Fast and Sensitive Determination of Iodide Based on Ternary Chalcogenides Nanoparticles
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
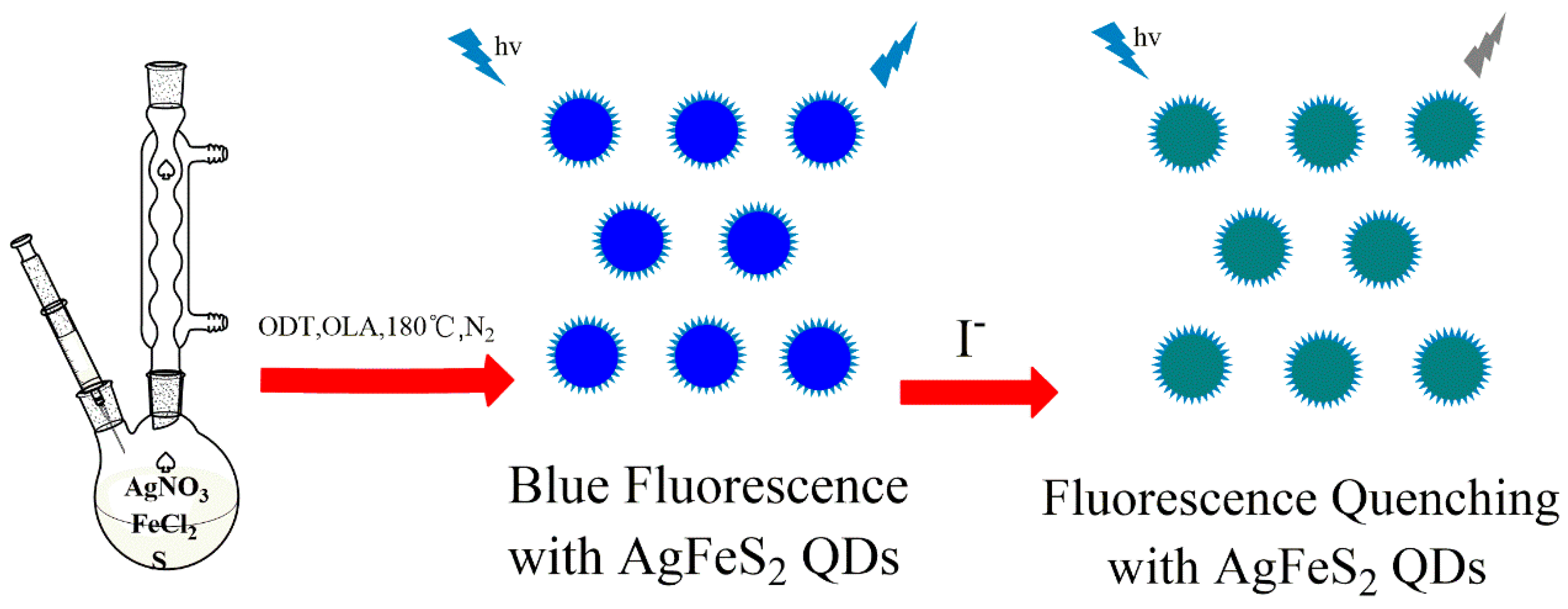
2.3. Synthesis of AgFeS2 Quantum Dots
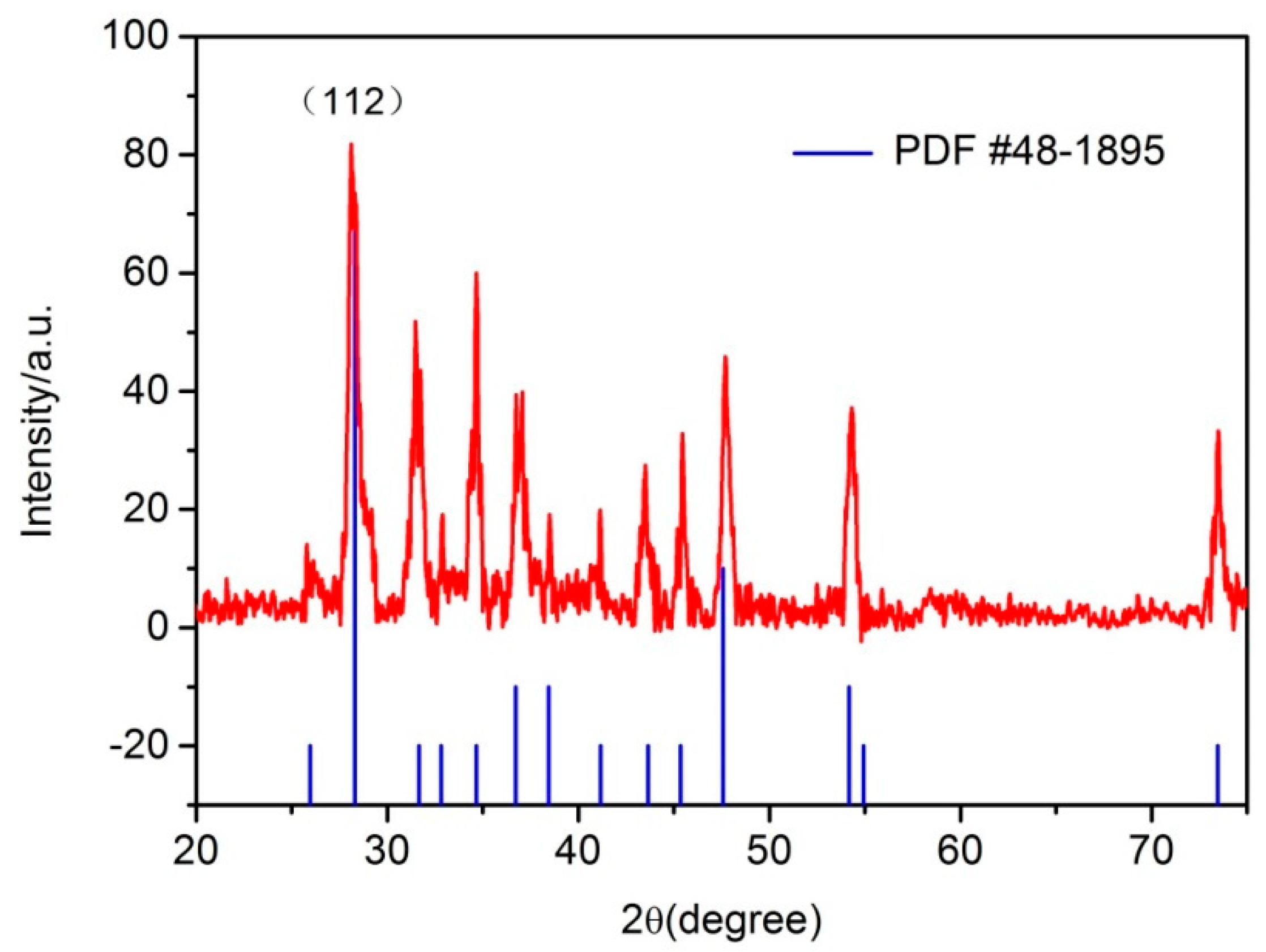
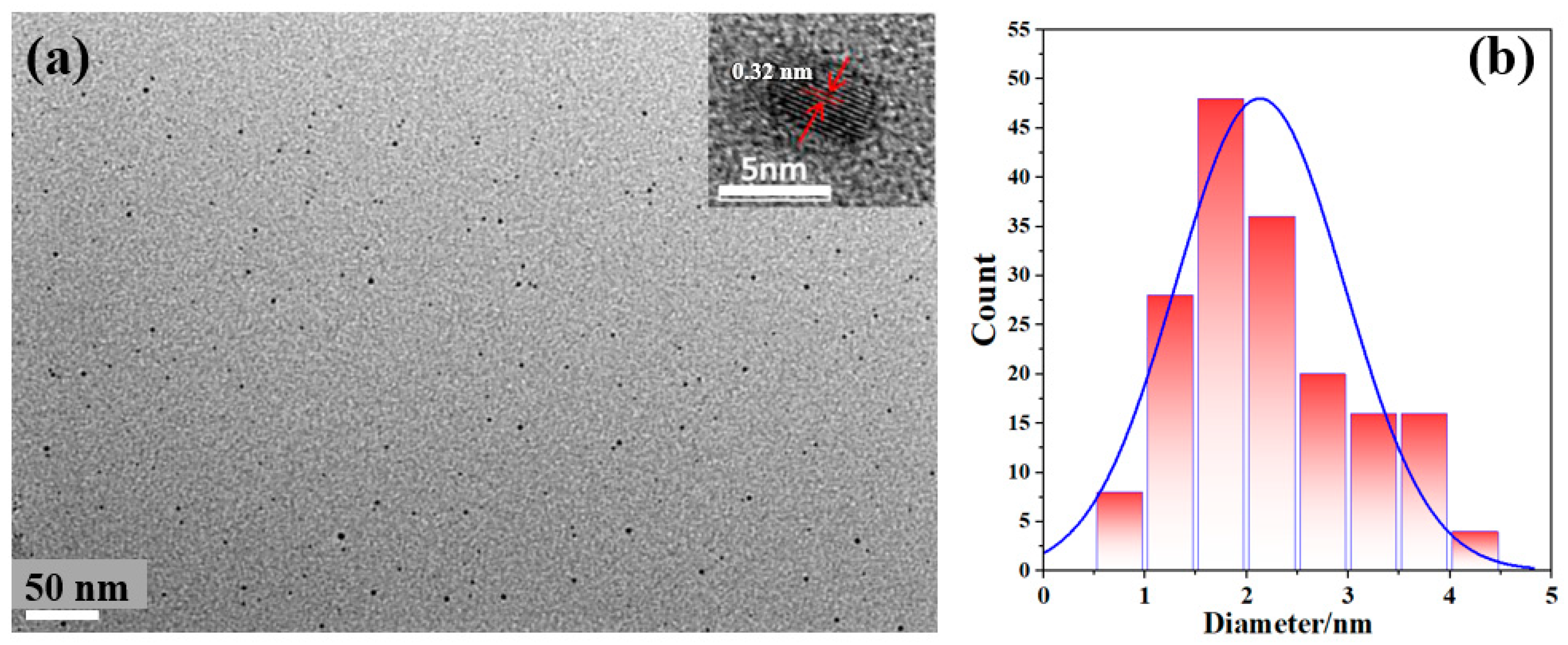
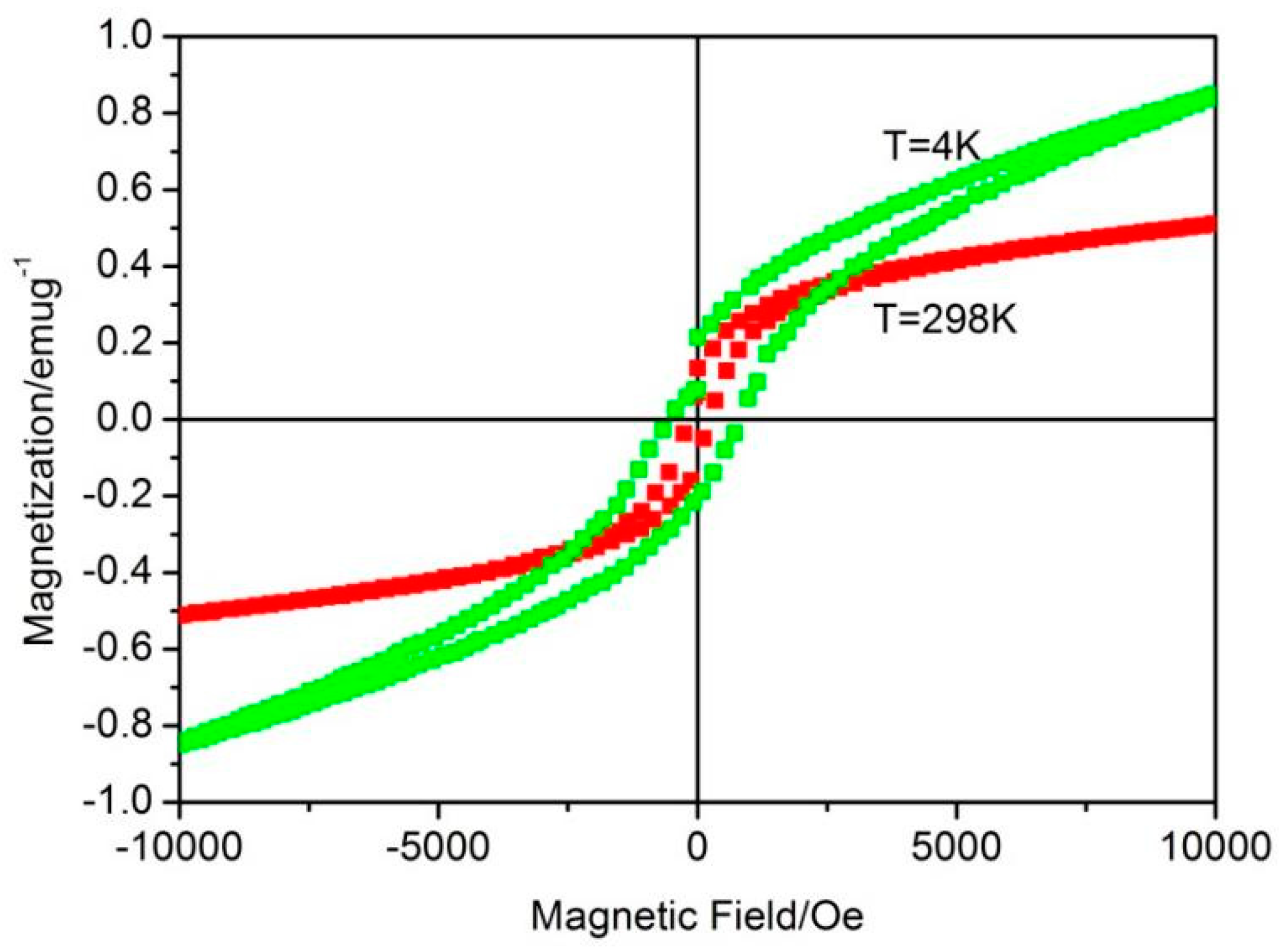
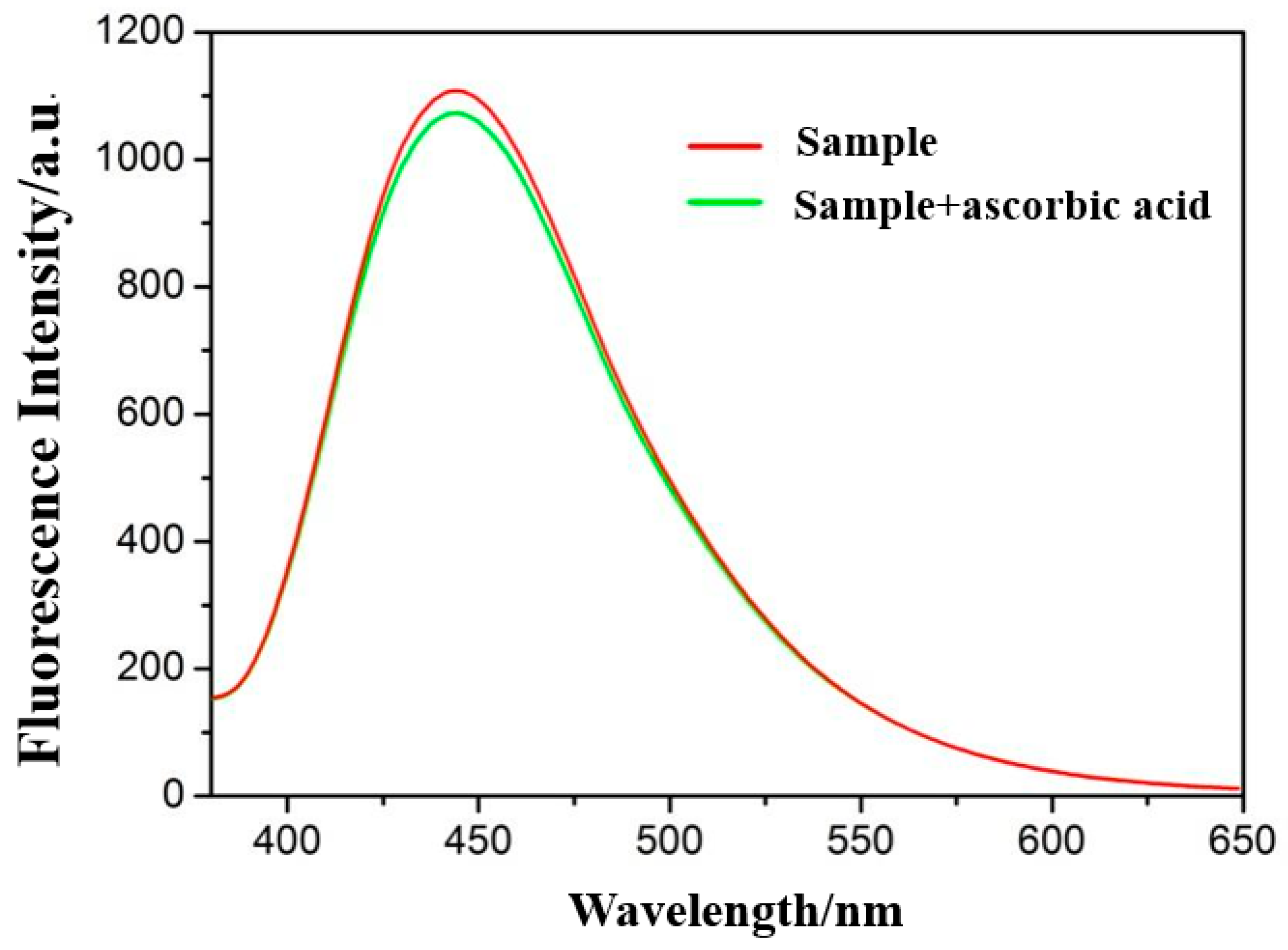
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreda-Piñeiro, A.; Romarís-Hortas, V.; Bermejo-Barrera, P. A review on iodine speciation for environmental, biological and nutrition fields. J. Anal. At. Spectrom. 2011, 26, 2107–2152. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Boelaert, K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015, 3, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Frizzarin, R.M.; Aguado, E.; Portugal, L.A.; Moreno, D.; Estela, J.M.; Rocha, F.R.P.; Cerdà, V. A portable multi-syringe flow system for spectrofluorimetric determination of iodide in seawater. Talanta 2015, 144, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Hetzel, B.S. Eliminating iodine deficiency disorders--the role of the International Council in the global partnership. Bull. World Health Organ. 2002, 80, 410–417. [Google Scholar] [PubMed]
- Zimmermann, M.B. Iodine Deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef]
- Rani, P.; Sindhu, J.; Kumar, S. 5-Hydroxydibenzo[a,i]phenazine-8,13-dione: A selective and sensitive colorimetric and fluorescent ‘turn-off’ sensor for iodide ion. J. Mol. Struct. 2023, 1275, 134621. [Google Scholar] [CrossRef]
- Hwang, C.; Kwak, T.; Kim, C.H.; Kim, J.H.; Park, S.J. Quantitative and rapid detection of iodide ion via electrolyte-gated IGZO thin-film transistors. Sens. Actuators B Chem. 2022, 353, 131144. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Wang, Y.; Xiao, S.; Wang, H.; Wang, J.-H.; Feng, L. Rapid detection of Cr(VI) ions based on cobalt(II)-doped carbon dots. Biosens. Bioelectron. 2017, 87, 46–52. [Google Scholar] [CrossRef]
- Kumari, N.; Hasan, M.A.; Ward, B.D.; Mishra, L. Reactivity of Tetrabutylammonium Iodide with a Heteronuclear 6Copper(II)–4Na(I) Complex: Selective Recognition of Iodide Ion. Ind. Eng. Chem. Res. 2013, 52, 15007–15014. [Google Scholar] [CrossRef]
- Chen, J.; Lin, Q.; Li, Q.; Li, W.-T.; Zhang, Y.-M.; Wei, T.-B. A highly selective colorimetric chemosensor for detection of iodide ions in aqueous solution. RSC Adv. 2016, 6, 86627–86631. [Google Scholar] [CrossRef]
- Mansha, M.; Khan, S.A.; Aziz, A.; Khan, A.Z.; Ali, S.; Khan, M. Optical Chemical Sensing of Iodide Ions: A Comprehensive Review for the Synthetic Strategies of Iodide Sensing Probes, Challenges, and Future Aspects. Chem. Rec. 2022, 22, e202200059. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Barrera, P.; Fernandez-Sanchez, L.M.; Aboal-Somoza, M.; Anllo-Sendin, R.M.; Bermejo-Barrera, A. Indirect atomic absorption spectrometry (IAAS) as a tool for the determination of iodide in infant formulas by precipitation of AgI and redissolution with cyanide. Microchem. J. 2001, 69, 205–211. [Google Scholar] [CrossRef]
- Bichsel, Y.; von Gunten, U. Determination of iodide and iodate by ion chromatography with postcolumn reaction and UV/visible detection. Anal. Chem. 1999, 71, 34–38. [Google Scholar] [CrossRef]
- Malon, A.; Radu, A.; Qin, W.; Qin, Y.; Ceresa, A.; Maj-Zurawska, M.; Bakker, E.; Pretsch, E. Improving the detection limit of anion-selective electrodes: An iodide-selective membrane with a nanomolar detection limit. Anal. Chem. 2003, 75, 3865–3871. [Google Scholar] [CrossRef]
- Ito, K.; Ichihara, T.; Zhuo, H.; Kumamoto, K.; Timerbaev, A.R.; Hirokawa, T. Determination of trace iodide in seawater by capillary electrophoresis following transient isotachophoretic preconcentration: Comparison with ion chromatography. Anal. Chim. Acta 2003, 497, 67–74. [Google Scholar] [CrossRef]
- Zhang, R.X.; Li, P.F.; Zhang, W.J.; Li, N.; Zhao, N. A highly sensitive fluorescent sensor with aggregation-induced emission characteristics for the detection of iodide and mercury ions in aqueous solution. J. Mater. Chem. C 2016, 4, 10479–10485. [Google Scholar] [CrossRef]
- Nabavi, S.; Alizadeh, N. A highly sensitive and selective turn-on fluorescence sensor for iodide detection based on newly synthesized oligopyrrole derivative and application to real samples. Sens. Actuators B Chem. 2014, 200, 76–82. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Q.; Liu, Y.; Li, H.; Zhang, Y.; Yao, S. A dual-signal readout sensor for highly sensitive detection of iodide ions in urine based on catalase-like reaction of iodide ions and N-doped C-dots. Sens. Actuators B Chem. 2017, 250, 429–435. [Google Scholar] [CrossRef]
- Chang, L.; Chen, Y.; Meng, Z.; Yang, Z.; Qin, J.; Zhou, J.; Dai, C.; Ji, X.; Qin, T.; Dou, X.; et al. Zinc Porphyrin Mixed with Metal Organic Framework Nanocomposites and Silver Nanoclusters for the Electrochemiluminescence Detection of Iodide. ACS Appl. Nano Mater. 2024, 7, 9031–9040. [Google Scholar] [CrossRef]
- Yuan, X.; Mi, X.; Liu, C.; Zhang, Z.; Wei, X.; Wang, D.; Tan, X.; Xiang, R.; Xie, W.; Zhang, Y. Ultrasensitive iodide detection in biofluids based on hot electron-induced reduction of p-Nitrothiophenol on Au@Ag core-shell nanoparticles. Biosens. Bioelectron. 2023, 235, 115365. [Google Scholar] [CrossRef]
- Fukushima, Y.; Aikawa, S. Colorimetric detection of iodide ion by a nuclear fast red-based Hg2+ complex in aqueous media. Tetrahedron Lett. 2021, 67, 152877. [Google Scholar] [CrossRef]
- Goh, H.; Nam, T.K.; Singh, A.; Singh, N.; Jang, D.O. Dipodal colorimetric sensor for Ag+ and its resultant complex for iodide sensing using a cation displacement approach in water. Tetrahedron Lett. 2017, 58, 1040–1045. [Google Scholar] [CrossRef]
- Joshi, R.J.; Varu, H.L.; Bhalodia, J.J.; Ambasana, M.A.; Bapodra, A.H.; Kapuriya, N.P. Highly selective fluorescence sensor based on azido pyrazole-chalcone conjugates for rapid detection of iodide ion. Results Chem. 2024, 7, 101409. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Zhang, J.; Huo, K.; Gu, J.; Zhou, Y.; Liu, Y.; Liu, Y.; Liu, X. Bimetallic-based colorimetric sensor for highly selective, stable and sensitive detection of iodide ions. Microchem. J. 2024, 199, 110098. [Google Scholar] [CrossRef]
- Saha, C.; Ghosh, S.K.; Kumari, P.; Perla, V.K.; Singh, H.; Mallick, K. Electrocatalytic efficiency of carbon nitride supported gold nanoparticle based sensor for iodide and cysteine detection. Anal. Biochem. 2024, 696, 115660. [Google Scholar] [CrossRef]
- Wei, M.-J.; Wei, Z.-Q.; Li, J.; Yu, L.; Zhang, S.-F.; Cheng, F.; Li, H.-Y.; Kong, F.-Y.; Wang, W. Covalent organic framework with extraordinary intrinsic catalytic activity for electrochemical sensing of iodide ions. Microchem. J. 2024, 200, 110399. [Google Scholar] [CrossRef]
- Zhong, X.; Li, C.; Chen, H.; Deng, P. Lanthanide doped metal−organic framework: Novel turn-on fluorescent sensing of iodide in kelp and seawater samples. Microchem. J. 2024, 202, 110758. [Google Scholar] [CrossRef]
- Silvi, S.; Credi, A. Luminescent sensors based on quantum dot–molecule conjugates. Chem. Soc. Rev. 2015, 44, 4275–4289. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, T.; Wang, S.; Hou, X. Semicondutor quantum dots-based metal ion probes. Nanoscale 2014, 6, 43–64. [Google Scholar] [CrossRef]
- Sheng, X.; Liu, Y.; Wang, Y.; Li, Y.; Wang, X.; Wang, X.; Dai, Z.; Bao, J.; Xu, X. Cesium Lead Halide Perovskite Quantum Dots as a Photoluminescence Probe for Metal Ions. Adv. Mater. 2017, 29, 1700150. [Google Scholar] [CrossRef]
- Wang, L.; Kang, X.; Pan, D. Gram-Scale Synthesis of Hydrophilic PEI-Coated AgInS2 Quantum Dots and Its Application in Hydrogen Peroxide/Glucose Detection and Cell Imaging. Inorg. Chem. 2017, 56, 6122–6130. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, M.; Zhu, T.; Tang, X.; Han, S.; Huang, W.; Shi, Y.; Liu, A. The synthesis of water-dispersible zinc doped AgInS2 quantum dots and their application in Cu2+ detection. J. Lumin. 2017, 192, 547–554. [Google Scholar] [CrossRef]
- Datt, G.; Manivel Raja, M.; Abhyankar, A.C. Steering of Magnetic Interactions in Ni0.5Zn0.5Fe2–x(Mn)xO4 Nanoferrites via Substitution-Induced Cationic Redistribution. J. Phys. Chem. C 2021, 125, 10693–10707. [Google Scholar] [CrossRef]
- Abdullah, M.; Alahmari, S.D.; Aman, S.; Ejaz, S.R.; Haleem, Y.A.; Gouadria, S.; Al-Sehemi, A.G.; Henaish, A.M.A.; Ahmad, Z.; Farid, H.M.T. Facile fabrication of AgFeS2 nanostructure via hydrothermal route for supercapacitor application. J. Energy Storage 2024, 77, 109875. [Google Scholar] [CrossRef]
- Foner, S. Versatile and Sensitive Vibrating-Sample Magnetometer. Rev. Sci. Instrum. 1959, 30, 548–557. [Google Scholar] [CrossRef]
- Faraji, M.; Yamini, Y.; Rezaee, M. Magnetic nanoparticles: Synthesis, stabilization, functionalization, characterization, and applications. J. Iran. Chem. Soc. 2010, 7, 1–37. [Google Scholar] [CrossRef]
- Wang, X.; Tang, H.; Tian, X.M.; Zeng, R.Y.; Jia, Z.J.; Huang, X.H. Sunlight and UV driven synthesis of Ag nanoparticles for fluorometric and colorimetric dual-mode sensing of ClO. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117996. [Google Scholar]
- Qiao, G.X.; Liu, L.; Hao, X.X.; Zheng, J.K.; Liu, W.Q.; Gao, J.W.; Zhang, C.C.; Wang, Q.M. Signal transduction from small particles: Sulfur nanodots featuring mercury sensing, cell entry mechanism and in vitro tracking performance. Chem. Eng. J. 2020, 382, 122907. [Google Scholar] [CrossRef]
- Gu, J.P.; Li, X.Q.; Zhou, G.F.; Liu, W.Q.; Gao, J.W.; Wang, Q.M. A novel self-calibrating strategy for real time monitoring of formaldehyde both in solution and solid phase. J. Hazard. Mater. 2020, 386, 121883. [Google Scholar] [CrossRef]
- Zhang, J.L.; Yang, H.J.; Shen, G.X.; Cheng, P.; Zhang, J.Y.; Guo, S.W. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef]

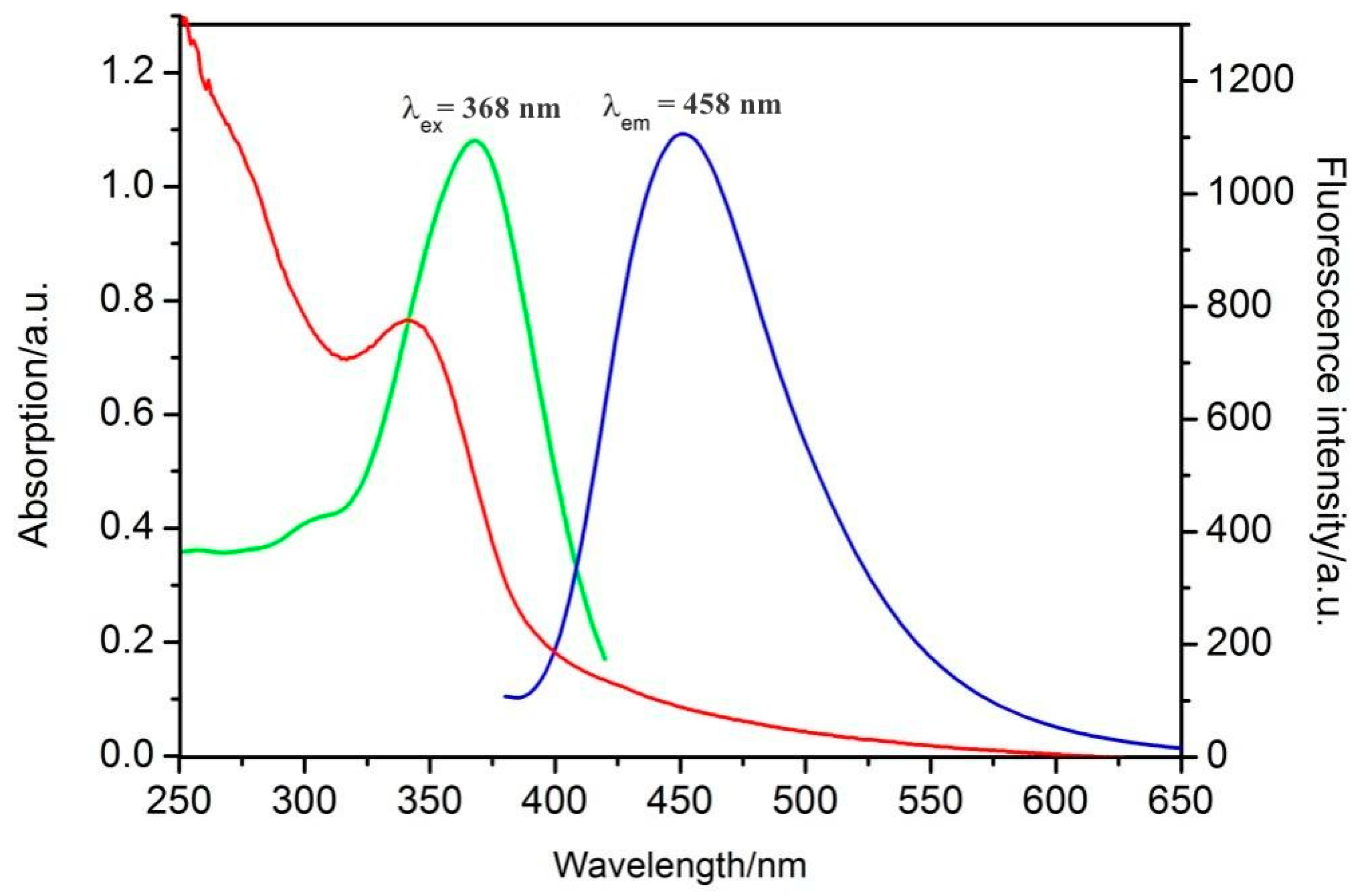
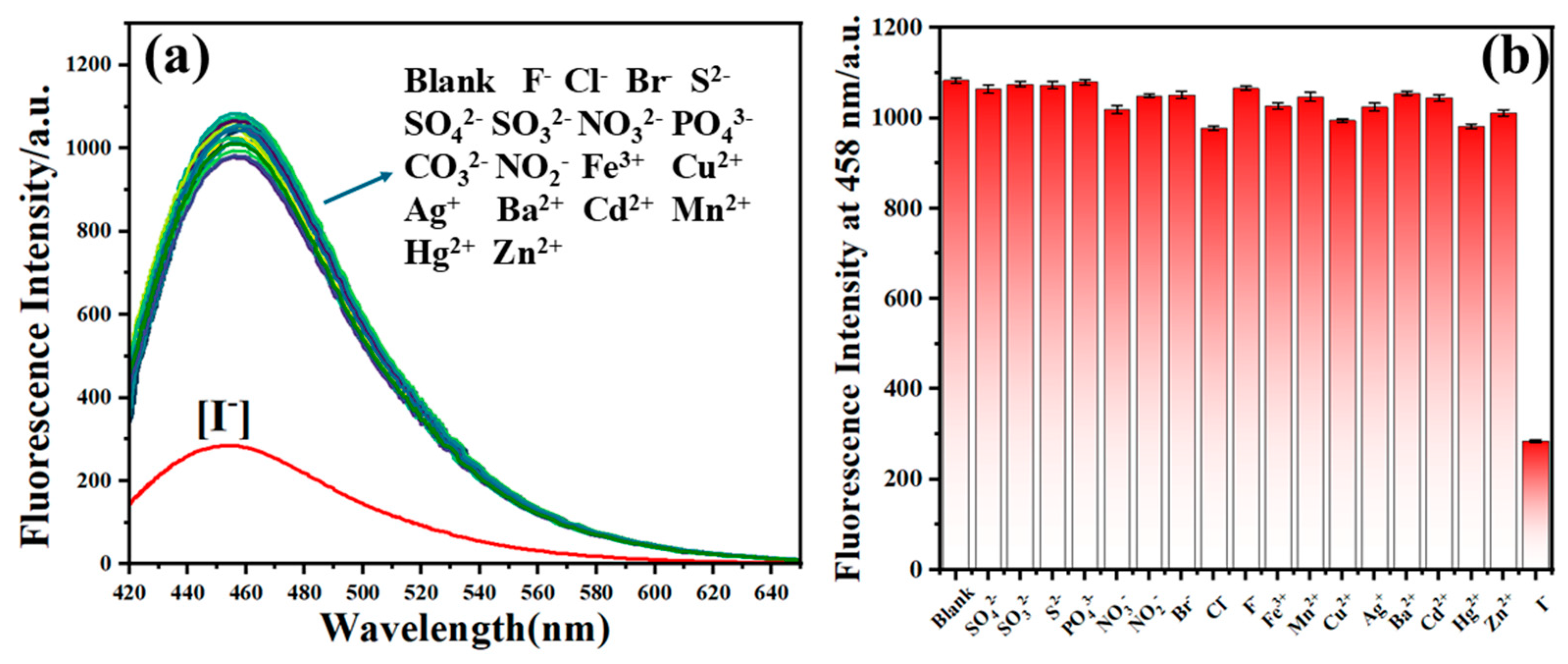
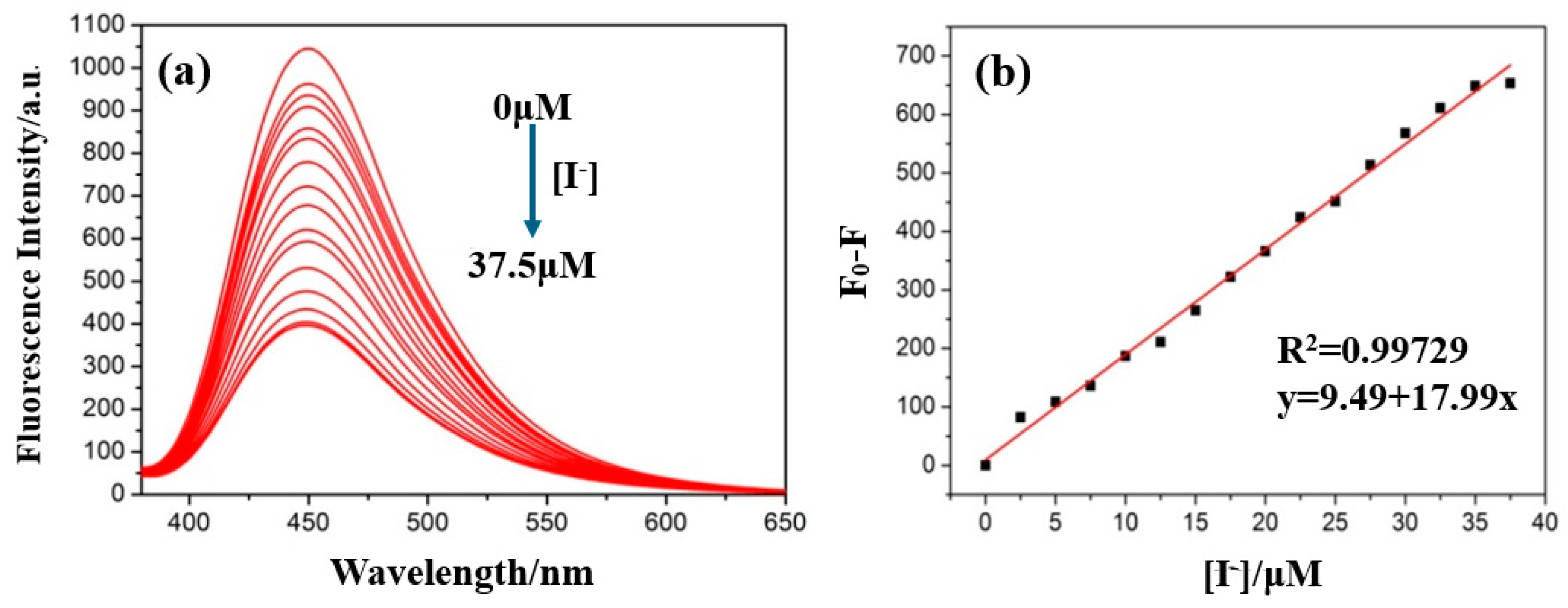
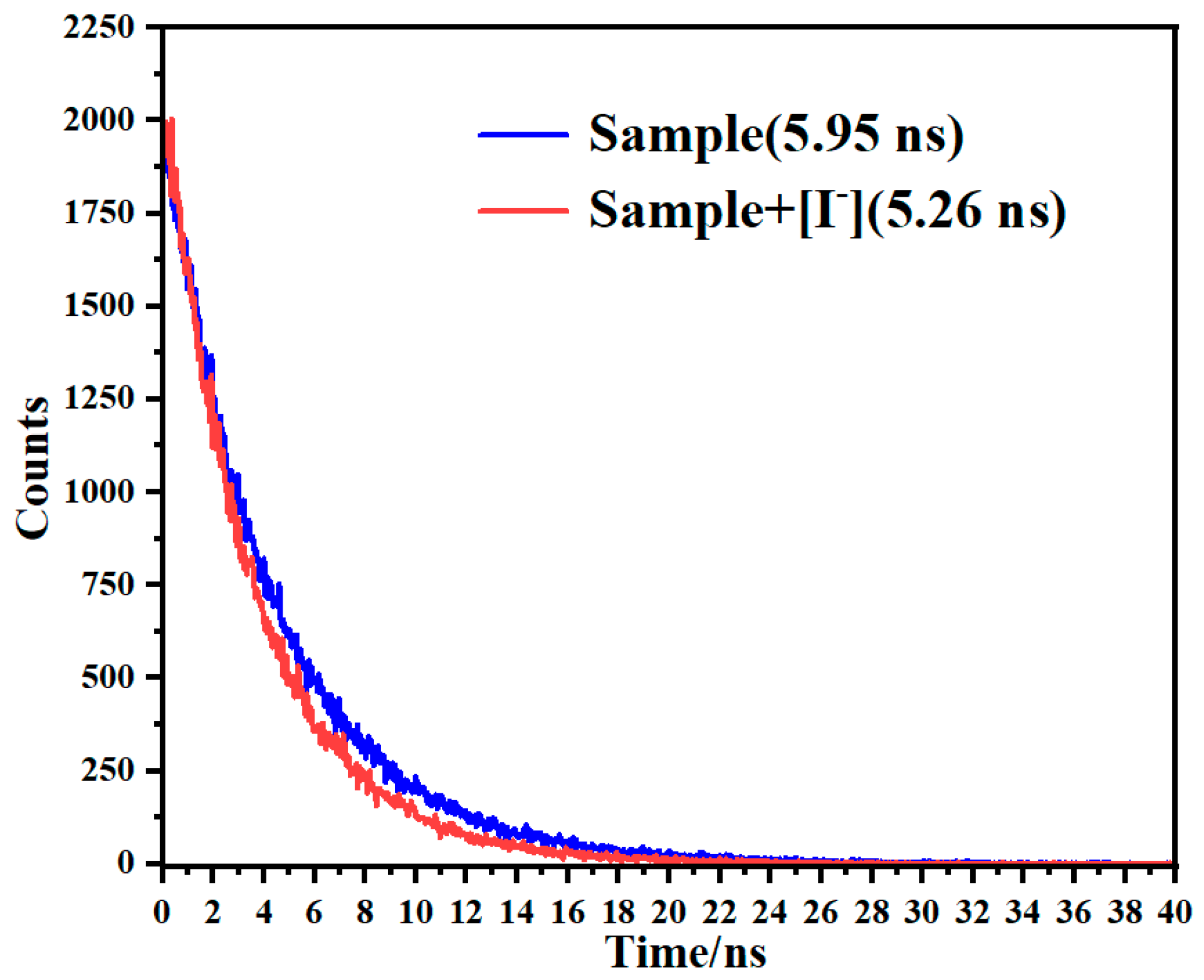

| Samples | Added (μM) | Found (μM) | Recovery [%] | RSD (n = 3, %) |
|---|---|---|---|---|
| 1 | 5 | 5.02 | 100.4% | 2.52% |
| 2 | 15 | 15.21 | 101.4% | 1.36% |
| 3 | 20 | 19.87 | 99.35% | 2.98% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wu, N.; Wang, W.; Hu, Y.; Luo, Z.; Zheng, Y.; Wang, Q. Fast and Sensitive Determination of Iodide Based on Ternary Chalcogenides Nanoparticles. Molecules 2024, 29, 4751. https://doi.org/10.3390/molecules29194751
Wang Z, Wu N, Wang W, Hu Y, Luo Z, Zheng Y, Wang Q. Fast and Sensitive Determination of Iodide Based on Ternary Chalcogenides Nanoparticles. Molecules. 2024; 29(19):4751. https://doi.org/10.3390/molecules29194751
Chicago/Turabian StyleWang, Zhitai, Nengtao Wu, Weihao Wang, Yaozheng Hu, Zhijie Luo, Yuhui Zheng, and Qianming Wang. 2024. "Fast and Sensitive Determination of Iodide Based on Ternary Chalcogenides Nanoparticles" Molecules 29, no. 19: 4751. https://doi.org/10.3390/molecules29194751
APA StyleWang, Z., Wu, N., Wang, W., Hu, Y., Luo, Z., Zheng, Y., & Wang, Q. (2024). Fast and Sensitive Determination of Iodide Based on Ternary Chalcogenides Nanoparticles. Molecules, 29(19), 4751. https://doi.org/10.3390/molecules29194751




