A Comprehensive Review on Deep Eutectic Solvents: Their Current Status and Potential for Extracting Active Compounds from Adaptogenic Plants
Abstract
1. Introduction
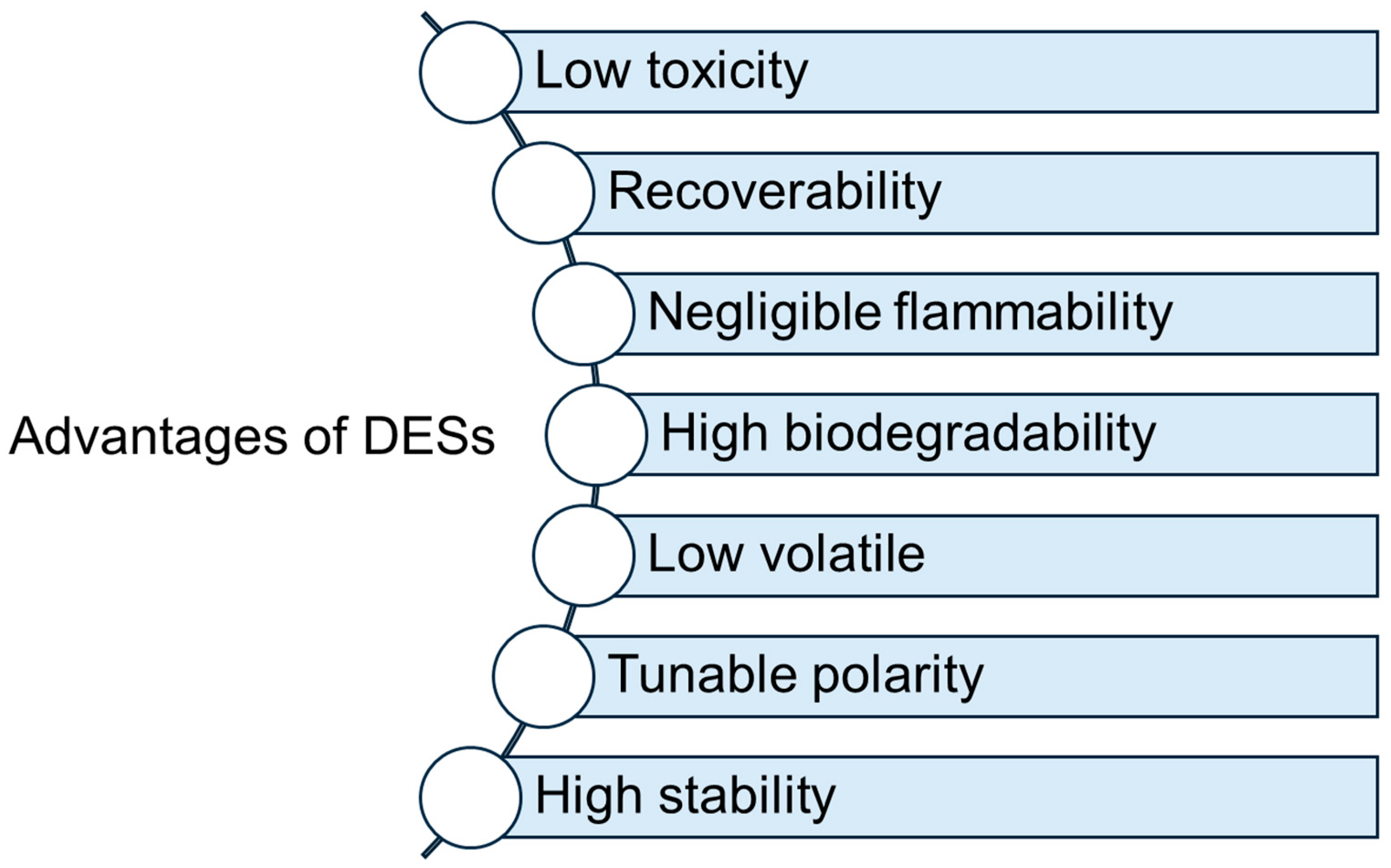
2. Deep Eutectic Solvents
3. Adaptogens
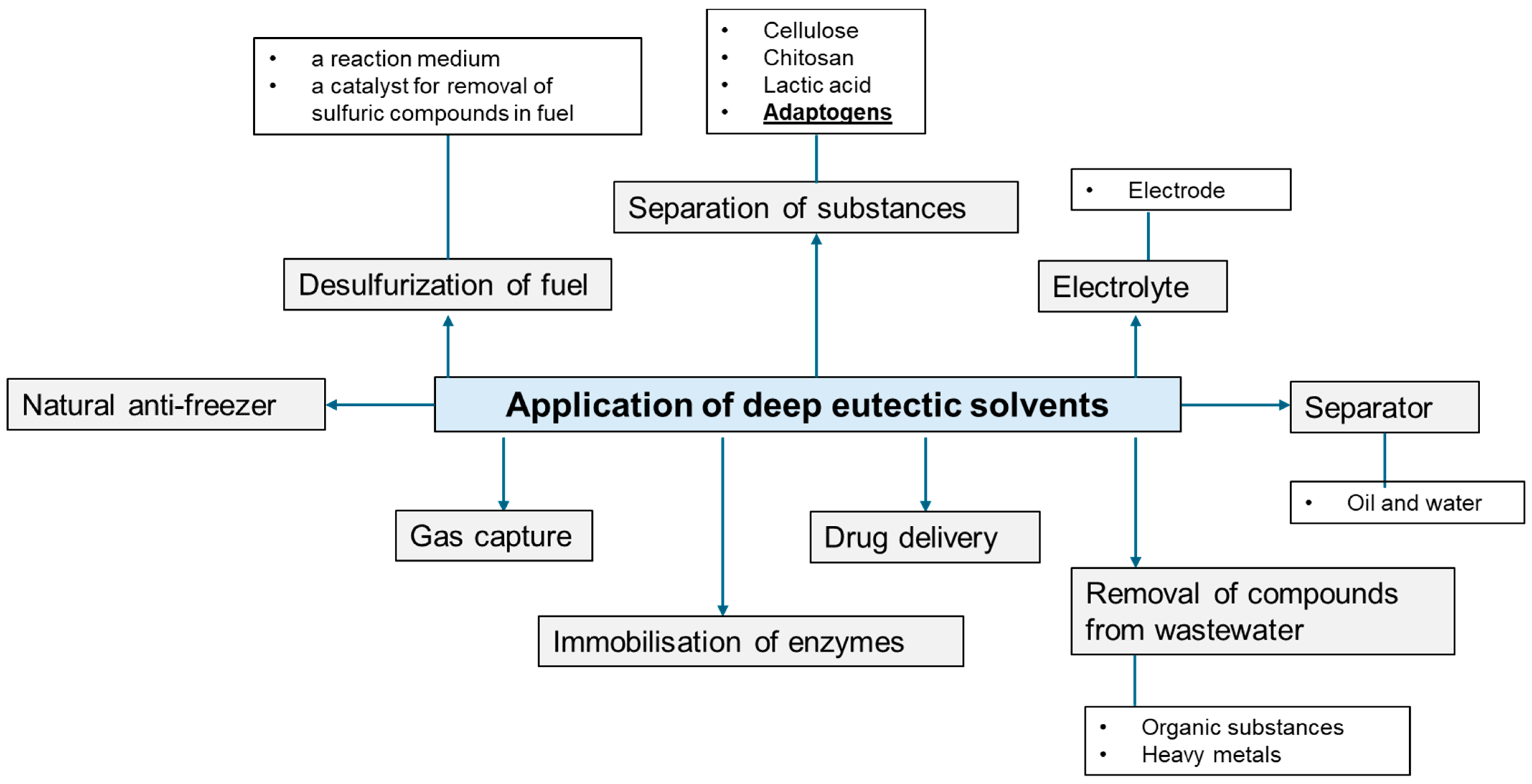
4. Application of Deep Eutectic Solvents
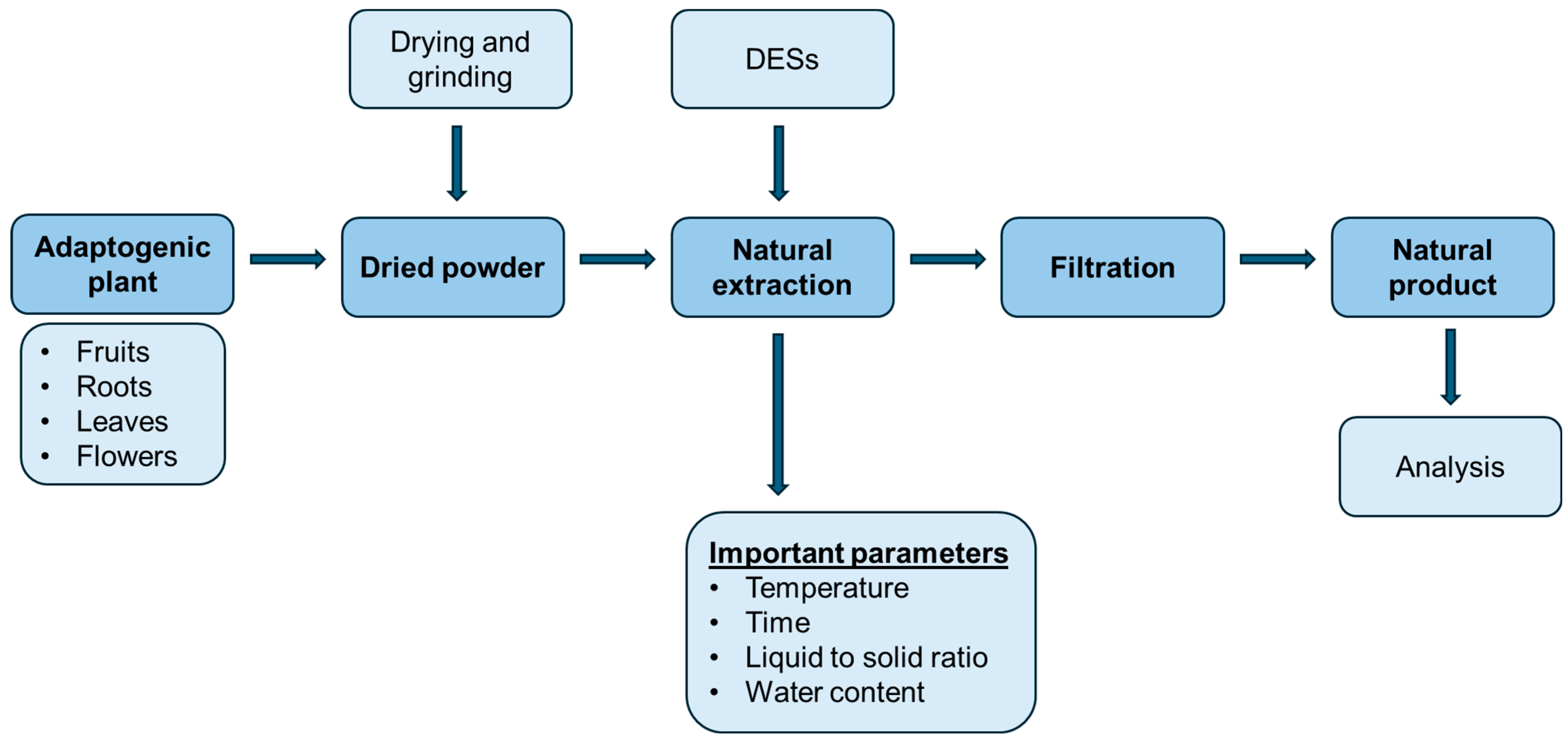
Extraction of Adaptogens with Deep Eutectic Solvents
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248. [Google Scholar] [CrossRef]
- Raisi, L.; Hashemi, S.H.; Jamali Keikha, A.; Kaykhaii, M. Application of a novel deep eutectic solvent modified carbon nanotube for pipette-tip micro solid phase extraction of 6-mercaptopurine. BMC Chem. 2024, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Elhamarnah, Y.; Qiblawey, H.; Nasser, M. A review on deep eutectic solvents as the emerging class of green solvents for membrane fabrication and separations. J. Mol. Liq. 2024, 398, 124250. [Google Scholar] [CrossRef]
- Janicka, P.; Płotka-Wasylka, J.; Jatkowska, N.; Chabowska, A.; Fares, M.Y.; Andruch, V.; Kaykhaii, M.; Gębicki, J. trends in the new generation of green solvents in extraction processes. Curr. Opin. Green Sustain. Chem. 2022, 37, 100670. [Google Scholar] [CrossRef]
- Hashemi, B.; Shiri, F.; Švec, F.; Nováková, L. Green solvents and approaches recently applied for extraction of natural bioactive compounds. TrAC Trends Anal. Chem. 2022, 157, 116732. [Google Scholar] [CrossRef]
- Javahershenas, R. Recent advances in the application of deep eutectic solvents for the synthesis of spiro heterocyclic scaffolds via multicomponent reactions. J. Mol. Liq. 2023, 385, 122398. [Google Scholar] [CrossRef]
- Hu, X.; Knibbs, L.D.; Zhou, Y.; Ou, Y.; Dong, G.-H.; Dong, H. The role of lifestyle in the association between long-term ambient air pollution exposure and cardiovascular disease: A national cohort study in China. BMC Med. 2024, 22, 93. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Park, H.; Ho, M.; Bhardwaj, K.; Sugimura, N.; Lee, H.W.; Meng, H.; Ebert, M.P.; Chao, K.; et al. Unveiling and harnessing the human gut microbiome in the rising burden of non-communicable diseases during urbanization. Gut Microb. 2023, 15, 2237645. [Google Scholar] [CrossRef]
- Kobayashi, J.; Ohtake, K.; Uchida, H. NO-Rich Diet for Lifestyle-Related Diseases. Nutrients 2015, 7, 4911–4937. [Google Scholar] [CrossRef]
- Husain, S.; Hillmann, K.; Hengst, K.; Englert, H. Effects of a lifestyle intervention on the biomarkers of oxidative stress in non-communicable diseases: A systematic review. Front. Aging 2023, 4, 1085511. [Google Scholar] [CrossRef]
- Liu, K.; Wang, H.; Wang, W.; Ming, L.; Wei, G.; Liang, H.; He, Z.; Liu, G.; Xu, C.; Liu, X. Urban chronic diseases management through promoting low carbon lifestyle and physical activities. Int. J. Low-Carbon Technol. 2022, 17, 950–961. [Google Scholar] [CrossRef]
- Nagakura, Y.; Hayashi, M.; Kajioka, S. Analysis of Japanese nationwide health datasets: Association between lifestyle habits and prevalence of neuropathic pain and fibromyalgia with reference to dementia-related diseases and Parkinson’s Disease. Scand. J. Pain 2023, 23, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Tandon, R.; Tandon, N. The rising status of edible seeds in lifestyle related diseases: A review. Food Chem. 2023, 402, 134220. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Funamoto, M.; Sunagawa, Y.; Shimizu, S.; Katanasaka, Y.; Miyazaki, Y.; Wada, H.; Hasegawa, K.; Morimoto, T. Anti-inflammatory action of curcumin and its use in the treatment of lifestyle-related diseases. Eur. Cardiol. Rev. 2019, 14, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Aluko, R.E.; Hati, S.; Solanki, D. Bioactive peptides in the management of lifestyle-related diseases: Current trends and future perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 4593–4606. [Google Scholar] [CrossRef]
- Wróbel-Biedrawa, D.; Podolak, I. Anti-neuroinflammatory effects of adaptogens: A mini-review. Molecules 2024, 29, 866. [Google Scholar] [CrossRef]
- Begum, S.N.; Ray, A.S.; Rahaman, C.H. A comprehensive and systematic review on potential anticancer activities of eugenol: From pre-clinical evidence to molecular mechanisms of action. Phytomedicine 2022, 107, 154456. [Google Scholar] [CrossRef]
- Bedair, H.M.; Samir, T.M.; Mansour, F.R. Antibacterial and antifungal activities of natural deep eutectic solvents. Appl. Microbiol. Biotechnol. 2024, 108, 198. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Development of deep eutectic solvents applied in extraction and separation. J. Sep. Sci. 2016, 39, 3505–3520. [Google Scholar] [CrossRef]
- Shah, P.A.; Chavda, V.; Hirpara, D.; Sharma, V.S.; Shrivastav, P.S.; Kumar, S. Exploring the potential of deep eutectic solvents in pharmaceuticals: Challenges and opportunities. J. Mol. Liq. 2023, 390, 123171. [Google Scholar] [CrossRef]
- Wu, K.; Ren, J.; Wang, Q.; Nuerjiang, M.; Xia, X.; Bian, C. Research progress on the preparation and action mechanism of natural deep eutectic solvents and their application in food. Foods 2022, 11, 3528. [Google Scholar] [CrossRef] [PubMed]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef] [PubMed]
- Koh, Q.Q.; Kua, Y.L.; Gan, S.; Tan, K.W.; Lee, T.Z.E.; Cheng, W.K.; Lau, H.L.N. Sugar-based natural deep eutectic solvent (NADES): Physicochemical properties, antimicrobial activity, toxicity, biodegradability and potential use as green extraction media for phytonutrients. Sustain. Chem. Pharm. 2023, 35, 101218. [Google Scholar] [CrossRef]
- Kalantri, S.; Vora, A. Eutectic solutions for healing: A comprehensive review on therapeutic deep eutectic solvents (TheDES). Drug Dev. Ind. Pharm. 2024, 50, 387. [Google Scholar] [CrossRef]
- Sekharan, T.R.; Chandira, R.M.; Tamilvanan, S.; Rajesh, S.C.; Venkateswarlu, B.S. Deep eutectic solvents as an alternate to other harmful solvents. Biointerface Res. Appl. Chem. 2022, 12, 847–860. [Google Scholar]
- Omar, K.A.; Sadeghi, R. Physicochemical properties of deep eutectic solvents: A review. J. Mol. Liq. 2022, 360, 119524. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, A.; Panesar, P.S.; Thakur, A. potential of deep eutectic solvents in the extraction of value-added compounds from agro-industrial by-products. Appl. Food Res. 2022, 2, 100211. [Google Scholar] [CrossRef]
- Negi, T.; Kumar, A.; Sharma, S.K.; Rawat, N.; Saini, D.; Sirohi, R.; Prakash, O.; Dubey, A.; Dutta, A.; Shahi, N.C. Deep eutectic solvents: Preparation, properties, and food applications. Heliyon 2024, 10, e28784. [Google Scholar] [CrossRef]
- Hao, Y.; Pei, F.; Huang, J.; Li, G.; Zhong, C. Application of deep eutectic solvents on extraction of flavonoids. J. Sep. Sci. 2024, 47, 2300925. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khodja, A.; Ibrahim, T.; Khamis, M.; Anwar, A.; Khan, N.A. The increasing importance of novel deep eutectic solvents as potential effective antimicrobials and other medicinal properties. World J. Microbiol. Biotechnol. 2023, 39, 330. [Google Scholar] [CrossRef] [PubMed]
- Nian, B.; Li, X. Can deep eutectic solvents be the best alternatives to ionic liquids and organic solvents: A perspective in enzyme catalytic reactions. Int. J. Biol. Macromol. 2022, 217, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Sonyeam, J.; Chaipanya, R.; Suksomboon, S.; Khan, M.J.; Amatariyakul, K.; Wibowo, A.; Posoknistakul, P.; Charnnok, B.; Liu, C.G.; Laosiripojana, N.; et al. Process design for acidic and alcohol based deep eutectic solvent pretreatment and high pressure homogenization of palm bunches for nanocellulose production. Sci. Rep. 2024, 14, 7550. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lu, S.; Wu, X.; Wang, L.; Wang, Z.; Zhao, L. Application of hydrophobic eutectic solvent in efficient biotransformation of total flavonoids of Herba epimedii. J. Biotechnol. 2024, 391, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, P.; Shinde, T.; Lokhande, K.; Bondarde, M.; Bhakare, M.; Some, S. Green and sustainable approach towards synthesis of polymeric composite for transparent cellulosic flame retardant. Colloids Surf. A Physicochem. Eng. Asp. 2024, 696, 134334. [Google Scholar] [CrossRef]
- Azmi, N.; Mun Lock, S.S.; Berghuis, N.T.; Sarwono, A.; Zahra, N.L.; Rahman, A.; Waqas, S.; Farooqi, A.S. Influence of synthesis approach and formulation on the physicochemical properties of chitin and chitosan from Black Soldier Fly. Results Eng. 2024, 23, 102401. [Google Scholar] [CrossRef]
- Yaakob, M.N.A.; Salim, N.; Mustapha, S.N.H.; Misnon, I.I.; Rahim, M.H.A.; Roslan, R. Efficient lignin extraction from oil palm empty fruit bunches using guanidine-based deep eutectic solvents under microwave assistance. Ind. Crops Prod. 2024, 218, 118968. [Google Scholar] [CrossRef]
- Brendler, T.; Al-Harrasi, A.; Bauer, R.; Gafner, S.; Hardy, M.L.; Heinrich, M.; Hosseinzadeh, H.; Izzo, A.A.; Michaelis, M.; Nassiri-Asl, M.; et al. Botanical drugs and supplements affecting the immune response in the time of COVID-19: Implications for research and clinical practice. Phytother. Res. 2021, 35, 3013–3031. [Google Scholar] [CrossRef]
- Esmaealzadeh, N.; Iranpanah, A.; Sarris, J.; Rahimi, R. A literature review of the studies concerning selected plant-derived adaptogens and their general function in body with a focus on animal studies. Phytomedicine 2022, 105, 154354. [Google Scholar] [CrossRef]
- Rybnikář, M.; Malaník, M.; Šmejkal, K.; Švajdlenka, E.; Shpet, P.; Babica, P.; Dall’Acqua, S.; Smištík, O.; Jurček, O.; Treml, J. Dibenzocyclooctadiene Lignans from Schisandra Chinensis with anti-inflammatory effects. Int. J. Mol. Sci. 2024, 25, 3465. [Google Scholar] [CrossRef]
- Todorova, V.; Ivanov, K.; Ivanova, S. Comparison between the biological active compounds in plants with adaptogenic properties (Rhaponticum carthamoides, Lepidium meyenii, Eleutherococcus senticosus and Panax ginseng). Plants 2021, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A. Herba Sideritis: A putative adaptogen for reducing the risk of age-related cognitive decline and neurodegenerative disorders. Phytomed. Plus 2024, 4, 100519. [Google Scholar] [CrossRef]
- Panossian, A.; Efferth, T. Network pharmacology of adaptogens in the assessment of their pleiotropic therapeutic activity. Pharmaceuticals 2022, 15, 1051. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Shekhar, N.; Thakur, A.K. An evidence-based review of medicinal plants in the overall management of chronic fatigue. Curr. Psychiatry Res. Rev. 2021, 17, 154–171. [Google Scholar] [CrossRef]
- Della Porta, M.; Maier, J.A.; Cazzola, R. Effects of Withania somnifera on cortisol levels in stressed human subjects: A systematic review. Nutrients 2023, 15, 5015. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, M.T.; Hegazy, M.A.; Mohamed, E.H. Major biochemical constituents of Withania somnifera (Ashwagandha) extract: A review of chemical analysis. Rev. Anal. Chem. 2023, 42, 20220055. [Google Scholar] [CrossRef]
- Sánchez, I.A.; Cuchimba, J.A.; Pineda, M.C.; Argüello, Y.P.; Kočí, J.; Kreider, R.B.; Petro, J.L.; Bonilla, D.A. Adaptogens on depression-related outcomes: A systematic integrative review and rationale of synergism with physical activity. Int. J. Environ. Res. Public Health 2023, 20, 5298. [Google Scholar] [CrossRef]
- Minich, D.M.; Ross, K.; Frame, J.; Fahoum, M.; Warner, W.; Meissner, H.O. Not all maca is created equal: A review of colors, nutrition, phytochemicals, and clinical uses. Nutrients 2024, 16, 530. [Google Scholar] [CrossRef]
- Greene, E.S.; Ardakani, M.A.; Dridi, S. Effects of an herbal adaptogen feed-additive on feeding-related hypothalamic neuropeptides in chronic cyclic heat-stressed chickens. Neuropeptides 2024, 106, 102439. [Google Scholar] [CrossRef]
- Ben-Azu, B.; Adebayo, O.G.; Adebesin, A.; Oparaji, K.C.; Ojiakor, V.O.; Pender, G.C.; Odeghe, B.O.; Omeiza, N.A.; Abdulrahim, H.A.; Ezieshi, V.; et al. Diosgenin reverses posttraumatic stress disorder in mice by augmenting neurochemical release and inhibiting HPA axis dysfunction, oxidative stress, and neuroinflammation. J. Affect. Disord. Rep. 2024, 17, 100814. [Google Scholar] [CrossRef]
- Pięta, E.; Chrabąszcz, K.; Pogoda, K.; Suchy, K.; Paluszkiewicz, C.; Kwiatek, W.M. Adaptogenic activity of Withaferin A on human cervical carcinoma cells using high-definition vibrational spectroscopic imaging. Biochim. Biophys. Acta—Mol. Basis Dis. 2023, 1869, 166615. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Jagim, A.R.; Potter, G.D.M.; Garner, D.; Galpin, A.J. Rhodiola rosea as an adaptogen to enhance exercise performance: A review of the literature. Br. J. Nutr. 2024, 131, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, L.; Song, Z.; Xu, L. Extraction and application of natural rutin from Sophora Japonica to prepare the novel fluorescent sensor for detection of copper ions. Front. Bioeng. Biotechnol. 2021, 9, 642138. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.R.; Huang, J.H.; He, D.; Yi, Z.Y.; Zhao, D.; Liu, Z.; Zhang, S.H.; Huang, L.Q. Green and efficient extraction of polysaccharide and ginsenoside from American ginseng (Panax quinquefolius L.) by deep eutectic solvent extraction and aqueous two-phase system. Molecules 2022, 27, 3132. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, G.; Han, Y.; Ma, J.; Bai, B.; Gao, J.; Zhang, Z. Ginsenosides and polysaccharides from ginseng co-fermented with multi-enzyme-coupling probiotics improve in vivo immunomodulatory effects. Nutrients 2023, 15, 2434. [Google Scholar] [CrossRef]
- An, H.M.; Choi, Y.S.; Bae, S.K.; Lee, Y.K. Effect of the combination of probiotics and Korean red ginseng on diabetic wound healing exposed to diesel exhaust particles (DEPs). Medicina 2023, 59, 1155. [Google Scholar] [CrossRef]
- Lee, G.; Lee, Y.J.; Kim, Y.J.; Park, Y. Synthesis of Au–Ag bimetallic nanoparticles using Korean red ginseng (Panax ginseng Meyer) root extract for chemo-photothermal anticancer therapy. Arch. Pharm. Res. 2023, 46, 659–678. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, J.; Gao, Y.; Gao, X.; Yan, P. Optimization of fermentation conditions and product identification of a saponin-producing endophytic fungus. Microorganisms 2023, 11, 2331. [Google Scholar] [CrossRef]
- Han, E.J.; Elbegbayar, E.; Baek, Y.; Lee, J.S.; Lee, H.G. taste masking and stability improvement of Korean red ginseng (Panax ginseng) by nanoencapsulation using chitosan and gelatin. Int. J. Biol. Macromol. 2023, 250, 126259. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Khakisahneh, S.; Han, S.Y.; Song, E.J.; Nam, Y.D.; Kim, H. Ginseng extracts improve circadian clock gene expression and reduce inflammation directly and indirectly through gut microbiota and PI3K signaling pathway. NPJ Biofilms Microbiomes 2024, 10, 24. [Google Scholar] [CrossRef]
- Meng, Q.; Pan, J.; Liu, Y.; Chen, L.; Ren, Y. Anti-tumour effects of polysaccharide extracted from Acanthopanax Senticosus and cell-mediated immunity. Exp. Ther. Med. 2018, 15, 1694–1701. [Google Scholar] [PubMed]
- Gou, D.; Qiu, P.; Wang, Y.; Hong, F.; Ren, P.; Cheng, X.; Wang, L.; Dou, X.; Liu, T.; Liu, J.; et al. Multifunctional chitosan-based hydrogel wound dressing loaded with Acanthopanax senticosus and Osmundastrum cinnamomeum: Preparation, characterization and coagulation mechanism. J. Mech. Behav. Biomed. Mater. 2024, 151, 106384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Guo, Z.; Zhang, P.; Liu, Z.; Song, L.; Zhang, Z.; Jia, Y.; Cao, Z.; Ma, J. Eleutheroside B, a selective late sodium current inhibitor, suppresses atrial fibrillation induced by sea anemone toxin II in rabbit hearts. Acta Pharmacol. Sin. 2021, 42, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Gao, X.; Lu, Y.; Lu, F.; Wang, Y.; Chen, P.; Wang, C.; Yuan, C.; Liu, S. Integrated proteomics and metabolomics reveals metabolism disorders in the α-syn mice and potential therapeutic effect of Acanthopanax senticosus extracts. J. Ethnopharmacol. 2024, 318, 116878. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jia, X.; Yang, S.; Zhang, G.; Li, A.; Du, P.; Liu, L.; Li, C. Optimization of ultrasonic-assisted extraction of flavonoids, polysaccharides, and eleutherosides from Acanthopanax senticosus using response surface methodology in development of health wine. LWT 2022, 165, 11372. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sinha, S.; Srivastava, J.; Varshney, A. Withania somnifera (L.) dunal whole-plant extract demonstrates acceptable non-clinical safety in rat 28-day subacute toxicity evaluation under GLP-compliance. Sci. Rep. 2022, 12, 11047. [Google Scholar] [CrossRef]
- Jepkorir, M.; Nyanjom, S.G.; Kamau, S.; Chepng’etich, J.; Kipkoech, G.; Mwitari, P.G. In vivo anti-inflammatory activity, safety and gene expression profiles of Carissa edulis, Withania somnifera, Prunus africana and Rhamnus prinoides for potential management of rheumatoid arthritis. Sci. Afr. 2023, 22, e01933. [Google Scholar] [CrossRef]
- Yerram, C.; Jillella, A.; Reddy, V. Effects of Withania somnifera root extract serum application on hair health in healthy adults: A prospective, double-blind, randomized, parallel, placebo-controlled study. J. Ayurveda Integr. Med. 2023, 14, 100817. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, X.; Xiao, S.; Lin, Y.; Xiao, Z.; Zhou, W.; Zhang, Y. Impact of molecular weight and gastrointestinal digestion on the immunomodulatory effects of Lycium barbarum polysaccharides. Int. J. Biol. Macromol. 2024, 274, 133500. [Google Scholar] [CrossRef]
- Jeong, E.; Eun, S.; Chae, S.; Lee, S. Prebiotic potential of goji berry (Lycium barbarum) in improving intestinal integrity and inflammatory profiles via modification of the gut microbiota in high-fat diet-fed rats. J. Med. Food. 2024, 1089, 704–712. [Google Scholar] [CrossRef]
- Sewelam, A.; Kabil, S.L.; Jarrar, B.; Sabry, M.; Morsy, M. Lycium barbarum polysaccharide attenuates acute toxicity caused by titanium dioxide nanoparticles in splenic and pulmonary tissues. Pharmaceutical Sci. 2024, 30, 339–354. [Google Scholar] [CrossRef]
- Liang, L.; Lin, L.; Zhao, M. Exploration of green preparation strategy for Lycium barbarum polysaccharide targeting bacteroides proliferative and immune-enhancing activities and its potential use in geriatric foods. Int. J. Biol. Macromol. 2024, 267, 131316. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, K.; Cao, X.; Rong, W.; Shi, W.; Yu, Q.; Deng, W.; Yu, J.; Xu, X. Plant-derived exosomes extracted from Lycium barbarum L. loaded with isoliquiritigenin to promote spinal cord injury repair based on 3D printed bionic scaffold. Bioeng. Transl. Med. 2024, 9, e10646. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, X.; Yang, X.; Lu, S.; Zhang, H.; Fan, Y. Stability protection of lutein emulsions by utilizing a functional conjugate of collagen and Lycium barbarum L. leaf flavonoid. Food Res. Int. 2024, 176, 113775. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sun, X.; Fang, T.; Ma, M.; Wu, Y.; Zha, X. Utilisation of flavonoids from Lycium Barbarum L. leaves cross-linked with whey protein for the stabilisation and delivery of β-carotene emulsions. Int. J. Food. Sci. Technol. 2023, 58, 249–258. [Google Scholar] [CrossRef]
- Long, L.F.; Zhao, Q.F.; Zhang, F.L.; Tang, R.; Wei, J.B.; Guan, S.; Chen, Y. Inhibitory Effect of benzocaine from Schisandra chinensis on Alternaria alternata. Sci. Rep. 2024, 14, 6691. [Google Scholar] [CrossRef]
- Wu, L.; Guo, X.; Gao, Y.; Yu, W.; Qin, W.; Kuang, H.; Su, Y. Untargeted metabolomics reveals intervention effects of wine-processed Schisandra chinensis polysaccharide on Alzheimer’s Disease mice. Int. J. Biol. Macromol. 2024, 267, 130804. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, C.; Zhang, J.; Xiao, W.; Yang, F.; Yu, Y.; Li, T.; Wang, Y. Schisandra chinensis polysaccharide protects against cyclosporin a-induced liver injury by promoting hepatocyte proliferation. J. Funct. Foods 2021, 87, 104799. [Google Scholar] [CrossRef]
- Wu, Z.; Li, K.; Tian, X.; Zhou, M.; Li, Z. Schisandra chinensis water extract protects ethanol-induced neurotoxicity in Caenorhabditis elegans. J. Food Biochem. 2020, 44, 13249. [Google Scholar] [CrossRef]
- Jin, J.; Chen, M.; Wang, H.; Li, S.; Ma, L.; Wang, B. Schizandrin A attenuates early brain injury following subarachnoid hemorrhage through suppressing neuroinflammation. Mol. Biol. Rep. 2024, 51, 236. [Google Scholar] [CrossRef]
- Yan, T.; Xu, M.; Wu, B.; Liao, Z.; Liu, Z.; Zhao, X.; Bi, K.; Jia, Y. The effect of Schisandra Chinensis extracts on depression by noradrenergic, dopaminergic, GABAergic and glutamatergic systems in the forced swim test in mice. Food Funct. 2016, 7, 2811–2819. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, Y.; Lee, H.; Yang, B.; Choi, C.-H.; Jeong, H.; Kim, H.; An, W. Prediction of the therapeutic mechanism responsible for the effects of sophora japonica flower buds on contact dermatitis by network-based pharmacological analysis. J. Ethnopharmacol. 2021, 271, 113843. [Google Scholar] [CrossRef] [PubMed]
- Thabit, S.; Handoussa, H.; ElSayed, N.S.; Breitinger, H.G.; Breitinger, U.; Wink, M. A fruit extract of Styphnolobium japonicum (L.) Counteracts oxidative stress and mediates neuroprotection in Caenorhabditis elegans. BMC Complement. Med. Ther. 2023, 23, 330. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, T.; Luo, X.; Jia, M.; Han, L.; Yu, H.; Bao, Y.; Wang, S.; Meng, X. Sophora japonica extract inhibits ulcerative colitis and colitis-associated colon cancer via regulation of lipid metabolism. J. Food Biochem. 2024, 2024, 5552747. [Google Scholar] [CrossRef]
- Zeng, W.; Cheng, N.; Liang, X.; Hu, H.; Luo, F.; Jin, J.; Li, Y. Electrospun polycaprolactone nanofibrous membranes loaded with baicalin for antibacterial wound dressing. Sci. Rep. 2022, 12, 10900. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska-Walendowska, M.; Koumentakou, I.; Lazaridou, M.; Bikiaris, D.; Miklaszewski, A.; Plech, T.; Cielecka-Piontek, J. 3D-printed chitosan-based scaffolds with Scutellariae baicalensis extract for dental applications. Pharmaceutics 2024, 16, 359. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, P.; Muthiah, P.; Louis, L.R.P.; Sambandam, R. Baicalin: A potential therapeutic agent for diabetes and renal protection. Bioact. Compd. Health Dis. 2023, 6, 185–201. [Google Scholar]
- Huang, Y.; Zhou, Y.; Shen, Y.; Wang, J.; Zhou, L.; Chen, Z. Preparation of baicalin colon-targeted granules and its intervention effect on ulcerative colitis in rats. Particuology 2024, 85, 13–21. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, H.J.; Lin, M.W.; Yang, X.R.; Lee, C.H.; Lin, C.S. Inhibitory efficacy of main components of Scutellaria baicalensis on the interaction between spike protein of SARS-CoV-2 and human angiotensin-converting enzyme II. Int. J. Mol. Sci. 2024, 25, 2935. [Google Scholar] [CrossRef]
- Tu, Y.; Li, L.; Fan, W.; Liu, L.; Wang, Z.; Yang, L. Development of green and efficient extraction of bioactive ginsenosides from Panax ginseng with deep eutectic solvents. Molecules 2022, 27, 4339. [Google Scholar] [CrossRef]
- Liu, X.; Ahlgren, S.; Korthout, H.A.A.J.; Salomé-Abarca, L.F.; Bayona, L.M.; Verpoorte, R.; Choi, Y.H. Broad range chemical profiling of natural deep eutectic solvent extracts using a high performance thin layer chromatography–based method. J. Chromatogr. A 2018, 1532, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.M.; Lee, M.S.; Nam, M.W.; Zhao, J.; Jin, Y.; Lee, D.K.; Kwon, S.W.; Jeong, J.H.; Lee, J. Tailoring and recycling of deep eutectic solvents as sustainable and efficient extraction media. J. Chromatogr. A 2015, 1424, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Su, J.; Wang, X.; Zhang, R.; Li, X.; Li, Y.; Ding, Y.; Chu, X. Eco-friendly and efficient extraction of polysaccharides from Acanthopanax senticosus by ultrasound-assisted deep eutectic solvent. Molecules 2024, 29, 942. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Xue, J.; Wang, X.; Zhang, R.; Zhang, X.; Yang, Y.; Chu, X. Modulation of cyclophosphamide-induced immunosuppression and intestinal flora in broiler by deep eutectic solvent extracted polysaccharides of Acanthopanax senticosus. Front. Vet. Sci. 2024, 11, 1415716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Su, J.; Chu, X.; Wang, X. A green method of extracting and recovering flavonoids from Acanthopanax senticosus using deep eutectic solvents. Molecules 2022, 27, 923. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; He, D.; Zhang, Y.; He, D.; Wang, X.; Zhou, J. Characterization of lignin extracted from Acanthopanax senticosus residue using different methods on UV-resistant behavior. Int. J. Biol. Macromol. 2021, 192, 498–505. [Google Scholar] [CrossRef]
- Shi, X.; Yang, Y.; Ren, H.; Sun, S.; Mu, L.t.; Chen, X.; Wang, Y.; Zhang, Y.; Wang, L.; Sun, C. Identification of multiple components in deep eutectic solvent extract of Acanthopanax senticosus root by ultra-high-performance liquid chromatography with quadrupole orbitrap mass spectrometry. Phytochem. Lett. 2020, 35, 175–185. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, Q.; Sun, S.; Liu, B.; Zhang, Y.; Zhang, X.; Sun, C. Study on the technology of efficient extraction of eleutheroside E from Acanthopanax senticosus by green solvent DES. Phytochem. Anal. 2022, 33, 879–885. [Google Scholar] [CrossRef]
- Li, J.H.; Li, W.; Luo, S.; Ma, C.H.; Liu, S.X. Alternate ultrasound/microwave digestion for deep eutectic hydro-distillation extraction of essential oil and polysaccharide from Schisandra chinensis (Turcz.) Baill. Molecules 2019, 24, 1288. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Chen, S.N.; Friesen, J.B.; Nikolić, D.; Choules, M.P.; McAlpine, J.B.; Lankin, D.C.; Gemeinhart, R.A.; Pauli, G.F. The influence of natural deep eutectic solvents on bioactive natural products: Studying interactions between a hydrogel model and Schisandra chinensis metabolites. Fitoterapia 2018, 127, 212–219. [Google Scholar] [CrossRef]
- Yan, X.-Y.; Cai, Z.-H.; Zhao, P.-Q.; Wang, J.-D.; Fu, L.-N.; Gu, Q.; Fu, Y.-J. Application of a novel and green temperature-responsive deep eutectic solvent system to simultaneously extract and separate different polar active phytochemicals from Schisandra chinensis (Turcz.) Baill. Food Res. Int. 2023, 165, 112541. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, S.; Zhi, H.; Huang, J.; Song, H.; Fan, J.; Fu, Y.; Liu, Z. Microwave-assisted deep eutectic solvent extraction of five lignans from Schisandra chinensis. Sep. Sci. Plus 2024, 7, 2400006. [Google Scholar] [CrossRef]
- Meng, Y.; Sui, X.; Pan, X.; Zhang, X.; Sui, H.; Xu, T.; Zhang, H.; Liu, T.; Liu, J.; Ge, P. Density-oriented deep eutectic solvent-based system for the selective separation of polysaccharides from Astragalus membranaceus Var. Mongholicus under ultrasound-assisted conditions. Ultrason. Sonochem. 2023, 98, 106522. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xia, M.; Li, X.; Wang, R.; Liu, W.; Zheng, R.; Wang, Z.; Yang, L.; Shi, Y. Characterization of carotenoids in Lycium barbarum fruit by using UPC2-PDA-Q-TOF-MSE couple with deep eutectic solvents extraction and evaluation of their 5α-reductase inhibitory activity. Front. Chem. 2022, 10, 10502000. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Xu, Y.; Cai, C.; Tan, Z. Extraction of Lycium barbarum polysaccharides using temperature-switchable deep eutectic solvents: A sustainable methodology for recycling and reuse of the extractant. J. Mol. Liq. 2023, 383, 122063. [Google Scholar] [CrossRef]
- Ali, M.C.; Chen, J.; Zhang, H.; Li, Z.; Zhao, L.; Qiu, H. Effective extraction of flavonoids from Lycium barbarum L. fruits by deep eutectic solvents-based ultrasound-assisted extraction. Talanta 2019, 203, 16–22. [Google Scholar] [CrossRef]
- Gu, J.; Lin, L.; Zhao, M. Demonstration of feasibility and effectiveness of deep eutectic solvent-water system extraction of RG-I type pectin from wolfberry based on target polysaccharide, solvent and their interactions. Food Hydrocoll. 2023, 144, 109027. [Google Scholar] [CrossRef]
- Peng, F.; Xu, P.; Zhao, B.Y.; Zong, M.H.; Lou, W.Y. The application of deep eutectic solvent on the extraction and in vitro antioxidant activity of rutin from Sophora japonica bud. J. Food Sci. Technol. 2018, 55, 2326–2333. [Google Scholar] [CrossRef]
- Zhao, B.Y.; Xu, P.; Yang, F.X.; Wu, H.; Zong, M.H.; Lou, W.Y. Biocompatible deep eutectic solvents based on choline chloride: Characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Le, N.T.; Nguyen, T.P.D.; Ho, D.V.; Phung, H.T.; Nguyen, H.T. Green solvents-based rutin extraction from Sophora japonica L. J. Appl. Res. Med. Aromat. Plants 2023, 36, 100508. [Google Scholar] [CrossRef]
- Zang, Y.Y.; Yang, X.; Chen, Z.G.; Wu, T. One-pot preparation of quercetin using natural deep eutectic solvents. Process Biochem. 2020, 89, 193–198. [Google Scholar] [CrossRef]
- Ni, Y.; Zhu, L.; Ye, S.; Xu, X.; Liang, X.; Fang, S. Biorefinery of flavonoid aglycones using acidic natural deep eutectic solvents: Role of bronsted acids and application in valorization of Sophora japonica buds waste. Food Bioprod. Process. 2024, 143, 80–89. [Google Scholar] [CrossRef]
- Sohail, F.; Ahmed, D. Comparison of deep eutectic solvent-based ultrasound and heat-assisted extraction of bioactive compounds from Withania somnifera and process optimization using response surface methodology. Acta Chim. Slov. 2023, 70, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, X.; Cheng, Q.; Xi, X.; Zhang, L. Deep Eutectic solvent-based microwave-assisted extraction of baicalin from Scutellaria baicalensis georgi. J. Chem. 2018, 2018, 9579872. [Google Scholar] [CrossRef]
- Wang, H.; Ma, X.; Cheng, Q.; Wang, L.; Zhang, L. Deep eutectic solvent-based ultrahigh pressure extraction of baicalin from Scutellaria baicalensis Georgi. Molecules 2018, 23, 3233. [Google Scholar] [CrossRef]
- Wang, D.; Luo, X.; Huang, Y.; Wang, M.; Xia, Z. Combined magnetic molecularly imprinted polymers with a ternary deep eutectic solvent to purify baicalein from the Scutellaria baicalensis georgi by magnetic separation. Microchem. J. 2020, 157, 105109. [Google Scholar] [CrossRef]
- Oomen, W.W.; Begines, P.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvent extraction of flavonoids of Scutellaria baicalensis as a replacement for conventional organic solvents. Molecules 2020, 25, 617. [Google Scholar] [CrossRef]
- Hao, C.; Chen, L.; Dong, H.; Xing, W.; Xue, F.; Cheng, Y. Extraction of flavonoids from Scutellariae radix using ultrasound-assisted deep eutectic solvents and evaluation of their anti-inflammatory activities. ACS Omega 2020, 5, 23140–23147. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, M.; Guo, H.; Xu, J.; Ye, J.; Zhao, J.; Zhao, L. Ultrasound-assisted deep eutectic solvent as green and efficient media for the extraction of flavonoids from Radix scutellariae. New J. Chem. 2019, 43, 644–650. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Xing, R.; Wang, R.; Chen, X.; Hu, S. A switchable deep eutectic solvent for the homogeneous liquid-liquid microextraction of flavonoids from “Scutellariae radix”. J. Chromatogr. A 2023, 1688, 463712. [Google Scholar] [CrossRef]
- Guo, X.; Ren, T.; Li, H.; Lu, Q.; Di, X. Antioxidant activity and mechanism exploration for microwave-assisted extraction of flavonoids from Scutellariae radix using natural deep eutectic solvent. Microchem. J. 2024, 200, 110300. [Google Scholar] [CrossRef]


| Synthesis Method | Characteristic |
|---|---|
| Heating and stirring | simple method; compounds are heated and mixed together under constant stirring for around 8 to 12 h with a magnetic stirrer; homogenous liquid is formed; synthesis is performed at temperature ranges from 50 to 100 °C; higher temperatures might lead to degradation of final products due to esterification reaction; |
| Freeze-drying | components are mixed first with water, then freeze-dried; and finally, the water is sublimated; |
| Evaporation | dissolution of compounds forming a DES and then evaporation of water at 50 °C; the substance should be placed in a desiccator; |
| Grinding | the compounds are mixed at room temperature, crushed with a pestle in a mortar; homogenous liquid is formed; |
| Ultrasound-assisted synthesis | very fast preparation of deep eutectic solvents; homogenization process for around 1 min and then the mixture is sonicated for 30 min; the steps are repeated several times to obtain a homogenous final product, which has to be stored in a desiccator at room temperature; HBD and HBA components interact through the cavitation effect, which was caused by ultrasonic waves; |
| Microwave-assisted synthesis | homogenization in vortex for approx. 1 min; mixture is treated in a microwave reactor at 850 W for 45 min with 600 rpm steering speed and at 80 °C; short synthesis time through dielectric heating and interaction with materials and dipole rotation; molecules of HBD and HBA components start to collide and result in fast and efficient synthesis; |
| Property | Characteristic |
|---|---|
| Melting point | the melting point of a DES is lower than the melting point of components through strong interaction between the HBD and HBA; significant impact of anion on the melting point; depends on the change of entropy, the interaction of hydrogen bond donor with hydrogen bond acceptor and lattice energies; |
| pH | pH is correlated with the temperature of a DES; with an increase in temperature, the pH of the deep eutectic solvent decreases linearly; the most important physical property of a DES; pH is influenced by the acidity and basicity of hydrogen bond acceptor and hydrogen bond donor; |
| Surface tension | surface tension is dependent on the intermolecular interactions between precursors; it varies with molar ratio, temperature, and the type of HBA and HBD; with the increase in the alkyl chain length of the cation, the surface tension of DES decreases; with the increase in organic salt, the hydrogen bond network can be disrupted and the surface tension also decreases; |
| Polarity | most of the DESs are polar; polarity increases with the increased interaction between hydrogen bonding donor and hydrogen bonding acceptor; |
| Density | only hydrophobic DESs show the densities lower than water; the reduction of density can occur with increasing temperature because of increasing ionic motion; it is dependent on the HBA and HBD molar ratio and the existence of holes and vacancies within the deep eutectic solvent; the density can be decreased due to the increase in the alkyl chain of cation resulting in the increase in free volume; |
| Viscosity | one of the most important factors—temperature. The breakdown of the hydrogen bond network between the HBD and HBA; with higher temperature, and lower viscosity; factors, which influence the viscosity: temperature, molar and mass ratios, and the nature of hydrogen bond acceptor and hydrogen bond donor; with higher viscosity the mobility in the small volume is lower; important are also the interactions including van der Waals and electrostatic forces between the HBD and HBA; |
| Refractive index (RI) | refractive index allows the information about the composition of deep eutectic solvent; the RI is higher due to larger sizes of molecules; decrease of RI results in less dense samples; there is a reduction of hydrogen bond interaction with the increase in temperature and the RI is inversely proportional to this parameter; |
| Conductivity | due to high viscosity, DESs exhibit low conductivity at room temperature; conductivity is dependent on the temperature, the alkyl chain length of the cation, and the molar ratio of the HBD and HBA; conductivity is strongly connected to the temperature; with higher temperatures, the ionic mobility increases, and the hydrogen bond network gets raptured; |
| Toxicity | DESs are mostly considered green solvents; the toxicity of DESs depends on its chemical structure and the precursors of the mixture; the acidity of DESs leads to more cytotoxic substances; moreover, the toxicity is correlated with the organic acid hydrogen bond donor, which has higher toxicity than different HBDs; the toxicity and cytotoxicity of bacteria, fungi, and viruses have been shown in several research; |
| Effect of water | there is a low possibility of drying DESs, because of their hygroscopic nature; physicochemical properties and biocompatibility are related to the water content and water addition to DESs and the polarity as well as solubilization capacity are influenced by the addition of water; the increased chain length of carboxylic acid results in the increased adsorption capacity and rate of water molecules adsorbed from air; dissolution of DESs in water increases the toxicity of the mixture; with the increase in water content, the parameters of the melting point, density, and viscosity are decreased by disruption of hydrogen bonds; moreover, there is an increase in ionic mobility; |
| Biodegradability | bacteria and fungi easily metabolize the DESs due to neutrality of hydrogen bond donors and acceptors; the most biodegradable donors are amines and the least ones are acids; |
| Plant | Active Substance | DESs | Water Content (wt%) | Liquid/Solid Ratio (mL/g) | Extraction Amount (mg/g) | Ref. |
|---|---|---|---|---|---|---|
| P. ginseng | Ginsenosides | Choline chloride–urea (1:2) | 20 | 15 | 11.41 | [90] |
| White ginseng | Glycerol–L-proline–sucrose (9:4:1) | - | - | 8.24 | [92] | |
| A. senticosus | Polysaccharides | L-proline:L-malic acid (4:1) | 32 | 31 | 35.45 | [94] |
| Flavonoids | Glycerol–levulinic acid (1:1) | 28 | 18 | 23.93 | [95] | |
| S. chinesis | Polysaccharides | Chlorine chloride–ethylene glycol (1:3) | 43 | 30 | 85.60 | [99] |
| Schizandrol A | Glycolic acid–chlorine chloride (1:4) | 30 | 20 | 10.89 | [102] | |
| Schizandrol B | 8.62 | |||||
| Schisantherin A | 4.02 | |||||
| Schisandrin A | 4.89 | |||||
| Schizandrin B | 5.32 | |||||
| A. membranaceus | Polysaccharides | Choline chloride–oxalic acid (1:2) | 55 | 24 | 61.4 | [104] |
| L. barbarum | All-trans-β-carotene | Choline chloride–malonic acid (1:1) | - | - | 9.98 | [104] |
| All-trans-β-zeaxanthin | 5.98 | |||||
| Β-cryptoxanthin monopalmitate | 55.34 | |||||
| Zeaxanthin monopalmitate | 44.99 | |||||
| Polysaccharides | Tetracaine–lauric acid (1:1) | 70 | 25 | 465.00 | [105] | |
| Morin | Choline chloride–p-toulene sulfonic acid | - | - | 12.70 | [106] | |
| Rutin | 9.10 | |||||
| Myricetin | 57.20 | |||||
| S. japonica | Rutin | Choline chloride–thiethylene glycol (1:4) | 18 | 10 | 279.80 | [108] |
| 2 | - | 194.17 | [109] | |||
| Choline chloride–glycerol (1:1) | 20 | - | 291.57 | [111] | ||
| Choline chloride–citric acid (1:1) | 10 | - | 10.10 | [112] | ||
| Quercetin | 38.70 | |||||
| W. somnifera | Flavonoids | Sodium acetate–glycerol (1:3) | 50 | - | 6.08 | [113] |
| S. baicalensis | Baicalin | Decanoic acid–tetrabutylammonium chloride (1:2) | 33 | 16 | 106.96 | [114] |
| Choline chloride–lactic acid (1:1) | 40 | 110 | 116.80 | [115] | ||
| Citric acid–β alanine (1:1) | 40 | - | 39.40 | [117] | ||
| Baicalein | 2.70 | |||||
| Scutellarein | 7.50 | |||||
| Wogonin | 18.60 | |||||
| Wogonoside | 59.40 | |||||
| Oroxylin A | 2.90 | |||||
| Oroxyloside | 5.40 | |||||
| Baicalin | Citric acid–proline (1:1) | 60 | - | 32.00 | ||
| Baicalein | 3.20 | |||||
| Scutellarein | 4.60 | |||||
| Wogonin | 10.90 | |||||
| Wogonoside | 82.40 | |||||
| Oroxylin A | 7.00 | |||||
| Oroxyloside | 12.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanisz, M.; Stanisz, B.J.; Cielecka-Piontek, J. A Comprehensive Review on Deep Eutectic Solvents: Their Current Status and Potential for Extracting Active Compounds from Adaptogenic Plants. Molecules 2024, 29, 4767. https://doi.org/10.3390/molecules29194767
Stanisz M, Stanisz BJ, Cielecka-Piontek J. A Comprehensive Review on Deep Eutectic Solvents: Their Current Status and Potential for Extracting Active Compounds from Adaptogenic Plants. Molecules. 2024; 29(19):4767. https://doi.org/10.3390/molecules29194767
Chicago/Turabian StyleStanisz, Malgorzata, Beata J. Stanisz, and Judyta Cielecka-Piontek. 2024. "A Comprehensive Review on Deep Eutectic Solvents: Their Current Status and Potential for Extracting Active Compounds from Adaptogenic Plants" Molecules 29, no. 19: 4767. https://doi.org/10.3390/molecules29194767
APA StyleStanisz, M., Stanisz, B. J., & Cielecka-Piontek, J. (2024). A Comprehensive Review on Deep Eutectic Solvents: Their Current Status and Potential for Extracting Active Compounds from Adaptogenic Plants. Molecules, 29(19), 4767. https://doi.org/10.3390/molecules29194767