Determination and Ecological Risk Assessment of Quinolone Antibiotics in Drinking and Environmental Waters Using Fully Automated Disk-Based SPE Coupled with UPLC–MS/MS
Abstract
1. Introduction
2. Results and Discussion
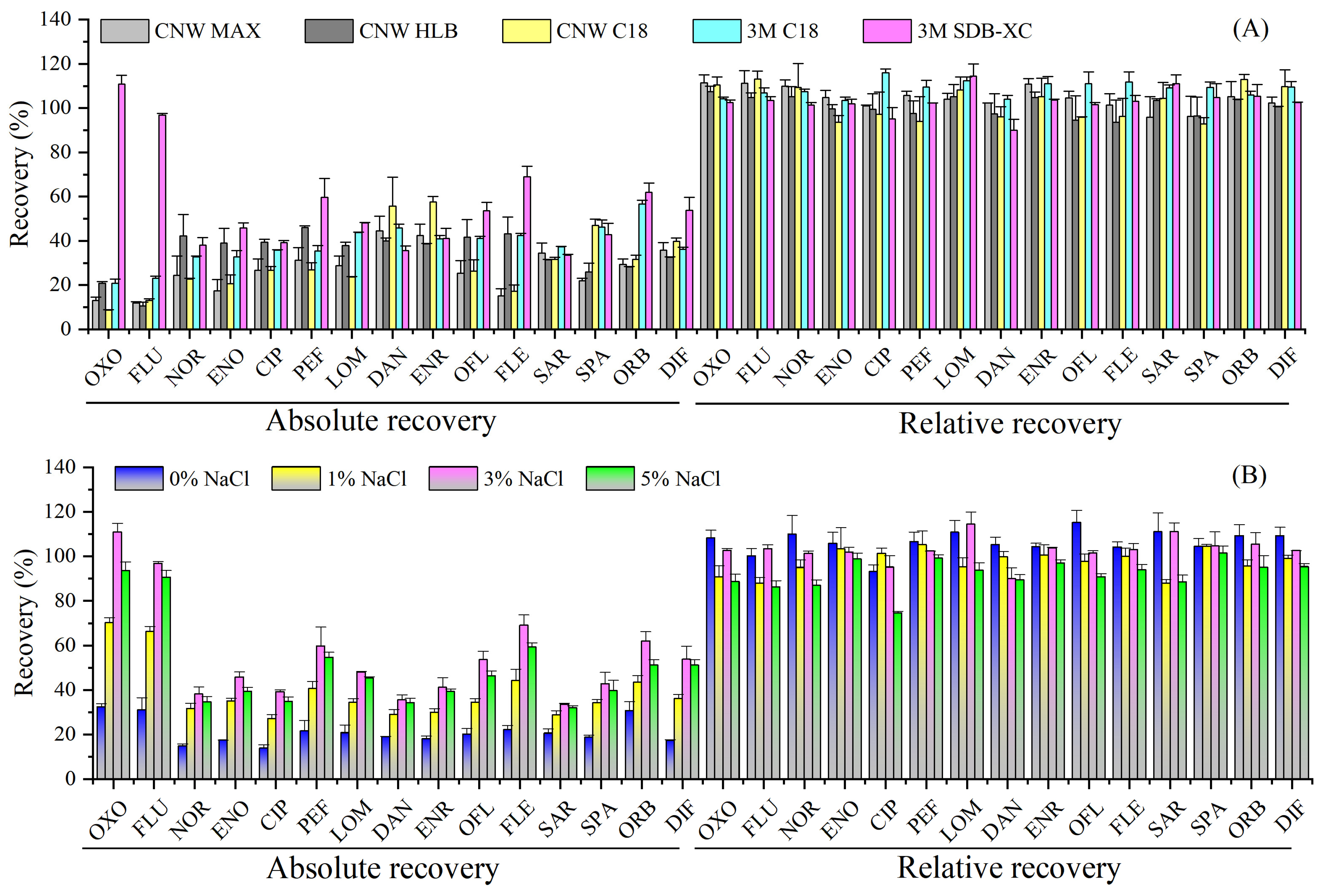
2.1. Optimization of Automated Disk-Based Solid-Phase Extraction Procedures
2.1.1. Selection of Disk Adsorbents
2.1.2. Effect of Ionic Strength
2.1.3. Effect of Na2EDTA and Sample pH
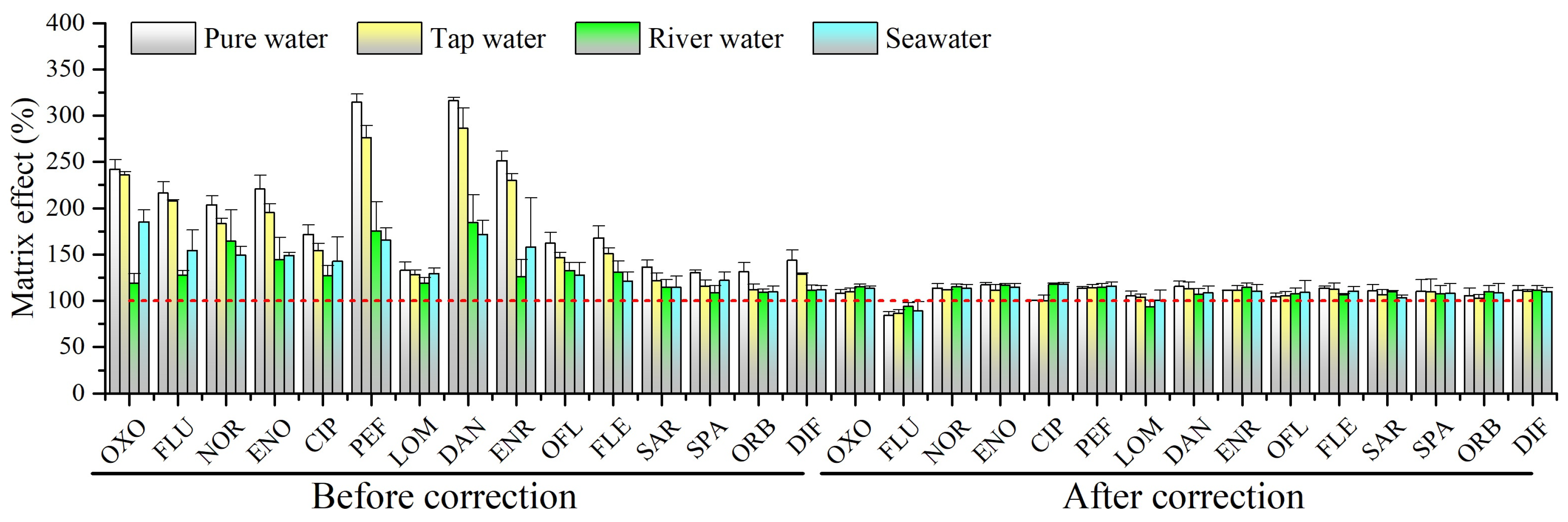
2.2. Matrix Effect
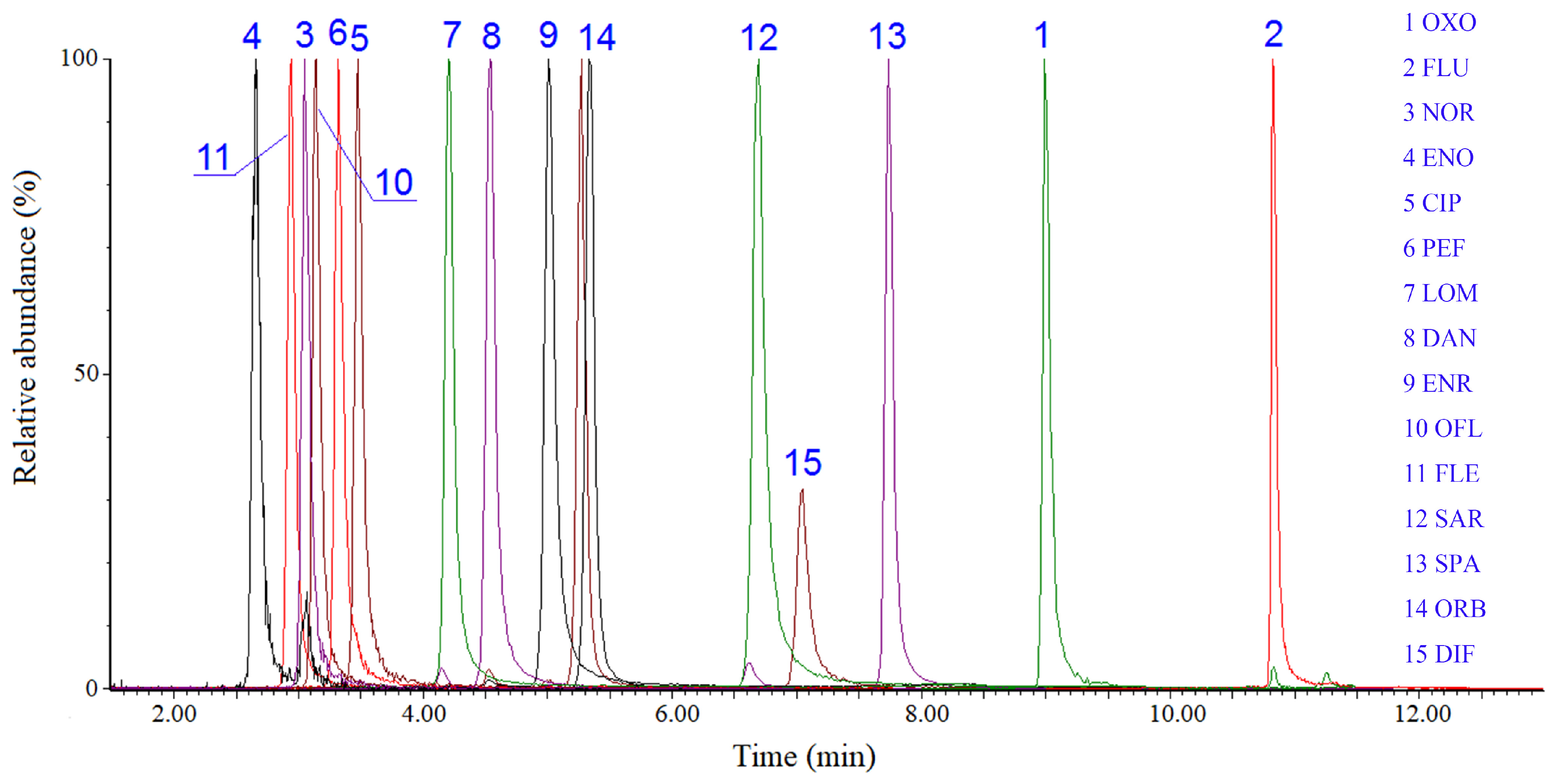
2.3. Evaluation of the Method Performance
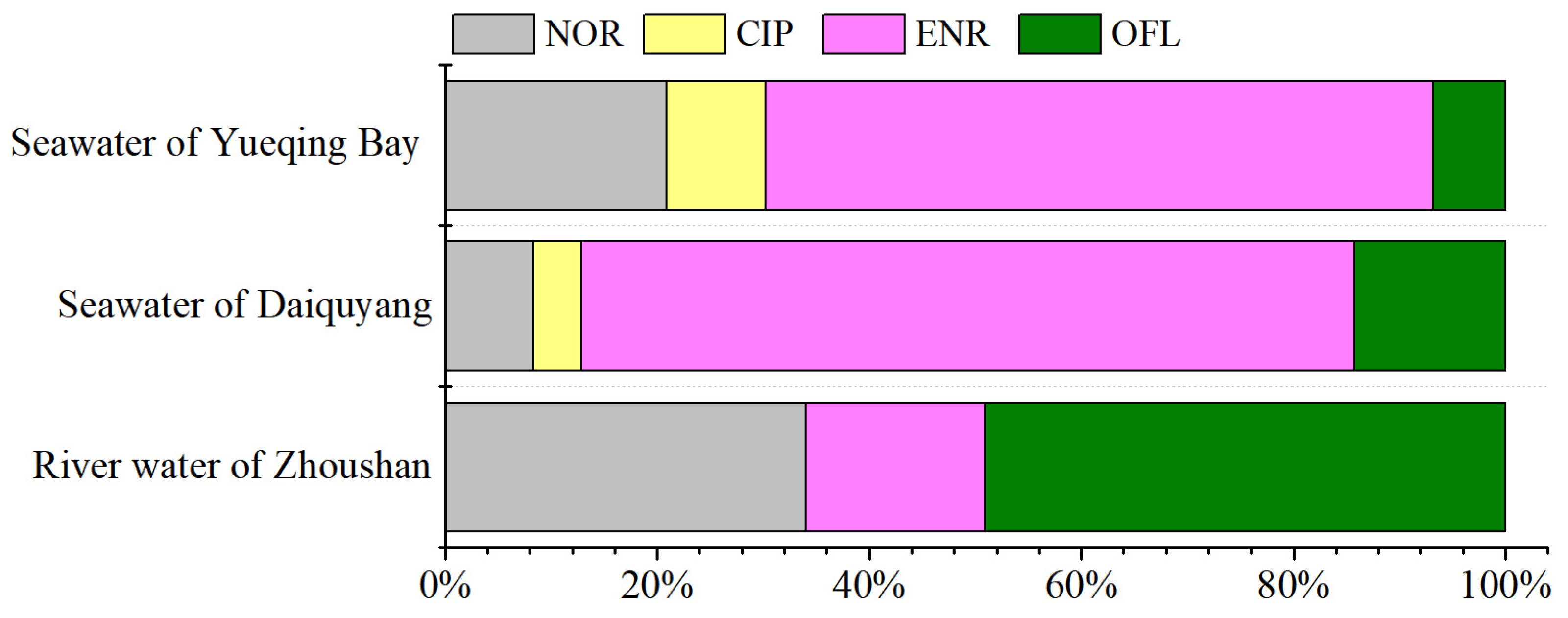
2.4. Real Water Analysis
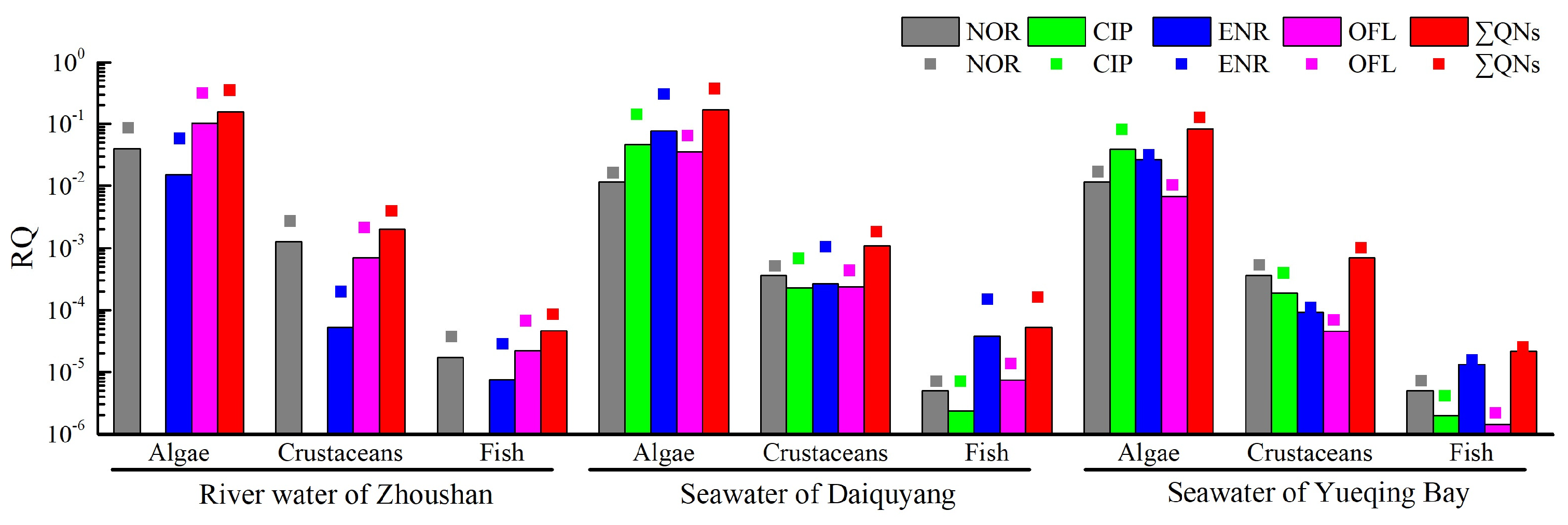
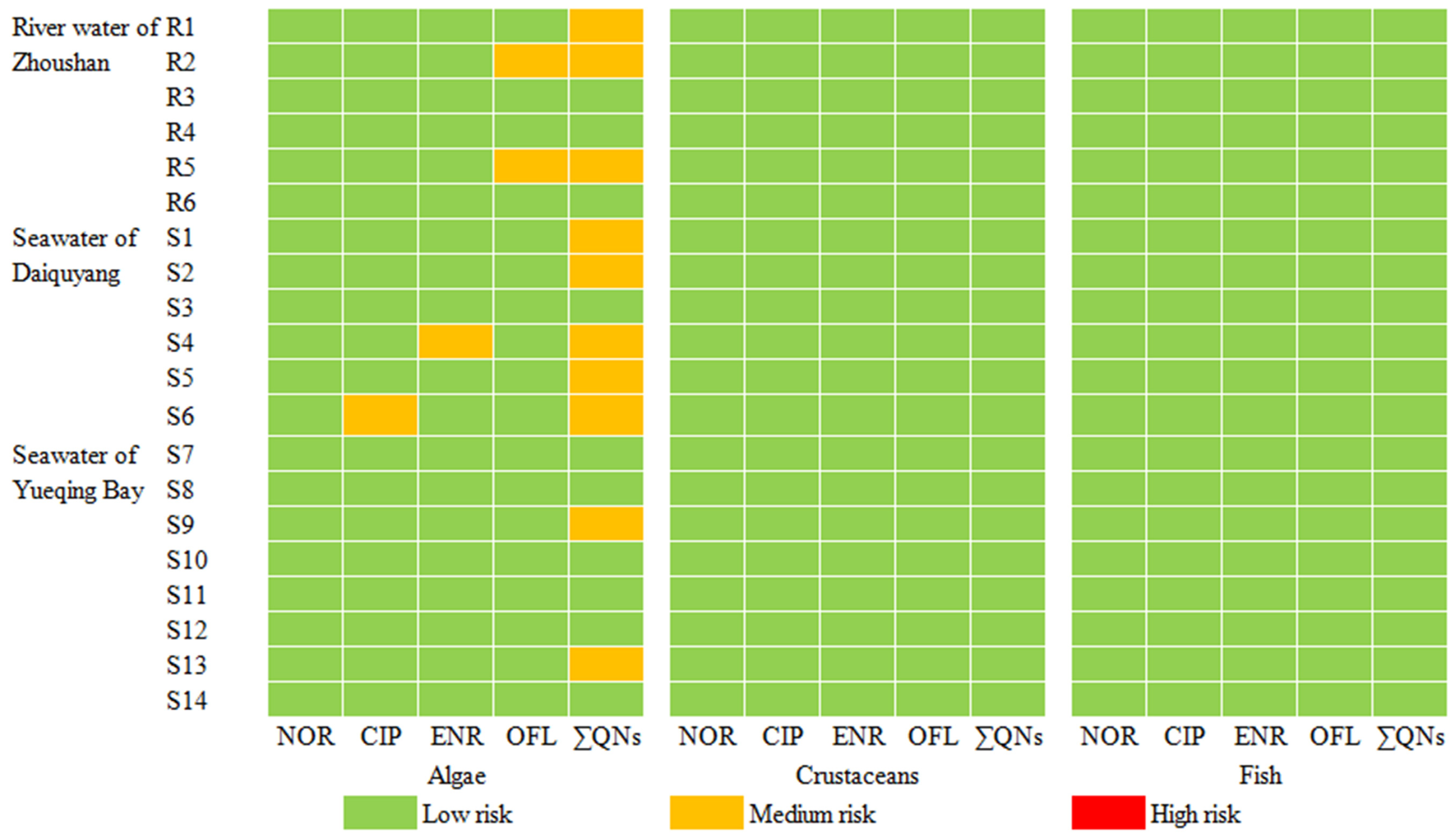
2.5. Ecological Risk Assessment
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sampling and Preparation
3.3. Automated Disk-Based Solid-Phase Extraction
3.4. Instrumental Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, L.; Chen, C.; Li, S.; Ye, P.; Shi, Y.; Sharma, G.; Sarkar, B.; Shaheen, S.M.; Lee, S.S.; Xiao, R.; et al. A comprehensive and global evaluation of residual antibiotics in agricultural soils: Accumulation, potential ecological risks, and attenuation strategies. Ecotox. Environ. Saf. 2023, 262, 115175. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial resistance (AMR). Brit. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, L.; Xu, L.; Gracia-Marín, E.; Pitarch, E.; Serrano, R.; Kasprzyk-Hordern, B. Understanding associations between antimicrobial agents usage and antimicrobial resistance genes prevalence at the community level using wastewater-based epidemiology: A Spanish pilot study. Sci. Total Environ. 2024, 926, 171996. [Google Scholar] [CrossRef]
- Rambliere, L.; Guillemot, D.; Delarocque-Astagneau, E.; Huynh, B.T. Impact of mass and systematic antibiotic administration on antibiotic resistance in low-and middle-income countries. A systematic review. Int. J. Antimicrob. Agents 2021, 58, 106364. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, T. A comprehensive review on quinolone contamination in environments: Current research progress. Environ. Sci. Pollut. R. 2023, 30, 48778–48792. [Google Scholar] [CrossRef] [PubMed]
- Jiwanti, P.K.; Wardhana, B.Y.; Sutanto, L.G.; Chanif, M.F. A Review on Carbon-based Electrodes for Electrochemical Sensor of Quinolone Antibiotics. ChemistrySelect 2022, 7, e202103997. [Google Scholar] [CrossRef]
- Meng, F.; Sun, S.; Geng, J.; Ma, L.; Jiang, J.; Li, B.; Yabo, S.D.; Lu, L.; Fu, D.; Shen, J.; et al. Occurrence, distribution, and risk assessment of quinolone antibiotics in municipal sewage sludges throughout China. J. Hazard. Mater. 2023, 453, 131322. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Chen, M.; Feng, Q.; Zhang, X.; Wang, S.; Zhao, R.; Jiang, T. Adsorption and photocatalytic degradation of quinolone antibiotics from wastewater using functionalized biochar. Environ. Pollut. 2023, 336, 122409. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Y.; Zhang, L.; Zhao, B.; Lu, M.; Cui, J.; Tang, W. Source apportionment and specific-source-site risk of quinolone antibiotics for effluent-receiving urban rivers and groundwater in a city, China. J. Environ. Sci. 2024, 144, 185–198. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Shan, X.; Yang, Y.; Song, L.; Teng, Y.; Chen, H. Meta-analysis addressing the characterization and risk identification of antibiotics and antibiotic resistance genes in global groundwater. Sci. Total Environ. 2023, 860, 160513. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Y.; Wang, Y.; Cui, F. Investigation on the fate of quinolone antibiotics in three drinking water treatment plants of China. Water Supply 2022, 22, 170–180. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, T.; Wang, Y.; Tao, H.; Liu, S.; Shi, W. The occurrence and removal of selected fluoroquinolones in urban drinking water treatment plants. Environ. Monit. Assess. 2015, 187, 729. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, B.; Liu, M.; Mao, S. Demand, status, and prospect of antibiotics detection in the environment. Sensor. Actuat. B Chem. 2022, 369, 132383. [Google Scholar] [CrossRef]
- Rusu, A.; Hancu, G.; Völgyi, G.; Tóth, G.; Noszál, B.; Gyéresi, Á. Separation and determination of quinolone antibacterials by capillary electrophoresis. J. Chromatogra. Sci. 2014, 52, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Zhu, X.; Zhao, P.; Ren, W. Determination of quinolone antibiotics in surface water by MnFe2O4@ TiO2 magnetic solid phase extraction-high performance liquid chromatography. Microchem. J. 2024, 204, 111090. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Mu, Y.; Liu, H. A method for simultaneously and accurately quantify seven quinolones in matrix reference materials by HPLC-MS. Microchem. J. 2024, 204, 110984. [Google Scholar] [CrossRef]
- Guidi, L.R.; Santos, F.A.; Ribeiro, A.C.S.; Fernandes, C.; Silva, L.H.; Gloria, M.B.A. A simple, fast and sensitive screening LC-ESI-MS/MS method for antibiotics in fish. Talanta 2017, 163, 85–93. [Google Scholar] [CrossRef]
- Maia, A.S.; Paíga, P.; Delerue-Matos, C.; Castro, P.M.; Tiritan, M.E. Quantification of fluoroquinolones in wastewaters by liquid chromatography-tandem mass spectrometry. Environ. Pollut. 2020, 259, 113927. [Google Scholar] [CrossRef]
- Ye, S.B.; Huang, Y.; Lin, D.Y. QuEChERS sample pre-processing with UPLC–MS/MS: A method for detecting 19 quinolone-based veterinary drugs in goat’s milk. Food Chem. 2022, 373, 131466. [Google Scholar]
- Li, Q.; Zhu, S.; Wu, F.; Chen, F.; Guo, C. Slice-layer COFs-aerogel: A regenerative dispersive solid-phase extraction adsorbent for determination of ultra-trace quinolone antibiotics. Microchim. Acta 2023, 190, 369. [Google Scholar] [CrossRef]
- Pang, J.L.; Liao, Y.M.; Huang, X.J.; Ye, Z.; Yuan, D.X. Metalorganic framework-monolith composite-based in-tube solid phase microextraction on-line coupled to high-performance liquid chromatography-fluorescence detection for the highly sensitive monitoring of fluoroquinolones in water and food samples. Talanta 2019, 99, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Selahle, S.K.; Nomngongo, P.N. Determination of fluoroquinolones in the environmental samples using vortex assisted dispersive liquid-liquid microextraction coupled with high performance liquid chromatography. Int. J. Environ. Anal. Chem. 2020, 100, 282–294. [Google Scholar] [CrossRef]
- Huang, X.; Qiu, N.; Yuan, D.; Lin, Q. Preparation of a mixed stir bar for sorptive extraction based on monolithic material for the extraction of quinolones from wastewater. J. Chromatogra. A 2010, 1217, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Mejías, C.; Santos, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Automatised on-line SPE-chiral LC-MS/MS method for the enantiomeric determination of main fluoroquinolones and their metabolites in environmental water samples. Microchem. J. 2023, 185, 108217. [Google Scholar] [CrossRef]
- Erger, C.; Schmidt, T.C. Disk-based solid-phase extraction analysis of organic substances in water. TrAC Trend. Anal. Chem. 2014, 61, 74–82. [Google Scholar] [CrossRef]
- Calderilla, C.; Maya, F.; Cerdà, V.; Leal, L.O. 3D printed device for the automated preconcentration and determination of chromium (VI). Talanta 2018, 184, 15–22. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; He, X.; Wang, Z.; Wu, D.; Zheng, X.; Zheng, L.; Wang, B. Simultaneous determination of neonicotinoids and fipronil and its metabolites in environmental water from coastal bay using disk-based solid-phase extraction and high-performance liquid chromatography–tandem mass spectrometry. Chemosphere 2019, 234, 224–231. [Google Scholar] [CrossRef]
- Peixoto, P.S.; Toth, I.V.; Barreiros, L.; Machado, A.; Bordalo, A.A.; Lima, J.L.; Segundo, M.A. Screening of fluoroquinolones in environmental waters using disk-based solid-phase extraction combined to microplate fluorimetric determination and LC-MS/MS. Int. J. Environ. Anal. Chem. 2019, 99, 258–269. [Google Scholar] [CrossRef]
- Qi, M.; He, P.; Hu, H.; Zhang, T.; Li, T.; Zhang, X.; Qin, Y.; Zhu, Y.; Guo, Y. An automated solid-phase extraction–UPLC–MS/MS method for simultaneous determination of sulfonamide antimicrobials in environmental water. Molecules 2023, 28, 4694. [Google Scholar] [CrossRef]
- Huber, C.; Klimant, I.; Krause, C.; Werner, T.; Mayr, T.; Wolfbeis, O.S. Optical sensor for seawater salinity. Fresenius J. Anal. Chem. 2000, 368, 196–202. [Google Scholar] [CrossRef]
- Belal, F.; El-Enany, N.; Wahba, M.E.K. Analytical methods for the determination of some selected 4-quinolone antibacterials. Rev. Anal. Chem. 2017, 36, 20150020. [Google Scholar] [CrossRef]
- Mokh, S.; El Khatib, M.; Koubar, M.; Daher, Z.; Al Iskandarani, M. Innovative SPE-LCMS/MS technique for the assessment of 63 pharmaceuticals and the detection of antibiotic-resistant-bacteria: A case study natural water sources in Lebanon. Sci. Total Environ. 2017, 609, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Seifrtová, M.; Nováková, L.; Lino, C.; Pena, A.; Solich, P. An overview of analytical methodologies for the determination of antibiotics in environmental waters. Anal. Chim. Acta 2009, 649, 158–179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Ying, G.G.; Liu, S.; Zhao, J.L.; Chen, F.; Zhang, R.Q.; Peng, F.Q.; Zhang, Q.Q. Simultaneous determination of human and veterinary antibiotics in various environmental matrices by rapid resolution liquid chromatography–electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2012, 1244, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Ellison, S.L.; Wood, R. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Xue, M.; Wu, H.; Liu, S.; Huang, X.; Jin, Q.; Ren, R. Simultaneous determination of 44 pharmaceutically active compounds in water samples using solid-phase extraction coupled with ultra-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 203–222. [Google Scholar] [CrossRef]
- Panditi, V.R.; Batchu, S.R.; Gardinali, P.R. Online solid-phase extraction–liquid chromatography–electrospray–tandem mass spectrometry determination of multiple classes of antibiotics in environmental and treated waters. Anal. Bioanal. Chem. 2013, 405, 5953–5964. [Google Scholar] [CrossRef]
- Dorival-García, N.; Labajo-Recio, C.; Zafra-Gómez, A.; Juárez-Jiménez, B.; Vílchez, J.L. Improved sample treatment for the determination of 17 strong sorbed quinolone antibiotics from compost by ultra high performance liquid chromatography tandem mass spectrometry. Talanta 2015, 138, 247–257. [Google Scholar] [CrossRef]
- Han, Q.F.; Zhao, S.; Zhang, X.R.; Wang, X.L.; Song, C.; Wang, S.G. Distribution, combined pollution and risk assessment of antibiotics in typical marine aquaculture farms surrounding the Yellow Sea, North China. Environ. Int. 2020, 138, 105551. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Xiong, L.; Song, G.; Liu, F. Changes of antibiotic occurrence and hydrochemistry in groundwater under the influence of the South-to-North Water Diversion (the Hutuo River, China). Sci. Total Environ. 2022, 832, 154779. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, H.; Du, J.; Qu, Y.; Shen, C.; Tan, F.; Chen, J.; Quan, X. Occurrence, removal, and risk assessment of antibiotics in 12 wastewater treatment plants from Dalian, China. Environ. Sci. Pollut. Res. 2017, 24, 16478–16487. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Zhuo, X.; Guo, Y. Occurrence and risk assessment of four typical fluoroquinolone antibiotics in raw and treated sewage and in receiving waters in Hangzhou, China. J. Agric. Food Chem. 2011, 59, 7303–7309. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.F.; Song, C.; Sun, X.; Zhao, S.; Wang, S.G. Spatiotemporal distribution, source apportionment and combined pollution of antibiotics in natural waters adjacent to mariculture areas in the Laizhou Bay, Bohai Sea. Chemosphere 2021, 279, 130381. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Bao, Y.; Xu, B. Seasonal variation of antibiotics in surface water of Pudong New Area of Shanghai, China and the occurrence in typical wastewater sources. Chemosphere 2020, 239, 124816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, L.; Qin, S.; Cui, J.; Liu, Y. Quinolones antibiotics in the Baiyangdian Lake, China: Occurrence, distribution, predicted no-effect concentrations (PNECs) and ecological risks by three methods. Environ. Pollut. 2020, 256, 113458. [Google Scholar] [CrossRef]
- Faleye, A.C.; Adegoke, A.A.; Ramluckan, K.; Fick, J.; Bux, F.; Stenström, T.A. Concentration and reduction of antibiotic residues in selected wastewater treatment plants and receiving waterbodies in Durban, South Africa. Sci. Total Environ. 2019, 678, 10–20. [Google Scholar] [CrossRef]
- Pereira, A.M.; Silva, L.J.; Meisel, L.M.; Pena, A. Fluoroquinolones and tetracycline antibiotics in a Portuguese aquaculture system and aquatic surroundings: Occurrence and environmental impact. J. Toxicol. Environ. Health Part A 2015, 78, 959–975. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, I.S.; Oh, J.E. Human and veterinary pharmaceuticals in the marine environment including fish farms in Korea. Sci. Total Environ. 2017, 579, 940–949. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, S.; Wang, Y.; Liu, X.; Liu, Y.; Xu, J.; Zhang, T.; Wang, Z.; Yang, Y. Occurrence of antibiotics and antibiotic resistance genes and their correlations in lower Yangtze River, China. Environ. Pollut. 2020, 257, 113365. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, Y.; Zhang, R.; Han, M.; Zeng, W.; Wang, Y.; Yu, K.; Yang, Y. Occurrence, source, and the fate of antibiotics in mariculture ponds near the Maowei Sea, South China: Storm caused the increase of antibiotics usage. Sci. Total Environ. 2021, 752, 141882. [Google Scholar] [CrossRef]
- Chen, J.; Huang, L.; Wang, Q.; Zeng, H.; Xu, J.; Chen, Z. Antibiotics in aquaculture ponds from Guilin, South of China: Occurrence, distribution, and health risk assessment. Environ. Res. 2022, 204, 112084. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Shi, H.; Liu, J.; Zheng, C.; Lu, G.; Hao, S.; Jin, Y.; He, C. Occurrence, source apportionment and ecological risk assessment of thirty antibiotics in farmland system. J. Environ. Manag. 2023, 335, 117546. [Google Scholar] [CrossRef]
- Khan, A.H.; Aziz, H.A.; Khan, N.A.; Hasan, M.A.; Ahmed, S.; Farooqi, I.H.; Dhingra, A.; Vambol, V.; Changani, F.; Yousefi, M.; et al. Impact, disease outbreak and the eco-hazards associated with pharmaceutical residues: A Critical review. Int. J. Environ. Sci. Technol. 2022, 19, 677–688. [Google Scholar] [CrossRef]
- Bhatt, S.; Chatterjee, S. Fluoroquinolone antibiotics: Occurrence, mode of action, resistance, environmental detection, and remediation–A comprehensive review. Environ. Pollut. 2022, 315, 120440. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Qi, M.; He, P.; Chen, X.; Li, Z.; Cheng, H. Occurrence and risk assessment of quinolones and sulfonamides in freshwater aquaculture ponds in Northeast Zhejiang, China. Sci. Total Environ. 2024, 953, 176066. [Google Scholar] [CrossRef]
- Ando, T.; Nagase, H.; Eguchi, K.; Hirooka, T.; Nakamura, T.; Miyamoto, K.; Hirata, K. A novel method using cyanobacteria for ecotoxicity test of veterinary antimicrobial agents. Environ. Toxicol. Chem. 2007, 26, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Li, Z.; Liu, J. Effects of selected pharmaceuticals on growth, reproduction and feeding of Daphnia Magna. Fresenius Environ. Bull. 2013, 22, 2588–2594. [Google Scholar]
- Liu, J.; Lu, G.; Wu, D.; Yan, Z. A multi-biomarker assessment of single and combined effects of norfloxacin and sulfamethoxazole on male goldfish (Carassius auratus). Ecotoxicol. Environ. Saf. 2014, 102, 12–17. [Google Scholar] [CrossRef]
- Halling-Sorensen, B.; Lützhøft, H.C.H.; Andersen, H.R.; Ingerslev, F. Environmental risk assessment of antibiotics: Comparison of mecillinam, trimethoprim and ciprofloxacin. J. Antimicrob. Chemother. 2000, 461, 53–58. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Kim, P.G.; Lee, C.; Choi, K.; Choi, K. Implication of global environmental changes on chemical toxicity-effect of water temperature, pH, and ultraviolet B irradiation on acute toxicity of several pharmaceuticals in Daphnia magna. Ecotoxicology 2010, 19, 662–669. [Google Scholar] [CrossRef]
- Shahnaz, T.; Priyan, V.V.; Pandian, S.; Narayanasamy, S. Use of Nanocellulose extracted from grass for adsorption abatement of ciprofloxacin and diclofenac removal with phyto, and fish toxicity studies. Environ. Pollut. 2021, 268, 115494. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.A.; Belden, J.B.; Lydy, M.J. Toxicity of fluoroquinolone antibiotics to aquatic organisms. Environ. Toxicol. Chem. 2005, 24, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.R.; Bell, T.A.; Lightner, D.V. Shrimp antimicrobial testing. II. Toxicity testing and safety determination for twelve antimicrobials with penaeid shrimp larvae. J. Aquat. Anim. Health 1992, 4, 262–270. [Google Scholar] [CrossRef]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Pascarella, L.; Parrella, A. Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci. Total Environ. 2005, 346, 87–98. [Google Scholar] [CrossRef] [PubMed]
| Analyte | ILIS | Linear Range (μg/L) | Calibration Curve | r2 | LOD a (ng/L) | LOQ b (ng/L) | EFs | Precision, RSD (%, n = 6) | |
|---|---|---|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | ||||||||
| OXO | OXO-D5 | 0.05−100 | y = 0.75x − 0.008 | 0.9998 | 0.014 | 0.045 | 1022 | 4.4 | 8.7 |
| FLU | FLU-13C3 | 0.05−100 | y = 1.10x − 0.001 | 0.9998 | 0.012 | 0.040 | 1032 | 7.7 | 12.3 |
| NOR | NOR-D5 | 0.05−100 | y = 1.14x − 0.012 | 0.9999 | 0.026 | 0.080 | 1009 | 7.9 | 9.8 |
| ENO | ENO-D8 | 0.05−100 | y = 1.24x − 0.052 | 0.9992 | 0.055 | 0.160 | 1012 | 10.8 | 11.6 |
| CIP | CIP-D8 | 0.05−100 | y = 1.01x + 0.025 | 0.9997 | 0.025 | 0.080 | 950 | 4.1 | 4.6 |
| PEF | PEF-D5 | 0.05−100 | y = 1.17x + 0.163 | 0.9996 | 0.018 | 0.055 | 1018 | 8.6 | 9.9 |
| LOM | LOM-D5 | 0.05−100 | y = 1.18x + 0.135 | 0.9992 | 0.022 | 0.065 | 1136 | 7.7 | 11.1 |
| DAN | DAN-D3 | 0.05−100 | y = 1.08x + 0.053 | 0.9999 | 0.008 | 0.025 | 897 | 6.9 | 9.8 |
| ENR | ENR-D5 | 0.05−100 | y = 1.13x + 0.011 | 0.9994 | 0.024 | 0.075 | 1031 | 3.9 | 8.2 |
| OFL | OFL-D3 | 0.05−100 | y = 1.14x + 0.075 | 0.9999 | 0.022 | 0.070 | 1012 | 2.9 | 7.7 |
| FLE | FLE-D3 | 0.05−100 | y = 1.19x + 0.008 | 0.9997 | 0.022 | 0.070 | 1028 | 2.7 | 7.8 |
| SAR | SAR-D8 | 0.05−100 | y = 1.21x + 0.016 | 0.9996 | 0.022 | 0.070 | 1108 | 4.9 | 9.7 |
| SPA | SPA-D4 | 0.05−100 | y = 1.06x − 0.006 | 0.9992 | 0.02 | 0.060 | 1039 | 4.4 | 8.1 |
| ORB | ORB-D4 | 0.05−100 | y = 0.59x + 0.001 | 0.9997 | 0.02 | 0.060 | 1048 | 4.0 | 7.6 |
| DIF | DIF-D3 | 0.05−100 | y = 0.79x − 0.008 | 0.9996 | 0.024 | 0.075 | 1023 | 5.0 | 8.3 |
| Analyte | Retention Time (min) | Precursor Ion (m/z) | Product Ion (m/z) | Cone Voltage (V) | Collision Energy (eV) |
|---|---|---|---|---|---|
| OXO | 9.01 | 262 | 216, 244 * | 23 | 30, 20 |
| FLU | 10.83 | 262.1 | 202, 244 * | 29 | 32, 18 |
| NOR | 3.06 | 320.1 | 233, 276.1 * | 30 | 25, 18 |
| ENO | 2.65 | 321.1 | 232, 303.1 * | 32 | 30, 35 |
| CIP | 3.48 | 332.2 | 288.2, 314.2 * | 28 | 16, 15 |
| PEF | 3.32 | 334.1 | 290.1, 316.1 * | 34 | 15, 20 |
| LOM | 4.20 | 352.1 | 265.1 *, 308.1 | 31 | 22, 15 |
| DAN | 4.53 | 358.2 | 96, 340.2 * | 34 | 25, 22 |
| ENR | 5.01 | 360.4 | 245.2, 316.2 * | 32 | 18, 20 |
| OFL | 3.14 | 362.1 | 261.1, 318.1 * | 30 | 20, 20 |
| FLE | 2.94 | 370.1 | 269.1, 326.1 * | 34 | 25, 19 |
| SAR | 6.69 | 386.2 | 299.1, 342.1 * | 33 | 27, 18 |
| SPA | 7.74 | 393.2 | 292.1 *, 349.1 | 37 | 24, 20 |
| ORB | 5.33 | 396.1 | 295.1 *, 352 | 36 | 22, 15 |
| DIF | 7.05 | 400.2 | 299, 356.1 * | 37 | 27, 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Da, X.; Li, Z.; Li, T.; Zhang, X.; Bian, T.; Jin, Y.; Xu, K.; Guo, Y. Determination and Ecological Risk Assessment of Quinolone Antibiotics in Drinking and Environmental Waters Using Fully Automated Disk-Based SPE Coupled with UPLC–MS/MS. Molecules 2024, 29, 4611. https://doi.org/10.3390/molecules29194611
Hu H, Da X, Li Z, Li T, Zhang X, Bian T, Jin Y, Xu K, Guo Y. Determination and Ecological Risk Assessment of Quinolone Antibiotics in Drinking and Environmental Waters Using Fully Automated Disk-Based SPE Coupled with UPLC–MS/MS. Molecules. 2024; 29(19):4611. https://doi.org/10.3390/molecules29194611
Chicago/Turabian StyleHu, Hongmei, Xingyu Da, Zhenhua Li, Tiejun Li, Xiaoning Zhang, Tianbin Bian, Yanjian Jin, Kaida Xu, and Yuanming Guo. 2024. "Determination and Ecological Risk Assessment of Quinolone Antibiotics in Drinking and Environmental Waters Using Fully Automated Disk-Based SPE Coupled with UPLC–MS/MS" Molecules 29, no. 19: 4611. https://doi.org/10.3390/molecules29194611
APA StyleHu, H., Da, X., Li, Z., Li, T., Zhang, X., Bian, T., Jin, Y., Xu, K., & Guo, Y. (2024). Determination and Ecological Risk Assessment of Quinolone Antibiotics in Drinking and Environmental Waters Using Fully Automated Disk-Based SPE Coupled with UPLC–MS/MS. Molecules, 29(19), 4611. https://doi.org/10.3390/molecules29194611






