1. Introduction
There is a global shift from using petroleum-based products to bio-based ones that are obtained from renewable resources like biomass. This is composed from organic matter such as vegetable or animal oils, garbage, waste, lignin, and cellulose [
1]. Utilizing bio-based products from biomass, such as bio-epoxides from seed oils, bio-polyols, and bio-based acrylates or polyesters, is crucial for introducing biogenic carbon into the product life cycle, ultimately leading to a reduction in the carbon footprint by sequestering atmospheric carbon dioxide through photosynthesis [
2]. Using biomass-derived raw materials for various polymer applications, such as coatings, adhesives, elastomers, and composites, is the most direct and effective approach. Examples include components made from seed oils or vegetable oils, bio-based polymers, UV-cured systems, polymers from recycled residues, and byproducts from secondary bio-based streams. The commitment to developing more sustainable products and processes constitutes a necessity for continuous innovation for the future industrial manufacturers [
3]. In this context, soybean oil (SO) is a great renewable resource that is abundant, versatile, and low cost. It was employed to obtain acrylated monomers that have the necessary reactivity to synthesize new bio-based polymers as a sustainable alternative to traditional petroleum-based platform molecules.
Acrylated oils constitute a very promising alternative to be employed in diverse coating applications. The lower viscosity of these materials compared to conventional epoxy acrylates eliminates the need for their reduction with a reactive diluent [
4]. They are primarily flexible and possess an aliphatic acrylic backbone, which can significantly improve the UV coating flexibility and adhesion of the polymer [
4,
5]. Special interest has been paid to UV-curable coatings, where acrylated oils react by means of UV-initiated free radical polymerizations in the presence of specific photoinitiators. As an example, new photocurable biocompatible liquid resins have been developed for 3D stereolithography-based bioprinting [
6,
7]. Acrylated coatings have also been developed to reduce the moisture sensitivity and permeability of bio-based films [
8].
The acrylation degree of the oil is an important factor that is very relevant for the mechanical properties of the polymer. In this way, vegetable oils are not always fully acrylated to obtain monomers with specific characteristics. Boucher et al. [
9] epoxidized and partially acrylated linseed oil and copolymerized it with (3,4-dihydroxyphenetyl)-acrylamide and N,N-dimethyl acrylamide to develop a coating for corrosion protection. A series of partially acrylated vegetable oils with different functional groups was synthesized and further polymerized with styrene [
10]. Several methyl esters of C16 to C24 derived from camelina oil and linseed oil were epoxidized, fully or partially acrylated, and then polymerized in emulsion with various amounts of bio-based derivatives (5–30 wt.% in monomer mixture) to obtain polymeric latexes for coating formulations [
11].
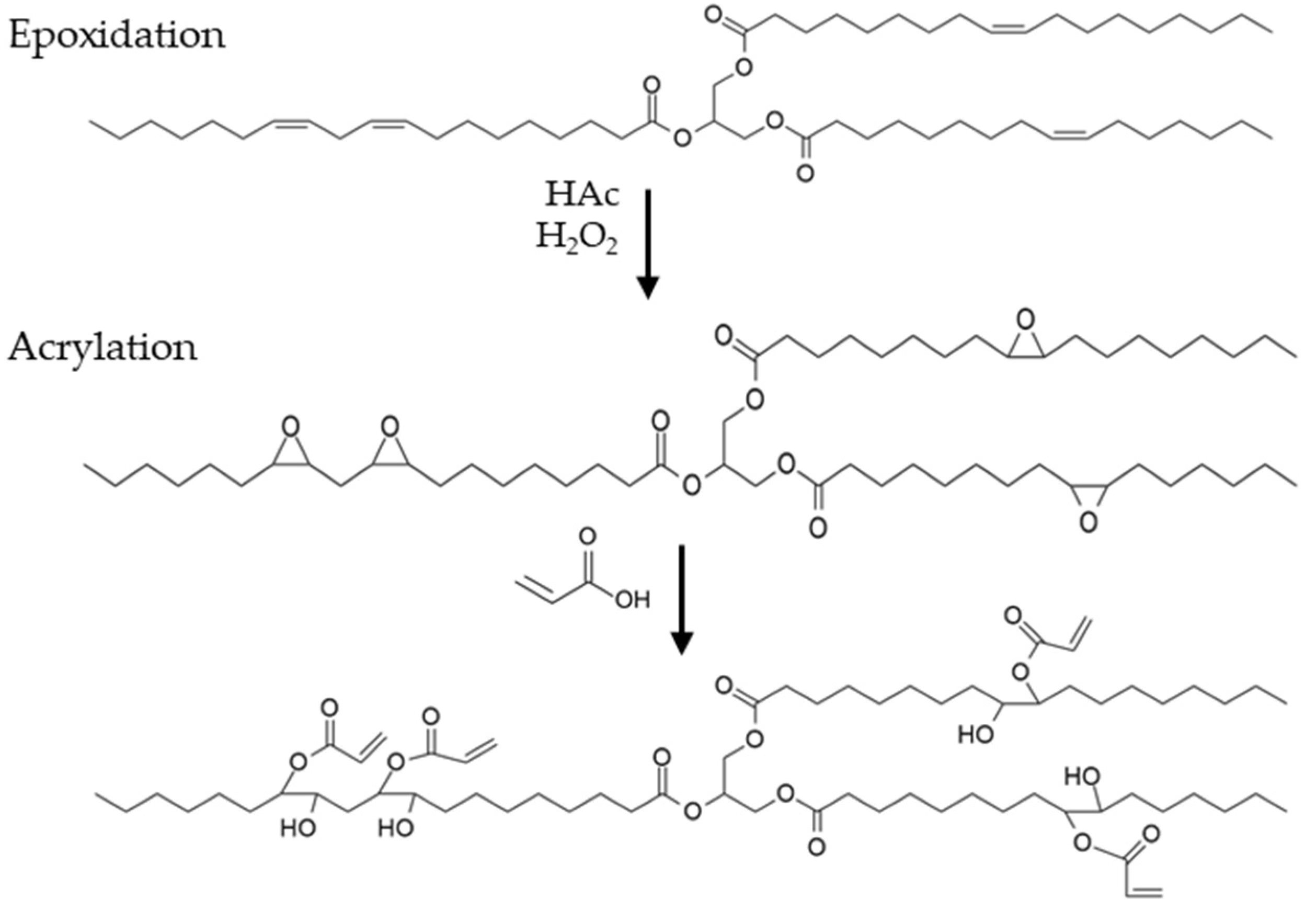
Typically, acrylated epoxidized oils are obtained by adding acrylic acid (AA) to epoxidized vegetable oils (ESO if they are obtained from SO), which are more reactive than the original raw materials. The commercial production of epoxidized oils is carried out with homogeneous acidic catalysts, carboxylic acids (principally acetic or formic acids), and an aqueous hydrogen peroxide solution, usually in concentrations between 30 and 50%
w/
w [
12].
The acrylation process is usually carried out with an excess of AA, hydroquinone as a free radical inhibitor, and different catalysts. 1,4-diazobicyclooctane with temperatures up to 95 °C and times up to 11 h has been used [
13]. Acrylated epoxidized soybean oil (AESO) was also synthesized using triphenylphosphine oxide as the catalyst and a temperature of 120 °C for 6 h, achieving a conversion of 95% [
5]. Triethylamine was also considered as an efficient catalyst for the acrylation of epoxidized oils. In this case, a temperature of 80 °C and 8 h were employed to achieve an average conversion of the epoxidized groups of 70% [
14]. Although they are common catalysts, they need to be handled with extra precautions due to their high oral and inhalation toxicity [
15]. A solution of 40–60% chromium (III) 2-ethylhexanoate in a mixture of di (heptyl, nonyl, undecyl) phthalates) was demonstrated to be one of the most effective catalysts in the acrylation of vegetable oils. Complete acrylation of camelina oil with AA was obtained at 80 °C with 2% of this catalyst in 12 h [
16].
Catalytic acrylation reactions offer several advantages, such as higher reaction rates at lower temperatures, but they also show some limitations. Once the acrylation process is finished, it is essential to purify the product to remove any traces of remaining AA and catalyst, which may negatively affect the product’s further applications. Spent catalyst residues must be properly handled.
Meléndez et al. [
17] studied the synthesis of an oligo-acrylated product from soya oil and acrylic acid without using any catalyst, which was obtained in 86% yield after 12 h at 120 °C. The acrylated monomers studied in this paper were obtained without the use of any catalyst to avoid the generation of any residual stream. The reactant ratio and operational conditions were selected to obtain a product with a high acrylation degree with lower reaction times to avoid the formation of undesired acrylic oil auto-polymerization.
On the other hand, the application of different analytical technologies is crucial for a deep analysis of the molecular structure of acrylated molecules. The most commonly used methods to characterize acrylated monomers include FTIR spectroscopy, which qualitatively describes the different functional groups present in triglyceride molecules, and
1HNMR to calculate the average inclusion degree of AA in the epoxidized fatty chains. Chemical titrations such as the iodine value (IV) and the oxirane content (COOe) are used to determine the double bond conversion in the epoxidation reaction and the remaining epoxide presence after the acrylation reaction, respectively [
18]. A better understanding of partially acrylated structures using mass spectrophotometric techniques would permit a visual identification of the different functionalities present in fatty chains of various lengths and original unsaturation degree. Triglycerides with no functionalities, those with a single functionality, and molecules with more than one acrylate group could be distinguished, which is interesting for controlling polymerization reactions.
Kuki et al. [
19] employed matrix-assisted laser desorption ionization and electrospray ionization mass spectrometry (MALDI-MS and ESI-MS) for the characterization of epoxidized soybean and linseed oils. These techniques allowed for the identification of different epoxidized triglyceride (TG) mass spectral peak series and the number of epoxide groups in the products without any complicated and time-consuming sample preparation.
In this study, different spectroscopy techniques, such as direct infusion, mass spectrometry with electrospray ionization, and ultra-high-performance liquid chromatography were combined with FTIR and 1HNMR to define a methodology for the characterization of the epoxy and acrylic functionalities in fatty chains with different numbers of carbon atoms.
2. Results and Discussion
First, SO was epoxidized with acetic acid and hydrogen peroxide (50% w/w) using H2SO4 (96%) as the acid catalyst. The purification process of the intermediates did not involve the use of any hazardous organic solvents, since impurities, unreacted materials, and catalyst residues were removed from the epoxidized intermediate through 4 washes with a warm brine solution (60 °C).
The epoxidized samples presented a COOe of 6.6 g O/100 g, and unsaturation degree of 8 g I2/100 g. These values were determined by measuring the oxirane content and the IV of ESO. According to this, the oxirane yield calculated for the ESO intermediate was 88%.
The FTIR spectra of the samples demonstrated the development of distinctive peaks for the epoxidized products. A peak at 830 cm−1, characteristic of the epoxide group, was observed, which evidenced that the reaction took place. Other representative peaks related to the fatty unsaturations =C–H at 3000 cm−1 and C=C at 1651 cm−1 appeared in the spectrum of SO but disappeared in the ESO spectrum, indicative that the internal double bonds of SO reacted with the peracid.
Table 1 displays the most relevant peaks found in the spectra of SO and ESO. It is noted that no peaks were present at wavelengths between 3300 and 3500 cm
−1, which are typical of -OH groups. This confirmed that no undesired reactions for producing glycols occurred. (
Figure 1).
It is well known that the oxirane rings of ESO consist of tensioned structures that easily react with nucleophiles such as alcohols, amines, or protic acids. These molecules interact with the electrophilic carbons of the epoxide rings, polarizing them, breaking the initial bonds, and creating new ones as ester and hydroxyl functionalities (
Scheme 1).
In the second step, the epoxidized intermediates were reacted with AA to produce acrylated monomers. A slight excess of acid was used (RM AA/epoxide groups 1.1:1) along with hydroquinone (0.2% w/w) to prevent the auto-polymerization of AA.
Additionally, the acrylation process was carried out without a catalyst to avoid the generation of effluents containing spent catalyst, which are difficult to recover. AESO was obtained by controlling both the reagent ratio and temperature. Previous references described that the synthesis of an acrylated oligomer could be performed at 120 °C without generating toxic effluents containing spent catalyst [
17]. Here, the formation of AESO was maintained for 4 h and monitored using FTIR spectroscopy to track the evolution of the different molecules that were being synthesized. The remaining epoxide groups were determined by the oxirane content measurement, which gave a value of 0.81 g O/100 g.
It was observed that longer reaction times led to undesired auto-polymerization of AA and/or AESO molecules, evidenced by a decrease in the acrylic peak length (
Figure 2). These could limit the overall product yield despite full conversion. When the oxirane ring is opened with AA, it produces an AESO molecule with a secondary hydroxyl group. However, this secondary hydroxyl group can further esterify with another acrylic acid and then produce water. Alternatively, water can react with other epoxide rings, yielding diols as a byproduct [
17].
The disappearance of peaks at 823–830 cm
−1 corresponding to epoxy groups indicated high reaction conversion. The remaining oxirane functionalities in the product could not be observed in the spectrum with enough precision. Additionally, new peaks attributed to acrylate groups (CH2=CH–COO–) appeared at 1619 cm
−1. The signals at 3400–3500 cm
−1 corresponding to the formation of hydroxyl groups confirmed the opening of oxirane rings to form acrylate groups and alcohols. Other notable peaks included the C=O stretching vibration of acrylate ester groups at 1721 cm
−1 close to the triglyceride ester peak at 1740 cm
−1. Other relevant peaks were C–O stretching at 1000 cm
−1, =C–H bending (twisting) at 1050 cm
−1, and the trans and cis C=C stretching vibration at 1640 cm
−1, all of which demonstrated the attachment of acrylate groups to fatty acid chains. The principal FTIR signals are listed in
Table 2.
Figure 3 shows the
1H NMR spectrum of thermically acrylated epoxidized soybean oil. The two sets of peaks from 4.0 to 4.4 ppm are produced by the four methylene hydrogen atoms attached to the glycerol center. The peak at 2.3 ppm is produced by the six methylene hydrogen atoms alpha to the carbonyl groups. The peak areas of the four methylene hydrogens in glycerol were used as the internal standard to determine the number of acrylates per triacylglycerol by comparing with the peak areas from the three acrylate protons (5.7–6.6 ppm). Considering an equivalent methodology to the ones described by Zhang et al. [
20] and Su et al. [
21], the acrylated molecule/TG ratio was determined to give a value of 2.1 using the following formula:
The authors defined an initial number of double bonds per SO molecule as 4.08. Therefore, the conversion of double bonds to acrylates was calculated to be 51%.
The signals of the protons [–CHOCH–] corresponding to the remaining epoxide groups in the acrylate monomers at 2.9 ppm could not be distinguished with high precision. Additional techniques were necessary for a more detailed analysis of the different functionalities present in the product.
A specific methodology based on electrospray ionization techniques was developed to clearly distinguish AESO molecules with similar masses but different geometries, which can cause different steric effects and reactivity and affect in a different way further polymerization processes. These technologies are favored over others due to their gentle ionization process, ensuring intact ionization and analysis of samples. They are most suitable for measuring lipid substances and can efficiently identify changes in their different structures, such as unsaturated, epoxidized, or acrylated functionalities. Additionally, ultra-high-performance liquid chromatography and mass spectrometry detection are used before ionization to improve detection. These steps ensure that the ionization of the main substances does not interfere with the ionization and detection of less abundant ones, also known as ion suppression.
Three different types of samples were analyzed by mass spectrometry techniques: (1) unreacted SO; (2) ESO intermediate, and (3) AESO sample obtained by a non-catalytic reaction. Two different approaches were carried out for the characterization of the functional groups present in the molecules.
Method 1: Direct sample infusion and mass spectrometry detection with an electrospray source and high-resolution time-of-flight analyzer (DI-ESI-TOF-MS).
Method 2: Ultra-high-performance liquid chromatography and mass spectrometry detection with an electrospray source and high-resolution time-of-flight analyzer (UHPLC/ESI-TOF-MS).
To make the identification of unsaturations, epoxidations, and acrylated groups easier, the diverse TG structures were named as shown in
Figure 4.
The possibilities of the TG distribution analyzed are very broad. Molecules of different fatty chain lengths, unreacted TGs (TGs), fully epoxidized TGs (ETG), partially epoxidized TGs (pETG), completely acrylated TGs (ATG), and partially epoxidized and acrylated TGs (pATG) could be detected. In
Figure 5, an example of the nomenclature used for the determination of sample molecules is shown.
It was evidenced that by using DI-ESI-TOF-MS (method 1), no epoxidation or acrylation functionalities appeared in the SO sample (
Figure 6a) due to the absence of signals corresponding to these structures in comparison to the corresponding commercial extracts. Different fully epoxidized molecules (even for the less abundant TGs) were found in the ESO intermediates thanks to the high resolution of the technique (
Figure 6b).
In the AESO sample synthesized without using any catalyst, epoxidized and acrylated functionalities were found in TGs with different chain lengths. This revealed the uncomplete conversion of the reaction (
Figure 7). Both functionalities were more clearly observed with this analytical technique than with others.
Taking into consideration a mass difference Δm/z = 72 between the epoxy and the acrylate groups, a series of TGs with several acrylation degrees was identified considering the mass differences between the epoxidized/acrylated groups.
Analyzing more in detail the series (54:4), in which the assignment was unambiguous, it was possible to calculate the epoxidation/acrylation ratio, assuming that all triglycerides, having very similar structures, ionize in a similar way in the mass spectrometer.
If m is defined as the total number of functionalized triglyceride unsaturations, either as epoxy or as acrylate, the total concentration of this group is given by the following formula:
Similarly, if n is defined as the number of acrylate groups in a sample, the concentration of this group can be calculated as follows:
And the concentration of the epoxy group in the sample is defined as follows:
Thus, the acrylation percentage %AC of a specific triglyceride defined by m functionalized unsaturations and n acrylate ones is determined by the following equation:
On the other hand, if we define ionizability obtained by mass spectrometry, i, as the ratio between the intensity (Int) obtained from a signal and its relationship to the concentration of the analyte, assuming that the ionizability of fully epoxidized, partially acrylated, or fully acrylated triglycerides is similar and proportional, the acrylation percentage can be defined as follows:
It is worth mentioning that making an unequivocal assignment between different lipids is quite difficult since the increase in mass due to one more carbon in a fatty acid and the presence of an epoxy group offer very similar signals (
m/
z 13.98, 14.02). However, it is possible to estimate the percentage of ionization based on the assigned signals corresponding to unique structures and assuming that the ionizability of these structures is similar concerning the number of epoxy/acrylate groups. Using the intensities for the different components Int (m,n) obtained in the ETG(54:4:4:0); ATG(54:4:4:4), in relation to the acrylation number (n) and the number of total epoxides groups (m) (
Figure 8 and
Table 3), it could be confidently approximated that the percentages of acrylation with respect to the epoxidized groups were around 61.1% (ec 7) and 53.7% of the initial double bonds of SO (taking in consideration that the oxirane yield calculated for the ESO intermediate was 88%).
The results obtained fit with the corresponding ones obtained by 1H NMR, taking into consideration that the oxirane yield calculated for the ESO intermediate was 88%.
On the other hand, different assignations were made using UPLC/MS (method 2) for the ESO and AESO molecules. Due to the sample’s complexity, only some TGs were unequivocally identified. The chromatograms corresponding to ETG(50:2) and ETG(50:3) showed that these compounds were not detectable by direct infusion (method 1) and were determined using this methodology instead. Some isomers could also be separated based on their polarity (
Figure 9).
The series of TGs with 52 and 54 carbons with different degrees of epoxidation were monitored by extracting the ion chromatograms. Thus, various isomers of these ETGs could be separated based on their different polarities (
Figure 10). ETG(52:2), ETG(52:3), ETG(52:4), ETG(52:5), and ETG(52:6) were successfully monitored in the following chromatograms (
Figure 9). The retention times were according to the higher polarities of more epoxy groups in the TGs.
Similar structures could be found in the AESO sample, but in this case, the signals corresponded to acrylated groups instead of epoxide rings (
Figure 11). It is worth mentioning that chromatographic separation allowed for the separation and detection of numerous isobaric structures of different acrylated TGs based on their polarity.