Oxidative Catalytic Depolymerization of Lignin into Value-Added Monophenols by Carbon Nanotube-Supported Cu-Based Catalysts
Abstract
1. Introduction
2. Result and Discussion
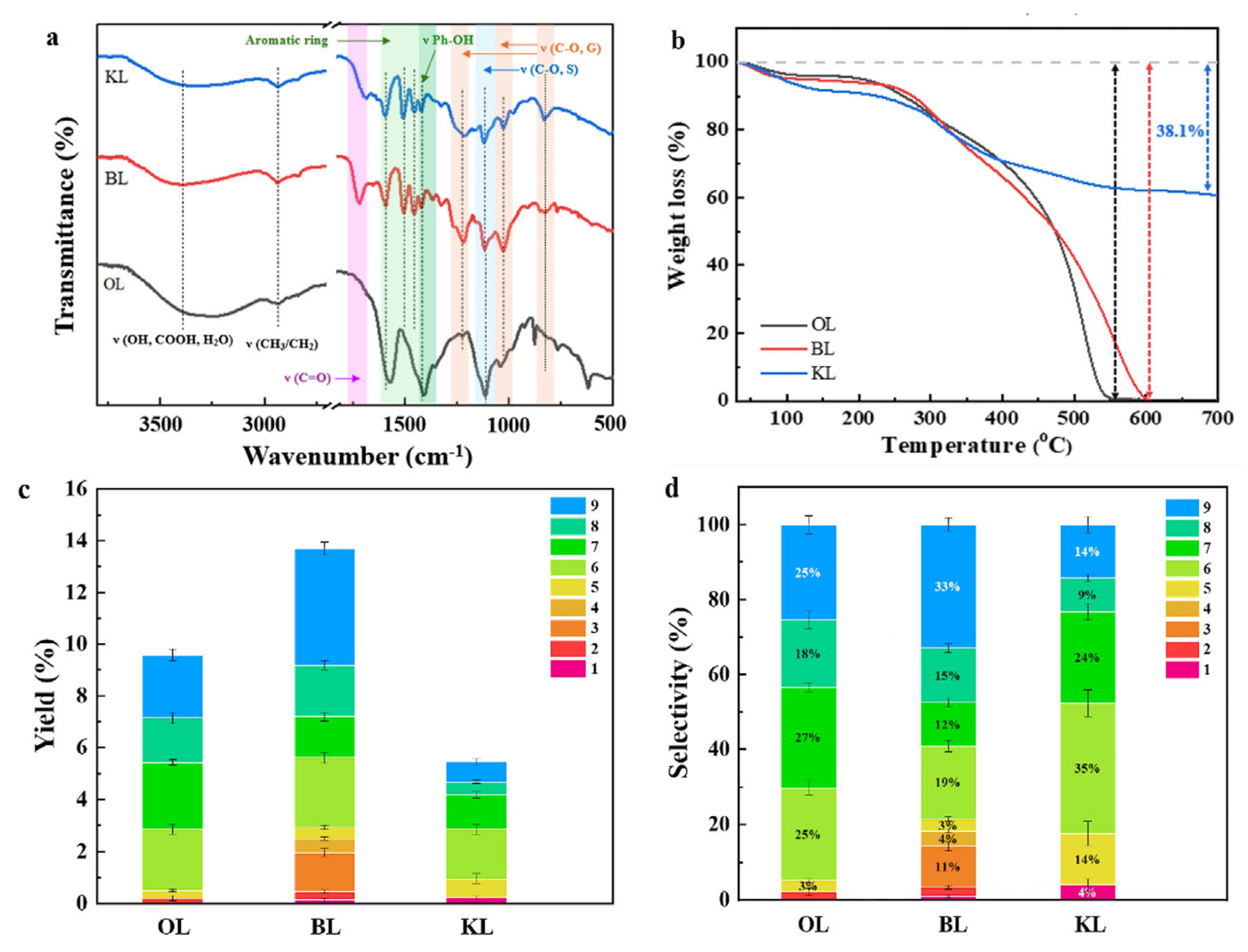
2.1. Characterization of CuO/CNT Catalysts
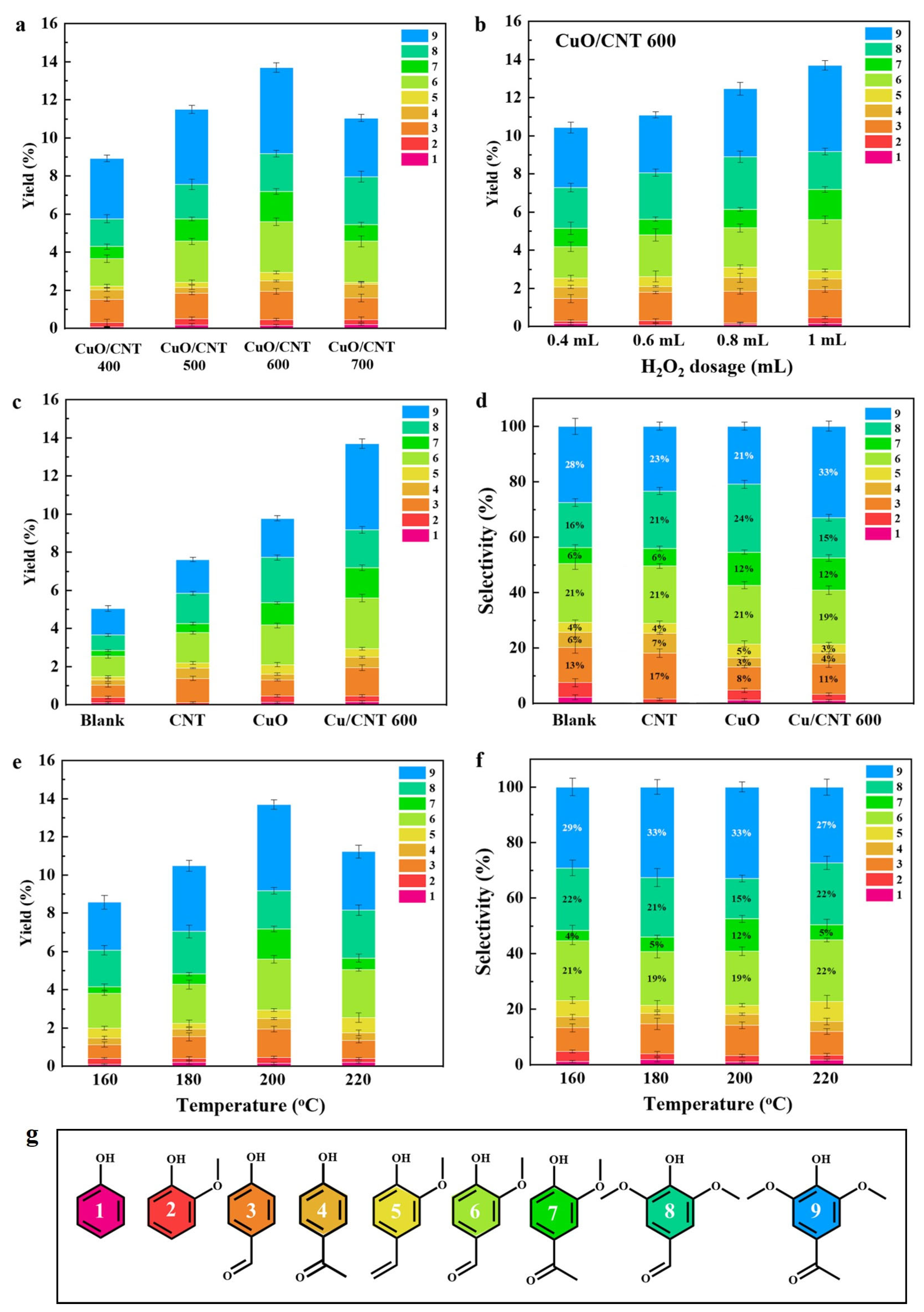
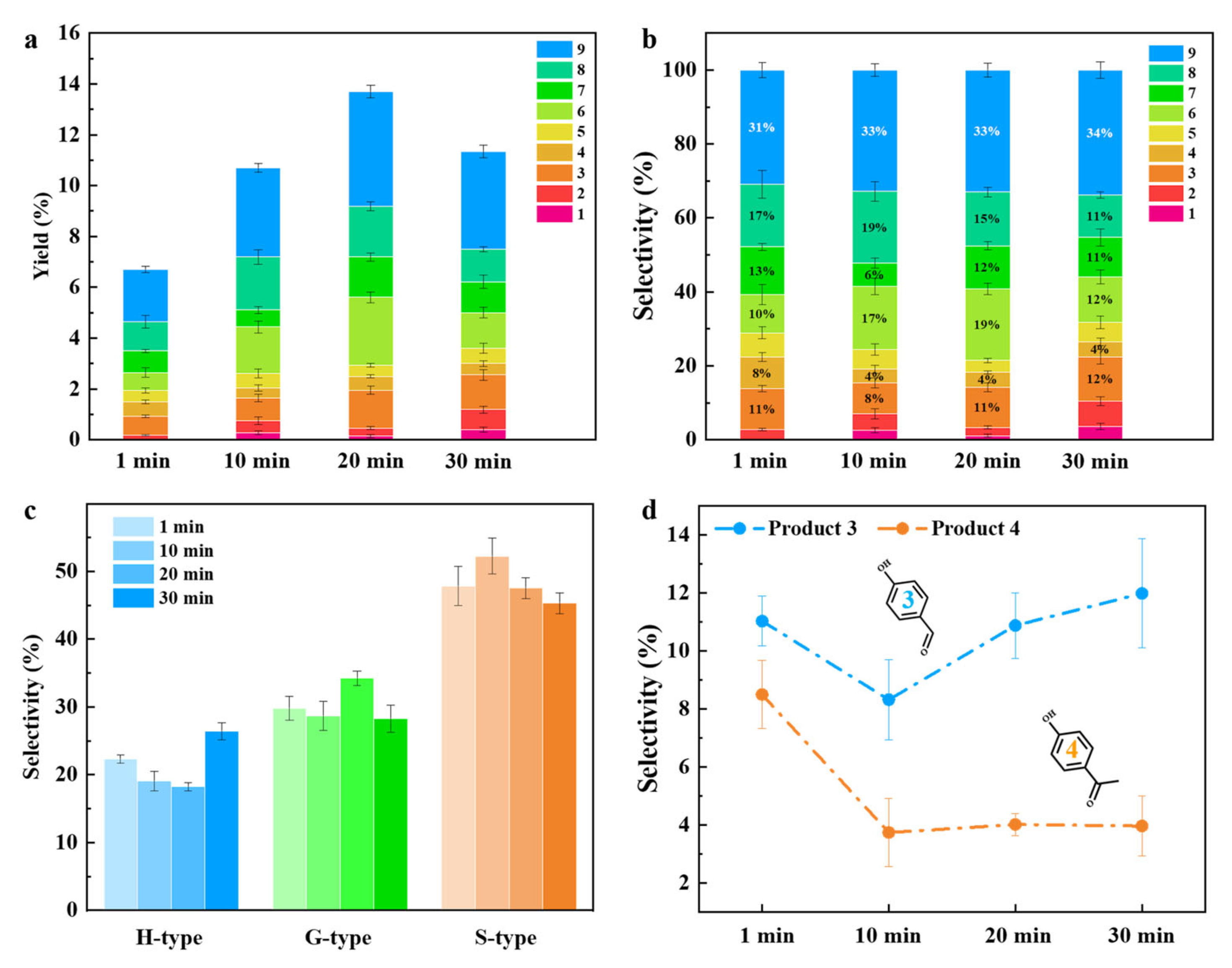
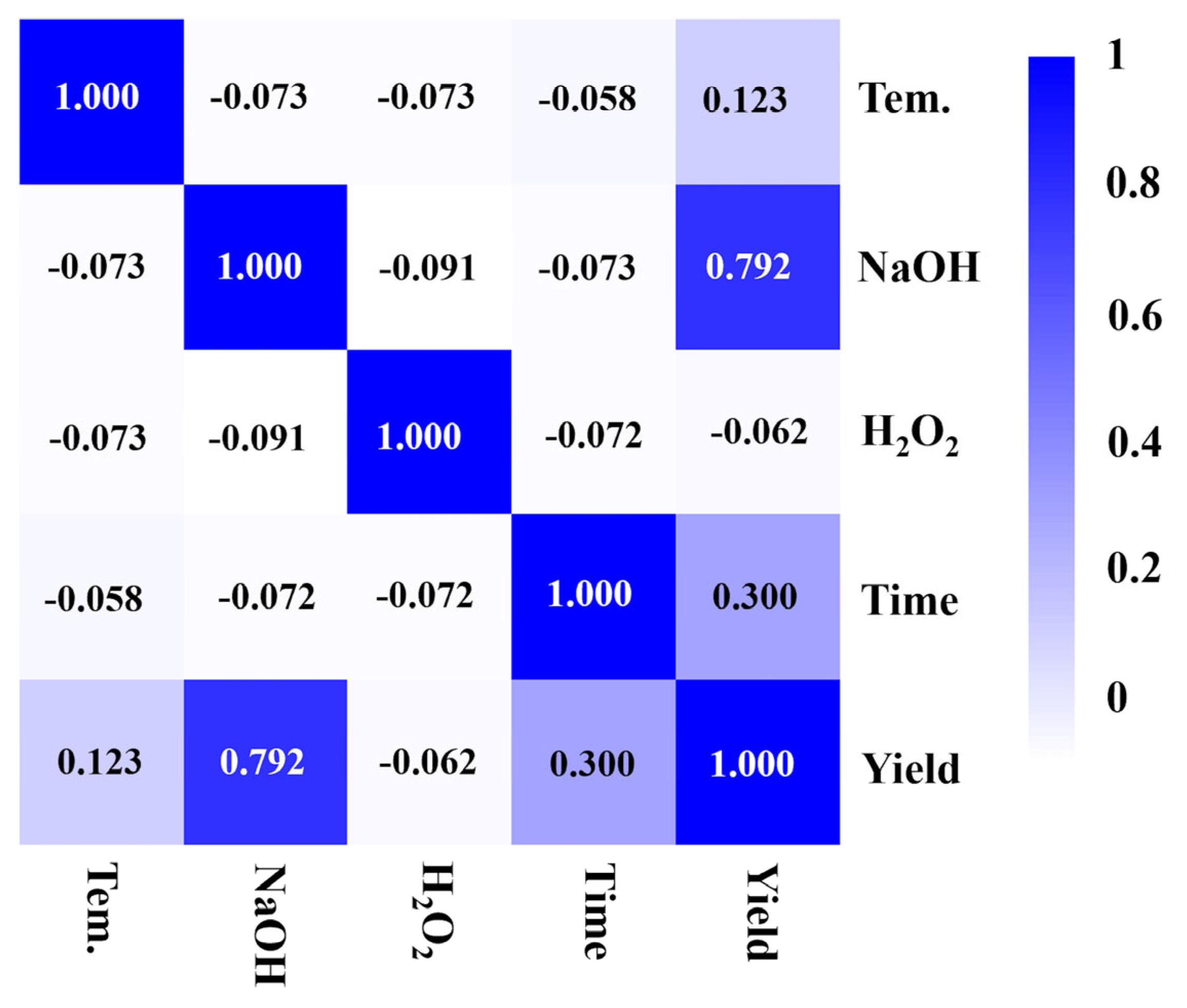
2.2. Catalytic Performance of CuO/CNT for Lignin Depolymerization
2.3. Production of Monophenols from Different Lignin Substrates
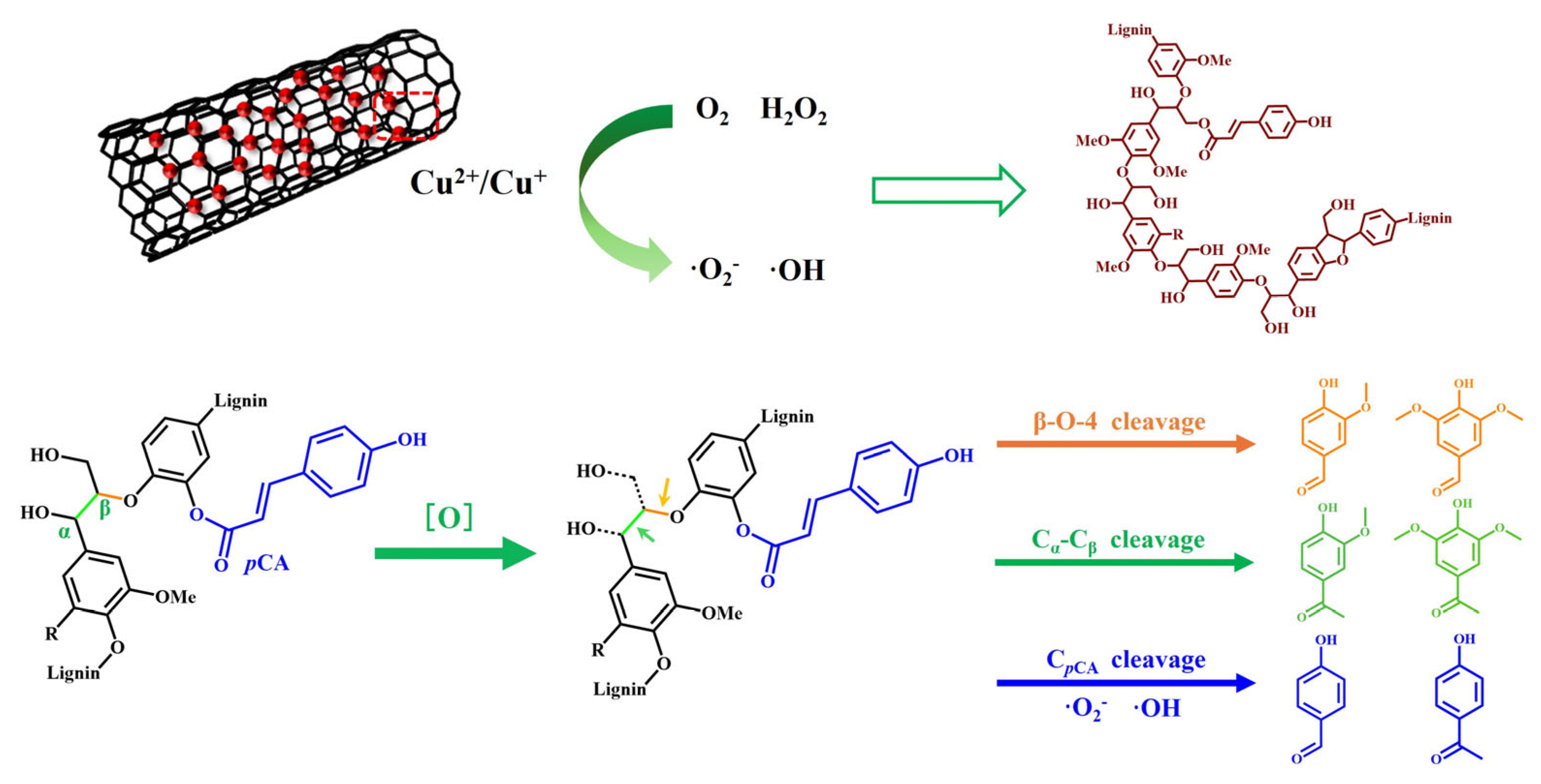
2.4. Mechanism Study
3. Methods
3.1. Lignin Samples
3.2. Preparation of CuO/CNT Catalyst
3.3. Microwave-Assisted Oxidative Depolymerization of Lignin
3.4. Qualitative and Quantitative Analysis of Products
3.5. Characterization of Catalyst and Lignin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Manikandan, S.; Vickram, S.; Sirohi, R.; Subbaiya, R.; Krishnan, R.Y.; Karmegam, N.; Sumathijones, C.; Rajagopal, R.; Chang, S.W.; Ravindran, B.; et al. Critical Review of Biochemical Pathways to Transformation of Waste and Biomass into Bioenergy. Bioresour. Technol. 2023, 372, 128679. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Sun, P.-P.; Singhania, R.R.; Patel, A.K.; Dong, C.-D. Journey of Lignin from a Roadblock to Bridge for Lignocellulose Biorefineries: A Comprehensive Review. Sci. Total Environ. 2023, 861, 160560. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic Conversion of Lignocellulosic Biomass into Chemicals and Fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Al-Azkawi, A.; Elliston, A.; Al-Bahry, S.; Sivakumar, N. Waste Paper to Bioethanol: Current and Future Prospective. Biofuels Bioprod. Biorefining 2019, 13, 1106–1118. [Google Scholar] [CrossRef]
- Latif, M.N.; Wan Isahak, W.N.R.; Samsuri, A.; Hasan, S.Z.; Manan, W.N.; Yaakob, Z. Recent Advances in the Technologies and Catalytic Processes of Ethanol Production. Catalysts 2023, 13, 1093. [Google Scholar] [CrossRef]
- Huynh, Q.T.; Zhong, C.-T.; Huang, Q.; Lin, Y.-C.; Chen, K.-F.; Liao, C.-S.; Dong, C.-D.; Chang, K.-L. Highly Effective Synthesis of 5-Hydroxymethylfurfural from Lignocellulosic Biomass over a Green and One-Pot Reaction in Biphasic System. Bioresour. Technol. 2023, 387, 129590. [Google Scholar] [CrossRef]
- Kumar Vaidyanathan, V.; Saikia, K.; Senthil Kumar, P.; Karanam Rathankumar, A.; Rangasamy, G.; Dattatraya Saratale, G. Advances in Enzymatic Conversion of Biomass Derived Furfural and 5-Hydroxymethylfurfural to Value-Added Chemicals and Solvents. Bioresour. Technol. 2023, 378, 128975. [Google Scholar] [CrossRef]
- Poveda-Giraldo, J.A.; Solarte-Toro, J.C.; Cardona Alzate, C.A. The Potential Use of Lignin as a Platform Product in Biorefineries: A Review. Renew. Sustain. Energy Rev. 2021, 138, 110688. [Google Scholar] [CrossRef]
- Zevallos Torres, L.A.; Lorenci Woiciechowski, A.; de Andrade Tanobe, V.O.; Karp, S.G.; Guimarães Lorenci, L.C.; Faulds, C.; Soccol, C.R. Lignin as a Potential Source of High-Added Value Compounds: A Review. J. Clean. Prod. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Raj, T.; Chen, C.-W.; Ponnusamy, V.K.; Tahir, N.; Kim, S.-H.; Dong, C.-D. Lignin Valorisation via Enzymes: A Sustainable Approach. Fuel 2022, 311, 122608. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, C.; Zhan, Y.; Han, S.; Wang, J.; Meng, X.; Yoo, C.G.; Fang, G.; Ragauskas, A.J. Effective Biomass Fractionation and Lignin Stabilization Using a Diol DES System. Chem. Eng. J. 2022, 443, 136395. [Google Scholar] [CrossRef]
- Lawoko, M.; Samec, J.S.M. Kraft Lignin Valorization: Biofuels and Thermoset Materials in Focus. Curr. Opin. Green Sustain. Chem. 2023, 40, 100738. [Google Scholar] [CrossRef]
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Pulkki, R.; Xu, C. Global Lignin Supply Overview and Kraft Lignin Potential as an Alternative for Petroleum-Based Polymers. Renew. Sustain. Energy Rev. 2020, 123, 109768. [Google Scholar] [CrossRef]
- Sheng, Y.; Lam, S.S.; Wu, Y.; Ge, S.; Wu, J.; Cai, L.; Huang, Z.; Le, Q.V.; Sonne, C.; Xia, C. Enzymatic Conversion of Pretreated Lignocellulosic Biomass: A Review on Influence of Structural Changes of Lignin. Bioresour. Technol. 2021, 324, 124631. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Meng, X.; Sun, F.; Zhang, J.; Tu, M.; Chang, J.-S.; Reungsang, A.; Xia, A.; Ragauskas, A.J. Advances and Perspectives on Mass Transfer and Enzymatic Hydrolysis in the Enzyme-Mediated Lignocellulosic Biorefinery: A Review. Biotechnol. Adv. 2023, 62, 108059. [Google Scholar] [CrossRef]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic Pyrolysis of Lignocellulosic Biomass for Bio-Oil Production: A Review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef]
- Zoppi, G.; Tito, E.; Bianco, I.; Pipitone, G.; Pirone, R.; Bensaid, S. Life Cycle Assessment of the Biofuel Production from Lignocellulosic Biomass in a Hydrothermal Liquefaction–Aqueous Phase Reforming Integrated Biorefinery. Renew. Energy 2023, 206, 375–385. [Google Scholar] [CrossRef]
- Wu, J.; Li, T.; Qiu, X.; Qin, Y.; Chen, L. γ-Valerolactone/H2O Binary Solvent for One-Pot Preparation and Functional Tailoring of Lignin-Based Carbon Dots. ACS Sustain. Chem. Eng. 2023, 11, 12256–12264. [Google Scholar] [CrossRef]
- Chen, R.; Huang, Y.; Rao, C.; Su, H.; Pang, Y.; Lou, H.; Yang, D.; Qiu, X. Enhanced Photocatalytic Degradation of Lignin by In2S3 with Hydrophobic Surface and Metal Defects. Appl. Surf. Sci. 2022, 600, 154110. [Google Scholar] [CrossRef]
- Wu, K.; Cao, M.; Zeng, Q.; Li, X. Radical and (Photo)Electron Transfer Induced Mechanisms for Lignin Photo- and Electro-Catalytic Depolymerization. Green Energy Environ. 2023, 8, 383–405. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Z.; Yin, Q.; Sun, T.; Liu, Y.; Ren, G.; Li, C. Waste to wealth: H2S-free fabrication of Fe-ZnS/NC by industrial lignin self S-doping for efficient lignin aerobic oxidation. Appl. Catal. B Environ. 2023, 339, 123129. [Google Scholar] [CrossRef]
- Tang, B.; Li, W.; Zhang, X.; Zhang, B.; Zhang, H.; Li, C. Depolymerization of Kraft lignin to liquid fuels with MoS2 derived oxygen-vacancy-enriched MoO3 in a hydrogen-donor solvent system. Fuel 2022, 324, 124674. [Google Scholar] [CrossRef]
- Sosa, F.H.B.; Bjelić, A.; Coutinho, J.A.P.; Costa, M.C.; Likozar, B.; Jasiukaitytė-Grojzdek, E.; Grilc, M.; Lopes, A.M.d.C. Conversion of Organosolv and Kraft lignins into value-added compounds assisted by an acidic deep eutectic solvent. Sustain. Energy Fuels 2022, 6, 4800–4815. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, S.; Ji, L.; Shi, H. Oligomeric Aromatic Oxides (OAO) Production From Sugarcane Bagasse Lignin by Acid-Catalyzed Solvothermal Liquefaction in Methanol. Front. Energy Res. 2020, 8, 608415. [Google Scholar] [CrossRef]
- Cai, W.; Wang, X.; Zhu, Z.; Kumar, R.; Nana Amaniampong, P.; Zhao, J.; Hu, Z.-T. Synergetic effects in the co-pyrolysis of lignocellulosic biomass and plastic waste for renewable fuels and chemicals. Fuel 2023, 353, 129210. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, W.; Pan, L.; Huang, Z.; Zhang, X.; Shi, C.; Zou, J.-J. Ni5Fe5/Al2O3 catalytic hydrogenolysis of lignin: Mechanism investigation and selectivity regulation. Green Chem. 2023, 25, 7782–7793. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, X.; Guo, Y.; Wang, Y. Self-Hydrogen Supplied Catalytic Fractionation of Raw Biomass into Lignin-Derived Phenolic Monomers and Cellulose-Rich Pulps. JACS Au 2023, 3, 1911–1917. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Clemmensen, I.; Meier, S.; Costa, C.A.E.; Rodrigues, A.E.; Hulteberg, C.P.; Riisager, A. On the Oxidative Valorization of Lignin to High-Value Chemicals: A Critical Review of Opportunities and Challenges. ChemSusChem 2022, 15, e202201232. [Google Scholar] [CrossRef]
- Hu, Y.; Cui, Y.; Zhao, S.; Zhao, X.; Hu, X.; Song, Z.; Fan, W.; Zhang, Q. Selective C–C bond cleavage of oxidized lignin in an aqueous phase under mild conditions. Green Chem. 2023, 25, 5150–5159. [Google Scholar] [CrossRef]
- Gao, D.; Ouyang, D.; Bai, Y.; Zhao, X. Synthesis and Computational Investigation of Antioxidants Prepared by Oxidative Depolymerization of Lignin and Aldol Condensation of Aromatic Aldehydes. ChemSusChem 2023, 16, e202300208. [Google Scholar] [CrossRef] [PubMed]
- Kärkäs, M.D.; Matsuura, B.S.; Monos, T.M.; Magallanes, G.; Stephenson, C.R.J. Transition-metal catalyzed valorization of lignin: The key to a sustainable carbon-neutral future. Org. Biomol. Chem. 2016, 14, 1853–1914. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Fu, X.; Wang, P.; Li, K.; Wei, L.; Zhai, S.; An, Q. Selective production of p-hydroxybenzaldehyde in the oxidative depolymerization of alkali lignin catalyzed by copper-containing imidazolium-based ionic liquids. J. Mol. Liq. 2023, 385, 122378. [Google Scholar] [CrossRef]
- Zhao, X.; Ding, Y.; Du, B.; Zhu, J.Y.; Liu, D. Polyoxometalate-Mediated Lignin Oxidation for Efficient Enzymatic Production of Sugars and Generation of Electricity from Lignocellulosic Biomass. Energy Technol. 2017, 5, 1179–1185. [Google Scholar] [CrossRef]
- Xiao, X.; Han, Y.; Liu, C.; Li, Y.; Sun, G.; Wang, X. Visible-light-activated TiO2 photocatalysis regionally modified by SiO2 for lignin depolymerization. Mater. Today Energy 2022, 30, 101190. [Google Scholar] [CrossRef]
- Gale, M.; Cai, C.M.; Gilliard-Abdul-Aziz, K.L. Heterogeneous Catalyst Design Principles for the Conversion of Lignin into High-Value Commodity Fuels and Chemicals. ChemSusChem 2020, 13, 1947–1966. [Google Scholar] [CrossRef]
- Ren, X.; Wang, P.; Han, X.; Zhang, G.; Gu, J.; Ding, C.; Zheng, X.; Cao, F. Depolymerization of Lignin to Aromatics by Selectively Oxidizing Cleavage of C–C and C–O Bonds Using CuCl2/Polybenzoxazine Catalysts at Room Temperature. ACS Sustain. Chem. Eng. 2017, 5, 6548–6556. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, S.S.; Tsang, D.C.W.; Clark, J.H.; Budarin, V.L.; Hu, C.; Wu, K.C.-W.; Zhang, S. Microwave-assisted depolymerization of various types of waste lignins over two-dimensional CuO/BCN catalysts. Green Chem. 2020, 22, 725–736. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, S.; Wang, B.; Shi, C.; Nie, Y. Microwave-assisted pyrolysis aspen wood for production of valuable products under different temperatures. Arab. J. Chem. 2023, 16, 105187. [Google Scholar] [CrossRef]
- Vega-Aguilar, C.A.; Costa, C.; Barreiro, M.F.; Rodrigues, A.E. Microwave-Assisted Lignin Wet Peroxide Oxidation to C4 Dicarboxylic Acids. Ind. Eng. Chem. Res. 2022, 61, 3570–3581. [Google Scholar] [CrossRef]
- Hamzah, W.S.W.; Kait, C.F.; Baharuddin, N.A.; Rahim, A.H.A.; Jumbri, K.; Wilfred, C.D.; Man, Z.; Idris, A. Microwave-assisted chemistry: Parametric optimization for catalytic degradation of lignin model compounds in imidazolium-based ILs. Biomass Convers. Biorefinery 2023, 13, 1793–1803. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Costa, M.; Pereira, C.; Bachiller-Baeza, B.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A.; Freire, C. Novel electrochemical sensor based on N-doped carbon nanotubes and Fe3O4 nanoparticles: Simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. J. Colloid Interface Sci. 2014, 432, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Liu, S.; Yang, G.; Jiang, P.; Luo, X.; Chen, Y.; Xu, M.; Lester, E.; Wu, T. Microwave-accelerated hydrolysis for hydrogen production over a cobalt-loaded multi-walled carbon nanotube-magnetite composite catalyst. Appl. Energy 2023, 333, 120538. [Google Scholar] [CrossRef]
- Araia, A.; Wang, Y.; Jiang, C.; Brown, S.; Caiola, A.; Robinson, B.; Li, W.; Hu, J. Insight into Enhanced Microwave Heating for Ammonia Synthesis: Effects of CNT on the Cs–Ru/CeO2 Catalyst. ACS Appl. Mater. Interfaces 2023, 15, 24296–24305. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, J.A.; Arenillas, A.; Fidalgo, B.; Fernández, Y.; Zubizarreta, L.; Calvo, E.G.; Bermúdez, J.M. Microwave heating processes involving carbon materials. Fuel Process. Technol. 2010, 91, 1–8. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, Y.; Li, Q.; Li, H.; Bian, Z. Microwave-Assisted Photocatalytic Degradation of Organic Pollutants via CNTs/TiO2. Catalysts 2022, 12, 940. [Google Scholar] [CrossRef]
- Chen, J.; Xue, S.; Song, Y.; Shen, M.; Zhang, Z.; Yuan, T.; Tian, F.; Dionysiou, D.D. Microwave-induced carbon nanotubes catalytic degradation of organic pollutants in aqueous solution. J. Hazard. Mater. 2016, 310, 226–234. [Google Scholar] [CrossRef]
- Davaritouchaee, M.; Hiscox, W.C.; Terrell, E.; Mancini, R.J.; Chen, S. Mechanistic studies of milled and Kraft lignin oxidation by radical species. Green Chem. 2020, 22, 1182–1197. [Google Scholar] [CrossRef]
- Ojeery, A.A.; Hassan, H.U.; Balawi, S.A.A.; Iqbal, M.W.; Afzal, A.M.; Hadia, N.M.A. Growth of AgCoS@CNTs Composite on Nickel Foam to Enrich the Redox Active Sites for Battery-Supercapacitor Hybrid Energy Storage Device. J. Phys. Chem. Solids 2023, 180, 111473. [Google Scholar] [CrossRef]
- Sun, H.; Wang, H.; Qu, Z. Construction of CuO/CeO2 Catalysts via the Ceria Shape Effect for Selective Catalytic Oxidation of Ammonia. ACS Catal. 2023, 13, 1077–1088. [Google Scholar] [CrossRef]
- Thanasamy, D.; Jesuraj, D.; Avadhanam, V.; Chinnadurai, K.; Kannan, S.K.K. Microstructural effect of various polyaniline-carbon nanotube core-shell nanocomposites on electrochemical supercapacitor electrode performance. J. Energy Storage 2022, 53, 105087. [Google Scholar] [CrossRef]
- Basit, R.A.; Abbasi, Z.; Hafeez, M.; Ahmad, P.; Khan, J.; Khandaker, M.U.; Al-Mugren, K.S.; Khalid, A. Successive Photocatalytic Degradation of Methylene Blue by ZnO, CuO and ZnO/CuO Synthesized from Coriandrum Sativum Plant Extract via Green Synthesis Technique. Crystals 2023, 13, 281. [Google Scholar] [CrossRef]
- Arfan, M.; Hussain, I.; Ahmad, Z.; Afzal, A.; Shahid, T.; Wattoo, A.G.; Rafi, M.; Zeb, A.; Shahzad, M.I.; Zhenlun, S. Facile Synthesis and Characterization of CuO–CeO2 Nanostructures for Photocatalytic Applications. Cryst. Res. Technol. 2022, 57, 2100230. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Vigul, D.O.; Kaygorodov, K.L.; Chelbina, Y.V.; Mazurova, E.V. Catalytic Oxidation of Flax Shives into Vanillin and Pulp. Catalysts 2022, 12, 1003. [Google Scholar] [CrossRef]
- Arulkumar, E.; Shree, S.S.; Thanikaikarasan, S. Synthesis and characterization of CuO–Mn3O4: Application to chromium (VI) photocatalytic reduction. J. Mater. Sci. Mater. Electron. 2024, 35, 198. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.; He, P.; Yuan, Y. Effect of boric oxide doping on the stability and activity of a Cu–SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2011, 277, 54–63. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, S.; Shen, H.; Xu, X.; Fang, X.; Wang, X. Studying the Structure-Reactivity Relationship of CuO/CeO2 for Catalytic Soot Particulate Combustion: On the Monolayer Dispersion Threshold Effect. ChemCatChem 2024, 16, e202301766. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Zhu, X.; Chen, B. Hollow multi-shelled Co3O4 as nanoreactors to activate peroxymonosulfate for highly effective degradation of Carbamazepine: A novel strategy to reduce nano-catalyst agglomeration. J. Hazard. Mater. 2022, 427, 127890. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.; Huang, D.; Jawad, A.; Chen, Z.; Yang, J.; Liu, W.; Shen, Y.; Wang, M.; Yin, G. Support-Dependent Active Species Formation for CuO Catalysts: Leading to Efficient Pollutant Degradation in Alkaline Conditions. J. Hazard. Mater. 2017, 328, 56–62. [Google Scholar] [CrossRef]
- Liu, C.; Lin, F.; Kong, X.; Fan, Y.; Xu, W.; Lei, M.; Xiao, R. Lignin-first biorefinery of corn stalk via zirconium(IV) chloride/sodium hydroxide-catalyzed aerobic oxidation to produce phenolic carbonyls. Bioresour. Technol. 2022, 354, 127183. [Google Scholar] [CrossRef]
- Sun, Y.-C.; Wang, M.; Sun, R.-C. Toward an Understanding of Inhomogeneities in Structure of Lignin in Green Solvents Biorefinery. Part 1: Fractionation and Characterization of Lignin. ACS Sustain. Chem. Eng. 2015, 3, 2443–2451. [Google Scholar] [CrossRef]
- Seo, J.H.; Jeong, H.; Lee, H.W.; Choi, C.S.; Bae, J.H.; Lee, S.M.; Kim, Y.S. Characterization of solvent-fractionated lignins from woody biomass treated via supercritical water oxidation. Bioresour. Technol. 2019, 275, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Chen, C.; Yoo, C.G.; Meng, X.; Li, M.; Pu, Y.; Ragauskas, A.J.; Dong, C.; Yang, H. Insights of Ethanol Organosolv Pretreatment on Lignin Properties of Broussonetia Papyrifera. ACS Sustain. Chem. Eng. 2018, 6, 14767–14773. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, S.S.; Zhang, S.; Ok, Y.S.; Matsagar, B.M.; Wu, K.C.-W.; Tsang, D.C.W. Advances in lignin valorization towards bio-based chemicals and fuels: Lignin biorefinery. Bioresour. Technol. 2019, 291, 121878. [Google Scholar] [CrossRef]
- Adamovic, T.; Zhu, X.; Perez, E.; Balakshin, M.; Cocero, M.J. Understanding sulfonated kraft lignin re-polymerization by ultrafast reactions in supercritical water. J. Supercrit. Fluids 2022, 191, 105768. [Google Scholar] [CrossRef]
- Happs, R.M.; Addison, B.; Doeppke, C.; Donohoe, B.S.; Davis, M.F.; Harman-Ware, A.E. Comparison of methodologies used to determine aromatic lignin unit ratios in lignocellulosic biomass. Biotechnol. Biofuels 2021, 14, 58. [Google Scholar] [CrossRef]
- Davaritouchaee, M.; Hiscox, W.C.; Martinez-Fernandez, J.; Fu, X.; Mancini, R.J.; Chen, S. Effect of reactive oxygen species on biomass structure in different oxidative processes. Ind. Crops Prod. 2019, 137, 484–494. [Google Scholar] [CrossRef]
- Hafezisefat, P.; Qi, L.; Brown, R.C. Lignin Depolymerization and Esterification with Carboxylic Acids to Produce Phenyl Esters. ACS Sustain. Chem. Eng. 2023, 11, 17053–17060. [Google Scholar] [CrossRef]
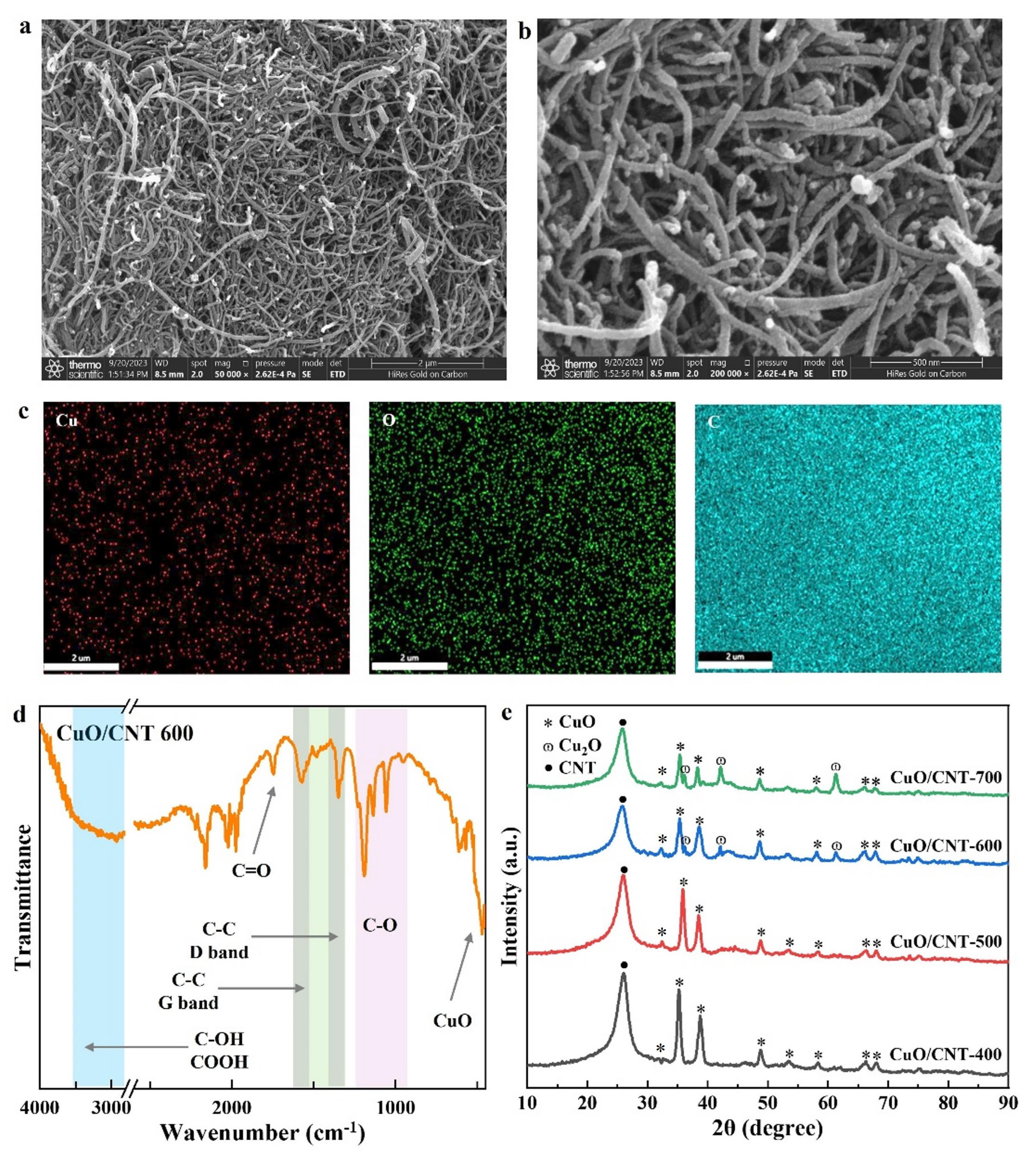
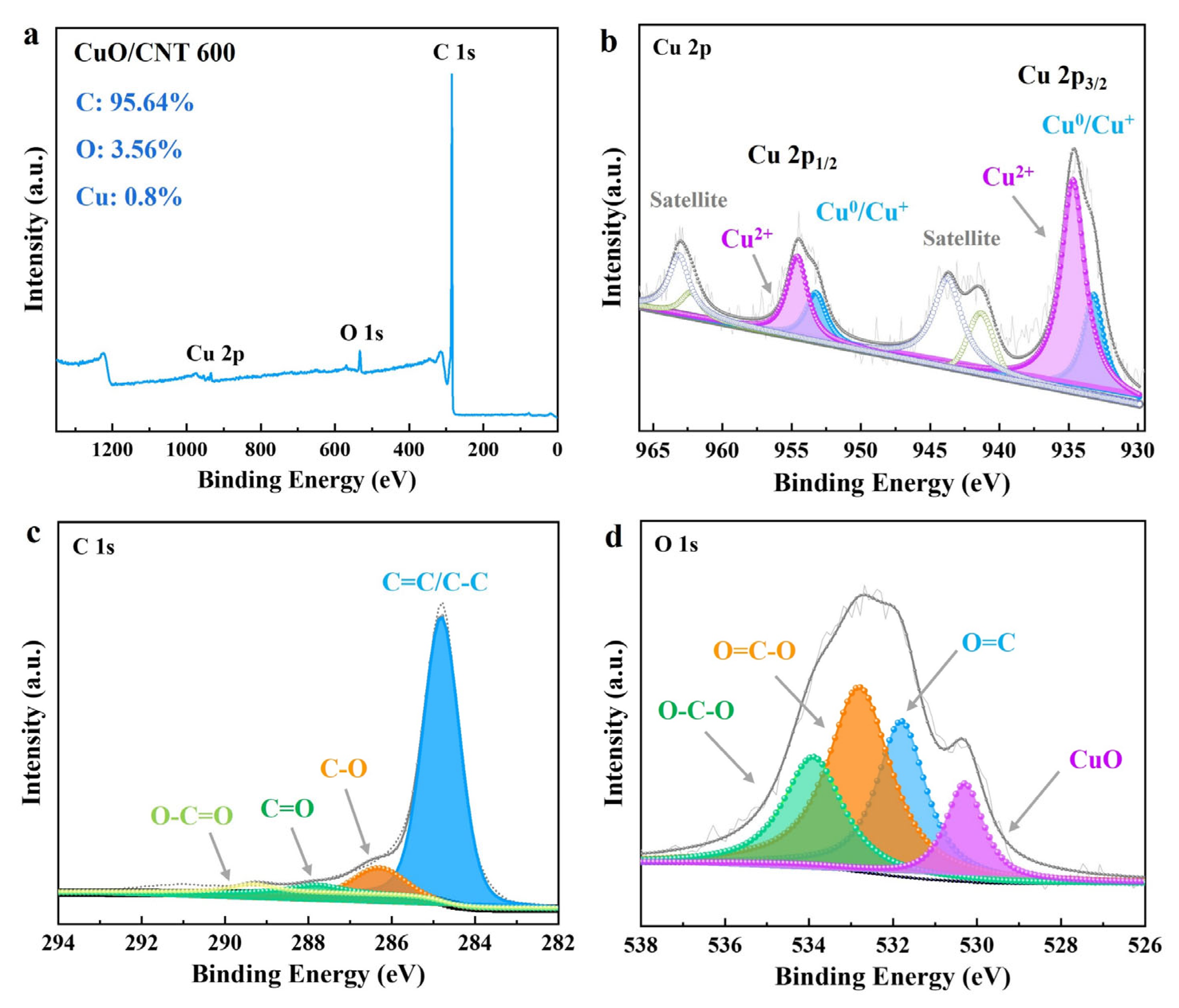



| Catalyst | SBET (m2/g) | V a (cm3/g) | D b (nm) |
|---|---|---|---|
| CuO/CNT 400 | 222.8 | 0.29 | 5.3 |
| CuO/CNT 500 | 201.5 | 0.19 | 2.4 |
| CuO/CNT 600 | 189.1 | 0.23 | 4.8 |
| CuO/CNT 700 | 70.0 | 0.22 | 3.6 |
| Lignin | N | C | H | S | O | Mw (Da) |
|---|---|---|---|---|---|---|
| OL | 0.14 | 55.34 | 5.72 | 0 | 38.8 | 1950 |
| BL | 0.77 | 52.92 | 5.54 | 0 | 40.77 | 925 |
| KL | 0.11 | 53.77 | 5.28 | 2.88 | 37.96 | 10,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Cao, Y.; Gao, J.; Luo, G.; Fan, J.; Clark, J.H.; Zhang, S. Oxidative Catalytic Depolymerization of Lignin into Value-Added Monophenols by Carbon Nanotube-Supported Cu-Based Catalysts. Molecules 2024, 29, 4762. https://doi.org/10.3390/molecules29194762
Tang C, Cao Y, Gao J, Luo G, Fan J, Clark JH, Zhang S. Oxidative Catalytic Depolymerization of Lignin into Value-Added Monophenols by Carbon Nanotube-Supported Cu-Based Catalysts. Molecules. 2024; 29(19):4762. https://doi.org/10.3390/molecules29194762
Chicago/Turabian StyleTang, Chen, Yang Cao, Jie Gao, Gang Luo, Jiajun Fan, James H. Clark, and Shicheng Zhang. 2024. "Oxidative Catalytic Depolymerization of Lignin into Value-Added Monophenols by Carbon Nanotube-Supported Cu-Based Catalysts" Molecules 29, no. 19: 4762. https://doi.org/10.3390/molecules29194762
APA StyleTang, C., Cao, Y., Gao, J., Luo, G., Fan, J., Clark, J. H., & Zhang, S. (2024). Oxidative Catalytic Depolymerization of Lignin into Value-Added Monophenols by Carbon Nanotube-Supported Cu-Based Catalysts. Molecules, 29(19), 4762. https://doi.org/10.3390/molecules29194762








