Solvent-Free Method of Polyacrylonitrile-Coated LLZTO Solid-State Electrolytes for Lithium Batteries
Abstract
1. Introduction
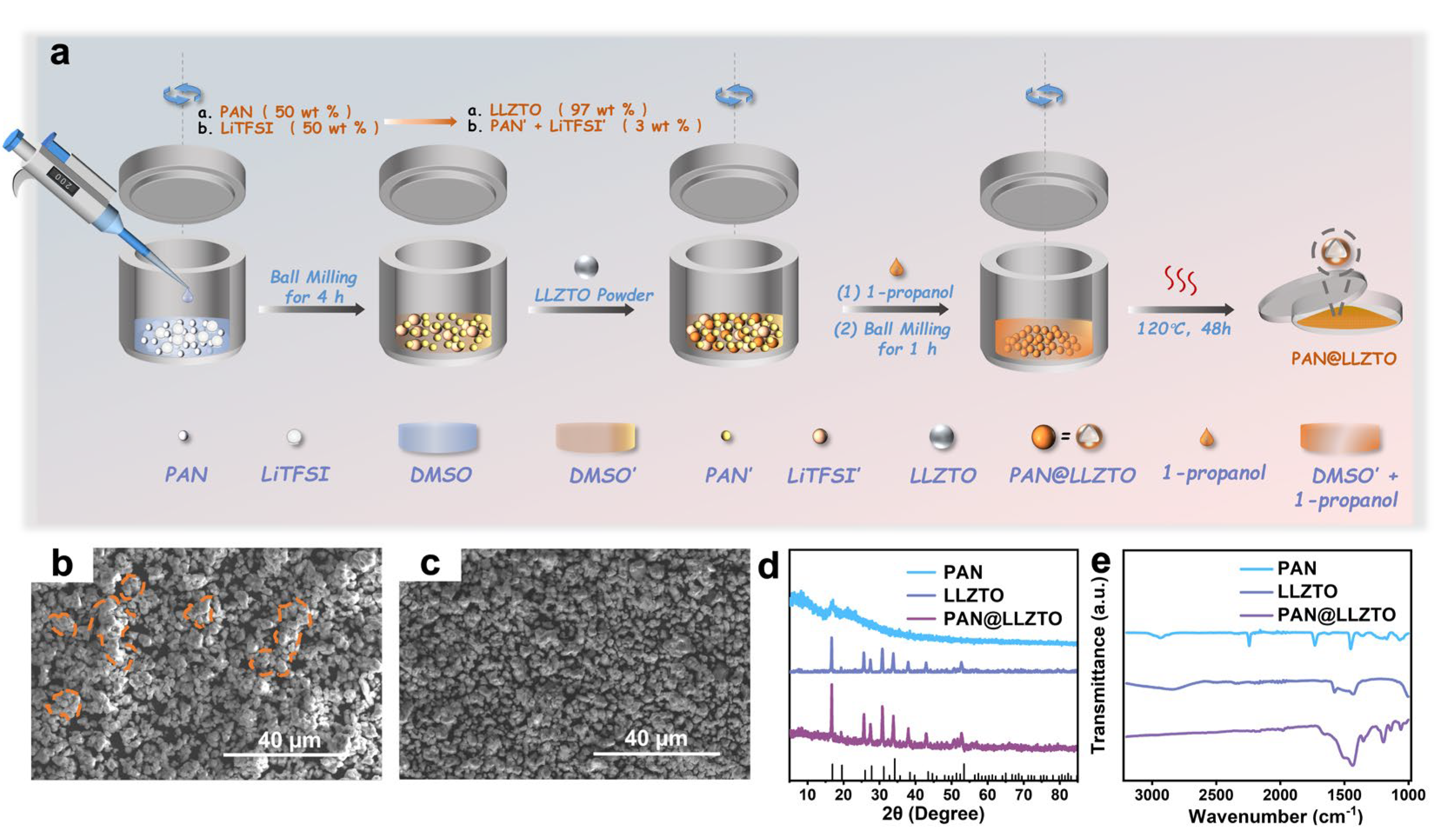
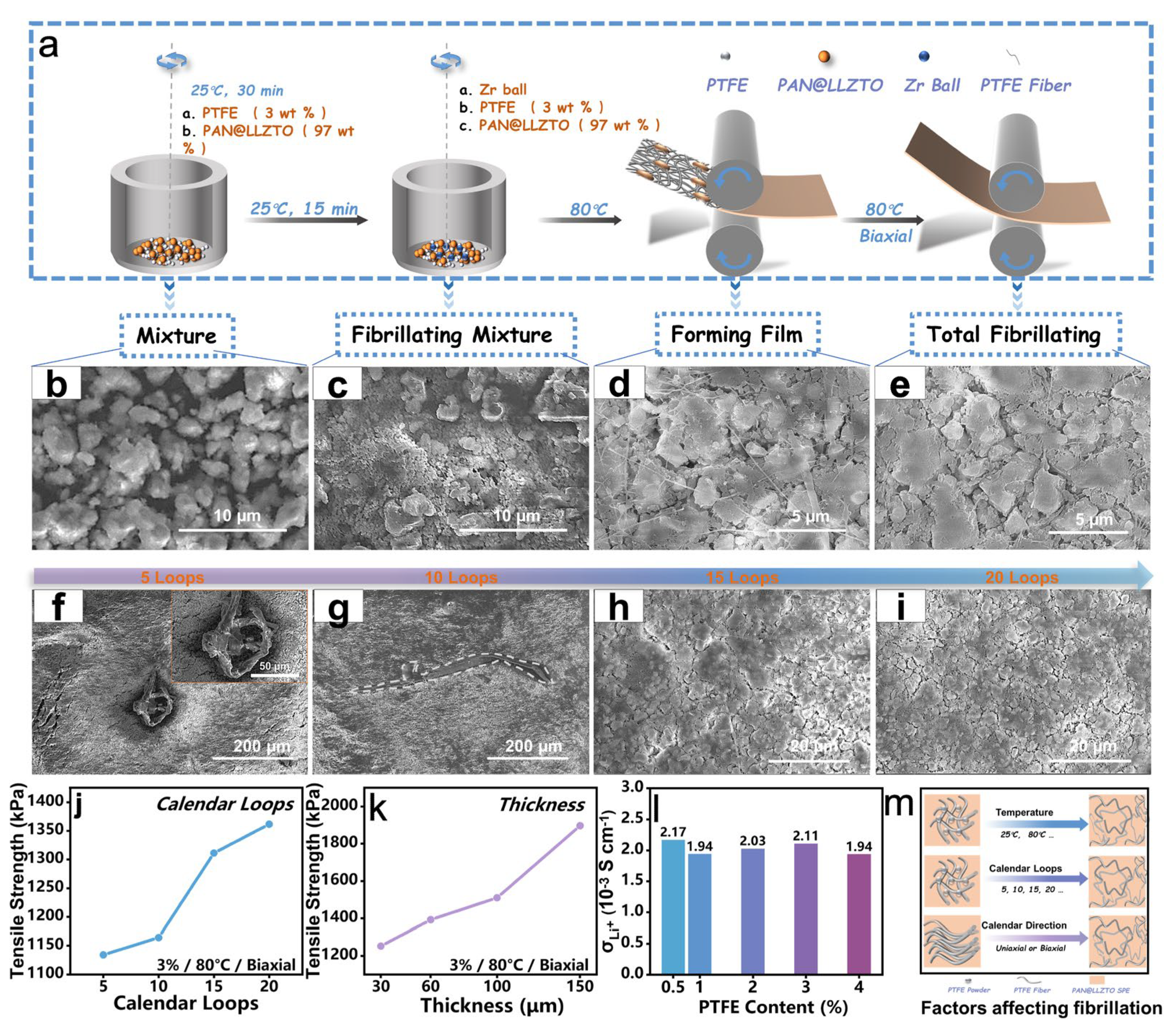
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of 3% PAN@LLZTO Powder
3.3. Preparation of PTFE-LLZTO Electrolyte Membrane
3.4. Preparation of LFP Electrodes and Cell Assembling
3.5. Characterizations and Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdul Razzaq, A.; Yao, Y.; Shah, R.; Qi, P.; Miao, L.; Chen, M.; Zhao, X.; Peng, Y.; Deng, Z. High-performance lithium sulfur batteries enabled by a synergy between sulfur and carbon nanotubes. Energy Storage Mater. 2019, 16, 194–202. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, H.; Kong, X.; Huang, W.; Tsao, Y.; Mackanic, D.G.; Wang, K.; Wang, X.; Huang, W.; Choudhury, S.; et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat. Energy 2020, 5, 526–533. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.; Weng, S.; Ding, F.; Qi, X.; Lu, J.; Li, Y.; Zhang, X.; Rong, X.; Lu, Y.; et al. Interfacial engineering to achieve an energy density of over 200 Wh kg−1 in sodium batteries. Nat. Energy 2022, 7, 511–519. [Google Scholar] [CrossRef]
- Zheng, X.; Cui, P.; Qian, Y.; Zhao, G.; Zheng, X.; Xu, X.; Cheng, Z.; Liu, Y.; Dou, S.X.; Sun, W. Multifunctional Active-Center-Transferable Platinum/Lithium Cobalt Oxide Heterostructured Electrocatalysts towards Superior Water Splitting. Angew. Chem. Int. Ed. 2020, 59, 14533–14540. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. Challenges in speeding up solid-state battery development. Nat. Energy 2023, 8, 230–240. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Ba, D.; Zhao, Y.; Ye, Y.; Li, Y.; Liu, J. Filler-Integrated Composite Polymer Electrolyte for Solid-State Lithium Batteries. Adv. Mater. 2022, 35, 2110423. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A.; Cavallaro, K.A.; Liu, Y.; McDowell, M.T. The promise of alloy anodes for solid-state batteries. Joule 2022, 6, 1418–1430. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-Type Solid-State Electrolytes: Materials, Interfaces, and Batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Liu, X.; Zhong, H.; Xu, H.; Xu, Z.; Shao, H.; Ding, F. Suppression of Lithium Dendrite Formation by Using LAGP-PEO (LiTFSI) Composite Solid Electrolyte and Lithium Metal Anode Modified by PEO (LiTFSI) in All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2017, 9, 13694–13702. [Google Scholar] [CrossRef]
- Xu, S.; Sun, Z.; Sun, C.; Li, F.; Chen, K.; Zhang, Z.; Hou, G.; Cheng, H.M.; Li, F. Homogeneous and Fast Ion Conduction of PEO-Based Solid-State Electrolyte at Low Temperature. Adv. Funct. Mater. 2020, 30, 2007172. [Google Scholar] [CrossRef]
- Xin, S.; Chang, Z.; Zhang, X.; Guo, Y.-G. Progress of rechargeable lithium metal batteries based on conversion reactions. Natl. Sci. Rev. 2017, 4, 54–70. [Google Scholar] [CrossRef]
- Wei, S.; Ma, L.; Hendrickson, K.E.; Tu, Z.; Archer, L.A. Metal–Sulfur Battery Cathodes Based on PAN–Sulfur Composites. J. Am. Chem. Soc. 2015, 137, 12143–12152. [Google Scholar] [CrossRef]
- Sha, L.; Sui, B.-B.; Wang, P.-F.; Gong, Z.; Zhang, Y.-H.; Wu, Y.-H.; Zhao, L.-N.; Tang, J.-J.; Shi, F.-N. 3D network of zinc powder woven into fibre filaments for dendrite-free zinc battery anodes. Chem. Eng. J. 2024, 481, 148393. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Zeng, X.-X.; Zhang, X.-D.; Zuo, T.-T.; Yan, M.; Yin, Y.-X.; Shi, J.-L.; Wu, X.-W.; Guo, Y.-G.; Wan, L.-J. Engineering Janus Interfaces of Ceramic Electrolyte via Distinct Functional Polymers for Stable High-Voltage Li-Metal Batteries. J. Am. Chem. Soc. 2019, 141, 9165–9169. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Westover, A.S.; Yue, J.; Fan, X.; Wang, F.; Chi, M.; Leonard, D.N.; Dudney, N.J.; Wang, H.; Wang, C. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 2019, 4, 187–196. [Google Scholar] [CrossRef]
- Zhang, W.; Nie, J.; Li, F.; Wang, Z.L.; Sun, C. A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy 2018, 45, 413–419. [Google Scholar] [CrossRef]
- Liu, Q.; Geng, Z.; Han, C.; Fu, Y.; Li, S.; He, Y.-B.; Kang, F.; Li, B. Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries. J. Power Sources 2018, 389, 120–134. [Google Scholar] [CrossRef]
- Tao, X.; Liu, Y.; Liu, W.; Zhou, G.; Zhao, J.; Lin, D.; Zu, C.; Sheng, O.; Zhang, W.; Lee, H.-W.; et al. Solid-State Lithium–Sulfur Batteries Operated at 37 °C with Composites of Nanostructured Li7La3Zr2O12/Carbon Foam and Polymer. Nano Lett. 2017, 17, 2967–2972. [Google Scholar] [CrossRef]
- Okumura, T.; Takeuchi, T.; Kobayashi, H. All-Solid-State Batteries with LiCoO2-Type Electrodes: Realization of an Impurity-Free Interface by Utilizing a Cosinterable Li3.5Ge0.5V0.5O4 Electrolyte. ACS Appl. Energy Mater. 2020, 4, 30–34. [Google Scholar] [CrossRef]
- Jian, Z.; Hu, Y.S.; Ji, X.; Chen, W. NASICON-Structured Materials for Energy Storage. Adv. Mater. 2017, 29, 1601925. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, N.; Zhang, M.; Li, Y.; Chu, P.K.; Guo, X.; Di, Z.; Wang, X.; Li, H. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy 2016, 28, 447–454. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, K.; Lang, J.; Zhuo, D.; Huang, Z.; Wang, C.; Wu, H.; Cui, Y. An intermediate temperature garnet-type solid electrolyte-based molten lithium battery for grid energy storage. Nat. Energy 2018, 3, 732–738. [Google Scholar] [CrossRef]
- Zhai, L.; Yang, K.; Jiang, F.; Liu, W.; Yan, Z.; Sun, J. High-performance solid-state lithium metal batteries achieved by interface modification. J. Energy Chem. 2023, 79, 357–364. [Google Scholar] [CrossRef]
- Shi, C.; Hamann, T.; Takeuchi, S.; Alexander, G.V.; Nolan, A.M.; Limpert, M.; Fu, Z.; O’Neill, J.; Godbey, G.; Dura, J.A.; et al. 3D Asymmetric Bilayer Garnet-Hybridized High-Energy-Density Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2022, 15, 751–760. [Google Scholar] [CrossRef]
- Chen, W.-P.; Duan, H.; Shi, J.-L.; Qian, Y.; Wan, J.; Zhang, X.-D.; Sheng, H.; Guan, B.; Wen, R.; Yin, Y.-X.; et al. Bridging Interparticle Li+ Conduction in a Soft Ceramic Oxide Electrolyte. J. Am. Chem. Soc. 2021, 143, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Cheng, S.H.S.; Hu, J.; Zhang, Y.; Yang, H.; Liu, Y.; Liao, W.; Chen, D.; Liao, C.; Cheng, X.; et al. In-Situ Intermolecular Interaction in Composite Polymer Electrolyte for Ultralong Life Quasi-Solid-State Lithium Metal Batteries. Angew. Chem. Int. Ed. 2021, 60, 12116–12123. [Google Scholar] [CrossRef]
- Yu, S.; Schmidt, R.D.; Garcia-Mendez, R.; Herbert, E.; Dudney, N.J.; Wolfenstine, J.B.; Sakamoto, J.; Siegel, D.J. Elastic Properties of the Solid Electrolyte Li7La3Zr2O12(LLZO). Chem. Mater. 2015, 28, 197–206. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, D.; Ma, Y.; Natan, A.; Aurora, P.; Zhu, H. Sulfide-Based Solid-State Electrolytes: Synthesis, Stability, and Potential for All-Solid-State Batteries. Adv. Mater. 2019, 31, 1901131. [Google Scholar] [CrossRef]
- Wu, N.; Chien, P.H.; Qian, Y.; Li, Y.; Xu, H.; Grundish, N.S.; Xu, B.; Jin, H.; Hu, Y.Y.; Yu, G.; et al. Enhanced Surface Interactions Enable Fast Li+ Conduction in Oxide/Polymer Composite Electrolyte. Angew. Chem. Int. Ed. 2020, 59, 4131–4137. [Google Scholar] [CrossRef]
- Bi, C.X.; Zhao, M.; Hou, L.P.; Chen, Z.X.; Zhang, X.Q.; Li, B.Q.; Yuan, H.; Huang, J.Q. Anode Material Options Toward 500 Wh kg−1 Lithium–Sulfur Batteries. Adv. Sci. 2021, 9, 2103910. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-K.; Yuan, H.; Yang, S.-J.; Lu, Y.; Sun, S.; Liu, J.; Liao, Y.-L.; Li, S.; Zhao, C.-Z.; Huang, J.-Q. Dry electrode technology for scalable and flexible high-energy sulfur cathodes in all-solid-state lithium-sulfur batteries. J. Energy Chem. 2022, 71, 612–618. [Google Scholar] [CrossRef]
- Zhang, Y.; Huld, F.; Lu, S.; Jektvik, C.; Lou, F.; Yu, Z. Revisiting Polytetrafluorethylene Binder for Solvent-Free Lithium-Ion Battery Anode Fabrication. Batteries 2022, 8, 57. [Google Scholar] [CrossRef]
- Suh, Y.; Koo, J.K.; Im, H.-j.; Kim, Y.-J. Astonishing performance improvements of dry-film graphite anode for reliable lithium-ion batteries. Chem. Eng. J. 2023, 476, 146299. [Google Scholar] [CrossRef]
- Tao, R.; Steinhoff, B.; Sun, X.-G.; Sardo, K.; Skelly, B.; Meyer, H.M.; Sawicki, C.; Polizos, G.; Lyu, X.; Du, Z.; et al. High-throughput and high-performance lithium-ion batteries via dry processing. Chem. Eng. J. 2023, 471, 144300. [Google Scholar] [CrossRef]
- Duan, H.; Chen, W.P.; Fan, M.; Wang, W.P.; Yu, L.; Tan, S.J.; Chen, X.; Zhang, Q.; Xin, S.; Wan, L.J.; et al. Building an Air Stable and Lithium Deposition Regulable Garnet Interface from Moderate-Temperature Conversion Chemistry. Angew. Chem. Int. Ed. 2020, 59, 12069–12075. [Google Scholar] [CrossRef]
- Lee, D.J.; Jang, J.; Lee, J.P.; Wu, J.; Chen, Y.T.; Holoubek, J.; Yu, K.; Ham, S.Y.; Jeon, Y.; Kim, T.H.; et al. Physio-Electrochemically Durable Dry-Processed Solid-State Electrolyte Films for All-Solid-State Batteries. Adv. Funct. Mater. 2023, 33, 2301341. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Chen, H.; Liu, H.; Li, L.; Song, J.; Xu, M.; Bao, S.-J. Dry approach production of a garnet solid electrolyte membrane for lithium batteries. Inorg. Chem. Front. 2023, 10, 6023–6031. [Google Scholar] [CrossRef]
- Wang, C.; Yu, R.; Duan, H.; Lu, Q.; Li, Q.; Adair, K.R.; Bao, D.; Liu, Y.; Yang, R.; Wang, J.; et al. Solvent-Free Approach for Interweaving Freestanding and Ultrathin Inorganic Solid Electrolyte Membranes. ACS Energy Lett. 2021, 7, 410–416. [Google Scholar] [CrossRef]
- Haripriya, M.; Manimekala, T.; Dharmalingam, G.; Minakshi, M.; Sivasubramanian, R. Asymmetric Supercapacitors Based on ZnCo2O4 Nanohexagons and Orange Peel Derived Activated Carbon Electrodes. Chem.–Asian J. 2024, 19, e202400202. [Google Scholar] [CrossRef]
- Vasudevan, S.; D, S.T.; Manickam, M.; Sivasubramanian, R. A sol–gel derived LaCoO3 perovskite as an electrocatalyst for Al–air batteries. Dalton Trans. 2024, 53, 3713–3721. [Google Scholar] [CrossRef] [PubMed]


| Solvent-Free Membrane | Thickness (µm) | PTFE (wt%) | Ionic Conductivity (10−3 S cm−1) 25 °C | Batteries | References |
|---|---|---|---|---|---|
| Li3InCl6/PTFE | 20 | 0.5 | 1.00 | Li3InCl6@LiCoO2|Li3InCl6/LPSC|Graphite/LPSC | [39] |
| LPSC/S/C/PTFE | 93 | 1.0 | 0.63 | S/C/LPSC|LPSC|Li | [32] |
| LPSC/PTFE | 300 | 2.0 | 1.28 | NCM811/LPSC|LPSC|Si | [37] |
| LLZTO/PTFE | 20 | 0.5 | 0.52 | NCM811|LLZTO/LiTFSI in DMSO with 10% FEC|Si-C-450 | [39] |
| LLZTO/PTFE | 20 | 3.0 | 0.66 | LFP|LLZTO/LiPF6 in EC:PC = 1:1 vol% with 5% FEC|Li | [38] |
| PAN@LLZTO/PTFE | 62 | 3.0 | 2.11 | LFP|PAN@ LLZTO/LiPF6 in EC:DEC = 1:1 vol% with 5% FEC|Li | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, K.; Shen, H.; Zhang, H.; Yao, H.; Chen, Z.; Jiang, Z. Solvent-Free Method of Polyacrylonitrile-Coated LLZTO Solid-State Electrolytes for Lithium Batteries. Molecules 2024, 29, 4452. https://doi.org/10.3390/molecules29184452
Wang X, Zhang K, Shen H, Zhang H, Yao H, Chen Z, Jiang Z. Solvent-Free Method of Polyacrylonitrile-Coated LLZTO Solid-State Electrolytes for Lithium Batteries. Molecules. 2024; 29(18):4452. https://doi.org/10.3390/molecules29184452
Chicago/Turabian StyleWang, Xuehan, Kaiqi Zhang, Huilin Shen, Hao Zhang, Hongyan Yao, Zheng Chen, and Zhenhua Jiang. 2024. "Solvent-Free Method of Polyacrylonitrile-Coated LLZTO Solid-State Electrolytes for Lithium Batteries" Molecules 29, no. 18: 4452. https://doi.org/10.3390/molecules29184452
APA StyleWang, X., Zhang, K., Shen, H., Zhang, H., Yao, H., Chen, Z., & Jiang, Z. (2024). Solvent-Free Method of Polyacrylonitrile-Coated LLZTO Solid-State Electrolytes for Lithium Batteries. Molecules, 29(18), 4452. https://doi.org/10.3390/molecules29184452






