Ascorbic Acid and Graphene Oxide Exposure in the Model Organism Acheta domesticus Can Change the Reproduction Potential
Abstract
1. Introduction
2. Results
2.1. Vg Gene Relative Expression
2.2. Total Vg Protein Expression
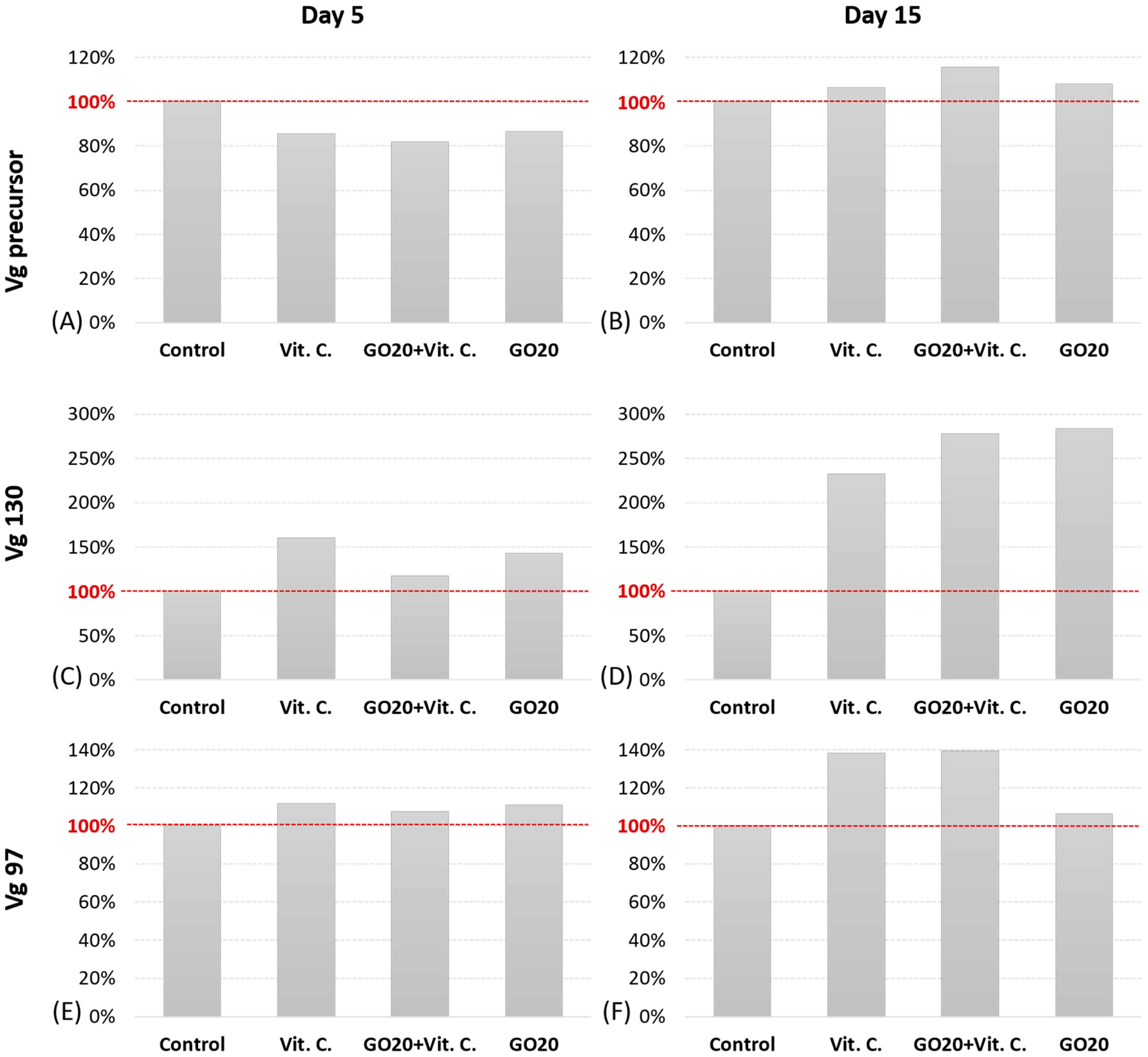
2.3. Vg Protein Precursor and Subunits Expression
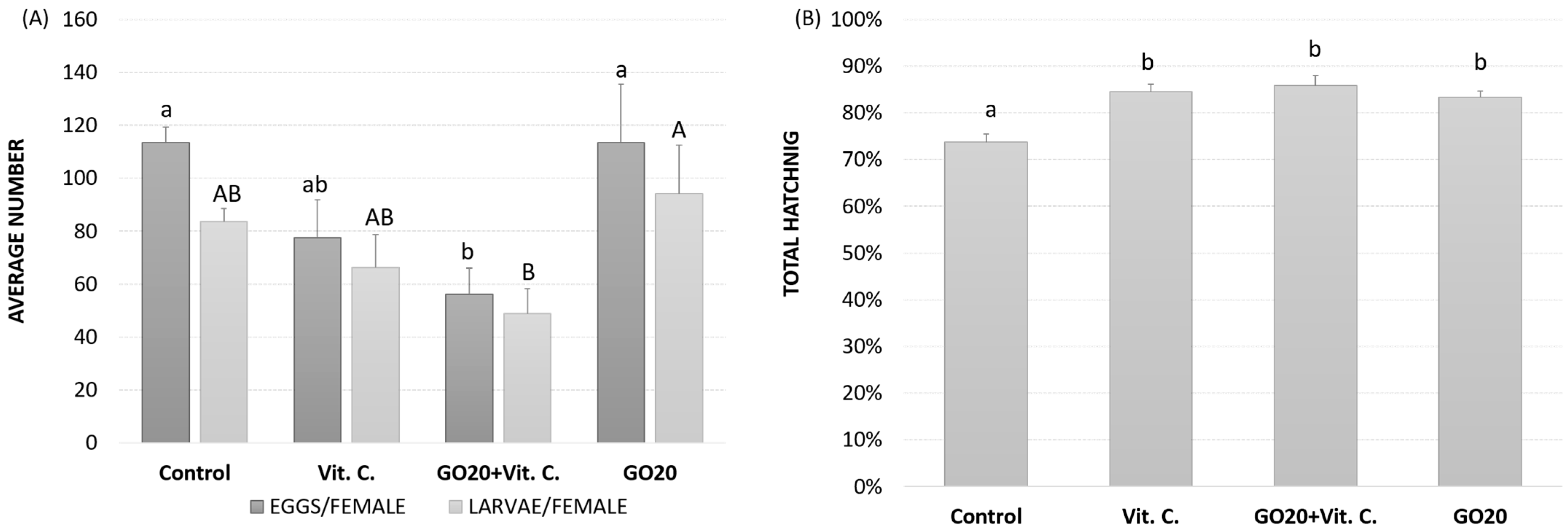
2.4. Egg Laying and Hatching Success
2.5. Major Energetic Components in Eggs
2.5.1. Lipids
2.5.2. Glucose
2.5.3. Glycogen
2.5.4. Total Protein
3. Discussion
4. Materials and Methods
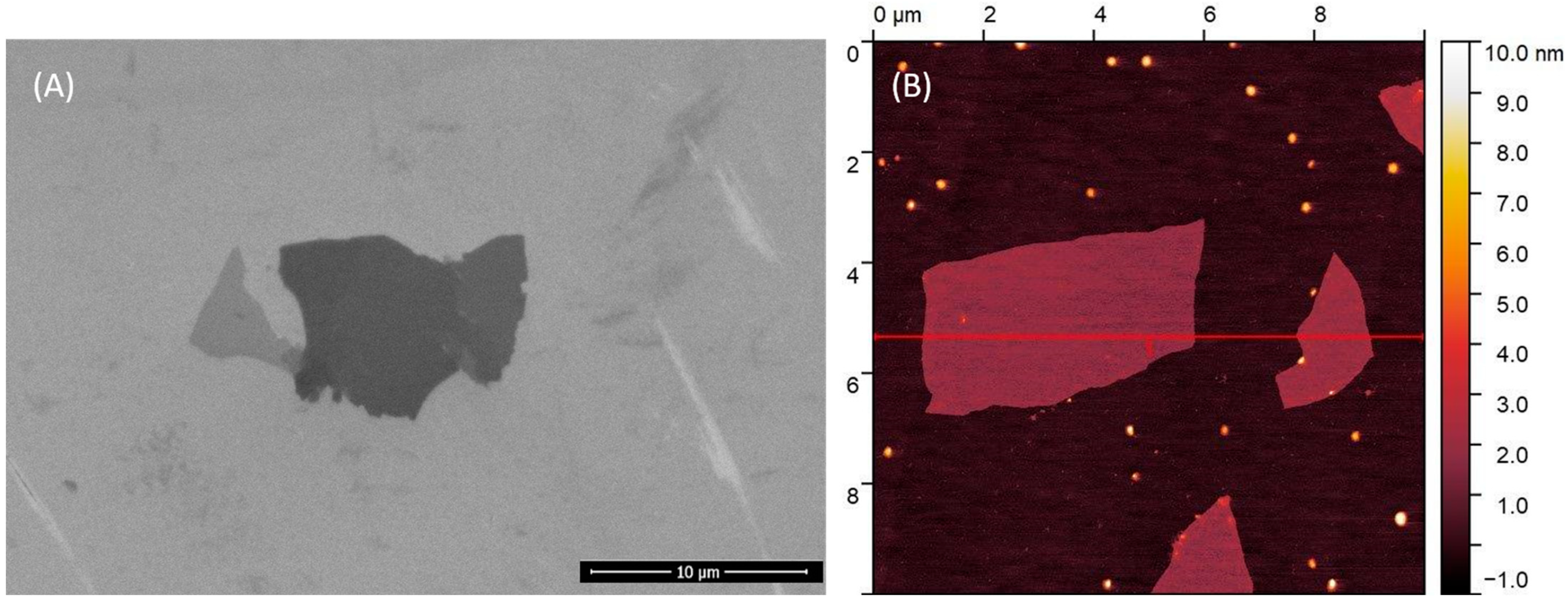
4.1. Graphene Oxide Characteristics
4.2. Characteristics of the Species
4.3. Food Preparation: GO Food, Vitamin C Food, GO + Vitamin C Food, Control Food
4.4. Experimental Model
4.5. Sample Preparation and Measurement of Selected Parameters
4.5.1. Vg Gene Relative Expression
4.5.2. Total Vg Protein Expression
4.5.3. Vg Protein Precursor and Subunits Expression
4.5.4. Egg Laying and Hatching Success: Eggs Laid per Female, Larvae Hatching Success per Female, Total Hatching Success
4.5.5. Major Energetic Components in Eggs: Lipids, Glucose, Glycogen, Total Protein
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhahebi, A.; Convergence, N.; Mohammed, A.; Dhahebi, A.; Chandra, S.; Gopinath, B. Graphene impregnated electrospun nanofiber sensing materials: A comprehensive overview on bridging laboratory set-up to industry. Nano Converg. 2020, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lu, F.; Tu, Y.; Ren, Z. Glucose Biosensors Based on Carbon Nanotube Nanoelectrode Ensembles. Nano Lett. 2004, 4, 191–195. [Google Scholar] [CrossRef]
- Timur, S.; Anik, U.; Odaci, D.; Gorton, L. Development of a microbial biosensor based on carbon nanotube (CNT) modified electrodes. Electrochem. Commun. 2007, 9, 1810–1815. [Google Scholar] [CrossRef]
- Maehashi, K.; Katsura, T.; Kerman, K.; Takamura, Y.; Matsumoto, K.; Tamiya, E. Label-Free Protein Biosensor Based on Aptamer-Modified Carbon Nanotube Field-Effect Transistors. Anal. Chem. 2007, 79, 782–787. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Markovic, M.; Kumar, A.; Andjelkovic, I.; Lath, S.; Kirby, J.K.; Losic, D.; Batley, G.E.; McLaughlin, M.J. Ecotoxicology of manufactured graphene oxide nanomaterials and derivation of preliminary guideline values for freshwater environments. Environ. Toxicol. Chem. 2018, 37, 1340–1348. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, S.; Ganguli, A.K.; Shanmugam, V. Anti-drift nano-stickers made of graphene oxide for targeted pesticide delivery and crop pest control. Carbon N. Y. 2017, 115, 781–790. [Google Scholar] [CrossRef]
- Shojaei, T.R.; Salleh, M.A.M.; Tabatabaei, M.; Mobli, H.; Aghbashlo, M.; Rashid, S.A.; Tan, T. Chapter 11—Applications of Nanotechnology and Carbon Nanoparticles in Agriculture. In Micro and Nano Technologies, Synthesis, Technology and Applications of Carbon Nanomaterials; Rashid, S.A., Raja Othman, R.N.I., Hussein, M.Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–277. ISBN 978-0-12-815757-2. [Google Scholar]
- Rhazouani, A.; Gamrani, H.; El Achaby, M.; Aziz, K.; Gebrati, L.; Uddin, M.S.; Aziz, F. Synthesis and Toxicity of Graphene Oxide Nanoparticles: A Literature Review of In Vitro and In Vivo Studies. Biomed. Res. Int. 2021, 2021, 5518999. [Google Scholar] [CrossRef]
- Chen, M.; Yin, J.; Liang, Y.; Yuan, S.; Wang, F.; Song, M.; Wang, H. Oxidative stress and immunotoxicity induced by graphene oxide in zebrafish. Aquat. Toxicol. 2016, 174, 54–60. [Google Scholar] [CrossRef]
- Mu, Q.; Su, G.; Li, L.; Gilbertson, B.O.; Yu, L.H.; Zhang, Q.; Sun, Y.-P.; Yan, B. Size-Dependent Cell Uptake of Protein-Coated Graphene Oxide Nanosheets. ACS Appl. Mater. Interfaces 2012, 4, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.C.; Bode, A.M. Biology of free radical scavengers: An evaluation of ascorbate. FASEB J. 1993, 7, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, O.; De Tullio, M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta Gen. Subj. 2002, 1569, 1–9. [Google Scholar] [CrossRef]
- Datta, M.; Kaviraj, A. Ascorbic acid supplementation of diet for reduction of deltamethrin induced stress in freshwater catfish Clarias gariepinus. Chemosphere 2003, 53, 883–888. [Google Scholar] [CrossRef]
- Yousef, M.; Abdallah, G.; Kamel, K. Effect of ascorbic acid and Vitamin E supplementation on semen quality and biochemical parameters of male rabbits. Anim. Reprod. Sci. 2003, 76, 99–111. [Google Scholar] [CrossRef]
- Somayeh, B.; Mohammad, F. Vitamin C can reduce toxic effects of Nano Zinc Oxide. Int. J. Biol. Sci. 2014, 3, 65–70. [Google Scholar]
- Fukui, H.; Iwahashi, H.; Endoh, S.; Nishio, K.; Yoshida, Y.; Hagihara, Y.; Horie, M. Ascorbic acid attenuates acute pulmonary oxidative stress and inflammation caused by zinc oxide nanoparticles. J. Occup. Health 2015, 57, 118–125. [Google Scholar] [CrossRef]
- Begum, P.; Fugetsu, B. Phytotoxicity of multi-walled carbon nanotubes on red spinach (Amaranthus tricolor L.) and the role of ascorbic acid as an antioxidant. J. Hazard. Mater. 2012, 243, 212–222. [Google Scholar] [CrossRef]
- Gupta, R.S.; Kim, J.; Gomes, C.; Oh, S.; Park, J.; Im, W.B.; Seong, J.Y.; Ahn, R.S.; Kwon, H.B.; Soh, J. Effect of ascorbic acid supplementation on testicular steroidogenesis and germ cell death in cadmium-treated male rats. Mol. Cell. Endocrinol. 2004, 221, 57–66. [Google Scholar] [CrossRef]
- Flasz, B.; Dziewięcka, M.; Kędziorski, A.; Tarnawska, M.; Augustyniak, J.; Augustyniak, M. Multigenerational selection towards longevity changes the protective role of vitamin C against graphene oxide-induced oxidative stress in house crickets. Environ. Pollut. 2021, 290, 117996. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, L.; Yao, C.; Ding, L.; Li, C.; Fang, J.; Sui, K.; Liu, Y.; Wu, M. A combined toxicity study of zinc oxide nanoparticles and vitamin C in food additives. Nanoscale 2014, 6, 15333–15342. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Hemmer, B.L.; Goodman, L.R.; Cripe, G.M. Multigenerational exposure of the estuarine sheepshead minnow (Cyprinodon variegatus) to 17β-estradiol. II. population-level effects through two life cycles. Environ. Toxicol. Chem. 2009, 28, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Sumpter, J.P.; Jobling, S. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ. Health Perspect. 1995, 103, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Kime, D.E.; Nash, J.P.; Scott, A.P. Vitellogenesis as a biomarker of reproductive disruption by xenobiotics. Aquaculture 1999, 177, 345–352. [Google Scholar] [CrossRef]
- Tufail, M.; Nagaba, Y.; Elgendy, A.M.; Takeda, M. Regulation of vitellogenin genes in insects. Entomol. Sci. 2014, 17, 269–282. [Google Scholar] [CrossRef]
- Tufail, M.; Takeda, M. Molecular characteristics of insect vitellogenins. J. Insect Physiol. 2008, 54, 1447–1458. [Google Scholar] [CrossRef]
- Bradley, J.T.; Edwards, J.S. Yolk proteins in the house cricket, Acheta domesticus: Identification, characterization, and effect of ovariectomy upon their synthesis. J. Exp. Zool. 1978, 204, 239–248. [Google Scholar] [CrossRef]
- Sarmento, N.L.A.F.; Martins, E.F.F.; Costa, D.C.; Mattioli, C.C.; da Costa Julio, G.S.; Figueiredo, L.G.; Luz, M.R.; Luz, R.K. Reproductive efficiency and egg and larvae quality of Nile tilapia fed different levels of vitamin C. Aquaculture 2018, 482, 96–102. [Google Scholar] [CrossRef]
- Waagbø, R.; Thorsen, T.; Sandnes, K. Role of dietary ascorbic acid in vitellogenesis in rainbow trout (Salmo gairdneri). Aquaculture 1989, 80, 301–314. [Google Scholar] [CrossRef]
- Grajeda-Cota, P.; Ramírez-Mares, M.V.; González De Mejía, E. Vitamin C protects against in vitro cytotoxicity of cypermethrin in rat hepatocytes. Toxicol. Vitr. 2004, 18, 13–19. [Google Scholar] [CrossRef]
- Pallauf, K.; Bendall, J.K.; Scheiermann, C.; Watschinger, K.; Hoffmann, J.; Roeder, T.; Rimbach, G. Vitamin C and lifespan in model organisms. Food Chem. Toxicol. 2013, 58, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Belin, S.; Kaya, F.; Burtey, S.; Fontes, M. Ascorbic Acid and Gene Expression: Another Example of Regulation of Gene Expression by Small Molecules? Curr. Genom. 2010, 11, 52–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chojkier, M.; Houglum, K.; Solis-Herruzo, J.; Brenner, D.A. Stimulation of Collagen Gene Expression by Ascorbic Acid in Cultured Human Fibroblasts: A role for lipid peroxidation?*. J. Biol. Chem. 1989, 264, 16957–16962. [Google Scholar] [CrossRef] [PubMed]
- Lyons, B.L.; Schwarz, R.I. Ascorbate stimulation of PAT cells causes an increase in transcription rates and a decrease in degradation rates of procollagen mRNA. Nucleic Acids Res. 1984, 12, 2569–2579. [Google Scholar] [CrossRef]
- Murad, S.; Grove, D.; Lindberg, K.A.; Reynolds, G.; Sivarajah, A.; Pinnell, S.R. Regulation of collagen synthesis by ascorbic acid. Proc. Natl. Acad. Sci. USA 1981, 78, 2879–2882. [Google Scholar] [CrossRef]
- Horovitz, O.; Knaack, D.; Podleski, T.R.; Salpeter, M.M. Acetylcholine receptor alpha-subunit mRNA is increased by ascorbic acid in cloned L5 muscle cells: Northern blot analysis and in situ hybridization. J. Cell Biol. 1989, 108, 1823–1832. [Google Scholar] [CrossRef]
- Horovitz, O.; Spitsberg, V.; Salpeter, M.M. Regulation of acetylcholine receptor synthesis at the level of translation in rat primary muscle cells. J. Cell Biol. 1989, 108, 1817–1822. [Google Scholar] [CrossRef]
- Sullivan, T.A.; Uschmann, B.; Hough, R.; Leboy, P.S. Ascorbate modulation of chondrocyte gene expression is independent of its role in collagen secretion. J. Biol. Chem. 1994, 269, 22500–22506. [Google Scholar] [CrossRef]
- Suzuki, H.; Torii, Y.; Hitomi, K.; Tsukagoshi, N. Ascorbate-dependent elevation of mRNA levels for cytochrome P450s induced by polychlorinated biphenyls. Biochem. Pharmacol. 1993, 46, 186–189. [Google Scholar] [CrossRef]
- TORII, Y.; HITOMI, K.; TSUKAGOSHI, N. L-Ascorbic Acid 2-Phosphate Promotes Osteoblastic Differentiation of MC3T3-E1 Mediated by Accumulation of Type I Collagen. J. Nutr. Sci. Vitaminol. 1994, 40, 229–238. [Google Scholar] [CrossRef]
- Tufail, M.; Lee, J.M.; Hatakeyama, M.; Oishi, K.; Takeda, M. Cloning of vitellogenin cDNA of the American cockroach, Periplaneta americana (Dictyoptera), and its structural and expression analyses. Arch. Insect Biochem. Physiol. 2000, 45, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Su, M.; Wang, H.; Zhou, L.; Meng, Z.; Xiong, G.; Liao, X.; Lu, H. Carboxyl graphene oxide nanoparticles induce neurodevelopmental defects and locomotor disorders in zebrafish larvae. Chemosphere 2021, 270, 128611. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, S.; Hinzmann, M.; Sawosz, E.; Grodzik, M.; Kutwin, M.; Wierzbicki, M.; Strojny, B.; Vadalasetty, K.P.; Lipińska, L.; Chwalibog, A. Interaction of different forms of graphene with chicken embryo red blood cells. Environ. Sci. Pollut. Res. 2017, 24, 21671–21679. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Q.; Zheng, G.; Han, S.; Zhao, F.; Hu, Q.; Fu, Z. Developmental neurotoxicity and immunotoxicity induced by graphene oxide in zebrafish embryos. Environ. Toxicol. 2019, 34, 415–423. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, X.; Lynch, I.; Cui, F. Comparative evaluation of the mechanisms of toxicity of graphene oxide and graphene oxide quantum dots to blue-green algae Microcystis aeruginosa in the aquatic environment. J. Hazard. Mater. 2022, 425, 127898. [Google Scholar] [CrossRef]
- Bartosz, G. Reactive oxygen species: Destroyers or messengers? Biochem. Pharmacol. 2009, 77, 1303–1315. [Google Scholar] [CrossRef]
- Forman, H.J.; Maiorino, M.; Ursini, F. Signaling Functions of Reactive Oxygen Species. Biochemistry 2010, 49, 835–842. [Google Scholar] [CrossRef]
- Krumova, K.; Cosa, G. Section I Fundamentals. In Singlet Oxygen: Applications in Biosciences and Nanosciences; Nonell, S., Flors, C., Nonell, S., Flors, C., Eds.; The Royal Society of Chemistry: London, UK, 2016; pp. 1–21. [Google Scholar]
- Bangeppagari, M.; Park, S.H.; Kundapur, R.R.; Lee, S.J. Graphene oxide induces cardiovascular defects in developing zebrafish (Danio rerio) embryo model: In-vivo toxicity assessment. Sci. Total Environ. 2019, 673, 810–820. [Google Scholar] [CrossRef]
- Chatterjee, N.; Kim, Y.; Yang, J.; Roca, C.P.; Joo, S.-W.; Choi, J. A systems toxicology approach reveals the Wnt-MAPK crosstalk pathway mediated reproductive failure in Caenorhabditis elegans exposed to graphene oxide (GO) but not to reduced graphene oxide (rGO). Nanotoxicology 2017, 11, 76–86. [Google Scholar] [CrossRef]
- D’Amora, M.; Camisasca, A.; Lettieri, S.; Giordani, S. Toxicity assessment of carbon nanomaterials in zebrafish during development. Nanomaterials 2017, 7, 414. [Google Scholar] [CrossRef]
- Flasz, B.; Dziewięcka, M.; Ajay, A.K.; Tarnawska, M.; Babczyńska, A.; Kędziorski, A.; Napora-Rutkowski, Ł.; Ziętara, P.; Świerczek, E.; Augustyniak, M. Age-and Lifespan-Dependent Differences in GO Caused DNA Damage in Acheta domesticus. Int. J. Mol. Sci. 2022, 2023, 290. [Google Scholar] [CrossRef] [PubMed]
- Flasz, B.; Dziewięcka, M.; Kędziorski, A.; Tarnawska, M.; Augustyniak, M. Vitellogenin expression, DNA damage, health status of cells and catalase activity in Acheta domesticus selected according to their longevity after graphene oxide treatment. Sci. Total Environ. 2020, 737, 140274. [Google Scholar] [CrossRef]
- Szmidt, M.; Sawosz, E.; Urbańska, K.; Jaworski, S.; Kutwin, M.; Hotowy, A.; Wierzbicki, M.; Grodzik, M.; Lipińska, L.; Chwalibog, A. Toxicity of different forms of graphene in a chicken embryo model. Environ. Sci. Pollut. Res. 2016, 23, 19940–19948. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2010, 6, 8. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.; Zhang, S.; Zhang, Y.; Lee, S.-T.; Liu, Z. In Vivo Pharmacokinetics, Long-Term Biodistribution, and Toxicology of PEGylated Graphene in Mice. ACS Nano 2011, 5, 516–522. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.; Huang, Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon N. Y. 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Maliszewska, J.; Wyszkowska, J.; Tęgowska, E. Hemolymph Ph as a marker of pesticide exposition. Folia Pomeranae Univ. Technol. Stetin. Agric. Aliment. Piscaria Zootech. 2017, 338, 101–108. [Google Scholar] [CrossRef]
- Wyatt, G.R.; Loughheed, T.C.; Wyatt, S.S. The chemistry of insect hemolymph bombyx mori, and two other species. J. Gen. Physiol. 1956, 39, 853–868. [Google Scholar] [CrossRef]
- Duan, G.; Kang, S.-G.; Tian, X.; Garate, J.A.; Zhao, L.; Ge, C.; Zhou, R. Protein corona mitigates the cytotoxicity of graphene oxide by reducing its physical interaction with cell membrane. Nanoscale 2015, 7, 15214. [Google Scholar] [CrossRef]
- Liu, X.; Yan, C.; Chen, K.L. Adsorption of Human Serum Albumin on Graphene Oxide: Implications for Protein Corona Formation and Conformation. Environ. Sci. Technol 2019, 53, 7. [Google Scholar] [CrossRef]
- Greenbaum, D.; Colangelo, C.; Williams, K.; Gerstein, M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003, 4, 117. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.; Güell, M.; Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009, 583, 3966–3973. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.H. The utility of protein and mRNA correlation. Trends Biochem. Sci. 2015, 40, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Dasmahapatra, A.K.; Powe, D.K.; Dasari, T.P.S.; Tchounwou, P.B. Assessment of reproductive and developmental effects of graphene oxide on Japanese medaka (Oryzias latipes). Chemosphere 2020, 259, 127221. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.K.; Awasthi, K.K.; John, P.J. Effects of Nano-Graphene Oxide on Testis, Epididymis and Fertility of Wistar Rats. Basic Clin. Pharmacol. Toxicol. 2017, 121, 202–210. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Sahoo, S.K.; Sahu, S.; Mukherjee, S.; Hota, G.; Mishra, M. Oral administration of graphene oxide nano-sheets induces oxidative stress, genotoxicity, and behavioral teratogenicity in Drosophila melanogaster. Environ. Sci. Pollut. Res. 2019, 26, 19560–19574. [Google Scholar] [CrossRef]
- Kong, C.; Aziz, A.I.; Kakarla, A.B.; Kong, I.; Kong, W. Toxicity Evaluation of Graphene and Poly(Lactic-Acid) Using a Nematode Model. Solid State Phenom. 2019, 290, 101–106. [Google Scholar] [CrossRef]
- Mesarič, T.; Sepčić, K.; Drobne, D.; Makovec, D.; Faimali, M.; Morgana, S.; Falugi, C.; Gambardella, C. Sperm exposure to carbon-based nanomaterials causes abnormalities in early development of purple sea urchin (Paracentrotus lividus). Aquat. Toxicol. 2015, 163, 158–166. [Google Scholar] [CrossRef]
- Skovmand, A.; Jacobsen Lauvås, A.; Christensen, P.; Vogel, U.; Sørig Hougaard, K.; Goericke-Pesch, S. Pulmonary exposure to carbonaceous nanomaterials and sperm quality. Part. Fibre Toxicol. 2018, 15, 10. [Google Scholar] [CrossRef]
- Dziewięcka, M.; Karpeta-Kaczmarek, J.; Augustyniak, M.; Rost-Roszkowska, M. Short-term in vivo exposure to graphene oxide can cause damage to the gut and testis. J. Hazard. Mater. 2017, 328, 80–89. [Google Scholar] [CrossRef]
- Dziewięcka, M.; Witas, P.; Karpeta-Kaczmarek, J.; Kwaśniewska, J.; Flasz, B.; Balin, K.; Augustyniak, M. Reduced fecundity and cellular changes in Acheta domesticus after multigenerational exposure to graphene oxide nanoparticles in food. Sci. Total Environ. 2018, 635, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Valle, D. Vitellogenesis in insects and other groups: A review. Mem. Inst. Oswaldo Cruz 1993, 88, 1–26. [Google Scholar] [CrossRef]
- Yaqoob, P. Role of Lipids in Human Nutrition. In Handbook of Olive Oil: Analysis and Properties; Aparicio, R., Harwood, J., Eds.; Springer: Boston, MA, USA, 2013; pp. 655–675. ISBN 978-1-4614-7777-8. [Google Scholar]
- Dziewięcka, M.; Pawlyta, M.; Majchrzycki, Ł.; Balin, K.; Barteczko, S.; Czerkawska, M.; Augustyniak, M. The Structure–Properties–Cytotoxicity Interplay: A Crucial Pathway to Determining Graphene Oxide Biocompatibility. Int. J. Mol. Sci. 2021, 22, 5401. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, M.; Babczyńska, A.; Dziewięcka, M.; Flasz, B.; Karpeta-Kaczmarek, J.; Kędziorski, A.; Mazur, B.; Rozpędek, K.; Seyed Alian, R.; Skowronek, M.; et al. Does age pay off? Effects of three-generational experiments of nanodiamond exposure and withdrawal in wild and longevity-selected model animals. Chemosphere 2022, 303, 135129. [Google Scholar] [CrossRef] [PubMed]
- Horch, H.W.; Mito, T.; Popadi, A.; Ohuchi, H.; Noji, S. The Cricket as a Model Organism; Springer: Tokyo, Japan, 2017; ISBN 9784431564768. [Google Scholar]
- PN-A-04019:1998; Food Products—Determination of Vitamin C. Polish Committee for Standardization: Warsaw, Poalnd, 1998.
- Zhou, C.; Liu, S.; Song, W.; Luo, S.; Meng, G.; Yang, C.; Yang, H.; Ma, J.; Wang, L.; Gao, S.; et al. Characterization of viral RNA splicing using whole-transcriptome datasets from host species. Sci. Rep. 2018, 8, 3273. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef]
- Ruan, W.; Lai, M. Actin, a reliable marker of internal control? Clin. Chim. Acta 2007, 385, 1–5. [Google Scholar] [CrossRef]
- Foray, V.; Pelisson, P.F.; Bel-Venner, M.C.; Desouhant, E.; Venner, S.; Menu, F.; Giron, D.; Rey, B. A handbook for uncovering the complete energetic budget in insects: The van Handel’s method (1985) revisited. Physiol. Entomol. 2012, 37, 295–302. [Google Scholar] [CrossRef]
- Flasz, B.; Dziewięcka, M.; Kędziorski, A.; Tarnawska, M.; Augustyniak, M. Multigenerational graphene oxide intoxication results in reproduction disorders at the molecular level of vitellogenin protein expression in Acheta domesticus. Chemosphere 2021, 280, 130772. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flasz, B.; Tarnawska, M.; Kędziorski, A.; Napora-Rutkowski, Ł.; Szczygieł, J.; Gajda, Ł.; Nowak, N.; Augustyniak, M. Ascorbic Acid and Graphene Oxide Exposure in the Model Organism Acheta domesticus Can Change the Reproduction Potential. Molecules 2024, 29, 4594. https://doi.org/10.3390/molecules29194594
Flasz B, Tarnawska M, Kędziorski A, Napora-Rutkowski Ł, Szczygieł J, Gajda Ł, Nowak N, Augustyniak M. Ascorbic Acid and Graphene Oxide Exposure in the Model Organism Acheta domesticus Can Change the Reproduction Potential. Molecules. 2024; 29(19):4594. https://doi.org/10.3390/molecules29194594
Chicago/Turabian StyleFlasz, Barbara, Monika Tarnawska, Andrzej Kędziorski, Łukasz Napora-Rutkowski, Joanna Szczygieł, Łukasz Gajda, Natalia Nowak, and Maria Augustyniak. 2024. "Ascorbic Acid and Graphene Oxide Exposure in the Model Organism Acheta domesticus Can Change the Reproduction Potential" Molecules 29, no. 19: 4594. https://doi.org/10.3390/molecules29194594
APA StyleFlasz, B., Tarnawska, M., Kędziorski, A., Napora-Rutkowski, Ł., Szczygieł, J., Gajda, Ł., Nowak, N., & Augustyniak, M. (2024). Ascorbic Acid and Graphene Oxide Exposure in the Model Organism Acheta domesticus Can Change the Reproduction Potential. Molecules, 29(19), 4594. https://doi.org/10.3390/molecules29194594





