A Quantitative Evaluation of the Influence of Chemical Variables of Biomasses of Poplar SRC Commercial Clones in Torrefaction
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Analysis of Biomass
2.2. Exploratory Data Analysis
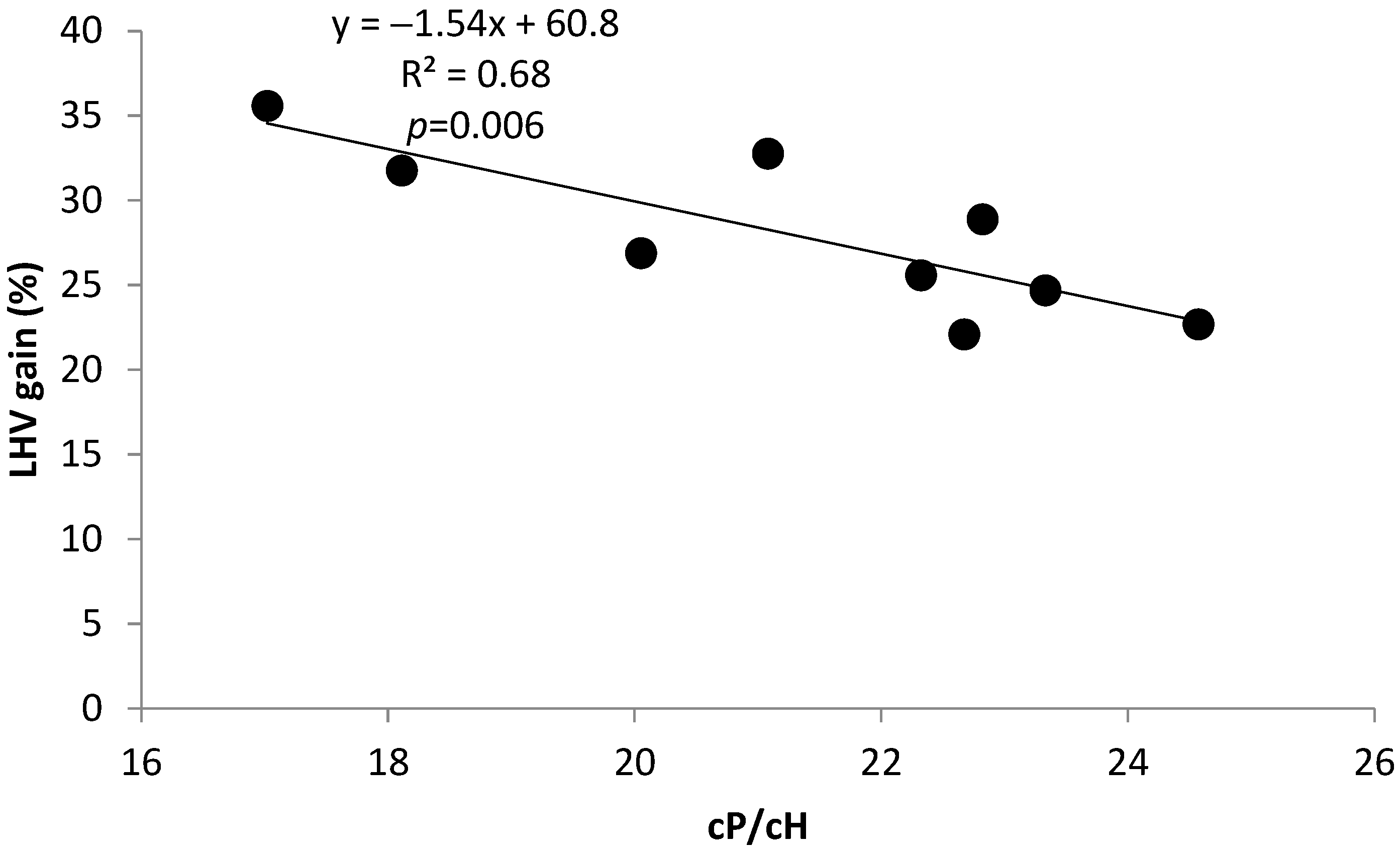
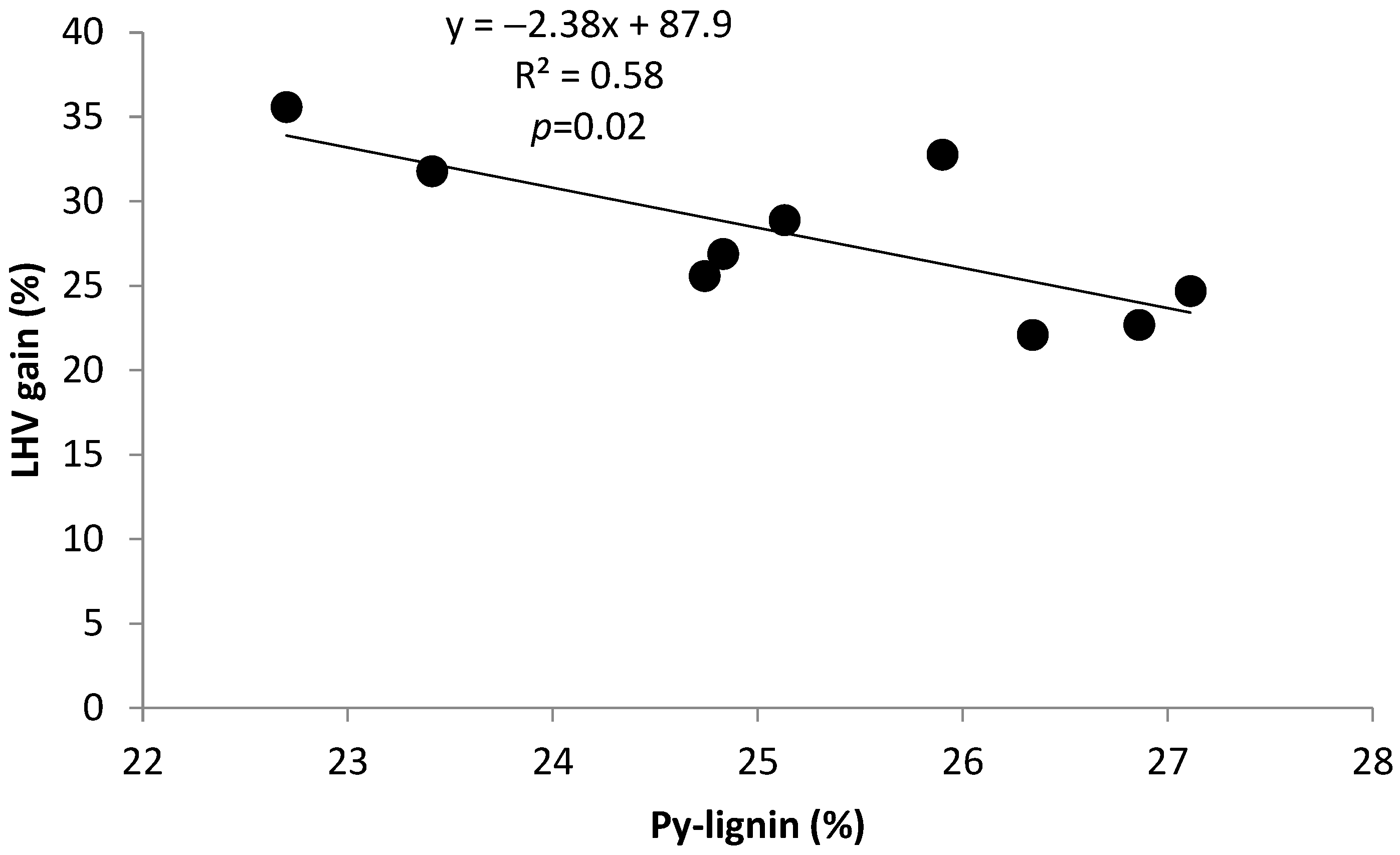
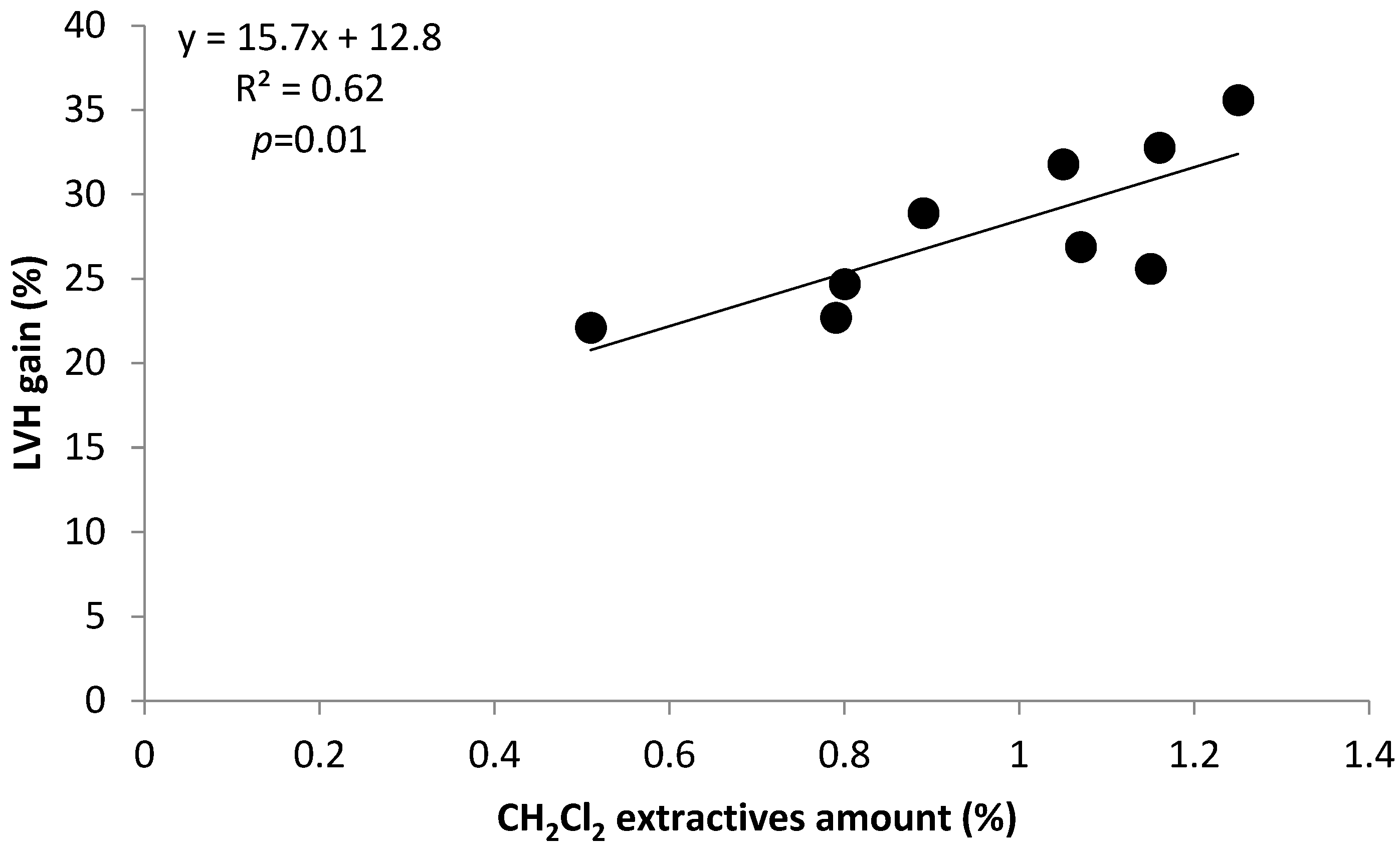
2.2.1. Correlation and Linear Regression
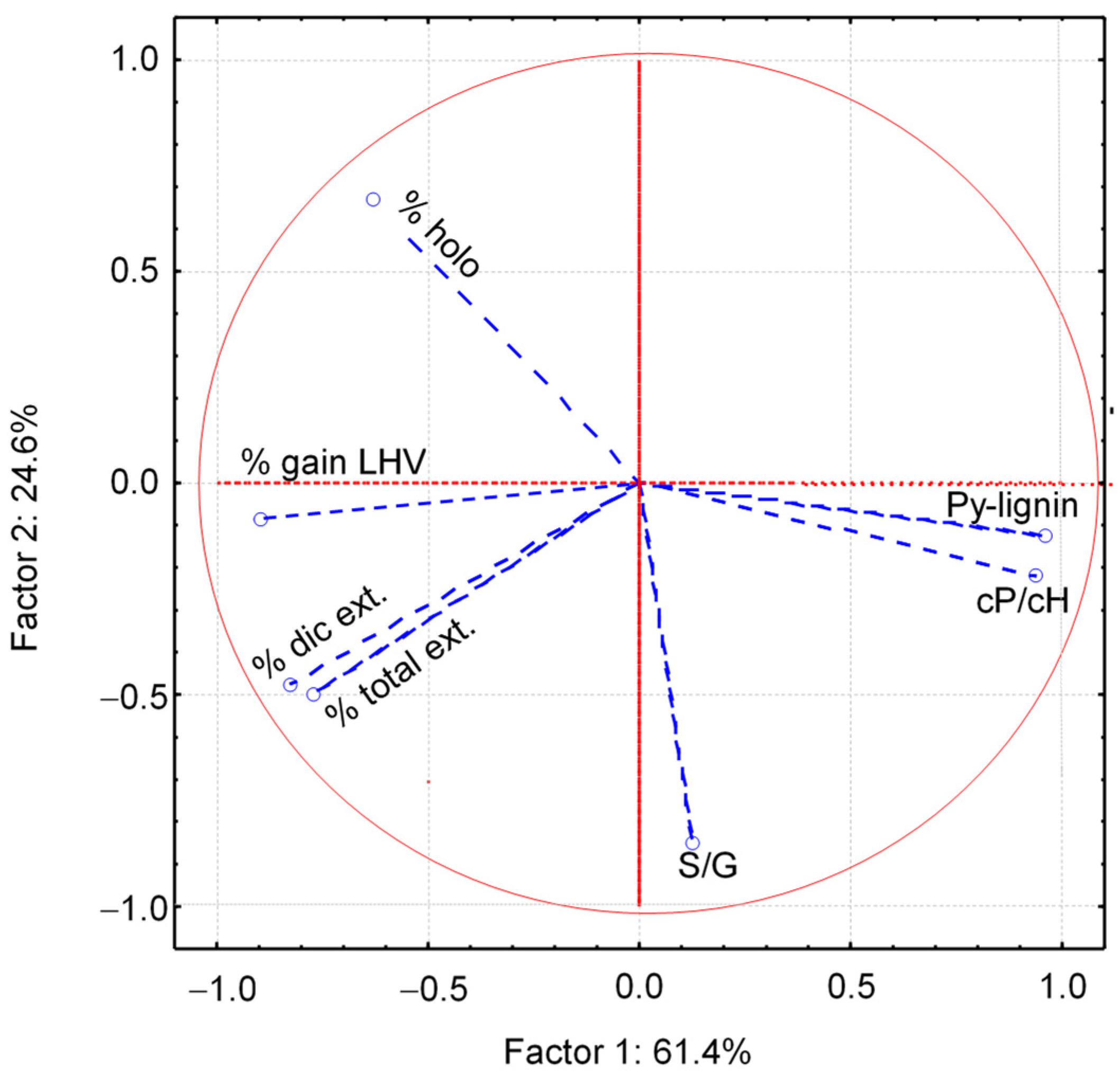
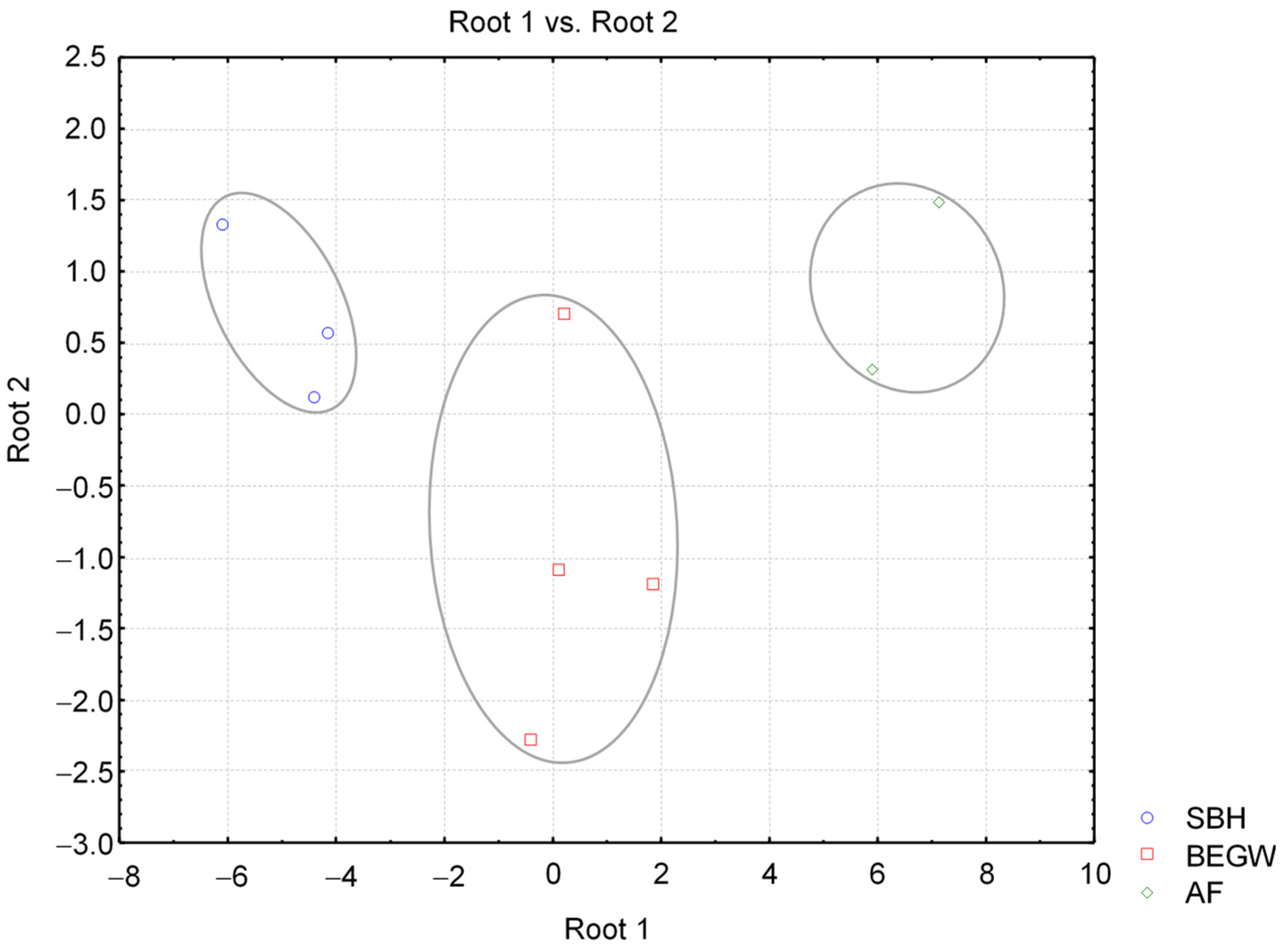
2.2.2. Multivariate Data Analysis
3. Materials and Methods
3.1. Extractives Content
3.2. Analytical Pyrolysis and Torrefaction Procedures
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubio-Varas, M.; Muñoz-Delgado, B. Long-term diversification paths and energy transitions in Europe. Ecol. Econ. 2019, 163, 158–168. [Google Scholar] [CrossRef]
- Sixto, H.; Hernandez, M.J.; Barrio, M.; Carrasco, J.; Cañellas, I. Plantaciones del género Populus para la producción de biomass com fins energéticos: Revision. Investig. Agrar. Sist. Recur. For. 2009, 16, 277–294. (In Spanish) [Google Scholar]
- Blanco, H.; Garasa, M.; Círia, M.; Garcia, J.; Vinãs, I. Manual de Cultivo de Populus spp. Para la Produccíon de Biomasa com Fines Energéticos; INITAA: Madrid, Spain, 2010; 55p. (In Spanish) [Google Scholar]
- Rodrigues, A.; Bordado, J.; Mateus, M. An evaluation of SRCs as a Potential carbon neutral source of biomass for energy and Chemicals. Adv. Environ. Ser. 2015, 43, 79–144. [Google Scholar]
- El Kasmioui, O.; Ceulemans, R. Financial analysis of the cultivation of poplar and willow for bioenergy. Biomass Bioenergy 2012, 43, 52–64. [Google Scholar] [CrossRef]
- Mohart, C.; Sheppard, J.; Spieker, H. Above Ground Leafless Woody Biomass and Nutrient Content within Different Compartments of a P. maximowicii × P. trichocarpa Poplar Clone. Forests 2013, 4, 471–487. [Google Scholar] [CrossRef]
- Rodrigues, A.; Loureiro, L.; Nunes, L. Torrefaction of woody biomasses from poplar SRC and Portuguese roundwood: Properties of torrefied products. Biomass Bioenergy 2018, 108, 55–65. [Google Scholar] [CrossRef]
- Ciolkosz, D.; Wallace, R. A review of torrefaction for bioenergy feedstock production. Biofuels Bioprod. Biorefining 2011, 5, 317–329. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Ragauskas, A.; Tuskan, G. Poplar as a feedstock for biofuels: A review of compositional characteristics. Biofuels Bioprod. Biorefining 2010, 4, 209–226. [Google Scholar] [CrossRef]
- Rego, F.; Dias, A.; Casquilho, M.; Rosa, F.; Rodrigues, A. Fast determination of lignocellulosic composition of poplar biomass by thermogravimetry. Biomass Bioenergy 2019, 122, 375–380. [Google Scholar] [CrossRef]
- Bergman, P.; Boersma, A.; Kiel, J.; Prins, M.; Ptasinski, K.; Janssen, F. Torrefaction for entrained-flow gasification of biomass. In Energy Research Centre of the Netherlands Scientific Report; Energy Research Centre of the Netherlands: Petten, The Netherlands, 2010; ECN-C--05-067; 50p. [Google Scholar]
- Giudiciani, P.; Gargiulo, V.; Grottola, C.; Alfe, M.; Ferreiro, A.; Mendes, M.; Fagnano, M.; Ragucci, R. Inherent Metal Elements in Biomass Pyrolysis: A Review. Energy Fuels 2021, 35, 5407–5478. [Google Scholar] [CrossRef]
- Davison, B.; Drescher, S.; Tuskan, G.; Davis, M.; Nghiem, N. Variation of S/G ratio and lignin content in a Populus family influences the release of xylose by dilute acid hydrolysis. Appl. Biochem. Biotechnol. 2005, 129–132, 427–435. [Google Scholar]
- Lupoi, J.; Seema, S.; Parthasarathi, R.; Simmons, B.; Henry, R. Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renew. Sustain. Energy Rev. 2015, 49, 871–906. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Rowell, R.; Pettersen, R.; Han, J.; Rowell, J.; Tshabalala, M. Cell Wall Chemistry. In Handbook of Wood Chemistry and Wood Composites; Rowell, R., Ed.; CRC Press: Madison, WI, USA, 2005; pp. 35–74. [Google Scholar]
- Rodrigues, J.; Meier, D.; Faix, O.; Pereira, H. Determination of tree-to-tree variation in syringyl/guaiacyl ratio of Eucalyptus globulus wood lignin by analytical pyrolysis. J. Anal. Appl. Pyrolysis 1999, 48, 121–128. [Google Scholar] [CrossRef]
- Alves, A.; Schwanninger, M.; Pereira, H.; Rodrigues, J. Analytical pyrolysis is a direct method to determine the lignin content in wood Part 1: Comparison of pyrolysis lignin with Klason lignin. J. Anal. Appl. Pyrolysis 2006, 76, 209–213. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Yang, H.; Wang, X.; Zhang, X.; Chen, H. Generalized two-dimensional correlation infrared spectroscopy to reveal mechanisms of lignocellulosic biomass pyrolysis. Proc. Combust. Inst. 2019, 37, 3013–3021. [Google Scholar] [CrossRef]
- Harvey, O.; Herbert, B.; Kuo, L.J.; Louchouarn, P. Generalized Two-Dimensional Perturbation Correlation Infrared Spectroscopy Reveals Mechanisms for the Developments of Surface Charge and Recalcitrance in Plant-Derived Biochars. Environ. Sci. Technol. 2012, 46, 10641–10650. [Google Scholar] [CrossRef]
- Kelly, J.; Helleur, R. Quantitative analysis of the major saccharides in sulfite-treated wood pulps by pyrolysis-gas chromatography: The effect of metal ions. J. Anal. Appl. Pyrolysis 1992, 23, 153–163. [Google Scholar] [CrossRef]
- Rodrigues, J.; Puls, J.; Faix, O.; Pereira, H. Determination of Monosaccharide Composition of Eucalyptus globulus Wood by FTIR Spectroscopy. Holzforschung 2001, 55, 265–269. [Google Scholar] [CrossRef]
- Meier, D.; Fortmann, I.; Odermatt, J.; Faix, O. Discrimination of genetically modified poplar clones by analytical pyrolysis–gas chromatography and principal component analysis. J. Anal. Appl. Pyrolysis 2005, 74, 129–137. [Google Scholar] [CrossRef]
- Alves, A.; Gierlinger, N.; Schwanninger, M.; Rodrigues, J. Analytical pyrolysis as a direct method to determine the lignin content in wood, Part 3: Evaluation of species-specific and tissue-specific differences in softwood lignin composition using principal component analysis. J. Anal. Appl. Pyrolysis 2009, 85, 30–37. [Google Scholar] [CrossRef]
- Lahive, C.; Kamer, P.; Lancefield, C.; Deuss, P. An Introduction to Model Compounds of Lignin Linking Motifs; Synthesis and Selection Considerations for Reactivity Studies. ChemSusChem 2020, 13, 4238–4265. [Google Scholar] [CrossRef] [PubMed]
- Ru, B.; Wang, S.; Dai, G.; Zhang, L. Effect of Torrefaction on Biomass Physicochemical characteristics and the resulting pyrolysis behavior. Energy Fuels 2015, 29, 5865–5874. [Google Scholar] [CrossRef]
- Acharya, B.; Sule, I.; Dutta, A. A review on advances of torrefaction technologies for biomass processing. Biomass Convers. Bioreferies 2012, 2, 349–369. [Google Scholar] [CrossRef]
- Tumurulu, J.; Ghiasi, B.; Soelberg, N.; Sokhansanj, S. Biomass Torrefaction Process, Product Properties, Reactor Types, and Moving Bed Reactor Design Concepts. Front. Energy Res. 2021, 9, 728140. [Google Scholar] [CrossRef]
- Nanou, P.; Huijgen, W.; Carbo, M.; Kiel, J. The role of lignin in the densification of torrefied wood in relation to the final product properties. Biomass Bioenergy 2018, 111, 248–262. [Google Scholar] [CrossRef]
- Gray, M.; Johnson, M.; Dragila, M.; Kleber, M. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass Bioenergy 2014, 61, 196–205. [Google Scholar] [CrossRef]
- Szadkowska, D.; Zawadzki, J.; Kozakiewicz, P.; Radomski, A. Identification of Extractives from Various Poplar Species. Forests 2021, 12, 647. [Google Scholar] [CrossRef]
- Johnson, R.; Wichern, D. Applied Multivariate Statistical Analysis, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2002; 767p, ISBN 0-13-092553-5. [Google Scholar]
- Dinus, R. Genetic improvement of poplar feedstock quality for ethanol production. Appl. Biochem. Biotechnol. 2001, 91–93, 23–34. [Google Scholar] [CrossRef]
- Dinus, R.; Payne, P.; Sewell, M.; Chiang, V.; Tuskan, G. Genetic modification of short rotation popular wood: Properties for ethanol fuel and fiber productions. Crit. Rev. Plant Sci. 2001, 20, 51–69. [Google Scholar] [CrossRef]
- Dixon, R.; Barros, J. Lignin biosynthesis: Old roads revisited and new roads explored. Open Biol. 2019, 9, 190215. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, L.; Verlinden, M.; Ceulemans, R. Establishment and two-year growth of a bio-energy plantation with fast-growing Populus trees in Flanders (Belgium): Effects of genotype and former land use. Biomass Bioenergy 2012, 42, 151–163. [Google Scholar] [CrossRef]
- Lazdina, D.; Bardule, A.; Lazdins, A.; Jansons, A. The first three-year development of ALASIA poplar clones AF2, AF6, AF7, AF8 in biomass short rotation coppice experimental cultures in Latvia. Agron. Res. 2014, 12, 543–552. [Google Scholar]
- Alves, A.; Rodrigues, J.; Wimmer, R.; Schwanninger, M. Analytical pyrolysis as a direct method to determine the lignin content in wood, Part 2: Evaluation of the common model and the influence of compression wood. J. Anal. Appl. Pyrolysis 2008, 81, 167–172. [Google Scholar] [CrossRef]
- EN 14918; Solid Biofuels—Determination of Calorific Value. Deutsches Institut für Normung e. V. [German Institute for Standardisation]: Berlin, Germany, 2009.
- ASTM D7582-12; Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis. America Society for Testing and Material: Philadelphia, PA, USA, 2012.
| Clone | % CH2Cl2 Extractives | % ethanol Extractives | % H2O Extractives | % Total Extractives | (cP/cH) Ratio | (S/G) Ratio | Py-Lignin (%) | (H/G) Ratio | |
| Skado | 1.25 | 2.41 | 2.26 | 5.92 | 17.02 | 0.85 | 22.70 | 0.13 | |
| Bakan | 1.05 | 2.86 | 2.62 | 6.53 | 18.11 | 0.78 | 23.41 | 0.20 | |
| Hees | 1.16 | 2.62 | 2.04 | 5.83 | 21.08 | 1.00 | 25.90 | 0.12 | |
| Brandaris | 1.15 | 2.71 | 2.47 | 6.33 | 22.32 | 0.94 | 24.74 | 0.15 | |
| Ellert | 0.89 | 2.23 | 1.89 | 5.01 | 22.82 | 0.94 | 25.13 | 0.10 | |
| Grimminge | 0.80 | 1.53 | 1.44 | 3.77 | 23.33 | 0.79 | 27.11 | 0.09 | |
| Wolterson | 1.07 | 2.51 | 1.67 | 5.26 | 20.05 | 0.99 | 24.83 | 0.11 | |
| AF8 | 0.79 | 2.57 | 1.76 | 5.13 | 24.57 | 0.96 | 26.86 | 0.14 | |
| AF2 | 0.51 | 1.90 | 1.30 | 3.71 | 22.67 | 0.85 | 26.34 | 0.16 | |
| Clone | % HoloPy | LHV (MJkg−1) (*) | % LHVGain (#) | % Volatiles Loss (#) | % Fixed Carbon Gain (#) | % Ratio (O/C) Loss (#) | % Carbon gain (#) | % Mass Loss Torrefaction (#) | % Oxygen Loss (#) |
| Skado | 71.38 | 18.48 ± 0.4 | 35.60 | 25.37 | 51.05 | 51.53 | 30.62 | 46.83 | 36.68 |
| Bakan | 70.06 | 18.53 ± 0.6 | 31.79 | 23.90 | 50.65 | 47.63 | 27.96 | 49.58 | 32.98 |
| Hees | 68.27 | 18.80 ± 0.9 | 32.78 | 29.90 | 53.16 | 50.80 | 29.92 | 45.14 | 36.03 |
| Brandaris | 68.93 | 18.70 ± 0.6 | 25.60 | 22.30 | 45.08 | 49.60 | 28.65 | 43.21 | 35.19 |
| Ellert | 69.86 | 18.70 ± 0.7 | 28.90 | 23.90 | 48.30 | 52.42 | 30.89 | 46.60 | 37.73 |
| Grimminge | 69.12 | 18.60 ± 1.1 | 24.70 | 24.70 | 48.48 | 52.80 | 30.84 | 49.77 | 38.30 |
| Wolterson | 69.92 | 18.70 ± 0.4 | 26.90 | 25.60 | 53.02 | 40.06 | 30.62 | 47.81 | 26.67 |
| AF8 | 68.01 | 18.50 ± 0.6 | 22.70 | 21.04 | 44.40 | 48.67 | 28.74 | 45.80 | 33.92 |
| AF2 | 69.96 | 18.50 ± 0.7 | 22.11 | 17.30 | 41.30 | 43.45 | 24.52 | 45.80 | 29.58 |
| CH2Cl2 Extr. | Ethanol Extr. | H2O Extr. | Total Extr. | cP/cH | S/G | Py-Lignin | H/G | HoloPy | LHV | Gain LHV | |
| CH2Cl2 extr. | 1.00 | 0.65 | 0.77 | 0.84 | −0.67 | 0.24 | −0.70 | 0.01 | 0.29 | 0.38 | 0.79 |
| Ethanol extr. | 0.65 | 1.00 | 0.79 | 0.92 | −0.42 | 0.40 | −0.55 | 0.56 | −0.08 | 0.19 | 0.43 |
| H2O extr. | 0.77 | 0.79 | 1.00 | 0.95 | −0.58 | −0.05 | −0.75 | 0.53 | 0.39 | 0.09 | 0.64 |
| Total extr. | 0.84 | 0.92 | 0.95 | 1.00 | −0.58 | 0.20 | −0.73 | 0.47 | 0.21 | 0.21 | 0.64 |
| cP/cH | −0.67 | −0.42 | −0.58 | −0.58 | 1.00 | 0.28 | 0.90 | −0.34 | −0.73 | 0.15 | −0.82 |
| S/G | 0.24 | 0.40 | −0.05 | 0.20 | 0.28 | 1.00 | 0.19 | −0.32 | −0.62 | 0.64 | −0.05 |
| Py-lignin | −0.70 | −0.55 | −0.75 | −0.73 | 0.90 | 0.19 | 1.00 | −0.40 | −0.69 | 0.14 | −0.76 |
| H/G | 0.01 | 0.56 | 0.53 | 0.47 | −0.34 | −0.32 | −0.40 | 1.00 | 0.20 | −0.47 | 0.08 |
| HoloPy | 0.29 | −0.08 | 0.39 | 0.21 | −0.73 | −0.62 | −0.69 | 0.20 | 1.00 | −0.16 | 0.41 |
| LHV | 0.38 | 0.19 | 0.09 | 0.21 | 0.15 | 0.64 | 0.14 | −0.47 | −0.16 | 1.00 | 0.15 |
| Gain LHV | 0.79 | 0.43 | 0.64 | 0.64 | −0.82 | −0.05 | −0.76 | 0.08 | 0.41 | 0.15 | 1.00 |
| Volatiles loss | 0.74 | 0.27 | 0.33 | 0.43 | −0.41 | 0.30 | −0.24 | −0.40 | −0.02 | 0.62 | 0.72 |
| Fixed carbon gain | 0.74 | 0.32 | 0.35 | 0.46 | −0.63 | 0.17 | −0.45 | −0.27 | 0.15 | 0.45 | 0.76 |
| Carbon gain | 0.59 | 0.02 | 0.16 | 0.22 | −0.16 | 0.24 | −0.17 | −0.69 | 0.00 | 0.43 | 0.46 |
| Volatiles Loss | Fixed Carbon Gain | Carbon Gain | |||||||||
| CH2Cl2 extr. | 0.74 | 0.74 | 0.59 | ||||||||
| Ethanol extr. | 0.27 | 0.32 | 0.02 | ||||||||
| H2O extr. | 0.33 | 0.35 | 0.16 | ||||||||
| Total extr. | 0.43 | 0.46 | 0.22 | ||||||||
| cP/cH | −0.41 | −0.63 | −0.16 | ||||||||
| S/G | 0.30 | 0.17 | 0.24 | ||||||||
| Py-lignin | −0.24 | −0.45 | −0.17 | ||||||||
| H/G | −0.40 | −0.27 | −0.69 | ||||||||
| HoloPy | −0.02 | 0.15 | 0.00 | ||||||||
| LHV | 0.62 | 0.45 | 0.43 | ||||||||
| Gain LHV | 0.72 | 0.76 | 0.46 | ||||||||
| Volatiles loss | 1.00 | 0.92 | 0.76 | ||||||||
| Fixed carbon gain | 0.92 | 1.00 | 0.71 | ||||||||
| Carbon gain | 0.76 | 0.71 | 1.00 | ||||||||
| Y = 50.2 + 10.1 x1 − 1.3 x2; R2 = 0.70 | p = 0.02 | Y: LHVgain; x1: ext. CH2Cl2; x2: Py-lignin | (1) |
| Y = 41.0 + 8.6 x1 − 1.0 x2; R2 = 0.78 | p = 0.01 | Y: LHVgain; x1: ext. CH2Cl2; x2: (cP/cH) ratio | (2) |
| Y = 23.9 + 16.9 x1 − 13.8 x2; R2 = 0.68 | p = 0.03 | Y: LHVgain; x1: ext. CH2Cl2; x2: (S/G) ratio | (3) |
| Y = 65.0 − 0.3 x1 − 1.4 x2; R2 = 0.68 | p = 0.03 | Y: LHVgain; x1: Py-lignin; x2: (cP/cH) ratio | (4) |
| Eigenvalue | % Total Variance | Cumulative Eigenvalue | Cumulative % Variance | |
|---|---|---|---|---|
| 1 | 4.29 | 61.39 | 4.29 | 61.39 |
| 2 | 1.71 | 24.48 | 6.01 | 85.88 |
| 3 | 0.47 | 6.82 | 6.48 | 92.71 |
| 4 | 0.31 | 4.46 | 6.80 | 97.17 |
| 5 | 0.11 | 1.59 | 6.91 | 98.77 |
| 6 | 0.08 | 1.22 | 7.00 | 100.0 |
| Factor 1 | Factor 2 | |
|---|---|---|
| %CH2Cl2 | −0.83 | 0.47 |
| %Total | −0.76 | 0.50 |
| LHV gain | −0.89 | 0.09 |
| Py-lignin (%) | 0.95 | 0.12 |
| HoloPy | −0.63 | 0.67 |
| S/G | 0.12 | −0.84 |
| cP/cH | 0.93 | −0.21 |
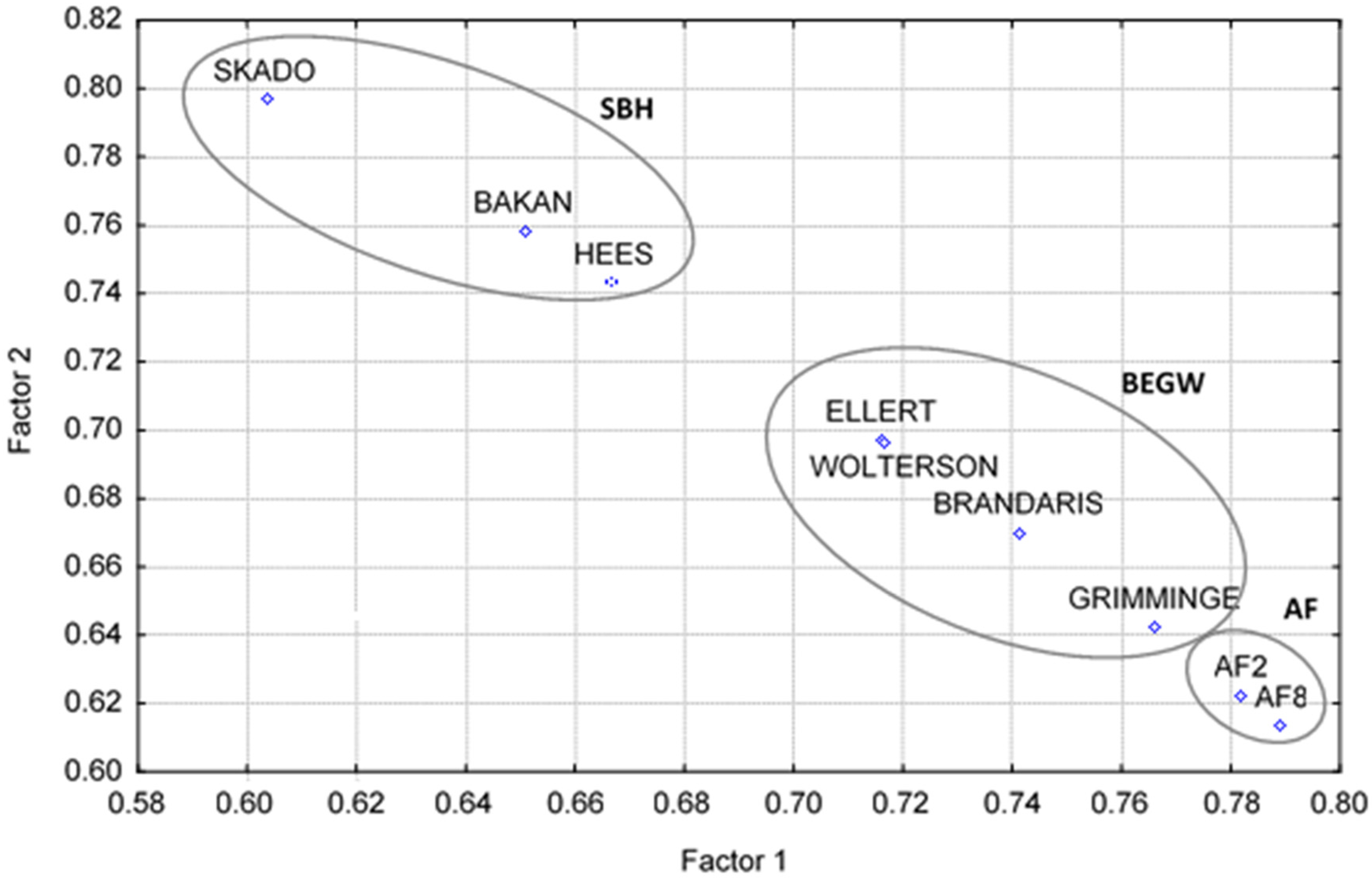
| Factor 1 | Factor 2 | |
|---|---|---|
| Skado | 0.60 | 0.79 |
| Bakan | 0.65 | 0.75 |
| Hees | 0.66 | 0.74 |
| Brandaris | 0.74 | 0.67 |
| Ellert | 0.71 | 0.69 |
| Grimminge | 0.76 | 0.64 |
| Wolterson | 0.71 | 0.69 |
| AF8 | 0.78 | 0.61 |
| AF2 | 0.78 | 0.62 |
| Expl.Var | 4.63 | 4.36 |
| Prop.Total | 0.51 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.M.; Alves, A.; Graça, J.; Rodrigues, J. A Quantitative Evaluation of the Influence of Chemical Variables of Biomasses of Poplar SRC Commercial Clones in Torrefaction. Molecules 2024, 29, 4542. https://doi.org/10.3390/molecules29194542
Rodrigues AM, Alves A, Graça J, Rodrigues J. A Quantitative Evaluation of the Influence of Chemical Variables of Biomasses of Poplar SRC Commercial Clones in Torrefaction. Molecules. 2024; 29(19):4542. https://doi.org/10.3390/molecules29194542
Chicago/Turabian StyleRodrigues, Abel Martins, Ana Alves, José Graça, and José Rodrigues. 2024. "A Quantitative Evaluation of the Influence of Chemical Variables of Biomasses of Poplar SRC Commercial Clones in Torrefaction" Molecules 29, no. 19: 4542. https://doi.org/10.3390/molecules29194542
APA StyleRodrigues, A. M., Alves, A., Graça, J., & Rodrigues, J. (2024). A Quantitative Evaluation of the Influence of Chemical Variables of Biomasses of Poplar SRC Commercial Clones in Torrefaction. Molecules, 29(19), 4542. https://doi.org/10.3390/molecules29194542








