Anti-Cancer Potential of Linear β-(1→6)-D-Glucan from Agaricus bisporus on Estrogen Receptor-Positive (ER+) Breast Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
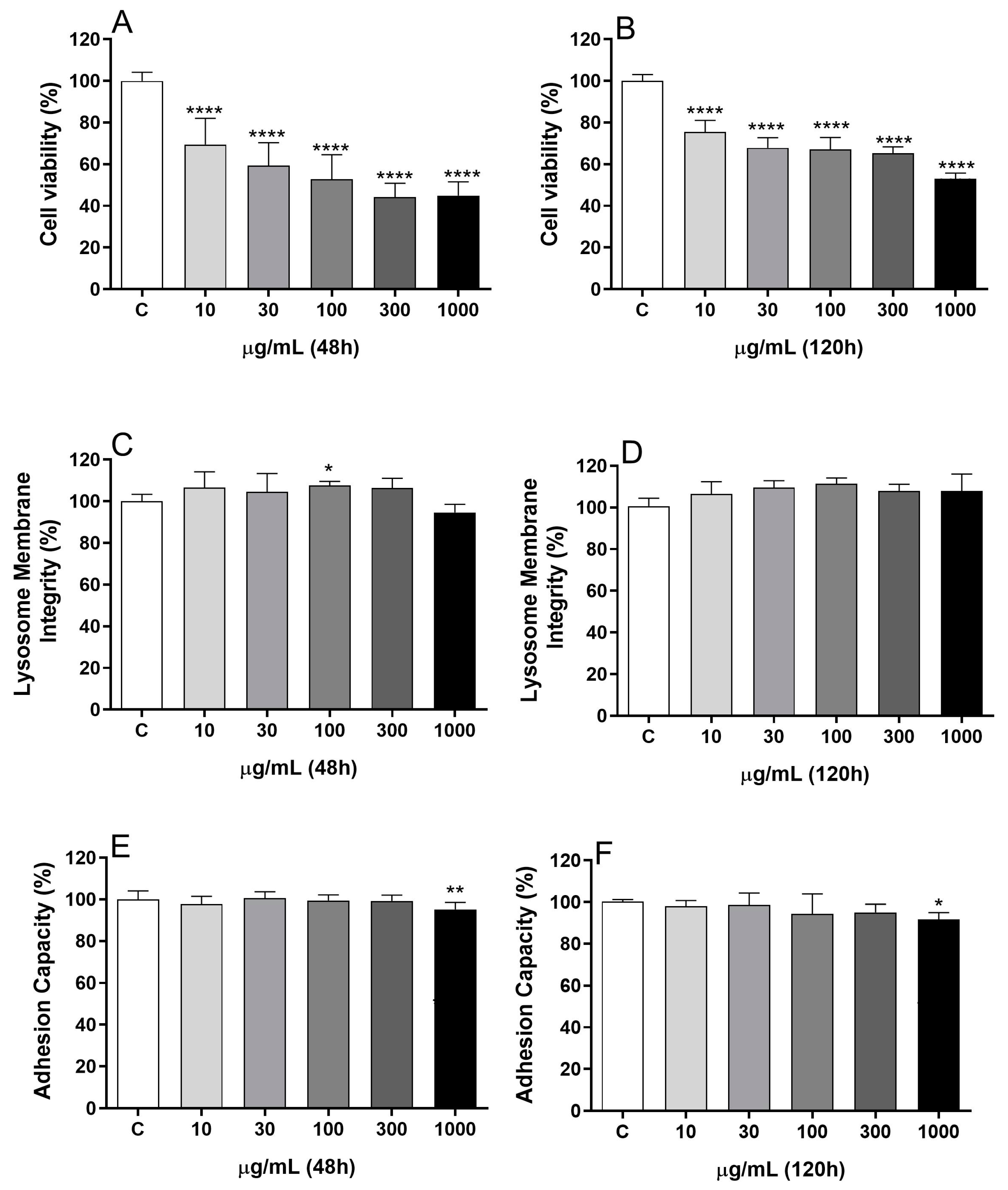
2.1. BDG16 Decreases the Metabolic Activity of MCF-7 Cells
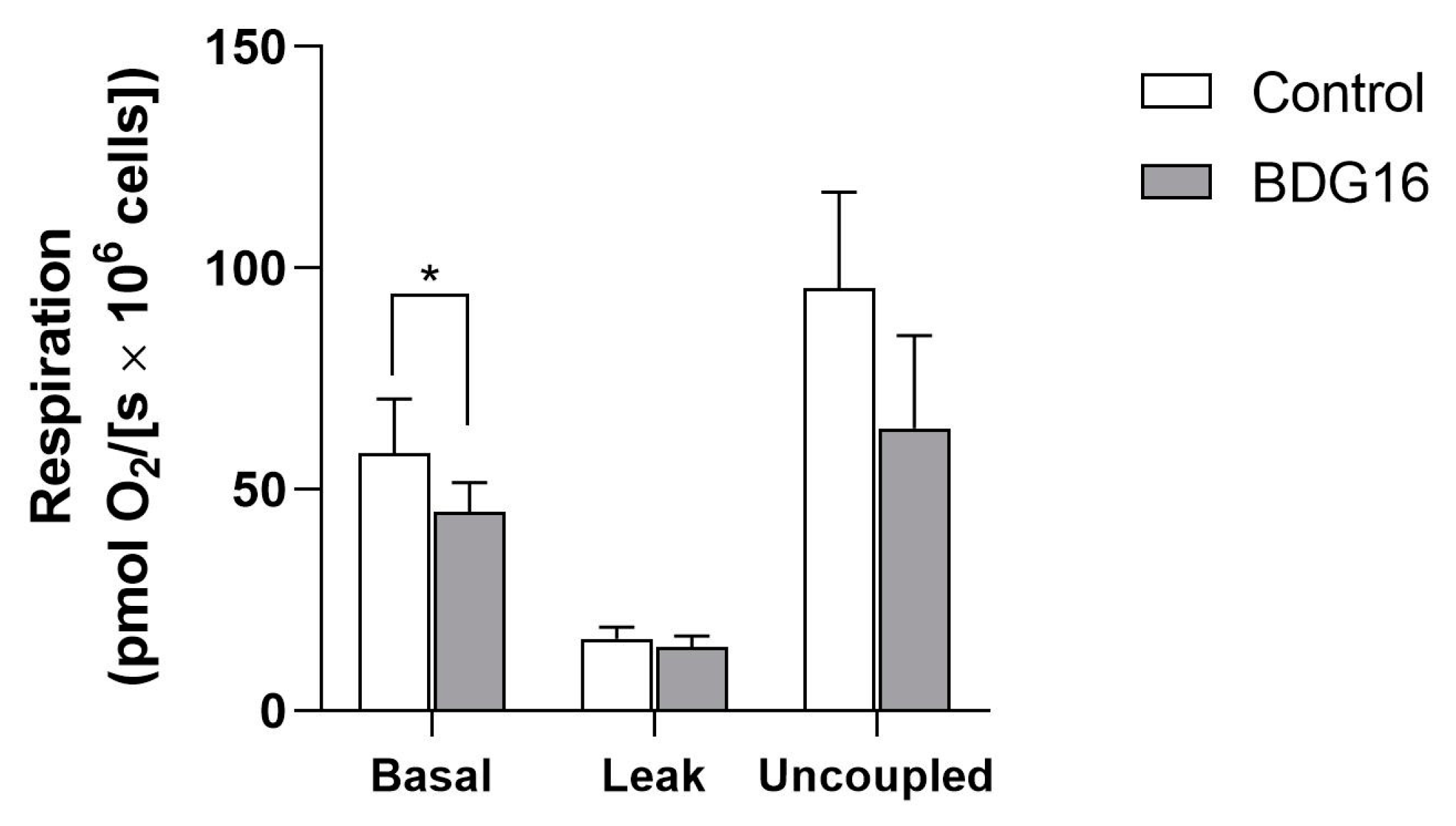
2.2. Cell Respiration
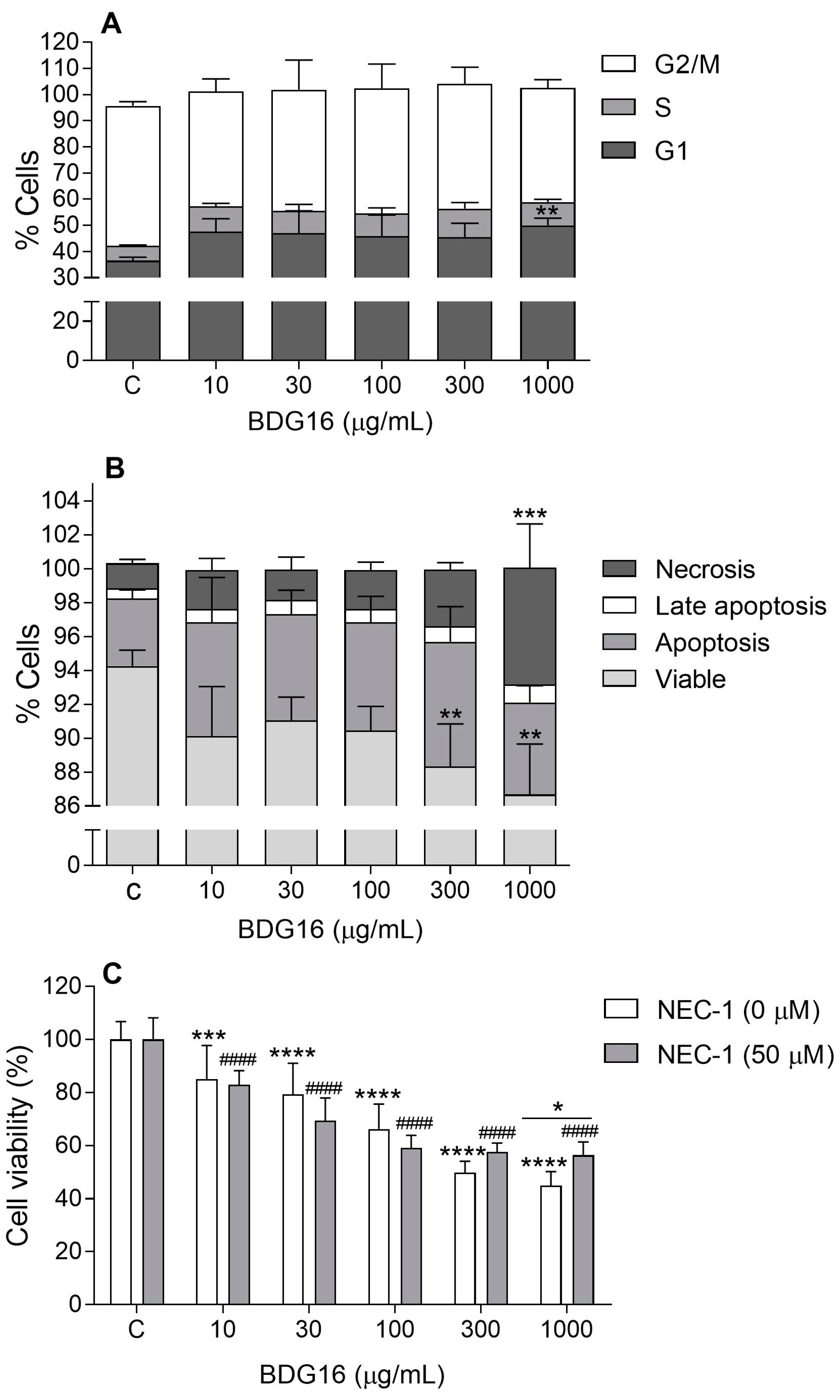
2.3. Cell-Cycle Evaluation
2.4. Apoptosis and Necroptosis Evaluation
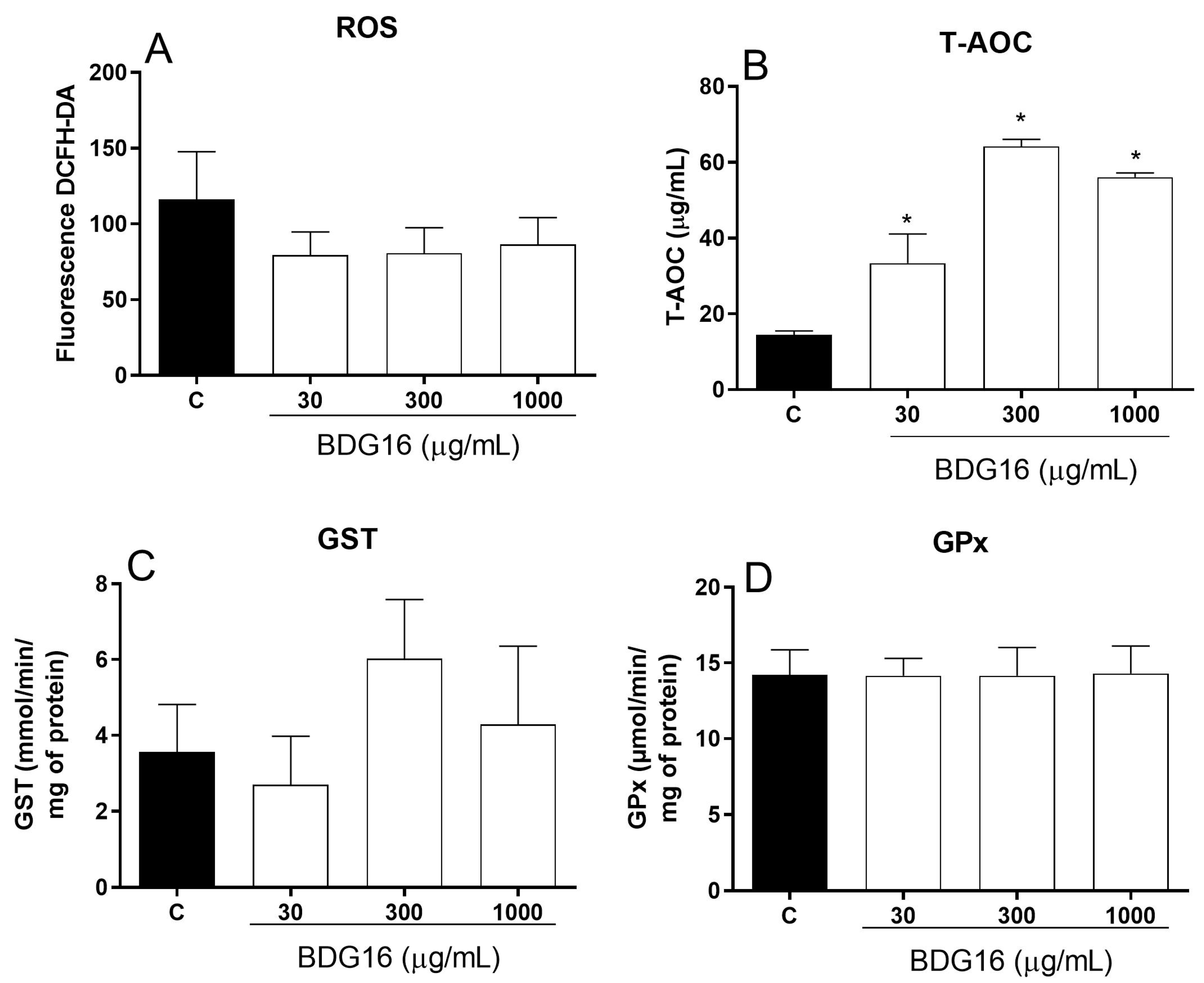
2.5. Oxidative Stress Analysis
3. Material and Methods
3.1. Reagents
3.2. Fungal Material and Preparation of β-Glucan
3.3. Estrogen Positive-Receptor (ER+) Breast Cancer Cell Line
3.4. Metabolic Activity Evaluation by MTT Assay
3.5. Lysosome Membrane Integrity Evaluation by Neutral Red Assay
3.6. Cell Adhesion Evaluation by Crystal Violet Assay
3.7. Measurement of Cell Respiration
3.8. Cell Cycle Analysis
3.9. Evaluation of Apoptosis
3.10. Necroptosis
3.11. RT-qPCR Analysis
3.12. Evaluation of Oxidative Stress Parameters
3.12.1. Total Reactive Oxygen Species (ROS)
3.12.2. Determination of Glutathione Peroxidase (GPx) Activity
3.12.3. Determination of Glutathione S-Transferase (GST) Activity
3.12.4. Determination of Total Antioxidant Activity (T-AOC)
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sima, P.; Vannucci, L.; Vetvicka, V. Glucans and Cancer: Historical Perspective. Cancer Transl. Med. 2015, 1, 209–214. [Google Scholar] [CrossRef]
- Zong, A.; Cao, H.; Wang, F. Anticancer Polysaccharides from Natural Resources: A Review of Recent Research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef]
- Rutckeviski, R.; Corso, C.R.; Román-Ochoa, Y.; Cipriani, T.R.; Centa, A.; Smiderle, F.R. Agaricus Bisporus β-(1→6)-D-Glucan Induces M1 Phenotype on Macrophages and Increases Sensitivity to Doxorubicin of Triple Negative Breast Cancer Cells. Carbohydr. Polym. 2022, 278, 118917. [Google Scholar] [CrossRef]
- Synytsya, A.; Novák, M. Structural Diversity of Fungal Glucans. Carbohydr. Polym. 2013, 92, 792–809. [Google Scholar] [CrossRef]
- Ramos, M.; Burgos, N.; Barnard, A.; Evans, G.; Preece, J.; Graz, M.; Ruthes, A.C.; Jiménez-Quero, A.; Martínez-Abad, A.; Vilaplana, F.; et al. Agaricus bisporus and its by-products as a source of valuable extracts and bioactive compounds. Food Chem. 2019, 292, 176–187. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Alquini, G.; Tadra-sfeir, M.Z.; Iacomini, M.; Wichers, H.J.; Van Griensven, L.J.L.D. Agaricus Bisporus and Agaricus Brasiliensis (1→6)-b-d-Glucans Show Immunostimulatory Activity on Human THP-1 Derived Macrophages. Carbohydr. Polym. 2013, 94, 91–99. [Google Scholar] [CrossRef]
- Cheng, H.; Sun, L.; Shen, D.; Ren, A.; Ma, F.; Tai, G.; Fan, L.; Zhou, Y. Beta-1,6 Glucan Converts Tumor-Associated Macrophages into an M1-like Phenotype. Carbohydr. Polym. 2020, 247, 116715. [Google Scholar] [CrossRef]
- Rossi, P.; Difrancia, R.; Quagliariello, V.; Savino, E.; Tralongo, P.; Randazzo, C.L.; Berretta, M. B-Glucans from Grifola Frondosa and Ganoderma Lucidum in Breast Cancer: An Example of Complementary and Integrative Medicine. Oncotarget 2018, 9, 24837–24856. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- INCA Instituto Nacional de Câncer José Alencar Gomes Da Silva. Available online: https://www.inca.gov.br/assuntos/cancer-de-mama (accessed on 2 May 2022).
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Popovic, V.; Zivkovic, J.; Davidovic, S.; Stevanovic, M.; Stojkovic, D. Mycotherapy of Cancer: An Update on Cytotoxic and Antitumor Activities of Mushrooms, Bioactive Principles and Molecular Mechanisms of Their Action. Curr. Top. Med. Chem. 2013, 13, 2791–2806. [Google Scholar] [CrossRef]
- Corso, C.R.; Mulinari Turin de Oliveira, N.; Moura Cordeiro, L.; Sauruk da Silva, K.; da Silva Soczek, S.H.; Frota Rossato, V.; Fernandes, E.S.; Maria-Ferreira, D. Polysaccharides with Antitumor Effect in Breast Cancer: A Systematic Review of Non-Clinical Studies. Nutrients 2021, 13, 2008. [Google Scholar] [CrossRef]
- Román, Y.; de Oliveira Barddal, H.P.; Iacomini, M.; Sassaki, G.L.; Cipriani, T.R. Anticoagulant and Antithrombotic Effects of Chemically Sulfated Fucogalactan and Citrus Pectin. Carbohydr. Polym. 2017, 174, 731–739. [Google Scholar] [CrossRef]
- Lee, H.-C.; Wei, Y.-H. Mitochondrial Role in Life and Death of the Cell. J. Biomed. Sci. 2000, 7, 2–15. [Google Scholar] [CrossRef]
- Timucin, A.C.; Basaga, H.; Kutuk, O. Selective Targeting of Antiapoptotic BCL-2 Proteins in Cancer. Med. Res. Rev. 2019, 39, 146–175. [Google Scholar] [CrossRef]
- Daba, A.S.; Ezeronye, O.U. Anti-Cancer Effect of Polysaccharides Isolated from Higher Basidiomycetes Mushrooms. Afr. J. Biotechnol. 2003, 2, 672–678. [Google Scholar]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The Role of Necroptosis in Cancer Biology and Therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef]
- Hernansanz-Agustín, P.; Enríquez, J.A. Generation of Reactive Oxygen Species by Mitochondria. Antioxidants 2021, 10, 415. [Google Scholar] [CrossRef]
- Dasari, S.; Ganjayi, M.S.; Yellanurkonda, P.; Basha, S.; Meriga, B. Role of Glutathione S-Transferases in Detoxification of a Polycyclic Aromatic Hydrocarbon, Methylcholanthrene. Chem. Biol. Interact. 2018, 294, 81–90. [Google Scholar] [CrossRef]
- Basit, F.; Van Oppen, L.M.P.E.; Schöckel, L.; Bossenbroek, H.M.; Van Emst-De Vries, S.E.; Hermeling, J.C.W.; Grefte, S.; Kopitz, C.; Heroult, M.; Willems, P.H.G.M.; et al. Mitochondrial Complex i Inhibition Triggers a Mitophagy-Dependent ROS Increase Leading to Necroptosis and Ferroptosis in Melanoma Cells. Cell Death Dis. 2017, 8, e2716. [Google Scholar] [CrossRef]
- Islam, T.; Afonso, M.B.; Rodrigues, C.M.P. The Role of RIPK3 in Liver Mitochondria Bioenergetics and Function. Eur. J. Clin. Investig. 2022, 52, e13648. [Google Scholar] [CrossRef]
- Tian, C.; Liu, Y.; Li, Z.; Zhu, P.; Zhao, M. Mitochondria Related Cell Death Modalities and Disease. Front. Cell Dev. Biol. 2022, 10, 832356. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, S.-Y.; Jiang, R.-L.; Wu, H.-D.; Ang Li, Y.; Long Lu, J.; Xiong, Y.; Han, B.; Lin, L. Necroptosis Signaling and Mitochondrial Dysfunction Cross-Talking Facilitate Cell Death Mediated by Chelerythrine in Glioma. Free Radic. Biol. Med. 2023, 202, 76–96. [Google Scholar] [CrossRef]
- Dhuriya, Y.K.; Sharma, D. Necroptosis: A Regulated Inflammatory Mode of Cell Death. J. Neuroinflamm. 2018, 15, 199. [Google Scholar] [CrossRef]
- Vetvicka, V.; Teplyakova, T.V.; Shintyapina, A.B.; Korolenko, T.A. Effects of Medicinal Fungi-derived Β-glucan on Tumor Progression. J. Fungi 2021, 7, 250. [Google Scholar] [CrossRef]
- Hsu, W.H.; Qiu, W.L.; Tsao, S.M.; Tseng, A.J.; Lu, M.K.; Hua, W.J.; Cheng, H.C.; Hsu, H.Y.; Lin, T.Y. Effects of WSG, a Polysaccharide from Ganoderma Lucidum, on Suppressing Cell Growth and Mobility of Lung Cancer. Int. J. Biol. Macromol. 2020, 165, 1604–1613. [Google Scholar] [CrossRef]
- Alonso, E.N.; Ferronato, M.J.; Gandini, N.A.; Fermento, M.E.; Obiol, D.J.; López Romero, A.; Arévalo, J.; Villegas, M.E.; Facchinetti, M.M.; Curino, A.C. Antitumoral Effects of D-Fraction from Grifola Frondosa (Maitake) Mushroom in Breast Cancer. Nutr. Cancer 2017, 69, 29–43. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Phase I/II Study to Assess the Effect of Soluble Beta-1,3/1,6-Glucan in Combination With Standard Therapy in Patients With Breast Cancer. Available online: https://clinicaltrials.gov/study/NCT00533364?cond=Cancer&intr=Beta-Glucan&rank=4 (accessed on 30 September 2024).
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Phillips, H.J. Dye Exclusion Tests for Cell Viability. In Tissue Culture: Methods and Applications; Kruse, P.F., Patterson, M.K., Eds.; Elsevier: New York, NY, USA, 1973; pp. 406–408. [Google Scholar]
- Djafarzadeh, S.; Jakob, S.M. High-Resolution Respirometry to Assess Mitochondrial Function in Permeabilized and Intact Cells. J. Vis. Exp. 2017, 2017, e54985. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, A.R.; Schmaldienst, S.; Stuhlmeier, K.M.; Chen, W.; Knapp, W.; Zlabinger, G.J. A Microplate Assay for the Detection of Oxidative Products Using 2′,7′-Dichlorofluorescin-Diacetate. J. Immunol. Methods 1992, 156, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.A.; Burk, R.F. Glutathione Peroxidase Activity in Selenium-Deficient Rat Liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. The First Enzymatic Step in Marcapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutckeviski, R.; Corso, C.R.; Fonseca, A.S.; Rodrigues, M.L.; Román-Ochoa, Y.; Cipriani, T.R.; Cavalli, L.R.; Cadena, S.M.S.C.; Smiderle, F.R. Anti-Cancer Potential of Linear β-(1→6)-D-Glucan from Agaricus bisporus on Estrogen Receptor-Positive (ER+) Breast Cancer Cells. Molecules 2024, 29, 4781. https://doi.org/10.3390/molecules29194781
Rutckeviski R, Corso CR, Fonseca AS, Rodrigues ML, Román-Ochoa Y, Cipriani TR, Cavalli LR, Cadena SMSC, Smiderle FR. Anti-Cancer Potential of Linear β-(1→6)-D-Glucan from Agaricus bisporus on Estrogen Receptor-Positive (ER+) Breast Cancer Cells. Molecules. 2024; 29(19):4781. https://doi.org/10.3390/molecules29194781
Chicago/Turabian StyleRutckeviski, Renata, Claudia Rita Corso, Aline Simoneti Fonseca, Mariane Londero Rodrigues, Yony Román-Ochoa, Thales Ricardo Cipriani, Luciane Regina Cavalli, Silvia Maria Suter Correia Cadena, and Fhernanda Ribeiro Smiderle. 2024. "Anti-Cancer Potential of Linear β-(1→6)-D-Glucan from Agaricus bisporus on Estrogen Receptor-Positive (ER+) Breast Cancer Cells" Molecules 29, no. 19: 4781. https://doi.org/10.3390/molecules29194781
APA StyleRutckeviski, R., Corso, C. R., Fonseca, A. S., Rodrigues, M. L., Román-Ochoa, Y., Cipriani, T. R., Cavalli, L. R., Cadena, S. M. S. C., & Smiderle, F. R. (2024). Anti-Cancer Potential of Linear β-(1→6)-D-Glucan from Agaricus bisporus on Estrogen Receptor-Positive (ER+) Breast Cancer Cells. Molecules, 29(19), 4781. https://doi.org/10.3390/molecules29194781







