Abstract
Reynoutria ciliinervis (Nakai) Moldenke (R. ciliinervis) root, a traditional Chinese medicine, was found to exhibit remarkable pharmacological properties through a series of comprehensive investigations. Our study commenced with a qualitative phytochemical analysis that identified 12 bioactive compounds within the plant. Subsequently, utilizing ultraviolet-visible spectrophotometry, the methanol extract emerged as the optimal solvent extract, which was abundant in diverse classes of compounds such as carbohydrates, phenolics, steroids, alkaloids, phenolic acids, and tannins. In vitro antioxidant assays underscored the exceptional free radical scavenging, metal ion chelation, hydrogen peroxide scavenging, singlet oxygen quenching, and β-carotene bleaching capabilities of the methanol extract, significantly outperforming other solvent extracts. Further ultra high-performance liquid chromatography–electrospray ionization–quadrupole time of flight–mass spectrometry analysis revealed the presence of 45 compounds, predominantly anthraquinones and phenolics, in the methanol extract. The extract demonstrated robust stability under various conditions, including high temperatures, varying pH levels, and simulated gastrointestinal digestion as well as efficacy in inhibiting the oxidation in edible oils. Acute toxicity tests in mice confirmed the safety of the methanol extract and provided a valuable dosage reference for future studies. Importantly, high-dose methanol extract exhibited a significant pre-protective effect against D-galactosamine-induced liver injury in rats, as evidenced by reduced alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase, malondialdehyde levels, and elevated catalase and albumin levels. These findings suggest a potential role for the methanol extract of R. ciliinervis root in treating oxidative stress-related disorders, highlighting the plant’s immense medicinal potential. Our research offers a thorough evaluation of the bioactive components, antioxidant properties, stability, and liver-protecting effects of the methanol extract, setting the stage for deeper investigation and potential clinical applications.
1. Introduction
Reynoutria ciliinervis (Nakai) Moldenke (R. ciliinervis), also known by its two important synonyms Polygonum ciliinerve and Pleuropterus ciliinervis, is a perennial herb. This versatile plant thrives abundantly in various regions of China, including Shaanxi, Gansu, Sichuan, Guizhou, and Jilin provinces, predominantly inhabiting valley shrubs and crevices of rocky mountain slopes at altitudes spanning from 700 to 1300 m. Its root (Figure 1), renowned for their medicinal properties, exhibits a color and luster strikingly similar to cinnabar, earning it the alias “Zhushaqi.” R. ciliinervis root, a celebrated traditional Chinese medicine hailing from the esteemed Taibai Mountain, boasts a rich historical background extending over a millennium. Its medicinal significance and application are first documented in the Song Dynasty’s renowned Bencao Tujing (Illustrated Classics of Materia Medica) [1]. Characterized by its cooling nature and bitter taste tinged with a subtle astringency, R. ciliinervis harmoniously aligns with the meridians of the stomach, spleen, heart, and liver. This herb possesses a multitude of therapeutic effects, including astringency and acid inhibition, fostering blood circulation to dispel stagnation, arresting bleeding, and facilitating tissue regeneration. Furthermore, it regulates qi to alleviate pain and purges heat and toxins from the body [2]. In the realm of folk medicine, R. ciliinervis is extensively employed to address a diverse array of ailments such as epigastric discomfort, diarrhea, swollen sore throats, rheumatic arthralgia, trauma and injuries resulting from falls and blows as well as external trauma with bleeding [3].
Figure 1.
Morphology of R. ciliinervis root.
R. ciliinervis root comprises a diverse array of chemical constituents, primarily anthraquinones, stilbenes, tannins, and polysaccharides, as evidenced by the literature [4]. In recent years, the most extensive research has centered on anthraquinones [5] and polysaccharides [6] within R. ciliinervis root. R. ciliinervis root boasts numerous pharmacological benefits, including antiviral, antibacterial, anti-inflammatory, antitumor, immunomodulatory, and antioxidant properties, as documented in various studies [7,8,9]. However, the primary research emphasis has been on its anti-inflammatory, antitumor, and antiviral activities [10] with relatively fewer reports focusing on its antioxidant effects. Furthermore, most studies tend to analyze a single phytochemical component in isolation. For instance, one study extracted crude polysaccharides from R. ciliinervis root to investigate their antioxidant potential both in vivo and in vitro [5]. Another report examined the impact of R. ciliinervis root’s total anthraquinone on the antioxidant capacity of S180 tumor-bearing mice. Additionally, researchers examined thoroughly the regulatory effects of R. ciliinervis root’s total anthraquinone on the immune and antioxidant functions of H22 tumor-bearing mice, revealing its significant antioxidant capacity [11,12]. Wang et al. further assessed the free radical scavenging abilities of R. ciliinervis root’s total tannin and polysaccharides through a chemical simulation system, emphasizing their potent antioxidant effects [13,14]. While these studies confirm R. ciliinervis root’s antioxidant activity, comprehensive and systematic research, encompassing both in vitro and in vivo evaluations, is crucial for its optimal development and utilization.
R. ciliinervis root possesses significant application potential and has garnered extensive attention in clinical practice. For instance, there are documented cases where R. ciliinervis root, as the primary component, was utilized in self-formulated stomach-protecting capsules to treat 100 patients with chronic atrophic gastritis, achieving a remarkable total effectiveness rate of 98% [15]. Additionally, a study investigating the use of traditional Chinese medicine primarily composed of R. ciliinervis root in treating 180 patients with chronic gastritis reported a staggering clinical cure rate of 95.60% [16]. Furthermore, R. ciliinervis root tablets were employed in treating 110 patients with acute bacterial dysentery, yielding a total effectiveness rate of 85.45% [17]. Despite these promising clinical outcomes, academic research on R. ciliinervis root remains scarce and is in its nascent stages. The present study aims to conduct a comprehensive analysis of R. ciliinervis root’s components, both qualitatively and quantitatively, ascertain its antioxidant prowess through in vitro experiments, and identify the extract with the optimal content and strongest antioxidant activity. Utilizing UHPLC-MS technology, the chemical composition of the extract is meticulously characterized, its oxidative stability is rigorously evaluated, and its potential hepatoprotective effects in rats is extensively explored. This endeavor contributes to broadening the application of R. ciliinervis root as an antioxidant and propels forward its therapeutic research in oxidative stress-related diseases.
2. Results and Discussion
2.1. Preliminary Identification of Phytochemicals
Table S1 showcases a qualitative phytochemical analysis of R. ciliinervis root, providing valuable insights into its chemical makeup. Notably, the analysis highlights the absence of saponins, alkaloids, and cyanogenic glycosides, emphasizing the exclusion of these potentially bioactive or potentially harmful compounds. However, two captivating findings emerged from the conducted experiments that warrant further scrutiny. Firstly, the detection of flavonoids presented an intriguing paradox with a mix of two positive and two negative results. This inconsistency might stem from the minute concentrations of flavonoids inherent in the plant material. Additionally, the varying sensitivity and detection limits of the utilized analytical methods—including the highly sensitive Shinoda test and lead acetate test, in contrast to the less sensitive AlCl3 test and alkaline reagent test—could have contributed to this discrepancy in results. To conclusively resolve this enigma, a quantitative assessment of flavonoid content is paramount. Secondly, the literature consistently cites R. ciliinervis root as a copious source of anthraquinones, predominantly present as anthraquinone glycosides, with lower concentrations of free anthraquinones [18]. The experiments were conducted with the existing literature, as all anthraquinone tests yielded positive outcomes, validating their presence in R. ciliinervis root. Nevertheless, the specific forms and chemical structures of these anthraquinones remain enigmatic. To unravel these mysteries, the employment of advanced analytical tools, such as UHPLC-MS, is indispensable.
2.2. Extraction Yields
It is universally recognized that the selection of solvents utilized in the extraction of chemical compounds from plants plays a pivotal role in determining both the yield and biological potency of the resulting extracts. The strategic use of solvents with varying polarities significantly enhances mass transfer efficiency, expanding the range of extraction capabilities [19]. In this research work, the extraction of bioactive compounds from R. ciliinervis root was undertaken utilizing four distinct solvents: water, methanol, ethanol, and an 80% ethanol solution. The primary objective of the study was to characterize the antioxidant activity of these extracts. The experimental findings revealed a diverse range of extraction yields, varying from 21.97 ± 0.09% to 25.50 ± 0.12%, as presented in Table 1. A meticulous comparison of the extraction efficiencies among the solvents emphasized the superiority of water as the solvent of choice, yielding the highest percentage of extract. Notably, the 80% ethanol extract closely followed suit, demonstrating a yield comparable to that of the water extract, highlighting its commendable extraction efficiency. In contrast, the methanol and ethanol extracts exhibited a consistent, albeit slightly lower, yield than the aforementioned solvents, maintaining a relatively high level overall. The exceptional yield of the water extract can be attributed to the abundance of highly polar and water-soluble compounds naturally present in R. ciliinervis root, such as carbohydrates and glycosides. However, it is crucial to acknowledge that water extracts may also contain a significant quantity of lesser-studied components, including pigments and pectin, which could potentially complicate the identification and accurate assessment of the biological activities of the targeted compounds. Therefore, while water emerges as an effective solvent for maximizing yield, it is imperative to consider the potential confounding factors when interpreting the results and evaluating the biological significance of the extracted compounds.

Table 1.
Extraction yields of R. ciliinervis root extracted with different solvents.
2.3. Content of Active Ingredients
2.3.1. Total Carbohydrate Content (TCC)
Carbohydrates are the cornerstone of living cell structures, functioning as the primary source of energy essential for sustaining cellular activities and regulating intricate biological processes. Beyond their fundamental energetic role, carbohydrates have garnered substantial attention for their myriad biological activities and potential therapeutic benefits in addressing various diseases. Notably, plant polysaccharides have emerged as a promising frontier in research, exhibiting remarkable pharmacological effects [20,21]. This comprehensive study presents a thorough evaluation of the TCC in diverse extracts. The adopted methodology allows for the simultaneous quantification of both carbohydrates and glycosides, offering a more comprehensive and nuanced analysis [22]. The results, as presented in Table 2, reveal a TCC range extending from 676.51 ± 3.63 to 749.5 ± 7.06 mg glucose equivalents (GE)/g extract, highlighting the substantial variation in the content of carbohydrates and glycosides among different extracts. These findings align with numerous reports that document the isolation of multiple glycosides, primarily anthraquinone glycosides, from R. ciliinervis root [23,24]. A meticulous study aimed at optimizing the extraction of anthraquinone from R. ciliinervis root has unequivocally demonstrated that 80% ethanol yields the highest total anthraquinone content [25]. This exceptional performance can be attributed to the high polarity of anthraquinone glycosides, which are abundant in R. ciliinervis root and are most efficiently extracted using 80% ethanol [26]. Consequently, the TCC of the 80% ethanol extract comprises both carbohydrates and glycosides, whereas the methanol extract primarily contains glycosides.

Table 2.
Total carbohydrate content (TCC), total protein content (TProC), total phenolic content (TPheC), total steroid content (TSC), total alkaloid content (TAC), total flavonoid content (TFC), total phenolic acid content (TPAC), total tannin content (TTanC), gallotannin content (GC), and condensed tannin content (CTC) of R. ciliinervis root extracted with different solvents.
2.3.2. Total Protein Content (TProC)
Plant-based proteins are exceedingly beneficial to health, owing to their low fat and cholesterol content, low calorie density, and abundant dietary fiber, making them an invaluable source of nutrition that is readily digested and absorbed by the human body. Furthermore, plant proteins possess antioxidant properties, effectively scavenging free radicals, and they play a pivotal role in maintaining gastrointestinal health and immune regulation [27]. The study has demonstrated that the aqueous extract of R. ciliinervis root exhibits a substantial TProC of 1233.90 ± 14.71 mg bovine serum albumin equivalents (BSAE)/g extract (Table 2). This may initially appear counterintuitive. However, it is crucial to understand that the extract is derived from raw medicinal material and is a concentrated product. The extraction efficiency achieved with water as the solvent is 25.50%, which means that 1 g of the extract is equivalent to 3.922 g of the raw medicinal material. To address the apparent concern of the content exceeding 100%, the calculation has been revised to express the TProC as 1233.90 mg BSAE per 3.922 g of raw medicinal material. This adjustment ensures accuracy and removes any confusion. Nevertheless, for the sake of brevity and clarity in presenting the experimental results, the extract is typically used as the basis for calculations.
This notable protein content not only has the potential to bolster patients’ overall health but also facilitates the absorption and resolution of inflammatory edema while mitigating pain responses associated with inflammation. Research indicates that the aqueous decoction of R. ciliinervis root exhibits significant analgesic, anti-inflammatory, and immunomodulatory effects on mouse ear edema induced by xylene or croton oil [8,28]. This may be attributed to the potent pharmacological effects derived from the high protein content in the decoction, and these findings further support the use of R. ciliinervis root in traditional Chinese medicine for treating conditions such as stomach pain, diarrhea, and rectal bleeding.
2.3.3. Total Phenolic Content (TPheC)
Plant polyphenols are replete with diverse pharmacological activities and are widely recognized for their myriad of health benefits in humans. These compounds possess a robust antioxidant capacity, which has been demonstrated to exert favorable effects on a wide range of health conditions, including antibacterial and anti-inflammatory properties. They have also shown promise as adjunctive therapies in the management of diseases such as cancer, diabetes, and cardiovascular disorders [29]. As demonstrated by the experimental findings presented in Table 2, the TPheC values of various extracts range from 89.18 ± 1.98 to 180.6 ± 1.60 mg gallic acid equivalents (GAE)/g extract. Notably, polyphenols derived from blueberries and grapes have been found to modulate intestinal flora, lower serum cholesterol levels, mitigate insulin resistance, and improve fatty liver degeneration and chronic inflammation [30,31]. Experimental data further suggest that solvents containing alcohol are more effective in extracting polyphenols. Specifically, the TPheC values of ethanol or 80% ethanol extracts are approximately twice those of water extracts, indicating that the inclusion of a certain proportion of ethanol during the extraction process can significantly enhance the yield and thus the potency of polyphenols.
2.3.4. Total Steroid Content (TSC)
Steroids, naturally abundant compounds, display a myriad of biological activities such as anti-inflammatory, antitumor, hormone-regulating, and immunosuppressive properties [32]. A comprehensive analysis was undertaken to assess the total steroidal content (TSC) present in four distinct solvent extracts of R. ciliinervis root. Experimental outcomes revealed notable variations in TSC among the tested solvent extracts (Table 2). Specifically, the methanol extract exhibited the highest TSC, reaching 3.44 ± 0.08 mg oleic acid equivalents (OAE)/g extract. Furthermore, the literature review illuminated that only a single steroid compound, β-sitosterol, has been isolated from R. ciliinervis root [10]. This limited repertoire of steroidal compounds in R. ciliinervis root provides a plausible explanation for the relatively low TSC levels observed in the study.
2.3.5. Total Alkaloid Content (TAC)
Alkaloids, also referred to as pseudo-bases due to their properties resembling those of alkalis, are ubiquitous in the plant kingdom and are a significant component of Chinese herbal medicine, which are known for their nitrogen-containing structure and potent bioactivity [33,34]. In the analysis presented in Table 2, notably, the water extract yielded the highest concentration of alkaloids, which was measured at 12.34 ± 0.89 mg berberine hydrochloride equivalents (BHE)/g extract. It is noteworthy that only two specific alkaloids: pyrrolezanthine-6-methyl-ether and N-trans-feruloyl-3-methoxytyramine—have been reported in the literature pertaining to the plant [35,36,37]. This finding not only validates the accuracy and precision of our experimental methodology but also emphasizes the fact that R. ciliinervis root contains alkaloids in relatively low concentrations.
2.3.6. Total Flavonoid Content (TFC)
Flavonoids, a versatile class of natural polyphenols, are prevalent in diets, and they are abundantly found in vegetables, fruits, grains, and even beverages such as tea. They serve as essential dietary components for humans, providing numerous health benefits [38]. The remarkable versatility of flavonoids extends far beyond their nutritional value, as they are also incorporated into health supplements, pharmaceutical formulations, and cosmetic products. Their diverse pharmacological effects, which encompass antibacterial, antioxidant, and anti-inflammatory properties, play a significant role in mitigating the risk of various diseases [39,40,41]. Experimental evaluations have yielded valuable insights into the flavonoid content of various extracts. Specifically, the TFC in these extracts ranged from 2.73 ± 0.16 to 10.84 ± 0.54 mg quercetin equivalents (QE)/g of extract, highlighting the substantial variation among different sources.
Free flavonoids, devoid of sugar moieties, generally tend to be insoluble in water but readily dissolve in organic solvents like methanol and ethanol. In contrast, flavonoid glycosides, which are flavonoids bound to sugar molecules, display enhanced solubility in solvents such as hot water, methanol, and ethanol. Upon perusing the current scientific literature, it becomes apparent that only a limited number of flavonoids—specifically, kaempferirin, annulatin 3′-O-β-D-xyloside [35,36], quercetin, and quercetin-3-O-glucoside [10], have been isolated from R. ciliinervis root. Based on the qualitative analysis results, the initial hypothesis regarding the low flavonoid content in R. ciliinervis root has been validated.
2.3.7. Total Phenolic Acid Content (TPAC)
This comprehensive study extends beyond the examination of TPheC to encompass an analysis of TPAC with a focus on diverse extracts sourced from R. ciliinervis root. The findings, as detailed in Table 2, reveal a considerable variation in TPAC values, spanning from 3.71 ± 0.23 to 11.05 ± 0.64 mg caffeic acid equivalents (CAE)/g extract. To emphasize the significance of the findings, it is imperative to highlight that the experimental data conclusively verify the presence of phenolic acids in R. ciliinervis root, albeit in relatively low concentrations.
2.3.8. Total Tannin Content (TTanC), Gallotannin Content (GC) and Condensed Tannin Content (CTC)
Tannins, inherently occurring polyphenol compounds found in plants, serve as vital secondary metabolites that bolster the chemical defense mechanisms of plants. They effectively shield plants from the threats posed by pathogens, insects, herbivores, and other potential adversaries. Additionally, tannins play a pivotal regulatory role in the growth and development of plants. Tannins exhibit a broad spectrum of pharmacological effects, and they are renowned for their antioxidant, antibacterial, anthelmintic, antiviral, and anti-inflammatory properties. Broadly speaking, tannins can be categorized into two main groups: hydrolyzable tannins and condensed tannins, with gallotannins being a notable representative of the former category [42,43,44]. To gain a deeper understanding of the tannin content in various extracts of R. ciliinervis root, measurements were conducted for GC, CTC, and TTanC, as outlined in Table 2. Condensed tannins, for instance, display limited solubility in polar organic solvents, whereas methanol or ethanol are commonly used solvents for the extraction of hydrolyzable tannins [45,46]. This explains the higher CTC content observed in the water extract. However, for the extraction of total tannins, the use of alcohol-based solvents is advantageous, ensuring a more efficient extraction process.
This study highlights the immense potential of R. ciliinervis root as a repository of bioactive compounds, such as proteins, carbohydrates, glycosides, and phenolics, each endowed with distinct pharmacological properties. The literature attests to R. ciliinervis root’s abundance in anthraquinones, tannins, and polysaccharides [4]. Notably, the anthraquinone content in R. ciliinervis root extracts has been reported to reach 80.81%, primarily in the form of bound anthraquinones [47]. Furthermore, the polysaccharide extraction rate from R. ciliinervis root purification has achieved 5.1% [6], while an ultrasound-assisted extraction method yielded a 2.0% tannin extraction rate [48]. To date, an impressive 86 compounds have been isolated and identified from R. ciliinervis root, encompassing anthraquinones, stilbenes, flavonoids, organic acids, lignans, and alkaloids [49], which confirms the accuracy of the content determination outcomes. Notably, our research has discovered that extraction with methanol, ethanol, and 80% ethanol yields higher concentrations of active ingredients compared to water extraction. It is crucial to emphasize that numerous qualitative analyses encompass chemical reactions that transcend the realm of any specific component type. These reactions often involve intricate interactions among various molecular entities, making them non-exclusive to any single component being studied. To ensure the authenticity of the results, at least two distinct experimental protocols were devised, minimizing the risk of false positives and enhancing the precision of the detection outcomes. Concurrently, these outcomes not only offer guidance but also provide robust support for quantitative analysis endeavors.
2.4. Antioxidant Activity In Vitro
2.4.1. 1,1-Diphenyl-2-Picrylhydrazyl Radical (DPPH) and 2,2′-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonicacid) Diammonium Salt (ABTS)
Evaluating the antioxidant potency of natural compounds and their extracts through their free radical scavenging capacity is crucial for understanding their therapeutic potential. DPPH and ABTS, two stable free radicals, serve as reliable benchmarks in assessing such capacity, as they undergo reduction and decolorization upon encountering antioxidants. This process is quantitatively measured by assessing the resultant absorbance, which inversely correlates with the antioxidant’s effectiveness [19]. In the DPPH assay, all extracts except the water extract demonstrated superior scavenging capabilities, surpassing the performance of the antioxidant butylated hydroxytoluene (BHT) and even approaching the efficacy of trolox and L-ascorbic acid (Table 3). These findings align with previous research identifying active components in R. ciliinervis root capable of neutralizing DPPH radicals [6,23,37,50], strengthening the observed results. Similar results were also observed in the ABTS assay (Table 3). Interestingly, studies on a closely related plant species, Polygonum multiflorum, have also documented significant ABTS free radical scavenging activity, particularly in its roots, highlighting the prevalence of potent antioxidants within this genus [51]. It is hypothesized that the robust free radical scavenging abilities of R. ciliinervis root may stem from its rich abundance of anthraquinones, their glycosides, and phenolic compounds.

Table 3.
Determination of antioxidant activity of various solvent extracts of R. ciliinervis root using DPPH, ABTS, hydroxyl, and superoxide radicals.
2.4.2. Hydroxyl Radicals and Superoxide Radicals
Evaluating the antioxidant capacity of a compound or plant extract necessitates a thorough examination of the hydroxyl and superoxide radicals generated within the biological system. These radicals, particularly hydroxyl radicals, possess highly reactive chemical properties with hydroxyl radicals exhibiting the most potent reactivity among them. An excess of these radicals in the human body can inflict oxidative damage on vital biomolecules, including proteins and DNA, ultimately disrupting the normal cellular structure and function. This, in turn, accelerates the aging process and predisposes individuals to a myriad of diseases. Consequently, the capacity to neutralize these free radicals serves as a crucial indicator of antioxidant detoxification, effectively mitigating cellular oxidative toxicity [13,52].
Our comprehensive investigation revealed that with the exception of the aqueous extract, all three tested extracts demonstrated remarkable hydroxyl radical scavenging abilities (Table 3). When discussing superoxide radicals, notably, the scavenging abilities of both 80% ethanol and ethanol extracts exceeded those of curcumin by a factor of ten (Table 3). This superior performance might be attributed to the rich content of tannins and polysaccharides present in the extracts, as prior research has emphasized the potent hydroxyl and superoxide radical scavenging abilities of these compounds in R. ciliinervis root [6,12,13,50].
2.4.3. Ferric Reducing Antioxidant Power (FRAP) and Cupric Reducing Antioxidant Capacity (CUPRAC)
The capacity of a sample to reduce iron and copper ions serves as an indicator of its antioxidant potential [53]. Two prevalent methods employed for this assessment are the FRAP and CUPRAC assays. Experimental findings from the FRAP assay reveal a pronounced positive correlation with the outcomes of the DPPH test. In contrast, during the CUPRAC test, the extracts, barring the water extract, manifested copper ion reduction capabilities that closely resembled that of trolox (Table 4). Furthermore, there is a documented report stating that Polygonum multiflorum demonstrates remarkable reduction abilities in FRAP experiments [51]. Additionally, some studies in the literature emphasize the iron ion reduction potential of R. ciliinervis root extracts [13], which is primarily attributed to the abundant phenol content present in both plant species.

Table 4.
Determination of antioxidant activity of various solvent extracts of R. ciliinervis root using FRAP, CUPRAC, and metal chelating.
2.4.4. Metal Chelation
The presence of ferrous and copper ions can amplify the Fenton reaction, resulting in heightened oxidative stress in the body [54]. Consequently, assessing the metal chelating capacity of samples is paramount for evaluating their antioxidant potential. Experimental findings revealed that various extracts demonstrated varying degrees of iron-chelating abilities. In terms of copper ion chelation, all extracts displayed commendable chelating abilities (Table 4). These outcomes emphasize the inferior metal chelating ability of the water extract in contrast to the superior metal ion chelating proficiency of the methanol extract. Furthermore, it was discovered that both Polygonum minus and Polygonum glabrum extracts possess chelating effects, which are intimately tied to the presence of phenolic compounds [19,55].
2.4.5. Hydrogen Peroxide (H2O2)
H2O2 is a potent oxidant that arises as a by-product during the body’s metabolic processes. While it plays a crucial role in cell signaling and immune responses, excessive levels of H2O2 can trigger oxidative stress, resulting in an elevation of intracellular reactive oxygen species (ROS) and subsequent damage to mitochondria, ultimately leading to apoptosis [56]. Consequently, the effective scavenging of H2O2 is paramount for preserving good health. Experimental findings have demonstrated that various extracts of R. ciliinervis root exhibit robust H2O2 scavenging capabilities (Table 5). Furthermore, research has indicated that the H2O2 scavenging activity of the methanol extract of Polygonum glabrum is comparable to that of vitamin E [55].

Table 5.
Determination of antioxidant activity of various solvent extracts of R. ciliinervis root using H2O2, singlet oxygen, and β-carotene bleaching assays.
2.4.6. Singlet Oxygen
Singlet oxygen, a highly reactive variant of molecular oxygen, is generated within mammalian cells under pathophysiological circumstances, posing a threat to health by oxidizing vital cellular macromolecules such as lipids, nucleic acids, and proteins [57]. In the singlet oxygen scavenging experiment conducted, all extracts except for the water extract exhibited commendable scavenging activity (Table 5).
2.4.7. β-Carotene Bleaching
β-Carotene is a natural pigment endowed with crucial physiological functions and biological activities. It is prevalent in plants but prone to fading upon oxidation [58]. In this experiment, linoleic acid was subjected to heating and oxidation, generating free radicals that subsequently caused the bleaching of β-carotene. However, in the presence of antioxidants, the formation of these free radicals was delayed, mitigating the bleaching rate of β-carotene. The antioxidant activity was quantitatively assessed by monitoring the decrease in β-carotene’s absorbance over time [59].
This study employed the β-carotene bleaching assay to evaluate the antioxidant potential of various extracts of R. ciliinervis root. Notably, the methanol, ethanol, and 80% ethanol extracts demonstrated robust antioxidant capabilities (Table 5), surpassing synthetic antioxidants like BHT, butyl hydroxyanisole, and tertiary butylhydroquinone (TBHQ) (Figure 2). Additionally, existing literature attests to the β-carotene bleaching inhibition activity exhibited by Polygonum minus, further reinforcing the significant potential of the Polygonum genus as natural antioxidants for food and pharmaceutical applications [60,61].

Figure 2.
Alterations in the absorbance of β-carotene in the presence of different extracts of R. ciliinervis root. BHT, BHA and TBHQ were employed as positive controls. BHT: butylated hydroxytoluene; BHA: butyl hydroxyanisole; TBHQ: tertiary butylhydroquinone.
The objective of this study is to investigate the antioxidant potential of R. ciliinervis. The research findings reveal that the plant contains active constituents, representing valuable natural sources of antioxidants. Notably, the methanol extract displays particularly promising outcomes, demonstrating superior values in TCC, TPheC, TAC, TPAC, TTanC, and GC. Furthermore, this extract exhibits robust scavenging capabilities against four distinct free radicals, strong reduction abilities toward iron and copper ions, moderate metal ion chelation, effective scavenging of singlet oxygen and H2O2, and it even surpasses synthetic antioxidants in inhibiting β-carotene bleaching. A previous report examining the antioxidant capacities of various solvent extracts from Polygonum minus concurred that the methanol extract possessed the highest antioxidant capacity [19], aligning with the research findings. Consequently, guided by these experimental results, the methanol extract was selected for further in-depth investigation, utilizing UHPLC-MS for compound identification with the aim of elucidating its active ingredients. Subsequently, the stability of the methanol extract under various environmental conditions will be evaluated with a focus on its ability to inhibit edible oil oxidation. This assessment aims to determine the stability and sustainability of its antioxidant capacity across various environments and conditions. Following this, acute toxicity tests were performed on mice to evaluate the extract’s acute toxicity and establish dosage guidelines for subsequent rat liver protection experiments. Ultimately, the focus shifted to examining the extract’s pre-protective effects against acute liver injury in rats with the ultimate goal of uncovering its therapeutic potential and protective mechanisms.
2.5. UHPLC-MS Identification
The current study examines the chemical composition of the methanol extract of R. ciliinervis root, utilizing UHPLC-ESI-Q-TOF-MS technology. Through meticulous comparison of molecular and fragment ions with reference data, the sources of the reference data are derived from the published literature (please refer to the analysis process of each peak for further details) and reputable public databases, including the Human Metabolome Database (https://hmdb.ca/, accessed on 10 August 2024) and MassBank (https://www.massbank.jp/, accessed on 10 August 2024). A comprehensive list of 45 bioactive compounds has been compiled (Table 6) with their structural representations illustrated in Figure S1. Additionally, Figure S2 presents the UHPLC-MS findings obtained in positive-ion mode, while detailed MS and MS/MS spectra are available in the Supplementary Materials (Figures S3–S100).

Table 6.
Compounds identified in methanol extract of R. ciliinervis root.
This thorough chemical characterization of the methanol extract provides a rich understanding of the diverse array of compounds it encompasses. Notably, several of the identified compounds have been previously isolated from R. ciliinervis root and have been implicated in its antioxidant properties. These findings emphasize the significant contribution of these compounds to the overall therapeutic potential of R. ciliinervis root, serving as a robust foundation for future research endeavors. By focusing on specific compounds responsible for the observed antioxidant effects, this study provides a foundation for targeted therapeutic developments and advancements in medicinal applications, setting the stage for future progress.
Peak 1 was identified as D-sucrose by matching fragment ions (m/z 343.1175 [M+H]+, 180.0820 [M−C6H11O5+H]+, and 163.0560 [M−C6H11O6]+) with reference [62]. Peak 2 at m/z 222.1066 had MS2 ions at m/z 163.0339 [M−C2H3N2O2+NH4]+ and 91.0519 [M−C4H7N2O3+NH4]+, and it was tentatively assigned as glutamylglycine [63]. The mass spectrum of peak 3 displayed an ion at m/z 292.1318 with its MS2 spectrum showing fragments at m/z 153.0145 and 136.0722. Peak 4 registered an m/z of 171.0241 and exhibited fragment ions at m/z 132.0983 [M−OH+H]+ and 103.0515 [M−COOH]+, and it was presumptively identified as citramalic acid [64]. Peak 5 displayed a [M+H]+ ion at m/z 307.0742 with its main fragment ions at m/z 289.0641 [M−OH]+ and 181.0462 [M−C6H5O3]+. These findings were characteristic of epigallocatechin [65] or gallocatechin [66]. Peak 6 (m/z 188.0657) was recognized as phenylalanine, which was based on the typical fragment ions at m/z 165.0507 [M]+ and 100.0730 [M−C3H6NO2+Na]+ [67]. Peak 7 had an m/z of 579.1385 and gave fragment ions at m/z 453.1047 and 289.0649, which correlated with the loss of −C6H5O2−OH+H and −C15H13O6, suggesting peak 7 was procyanidin B1 [68]. The precursor ion [M+H]+ of peak 8 appeared at m/z 291.0798, with its main fragment ions at m/z 273.0710 [M−OH]+ and 165.0512 [M−OH−C6H5O2]+, corresponding to D-(+)-catechin or (−)-epicatechin [10]. Peak 9 at m/z 365.0759 had MS2 ions at m/z 297.0541 [M−COOH]+ and 181.0453 [M−C6H11O6+NH4]+, and it was identified as caffeic acid 3-glucoside [69]. The main fragment ions of peak 10 (m/z 395.1254) were at m/z 227.1697 [M−C6H11O5+NH4]+ and 194.1131 [M−C6H11O6]+, and it was assigned as β-syringin [70].
Peak 11 had an m/z of 439.1509 and MS2 ions at m/z 340.2528 [M−C6H5+H]+ and 322.2425 [M−C6H5−H2O+H]+, and it was identified as 2-phenylethyl 6-O-β-D-xylopyranosyl-β-D-glucopyranoside [71]. Peak 12 showed an [M+NH4]+ ion at m/z 654.1180, producing major fragment ions at m/z 467.0745 [M−C7H5O5]+ and 449.0634 [M−C7H5O5−H2O]+, which is characteristic of 1,3,6-trigalloyl glucose [72]. Peak 13 was identified as N-acetylmethionine with an m/z of 191.0653. The MS2 spectrum displayed connections at m/z 177.0044 and 133.0623, corresponding to the loss of −CH3+H and −C2H4NO [73]. Peak 14, with an m/z of 245.0748, was identified as uridine, featuring fragment ions at m/z 227.1707 and 133.0612, corresponding to the loss of −OH and −C4H3N2O2 [74]. The mass spectrum of peak 15 exhibited an ion at m/z 463.1131, with its MS2 spectrum showing fragments at m/z 340.2531 [M−C7H7O2+H]+, 283.0540 [M−C6H11O6]+, and 163.0340 [M−C16H11O6]+, and it was recognized as chrysoeriol 7-O-glucoside [75]. Peak 16 was observed at m/z 600.2537 and characterized as (+)-lyoniresinol-3a-O-β-glucoside. Its MS2 ions at m/z 340.2528 and 163.0143 indicated the loss of −C8H9O3+H and −C22H27O8, respectively [76]. Peak 17 had an [M+H]+ ion at m/z 391.1310, producing major fragment ions at m/z 211.0708 [M−C6H11O6]+ and 107.0461 [M−C13H16O7+H]+, which are characteristics of polydatin [37]. Peak 18 was detected at m/z 419.1234 with fragment ions at m/z 257.0745 [M−C6H11O6+NH4]+ and 163.0150 [M−C15H11O4]+, and it was assigned as polygonimitin B [37]. Peak 19 (m/z 419.1248) was recognized as liquiritin based on the typical fragment ions at m/z 257.0747 [M−C6H11O6+NH4]+ and 222.1077 [M−C6H11O6−OH]+ [77]. The mass spectrum of peak 20 revealed an ion at m/z 183.0729 with its MS2 spectrum showing fragments at m/z 133.0826 and 89.0570.
Peak 21 had an m/z of 229.0796 with MS2 ions at m/z 229.0810 [M−OH+NH4]+ and 127.0123 [M−C8H7O+NH4]+, and it was tentatively identified as resveratrol based on the literature [37]. Peak 22, with an m/z of 233.0745 and MS2 ions at m/z 215.0650 [M−OH]+ and 205.0547 [M−CO+H]+, was tentatively characterized as cassiachromone [37]. Peak 23 at m/z 455.0837 was proposed as emodin 8-β-D-glucopyranoside with the main MS2 ions at m/z 271.0545 and 197.0547 corresponding to the loss of −C9H6O3+H and −C15H9O4+NH4 [37]. Peak 24 had an m/z of 543.1372 with major fragments including m/z 245.1807 [M−C13H15O9+NH4]+ and 192.1337 [M−C20H21O7+Na]+, and it was identified as resveratrol-3-O-(2″-O-galloyl)-β-D-glucopyranoside [24]. Peak 25 exhibited a precursor ion [M+H]+ at m/z 409.1396, generating major fragment ions at m/z 431.1235 [M+Na]+, 391.1313 [M−OH]+, and 229.0809 [M−C6H11O6]+, corresponding to torachrysone 8-O-glucoside [37]. Peak 26 had an m/z of 271.0528 with MS2 ions at m/z 225.0495 [M−CO−OH]+ and 197.0546 [M−C5H4O2+Na]+, and it was identified as aloe emodin [78]. The mass spectrum of peak 27 displayed an ion at m/z 309.0892 with its MS2 spectrum showing fragments at m/z 291.0800 and 147.0401. Peak 28 showed an m/z of 177.0495 and was recognized as 7-methoxycoumarin based on the characteristic fragment ions at m/z 149.0557 [M−CO+H]+, 145.0244 [M−OCH3]+, and 117.0301 [M−OCH3−CO]+ [79]. Peak 29, with an m/z of 256.0662, was identified as piscidic acid based on the characteristic fragment ions at m/z 222.1073 [M−2OH]+ and 194.1128 [M−OH−COOH]+ [35]. Peak 30 had [M+H]+ at m/z 271.0531 and produced significant fragment ions at m/z 163.0348 [M−C6H4O2+H]+ and 107.0464 [M−C8H4O4+H]+, which are characteristic of emodin [37].
Peak 31 presented an m/z of 285.0684 with fragment ions at m/z 201.0858 and 143.0363 after the elimination of −C4H4O2+H and −C8H4O4+Na, leading to the identification of 1-methyl emodin [37]. Peak 32 appeared at m/z 285.0686 with MS2 ions at m/z 242.0522 [M−CO−CH3+H]+ and 222.1077 [M−CH3−OH−OCH3+H]+, and it was identified as emodin 3-methyl ether [37]. Peak 33, observed at m/z 256.0666, was characterized as emodin anthrone with its MS2 ions at m/z 224.1228 and 194.1128 indicating the loss of −OH−CH3 and −2OH−CO, respectively [80]. Peak 34 at m/z 511.1279 exhibited fragment ions at m/z 453.3350 and 205.0554, indicating losses of −OH−H2O and −C16H11O5, and it was thus determined to be physicon-8-β-D-(6′-O-acetyl)glucoside [23]. The mass spectrum of peak 35 displayed an ion at m/z 285.0686 with its MS2 spectrum showing fragments at m/z 270.0827 and 222.1075. Peak 36, a [M+H]+ ion at m/z 521.1683, was suggested to be piceid-2″-O-conmarate, with its MS2 ions at m/z 293.0960 [M−C14H11O2−OH+H]+ and 275.0852 [M−C14H11O2−OH−H2O+H]+ corresponding to the literature [24]. Peak 37 was identified as palmitic acid at m/z 274.2673, with characteristic fragment ions at m/z 212.2320 [M−COOH+H]+, 71.0831 [C5H11]+, and 57.0679 [C4H9]+ [81]. Peak 38 at m/z 318.2925, with MS2 ions at m/z 183.0735 [C13H27]+, 155.0428 [C11H23]+, and 127.0123 [C9H19]+, was tentatively identified as phytosphingosine based on the literature [82]. Peak 39 displayed [M+NH4]+ at m/z 298.2663 and produced significant fragment ions at m/z 235.1866 [M−COOH]+ and 222.1074 [C16H29+H]+, which is characteristic of linoleic acid [83]. The mass spectrum of peak 40 displayed an ion at m/z 415.2021 with its MS2 spectrum showing fragments at m/z 340.2524 and 183.0737.
Peak 41 had [M+H]+ at m/z 302.2980 and yielded significant fragment ions at m/z 276.1902 [M−C3H7+NH4]+, 183.0741 [C13H27]+, and 100.0730 [C7H15+H]+, which are characteristic of sphinganine [84]. The mass spectrum of peak 42 displayed an ion at m/z 280.2560 with its MS2 spectrum showing fragments at m/z 263.2314 and 222.1071. Peak 43 exhibited a [M+NH4]+ peak at m/z 294.2350, which produced primary fragment ions at m/z 277.2105 [M−OH+NH4]+ and 249.2150 [M−COOH+NH4]+. These fragment ions are indicative of stearidonic acid [85]. The precursor ion [M+NH4]+ of peak 44 appeared at m/z 320.2480 with its main fragment ions at m/z 280.2576 [M−COOH+Na]+, 135.0404 [C10H15]+, and 123.0885 [C9H14+H]+, corresponding to eicosapentanoic acid [86]. Peak 45 registered an m/z of 296.2528 and exhibited fragment ions at m/z 279.2264 [M+H]+, 149.1033 [C11H17]+, and 135.0404 [C10H15]+, and it was presumptively identified as α-linolenic acid [87]. Peak 46 appeared at m/z 279.2245 with MS2 ions at m/z 222.1077 [M−C4H9+Na]+, 183.0734 [C13H27]+, and 127.0123 [C9H19]+, and it was identified as 14-methylpentadecanoic acid [88]. Peak 47 displayed [M+NH4]+ at m/z 471.3196 and produced significant fragment ions at m/z 155.0432 [C3H9NO4P+H]+ and 140.1148 [C2H7NO4P]+, which is characteristic of 1-palmitoyl-2-hydroxy-sn-glycero-3-PE [89]. The mass spectrum of peak 48 exhibited an ion at m/z 338.3331, with its MS2 spectrum showing fragments at m/z 279.1536 [C20H39]+, 237.2582 [C17H33]+, and 149.0196 [M−C13H25NO+Na]+, and it was recognized as erucamide [90]. Peak 49 presented an m/z of 437.3736 with fragment ions at m/z 407.3290 and 338.3350 after the elimination of −CH2OH+Na and −C7H15+Na, leading to the identification of β-sitosterol [10].
2.6. Stability of Methanol Extract
This study aims to comprehensively investigate the stability and antioxidant properties of phenolics present in R. ciliinervis root methanol extract. The results presented in Figure 3, Figure 4 and Figure 5 offer significant insights into the stability of the methanol extract under varying conditions, adding substantial value to the exploration of the properties.
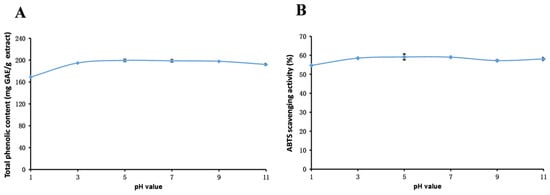
Figure 3.
Total phenolic content (A) and ABTS (B) assays to assess the stability of the methanol extract of R. ciliinervis root at various pH values. ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonicacid) diammonium salt.

Figure 4.
Total phenolic content (A) and ABTS (B) assays to assess the thermal stability of the methanol extract of R. ciliinervis root. ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonicacid) diammonium salt.
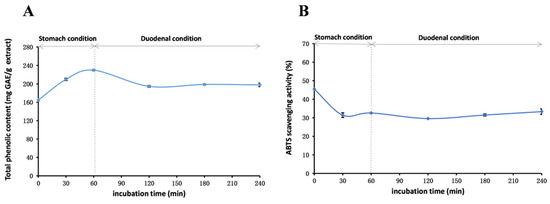
Figure 5.
Total phenolic content (A) and ABTS (B) assays to assess the stability of the methanol extract of R. ciliinervis root an in vitro simulation of the human digestive system. ABTS: 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonicacid) diammonium salt.
2.6.1. pH Stability
As depicted in Figure 3, the scavenging capacity of total phenolics and ABTS radicals in the methanol extract exhibits a similar fluctuation pattern under varying pH conditions. With an increase in pH, both parameters initially rise and then decline, peaking at pH 5. This trend is likely attributed to the hydrolysis of bound phenolics under these conditions, leading to an augmentation in total phenolic content and an enhancement in the ABTS radical scavenging capability. However, as the pH further increases, the total phenolic content diminishes, which is potentially due to the degradation of certain polyphenolic compounds under alkaline conditions [91]. These findings highlight the significant influence of pH on the stability of phenolic compounds in R. ciliinervis root, and it is advisable to store the methanol extract of R. ciliinervis root in neutral or slightly acidic environments.
2.6.2. Thermal Stability
Upon investigating the impact of heating time on the methanol extract, as illustrated in Figure 4, it was discernible that the TPheC value and ABTS radical scavenging activity initially decreased at 30 min, which was followed by an increase to their peak values when the heating time was extended to 60 min. Subsequently, no notable alterations were observed with further heating. The findings indicate that heating time exerts a minimal influence on the TPheC of R. ciliinervis root, while the antioxidant activity diminishes concurrently with the decrease in TPheC.
2.6.3. In Vitro Gastrointestinal Stability
To assess the stability of the methanol extract under gastrointestinal conditions, in vitro stability experiments that mimic the human digestive system were conducted. As depicted in Figure 5, over time, the TPheC values gradually augmented in the simulated oral and gastric environments, whereas in the simulated duodenal environment, the TPheC values abruptly declined and subsequently stabilized. This phenomenon might stem from the hydrolysis of bound phenolics triggered by the enzymatic system and the strong acidic environment of the gastrointestinal tract, leading to an elevation in TPheC values. However, upon the introduction of pancreatic enzymes, trypsin, and bile, the gastric acid is neutralized, creating a mildly alkaline pH. In this alkaline environment, a select few polyphenolic compounds undergo degradation, initially causing a transient dip in TPheC values before stabilizing. This observation is consistent with previously reported findings regarding pH stability. Additionally, excessive acidity had a pronounced effect on the ABTS radical scavenging capacity, which was partially restored under duodenal conditions, consistent with existing literature reports [92].
This study comprehensively analyzed the stability and antioxidant properties of phenolics in methanol extract of R. ciliinervis root under varying conditions. The results indicate that pH, heating time, and gastrointestinal conditions significantly impact the stability and antioxidant activity of these compounds. Optimal storage and handling conditions should consider maintaining a neutral or slightly acidic environment to preserve phenolic content and antioxidant activity.
2.7. Oxidative Stability of Oils
Fried foods are favored for their enticing taste and flavor. Nevertheless, during the frying process, exposure to high temperatures can encourage the oxidation and polymerization of oils, adversely impacting the quality of these foods [93]. To stabilize oils, the food industry frequently incorporates synthetic antioxidants into the oil. However, the utilization of synthetic antioxidants may pose safety concerns [94]. As a result, there is a mounting demand for natural antioxidants within the food industry. This experiment serves as a pivotal tool for assessing the antioxidant capability of methanol extract, emphasizing its medicinal value and potential to safeguard oils from oxidative degradation. The primary oxidation level of oils is typically gauged by measuring peroxide and acid values. Furthermore, K232 and K270 values are extensively employed to evaluate the primary and secondary stages of oil oxidation, respectively [95]. These indicators facilitate monitoring the oxidation status of oils, ensuring the quality and safety of fried foods.
The study reveals that the inclusion of two test doses of methanol extract in extra virgin olive oil (EVOO) significantly diminished K270 values and outperformed BHT (Figure 6). As for acidity values, the addition of methanol extracts at these doses yielded superior results with acidity levels notably lower than those achieved with TBHQ (Figure 7). In cold-pressed sunflower oil (CPSO), the addition of two experimental doses of methanol extract produced a better K232 value than TBHQ, which was accompanied by a marked reduction in acidity values comparable to BHT’s effect (Figure 6). In the comprehensive study aimed at stabilizing peanut oil, as documented in reference [96], resveratrol has emerged as a standout performer, showcasing its prowess by decreasing peroxide and acidity values by an impressive 30%. This remarkable feat not only significantly extends the shelf life of peanut oil by more than twofold but also fortifies its thermal stability, ensuring a higher level of preservation. The exceptional attributes of resveratrol within this context elucidate its pivotal role as a primary component in the methanol extract, which has yielded outstanding outcomes for the two edible oils under investigation in the experiment. This research underscores the profound antioxidant potential of methanol extract when applied to edible oils, highlighting its effectiveness in mitigating acidity levels, which is a pivotal quality indicator that directly correlates with the extent of oil oxidation [97]. The reduction in acidity signifies a slower rate of oxidation, preserving the freshness, flavor, and nutritional value of the oil. Consequently, the findings of this study underscore the need for further exploration into the utilization of methanol extract as a potent antioxidant in edible oils.
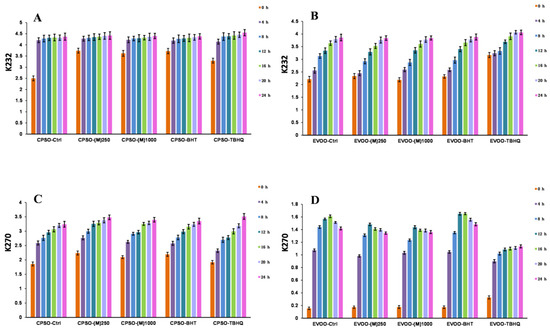
Figure 6.
Variations in the levels of conjugated dienes (K232) and trienes (K270) in CPSO (A,C) and EVOO (B,D) supplemented with the synthetic antioxidants, BHT and TBHQ, and different concentrations of the methanol extract of R. ciliinervis root, at 160 °C. CPSO: cold-pressed sunflower oil; EVOO: extra virgin olive oil; BHT: butylated hydroxytoluene; TBHQ: tertiary butylhydroquinone.
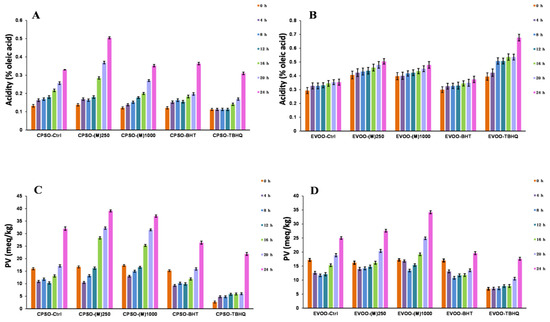
Figure 7.
Variations in the levels of acidity values and PV in CPSO (A,C) and EVOO (B,D) supplemented with the BHT and TBHQ, and different concentrations of the methanol extract of R. ciliinervis root, at 160 °C. CPSO: cold-pressed sunflower oil; EVOO: extra virgin olive oil; PV: peroxide values; BHT: butylated hydroxytoluene; TBHQ: tertiary butylhydroquinone.
2.8. Oral Acute Toxicity
An oral acute toxicity study was performed on mice to assess the acute toxicity of the methanol extract. Twenty mice were administered a single oral dose of 2000 mg/kg of the methanol extract. Encouragingly, none of the mice succumbed to any adverse effects within 24 h, suggesting that the methanol extract of R. ciliinervis root does not harbor highly toxic components and lacks significant acute toxicity. A previous report indicated that the intraperitoneal injection of R. ciliinervis root into mice yielded an LD50 of 2678 mg/kg over a 7 d observation period [28]. This LD50 value highlights the relatively low toxicity profile of R. ciliinervis root. “Chinese Materia Medica” documents that R. ciliinervis root possesses mild toxicity with a minority of patients reporting symptoms such as abdominal distension, nausea, vomiting, and hand numbness. Excessive consumption may also induce dizziness, which typically resolves upon cessation of use [98]. These effects may be attributed to the high anthraquinone content in R. ciliinervis root, notably emodin methyl ether, which in substantial doses can elicit diarrhea, headache, nausea, vomiting, muscle paralysis, and other symptoms [99]. Despite the widespread traditional usage of R. ciliinervis root, there exist a limited number of clinical research reports on its safety and efficacy. Consequently, there remains a dearth of extensive clinical research to fully support its safety and effectiveness. To comprehensively evaluate R. ciliinervis root’s suitability and potential health impacts on humans, further in-depth research, encompassing preclinical studies and comprehensive clinical trials, is imperative. However, it is crucial to note that the presence of Aloe emodin in methanol extract has been identified as a potential source of phototoxicity, hepatotoxicity, and nephrotoxicity, particularly when administered in high doses or over extended periods of time, as reported in [100]. Consequently, these aspects necessitate special attention and consideration in future toxicity research pertaining to the use of methanol extract in R. ciliinervis root.
2.9. Hepatoprotective Activity
The liver is a versatile organ with pivotal roles in numerous bodily functions. Chief among them are metabolizing carbohydrates, fats, and proteins, maintaining stable blood sugar levels, and synthesizing essential plasma proteins. Additionally, it serves as a crucial detoxification hub, breaking down harmful substances like alcohol and drugs. The liver secretes bile, which is vital for fat digestion and absorption, and it acts as a reservoir for iron, vitamins, and glycogen. It also synthesizes clotting factors, ensuring proper blood coagulation. Moreover, the liver possesses remarkable regenerative and immune defense capabilities, contributing to overall well-being. However, it is vulnerable to the adverse effects of conditions such as hepatitis, alcoholism, and fatty liver disease, leading to the release of enzymes when liver cells are compromised [101]. Thus, safeguarding liver health is paramount for preserving overall health and well-being, as it underpins numerous vital bodily processes.
R. ciliinervis root is primarily utilized as the primary medication in prescriptions and has been proven to exhibit therapeutic benefits in treating hepatitis, particularly with notable therapeutic effects. The underlying mechanism has been analyzed and attributed to the anthraquinone structure’s potent inhibitory action on the biosynthesis of viral cell DNA, RNA, and proteins [102]. Nevertheless, there remains a scarcity of extensive research reports specifically focusing on R. ciliinervis root therapy for liver diseases. Consequently, an experimental study was conducted using rats, wherein D-galactosamine was administered to induce liver injury with the aim of assessing the pre-protective effects of methanol extract on the liver. To quantify the severity of acute liver injury in the rats, various biochemical parameters were evaluated, including viscera index, liver enzyme activities, catalase (CAT) activities, malondialdehyde (MDA) and albumin levels. Notably, D-galactosamine is a well-established hepatotoxic agent that is capable of inducing liver damage (necrosis and inflammation) that closely mimics the pathology observed in human viral hepatitis [103].
To evaluate the impact of methanol extract on liver injury, 40 rats were randomly assigned to five groups (n = 8) with each group receiving a distinct oral treatment regimen. The control group (G1) received 0.5% sodium carboxymethyl cellulose, while the model group (G2) was also administered 0.5% sodium carboxymethyl cellulose but served as the baseline for injury comparison. The positive control group (G3) was treated with silymarin at 100 mg/kg body weight, which is a known liver protectant. The high-dose group (G4) and low-dose group (G5) were administered methanol extract at 300 mg/kg and 150 mg/kg body weight, respectively, for seven consecutive days. Following this treatment period, all groups were administered orally for 7 d with the subsequent injection of D-galactosamine in G2 through G5. The results indicate that pretreatment with both high-dose and low-dose methanol extract significantly reduced the viscera index in rats compared to G2 (p < 0.001, Figure 8). Remarkably, there was no statistically significant difference in the pre-protective effects on liver function between the methanol extract (both high and low doses) and silymarin, highlighting the potential therapeutic value of methanol extract in mitigating liver injury.
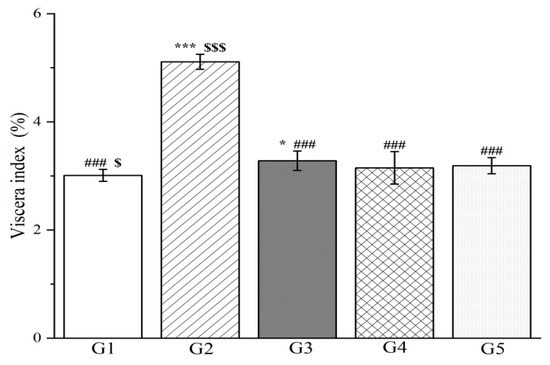
Figure 8.
The outcomes of treatment with the methanol extract of R. ciliinervis root on the hepatic viscera index in rats with liver injury. Values are expressed as the mean ± standard error of the mean (n = 8). G1: control group, G2: D-GalN group, G3: D-GalN + SMN group, G4: D-GalN + R. ciliinervis root300 group, G5: D-GalN + R. ciliinervis root150 group. D-GalN: D-galactosamine; SMN: silymarin. Significantly different from the control group at * p < 0.05 and *** p < 0.001. Significantly different from the D-GalN group at ### p < 0.001. Significantly different from the D-GalN + SMN group at $ p < 0.05 and $$$ p < 0.001.
Moreover, the research findings clearly demonstrate that the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyl transpeptidase (γ-GT) in both the liver tissue and serum of the model group were markedly elevated compared to the control group (p < 0.001, Figure 9 and Figure 10), confirming the successful induction of an acute liver injury rat model. In terms of liver tissue, when compared with G2, the administration of methanol extract in the treatment groups led to a significant reduction in the activities of key liver enzymes, including ALT and AST (p < 0.001, Figure 9). Specifically, the ALT levels in G4 and G5 exhibited reductions of 69.9% and 65.3%, respectively. Similarly, AST levels decreased by 45.3% and 43.0% in these groups. These outcomes are comparable to those achieved with silymarin treatment, emphasizing the potential efficacy of the methanol extract in mitigating acute liver injury.
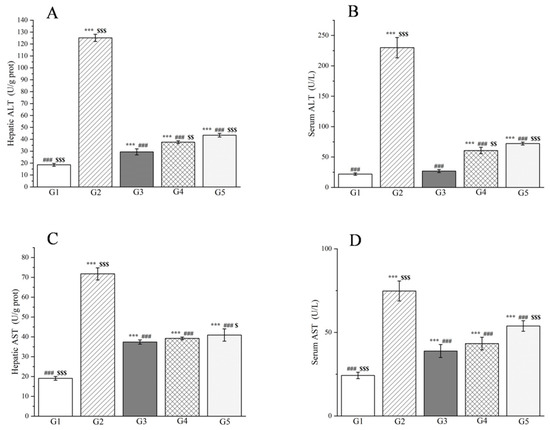
Figure 9.
Effects of the methanol extract of R. ciliinervis root on hepatic ALT (A), AST (C) and serum ALT (B), AST (D) in rats with liver injury. Values are expressed as the mean ± standard error of the mean (n = 8). G1: control group, G2: D-GalN group, G3: D-GalN + SMN group, G4: D-GalN + R. ciliinervis root300 group, G5: D-GalN + R. ciliinervis root150 group. D-GalN: D-galactosamine; SMN: silymarin; ALT: alanine aminotransferase; AST: aspartate aminotransferase. Significantly different from the control group at *** p < 0.001. Significantly different from the D-GalN group at ### p < 0.001. Significantly different from the D-GalN + SMN group at $ p < 0.05, $$ p < 0.01 and $$$ p < 0.001.

Figure 10.
Effects of the methanol extract of R. ciliinervis root on serum γ-GT (A), ALB (B) in rats with liver injury. Values are expressed as the mean ± standard error of the mean (n = 8). G1: control group, G2: D-GalN group, G3: D-GalN + SMN group, G4: D-GalN + R. ciliinervis root300 group, G5: D-GalN + R. ciliinervis root150 group. D-GalN: D-galactosamine; SMN: silymarin; γ-GT: γ-glutamyl transpeptidase. Significantly different from the control group at *** p < 0.001. Significantly different from the D-GalN group at # p < 0.05, ## p < 0.01 and ### p < 0.001. Significantly different from the D-GalN + SMN group at $$$ p < 0.001.
Simultaneously, the levels of ALT, AST, and γ-GT were measured in rat serum, which are enzymes that are predominantly localized within liver cells. However, upon liver cell damage, they are liberated into the bloodstream, resulting in an elevation of their serum levels. Experimental findings reveal that in comparison to G2, the methanol extract treatment groups significantly lowered the serum levels of both ALT and AST (p < 0.001, Figure 9) as well as the serum levels of γ-GT (p < 0.05, Figure 10). Specifically, the ALT levels in G4 and G5 decreased by 73.6% and 68.6%, respectively, when compared to G2. Analogously, the AST levels declined by 42.1% and 28.0%, respectively. Additionally, the γ-GT levels decreased by 26.4% and 12.7%, respectively. This reduction in liver enzyme activity serves as an indication of a decrease in liver injury and inflammation [104]. Our results affirm that the methanol extract from R. ciliinervis root exerts a pre-protective effect against D-galactosamine-induced liver injury, and this effect is dose-dependent.
In addition to liver enzymes, the albumin level in serum serves as a crucial indicator of liver function. The albumin level is directly correlated with the extent of liver function impairment; as the liver becomes damaged, the synthesis of albumin diminishes, leading to a decline in serum albumin levels. The study revealed that in comparison to G1, the albumin levels in rats from G2 were significantly reduced (p < 0.001, Figure 10). Nonetheless, when compared to G2, the administration of silymarin and a high-dose methanol extract resulted in a notable increase in albumin levels after 24 h (p < 0.001, Figure 10) with no discernible difference between the two treatments. Notably, the high-dose methanol extract demonstrated superior efficacy in enhancing albumin levels compared to the low-dose methanol extract.
D-galactosamine not only triggers liver damage but also enhances the generation of ROS in the body while diminishing the efficacy of antioxidants, resulting in elevated MDA levels and CAT activity [105]. When compared to G1, the MDA levels in the liver and serum of G2 rats were markedly elevated subsequent to D-galactosamine treatment (p < 0.001, Figure 11). Notably, treatment with two doses of methanol extract in G2 rats significantly inhibited the increase in MDA levels in both liver tissue and serum, which were induced by liver injury (p < 0.001, Figure 11). Furthermore, this inhibitory effect was observed to intensify with an increase in the administered dose.

Figure 11.
Effects of the methanol extract of R. ciliinervis root on hepatic MDA (A) and serum MDA (B) in rats with liver injury. Values are expressed as the mean ± standard error of the mean (n = 8). G1: control group, G2: D-GalN group, G3: D-GalN + SMN group, G4: D-GalN + R. ciliinervis root300 group, G5: D-GalN + R. ciliinervis root150 group. D-GalN: D-galactosamine; SMN: silymarin; MDA: malondialdehyde. Significantly different from the control group at * p < 0.05 and *** p < 0.001. Significantly different from the D-GalN group at ### p < 0.001. Significantly different from the D-GalN + SMN group at $ p < 0.05 and $$$ p < 0.001.
After the injection of D-galactosamine, the CAT levels in the liver and serum of G2 rats were significantly reduced (p < 0.001, Figure 12). In contrast, the CAT levels in the liver of rats treated with silymarin and the two doses of methanol extract were significantly increased compared to the G2 (p < 0.001, Figure 12). Notably, the therapeutic effect observed in the high-dose methanol extract group was comparable to that of silymarin. However, while both doses of methanol extract led to an increase in CAT levels in rat serum, the changes in the low-dose group were not statistically significant (p > 0.05, Figure 12). These findings suggest that the methanol extract of R. ciliinervis root can mitigate oxidative stress induced by D-galactosamine injection in rats to a certain degree, and the efficacy appears to correlate positively with the dose administered. Based on the aforementioned experimental results, it has been conclusively demonstrated that the methanol extract of R. ciliinervis root exerts a pre-protective effect against rat liver injury. This is achieved by reducing the levels of ALT, AST, γ-GT, and MDA while simultaneously increasing the levels of CAT and albumin. Furthermore, the high-dose methanol extract was found to be more effective than the low-dose extract, and its efficacy was comparable to that of silymarin.

Figure 12.
Effects of the methanol extract of R. ciliinervis root on hepatic CAT (A) and serum CAT (B) in rats with liver injury. Values are expressed as the mean ± standard error of the mean (n = 8). G1: control group, G2: D-GalN group, G3: D-GalN + SMN group, G4: D-GalN + R. ciliinervis root300 group, G5: D-GalN + R. ciliinervis root150 group. D-galactosamine; SMN: silymarin; CAT: catalase. Significantly different from the control group at ** p < 0.01 and *** p < 0.001. Significantly different from the D-GalN group at ## p < 0.01 and ### p < 0.001. Significantly different from the D-GalN + SMN group at $$ p < 0.01 and $$$ p < 0.001.
There are reports indicating that the total anthraquinone content of R. ciliinervis root has the ability to reduce lactate dehydrogenase activity and MDA levels in tumor-bearing mice, inhibiting tumor growth [12,106]. Additionally, another study has reported that R. ciliinervis root polysaccharides can enhance the levels of CAT, SOD, and GSH-Px in mice with immune dysfunction induced by cyclophosphamide while concurrently inhibiting MDA production, demonstrating noteworthy antioxidant effects [6,107]. Notably, the primary constituent of R. ciliinervis root, polydatin, has been found to alleviate liver steatosis and damage caused by a methionine-choline deficient diet in mice. It significantly reduces serum ALT and AST levels, inhibits liver oxidative stress, decreases inflammation, and achieves hepatoprotective effects [108]. Resveratrol, a natural plant-derived antitoxin, possesses potent anti-inflammatory properties that have been demonstrated to exert beneficial effects on the liver. In the context of carbon tetrachloride-induced liver injury in mice, resveratrol has been shown to effectively reduce ALT, AST levels, necrosis, and 4-hydroxynonenal, mitigating liver damage. Furthermore, it reduces liver cell apoptosis while carefully modulating liver cell regeneration, contributing to the overall protection of liver function [109]. In summary, the aforementioned observations suggest that R. ciliinervis holds potential as a natural source of liver protectants and antioxidants.
The results of the histopathological examination are presented in Figure 13. In the control group (Figure 13A), the liver tissue structure of the rats remains intact, showcasing morphologically normal hepatocytes that are neatly arranged. The portal area is clearly visible, and there is no evidence of inflammatory cell infiltration. Conversely, the model group (Figure 13B) exhibits a disrupted liver tissue structure with the disappearance of hepatic cords. There is an increased cell density, and various-sized lipid droplets are observed within the cells, which are accompanied by cytoplasmic vacuoles. Notably, in regions adjacent to blood vessels, there is a pronounced infiltration of inflammatory cells (Figure 13B, long-tailed arrow) along with scattered single-cell necrosis (Figure 13B, no-tailed arrow). These necrotic cells exhibit reduced volume, enhanced cytoplasmic eosinophilia, and nuclear pyknosis with fragmentation. When compared to the model group, the positive group (Figure 13C) demonstrates significant improvement in hepatocyte damage, which manifested as scattered mild swelling and minimal fatty degeneration in a few hepatocytes. There is also a limited amount of inflammatory cell infiltration around the portal areas (Figure 13C, long-tailed arrow), although individual hepatocyte necrosis can still be observed (Figure 13C, no-tailed arrow). The high-dose group displays a similar pattern of inflammatory cell infiltration around the portal area (Figure 13D, long-tailed arrow), which is accompanied by mild hepatocyte swelling, reduced lipid droplet vacuoles in the cytoplasm, scattered hepatocyte degeneration, and single hepatocyte necrosis (Figure 13D, no-tailed arrow). Likewise, the low-dose group exhibits a small amount of inflammatory cell infiltration near the portal area (Figure 13E, long-tailed arrow), along with mild hepatocyte swelling, scattered hepatocyte degeneration, and individual hepatocyte necrosis (Figure 13E, no-tailed arrow). Notably, both dose groups show a reduction in the degree and extent of lesions compared to the model group with the high-dose group exhibiting a lesser lesion range akin to the positive group. In conclusion, the histopathological examination of the tissue provides a clear illustration of the damage inflicted by D-galactosamine on rat livers. It also offers an intuitive view of the protective effects of the positive group and the two dose groups on rat liver health, further emphasizing the potential therapeutic application of methanol extract of R. ciliinervis root in liver diseases.
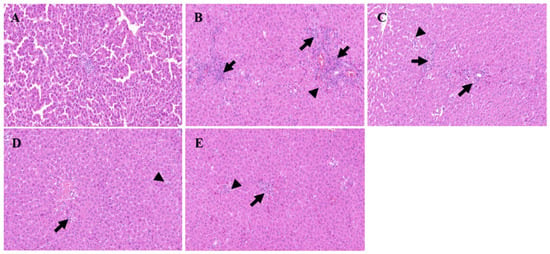
Figure 13.
Histological examination of liver sections in different groups (200× magnification). (A): Control group (200× magnification); (B): D-GalN group (200× magnification); (C): D-GalN + SMN group (200× magnification); (D): D-GalN + R. ciliinervis root300 group (200× magnification); (E): D-GalN + R. ciliinervis root150 group (200× magnification). D-GalN: D-galactosamine; SMN: silymarin; no-tailed arrow: single-cell necrosis; long-tailed arrow: inflammatory cells.
3. Material and Methods
3.1. Materials
The R. ciliinervis root (voucher specimen number: 2023-08-23-002) was collected in Tonghua, which is located at a latitude of 41°13′39.8″ N, longitude of 126°18′54.5″ E, and an altitude of 206.1 m, in Jilin Province, China, in August 2023. The identity of the specimen was verified by a plant taxonomist, Professor Junlin Yu. The voucher specimen is currently being stored in the Herbarium of Tonghua Normal University.
3.2. Methods
3.2.1. Qualitative Phytochemical Analysis
A qualitative phytochemical analysis was conducted on 15 distinct chemical components, adhering to a previously established methodology [110]. This analysis aimed to identify the various chemical constituents present within the sample. For detailed experimental procedures, please refer to the Supplementary Materials.
3.2.2. Preparation of Different Extracts of R. ciliinervis Root
To investigate the effect of various solvents on the extraction efficiency of active ingredients from R. ciliinervis root, this experiment utilized four different polar solvents: methanol, water, ethanol, and 80% ethanol. The extraction process was carried out according to the previously established methodology [110]. For detailed experimental procedures, please refer to the Supplementary Materials.
3.2.3. Quantitative Phytochemical Analysis
A quantitative phytochemical analysis was performed to ascertain the concentrations of various compounds, including TCC, TProC, TPheC, TSC, TAC, TFC, TPAC, TTanC, GC, and CTC. These measurements were conducted using methodologies previously outlined in references [110,111]. For detailed experimental procedures, please refer to the Supplementary Materials.
3.2.4. Antioxidant Activity Assays
Antioxidant activity assays were conducted utilizing a diverse array of methods, including DPPH, ABTS, hydroxyl radical, superoxide radical, FRAP, CUPRAC, metal chelation, H2O2, singlet oxygen, and β-carotene bleaching assays. These assays were performed in accordance with previously established protocols [111]. For detailed experimental procedures, please refer to the Supplementary Materials.
3.2.5. UHPLC-MS Analysis
The experimental conditions for UHPLC-MS were implemented according to the previously described methodology [95], ensuring the precision and reproducibility of the analytical process. For detailed experimental procedures, please refer to the Supplementary Materials.
3.2.6. Stability Studies of Methanol Extract
The pH stability, thermal stability, and stability in a gastrointestinal tract model system of the methanol extract of R. ciliinervis root were evaluated using methods previously outlined in the research [95]. For detailed experimental procedures, please refer to the Supplementary Materials.
3.2.7. Oxidative Stability Studies of Oils
The oxidative stability of EVOO and CPSO was assessed using methods previously described in the research [95]. For detailed experimental procedures, please refer to the Supplementary Materials.
3.2.8. Oral Acute Toxicity Study
An acute oral toxicity study was conducted in accordance with the established protocols outlined in the previous methods [95], aiming to evaluate the potential adverse effects of the methanol extract under investigation when administered orally in a single dose. For detailed experimental procedures, please refer to the Supplementary Materials.
3.2.9. Hepatoprotective Experiments
The hepatoprotective experiments were conducted in adherence to the procedures outlined in reference [95], encompassing animal selection, experimental protocol design, histopathological examination, and biochemical analyses. For detailed experimental procedures, please refer to the Supplementary Materials.
3.2.10. Statistical Analysis
Statistical significance between groups was determined using a one-way analysis of variance, which was followed by post hoc least significant difference tests and DUNCAN tests. p-values of 0.05, 0.01, and 0.001 were considered as indicative of significance, high significance, and very high significance, respectively.
4. Conclusions
This study systematically investigated R. ciliinervis root through a combination of qualitative and quantitative phytochemical analysis methods along with in vitro antioxidant activity assays. The findings revealed that the methanol extract of R. ciliinervis root was abundant in diverse active constituents and demonstrated remarkable performance in antioxidant tests, prompting its selection for further examination. Utilizing UHPLC-MS technology, a total of 45 compounds were identified within the methanol extract. Additionally, stability assessments of the methanol extract under various conditions, including heating, varying pH levels, and simulated human digestive system conditions, demonstrated significant stability of its antioxidant active ingredients and robust antioxidant capacity. Furthermore, oil stability experiments highlighted the potential of this extract as an effective antioxidant for stabilizing edible oils, positioning it as a promising candidate for applications in food preservation, particularly in the stabilization of edible oils.
In the experiment involving D-galactosamine-induced liver injury in rats, histopathological evaluations and biochemical markers confirmed a significant pre-protective effect of the methanol extract on the rat liver. In conclusion, our research emphasizes the potent antioxidant properties and protective effects of methanol extract of R. ciliinervis root against liver damage stemming from oxidative stress. These findings serve as a foundation for future investigations into the therapeutic potential of this plant in addressing oxidative stress-related disorders, such as neurodegenerative conditions, and cardiovascular complications. Looking ahead, the acute toxicity study of serves as an initial safety assessment, emphasizing the need for more extensive toxicological evaluations to ensure the safe and efficacious integration of this extract into clinical practice or consumer products. Future research should also delve into the mechanisms underlying its antioxidant and protective effects, exploring potential synergistic interactions among the identified compounds.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules29194701/s1. Table S1: Qualitative phytochemical analysis of R. ciliinervis root; Figure S1: Chemical structures of the compounds identified in methanol extract of R. ciliinervis root; Figure S2: Positive-ion mode UHPLC-MS findings of methanol extract of R. ciliinervis root; Figure S3–Figure S100: Presentation of MS and MS/MS spectra for peaks 1 through 49.
Author Contributions
J.H. and H.Z. conceived, designed, and supervised the research project as well as prepared and edited the manuscript. Z.X. and Y.H. collected plant material and prepared different solvent extracts and accomplished the biological evaluation experiments. H.P. and L.L. accomplished the statistical analysis. G.X. and J.Z. performed the biological evaluation experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Development Plan Project of Jilin Province, China [No. YDZJ202201ZYTS186]; and the independently selected topic project for basic scientific research of the Education Department of Liaoning Province in 2024, China.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The animal study protocol was approved by the Institutional Animal Care and Use Committee of Tonghua Normal University approved the experimental protocol (Ethic approval code: 20240035).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank Junlin Yu from Tonghua Normal University for the collection, identification, and provision of photographs of R. ciliinervis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, C.Y.; Han, L.P.; Zhou, T.J.; Wang, L.J.; Cui, T.T.; Wu, Y.F. Effects of different extracting methods on the content of total flavines in Polygonum cillinerve. Strait Pharm. J. 2018, 30, 23–25. [Google Scholar]
- Qiao, Y.; Mu, Y.; He, F.; Mu, K.F.; Mu, K.; Mu, K.X.; Yuan, R.H. Preliminary discussion on clinical medication law of taibai herbaceous medicine. J. Shaanxi Univ. Chin. Med. 2021, 44, 21–26. [Google Scholar] [CrossRef]
- Song, X.M.; Liu, H.J. Research and Application of Taibai Qi Yao; People’s Medical Publishing House: Beijing, China, 2011; pp. 114–117. [Google Scholar]
- Gang, A.S. Studies on Structural Development and Their Correlation with Accumulation of Effectual Components in Polygonum ciliinerve. Master’s Thesis, Northwest University, Xi’an, China, 2008. [Google Scholar]
- Zhao, Y.Q.; Zhao, Y.Q.; Yang, L.R.; Li, W.H.; Du, J.Y.; Hou, Q.D.; Fan, Y.P. Overview of research on total anthraquinones in Zhushaqi. Prog. Vet. Med. 2022, 43, 121–124. [Google Scholar] [CrossRef]
- Cui, J.J.; Yuan, J.F.; Zhang, Z.Q. Extraction and biological activity of crude polysaccharide from Polygonum ciliinerve. Nat. Prod. Res. Dev. 2007, 19, 960–964. [Google Scholar] [CrossRef]
- Zhang, W.M. Study on Antiviral and Antibacterial Active Components in Polygonum taipaishanense and Polygonum ciliinerve. Ph.D. Thesis, Northwest A&F University, Yangling, China, 2010. [Google Scholar]
- Chen, J.Y.; Du, Y.H.; Cai, Q. Experimental studies on the pharmacodynamics of Polygonum Cillinerve (Nakai) Ohwi. Hubei J. Tradit. Chin. Med. 1998, 20, 56–57. [Google Scholar]
- Zhao, Q.; Guo, H.L.; Guo, L.; Xu, B.; Zhang, X.S. Total anthraquinone in Zhushaqi (Polygonum cillinerve Ohwi): Its effects on xenograft tumor in S180, H22 and EAC animals. J. Shaanxi Univ. Chin. Med. 2008, 31, 61–63. [Google Scholar]
- Li, X.; Xu, H.N.; Li, S.X.; Zhang, H.W.; Jiang, Y.; Li, Y.Z.; Song, X.M.; Zhang, D.D.; Wang, W. Study on chemical constituents of “Taibai Qi Yao” Polygonum Cillinerve (Nakai) Ohwi. Chin. Tradit. Herb. Drugs 2023, 54, 1043–1050. [Google Scholar] [CrossRef]
- Zhao, Q.; Hu, R.; Wei, H.; Guo, H.L.; Lv, T.; Sun, F.Y. Study of total anthraquinone from Zhushaqi on the antioxidant activity influence in S180 bearing mice. Pharmacol. Clin. Chin. Mater. Med. 2013, 29, 72–74. [Google Scholar] [CrossRef]
- Lv, J.; Cao, L.X.; Zhao, X.; Wang, J.; Ma, L. Effects of different doses of total anthraquinone from Zhushaqi on immune function and antioxidant capacity in H22 tumor bearing mice. Prog. Mod. Biomed. 2018, 18, 2851–2854, 2877. [Google Scholar] [CrossRef]
- Wang, X.M.; Li, Z.X.; Ren, L.J. Study on antioxidative activity of polysaccharide in Polygonum cillinerve (Nakai) Ohwi. J. Anhui Agric. Sci. 2010, 38, 19346–19347, 19510. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhao, L.F.; Zhi, J.J.; Guo, J.B. Extraction and antioxidant activity of total tannins from Polygonum cillinerve (Nakai) Ohwi. Chem. Ind. Times 2011, 25, 25–28. [Google Scholar] [CrossRef]
- Feng, Z.L.; Wang, D.P.; Zhuang, S.T. Treatment of 100 cases of chronic atrophic gastritis with Hugailing capsule. Shaanxi J. Tradit. Chin. Med. 1995, 16, 296. [Google Scholar]
- Zeng, S.H.; Huang, L.Y. Treatment of 180 cases of chronic gastritis with Compound Zhushaqi Decoction. Shaanxi J. Tradit. Chin. Med. 1995, 16, 295. [Google Scholar]
- Internal Medicine Research Room of Shaanxi Institute of Traditional Chinese Medicine. Observation on the therapeutic effect of Zhushaqi in the treatment of 110 cases of acute bacterial dysentery. Shaanxi Med. J. 1972, 1, 17–19, 35. [Google Scholar]
- Qi, H.Y. Analysis of active components in traditional Chinese medicine Zhushaqi. Strait Pharm. J. 2019, 31, 50–52. [Google Scholar]
- Abdullah, M.Z.; Mohd Ali, J.; Abolmaesoomi, M.; Abdul-Rahman, P.S.; Hashim, O.H. Anti-proliferative, in vitro antioxidant, and cellular antioxidant activities of the leaf extracts from Polygonum minus huds: Effects of solvent polarity. Int. J. Food Prop. 2017, 20, 846–862. [Google Scholar] [CrossRef]
- Wang, W.L.; Zhu, X.M.; Liu, X.L. Research progress in factors influencing biological activity of polysaccharides. Chem. Bioeng. 2024, 41, 9–17. [Google Scholar] [CrossRef]
- Liang, L.H.; Su, Q.H.; Ma, Y.; Zhao, S.Z.; Zhang, H.J.; Gao, X.F. Research progress on the polysaccharide extraction and antibacterial activity. Ann. Microbiol. 2024, 74, 17. [Google Scholar] [CrossRef]
- Chen, M.H.; He, X.; Sun, H.; Sun, Y.; Li, L.; Zhu, J.Y.; Xia, G.Q.; Guo, X.; Zang, H. Phytochemical analysis, UPLC-ESI-Orbitrap-MS analysis, biological activity, and toxicity of extracts from Tripleurospermum limosum (Maxim.) Pobed. Arabian J. Chem. 2022, 15, 103797. [Google Scholar] [CrossRef]
- Qi, H.Y.; Zhang, C.F.; Zhang, M.; Liu, J.Q.; Wang, Z.T. Three new anthraquinones from Polygonum cillinerve. Chin. Chem. Lett. 2005, 16, 1050–1052. [Google Scholar]
- Qi, H.Y.; Zhang, C.F.; Zhang, M.; Wang, Z.T. Studies on constituents and antifungal activity of Polygonum cillinerve. Chin. Pharm. J. 2005, 40, 819–822. [Google Scholar]
- Hu, L.L. Screening and optimization of ethanol extraction process for active constituents from Polygonum cillinerve. Guizhou Agric. 2019, 47, 118–120, 124. [Google Scholar]
- Li, X.L. Extraction and In Vivo Pharmacokinetics of Anthraquinones from Polygonum Cillinerve (Nakai) Ohwi in Rats. Master’s Thesis, Shaanxi Normal University, Xi’an, China, 2008. [Google Scholar]
- Jung, M.; Lee, Y.; Han, S.O.; Hyeon, J.E. Advancements in sustainable plant-based alternatives: Exploring proteins, fats, and manufacturing challenges in alternative meat production. J. Microbiol. Biotechnol. 2024, 34, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.Y.; Wu, X.T. Experimental study on the anti-inflammatory and anti-ulcer effects of Polygonum Cillinerve (Nakai) Ohwi. J. Shaanxi Coll. Tradit. Chin. Med. 2003, 26, 48–49. [Google Scholar] [CrossRef]
- Susanti, I.; Pratiwi, R.; Rosandi, Y.; Hasanah, A.N. Separation methods of phenolic compounds from plant extract as antioxidant agents candidate. Plants 2024, 13, 965. [Google Scholar] [CrossRef]
- Jiao, X.Y.; Wang, Y.H.; Lin, Y.; Lang, Y.X.; Li, E.H.; Zhang, X.Y.; Zhang, Q.; Feng, Y.; Meng, X.J.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Collins, B.; Hoffman, J.; Martinez, K.; Grace, M.; Lila, M.A.; Cockrell, C.; Nadimpalli, A.; Chang, E.; Chuang, C.C.; Zhong, W.; et al. A polyphenol-rich fraction obtained from table grapes decreases adiposity, insulin resistance and markers of inflammation and impacts gut microbiota in high-fat-fed mice. J. Nutr. Biochem. 2016, 31, 150–165. [Google Scholar] [CrossRef]
- Yao, R.H. The current research status and progress of steroidal drugs. Technol. Wind 2016, 29, 260–261. [Google Scholar] [CrossRef]
- Wang, S.; Wen, B.Y.; Wang, N.; Liu, J.T.; He, L.C. Fluorenone alkaloid from Caulophyllum robustum Maxim. with anti-myocardial ischemia activity. Arch. Pharm. Res. 2009, 32, 521–526. [Google Scholar] [CrossRef]
- Si, K.W.; Liu, J.T.; He, L.C.; Li, X.K.; Gou, W.; Liu, C.H.; Li, X.Q. Effects of caulophine on caffeine-induced cellular injury and calcium homeostasis in rat cardiomyocytes. Basic Clin. Pharmacol. 2010, 107, 976–981. [Google Scholar] [CrossRef]
- Cao, X.H. Studies the Chemical Composition of Polygonum Cillinerve (Nakai) Ohwi and Its Biological Activities. Master’s Thesis, Shaanxi University of Science & Technology, Xi’an, China, 2018. [Google Scholar]
- Cao, X.H.; Ma, Y.M.; Wu, Y.; Xue, L.A. Studies on the chemical composition of Polygonum Cillinerve (Nakai) Ohwi. Chin. Tradit. Pat. Med. 2018, 40, 862–865. [Google Scholar]
- Zhang, Z.L. Study on the Chemical Composition of Polygonum cillinerve. Master’s Thesis, Shaanxi University of Chinese Medicine, Xianyang, China, 2022. [Google Scholar] [CrossRef]
- Patil, V.M.; Masand, N. Anticancer potential of flavonoids: Chemistry, biological activities, and future perspectives. Stud. Nat. Prod. Chem. 2018, 59, 401–430. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Tapas, A.; Sakarkar, D.; Kakde, R. Flavonoids as nutraceuticals: A review. Trop. J. Pharm. Res. 2018, 7, 1089–1099. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological application of tannin-based extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.; Jakovljević Kovač, M.; Pavić, V. A comprehensive analysis of diversity, structure, biosynthesis and extraction of biologically active tannins from various plant-based materials using deep eutectic solvents. Molecules 2024, 29, 2615. [Google Scholar] [CrossRef]
- Dehghanian, Z.; Habibi, K.; Dehghanian, M.; Aliyar, S.; Asgari Lajayer, B.; Astatkie, T.; Minkina, T.; Keswani, C. Reinforcing the bulwark: Unravelling the efficient applications of plant phenolics and tannins against environmental stresses. Heliyon 2022, 8, e09094. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Analysis of hydrolysable tannins. Anim. Feed Sci. Tech. 2001, 91, 3–20. [Google Scholar] [CrossRef]
- Boulekbache-Makhlouf, L.; Meudec, E.; Mazauric, J.P.; Madani, K.; Cheynier, V. Qualitative and semi-quantitative analysis of phenolics in Eucalyptus globulus leaves by high-performance liquid chromatography coupled with diode array detection and electrospray ionisation mass spectrometry. Phytochem. Anal. 2013, 24, 162–170. [Google Scholar] [CrossRef]
- Chen, B.; Wang, F. Colorimetry in determining the content of anthraquinone in Antenoron filiforme extractives. Mod. Chin. Med. 2008, 10, 56–57. [Google Scholar]
- Wang, X.M.; Zhao, W.X.; Wang, Y.S. Study on extraction of tannin from Polygonum Cillinerve (Nakai) Ohwi by ultrasonic method. Chem. Bioeng. 2011, 28, 34–36. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, Y.; Zhao, X.M.; Zhang, X.B.; Wei, X.; Tang, Y.Y.; Lei, X.; Song, X. Polygonum ciliinerve (Nakai) Ohwi: A review of its botany, traditional uses, phytochemistry, pharmacology, pharmacokinetics and toxicology. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 5403–5420. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ma, W.; Wang, L.; Sun, W.; Li, M.; Zhang, W.; Liu, Y.; Song, X.; Fan, Y. Characterization and antioxidant activity of the oligo-maltose fraction from Polygonum Cillinerve. Carbohydr. Polym. 2019, 226, 115307. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.T.; Nah, S.L.; Huang, Y.Y.; Wu, S.C. Potential antioxidant components and characteristics of fresh Polygonum Multiflorum. J. Food Drug Anal. 2010, 18, 120–127. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Tunnisa, F.; Nur, F.D.; Afriyanti, A.; Rosalina, D.; Ana, S.M.; Darmawan, N.; Dewi, Y.N. Antioxidant and antidiabetic compounds identification in several Indonesian underutilized Zingiberaceae spices using SPME-GC/MS-based volatilomics and in silico methods. Food Chem. X 2022, 14, 100285. [Google Scholar] [CrossRef]
- Lomozová, Z.; Hrubša, M.; Conte, P.F.; Papastefanaki, E.; Moravcová, M.; Catapano, M.C.; Proietti, S.I.; Karlíčková, J.; Kučera, R.; Macáková, K.; et al. The effect of flavonoids on the reduction of cupric ions, the copper-driven fenton reaction and copper-triggered haemolysis. Food Chem. 2022, 394, 133461. [Google Scholar] [CrossRef]
- Raja, S.; Ramya, I. In vitro antioxidant activity of Polygonum Glabrum. Int. J. Phytomed. 2017, 9, 351–363. [Google Scholar] [CrossRef][Green Version]
- Chen, Q.Y.; Yu, M.; Tian, Z.M.; Cui, Y.Y.; Deng, D.; Rong, T.; Liu, Z.C.; Song, M.; Li, Z.M.; Ma, X.Y.; et al. Exogenous glutathione protects IPEC-J2 cells against oxidative stress through a mitochondrial mechanism. Molecules 2022, 27, 2416. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, S.M.; Tak, J.K.; Choi, K.S.; Kwon, T.K.; Park, J.W. Regulation of singlet oxygen-induced apoptosis by cytosolic NADP+-dependent isocitrate dehydrogenase. Mol. Cell. Biochem. 2007, 302, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.Q.; Wang, F.; Lu, S.H.; Ma, F.T. Research progress on production, extraction technology and functions of β-carotene. Shandong Chem. Ind. 2023, 52, 69–72. [Google Scholar] [CrossRef]
- Cao, Y.N.; Song, Z.Q.; Wei, Z.; Zeng, L.Y.; Zhang, L.L.; Liu, Z.L. Research progress on measurement methods of antioxidant activity of antioxidants. China Pharm. 2013, 24, 86–88. [Google Scholar]
- Othman, A.; Mukhtar, N.J.; Ismail, N.S.; Chang, S.K. Phenolics, flavonoids content and antioxidant activities of 4 Malaysian herbal plants. Int. Food Res. J. 2014, 21, 759–766. [Google Scholar]
- Sumazian, Y.; Syahida, A.; Hakiman, M.; Maziah, M. Antioxidant activities, flavonoids, ascorbic acid and phenolic contents of Malaysian vegetables. J. Med. Plants Res. 2010, 4, 881–890. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Chi, H.; Li, Z.; Wang, C.; Wang, Q.; Feng, H.; Li, P. A qualitative analysis of cultured adventitious ginseng root’s chemical composition and immunomodulatory effects. Molecules 2024, 29, 111. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, Q.; Lametsch, R. Comparative analysis of substrate affinity and catalytic efficiency of γ-glutamyltransferase from bovine milk and Bacillus amyloliquefaciens. Food Chem. 2023, 405, 134930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; You, L.Y.; Liu, Y.F.; Zhang, S.J.; Ruan, Y.P.; Zhang, X.; Hu, L.L. Mechanism of action of the Banxia-Xiakucao herb pair in sleep deprivation: New comprehensive evidence from network pharmacology, transcriptomics and molecular biology experiments. J. Ethnopharmacol. 2024, 334, 118534. [Google Scholar] [CrossRef]
- Baron, G.; Ferrario, G.; Marinello, C.; Carini, M.; Morazzoni, P.; Aldini, G. Effect of extraction solvent and temperature on polyphenol profiles, antioxidant and anti-inflammatory effects of red grape skin by-product. Molecules 2021, 26, 5454. [Google Scholar] [CrossRef]
- Feng, Y.; Lin, J.; He, G.; Liang, L.; Liu, Q.; Yan, J.; Yao, Q. Compositions and biological activities of pomegranate peel polyphenols extracted by different solvents. Molecules 2022, 27, 4796. [Google Scholar] [CrossRef]
- Yao, Y.; Yu, Y.C.; Cai, M.R.; Zhang, Z.Q.; Bai, J.; Wu, H.M.; Li, P.; Zhao, T.T.; Ni, J.; Yin, X.B. UPLC-MS/MS method for the determination of the herb composition of Tangshen formula and the in vivo pharmacokinetics of its metabolites in rat plasma. Phytochem. Anal. 2022, 33, 402–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.B.; Yan, Y.; Ma, R.; Li, D.X.; Yin, W.F.; Tao, Q.W.; Xu, Y. Integrated phytochemistry and network pharmacology analysis to reveal effective substances and mechanisms of Bushen Quhan Zhiwang decoction in the treatment of rheumatoid arthritis. J. Ethnopharmacol. 2024, 325, 117897. [Google Scholar] [CrossRef] [PubMed]
- Żurek, N.; Pycia, K.; Pawłowska, A.; Potocki, L.; Kapusta, I.T. Chemical profiling, bioactive properties, and anticancer and antimicrobial potential of Juglans regia L. leaves. Molecules 2023, 28, 1989. [Google Scholar] [CrossRef]
- Liu, M.; Li, Z.; Cui, Q.; Yan, B.; Achi, J.G.; Zhao, Y.; Rong, L.; Du, R. Integrated serum pharmacochemistry and investigation of the anti-influenza A virus pneumonia effect of Qingjin Huatan decoction. J. Ethnopharmacol. 2024, 323, 117701. [Google Scholar] [CrossRef]
- Pande, J.; Chanda, S. Determination of phytochemical profile and antioxidant efficacy of Lavendula bipinnata leaves collected during Magha Nakshatra days and normal days using LC-QTOF-MS technique. J. Pharm. Biomed. Anal. 2020, 186, 113347. [Google Scholar] [CrossRef]
- Shah, S.L.; Bashir, K.; Rasheed, H.M.; Rahman, J.U.; Ikram, M.; Shah, A.J.; Majrashi, K.A.; Alnasser, S.M.; Menaa, F.; Khan, T. LC-MS/MS-based metabolomic profiling of constituents from Glochidion velutinum and its activity against cancer cell lines. Molecules 2022, 27, 9012. [Google Scholar] [CrossRef]
- Ramunaidu, A.; Pavankumar, P.; Ragi, N.; Ramesh, R.; Jagannatham, M.V.; Sripadi, P. Characterization of isomeric acetyl amino acids and di-acetyl amino acids by LC/MS/MS. J. Mass Spectrom. 2023, 58, e4982. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; You, X.; Chen, J.; Zhang, K.; Li, X. Metabolites of the marine fungus Ochroconis humicola (Y9-1). Chem. Nat. Compd. 2022, 58, 780–781. [Google Scholar] [CrossRef]
- Della Vedova, L.; Gado, F.; Vieira, T.A.; Grandini, N.A.; Palácio, T.L.N.; Siqueira, J.S.; Carini, M.; Bombardelli, E.; Correa, C.R.; Aldini, G.; et al. Chemical, nutritional and biological evaluation of a sustainable and scalable complex of phytochemicals from bergamot by-products. Molecules 2023, 28, 2964. [Google Scholar] [CrossRef]
- Lian, D.; Chen, T.; Yan, L.; Hou, H.; Gao, S.; Hu, Q.; Zhang, G.; Li, H.; Song, L.; Gao, Y.; et al. Protective effect of compatible herbs in Jin-Gu-Lian formula against Alangium chinense-induced neurotoxicity via oxidative stress, neurotransmitter metabolisms, and pharmacokinetics. Front. Pharmacol. 2023, 14, 1133982. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; He, J.; Jiang, J.; Zhao, Z.; Shu, Y. Systematic investigation of the material basis, multiple mechanisms and quality control of Simiao Yong’an decoction combined with antibiotic in the treatment of sepsis. Phytomedicine 2023, 116, 154910. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Pu, X.Y.; Pu, X.M.; Li, X.; Liu, Z.B.; Mei, M.J.; Wang, X.G.; Zhang, F.; Qiu, B.; Yu, J. Extracts of Knoxia roxburghii (Spreng.) M. A. Rau induce apoptosis in human MCF-7 breast cancer cells via mitochondrial pathways. Molecules 2022, 27, 6435. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fang, C.; Liu, Q.; Chen, X.; Chen, H.; Tian, S.; Peng, Q.; Yao, X. Mechanistic elucidation of QiJu-DiHuang Wan in management of age-related dry eye through metabolomics and network pharmacology. Phytomedicine 2024, 132, 155884. [Google Scholar] [CrossRef] [PubMed]
- Benamar, H.; Rarivoson, E.; Tomassini, L.; Frezza, C.; Marouf, A.; Bennaceur, M.; Nicoletti, M. Phytochemical profiles, antioxidant and anti-acetylcholinesterasic activities of the leaf extracts of Rhamnus lycioides subsp. oleoides (L.) Jahand. & Maire in different solvents. Nat. Prod. Res. 2019, 33, 1456–1462. [Google Scholar] [CrossRef]
- Dolma, S.K.; Singh, P.P.; Reddy, S.G.E. Insecticidal and enzyme inhibition activities of leaf/bark extracts, fractions, seed oil and isolated compounds from Triadica sebifera (L.) small against Aphis craccivora Koch. Molecules 2022, 27, 1967. [Google Scholar] [CrossRef]
- Cao, X.; Miao, X.; Ge, M.; Zhang, M.; Lv, Z.; Wang, W.; Chang, Y.; Ouyang, H.; He, J. Exploration of habitat-related chemical markers for Stephania tetrandra applying multiple chromatographic and chemometric analysis. Molecules 2022, 27, 7224. [Google Scholar] [CrossRef]
- Yang, S.; Shan, L.; Luo, H.; Sheng, X.; Du, J.; Li, Y. Rapid classification and identification of chemical components of Schisandra chinensis by UPLC-Q-TOF/MS combined with data post-processing. Molecules 2017, 22, 1778. [Google Scholar] [CrossRef]
- Ji, T.; Wang, J.; Xu, Z.; Cai, H.D.; Su, S.L.; Peng, X.; Ruan, H.S. Combination of mulberry leaf active components possessed synergetic effect on SD rats with diabetic nephropathy by mediating metabolism, Wnt/β-catenin and TGF-β/Smads signaling pathway. J. Ethnopharmacol. 2022, 292, 115026. [Google Scholar] [CrossRef]
- Saied, D.B.; Farag, M.A. How does maturity stage affect seeds metabolome via UPLC/MS based molecular networking and chemometrics and in relation to antioxidant effect? A case study in 4 major cereals and legumes. Food Chem. 2023, 426, 136491. [Google Scholar] [CrossRef]
- Yuan, Z.; Yang, L.; Zhang, X.; Ji, P.; Hua, Y.; Wei, Y. Mechanism of Huang-lian-Jie-du decoction and its effective fraction in alleviating acute ulcerative colitis in mice: Regulating arachidonic acid metabolism and glycerophospholipid metabolism. J. Ethnopharmacol. 2020, 259, 112872. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, H.; Zhao, H.; Geng, Y.; Ren, Y.; Guo, L.; Shi, J.; Xu, Z. Edgeworthia gardneri (Wall.) Meisn. water extract improves diabetes and modulates gut microbiota. J. Ethnopharmacol. 2019, 239, 111854. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hao, E.; Du, C.; Wei, W.; Wang, X.; Liu, T.; Deng, J. Investigating the underlying mechanisms of Ardisia japonica extract’s anti-blood-stasis effect via metabolomics and network pharmacology. Molecules 2023, 28, 7301. [Google Scholar] [CrossRef]
- Negro, E.J.; Sendker, J.; Stark, T.; Lipowicz, B.; Hensel, A. Phytochemical and functional analysis of horseradish (Armoracia rusticana) fermented and non-fermented root extracts. Fitoterapia 2022, 162, 105282. [Google Scholar] [CrossRef]
- Lai, Y.; Hua, L.; Yang, J.; Xu, J.; Chen, J.; Zhang, S.; Zhu, S.; Li, J.; Shi, S. The effect of Chinese agarwood essential oil with cyclodextrin inclusion against PCPA-induced insomnia rats. Molecules 2023, 28, 635. [Google Scholar] [CrossRef] [PubMed]
- LI, J.; Wang, Y.W.; Zheng, H.Y.; Zhong, Y.; Liu, X.Y.; Chen, A.J. Optimization of microwave-hot water extraction process of polyphenols from Phyllanthus emblica L. and its pH stability. Jiangsu J. Agr. Sci. 2020, 36, 733–742. [Google Scholar] [CrossRef]
- Mihailović, V.; Mišić, D.; Matić, S.; Mihailović, M.; Stanić, S.; Vrvić, M.M.; Katanić, J.; Mladenović, M.; Stankovi, N.; Boroja, T.; et al. Comparative phytochemical analysis of Gentiana Cruciata L. roots and aerial parts, and their biological activities. Ind. Crops Prod. 2015, 73, 49–62. [Google Scholar] [CrossRef]
- Aladedunye, F.A.; Przybylski, R. Degradation and nutritional quality changes of oil during frying. J. Am. Oil Chem. Soc. 2009, 86, 149–156. [Google Scholar] [CrossRef]
- Xu, X.Q.; Liu, A.M.; Hu, S.Y.; Ares, I.; Martínez-Larrañaga, M.R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Feng, J.X.; Sun, Y.; Wei, Z.B.; Sun, H.; Li, L.; Zhu, J.Y.; Xia, G.Q.; Zang, H. Screening the extract of Laportea bulbifera (Sieb. et Zucc.) Wedd. based on active component content, its antioxidant capacity and exploration of hepatoprotective activity in rats. Molecules 2023, 28, 6256. [Google Scholar] [CrossRef]
- Li, T.; Guo, Q.; Qu, Y.; Li, Y.; Liu, H.; Liu, L.; Zhang, Y.; Jiang, Y.; Wang, Q. Solubility and physicochemical properties of resveratrol in peanut oil. Food Chem. 2022, 368, 130687. [Google Scholar] [CrossRef]
- Endo, Y. Analytical methods to evaluate the quality of edible fats and oils: The JOCS standard methods for analysis of fats, oils and related materials (2013) and advanced methods. J. Oleo Sci. 2018, 67, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Editorial Committee of Chinese Materia Medica, State Administration of TCM. Chinese Materia Medica (Zhonghua Bencao); Shanghai Scientific and Technical Publishers: Shanghai, China, 2000; Volume 2, pp. 650–651. [Google Scholar]
- Shukla, V.; Asthana, S.; Gupta, P.; Dwivedi, P.D.; Tripathi, A.; Das, M. Toxicity of naturally occurring anthraquinones. Adv. Mol. Toxicol. 2017, 11, 1–50. [Google Scholar] [CrossRef]
- Dong, X.; Zeng, Y.; Liu, Y.; You, L.; Yin, X.; Fu, J.; Ni, J. Aloe-emodin: A review of its pharmacology, toxicity, and pharmacokinetics. Phytother. Res. 2020, 34, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K.; Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.C.; Zhou, Y.X. A summary of the chemical pharmacologic and clinical study of Zhushaqi—A herbal in the Taibai mountain. Chin. J. Ethnomed. Ethnopharm. 1999, 36, 42–43. [Google Scholar]
- Williams, R. Global challenges in liver disease. Hepatology 2006, 44, 521–526. [Google Scholar] [CrossRef]
- Chinnappan, R.; Mir, T.A.; Alsalameh, S.; Makhzoum, T.; Adeeb, S.; Al-Kattan, K.; Yaqinuddin, A. Aptasensors are conjectured as promising ALT and AST diagnostic tools for the early diagnosis of acute liver injury. Life 2023, 13, 1273. [Google Scholar] [CrossRef]
- Peng, X.W.; Yang, Y.Q.; Tang, L.; Wan, J.Y.; Dai, J.; Li, L.J.; Huang, J.Y.; Shen, Y.; Lin, L.; Gong, X.Q.; et al. Therapeutic benefits of apocynin in mice with lipopolysaccharide/d-galactosamine-induced acute liver injury via suppression of the late stage pro-apoptotic AMPK/JNK pathway. Biomed. Pharmacother. 2020, 125, 110020. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, F.Y.; Li, J.; Guo, H.L. Tumor-suppressive effect of total anthraquinone of Polygonum Cillinerve (Nakai) Ohwi on H22 hormonal mice and its effect on glucose metabolism. Chin. Tradit. Pat. Med. 2013, 35, 2508–2511. [Google Scholar] [CrossRef]
- Cui, J.J. Extraction of Crude Polysaccharides from Polygonum Cillinerve (Nakai) Ohwi and Their Biological Activities. Master’s Thesis, Shaanxi Normal University, Xi’an, China, 2008. [Google Scholar]
- Li, R.; Li, J.; Huang, Y.; Li, H.; Yan, S.; Lin, J.; Chen, Y.; Wu, L.; Liu, B.; Wang, G.; et al. Polydatin attenuates diet-inducednonalcoholic steatohepatitis and fibrosis in mice. Int. J. Biol. Sci. 2018, 14, 1411–1425. [Google Scholar] [CrossRef]
- Chan, C.C.; Lee, K.C.; Huang, Y.H.; Chou, C.K.; Lin, H.C.; Lee, F.Y. Regulation by resveratrol of the cellular factors mediating liver damage and regeneration after acute toxic liver injury. J. Gastroenterol. Hepatol. 2014, 29, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, M.H.; He, X.; Zhao, Y.M.; Yu, J.H.; Zhu, J.Y.; Li, L.; Xia, G.Q.; Zang, H. Phytochemical profiling and antioxdant, enzyme-inhibitory, and toxic activities of extracts from Adonis ramosa Franch. Nat. Prod. Res. 2023, 37, 2269–2273. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Feng, J.X.; Wei, Z.B.; Sun, H.; Li, L.; Zhu, J.Y.; Xia, G.Q.; Zang, H. Phytochemical analysis, antioxidant activities in vitro and in vivo, and theoretical calculation of different extracts of Euphorbia fischeriana. Molecules 2023, 28, 5172. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).