Design, Synthesis, and Antimicrobial Evaluation of New Thiopyrimidine–Benzenesulfonamide Compounds
Abstract
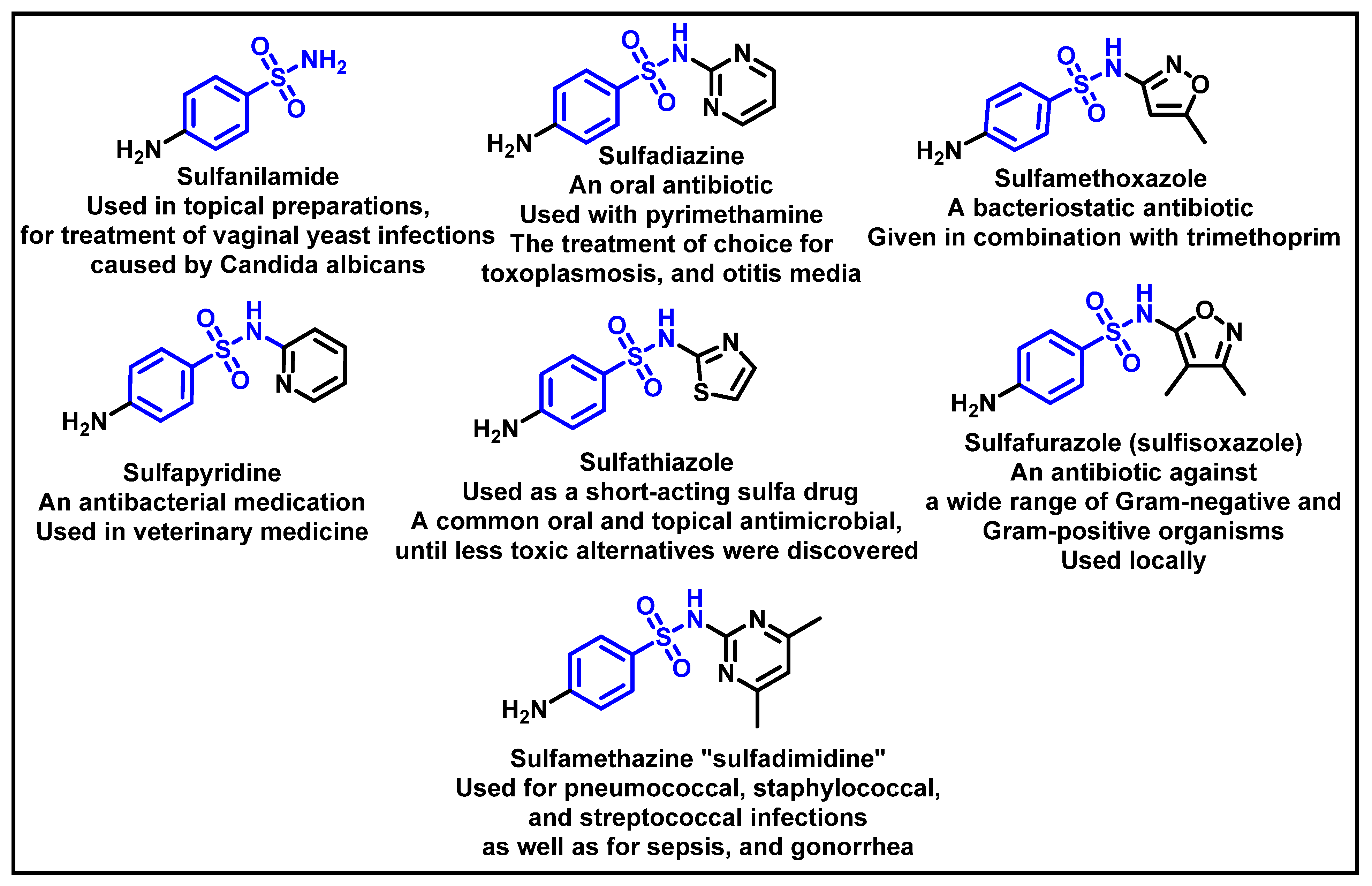
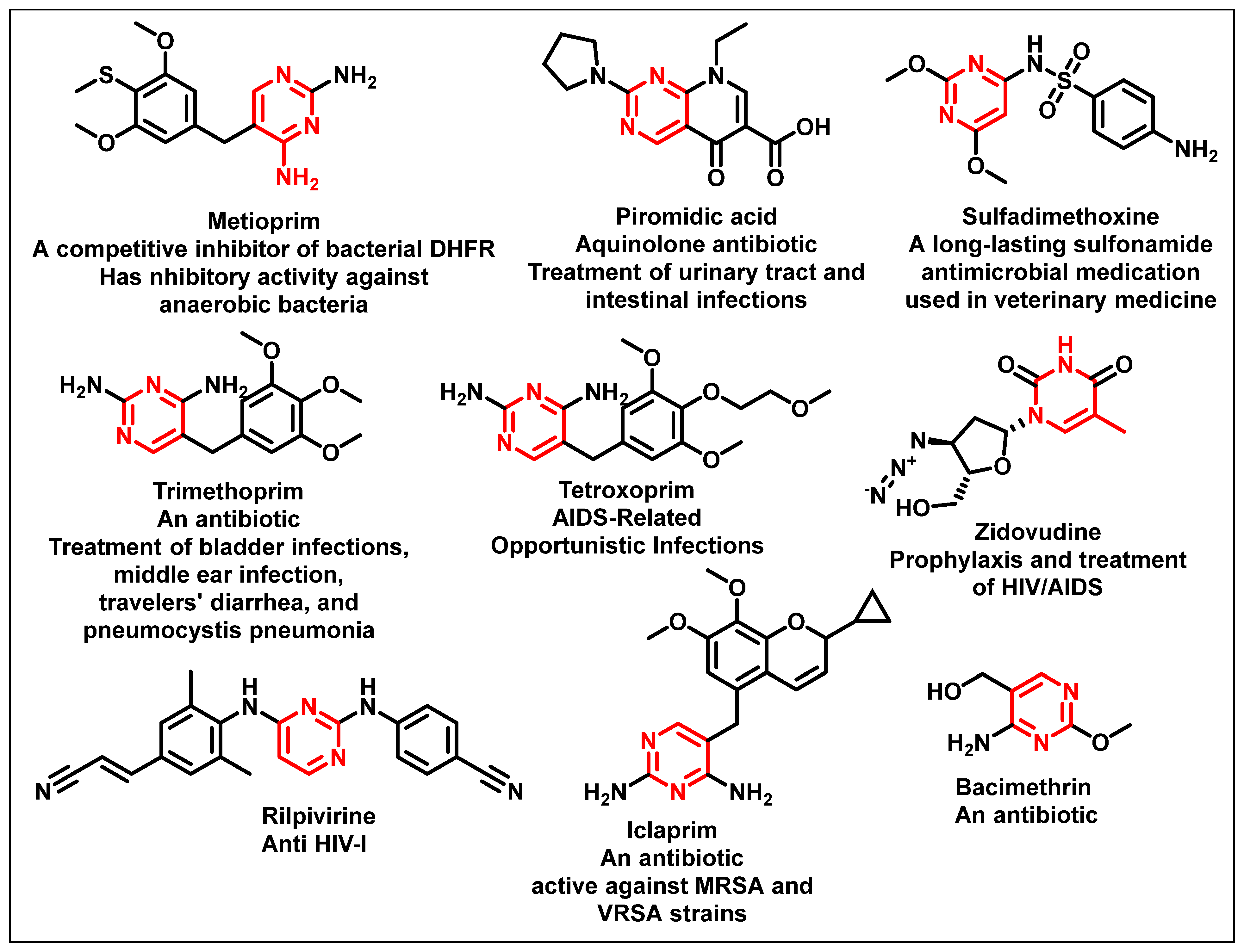
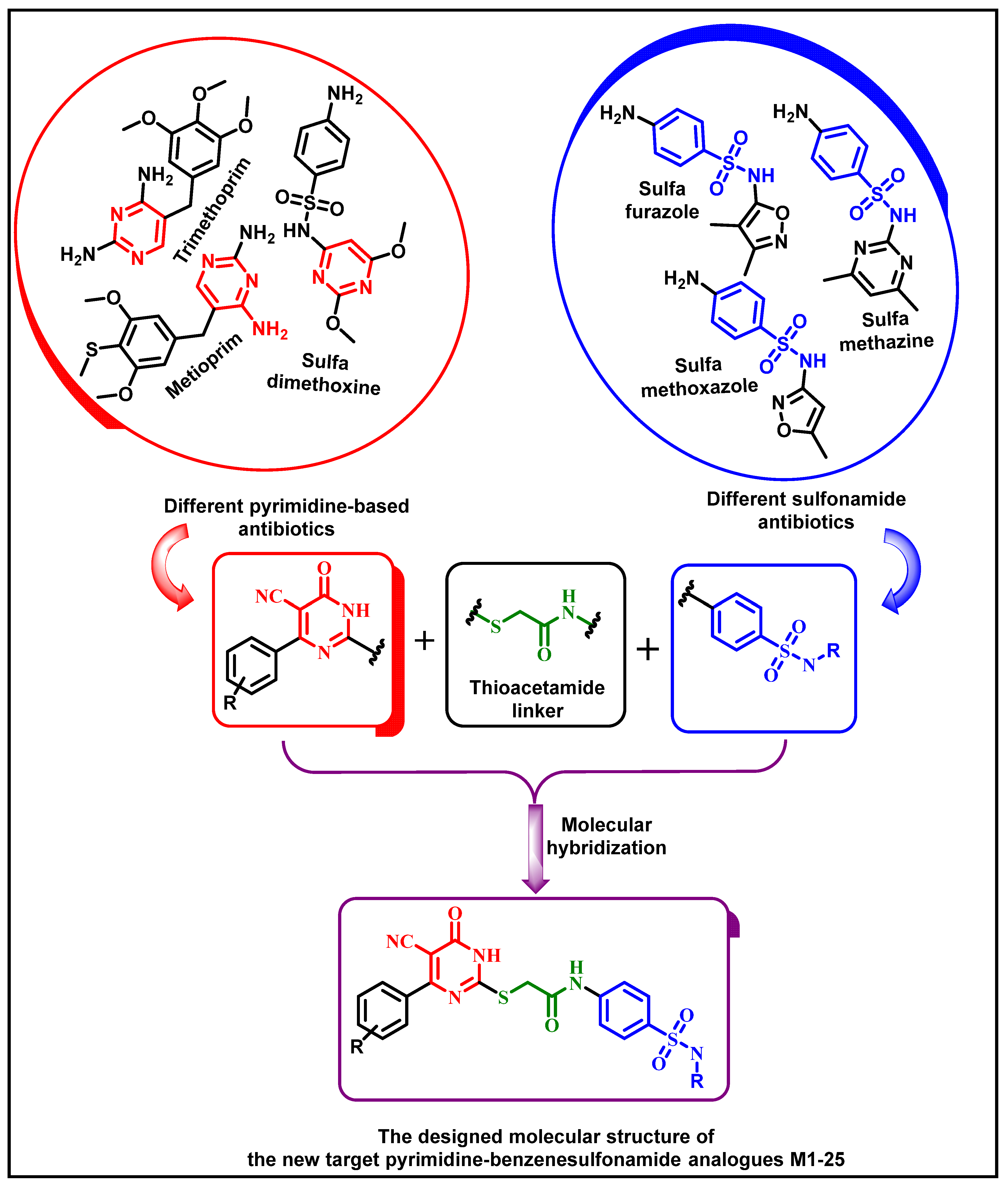
1. Introduction
2. Results and Discussion
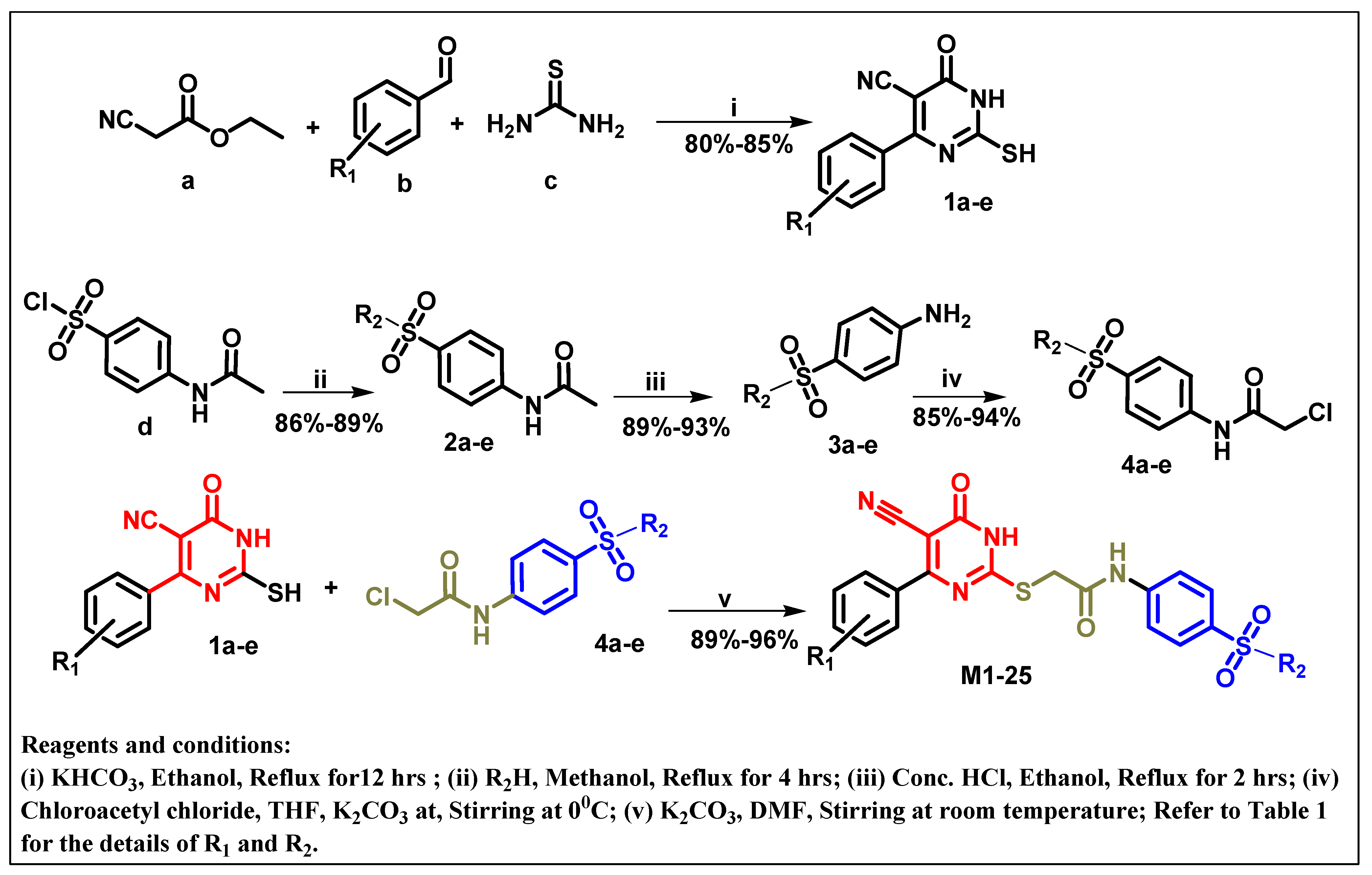
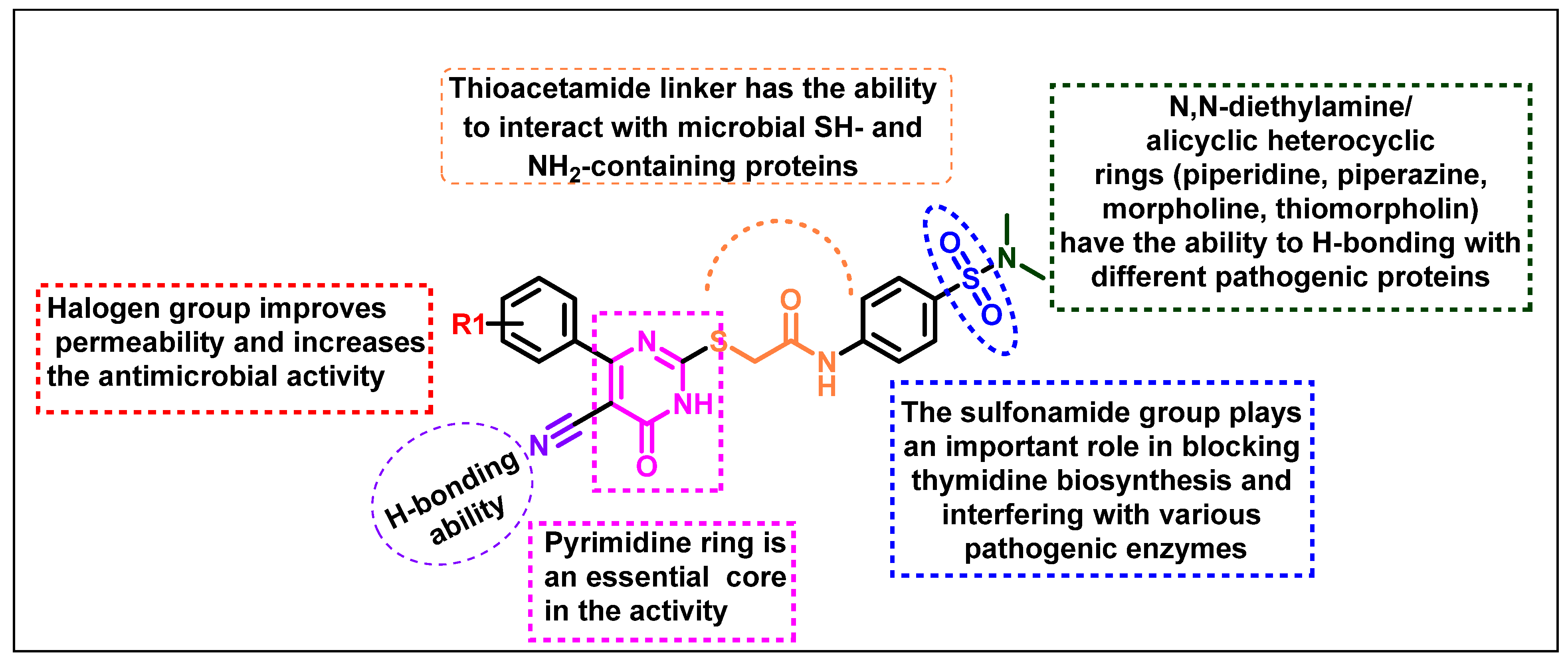
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Antimicrobial Activity Determination
2.2.2. MIC and MBC of Selected Compounds against More Susceptible Bacteria
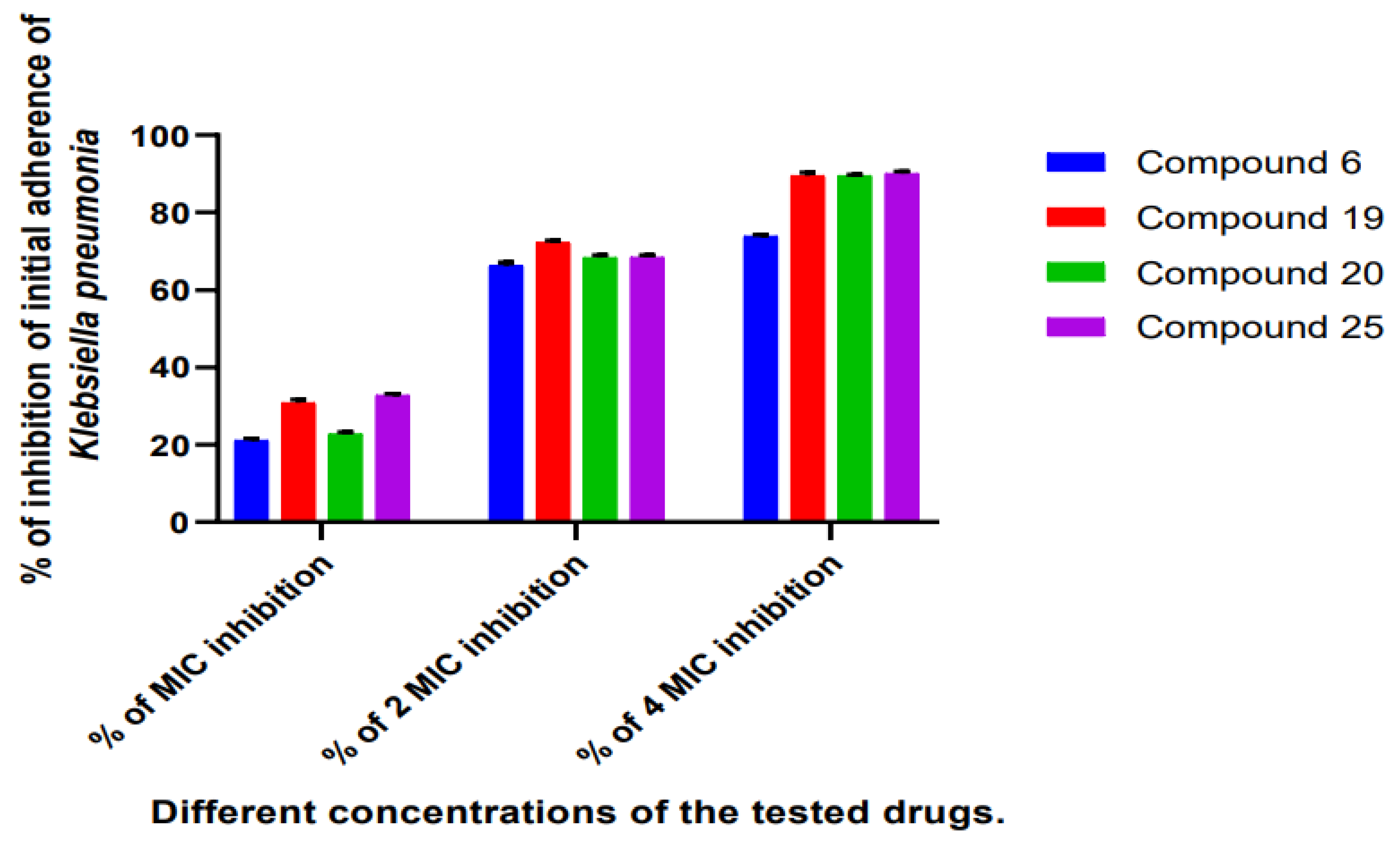
2.2.3. Determination of the Antibiofilm Effect of the Most Promising Compounds Using TCP Method
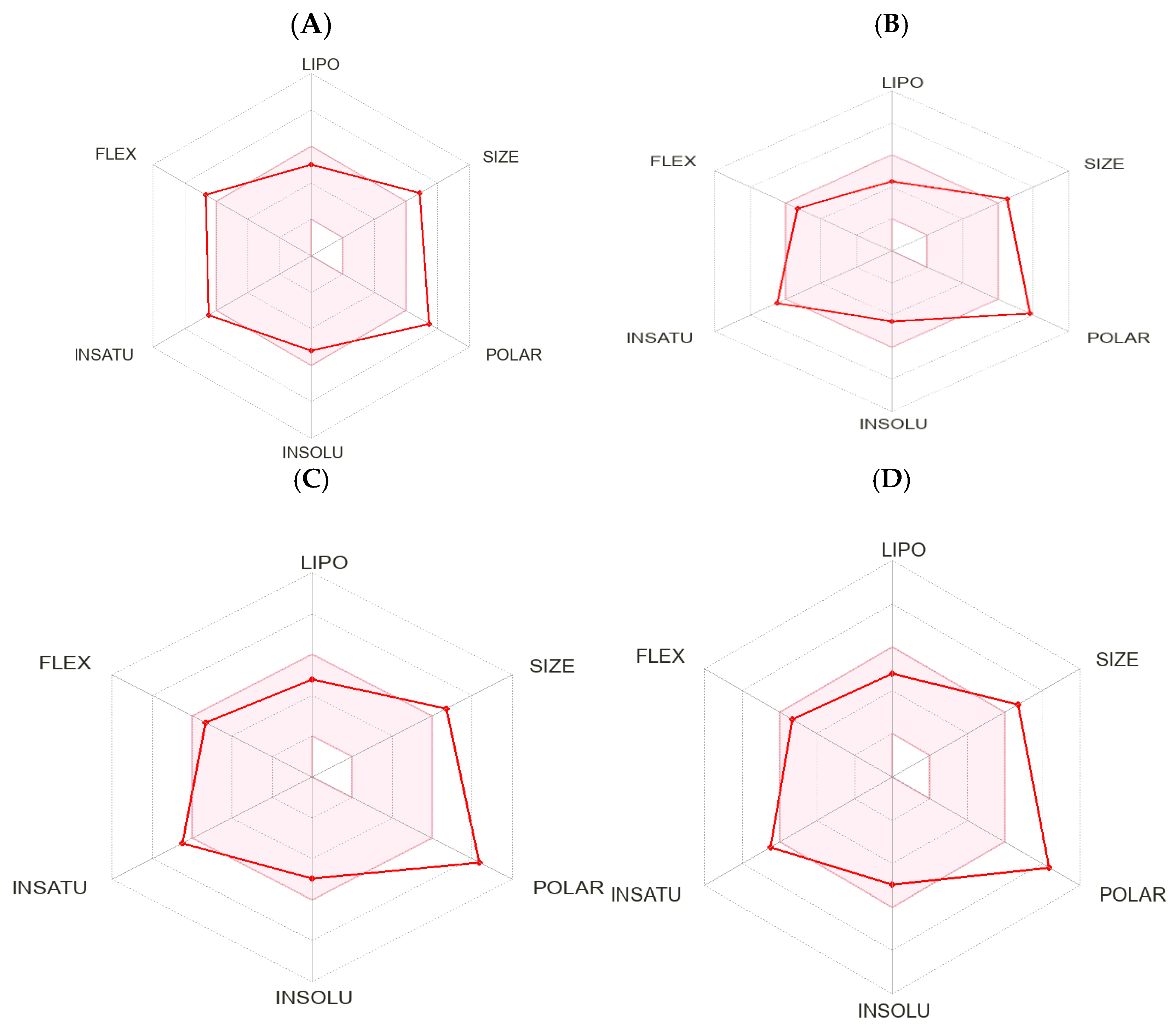
2.3. Calculated Physicochemical Properties and ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity)
Challenges and Future Directions
3. Experimental Section
3.1. Chemistry
3.1.1. General Procedure for Preparation of 6-Substituted-4-Oxo-2-Thioxo-1,2,3,4-Tetrahydropyrimidine-5-Carbonitriles [32]
2-Mercapto-6-Oxo-4-Phenyl-1,6-Dihydropyrimidine-5-Carbonitrile (1a)
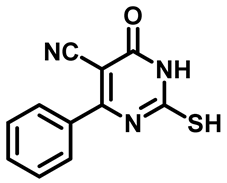
4-(4-Bromophenyl)-2-Mercapto-6-Oxo-1,6-Dihydropyrimidine-5-Carbonitrile (1b)
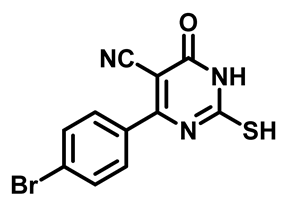
4-(4-Fluorophenyl)-2-Mercapto-6-Oxo-1,6-Dihydropyrimidine-5-Carbonitrile (1c)
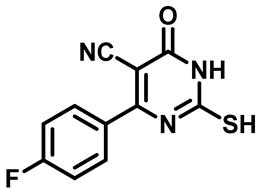
4-(4-Chlorophenyl)-2-Mercapto-6-Oxo-1,6-Dihydropyrimidine-5-Carbonitrile (1d)
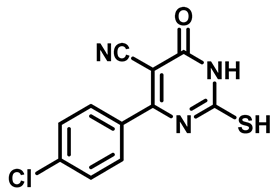
4-(3-Chlorophenyl)-2-Mercapto-6-Oxo-1,6-Dihydropyrimidine-5-Carbonitrile (1e)
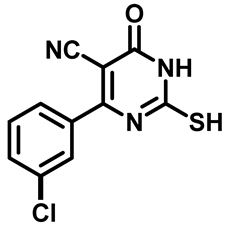
3.1.2. General Procedure for Preparation of N-Substituted Sulfonyl Phenyl Acetamides 2
N-(4-(N,N-Diethylsulfamoyl)Phenyl)Acetamide (2a)
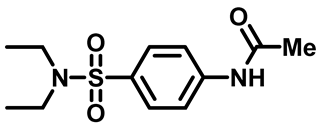
N-(4-(Piperidin-1-Ylsulfonyl)Phenyl)Acetamide (2b)
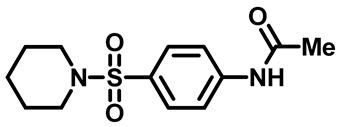
N-(4-((4-Methylpiperazin-1-Yl)Sulfonyl)Phenyl)Acetamide (2c)

N-(4-(Morpholinosulfonyl)Phenyl)Acetamide (2d)
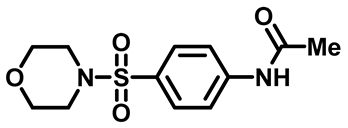
N-(4-(Thiomorpholinosulfonyl)Phenyl)Acetamide (2e)

3.1.3. General Procedure for Preparation of 4-Amino-Benzenesulfonamides 3
4-Amino-N,N-Diethylbenzenesulfonamide (3a)

4-(Piperidin-1-Ylsulfonyl)Aniline (3b)
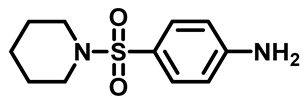
4-((4-Methylpiperazin-1-Yl)Sulfonyl)Aniline (3c)
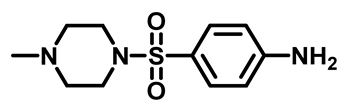
4-(Morpholinosulfonyl)Aniline (3d)
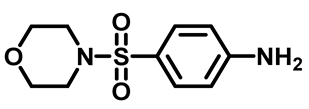
4-(Thiomorpholinosulfonyl)Aniline (3e)
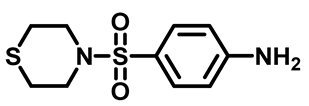
3.1.4. General Procedure for Preparation of N-Substituted Sulfonyl Phenyl Chloro-Acetamides [33]
2-Chloro-N-(4-(N,N-Diethylsulfamoyl)Phenyl)Acetamide (4a)
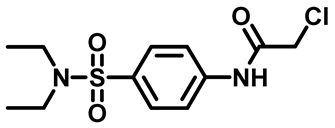
2-Chloro-N-(4-(Piperidin-1-Ylsulfonyl)Phenyl)Acetamide (4b)
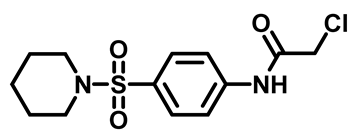
2-Chloro-N-(4-((4-Methylpiperazin-1-yl)Sulfonyl)Phenyl)Acetamide (4c)
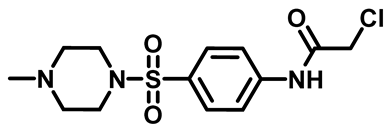
2-Chloro-N-(4-(Morpholinosulfonyl)Phenyl)Acetamide (4d)

2-Chloro-N-(4-(Thiomorpholinosulfonyl)Phenyl)Acetamide (4e)
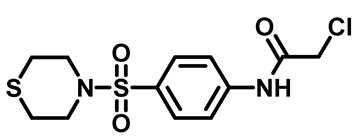
3.1.5. General Procedure for Preparation of Final Target Compounds M1–25
2-((5-Cyano-6-Oxo-4-Phenyl-1,6-Dihydropyrimidin-2-yl)thio)-N-(4-(N,N-Diethylsulfamoyl)Phenyl)Acetamide (M1)
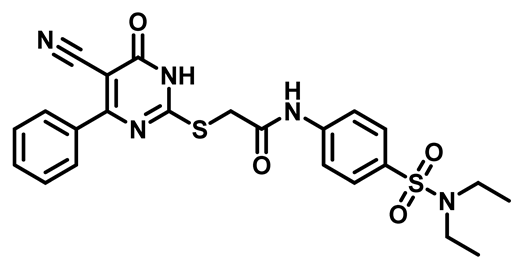
2-((5-Cyano-6-Oxo-4-Phenyl-1,6-Dihydropyrimidin-2-yl)Thio)-N-(4-(Piperidin-1-Ylsulfonyl)Phenyl)Acetamide (M2)
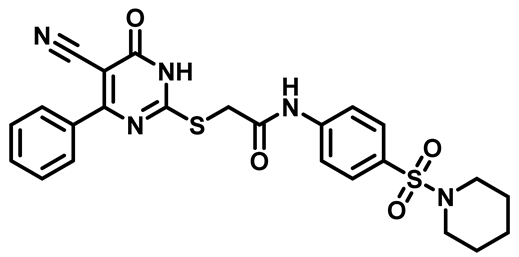
2-((5-Cyano-6-Oxo-4-Phenyl-1,6-Dihydropyrimidin-2-yl)Thio)-N-(4-((4-Methylpiperazin-1-yl)Sulfonyl)Phenyl)Acetamide (M3)
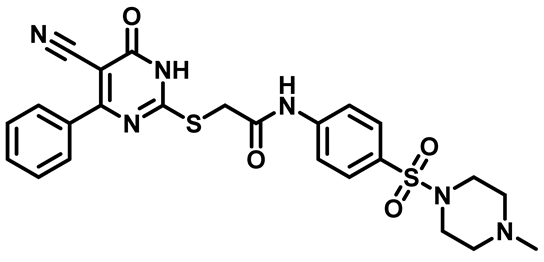
2-((5-Cyano-6-Oxo-4-Phenyl-1,6-Dihydropyrimidin-2-yl)Thio)-N-(4-(Morpholinosulfonyl)Phenyl)Acetamide (M4)

2-((5-Cyano-6-Oxo-4-Phenyl-1,6-Dihydropyrimidin-2-yl)thio)-N-(4-(Thiomorpholinosulfonyl)Phenyl)Acetamide (M5)
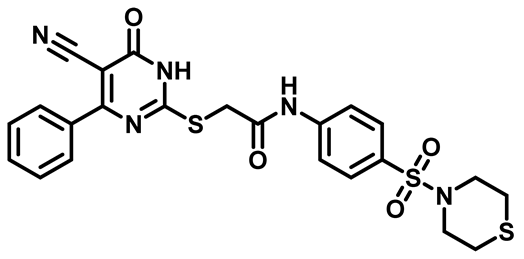
2-((4-(4-Bromophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)Thio)-N-(4-(N,N-Diethylsulfamoyl)Phenyl)Acetamide (M6)
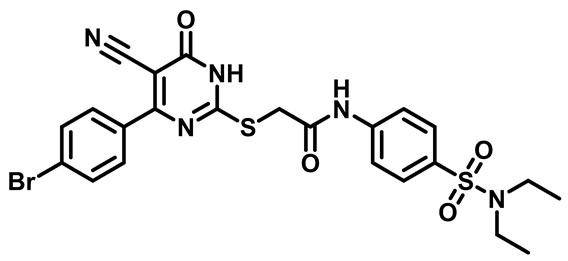
2-((4-(4-Bromophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)thio)-N-(4-(Piperidin-1-Ylsulfonyl)Phenyl)Acetamide (M7)
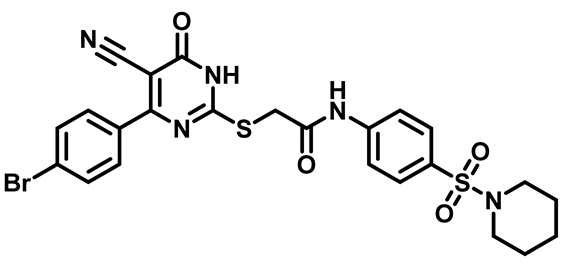
2-((4-(4-Bromophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)thio)-N-(4-((4-Methylpiperazin-1-yl)Sulfonyl)Phenyl)Acetamide (M8)
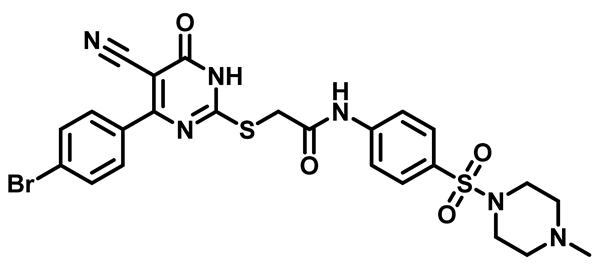
2-((4-(4-Bromophenyl)-5-Cyano-6-oxo-1,6-Dihydropyrimidin-2-yl)thio)-N-(4-(Morpholinosulfonyl)Phenyl)Acetamide (M9)
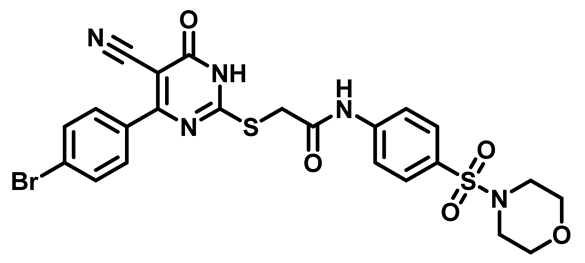
2-((4-(4-Bromophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)Thio)-N-(4-(Thiomorpholinosulfonyl)Phenyl)Acetamide (M10)
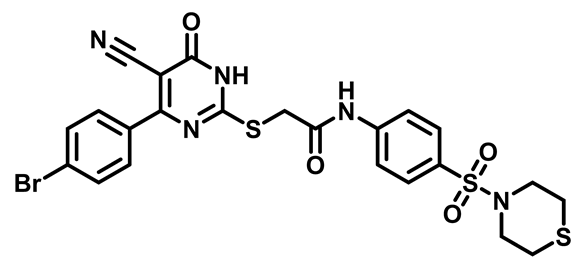
2-((4-(4-Chlorophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)thio)-N-(4-(N,N-Diethylsulfamoyl)Phenyl)Acetamide (M11)
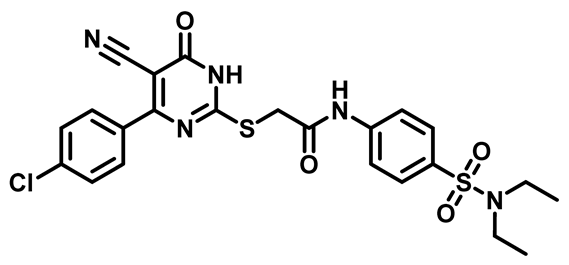
2-((4-(4-Chlorophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)Thio)-N-(4-(Piperidin-1-ylsulfonyl)Phenyl)Acetamide (M12)

2-((4-(4-Chlorophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)thio)-N-(4-((4-Methylpiperazin-1-yl)Sulfonyl)Phenyl)Acetamide (M13)

2-((4-(4-Chlorophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)thio)-N-(4-(Morpholinosulfonyl)Phenyl)Acetamide (M14)
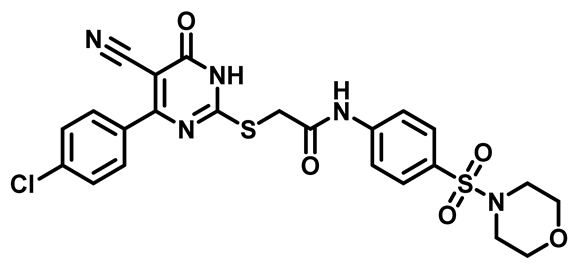
2-((4-(4-Chlorophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)Thio)-N-(4-(Thiomorpholinosulfonyl)Phenyl)Acetamide (M15)
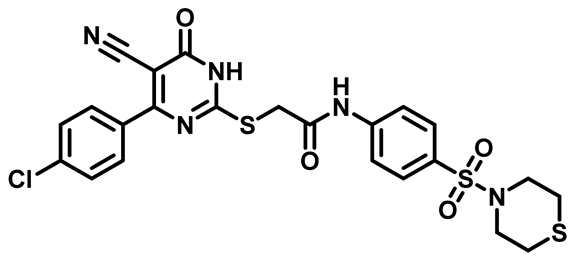
2-((4-(4-Fluorophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)Thio)-N-(4-(N,N-Diethylsulfamoyl)Phenyl)Acetamide (M16)
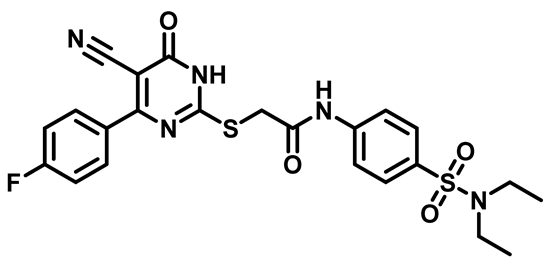
2-((4-(4-Fluorophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)Thio)-N-(4-(Piperidin-1-Ylsulfonyl)Phenyl)Acetamide (M17)
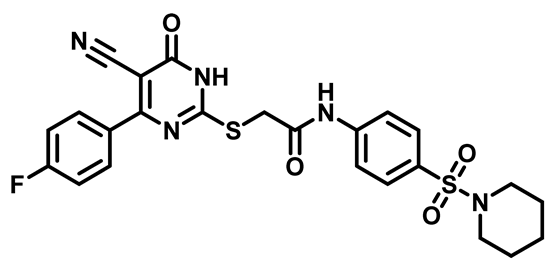
2-((4-(4-Fluorophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)Thio)-N-(4-((4-Methylpiperazin-1-yl)Sulfonyl)Phenyl)Acetamide (M18)
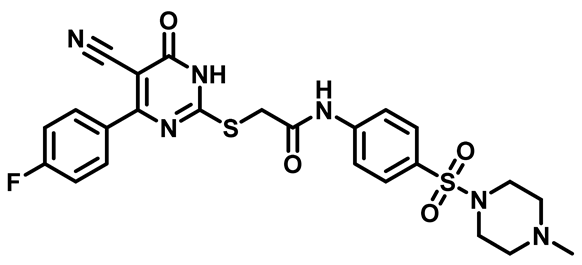
2-((4-(4-Fluorophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)Thio)-N-(4-(Morpholinosulfonyl)Phenyl)Acetamide (M19)

2-((4-(4-Fluorophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)Thio)-N-(4-(Thiomorpholinosulfonyl)Phenyl)Acetamide (M20)

2-((4-(3-Chlorophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)thio)-N-(4-(N,N-Diethylsulfamoyl)Phenyl)Acetamide (M21)
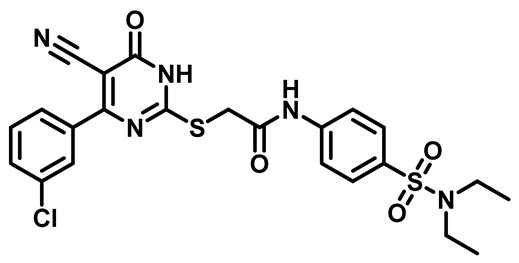
2-((4-(3-Chlorophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)thio)-N-(4-(Piperidin-1-ylsulfonyl)Phenyl)Acetamide (M22)
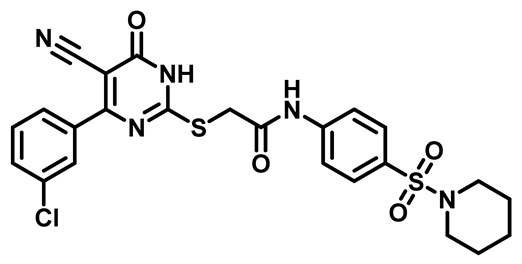
2-((4-(3-Chlorophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)thio)-N-(4-((4-Methylpiperazin-1-yl)Sulfonyl)Phenyl)Acetamide (M23)
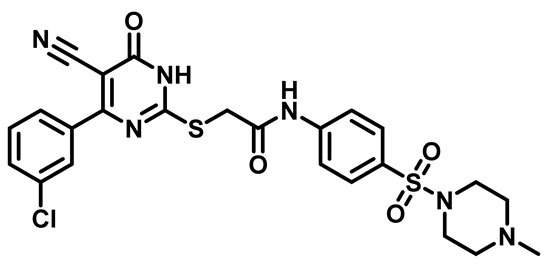
2-((4-(3-Chlorophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)thio)-N-(4-(Morpholinosulfonyl)Phenyl)Acetamide (M24)
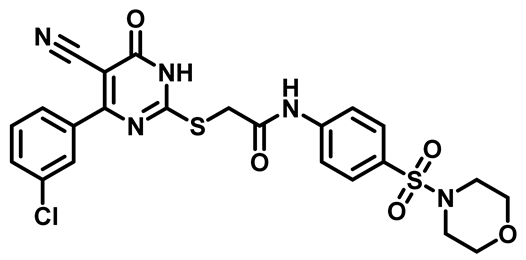
2-((4-(3-Chlorophenyl)-5-Cyano-6-Oxo-1,6-Dihydropyrimidin-2-yl)thio)-N-(4-(Thiomorpholinosulfonyl)Phenyl)Acetamide (M25)
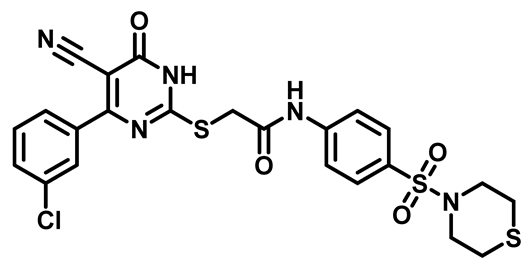
3.2. Biological Evaluation
3.2.1. Materials
3.2.2. Bacterial Strains
3.2.3. Agar Diffusion-Based Screening of Antimicrobial Activity
3.2.4. Measurement of Inhibition Zones
3.2.5. Determination of Minimum Inhibitory Concentration (MIC)
3.2.6. Determination of Minimum Bactericidal Concentration (MBC)
3.2.7. Antibiofilm Assay of the Selected Compounds by Tissue Culture Plate Method (TCP)
3.3. Calculation Physicochemical Properties and ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xue, Y.J.; Li, M.Y.; Jin, X.J.; Zheng, C.J.; Piao, H.R. Design, synthesis and evaluation of carbazole derivatives as potential antimicrobial agents. J. Enzym. Inhib. Med. Chem. 2021, 36, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Singh, V.K.; Mishra, R.; Sharma, A.; Pandey, A.; Srivastava, S.K.; Chaurasia, H. Design, Synthesis, DFT, docking Studies, and antimicrobial evaluation of novel benzimidazole containing sulphonamide derivatives. Bioorganic Chem. 2024, 149, 107473. [Google Scholar] [CrossRef] [PubMed]
- García Altares, M. Structural Diversity of Microalgal Marine Toxins. In Recent Advances in the Analysis of Marine Toxins; Elsevier: Amsterdam, The Netherlands, 2017; pp. 35–88. [Google Scholar]
- Rojas, V.; Rivas, L.; Cárdenas, C.; Guzmán, F. Cyanobacteria and Eukaryotic Microalgae as Emerging Sources of Antibacterial Peptides. Molecules 2020, 25, 5804. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Almalki, A.J.; Ibrahim, T.S.; Taher, E.S.; Mohamed, M.F.A.; Youns, M.; Hegazy, W.A.H.; Al-Mahmoudy, A.M.M. Synthesis, Antimicrobial, Anti-Virulence and Anticancer Evaluation of New 5(4H)-Oxazolone-Based Sulfonamides. Molecules 2022, 27, 671. [Google Scholar] [CrossRef]
- Bsharat, I.; Abdalla, L.; Sawafta, A.; Abu-Reidah, I.M.; Al-Nuri, M.A. Synthesis, characterization, antibacterial and anticancer activities of some heterocyclic imine compounds. J. Mol. Struct. 2023, 1289, 135789. [Google Scholar] [CrossRef]
- Vornhagen, J.; Burnside, K.; Whidbey, C.; Berry, J.; Qin, X.; Rajagopal, L. Kinase Inhibitors that Increase the Sensitivity of Methicillin Resistant Staphylococcus aureus to β-Lactam Antibiotics. Pathogens 2015, 4, 708–721. [Google Scholar] [CrossRef]
- Krátký, M.; Konečná, K.; Šimková, A.; Janďourek, O.; Maixnerová, J.; Stolaříková, J.; Vejsová, M.; Voxová, B.; Trejtnar, F.; Vinšová, J. Improving the antimicrobial activity of old antibacterial drug mafenide: Schiff bases and their bioactivity targeting resistant pathogens. Future Med. Chem. 2023, 15, 255–274. [Google Scholar] [CrossRef]
- Zhang, X.J.; Heggers, J.P.; Chinkes, D.L.; Wolf, S.E.; Hawkins, H.K.; Wolfe, R.R. Topical Sulfamylon cream inhibits DNA and protein synthesis in the skin donor site wound. Surgery 2006, 139, 633–639. [Google Scholar] [CrossRef]
- Krátký, M. Novel Sulfonamide Derivatives as a Tool to Combat Methicillin-Resistant Staphylococcus aureus. Future Med. Chem. 2024, 16, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Ge, H.-T.; Zhao, Y.; Zhao, D.; Fan, J.; Liao, L.-S. Novel carbazole derivatives designed by an ortho-linkage strategy for efficient phosphorescent organic light-emitting diodes. J. Mater. Chem. C 2018, 6, 4300–4307. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A.; Moradi, P.; Tahmasbi, B. Ni-SMTU@boehmite: As an efficient and recyclable nanocatalyst for oxidation reactions. RSC Adv. 2016, 6, 56458–56466. [Google Scholar] [CrossRef]
- Keypour, H.; Mahmoudabadi, M.; Shooshtari, A.; Bayat, M.; Mohsenzadeh, F.; Gable, R.W. Cadmium (II) macrocyclic Schiff-base complexes containing piperazine moiety: Synthesis, spectroscopic, X-ray structure, theoretical and antibacterial studies. J. Mol. Struct. 2018, 1155, 196–204. [Google Scholar] [CrossRef]
- Zayed, E.M.; Ewies, E.F.; Hassaballah, A.I.; Mohamed, G.G. Synthesis, Characterization, DFT, Docking, Antimicrobial and Thermal study of Pyrimidine—Carbonitrile ligand and its Metal Complexes. J. Mol. Struct. 2023, 1284, 135396. [Google Scholar] [CrossRef]
- Sharma, V.; Chitranshi, N.; Agarwal, A.K. Significance and biological importance of pyrimidine in the microbial world. Int. J. Med. Chem. 2014, 2014, 202784. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef]
- Rajabi, F.; De, S.; Luque, R. An Efficient and Green Synthesis of Benzimidazole Derivatives Using SBA-15 Supported Cobalt Nanocatalysts. Catal. Lett. 2015, 145, 1566–1570. [Google Scholar] [CrossRef]
- Lal, K.; Lalitmohan, J.P.; Mahendra, B.B. A Green Protocol for One-Pot Biginelli Condensation Catalyzed by Para Toulene Sulfonic Acid under Microwave Irradiation. Lett. Org. Chem. 2016, 13, 255–262. [Google Scholar] [CrossRef]
- Kalčic, F.; Kolman, V.; Zídek, Z.; Janeba, Z. Polysubstituted Pyrimidines as Potent Inhibitors of Prostaglandin E(2) Production: Increasing Aqueous Solubility. ChemMedChem 2021, 16, 2802–2806. [Google Scholar] [CrossRef]
- Kasralikar, H.M.; Jadhavar, S.C.; Bhansali, S.G.; Patwari, S.B.; Bhusare, S.R. Design and Synthesis of Novel 1,2,3-triazolyl-pyrimidinone Hybrids as Potential Anti-HIV-1 NNRT Inhibitors. J. Heterocycl. Chem. 2018, 55, 821–829. [Google Scholar] [CrossRef]
- Zayed, E.M.; Zayed, M.A.; El-Desawy, M. Preparation and structure investigation of novel Schiff bases using spectroscopic, thermal analyses and molecular orbital calculations and studying their biological activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Zayed, E.M.; Zayed, M.A.; Radwan, M.A.A.; Alminderej, F.M. Synthesis, characterization, antimicrobial, and docking study of novel 1-(furanyl)-3-(pyrrolyl)propenone-based ligand and its chelates of 3d-transition metal ions. Appl. Organomet. Chem. 2022, 36, e6489. [Google Scholar] [CrossRef]
- Kamal, R.; Kumar, R.; Kumar, V.; Kumar, V.; Bansal, K.K.; Sharma, P.C. Synthesis, Anthelmintic and Antimicrobial Evaluation of New 2-Arylidene-1-(4-methyl-6-phenylpyrimidin-2-yl)hydrazines. ChemistrySelect 2019, 4, 713–717. [Google Scholar] [CrossRef]
- Farag, A.M.; Sokker, H.H.; Zayed, E.M.; Nour Eldien, F.A.; Abd Alrahman, N.M. Removal of hazardous pollutants using bifunctional hydrogel obtained from modified starch by grafting copolymerization. Int. J. Biol. Macromol. 2018, 120 (Pt B), 2188–2199. [Google Scholar] [CrossRef]
- Kalčic, F.; Kolman, V.; Ajani, H.; Zídek, Z.; Janeba, Z. Polysubstituted Pyrimidines as mPGES-1 Inhibitors: Discovery of Potent Inhibitors of PGE2 Production with Strong Anti-inflammatory Effects in Carrageenan-Induced Rat Paw Edema. ChemMedChem 2020, 15, 1398–1407. [Google Scholar] [CrossRef]
- Mahmoudi-Gom Yek, S.; Azarifar, D.; Khaleghi-Abbasabadi, M.; Keypour, H.; Mahmoudabadi, M. Heterogenized magnetic graphene oxide-supported N-Schiff base Cu(II) complex as an exclusive nanocatalyst for synthesis of new pyrido[2,3-d]pyrimidine-7-carbonitrile derivatives. Appl. Organomet. Chem. 2020, 34, e5989. [Google Scholar] [CrossRef]
- Zhao, C.; Rakesh, K.P.; Ravidar, L.; Fang, W.Y.; Qin, H.L. Pharmaceutical and medicinal significance of sulfur (S(VI))-Containing motifs for drug discovery: A critical review. Eur. J. Med. Chem. 2019, 162, 679–734. [Google Scholar] [CrossRef]
- Dharuman, S.; Wallace, M.J.; Reeve, S.M.; Bulitta, J.B.; Lee, R.E. Synthesis and Structure-Activity Relationship of Thioacetamide-Triazoles against Escherichia coli. Molecules 2022, 27, 1518. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Alqahtani, A.S.; Soliman, A.M.; Askar, A.A. Novel N-(Substituted) Thioacetamide Quinazolinone Benzenesulfonamides as Antimicrobial Agents. Int. J. Nanomed. 2020, 15, 3161–3180. [Google Scholar] [CrossRef]
- Khalifa, A.; Khalil, A.; Abdel-Aziz, M.M.; Albohy, A.; Mohamady, S. Isatin-pyrimidine hybrid derivatives as enoyl acyl carrier protein reductase (InhA) inhibitors against Mycobacterium tuberculosis. Bioorganic Chem. 2023, 138, 106591. [Google Scholar] [CrossRef]
- Altamimi, A.-M.S.; Alafeefy, A.M.; Balode, A.; Vozny, I.; Pustenko, A.; El Shikh, M.E.; Alasmary, F.A.S.; Abdel-Gawad, S.A.; Žalubovskis, R. Symmetric molecules with 1,4-triazole moieties as potent inhibitors of tumour-associated lactate dehydrogenase-A. J. Enzym. Inhib. Med. Chem. 2018, 33, 147–150. [Google Scholar] [CrossRef]
- Jondle, C.N.; Gupta, K.; Mishra, B.B.; Sharma, J. Klebsiella pneumoniae infection of murine neutrophils impairs their efferocytic clearance by modulating cell death machinery. PLoS Pathog. 2018, 14, e1007338. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammad, S.; Badmasti, F.; Solgi, H.; Aminzadeh, Z.; Khodabandelo, Z.; Shahcheraghi, F. First Report of Extended-Spectrum Betalactamase-Producing Klebsiella pneumoniae Among Fecal Carriage in Iran: High Diversity of Clonal Relatedness and Virulence Factor Profiles. Microb. Drug Resist. 2020, 26, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Bodey, G.P.; Bolivar, R.; Fainstein, V.; Jadeja, L. Infections caused by Pseudomonas aeruginosa. Rev. Infect. Dis. 1983, 5, 279–313. [Google Scholar] [CrossRef]
- Aspatwar, A.; Kairys, V.; Rala, S.; Parikka, M.; Bozdag, M.; Carta, F.; Supuran, C.T.; Parkkila, S. Mycobacterium tuberculosis β-Carbonic Anhydrases: Novel Targets for Developing Antituberculosis Drugs. Int. J. Mol. Sci. 2019, 20, 5153. [Google Scholar] [CrossRef]
- Faleye, O.S.; Boya, B.R.; Lee, J.-H.; Choi, I.; Lee, J. Halogenated Antimicrobial Agents to Combat Drug-Resistant Pathogens. Pharmacol. Rev. 2024, 76, 90–141. [Google Scholar] [CrossRef]
- Khalaf, H.S.; Naglah, A.M.; Al-Omar, M.A.; Moustafa, G.O.; Awad, H.M.; Bakheit, A.H. Synthesis, Docking, Computational Studies, and Antimicrobial Evaluations of New Dipeptide Derivatives Based on Nicotinoylglycylglycine Hydrazide. Molecules 2020, 25, 3589. [Google Scholar] [CrossRef]
- Princiotto, S.; Casciaro, B.; Temprano, A.G.; Musso, L.; Sacchi, F.; Loffredo, M.R.; Cappiello, F.; Sacco, F.; Raponi, G.; Fernandez, V.P.; et al. The antimicrobial potential of adarotene derivatives against Staphylococcus aureus strains. Bioorganic Chem. 2024, 145, 107227. [Google Scholar] [CrossRef]
- Tangadanchu, V.K.R.; Sui, Y.F.; Zhou, C.H. Isatin-derived azoles as new potential antimicrobial agents: Design, synthesis and biological evaluation. Bioorganic Med. Chem. Lett. 2021, 41, 128030. [Google Scholar] [CrossRef]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef]
- Riquelme, S.A.; Ahn, D.; Prince, A. Pseudomonas aeruginosa and Klebsiella pneumoniae Adaptation to Innate Immune Clearance Mechanisms in the Lung. J. Innate Immun. 2018, 10, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Mohsenipour, Z.; Hassanshahian, M. The Effects of Allium sativum Extracts on Biofilm Formation and Activities of Six Pathogenic Bacteria. Jundishapur. J. Microbiol. 2015, 8, e18971. [Google Scholar] [CrossRef] [PubMed]
- Mounir, R.; Alshareef, W.A.; El Gebaly, E.A.; El-Haddad, A.E.; Ahmed, A.M.S.; Mohamed, O.G.; Enan, E.T.; Mosallam, S.; Tripathi, A.; Selim, H.M.R.M.; et al. Unlocking the Power of Onion Peel Extracts: Antimicrobial and Anti-Inflammatory Effects Improve Wound Healing through Repressing Notch-1/NLRP3/Caspase-1 Signaling. Pharmaceuticals 2023, 16, 1379. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol. Res. 2019, 144, 19–50. [Google Scholar] [CrossRef]
- López-López, E.; Naveja, J.J.; Medina-Franco, J.L. DataWarrior: An evaluation of the open-source drug discovery tool. Expert Opin. Drug Discov. 2019, 14, 335–341. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Vollaro, A.; Esposito, A.; Esposito, E.P.; Zarrilli, R.; Guaragna, A.; De Gregorio, E. PYED-1 Inhibits Biofilm Formation and Disrupts the Preformed Biofilm of Staphylococcus aureus. Antibiotics 2020, 9, 240. [Google Scholar] [CrossRef]
- Delafield, F.P.; Doudoroff, M.; Palleroni, N.J.; Lusty, C.J.; Contopoulos, R. Decomposition of poly-beta-hydroxybutyrate by pseudomonads. J. Bacteriol. 1965, 90, 1455–1466. [Google Scholar] [CrossRef]


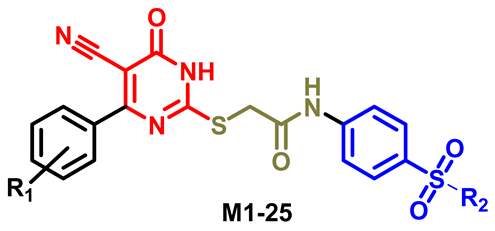 | |||||
|---|---|---|---|---|---|
| Compounds | R1 | R2 | Compounds | R1 | R2 |
| M1 | H | 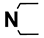 | M14 | 4-Cl |  |
| M2 | H | 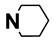 | M15 | 4-Cl |  |
| M3 | H | 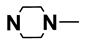 | M16 | 4-F | 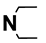 |
| M4 | H | 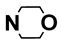 | M17 | 4-F |  |
| M5 | H |  | M18 | 4-F | 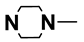 |
| M6 | 4-Br | 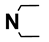 | M19 | 4-F | 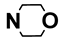 |
| M7 | 4-Br | 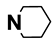 | M20 | 4-F |  |
| M8 | 4-Br | 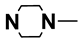 | M21 | 3-Cl | 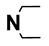 |
| M9 | 4-Br | 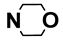 | M22 | 3-Cl |  |
| M10 | 4-Br |  | M23 | 3-Cl | 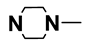 |
| M11 | 4-Cl | 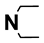 | M24 | 3-Cl | 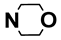 |
| M12 | 4-Cl | 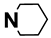 | M25 | 3-Cl | 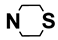 |
| M13 | 4-Cl | 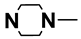 | |||
| Microbial Organisms | M 1 | M2 | M3 | M 4 | M 5 | M 6 | M 7 | M 8 | M 9 | M 10 | M 11 | M 12 | M 13 | M 14 | M 15 | M 16 | M 17 | M 18 | M 19 | M 20 | M 21 | M 22 | M 23 | M 24 | M 25 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli ATCC-25922 | 0 | 16 | 18 | 20 | 0 | 20 | 20 | 18 | 25 | 22 | 0 | 20 | 20 | 22 | 19 | 0 | 0 | 0 | 0 | 20 | 0 | 20 | 20 | 24 | 26 |
| K. pneumoniae | 20 | 22 | 24 | 22 | 22 | 26 | 22 | 22 | 24 | 24 | 20 | 17 | 15 | 22 | 22 | 24 | 24 | 22 | 28 | 26 | 20 | 20 | 19 | 26 | 26 |
| P. aeruginosa ATCC 27853 | 20 | 22 | 20 | 22 | 24 | 26 | 18 | 18 | 20 | 20 | 22 | 18 | 22 | 22 | 22 | 16 | 20 | 22 | 26 | 30 | 20 | 18 | 20 | 22 | 30 |
| S. aureus ATCC 6538 | 0 | 0 | 0 | 0 | 17 | 21 | 21 | 0 | 21 | 21 | 15 | 19 | 0 | 15 | 20 | 22 | 16 | 22 | 20 | 26 | 22 | 17 | 18 | 0 | 24 |
| S. epidermidis ATCC 35984 | 0 | 15 | 0 | 0 | 22 | 15 | 21 | 20 | 25 | 17 | 0 | 0 | 0 | 0 | 18 | 20 | 20 | 20 | 22 | 22 | 20 | 20 | 0 | 13 | 20 |
| B. subtilis ATCC 6633 | 18 | 22 | 0 | 0 | 22 | 20 | 22 | 17 | 26 | 15 | 18 | 22 | 0 | 13 | 18 | 23 | 25 | 0 | 24 | 15 | 15 | 20 | 0 | 13 | 28 |
| C. albicans ATCC-10231 | 0 | 0 | 0 | 0 | 17 | 21 | 21 | 0 | 21 | 21 | 15 | 19 | 0 | 15 | 18 | 23 | 16 | 23 | 20 | 26 | 22 | 17 | 18 | 0 | 24 |
| Chemical Compounds | Bacterial Strains | MIC μg/mL | MBC μg/mL |
|---|---|---|---|
| M6 | Klebsiella pneumoniae | 375 ± 0.00 | 1500 ± 0.45 |
| Pseudomonas aeruginosa | 375 ± 0.00 | 1500 ± 0.00 | |
| M19 | Klebsiella pneumoniae | 375 ± 0.00 | 1500 ± 0.29 |
| Pseudomonas aeruginosa | 375 ± 0.00 | 1500 ± 0.00 | |
| M20 | Klebsiella pneumoniae | 375 ± 0.00 | 7500 ± 0.00 |
| Pseudomonas aeruginosa | 375 ± 0.00 | 1500 ± 0.00 | |
| M25 | Klebsiella pneumoniae | 375 ± 0.00 | 7500 ± 0.00 |
| Pseudomonas aeruginosa | 375 ± 0.00 | 1500 ± 0.00 |
| Compound No. | MW | HBA | HBD | logP (o/w) | TPSA Å2 | Num. Rotatable Bonds | Lipinski |
|---|---|---|---|---|---|---|---|
| M6 | 576.49 | 6 | 2 | 3.04 | 169.7 | 8 | Yes; 1 violation: MW > 500 |
| M19 | 546.02 | 7 | 2 | 1.72 | 178.9 | 6 | Yes; 1 violation: MW > 500 |
| M20 | 562.09 | 6 | 2 | 2.35 | 195 | 6 | Yes; 1 violation: MW > 500 |
| M25 | 562.09 | 6 | 2 | 2.39 | 195 | 6 | Yes; 1 violation: MW > 500 |
| Compound M6 | Compound M19 | Compound M20 | Compound M25 | |
|---|---|---|---|---|
| Absorption Water solubility (log mol/L) Intestinal absorption Skin permeability (log Kp) | −4.0 82.4 −2.7 | −3.8 78.7 −2.7 | −3.8 85.1 −2.7 | −3.8 85.2 −2.7 |
| Distribution Blood–brain permeability(log BB) CNS permeability (log PS) | −1.46 −2.78 | −1.43 −3.45 | −1.42 −2.73 | −1.42 −2.73 |
| Metabolism CYP2D6 substrate CYP3A4 substrate CYP1A2 inhibitor | No Yes No | No Yes No | No Yes No | No Yes No |
| Excretion Total clearance (log mL/min/Kg) Renal OCT2 substrate | 0.1 No | 0.2 No | 0.1 No | 0.1 No |
| Toxicity AMES toxicity hERG inhibitor Tumorigenic Irritant | No No No No | No No No No | No No No No | No No No No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, A.; Anwar, M.M.; Alshareef, W.A.; El-Gebaly, E.A.; Elseginy, S.A.; Abdelwahed, S.H. Design, Synthesis, and Antimicrobial Evaluation of New Thiopyrimidine–Benzenesulfonamide Compounds. Molecules 2024, 29, 4778. https://doi.org/10.3390/molecules29194778
Khalifa A, Anwar MM, Alshareef WA, El-Gebaly EA, Elseginy SA, Abdelwahed SH. Design, Synthesis, and Antimicrobial Evaluation of New Thiopyrimidine–Benzenesulfonamide Compounds. Molecules. 2024; 29(19):4778. https://doi.org/10.3390/molecules29194778
Chicago/Turabian StyleKhalifa, Abdalrahman, Manal M. Anwar, Walaa A. Alshareef, Eman A. El-Gebaly, Samia A. Elseginy, and Sameh H. Abdelwahed. 2024. "Design, Synthesis, and Antimicrobial Evaluation of New Thiopyrimidine–Benzenesulfonamide Compounds" Molecules 29, no. 19: 4778. https://doi.org/10.3390/molecules29194778
APA StyleKhalifa, A., Anwar, M. M., Alshareef, W. A., El-Gebaly, E. A., Elseginy, S. A., & Abdelwahed, S. H. (2024). Design, Synthesis, and Antimicrobial Evaluation of New Thiopyrimidine–Benzenesulfonamide Compounds. Molecules, 29(19), 4778. https://doi.org/10.3390/molecules29194778







