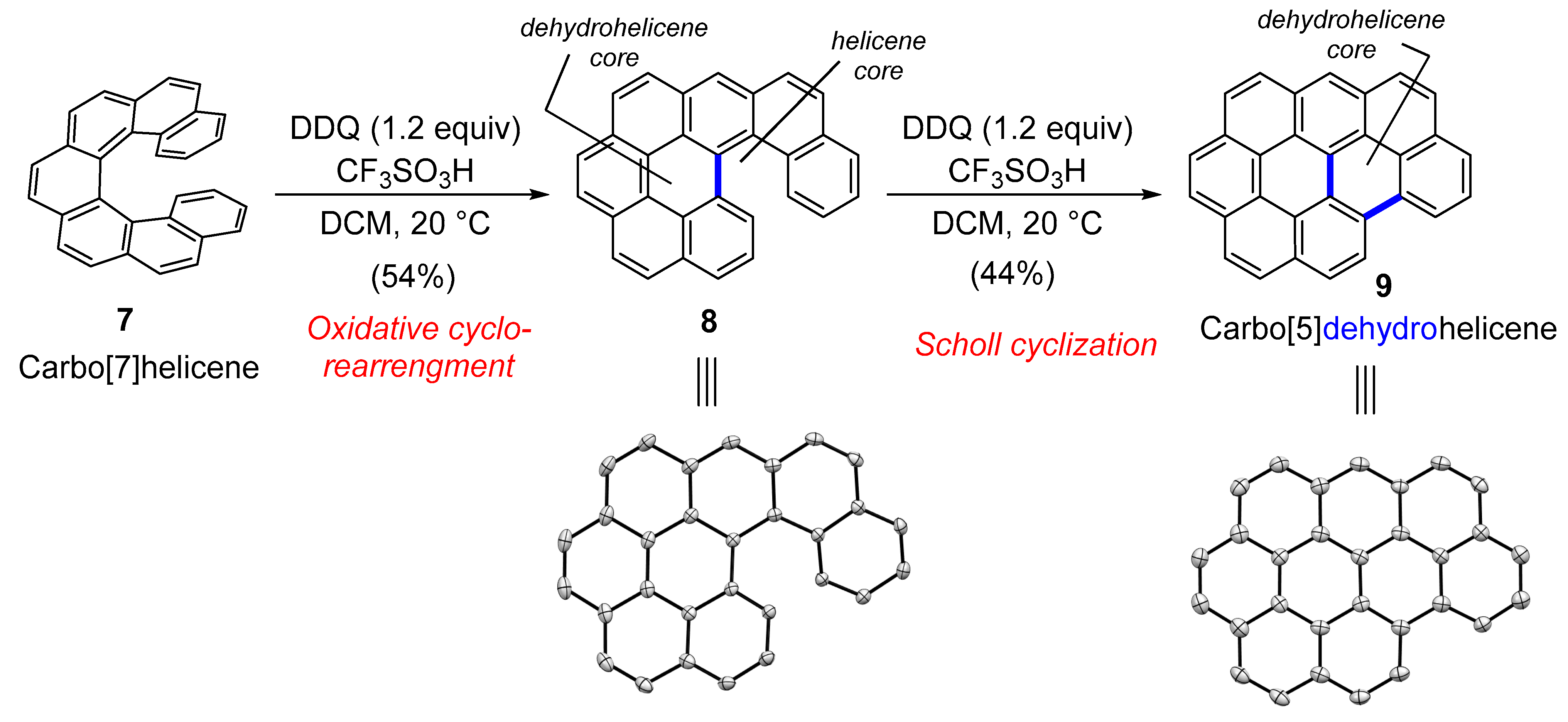
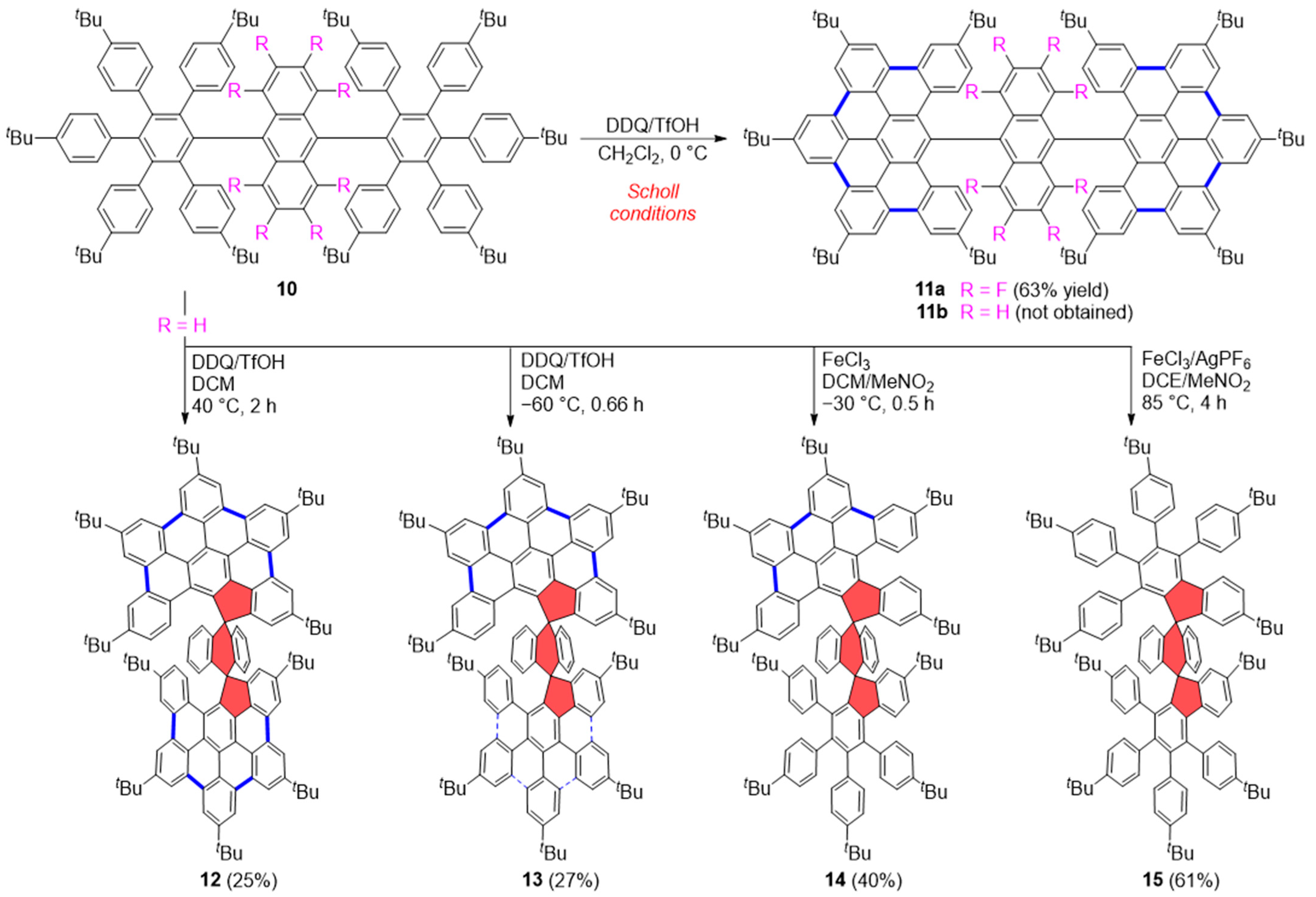
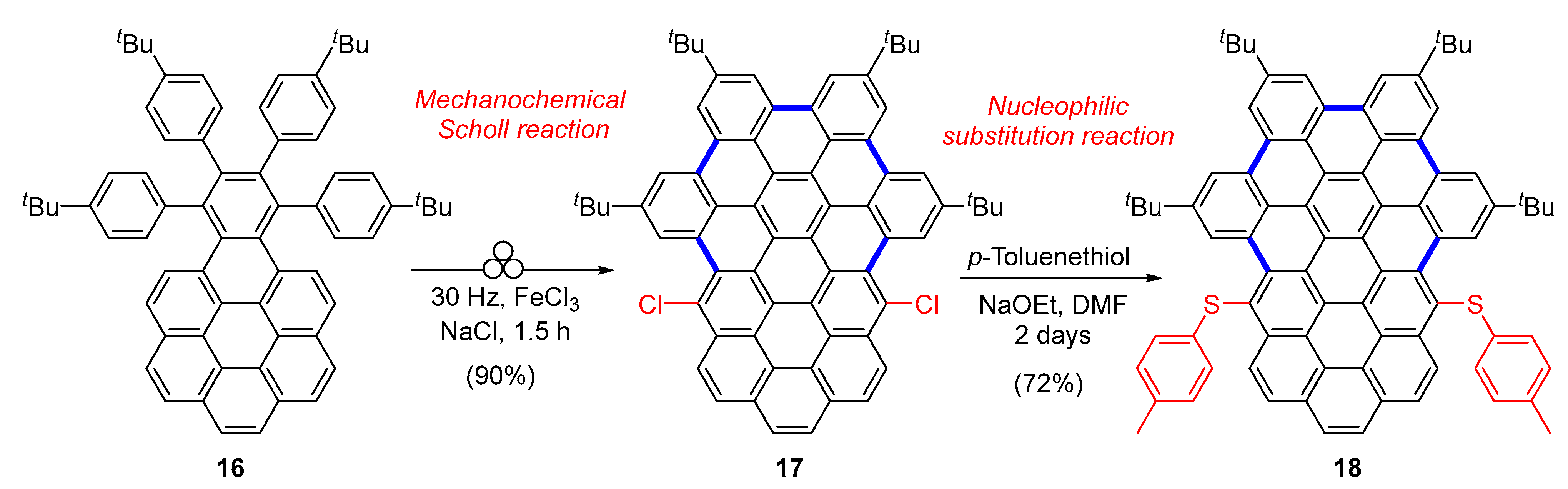
2.1. Carbo[5]dehydrohelicenes
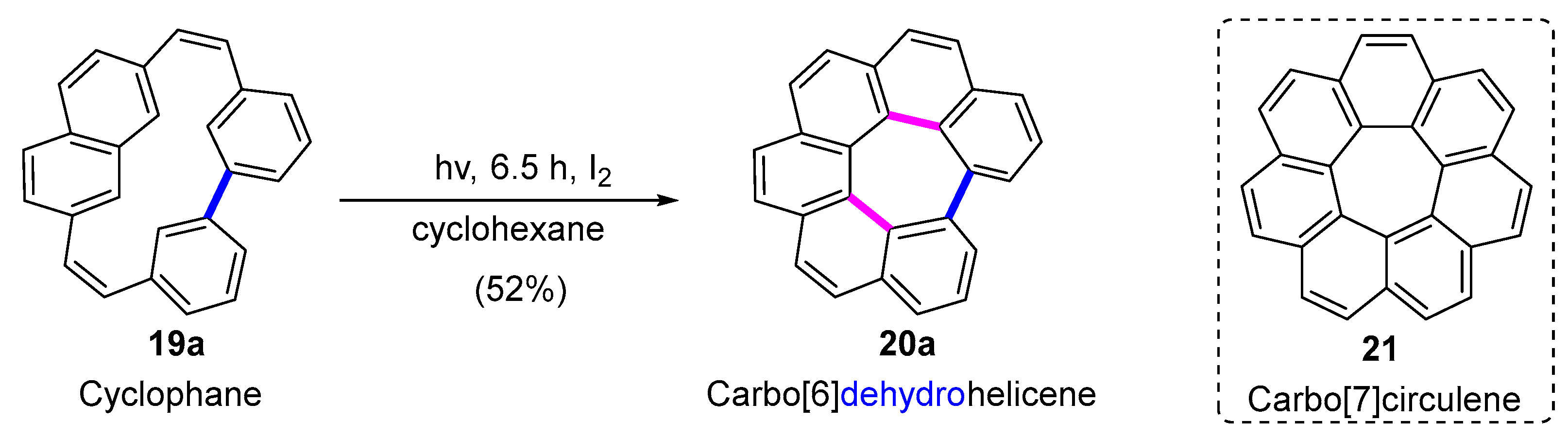
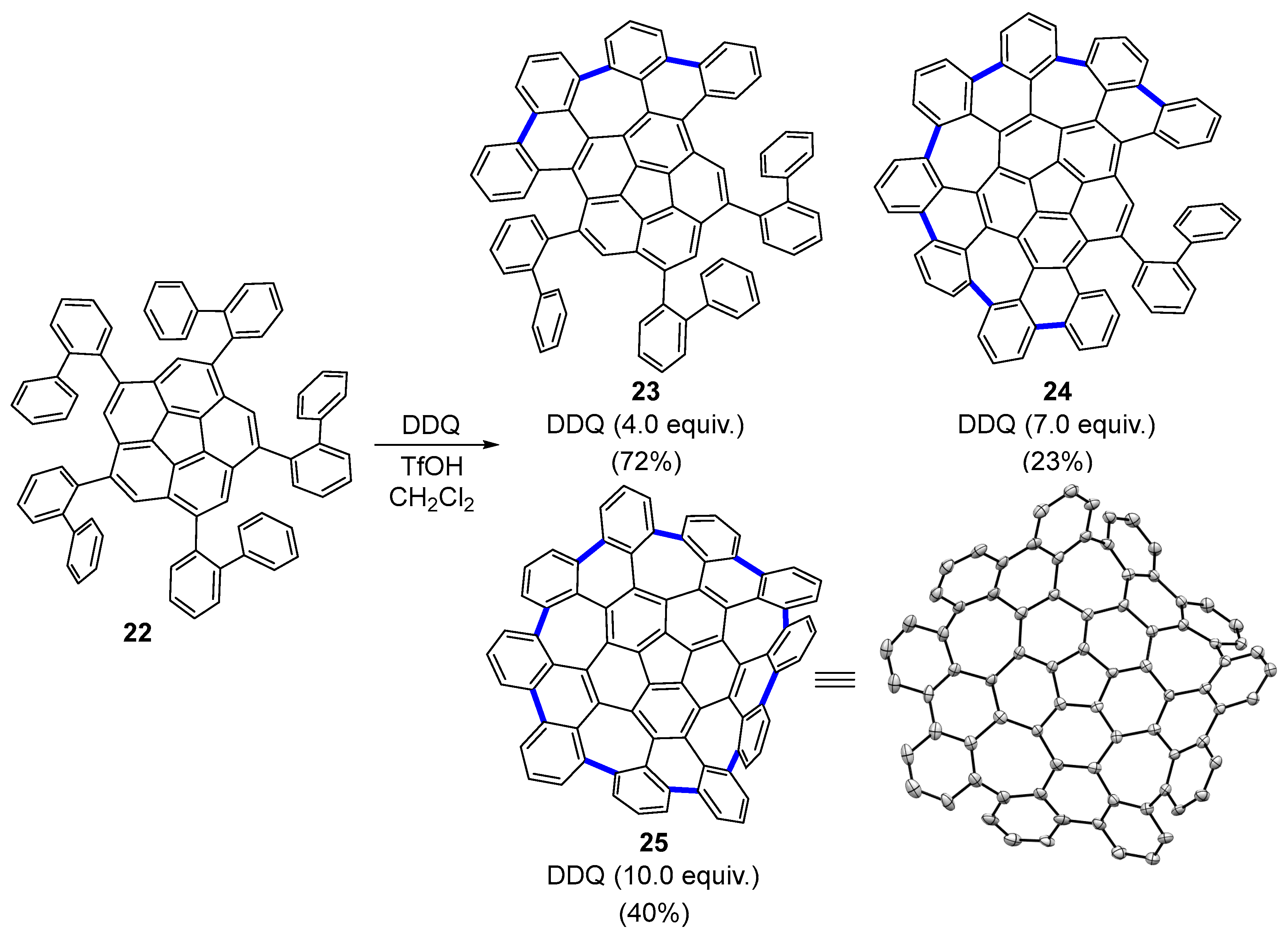
2.2. Carbo[6]dehydrohelicenes
3. Hetero[n]dehydrohelicenes
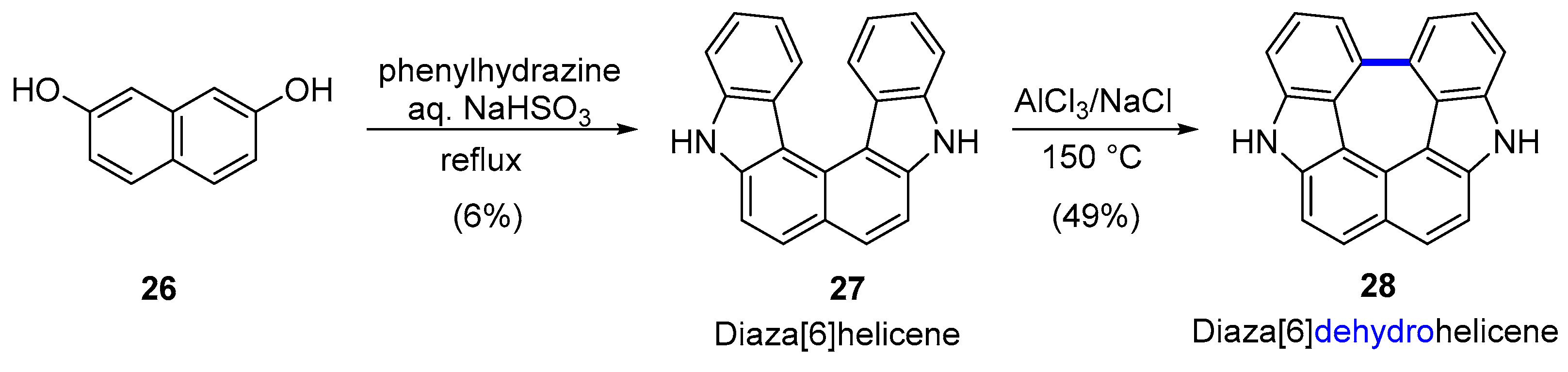
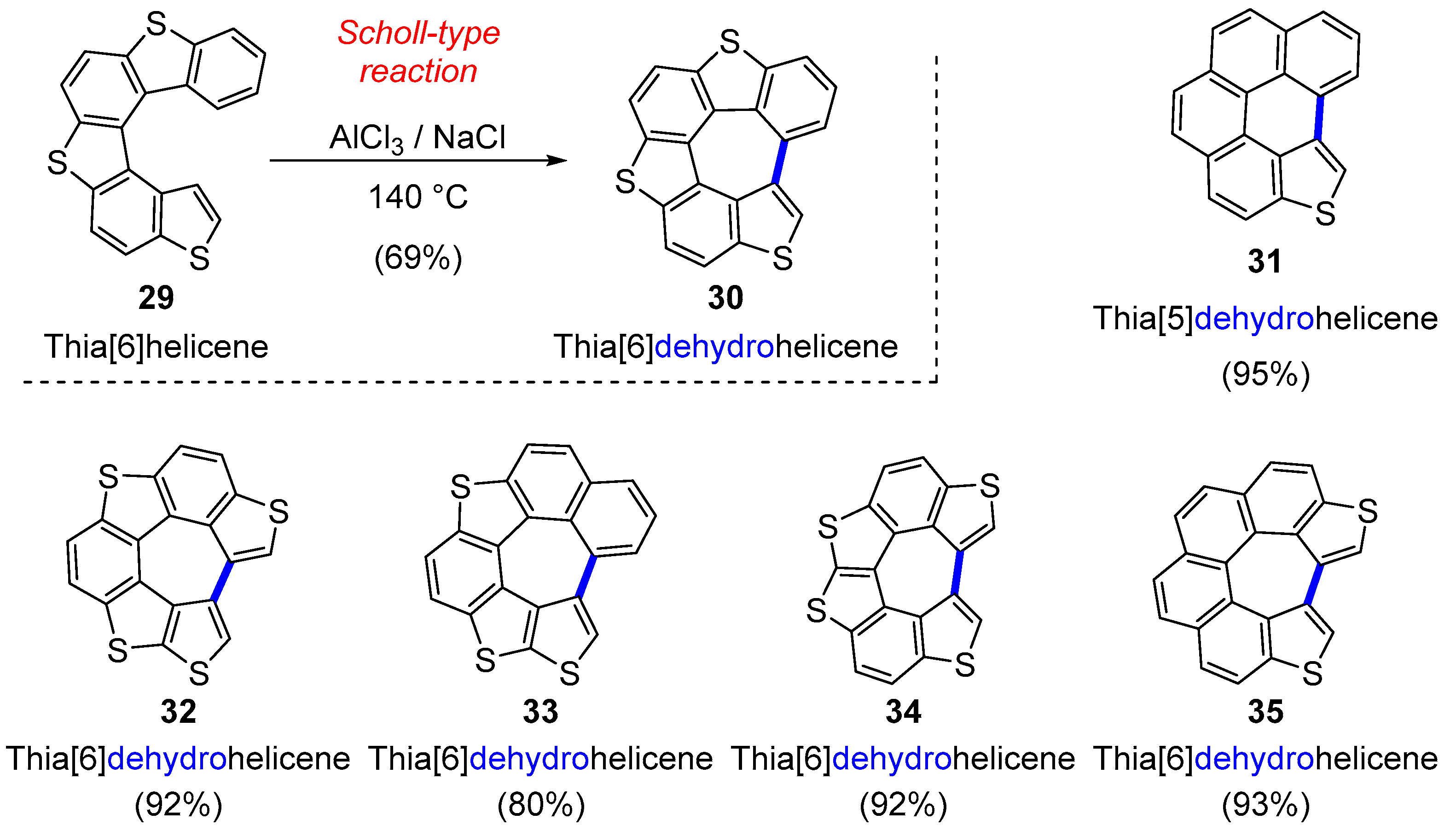
3.1. Hetero[5]dehydrohelicenes and Hetero[6]dehydrohelicenes
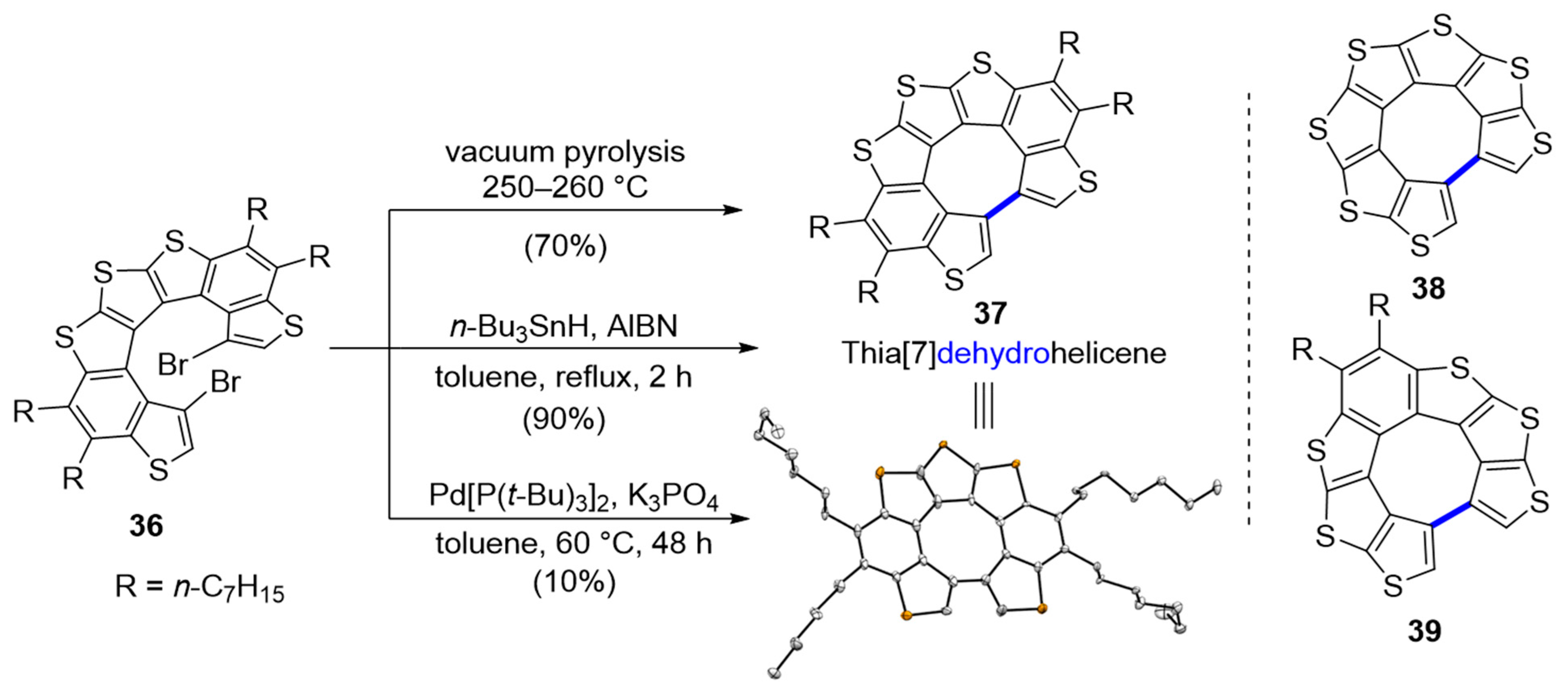
3.2. Hetero[7]dehydrohelicenes
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pasteur, L. Memoires sur la relation qui peut exister entre la forme crystalline et al. composition chimique, et sur la cause de la polarization rotatoire. Compt. Rend. 1848, 26, 535–538. [Google Scholar]
- van’t Hoff, J.H. A suggestion looking to the extension into space of the structural formulas at present used in chemistry, and a note upon the relation between the optical activity and the chemical constitution of organic compounds. Arch. Neerl. Sci. Exactes Nat. 1874, 9, 445–454. [Google Scholar]
- Le Bel, J.A. Sur les relations qui existent entre les formules atomiques des corps organiques et le pouvoir rotatoire de leurs dissolutions. Bull. Soc. Chim. Fr. 1874, 22, 337–347. [Google Scholar]
- Cahn, R.S.; Ingold, C.; Prelog, V. Specification of molecular chirality. Angew. Chem. Int. Ed. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Bedi, A.; Gidron, O. The consequences of twisting nanocarbons: Lessons from tethered twisted acenes. Acc. Chem. Res. 2019, 52, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Bedi, A.; Shimon, L.J.W.; Gidron, O. Helically locked tethered twistacenes. J. Am. Chem. Soc. 2018, 140, 8086–8090. [Google Scholar] [CrossRef]
- Bedi, A.; Gidron, O. Chiroptical properties of twisted acenes: Experimental and computational study. Chem. Eur. J. 2019, 25, 3279–3285. [Google Scholar] [CrossRef]
- Rickhaus, M.; Mayor, M.; Juríček, M. Chirality in curved polyaromatic systems. Chem. Soc. Rev. 2017, 46, 1643–1660. [Google Scholar] [CrossRef]
- Dopper, J.H.; Oudman, D.; Wynberg, H. Dehydrogenation of heterohelicenes by a Scholl type reaction. Dehydrohelicenes. J. Org. Chem. 1975, 40, 3398–3401. [Google Scholar] [CrossRef]
- Khalid, M.I.; Salem, M.S.H.; Sako, M.; Kondo, M.; Sasai, H.; Takizawa, S. Electrochemical synthesis of heterodehydro[7]helicenes. Commun. Chem. 2022, 5, 166. [Google Scholar] [CrossRef]
- Rajca, A.; Miyasaka, M.; Xiao, S.; Boratyński, P.J.; Pink, M.; Rajca, S. Intramolecular cyclization of thiophene-based [7]helicenes to quasi-[8]circulenes. J. Org. Chem. 2009, 74, 9105–9111. [Google Scholar] [CrossRef]
- Gingras, M. One hundred years of helicene chemistry. Part 1: Non-stereoselective syntheses of carbohelicenes. Chem. Soc. Rev. 2013, 42, 968–1006. [Google Scholar] [CrossRef] [PubMed]
- Gingras, M. One hundred years of helicene chemistry. Part 3: Applications and properties of carbohelicenes. Chem. Soc. Rev. 2013, 42, 1051–1095. [Google Scholar] [CrossRef] [PubMed]
- Gingras, M.; Félix, G.; Peresutti, R. One hundred years of helicene chemistry. Part 2: Stereoselective syntheses and chiral separations of carbohelicenes. Chem. Soc. Rev. 2013, 42, 1007–1050. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, C.-F. Helicenes: Synthesis and applications. Chem. Rev. 2012, 112, 1463–1535. [Google Scholar] [CrossRef]
- Salem, M.S.H.; Khalid, M.I.; Sasai, H.; Takizawa, S. Two-pot synthesis of unsymmetrical hetero[7]helicenes with intriguing optical properties. Tetrahedron 2023, 133, 133266. [Google Scholar] [CrossRef]
- Borissov, A.; Maurya, Y.K.; Moshniaha, L.; Wong, W.-S.; Żyła-Karwowska, M.; Stępień, M. Recent advances in heterocyclic nanographenes and other polycyclic heteroaromatic compounds. Chem. Rev. 2021, 122, 565–788. [Google Scholar] [CrossRef]
- Stępień, M.; Gońka, E.; Żyła, M.; Sprutta, N. Heterocyclic nanographenes and other polycyclic heteroaromatic compounds: Synthetic routes, properties, and applications. Chem. Rev. 2017, 117, 3479–3716. [Google Scholar] [CrossRef]
- Zander, M.; Franke, W.H. Über Carbazolo-carbazole. Chem. Berichte 1969, 102, 2728–2738. [Google Scholar] [CrossRef]
- Jessup, P.J.; Reiss, J.A.; Cyclophanes, V. Biphenylnaphthalenophanes and the synthesis of Hexa[7]circulene. Aust. J. Chem. 1976, 29, 173–178. [Google Scholar] [CrossRef]
- Roncali, J.; Leriche, P.; Blanchard, P. Molecular materials for organic photovoltaics: Small is beautiful. Adv. Mater. 2014, 26, 3821–3838. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-L.; Peng, Q.; Zhao, C.-H. Circularly polarized luminescence switching in small organic molecules. Chem. Eur. J. 2019, 25, 15441–15454. [Google Scholar] [CrossRef] [PubMed]
- Mori, T. Frontiers of circularly polarized luminescence Chemistry of isolated small organic molecules. In Circularly Polarized luminescence of Isolated Small Organic Molecules; Springer: Singapore, 2020; pp. 1–10. [Google Scholar]
- Wu, Z.-Y.; Xu, S.-L.; Yan, Q.-Q.; Chen, Z.-Q.; Ding, Y.-W.; Li, C.; Liang, H.-W.; Yu, S.-H. Transition metal–assisted carbonization of small organic molecules toward functional carbon materials. Sci. Adv. 2018, 4, eaat0788. [Google Scholar] [CrossRef] [PubMed]
- Barron, L.D.; Hecht, L.; McColl, I.H.; Blanch, E.W. Raman optical activity comes of age. Mol. Phys. 2004, 102, 731–744. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Pan, J.-J. The determination of the absolute configurations of chiral molecules using vibrational circular dichroism (VCD) spectroscopy. Chirality 2008, 20, 643–663. [Google Scholar] [CrossRef] [PubMed]
- Kurouski, D. Advances of vibrational circular dichroism (VCD) in bioanalytical chemistry. A review. Anal. Chim. Acta 2017, 990, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, A.; Malon, P.; Keiderling, T.A. Comparison of vibrational circular dichroism instruments: Development of a new dispersive VCD. Appl. Spectrosc. 2009, 63, 775–785. [Google Scholar] [CrossRef]
- Sánchez-Carnerero, E.M.; Agarrabeitia, A.R.; Moreno, F.; Maroto, B.L.; Muller, G.; Ortiz, M.J.; de la Moya, S. Circularly polarized luminescence from simple organic molecules. Chem. Eur. J. 2015, 21, 13488–13500. [Google Scholar] [CrossRef]
- Chen, N.; Yan, B. Recent theoretical and experimental progress in circularly polarized luminescence of small organic molecules. Molecules 2018, 23, 3376. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Li, Z. The progress of circularly polarized luminescence in chiral purely organic materials. Adv. Photonics Res. 2021, 2, 2000136. [Google Scholar] [CrossRef]
- Jakubec, M.; Storch, J. Recent advances in functionalizations of helicene backbone. J. Org. Chem. 2020, 85, 13415–13428. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, S.; Liu, X.; Narita, A.; Samorì, P.; Bonn, M.; Wang, H.I. Small Size, Big Impact: Recent Progress in Bottom-Up Synthesized Nanographenes for Optoelectronic and Energy Applications. Adv. Sci. 2022, 9, 2106055. [Google Scholar] [CrossRef]
- Xu, X.; Müllen, K.; Narita, A. Syntheses and characterizations of functional polycyclic aromatic hydrocarbons and graphene nanoribbons. Bull. Chem. Soc. Jpn. 2020, 93, 490–506. [Google Scholar] [CrossRef]
- Kawasumi, K.; Zhang, Q.; Segawa, Y.; Scott, L.T.; Itami, K. A grossly warped nanographene and the consequences of multiple odd-membered-ring defects. Nat. Chem. 2013, 5, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Hemminger, J.C.; Sarrao, J.; Crabtree, G.; Flemming, G.; Ratner, M. Challenges at the Frontiers of Matter and Energy: Transformative Opportunities for Discovery Science; USDOE Office of Science (SC): Germantown, MD, USA, 2015. [Google Scholar]
- Gupta, A.; Sakthivel, T.; Seal, S. Recent development in 2D materials beyond graphene. Prog. Mater. Sci. 2015, 73, 44–126. [Google Scholar] [CrossRef]
- Itami, K. Toward controlled synthesis of carbon nanotubes and graphenes. Pure Appl. Chem. 2012, 84, 907–916. [Google Scholar] [CrossRef]
- Pozo, I.; Guitián, E.; Pérez, D.; Peña, D. Synthesis of nanographenes, starphenes, and sterically congested polyarenes by aryne cyclotrimerization. Acc. Chem. Res. 2019, 52, 2472–2481. [Google Scholar] [CrossRef]
- Narita, A.; Wang, X.-Y.; Feng, X.; Müllen, K. New advances in nanographene chemistry. Chem. Soc. Rev. 2015, 44, 6616–6643. [Google Scholar] [CrossRef]
- Jassas, R.S.; Mughal, E.U.; Sadiq, A.; Alsantali, R.I.; Al-Rooqi, M.M.; Naeem, N.; Moussa, Z.; Ahmed, S.A. Scholl reaction as a powerful tool for the synthesis of nanographenes: A systematic review. RSC Adv. 2021, 11, 32158–32202. [Google Scholar] [CrossRef]
- Yamamoto, K.; Harada, T.; Okamoto, Y.; Chikamatsu, H.; Nakazaki, M.; Kai, Y.; Nakao, T.; Tanaka, M.; Harada, S.; Kasai, N. Synthesis and molecular structure of [7]circulene. J. Am. Chem. Soc. 1988, 110, 3578–3584. [Google Scholar] [CrossRef]
- Toya, M.; Omine, T.; Ishiwari, F.; Saeki, A.; Ito, H.; Itami, K. Expanded [2, 1][n]Carbohelicenes with 15-and 17-Benzene Rings. J. Am. Chem. Soc. 2023, 145, 11553–11565. [Google Scholar] [CrossRef] [PubMed]
- Chaolumen; Stepek, I.A.; Yamada, K.E.; Ito, H.; Itami, K. Construction of heptagon-containing molecular nanocarbons. Angew. Chem. Int. Ed. 2021, 60, 23508–23532. [Google Scholar] [CrossRef] [PubMed]
- Miera, G.G.; Matsubara, S.; Kono, H.; Murakami, K.; Itami, K. Synthesis of octagon-containing molecular nanocarbons. Chem. Sci. 2022, 13, 1848–1868. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-D.; Lu, H.-Y.; Chen, C.-F. Synthesis and structures of multifunctionalized helicenes and dehydrohelicenes: An efficient route to construct cyan fluorescent molecules. Chem. Eur. J. 2010, 16, 11843–11846. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Shukla, R.; Rathore, R. Oxidative C−C bond formation (Scholl reaction) with DDQ as an efficient and easily recyclable oxidant. Org. Lett. 2009, 11, 3474–3477. [Google Scholar] [CrossRef] [PubMed]
- Pieters, G.; Gaucher, A.; Prim, D.; Besson, T.; Planas, J.G.; Teixidor, F.; Viñas, C.; Light, M.E.; Hursthouse, M.B. Binaphthyl platform as starting materials for the preparation of electron rich benzo [g, h, i] perylenes. Application to molecular architectures based on amino benzo [g, h, i] perylenes and carborane combinations. Chem. Commun. 2011, 47, 7725–7727. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhang, G.; Ding, Y.; Yang, N.; Gan, F.; Crassous, J.; Qiu, H. Oxidative cyclo-rearrangement of helicenes into chiral nanographenes. Nat. Commun. 2021, 12, 2786. [Google Scholar] [CrossRef] [PubMed]
- Chillier, X.; Boulet, P.; Chermette, H.; Salama, F.; Weber, J. Absorption and emission spectroscopy of matrix-isolated benzo [g, h, i] perylene: An experimental and theoretical study for astrochemical applications. J. Chem. Phys. 2001, 115, 1769–1776. [Google Scholar] [CrossRef]
- Izquierdo-García, P.; Fernández-García, J.M.; Perles, J.; Fernández, I.; Martín, N. Electronic Control of the Scholl Reaction: Selective Synthesis of Spiro vs Helical Nanographenes. Angew. Chem. Int. Ed. 2023, 62, e202215655. [Google Scholar] [CrossRef]
- Stanojkovic, J.; William, R.; Zhang, Z.; Fernández, I.; Zhou, J.; Webster, R.D.; Stuparu, M.C. Synthesis of precisely functionalizable curved nanographenes via graphitization-induced regioselective chlorination in a mechanochemical Scholl Reaction. Nat. Commun. 2023, 14, 803. [Google Scholar] [CrossRef]
- Bruckmann, A.; Krebs, A.; Bolm, C. Organocatalytic reactions: Effects of ball milling, microwave and ultrasound irradiation. Green Chem. 2008, 10, 1131–1141. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Ralphs, K.; Hardacre, C.; James, S.L. Application of heterogeneous catalysts prepared by mechanochemical synthesis. Chem. Soc. Rev. 2013, 42, 7701–7718. [Google Scholar] [CrossRef]
- Hernández, J.G.; Bolm, C. Altering Product Selectivity by Mechanochemistry. J. Org. Chem. 2017, 82, 4007–4019. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.; Mack, J. Mechanochemistry and organic synthesis: From mystical to practical. Green Chem. 2018, 20, 1435–1443. [Google Scholar] [CrossRef]
- Andersen, J.M.; Mack, J. Decoupling the Arrhenius equation via mechanochemistry. Chem. Sci. 2017, 8, 5447–5453. [Google Scholar] [CrossRef]
- Ardila-Fierro, K.J.; Hernández, J.G. Sustainability assessment of mechanochemistry by using the twelve principles of green chemistry. ChemSusChem 2021, 14, 2145–2162. [Google Scholar] [CrossRef]
- Rasmussen, M.O.; Axelsson, O.; Tanner, D. A Practical Procedure for the Solid-Phase Synthesis of Racemic 2,2′-Dihydroxy-1,1′-binaphthyl. Synth. Commun. 1997, 27, 4027–4030. [Google Scholar] [CrossRef]
- Zhu, X.; Hua, Y.; Tian, C.; Abney, C.W.; Zhang, P.; Jin, T.; Liu, G.; Browning, K.L.; Sacci, R.L.; Veith, G.M.; et al. Accelerating Membrane-based CO2 Separation by Soluble Nanoporous Polymer Networks Produced by Mechanochemical Oxidative Coupling. Angew. Chem. Int. Ed. 2018, 57, 2816–2821. [Google Scholar] [CrossRef]
- Howard, J.L.; Brand, M.C.; Browne, D.L. Switching chemoselectivity: Using mechanochemistry to alter reaction kinetics. Angew. Chem. Int. Ed. 2018, 57, 16104–16108. [Google Scholar] [CrossRef]
- Báti, G.; Csókás, D.; Yong, T.; Tam, S.M.; Shi, R.R.S.; Webster, R.D.; Pápai, I.; García, F.; Stuparu, M.C. Mechanochemical Synthesis of Corannulene-Based Curved Nanographenes. Angew. Chem. Int. Ed. 2020, 59, 21620–21626. [Google Scholar] [CrossRef] [PubMed]
- Mori, T. Chiroptical properties of symmetric double, triple, and multiple helicenes. Chem. Rev. 2021, 121, 2373–2412. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.-H.; Park, G.; Kertesz, M. Electronic structure of helicenes, C2S helicenes, and thiaheterohelicenes. Chem. Mater. 2008, 20, 3266–3277. [Google Scholar] [CrossRef]
- Hong, J.; Xiao, X.; Liu, H.; Dmitrieva, E.; Popov, A.A.; Yu, Z.; Li, M.-D.; Ohto, T.; Liu, J.; Narita, A.; et al. Controlling the emissive, chiroptical, and electrochemical properties of double [7] helicenes through embedded aromatic rings. Chem. Eur. J. 2022, 28, e202202243. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.S.H.; Sabri, A.; Khalid, M.I.; Sasai, H.; Takizawa, S. Two-Step Synthesis, Structure, and Optical Features of a Double Hetero[7]helicene. Molecules 2022, 27, 9068. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-W.; Shen, J.-J. Multiple Heterohelicenes: Synthesis, Properties and Applications. Chem. Eur. J. 2022, 28, e202202069. [Google Scholar] [CrossRef]
- Oda, S.; Kawakami, B.; Yamasaki, Y.; Matsumoto, R.; Yoshioka, M.; Fukushima, D.; Nakatsuka, S.; Hatakeyama, T. One-shot synthesis of expanded heterohelicene exhibiting narrowband thermally activated delayed fluorescence. J. Am. Chem. Soc. 2021, 144, 106–112. [Google Scholar] [CrossRef]
- Matsuo, Y.; Maeda, C.; Tsutsui, Y.; Tanaka, T.; Seki, S. Synthesis of closed-Heterohelicenes Interconvertible between Their Monomeric and Dimeric Forms. Angew. Chem. Int. Ed. 2023, 62, e202314968. [Google Scholar] [CrossRef]
- Wynberg, H.; Groen, M.B.; Schadenberg, H. Synthesis and resolution of some heterohelicenes. J. Org. Chem. 1971, 36, 2797–2809. [Google Scholar] [CrossRef]
- Fujikawa, T.; Segawa, Y.; Itami, K. Laterally π-extended dithia[6]helicenes with heptagons: Saddle-helix hybrid molecules. J. Org. Chem. 2017, 82, 7745–7749. [Google Scholar] [CrossRef]
- Chen, F.; Tanaka, T.; Mori, T.; Osuka, A. Synthesis, structures, and optical properties of azahelicene derivatives and unexpected formation of azahepta[8]circulenes. Chem. Eur. J. 2018, 24, 7489–7497. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Chen, F.; Kise, K.; Tanaka, T.; Osuka, A. Facile synthesis of fluorescent hetero [8] circulene analogues with tunable solubilities and optical properties. Chem. Sci. 2019, 10, 11006–11012. [Google Scholar] [CrossRef] [PubMed]
- Lousen, B.; Pedersen, S.K.; Bols, P.S.; Hansen, K.H.; Pedersen, M.R.; Hammerich, O.; Bondarchuk, S.; Minaev, B.; Baryshnikov, G.V.; Ågren, H.; et al. Compressing a non-planar aromatic heterocyclic [7]helicene to a planar hetero[8]circulene. Chem. Eur. J. 2020, 26, 4935–4940. [Google Scholar] [CrossRef]
- Maeda, C.; Nomoto, S.; Akiyama, K.; Tanaka, T.; Ema, T. Facile synthesis of azahelicenes and diaza[8]circulenes through the intramolecular Scholl reaction. Chem. Eur. J. 2021, 27, 15699–15705. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sasai, H.; Takizawa, S. Atroposelective Synthesis of C–C Axially Chiral Compounds via Mono-and Dinuclear Vanadium Catalysis. Acc. Chem. Res. 2022, 55, 2949–2965. [Google Scholar] [CrossRef] [PubMed]
- Sako, M.; Higashida, K.; Kamble, G.T.; Kaut, K.; Kumar, A.; Hirose, Y.; Zhou, D.-Y.; Suzuki, T.; Rueping, M.; Maegawa, T.; et al. Chemo-and enantioselective hetero-coupling of hydroxycarbazoles catalyzed by a chiral vanadium(v) complex. Org. Chem. Front. 2021, 8, 4878–4885. [Google Scholar] [CrossRef]
- Kamble, G.T.; Salem, M.S.H.; Abe, T.; Park, H.; Sako, M.; Takizawa, S.; Sasai, H. Chiral Vanadium(V)-catalyzed Oxidative Coupling of 4-Hydroxycarbazoles. Chem. Lett. 2021, 50, 1755–1757. [Google Scholar] [CrossRef]
- Salem, M.S.H.; Kumar, A.; Sako, M.; Abe, T.; Takizawa, S.; Sasai, H. Preparation of optically pure dinuclear cobalt(III) complex with Λ-configuration as a dianionic chiral catalyst. Heterocycles 2021, 103, 225–230. [Google Scholar]
- Salem, M.S.H.; Takizawa, S. New anionic cobalt(III) complexes enable enantioselective synthesis of spiro-fused oxazoline and iodoacetal derivatives. Front. Chem. 2022, 10, 34291. [Google Scholar] [CrossRef]
- Salem, M.S.H.; Dubois, C.; Takamura, Y.; Kitajima, A.; Kawai, T.; Takizawa, S.; Kirihara, M. Light-induced autoxidation of aldehydes to peracids and carboxylic acids. Green Chem. 2024, 26, 375–383. [Google Scholar] [CrossRef]
- Salem, M.S.H.; Khalid, M.I.; Sako, M.; Higashida, K.; Lacroix, C.; Kondo, M.; Takishima, R.; Taniguchi, T.; Miura, M.; Vo-Thanh, G.; et al. Electrochemical synthesis of hetero[7]helicenes containing pyrrole and furan rings via an oxidative heterocoupling and dehydrative cyclization sequence. Adv. Synth. Catal. 2023, 365, 373–380. [Google Scholar] [CrossRef]
- Salem, M.S.H.; Sharma, R.; Khalid, M.I.; Sasi, M.; Amasaki, R.; Imai, Y.; Arisawa, M.; Takizawa, S. Data-driven Electrochemical One-pot Synthesis of Double Hetero[7]dehydrohelicene. Electrochemistry 2023, 91, 112015. [Google Scholar] [CrossRef]
- Kondo, M.; Wathsala, H.D.P.; Salem, M.S.H.; Ishikawa, K.; Hara, S.; Takaai, T.; Washio, T.; Sasai, H.; Takizawa, S. Bayesian optimization-driven parallel-screening of multiple parameters for the flow synthesis of biaryl compounds. Commun. Chem. 2022, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Sugizaki, A.; Khalid, M.I.; Wathsala, H.D.P.; Ishikawa, K.; Hara, S.; Takaai, T.; Washio, T.; Takizawa, S.; Sasai, H. Energy-, time-, and labor-saving synthesis of α-ketiminophosphonates: Machine-learning-assisted simultaneous multiparameter screening for electrochemical oxidation. Green Chem. 2021, 23, 5825–5831. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, S.; Li, J.; Wei, J.; Müllen, K.; Yin, M. A Water-Soluble, NIR-Absorbing Quaterrylenediimide Chromophore for Photoacoustic Imaging and Efficient Photothermal Cancer Therapy. Angew. Chem. Int. Ed. 2019, 58, 1638–1642. [Google Scholar] [CrossRef]
- Lin, H.-A.; Sato, Y.; Segawa, Y.; Nishihara, T.; Sugimoto, N.; Scott, L.T.; Higashiyama, T.; Itami, K. A Water-Soluble Warped Nanographene: Synthesis and Applications for Photoinduced Cell Death. Angew. Chem. Int. Ed. 2018, 57, 2874–2878. [Google Scholar] [CrossRef]
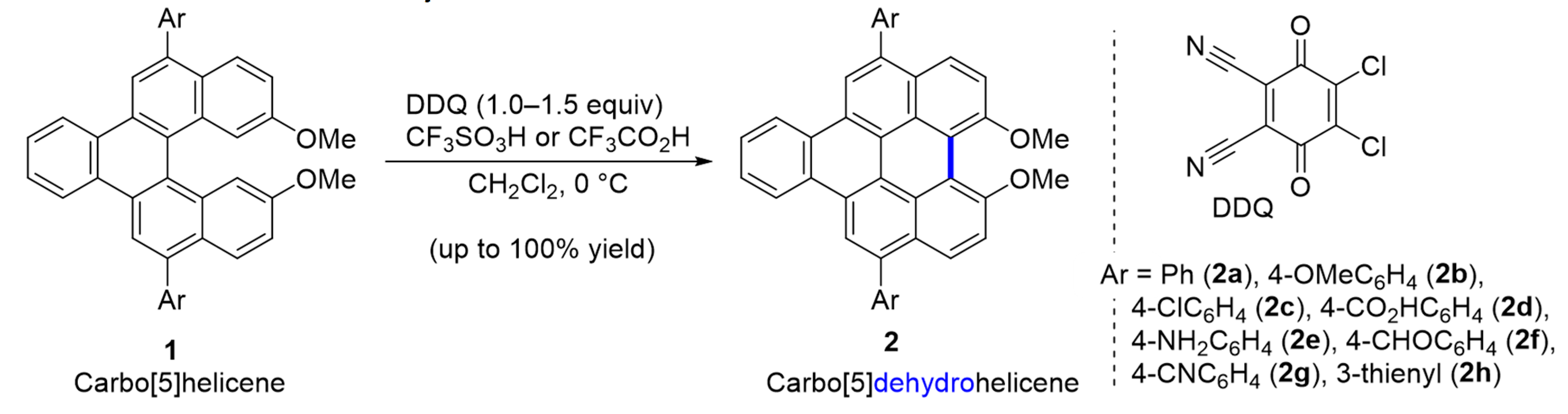


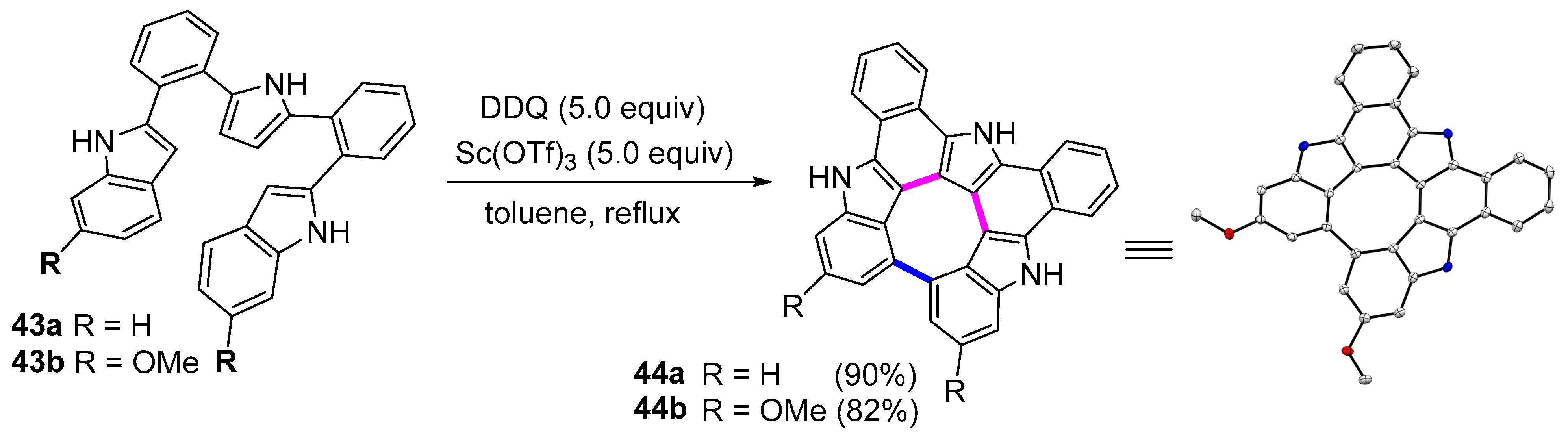

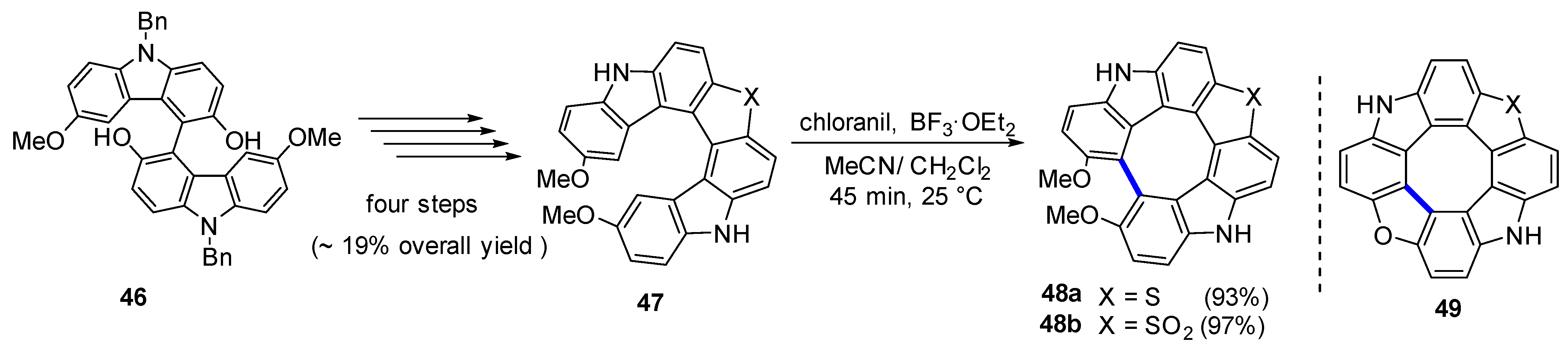

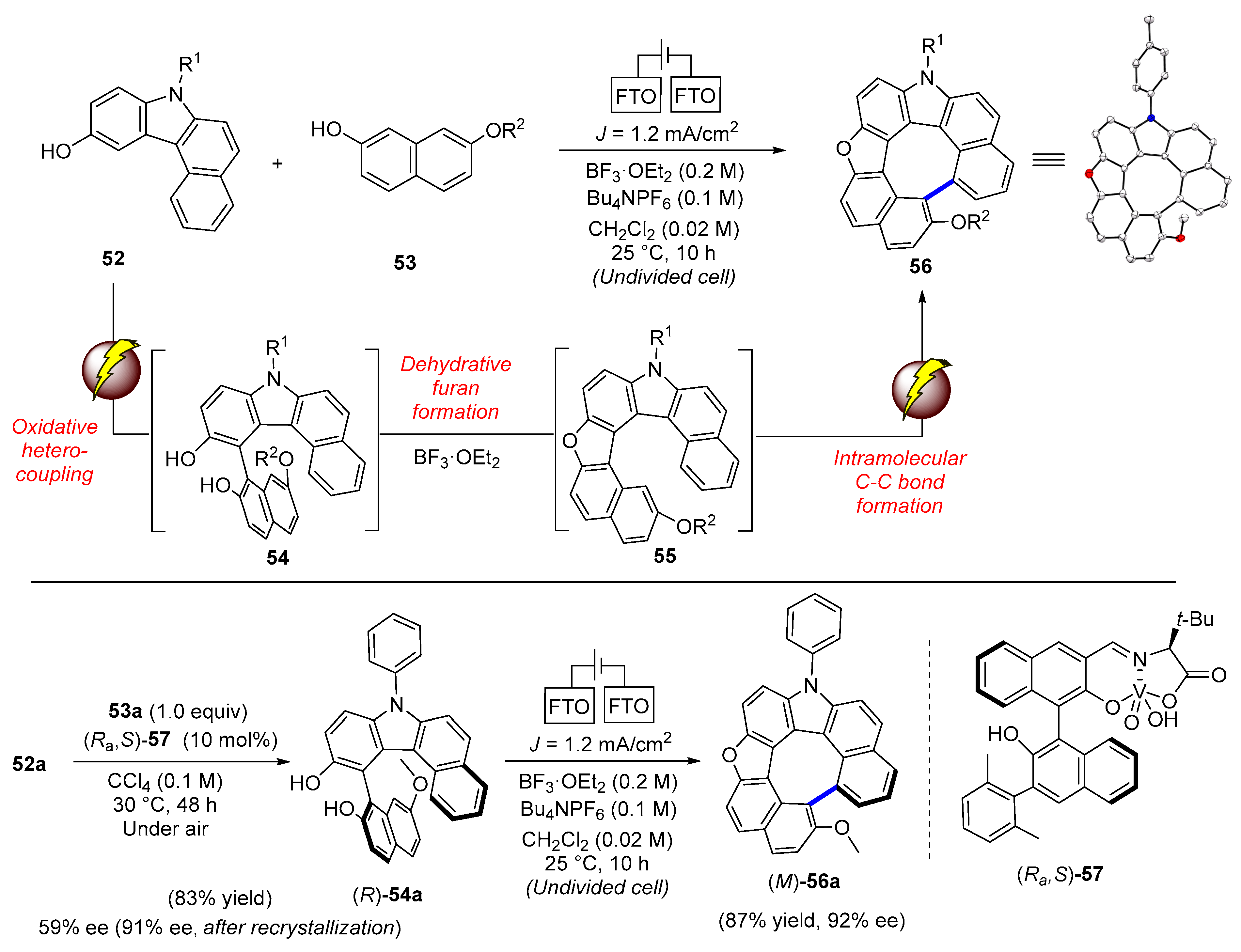

| Compound | Ar | Abs (nm) | Em (nm) | Quantum Yield ϕ |
|---|---|---|---|---|
| 2a | phenyl | 436 | 478 | 0.51 |
| 2b | 4-MeOC6H4 | 436 | 489 | 0.78 |
| 2c | 4-ClC6H4 | 435 | 480 | 0.45 |
| 2d | 4-CO2HC6H4 | 432 | 480 | 0.33 |
| 2e | 4-NH2C6H4 | 436 | 478 | 0.58 |
| 2f | 4-CHOC6H4 | 433 | 489 | 0.40 |
| 2g | 4-CNC6H4 | 433 | 480 | 0.47 |
| 2h | 3-thienyl | 438 | 481 | 0.75 |
| Compound | Abs (nm) | Em (nm) | ε/M−1 cm−1 | Stokes Shift |
|---|---|---|---|---|
| 4a | 311 | 481 | 48,300 | 170 |
| 4b | 309 | 475 | 17,300 | 166 |
| 4c | 308 | 474 | 43,100 | 166 |
| Compound | λmax/nm (ε/104 mol−1 dm3 cm−1) | |||
|---|---|---|---|---|
| 37 | 244 (5.9) | 326 (1.9) | 338 (2.1) | 348 (1.4) |
| 38 | 265 | - | - | - |
| 39 | 256 (~3.3) | 319 (~0.8) | 333 (~0.8) | - |
| Synthetic Approaches | Conditions | Compounds | Chiroptical Features gabs/glum |
|---|---|---|---|
| Scholl reaction | DDQ-mediated Scholl reaction | 2, 6, 11–14, 23–25 | - |
| Chloranil-mediated Scholl reaction | 48 | - | |
| FeCl3-mediated Scholl reaction | 51 | |glum| value of 2.5 × 10−4 at λem = 435 nm | |
| Phosphoric acid at 110 °C | 4 | - | |
| AlCl3/NaCl at 140–150 °C | 28, 30–35 | - | |
| Mechanochemical Scholl reaction | 17, 18 | - | |
| photochemical oxidative ring closure (hv, I2) | 20 | - | |
| Vacuum pyrolysis | 180–290 °C under a vacuum ≤ 0.1 mTorr | 37 | - |
| Metal-mediated oxidative coupling | n-Bu3SnH or Pd[P(t-Bu)3]2 | 37 | - |
| MoCl2 | 41, 42 | Cotton effects at 501, 471, 421 nm | |
| Oxidative fusion reaction | DDQ-Sc(OTf)3 | 44a, 44b | - |
| Hypervalent Iodine-induced Cyclization | PIFA/NaBH4 | 44c | Cotton effects at 450, and 340 nm |
| Electrochemical oxidation | 56, 59 | |glum| of 56 = 2.5 × 10−3 |glum| of 59 = 1.5 × 10−3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, M.I.; Salem, M.S.H.; Takizawa, S. Synthesis and Structural and Optical Behavior of Dehydrohelicene-Containing Polycyclic Compounds. Molecules 2024, 29, 296. https://doi.org/10.3390/molecules29020296
Khalid MI, Salem MSH, Takizawa S. Synthesis and Structural and Optical Behavior of Dehydrohelicene-Containing Polycyclic Compounds. Molecules. 2024; 29(2):296. https://doi.org/10.3390/molecules29020296
Chicago/Turabian StyleKhalid, Md. Imrul, Mohamed S. H. Salem, and Shinobu Takizawa. 2024. "Synthesis and Structural and Optical Behavior of Dehydrohelicene-Containing Polycyclic Compounds" Molecules 29, no. 2: 296. https://doi.org/10.3390/molecules29020296
APA StyleKhalid, M. I., Salem, M. S. H., & Takizawa, S. (2024). Synthesis and Structural and Optical Behavior of Dehydrohelicene-Containing Polycyclic Compounds. Molecules, 29(2), 296. https://doi.org/10.3390/molecules29020296








