Identification of Key Volatile Compounds in Tilapia during Air Frying Process by Quantitative Gas Chromatography–Ion Mobility Spectrometry
Abstract
1. Introduction
2. Results and Discussion
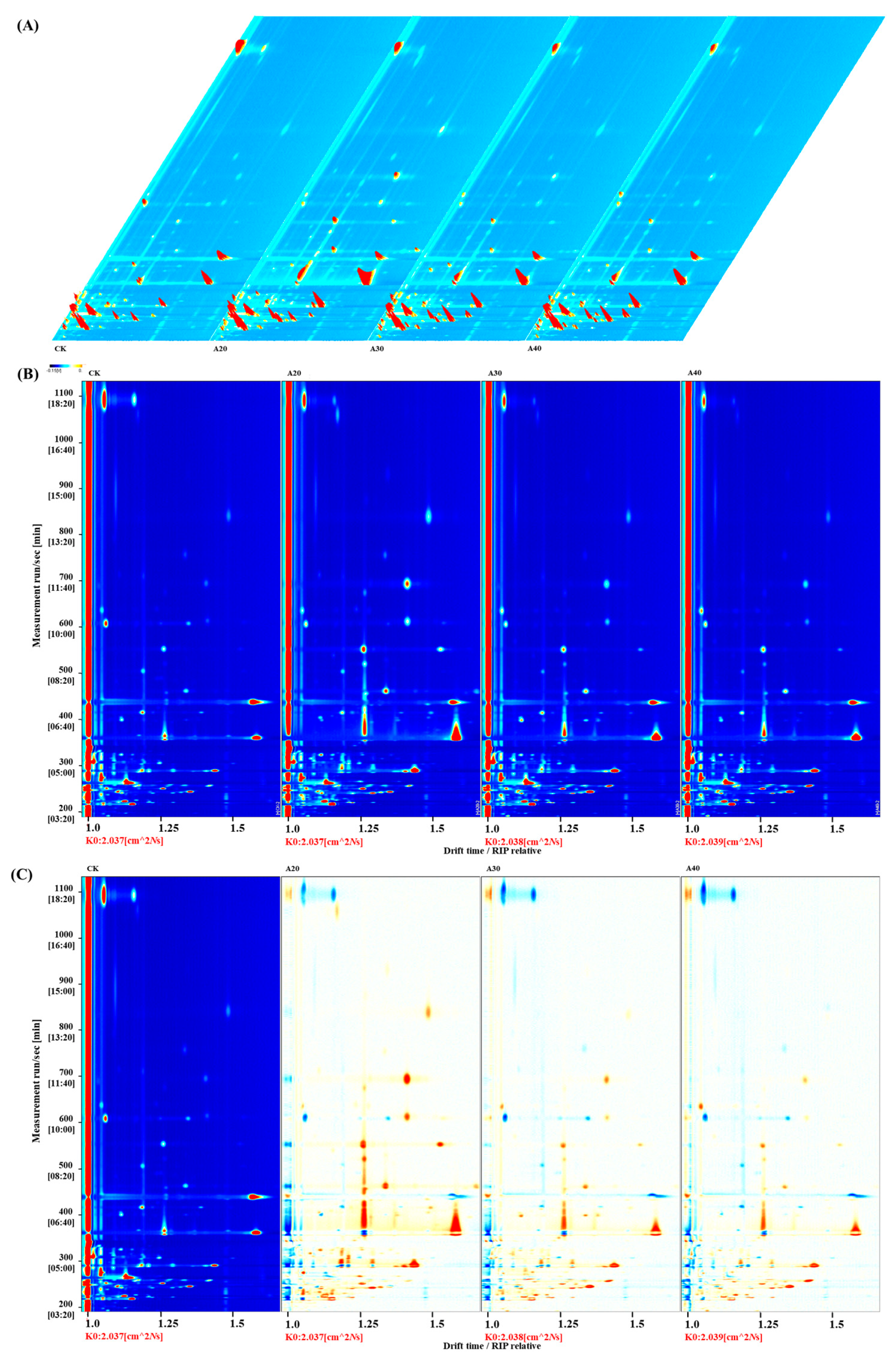
2.1. Change in VCs during Air Frying by Quantitative GC-IMS
2.2. Identification of Key VCs during Air Frying
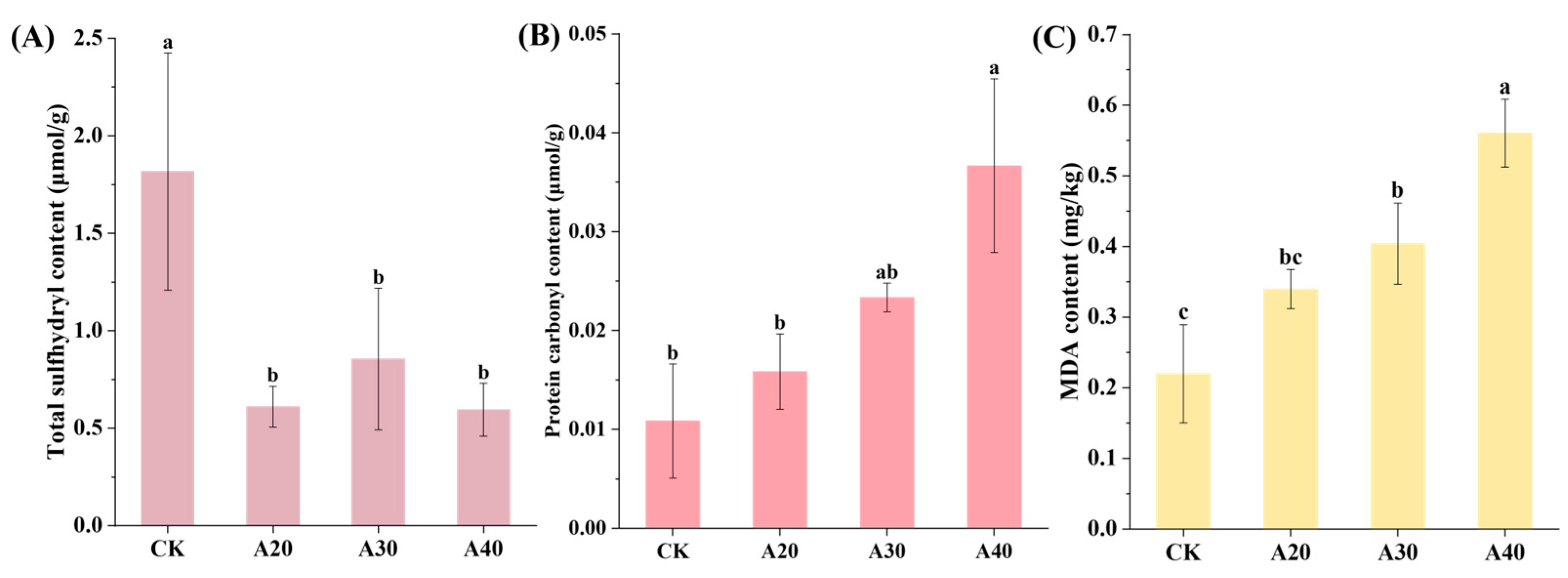
2.3. Change in Chemical Properties of Tilapia during Air Frying
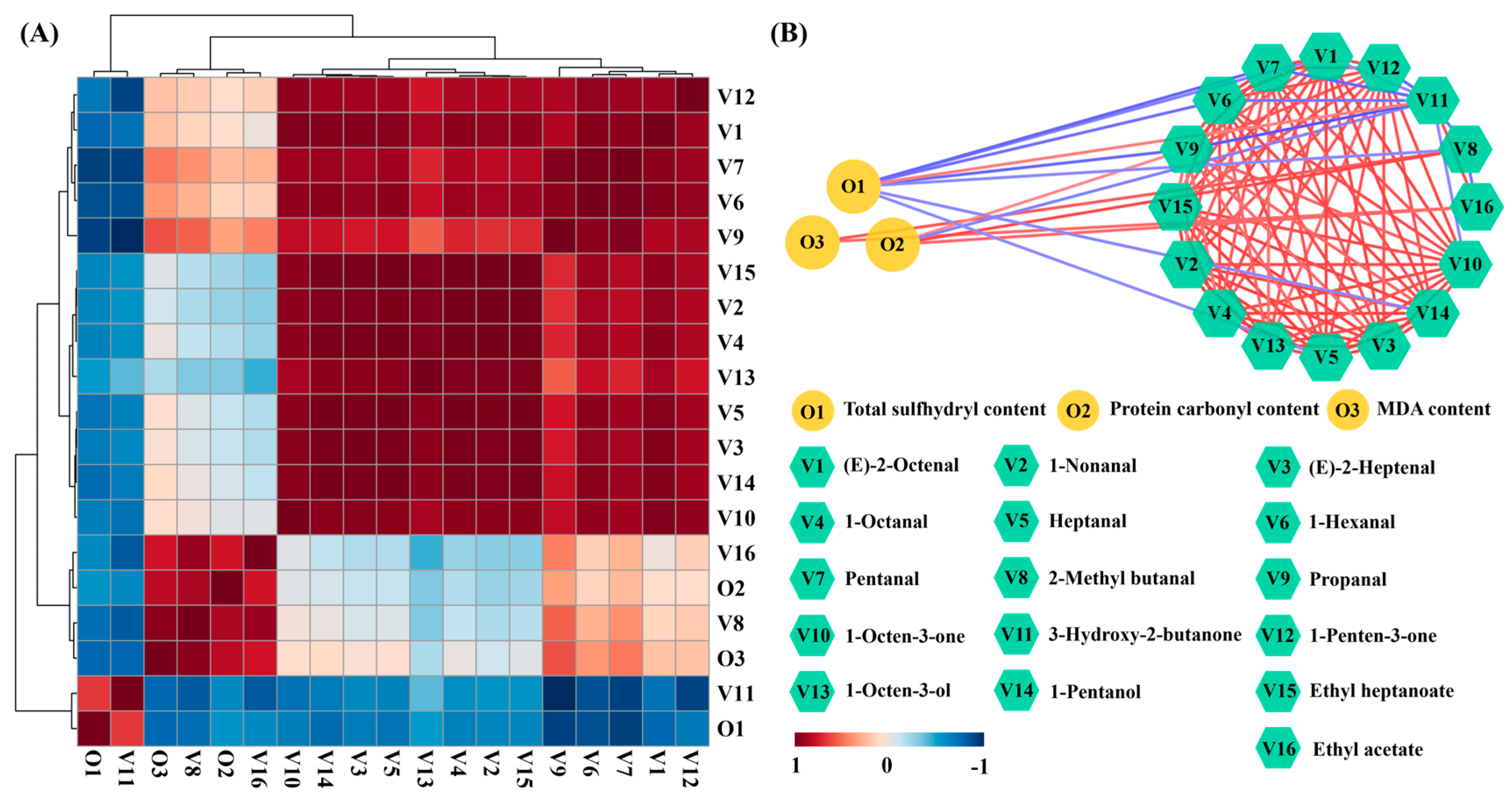
2.4. Correlation Analysis among Chemical Properties and Key VCs during Air Frying
3. Materials and Methods
3.1. Air Frying of Tilapia
3.2. Determination of VCs by Quantitative HS-GC-IMS
3.3. Analysis of Oxidation of Proteins and Lipids
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Wu, Z.; Ren, Y.; Zhou, Z.; Wang, W.-X.; Huang, Y.; Shu, X. Improving heat resistance of nile tilapia (Oreochromis niloticus) by dietary zinc supplementation. Aquac. Nutr. 2022, 2022, 6323789. [Google Scholar] [CrossRef]
- Jinagool, P.; Wipassa, V.; Chaiyasing, R.; Chukanhom, K.; Aengwanich, W. Effect of increasing ambient temperature on physiological changes, oxidative stress, nitric oxide, Total Antioxidant Power, and Mitochondrial Activity of Nile Tilapia (Oreochromis niloticus Linn.). Aquaculture 2024, 589, 741017. [Google Scholar] [CrossRef]
- Li, C.; Chen, S.; Huang, H.; Li, J.; Zhao, Y. Improvement mechanism of volatile flavor in fermented tilapia surimi by cooperative fermentation of Pediococcus acidilactici and Latilactobacillus sakei: Quantization of microbial contribution through influence of genus. Food Chem. 2024, 449, 139239. [Google Scholar] [CrossRef]
- Iwegbue, C.M.A.; Osijaye, K.O.; Igbuku, U.A.; Egobueze, F.E.; Tesi, G.O.; Bassey, F.I.; Martincigh, B.S. Effect of the number of frying cycles on the composition, concentrations and risk of polycyclic aromatic hydrocarbons (pahs) in vegetable oils and fried fish. J. Food Compos. Anal. 2020, 94, 103633. [Google Scholar] [CrossRef]
- Shi, H.; Qin, R.; Wu, R.; Rong, J.; Jia, C.; Liu, R. Effect of cryoprotectants on the formation of advanced glycation end products and acrylamide in fried fish cakes. Food Biosci. 2021, 44, 101433. [Google Scholar] [CrossRef]
- Shi, H.; Gao, R.; Liu, H.; Wang, Z.; Zhang, C.; Zhang, D. Qualitative and quantitative assessment of key aroma compounds, advanced glycation end products and heterocyclic amines in different varieties of commercially roasted meat products. Food Chem. 2024, 436, 137742. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, V.S.; Viana, D.S.B.; Keller, L.M.; de Melo, M.T.T.; Mulandeza, O.F.; Barbosa, M.I.M.J.; Barbosa Júnior, J.L.; Saldanha, T. Impact of air frying on food lipids: Oxidative evidence, current research, and insights into domestic mitigation by natural antioxidants. Trends Food Sci. Technol. 2024, 147, 104465. [Google Scholar] [CrossRef]
- Téllez-Morales, J.A.; Rodríguez-Miranda, J.; Aguilar-Garay, R. Review of the influence of hot air frying on food quality. Meas. Food 2024, 14, 100153. [Google Scholar] [CrossRef]
- Ghaitaranpour, A.; Koocheki, A.; Mohebbi, M.; Ngadi, M.O. Effect of deep fat and hot air frying on doughnuts physical properties and kinetic of crust formation. J. Cereal Sci. 2018, 83, 25–31. [Google Scholar] [CrossRef]
- Dehghannya, J.; Ngadi, M. Recent advances in microstructure characterization of fried foods: Different frying techniques and process modeling. Trends Food Sci. Technol. 2021, 116, 786–801. [Google Scholar] [CrossRef]
- Gouyo, T.; Rondet, É.; Mestres, C.; Hofleitner, C.; Bohuon, P. Microstructure analysis of crust during deep-fat or hot-air frying to understand french fry texture. J. Food Eng. 2021, 298, 110484. [Google Scholar] [CrossRef]
- Castro-López, R.; Mba, O.I.; Gómez-Salazar, J.A.; Cerón-García, A.; Ngadi, M.O.; Sosa-Morales, M.E. Evaluation of chicken nuggets during air frying and deep-fat frying at different temperatures. Int. J. Gastron. Food Sci. 2023, 31, 100631. [Google Scholar] [CrossRef]
- Qin, R.; Wu, R.; Shi, H.; Jia, C.; Rong, J.; Liu, R. Formation of ages in fish cakes during air frying and other traditional heating methods. Food Chem. 2022, 391, 133213. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, L.; Xue, J.; Wang, J.; Song, G.; Zhang, Y.; Shen, Q. Effect of air-frying conditions on the quality attributes and lipidomic characteristics of surimi during processing. Innov. Food Sci. Emerg. Technol. 2020, 60, 102305. [Google Scholar] [CrossRef]
- Chen, T.; Li, C.; Huang, H.; Zhao, Y.; Xiang, H.; Wang, D.; Feng, Y.; Yang, S.; Chen, S. Identification of key physicochemical properties and volatile flavor compounds for the sensory formation of roasted tilapia. Food Chem. 2024, 460, 140636. [Google Scholar] [CrossRef]
- He, J.; Qi, M. A new type of triptycene-based stationary phases with alkylated benzimidazolium cations for gas chromatographic separations. Chin. Chem. Lett. 2019, 32, 949–1266. [Google Scholar] [CrossRef]
- Wei, S.; Wu, Q.; Wang, Z.; Yu, X.; Jiao, J.; Dong, X. Determination of key volatile fishy substances of sea cucumber powder during the processing and their removal by supercritical fluid extraction. Food Res. Int. 2024, 190, 114603. [Google Scholar] [CrossRef]
- Li, C.; Cui, Q.; Li, L.; Huang, H.; Chen, S.; Zhao, Y.; Wang, Y. Formation and improvement mechanism of physical property and volatile flavor of fermented tilapia surimi by newly isolated lactic acid bacteria based on two dimensional correlation networks. Food Chem. 2024, 440, 138260. [Google Scholar] [CrossRef]
- Nie, R.; Zhang, C.; Liu, H.; Wei, X.; Gao, R.; Shi, H.; Zhang, D.; Wang, Z. Characterization of key aroma compounds in roasted chicken using SPME, SAFE, GC-O, GC–MS, AEDA, OAV, recombination-omission tests, and sensory evaluation. Food Chem. X 2024, 21, 101167. [Google Scholar] [CrossRef]
- Wu, T.; Wang, P.; Zhang, Y.; Zhan, P.; Zhao, Y.; Tian, H.; He, W. Identification of muttony-related compounds in cooked mutton tallows and their flavor intensities subjected to phenolic extract from thyme (Thymus vulgaris L.). Food Chem. 2023, 427, 136666. [Google Scholar] [CrossRef]
- Huang, Q.; Dong, K.; Wang, Q.; Huang, X.; Wang, G.; An, F.; Luo, Z.; Luo, P. Changes in volatile flavor of yak meat during oxidation based on multi-omics. Food Chem. 2022, 371, 131103. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Lin, L.; Zhu, Y.; Jiang, S.; Lu, J. Comparative study between surimi gel and surimi/crabmeat mixed gel on nutritional properties, flavor characteristics, color, and texture. J. Aquat. Food Prod. Technol. 2020, 29, 681–692. [Google Scholar] [CrossRef]
- Ding, T.; Li, Y. Biogenic amines are important indices for characterizing the freshness and hygienic quality of aquatic products: A review. LWT 2024, 194, 115793. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Lin, X.-N.; Ji, Y.-Q.; He, H.-J.; Yang, H.-Z.; Tang, X.-J.; Liu, Y.-G. Characterization and correlation of dominant bacteria and volatile compounds in post-fermentation process of ba-bao douchi. Food Res. Int. 2022, 160, 111688. [Google Scholar] [CrossRef]
- Shen, Z.; Tian, M.; Wang, F.; Liu, Y.; Wu, J.; Li, X. Enhancing freeze-thaw stability and flavor in surimi products: Impact of virgin coconut oil and fish oil incorporation. Food Biosci. 2024, 57, 103515. [Google Scholar] [CrossRef]
- Chen, X.; Du, B.; Liu, J.; Zhang, C.; Zhu, H.; Wang, K.; Sun, B.; Li, X. Exploring the significance of the 2nd and 4th round fermentations in the brewing process of sauce-flavor baijiu. Food Biosci. 2024, 59, 104114. [Google Scholar] [CrossRef]
- Li, Q.; Du, B.; Chen, X.; Zhao, Y.; Zhu, L.; Ma, H.; Sun, B.; Hao, J.; Li, X. Microbial community dynamics and spatial distribution of flavor compound metabolism during solid-state fermentation of baijiu enhanced by Wickerhamomyces anomalus. Food Biosci. 2024, 59, 103909. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Sun, Y.; Ji, L.; Liu, T.; Li, H.; Jiang, X. Nutritional evaluation, flavor characteristics and microbial community of shrimp paste made from different materials and variance analysis. Food Chem. Adv. 2023, 2, 100268. [Google Scholar] [CrossRef]
- Xiao, N.; Xu, H.; Jiang, X.; Sun, T.; Luo, Y.; Shi, W. Evaluation of aroma characteristics in grass carp mince as affected by different washing processes using an E-Nose, HS-SPME-GC-MS, HS-GC-IMS, and sensory analysis. Food Res. Int. 2022, 158, 111584. [Google Scholar] [CrossRef]
- Dong, X.; Li, D.; Huang, Y.; Wu, Q.; Liu, W.; Qin, L.; Zhou, D.; Prakash, S.; Yu, C. Nutritional value and flavor of turbot (Scophthalmus maximus) muscle as affected by cooking methods. Int. J. Food Prop. 2018, 21, 1972–1985. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Li, Q.; Zhang, L.; Wang, Z.; Han, L.; Yu, Q. Oxidation of myofibrillar protein and crosslinking behavior during processing of traditional air-dried yak (Bos grunniens) meat in relation to digestibility. LWT 2021, 142, 110984. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein oxidation: Basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef]
- Estévez, M. Protein Carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, X.; Liu, X.; Zhang, Y.; Zhao, K.; Zhang, K.; Wang, W. Effects of different cooking methods on physicochemical, textural properties of yak meat and its changes with intramuscular connective tissue during in vitro digestion. Food Chem. 2023, 422, 136188. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shi, C.; Xu, J.; Wang, X.; Zhong, J. Correlation between physicochemical properties and volatile compound profiles in tilapia muscles subjected to four different thermal processing techniques. Food Chem. X 2023, 18, 100748. [Google Scholar] [CrossRef]
- De Carvalho, I.O.A.M.; de Oliveira, V.S.; Chávez, D.W.H.; Gamallo, O.D.; Castro, R.N.; Sawaya, A.C.H.F.; Sampaio, G.R.; da Silva Torres, E.A.F.; Saldanha, T. The use of lemon juice and its role on polyunsaturated fatty acids and cholesterol oxides formation in thermally prepared sardines. J. Food Compos. Anal. 2021, 104, 104087. [Google Scholar] [CrossRef]
- Vieira, E.C.S.; Mársico, E.T.; Conte-Junior, C.A.; Damiani, C.; Canto, A.C.V.C.S.; Monteiro, M.L.G.; da Silva, F.A. Effects of different frying techniques on the color, fatty acid profile, and lipid oxidation of arapaima gigas. J. Food Process. Preserv. 2018, 42, e13820. [Google Scholar] [CrossRef]
- Liu, Y.; Al-Dalali, S.; Hu, Y.; Zhao, D.; Wang, J.; He, Z. Effect of different processing steps in the production of beer fish on volatile flavor profile and their precursors determined by hs-gc-ims, hplc, e-nose, and E-Tongue. Food Chem. X 2024, 23, 101623. [Google Scholar] [CrossRef]
- Yang, L.; Li, Z.; Xie, T.; Feng, J.; Xu, X.; Zhao, Y.; Gao, X. Effects of Sous-Vide on Quality, Structure and Flavor Characteristics of Tilapia Fillets. Molecules 2023, 28, 8075. [Google Scholar] [CrossRef]


| Volatile Compounds | OAV | Threshold (mg/kg) | Odor | |||
|---|---|---|---|---|---|---|
| CK | A20 | A30 | A40 | |||
| (E)-2-Octenal | 4.24 | 10.12 | 6.87 | 6.98 | 0.003 | Fatty, Cucumber |
| 1-Nonanal | 73.77 | 232.29 | 126.49 | 98.26 | 0.001 | Fatty, Floral, Lemon |
| (E)-2-Heptenal | 0.99 | 3.93 | 2.04 | 1.80 | 0.013 | Fatty, Fruity, Almond |
| 1-Octanal | 41.54 | 232.37 | 101.09 | 85.28 | 0.0007 | Fruity, Fatty |
| Heptanal | 21.52 | 119.59 | 56.98 | 50.16 | 0.003 | Citrus, Green, Herbal |
| 1-Hexanal | 205.12 | 620.18 | 442.24 | 431.40 | 0.0045 | Grassy, Fruity |
| Pentanal | 13.88 | 44.24 | 33.50 | 33.46 | 0.02 | Almond, Malty, Oil |
| 2-Methyl butanal | 74.52 | 117.42 | 180.35 | 231.05 | 0.001 | Malty, Almond |
| Propanal | 5.22 | 8.57 | 8.04 | 7.82 | 0.07 | Floral |
| 1-Octen-3-ol | 27.65 | 78.67 | 34.10 | 32.02 | 0.0012 | Mushroom |
| 1-Pentanol | 0.54 | 2.75 | 1.37 | 1.29 | 0.0015 | Green |
| 1-Octen-3-one | 1725.71 | 4168.56 | 2965.51 | 2544.49 | 0.000005 | Mushroom |
| 3-Hydroxy-2-butanone | 14.71 | 9.70 | 8.41 | 9.65 | 0.014 | Buttery, Green |
| 1-Penten-3-one | 14.49 | 32.55 | 26.64 | 22.47 | 0.6 | Green, Pungent |
| Ethyl heptanoate | 0.25 | 2.04 | 0.80 | 0.58 | 0.17 | Brandy, Fruity, Wine |
| Ethyl acetate | 39.08 | 48.22 | 91.55 | 90.47 | 0.005 | Fruity, Waxy, Floral |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Xue, Y.; Li, C.; Zhao, Y.; Huang, H.; Feng, Y.; Xiang, H.; Chen, S. Identification of Key Volatile Compounds in Tilapia during Air Frying Process by Quantitative Gas Chromatography–Ion Mobility Spectrometry. Molecules 2024, 29, 4516. https://doi.org/10.3390/molecules29184516
Chen T, Xue Y, Li C, Zhao Y, Huang H, Feng Y, Xiang H, Chen S. Identification of Key Volatile Compounds in Tilapia during Air Frying Process by Quantitative Gas Chromatography–Ion Mobility Spectrometry. Molecules. 2024; 29(18):4516. https://doi.org/10.3390/molecules29184516
Chicago/Turabian StyleChen, Tianyu, Yong Xue, Chunsheng Li, Yongqiang Zhao, Hui Huang, Yang Feng, Huan Xiang, and Shengjun Chen. 2024. "Identification of Key Volatile Compounds in Tilapia during Air Frying Process by Quantitative Gas Chromatography–Ion Mobility Spectrometry" Molecules 29, no. 18: 4516. https://doi.org/10.3390/molecules29184516
APA StyleChen, T., Xue, Y., Li, C., Zhao, Y., Huang, H., Feng, Y., Xiang, H., & Chen, S. (2024). Identification of Key Volatile Compounds in Tilapia during Air Frying Process by Quantitative Gas Chromatography–Ion Mobility Spectrometry. Molecules, 29(18), 4516. https://doi.org/10.3390/molecules29184516









