Unraveling the Dynamic Properties of New-Age Energy Materials Chemistry Using Advanced In Situ Transmission Electron Microscopy
Abstract
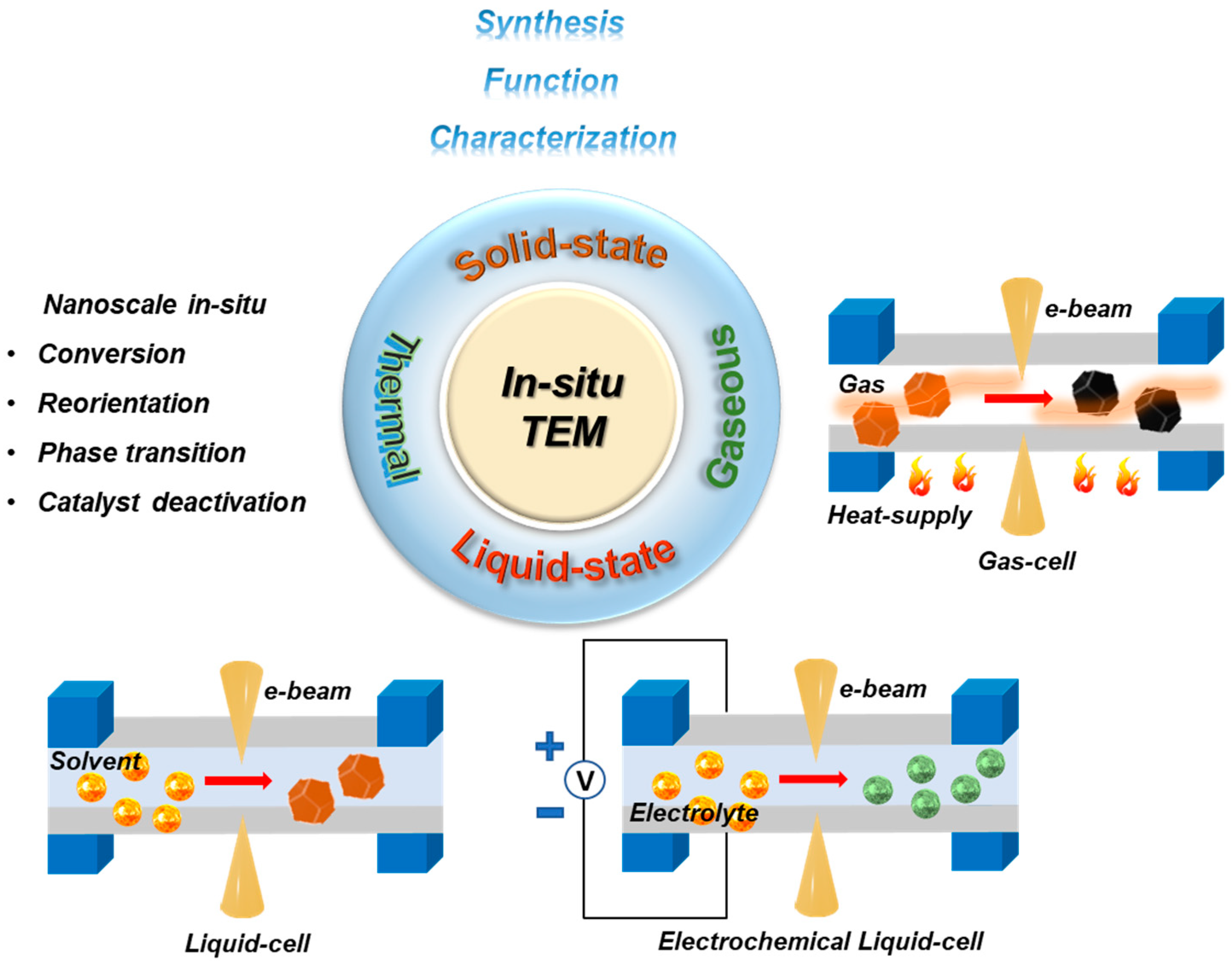
1. Introduction
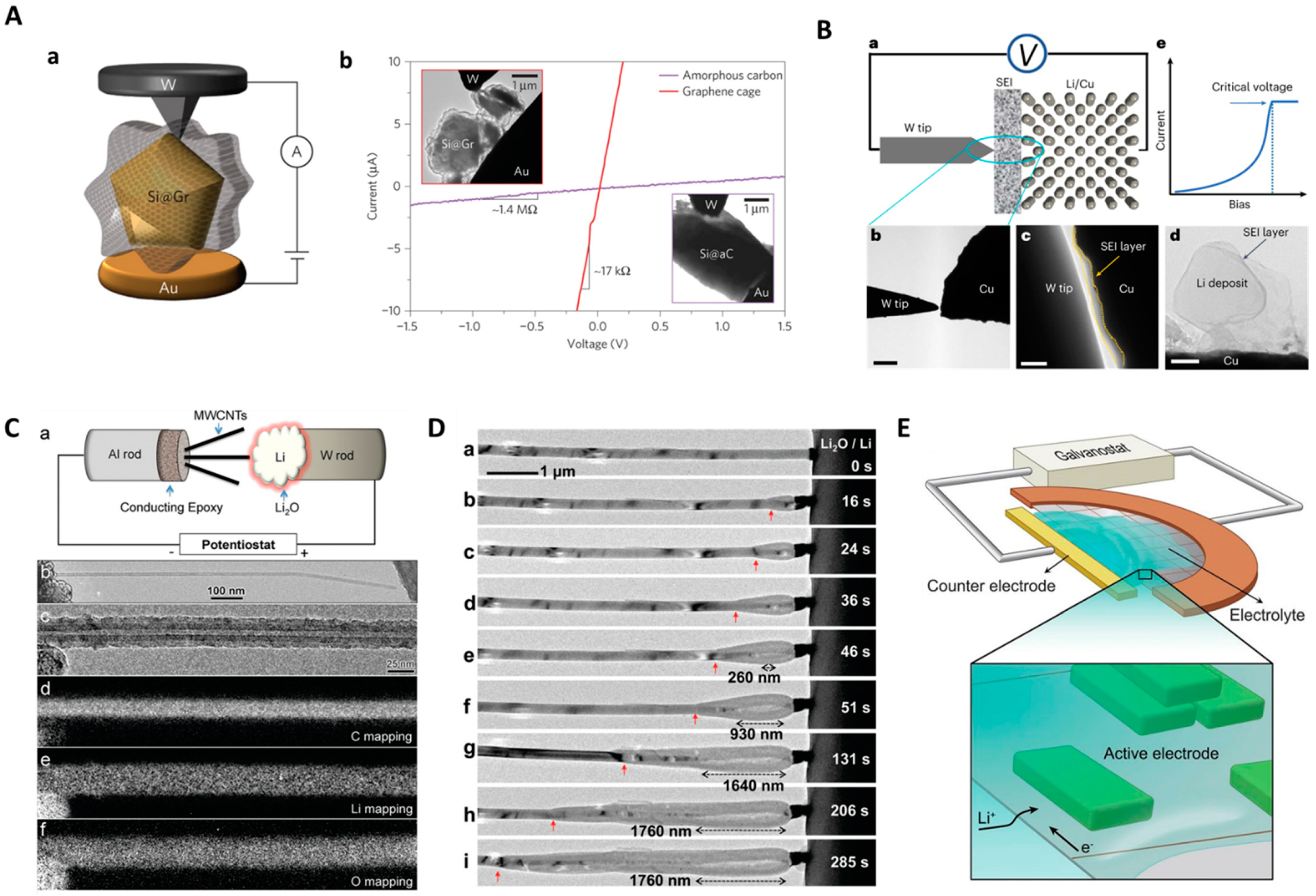
2. In Situ Solid-State and Electrochemical Biasing Integrated TEM Characterization for Energy Materials
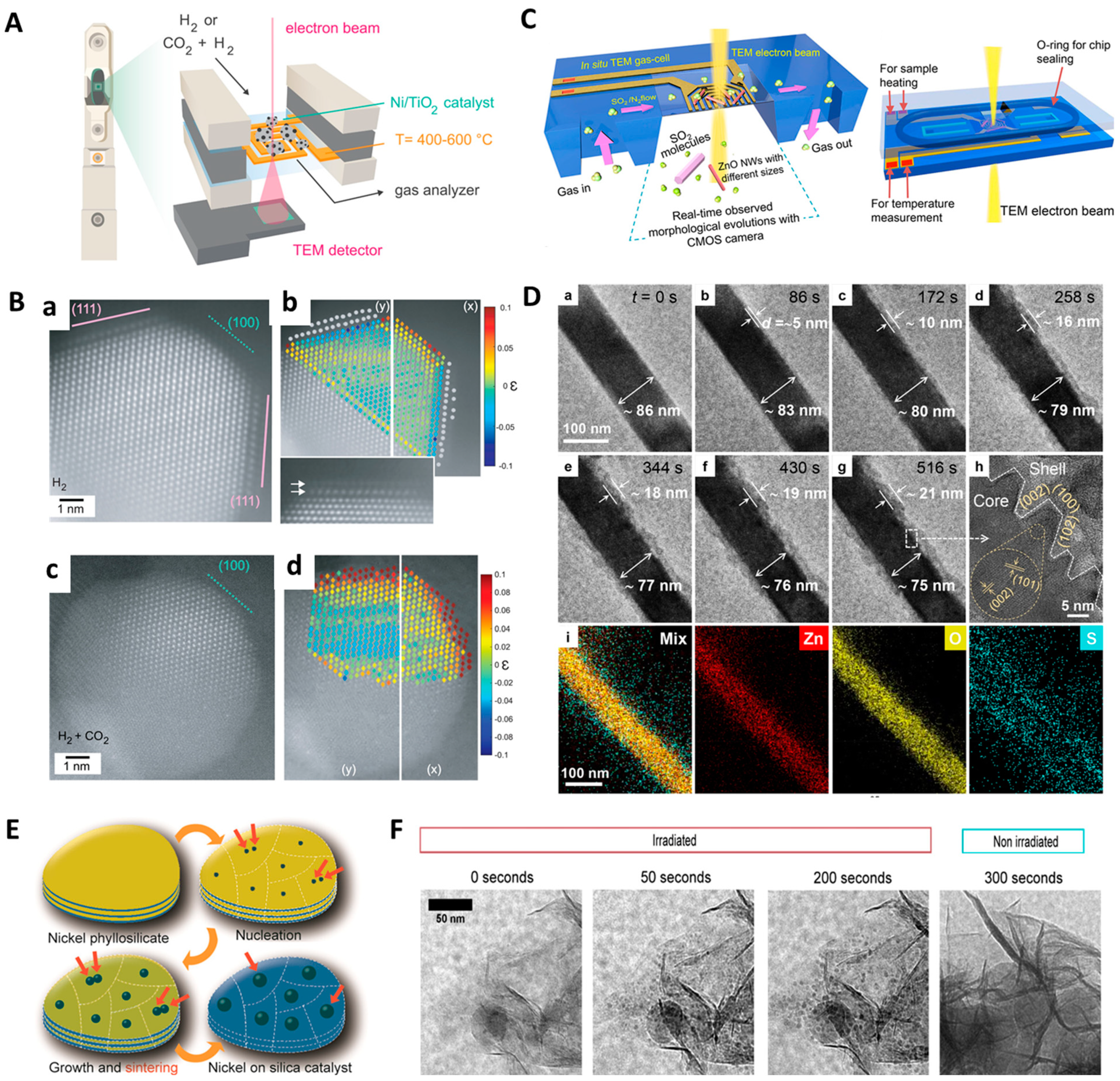
3. In Situ Gas-Phase/Environmental TEM (ETEM) and Integrated Thermal TEM Characterization for Energy Materials
3.1. Thermal (or Heat)-Induced In Situ TEM-Based Characterization
3.2. In Situ Gas Phase/ETEM-Based Characterization
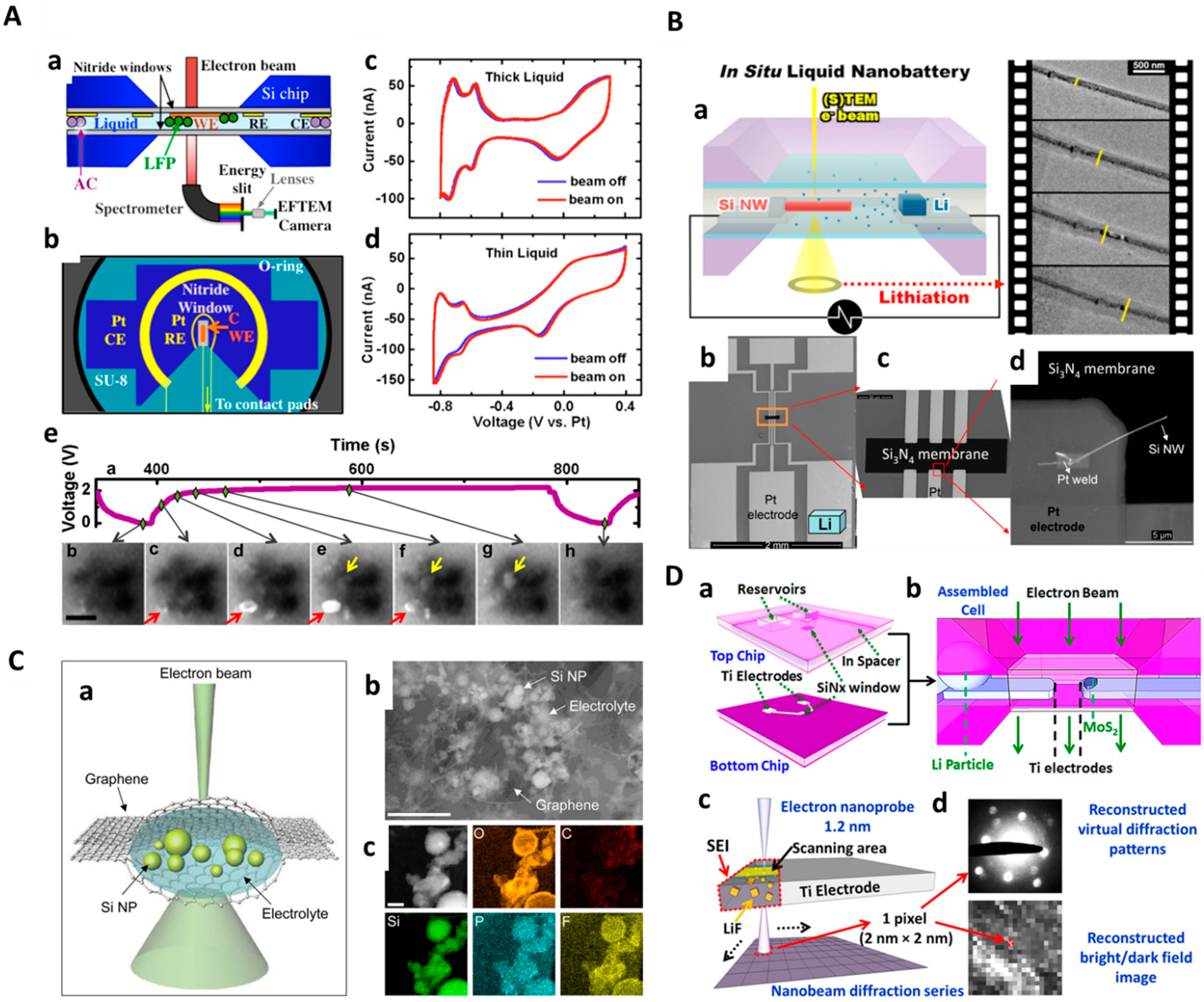
4. In Situ Liquid-Cell TEM Setup for Characterizing Energy Materials
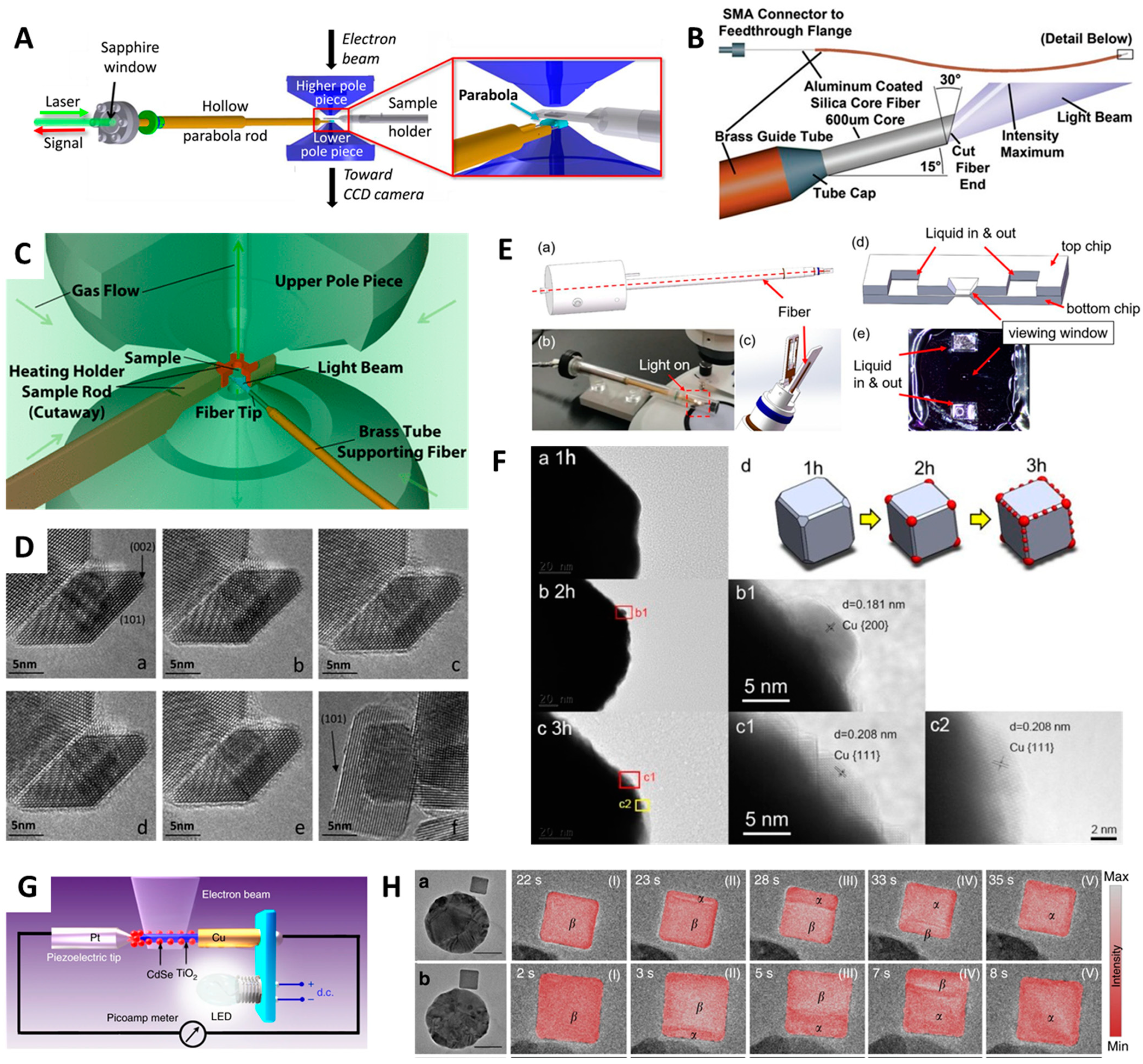
5. In Situ Light-Induced TEM Characterization for Energy Materials
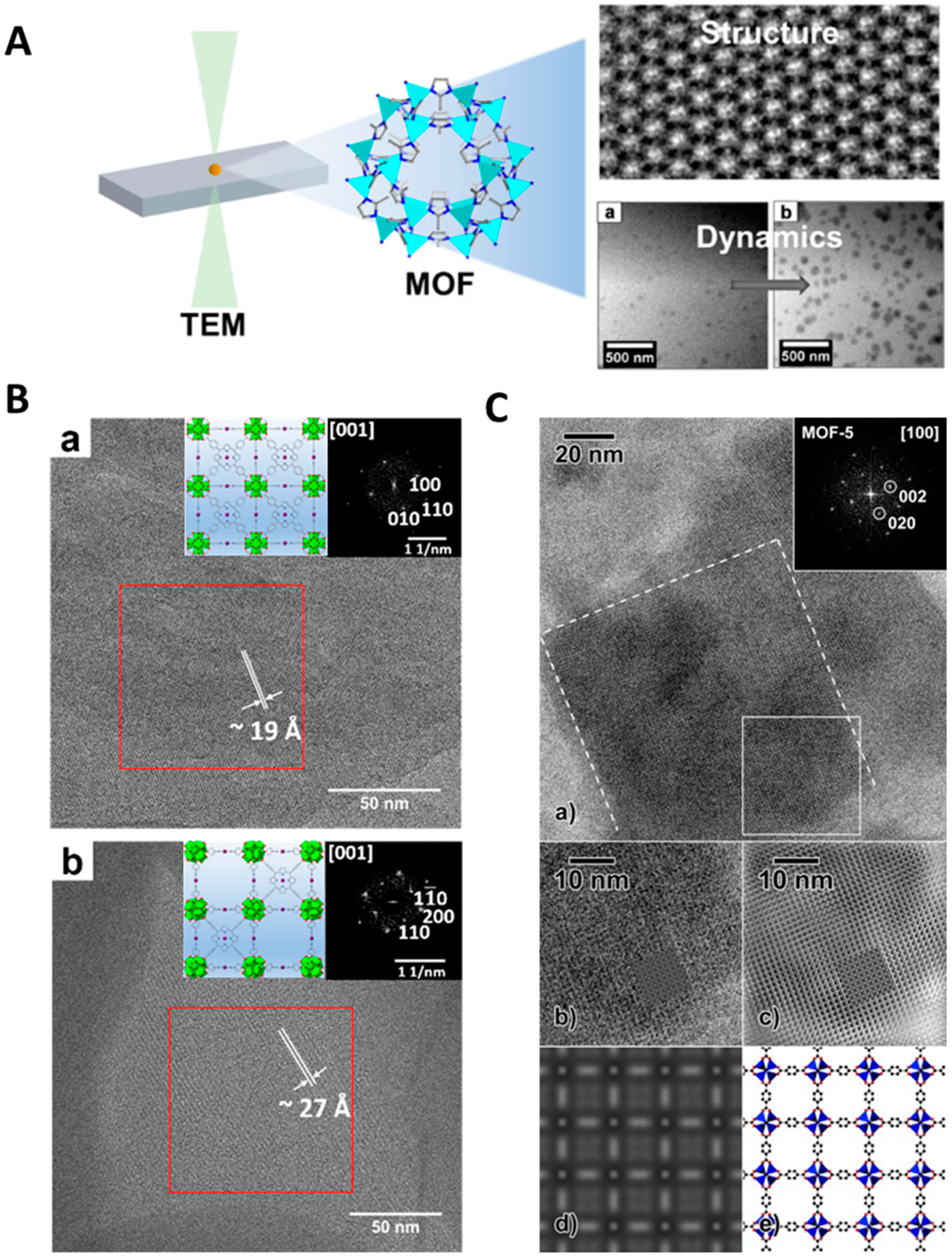
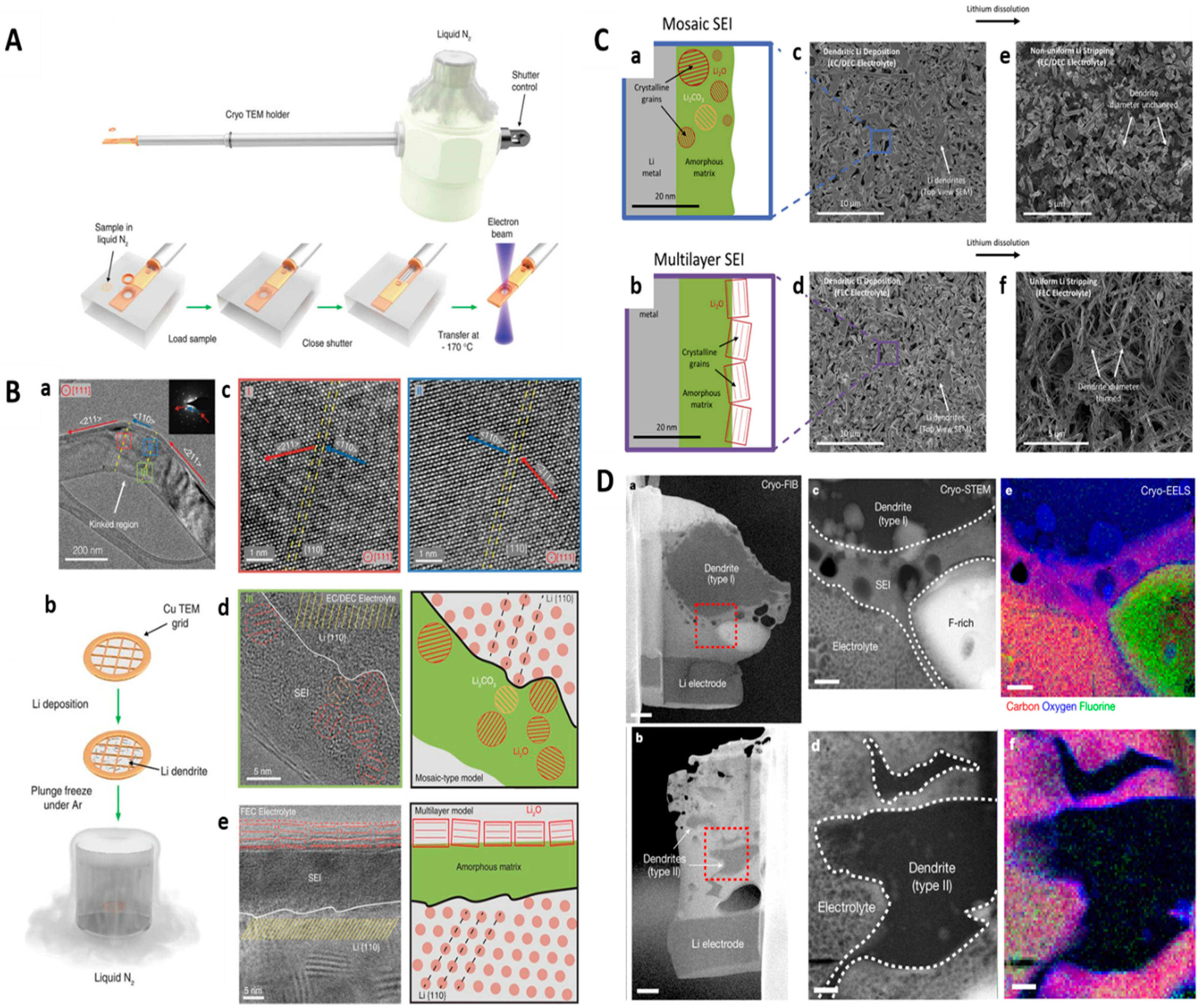
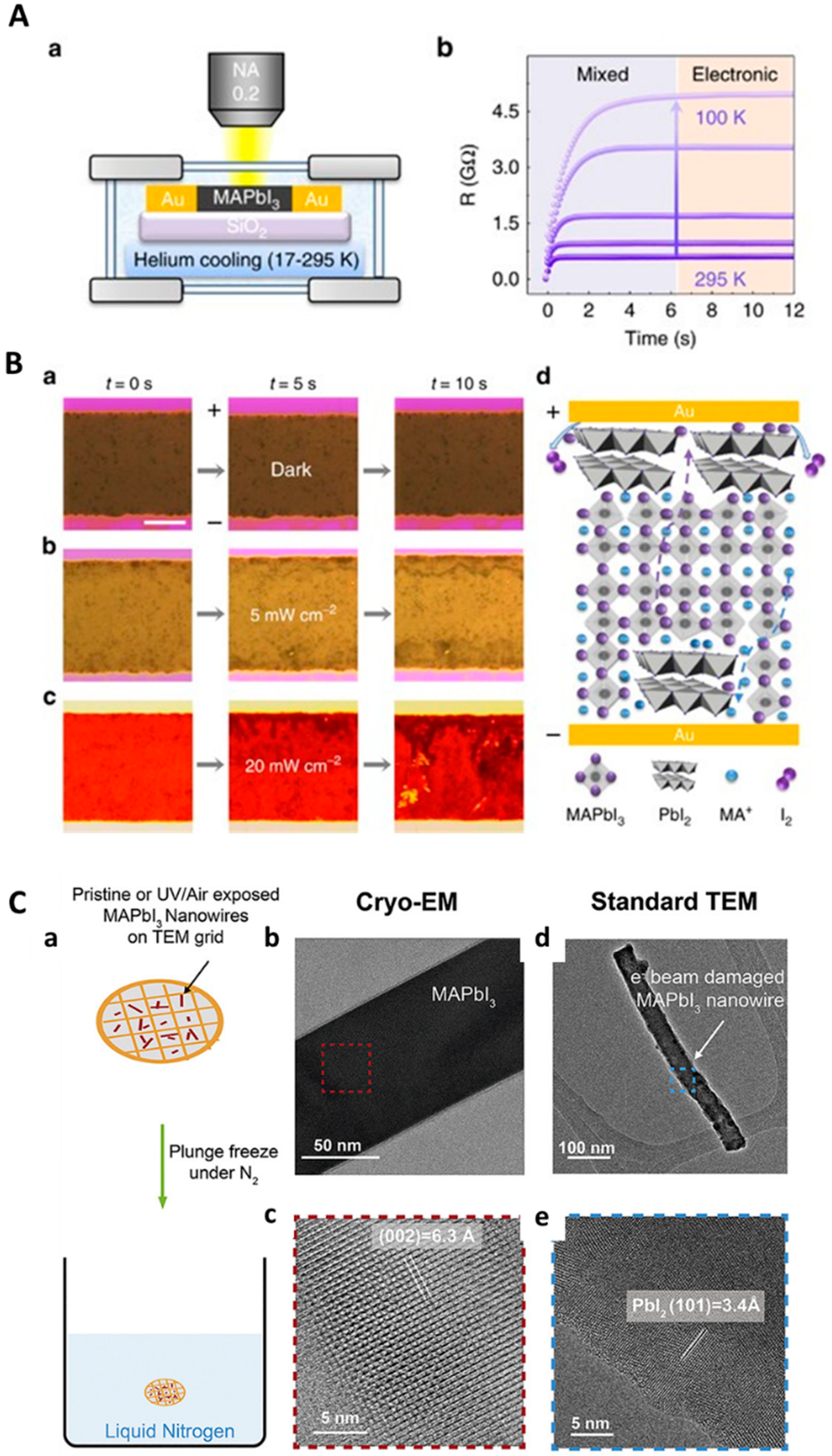
6. In Situ Cryo-TEM Characterization for Energy Materials
7. Future Prospects and Advancements in TEM Characterization Techniques for Energy Materials
8. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Divitini, G.; Cacovich, S.; Matteocci, F.; Cinà, L.; Di Carlo, A.; Ducati, C. In Situ Observation of Heat-Induced Degradation of Perovskite Solar Cells. Nat. Energy 2016, 1, 15012. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining Theory and Experiment in Electrocatalysis: Insights into Materials Design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, W.; Li, Y.; Pei, A.; Boyle, D.T.; Cui, Y. Correlating Structure and Function of Battery Interphases at Atomic Resolution Using Cryoelectron Microscopy. Joule 2018, 2, 2167–2177. [Google Scholar] [CrossRef]
- Zachman, M.J.; Tu, Z.; Choudhury, S.; Archer, L.A.; Kourkoutis, L.F. Cryo-STEM Mapping of Solid–Liquid Interfaces and Dendrites in Lithium-Metal Batteries. Nature 2018, 560, 345–349. [Google Scholar] [CrossRef]
- van der Wal, L.I.; Turner, S.J.; Zečević, J. Developments and Advances in in Situ Transmission Electron Microscopy for Catalysis Research. Catal. Sci. Technol. 2021, 11, 3634–3658. [Google Scholar] [CrossRef]
- De Alwis Goonatilleke, M.; Thomas, M.P.; Ullah, A.; Pham, R.H.; Guiton, B.S. Direct Observation of Sample Dynamics in the Gaseous Environment: A Perspective on Current Trends and Future Directions of In Situ Gas Cell Transmission Electron Microscopy. J. Phys. Chem. C 2023, 127, 18791–18808. [Google Scholar] [CrossRef]
- Chen, Q.; Dwyer, C.; Sheng, G.; Zhu, C.; Li, X.; Zheng, C.; Zhu, Y. Imaging Beam-Sensitive Materials by Electron Microscopy. Adv. Mater. 2020, 32, 1907619. [Google Scholar] [CrossRef]
- Dong, H.; Xu, F.; Sun, Z.; Wu, X.; Zhang, Q.; Zhai, Y.; Tan, X.D.; He, L.; Xu, T.; Zhang, Z.; et al. In Situ Interface Engineering for Probing the Limit of Quantum Dot Photovoltaic Devices. Nat. Nanotechnol. 2019, 14, 950–956. [Google Scholar] [CrossRef]
- Zheng, H.; Lu, X.; He, K. In Situ Transmission Electron Microscopy and Artificial Intelligence Enabled Data Analytics for Energy Materials. J. Energy Chem. 2022, 68, 454–493. [Google Scholar] [CrossRef]
- Gao, C.; Zhuang, C.; Li, Y.; Qi, H.; Chen, G.; Sun, Z.; Zou, J.; Han, X. In situ liquid cell transmission electron microscopy guiding the design of large-sized cocatalysts coupled with ultra-small photocatalysts for highly efficient energy harvesting. J. Mater. Chem. A. 2021, 9, 13057–13064. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, L.; Baumann, D.; Mei, L.; Yao, Y.; Duan, X.; Shi, Y.; Huang, J.; Huang, Y.; Duan, X. In Situ Transmission Electron Microscopy for Energy Materials and Devices. Adv. Mater. 2019, 31, 1900608. [Google Scholar] [CrossRef]
- Chen, L.J.; Wu, W.W. In Situ TEM Investigation of Dynamical Changes of Nanostructures. Mater. Sci. Eng. R Rep. 2010, 70, 303–319. [Google Scholar] [CrossRef]
- Żak, A.M. Light-Induced In Situ Transmission Electron Microscopy─Development, Challenges, and Perspectives. Nano Lett. 2022, 22, 9219–9226. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, Y.; Song, C.; Tao, P.; Shang, W.; Deng, T.; Zeng, X.; Wu, J. In-Situ Monitoring of Dynamic Behavior of Catalyst Materials and Reaction Intermediates in Semiconductor Catalytic Processes. J. Semicond. 2022, 43, 041104. [Google Scholar] [CrossRef]
- Liu, X.H.; Liu, Y.; Kushima, A.; Zhang, S.; Zhu, T.; Li, J.; Huang, J.Y. In Situ TEM Experiments of Electrochemical Lithiation and Delithiation of Individual Nanostructures. Adv. Energy Mater. 2012, 2, 722–741. [Google Scholar] [CrossRef]
- Gao, D.; Li, W.; Wang, H.; Wang, G.; Cai, R. Heterogeneous Catalysis for CO2 Conversion into Chemicals and Fuels. Trans. Tianjin Univ. 2022, 28, 245–264. [Google Scholar] [CrossRef]
- Chao, H.-Y.; Venkatraman, K.; Moniri, S.; Jiang, Y.; Tang, X.; Dai, S.; Gao, W.; Miao, J.; Chi, M. In Situ and Emerging Transmission Electron Microscopy for Catalysis Research. Chem. Rev. 2023, 123, 8347–8394. [Google Scholar] [CrossRef]
- Wu, J.; Shan, H.; Chen, W.; Gu, X.; Tao, P.; Song, C.; Shang, W.; Deng, T. In Situ Environmental TEM in Imaging Gas and Liquid Phase Chemical Reactions for Materials Research. Adv. Mater. 2016, 28, 9686–9712. [Google Scholar] [CrossRef]
- Wei, L.; Xu, Z.; Wu, X.; Ke, X.; Sui, M. Emerging Transmission Electron Microscopy Solutions for Electrocatalysts: From Synthesis to Deactivation. Mater. Chem. Front. 2024, 8, 2078–2108. [Google Scholar] [CrossRef]
- Ro, I.; Resasco, J.; Christopher, P. Approaches for Understanding and Controlling Interfacial Effects in Oxide-Supported Metal Catalysts. ACS Catal. 2018, 8, 7368–7387. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, Y.; Vila, R.; Li, Y.; Zhang, W.; Zhou, W.; Chiu, W.; Cui, Y. Cryogenic Electron Microscopy for Energy Materials. Acc. Chem. Res. 2021, 54, 3505–3517. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, J.; Mai, W.; Hong, G. A Decade of Advanced Rechargeable Batteries Development Guided by in Situ Transmission Electron Microscopy. Nano Energy 2021, 83, 105780. [Google Scholar] [CrossRef]
- Wu, F.; Yao, N. Advances in Sealed Liquid Cells for In-Situ TEM Electrochemial Investigation of Lithium-Ion Battery. Nano Energy 2015, 11, 196–210. [Google Scholar] [CrossRef]
- Han, C.; Islam, M.T.; Ni, C. In Situ TEM of Electrochemical Incidents: Effects of Biasing and Electron Beam on Electrochemistry. ACS Omega 2021, 6, 6537–6546. [Google Scholar] [CrossRef]
- Liu, X.H.; Huang, J.Y. In Situ TEM Electrochemistry of Anode Materials in Lithium Ion Batteries. Energy Environ. Sci. 2011, 4, 3844. [Google Scholar] [CrossRef]
- Wang, C.M.; Xu, W.; Liu, J.; Choi, D.W.; Arey, B.; Saraf, L.V.; Zhang, J.G.; Yang, Z.G.; Thevuthasan, S.; Baer, D.R.; et al. In Situ Transmission Electron Microscopy and Spectroscopy Studies of Interfaces in Li Ion Batteries: Challenges and Opportunities. J. Mater. Res. 2010, 25, 1541–1547. [Google Scholar] [CrossRef]
- Wheatcroft, L.; Özkaya, D.; Cookson, J.; Inkson, B.J. Towards In-Situ TEM for Li-Ion Battery Research. Energy Procedia 2018, 151, 163–167. [Google Scholar] [CrossRef]
- Serve, A.; Epicier, T.; Aouine, M.; Cadete Santos Aires, F.J.; Obeid, E.; Tsampas, M.; Pajot, K.; Vernoux, P. Investigations of Soot Combustion on Yttria-Stabilized Zirconia by Environmental Transmission Electron Microscopy (ETEM). Appl. Catal. A Gen. 2015, 504, 74–80. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Z.; Wang, D.; Wang, J.-X.; Pu, Y.; Chen, J.-F. CFD Modelling of Gas Flow Characteristics for the Gas-Heating Holder in Environmental Transmission Electron Microscope. Can. J. Chem. Eng. 2019, 97, 777–784. [Google Scholar] [CrossRef]
- Canavan, M.; Daly, D.; Rummel, A.; McCarthy, E.K.; McAuley, C.; Nicolosi, V. Novel in-Situ Lamella Fabrication Technique for in-Situ TEM. Ultramicroscopy 2018, 190, 21–29. [Google Scholar] [CrossRef]
- McDowell, M.T.; Ryu, I.; Lee, S.W.; Wang, C.; Nix, W.D.; Cui, Y. Studying the Kinetics of Crystalline Silicon Nanoparticle Lithiation with In Situ Transmission Electron Microscopy. Adv. Mater. 2012, 24, 6034–6041. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, K.; Lee, H.-W.; Lu, Z.; Liu, N.; Cui, Y. Growth of Conformal Graphene Cages on Micrometre-Sized Silicon Particles as Stable Battery Anodes. Nat. Energy 2016, 1, 15029. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, H.; Gao, P.; Galvez-Aranda, D.E.; Beltran, S.P.; Cao, X.; Le, P.M.L.; Liu, J.; Engelhard, M.H.; Li, S.; et al. Direct in Situ Measurements of Electrical Properties of Solid–Electrolyte Interphase on Lithium Metal Anodes. Nat. Energy 2023, 8, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, H.; Liu, X.H.; Huang, S.; Zhu, T.; Wang, J.; Kushima, A.; Hudak, N.S.; Huang, X.; Zhang, S.; et al. Lithiation-Induced Embrittlement of Multiwalled Carbon Nanotubes. ACS Nano 2011, 5, 7245–7253. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, L.Q.; Zhong, L.; Liu, Y.; Zheng, H.; Wang, J.W.; Cho, J.-H.; Dayeh, S.A.; Picraux, S.T.; Sullivan, J.P.; et al. Ultrafast Electrochemical Lithiation of Individual Si Nanowire Anodes. Nano Lett. 2011, 11, 2251–2258. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L. Structural Changes in Silicon Anodes during Lithium Insertion/Extraction. Electrochem. Solid-State Lett. 2004, 7, A93. [Google Scholar] [CrossRef]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; van Schalkwijk, W. Nanostructured Materials for Advanced Energy Conversion and Storage Devices. Nat. Mater 2005, 4, 366–377. [Google Scholar] [CrossRef]
- Liu, X.H.; Zheng, H.; Zhong, L.; Huang, S.; Karki, K.; Zhang, L.Q.; Liu, Y.; Kushima, A.; Liang, W.T.; Wang, J.W.; et al. Anisotropic Swelling and Fracture of Silicon Nanowires during Lithiation. Nano Lett. 2011, 11, 3312–3318. [Google Scholar] [CrossRef]
- Yi, T.-F.; Yang, S.-Y.; Xie, Y. Recent Advances of Li4Ti5O12 as a Promising next Generation Anode Material for High Power Lithium-Ion Batteries. J. Mater. Chem. A 2015, 3, 5750–5777. [Google Scholar] [CrossRef]
- Wang, F.; Wu, L.; Ma, C.; Su, D.; Zhu, Y.; Graetz, J. Excess Lithium Storage and Charge Compensation in Nanoscale Li4+xTi5O12. Nanotechnology 2013, 24, 424006. [Google Scholar] [CrossRef]
- Zhang, W.; Seo, D.-H.; Chen, T.; Wu, L.; Topsakal, M.; Zhu, Y.; Lu, D.; Ceder, G.; Wang, F. Kinetic Pathways of Ionic Transport in Fast-Charging Lithium Titanate. Science 2020, 367, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Masliuk, L.; Swoboda, M.; Algara-Siller, G.; Schlögl, R.; Lunkenbein, T. A Quasi in Situ TEM Grid Reactor for Decoupling Catalytic Gas Phase Reactions and Analysis. Ultramicroscopy 2018, 195, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, S.; Chen, G.; Liu, Z.; Caracciolo, D.; Madiou, M.; Shan, S.; Zhang, J.; He, H.; Che, R.; et al. Insights into Heterogeneous Catalysts under Reaction Conditions by In Situ/Operando Electron Microscopy. Adv. Energy Mater. 2022, 12, 2202097. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Q.; Gao, Z.; Geng, L.; Li, Y.; Zhang, F.; Yan, J.; Gao, Y.; Suenaga, K.; Zhang, L.; et al. In Situ TEM Visualization of Single Atom Catalysis in Solid-State Na–O2 Nanobatteries. J. Mater. Chem. A 2022, 10, 6096–6106. [Google Scholar] [CrossRef]
- Wang, C.-M. In Situ Transmission Electron Microscopy and Spectroscopy Studies of Rechargeable Batteries under Dynamic Operating Conditions: A Retrospective and Perspective View. J. Mater. Res. 2015, 30, 326–339. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Y.; Zhang, L.; Li, M.; Shan, Z.; Huang, J. In Situ TEM Observations of Discharging/Charging of Solid-State Lithium-Sulfur Batteries at High Temperatures. Small 2020, 16, 2001899. [Google Scholar] [CrossRef]
- Takeda, S.; Kuwauchi, Y.; Yoshida, H. Environmental Transmission Electron Microscopy for Catalyst Materials Using a Spherical Aberration Corrector. Ultramicroscopy 2015, 151, 178–190. [Google Scholar] [CrossRef]
- Wu, F.; Yao, N. Advances in Windowed Gas Cells for In-Situ TEM Studies. Nano Energy 2015, 13, 735–756. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Zhao, J.; Zhang, B.; Liu, L.; Bao, R.; Jing, Z. Sn-Based Anode Materials for Lithium-Ion Batteries: From Mechanism to Modification. J. Energy Storage 2024, 80, 109862. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Li, H.; Ma, C.; Zhao, Y.; Zhang, H.; Peng, Y. In Situ Transmission Electron Microscopy (TEM) Study on the Structural Evolution Behavior of Nano Sn Sheets under a Thermal Field. J. Mater. Chem. C 2024, 12, 4618–4626. [Google Scholar] [CrossRef]
- Fan, Z.; Xiao, H.; Wang, Y.; Zhao, Z.; Lin, Z.; Cheng, H.-C.; Lee, S.-J.; Wang, G.; Feng, Z.; Goddard, W.A.; et al. Layer-by-Layer Degradation of Methylammonium Lead Tri-Iodide Perovskite Microplates. Joule 2017, 1, 548–562. [Google Scholar] [CrossRef]
- Yang, B.; Dyck, O.; Ming, W.; Du, M.-H.; Das, S.; Rouleau, C.M.; Duscher, G.; Geohegan, D.B.; Xiao, K. Observation of Nanoscale Morphological and Structural Degradation in Perovskite Solar Cells by in Situ TEM. ACS Appl. Mater. Interfaces 2016, 8, 32333–32340. [Google Scholar] [CrossRef]
- Jinschek, J.R. Advances in the Environmental Transmission Electron Microscope (ETEM) for Nanoscale in Situ Studies of Gas–Solid Interactions. Chem. Commun. 2014, 50, 2696–2706. [Google Scholar] [CrossRef]
- Xiao, M.; Sun, H.; Meng, Y.; Zhu, F. Advances of in Situ Transmission Electron Microscopy Research on Gas Phase Catalyst Particles. Catal. Sci. Technol. 2024, 14, 2040–2063. [Google Scholar] [CrossRef]
- Wang, Q.; Xia, G.-J.; Zhao, Z.L.; Zhu, Y.; Shi, X.; Huang, L.; Wang, Y.-G.; Gu, M. Atomic Origin of CO-Interaction Effect of PtPb@Pt Catalyst Revealed by in Situ Environmental Transmission Electron Microscopy. Nano Energy 2020, 76, 105099. [Google Scholar] [CrossRef]
- Vogt, C.; Meirer, F.; Monai, M.; Groeneveld, E.; Ferri, D.; van Santen, R.A.; Nachtegaal, M.; Unocic, R.R.; Frenkel, A.I.; Weckhuysen, B.M. Dynamic Restructuring of Supported Metal Nanoparticles and Its Implications for Structure Insensitive Catalysis. Nat. Commun. 2021, 12, 7096. [Google Scholar] [CrossRef]
- Puga, A.V. On the Nature of Active Phases and Sites in CO and CO2 Hydrogenation Catalysts. Catal. Sci. Technol. 2018, 8, 5681–5707. [Google Scholar] [CrossRef]
- Vendelbo, S.B.; Elkjær, C.F.; Falsig, H.; Puspitasari, I.; Dona, P.; Mele, L.; Morana, B.; Nelissen, B.J.; van Rijn, R.; Creemer, J.F.; et al. Visualization of Oscillatory Behaviour of Pt Nanoparticles Catalysing CO Oxidation. Nat. Mater. 2014, 13, 884–890. [Google Scholar] [CrossRef]
- Monai, M.; Jenkinson, K.; Melcherts, A.E.M.; Louwen, J.N.; Irmak, E.A.; Van Aert, S.; Altantzis, T.; Vogt, C.; van der Stam, W.; Duchoň, T.; et al. Restructuring of Titanium Oxide Overlayers over Nickel Nanoparticles during Catalysis. Science 2023, 380, 644–651. [Google Scholar] [CrossRef]
- Epicier, T.; Koneti, S.; Avenier, P.; Cabiac, A.; Gay, A.-S.; Roiban, L. 2D & 3D in Situ Study of the Calcination of Pd Nanocatalysts Supported on Delta-Alumina in an Environmental Transmission Electron Microscope. Catal. Today 2019, 334, 68–78. [Google Scholar] [CrossRef]
- Wang, X.; Yao, F.; Xu, P.; Li, M.; Yu, H.; Li, X. Quantitative Structure–Activity Relationship of Nanowire Adsorption to SO2 Revealed by In Situ TEM Technique. Nano Lett. 2021, 21, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.-Y.; Jiang, H.; Ospina-Acevedo, F.; Balbuena, P.B.; Kauppinen, E.I.; Cumings, J.; Sharma, R. A Structure and Activity Relationship for Single-Walled Carbon Nanotube Growth Confirmed by in Situ Observations and Modeling. Nanoscale 2020, 12, 21923–21931. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.J.; Wezendonk, D.F.L.; Terorde, R.J.A.M.; de Jong, K.P. In Situ TEM Study of the Genesis of Supported Nickel Catalysts. J. Phys. Chem. C 2023, 127, 7772–7783. [Google Scholar] [CrossRef]
- Pinna, F. Supported Metal Catalysts Preparation. Catal. Today 1998, 41, 129–137. [Google Scholar] [CrossRef]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Albarazi, A.; Gálvez, M.E.; Da Costa, P. Synthesis Strategies of Ceria–Zirconia Doped Ni/SBA-15 Catalysts for Methane Dry Reforming. Catal. Commun. 2015, 59, 108–112. [Google Scholar] [CrossRef]
- Shi, H.; Tian, C.; Liu, X.; Sun, N.; Song, C.; Zheng, H.; Gao, K.; Wang, X.; Jiang, Z.; Xuan, Y.; et al. Ni-Phyllosilicate Nanotubes Coated by CeO2 for Ultra-Efficiency of 36.9% and near-Limit CO2 Conversion in Solar-Driven Conversion of CO2-to-Fuel. Chem. Eng. J. 2023, 454, 140063. [Google Scholar] [CrossRef]
- Hwang, S.; Chen, X.; Zhou, G.; Su, D. In Situ Transmission Electron Microscopy on Energy-Related Catalysis. Adv. Energy Mater. 2020, 10, 1902105. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, R.; Pekin, T.C.; Gammer, C.; Ciston, J.; Ercius, P.; Ophus, C.; Bustillo, K.; Song, C.; Zhao, S.; et al. Functional Materials Under Stress: In Situ TEM Observations of Structural Evolution. Adv. Mater. 2020, 32, 1906105. [Google Scholar] [CrossRef]
- Zhang, C.; Firestein, K.L.; Fernando, J.F.S.; Siriwardena, D.; Von Treifeldt, J.E.; Golberg, D. Recent Progress of In Situ Transmission Electron Microscopy for Energy Materials. Adv. Mater. 2020, 32, 1904094. [Google Scholar] [CrossRef]
- Zeng, Z.; Liang, W.-I.; Chu, Y.-H.; Zheng, H. In Situ TEM Study of the Li–Au Reaction in an Electrochemical Liquid Cell. Faraday Discuss. 2014, 176, 95–107. [Google Scholar] [CrossRef]
- De Yoreo, J.J. In-Situ Liquid Phase TEM Observations of Nucleation and Growth Processes. Prog. Cryst. Growth Charact. Mater. 2016, 62, 69–88. [Google Scholar] [CrossRef]
- Xu, T.; Sun, L. Investigation on Material Behavior in Liquid by in Situ TEM. Superlattices Microstruct. 2016, 99, 24–34. [Google Scholar] [CrossRef]
- Rehn, S.M.; Jones, M.R. New Strategies for Probing Energy Systems with In Situ Liquid-Phase Transmission Electron Microscopy. ACS Energy Lett. 2018, 3, 1269–1278. [Google Scholar] [CrossRef]
- Holtz, M.E.; Yu, Y.; Gunceler, D.; Gao, J.; Sundararaman, R.; Schwarz, K.A.; Arias, T.A.; Abruña, H.D.; Muller, D.A. Nanoscale Imaging of Lithium Ion Distribution During In Situ Operation of Battery Electrode and Electrolyte. Nano Lett. 2014, 14, 1453–1459. [Google Scholar] [CrossRef]
- Gu, M.; Parent, L.R.; Mehdi, B.L.; Unocic, R.R.; McDowell, M.T.; Sacci, R.L.; Xu, W.; Connell, J.G.; Xu, P.; Abellan, P.; et al. Demonstration of an Electrochemical Liquid Cell for Operando Transmission Electron Microscopy Observation of the Lithiation/Delithiation Behavior of Si Nanowire Battery Anodes. Nano Lett. 2013, 13, 6106–6112. [Google Scholar] [CrossRef]
- Yuk, J.M.; Seo, H.K.; Choi, J.W.; Lee, J.Y. Anisotropic Lithiation Onset in Silicon Nanoparticle Anode Revealed by in Situ Graphene Liquid Cell Electron Microscopy. ACS Nano 2014, 8, 7478–7485. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, X.; Bustillo, K.; Niu, K.; Gammer, C.; Xu, J.; Zheng, H. In Situ Study of Lithiation and Delithiation of MoS2 Nanosheets Using Electrochemical Liquid Cell Transmission Electron Microscopy. Nano Lett. 2015, 15, 5214–5220. [Google Scholar] [CrossRef]
- Liu, Q.; Levin, B.D.A.; Haiber, D.M.; Crozier, P.A. An In Situ Light Illumination System for an Aberration- Corrected Environmental Transmission Electron Microscope. arXiv 2021, arXiv:2104.02006. [Google Scholar]
- Duan, T.; Wang, W.; Cai, S.; Zhou, Y. On-Chip Light-Incorporated In Situ Transmission Electron Microscopy of Metal Halide Perovskite Materials. ACS Energy Lett. 2023, 8, 3048–3053. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Jiang, P.; Miao, H.; Wang, G.; Zhang, J. In Situ Light-Assisted Preparation of MoS2 on Graphitic C3N4 Nanosheets for Enhanced Photocatalytic H2 Production from Water. J. Mater. Chem. A 2015, 3, 7375–7381. [Google Scholar] [CrossRef]
- Cavalca, F.; Laursen, A.B.; Kardynal, B.E.; Dunin-Borkowski, R.E.; Dahl, S.; Wagner, J.B.; Hansen, T.W. In Situ Transmission Electron Microscopy of Light-Induced Photocatalytic Reactions. Nanotechnology 2012, 23, 075705. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Sui, M.; Li, R. Recent advances in in-situ transmission electron microscopy techniques for heterogeneous catalysis. iScience. 2023, 26, 107072. [Google Scholar] [CrossRef]
- Suzuki, K.; Ichihara, M.; Takeuchi, S.; Nakagawa, K.; Maeda, K.; Iwanaga, H. In Situ TEM Observation of Dislocation Motion in II–VI Compounds. Philos. Mag. A 1984, 49, 451–461. [Google Scholar] [CrossRef]
- Ohno, Y.; Takeda, S. A New Apparatus for in Situ Photoluminescence Spectroscopy in a Transmission Electron Microscope. Rev. Sci. Instrum. 1995, 66, 4866–4869. [Google Scholar] [CrossRef]
- Liu, C.; Ma, C.; Xu, J.; Qiao, R.; Sun, H.; Li, X.; Xu, Z.; Gao, P.; Wang, E.; Liu, K.; et al. Development of in Situ Optical Spectroscopy with High Temporal Resolution in an Aberration-Corrected Transmission Electron Microscope. Rev. Sci. Instrum. 2021, 92, 013704. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamasaki, J.; Tanaka, N. In Situ High-Resolution Transmission Electron Microscopy Observation of Photodecomposition Process of Poly-Hydrocarbons on Catalytic TiO2 Films. Appl. Phys. Lett. 2004, 84, 2542–2544. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst Releasing Hydrogen from Water. Nature 2006, 440, 295. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Domen, K. Effect of Post-Calcination on Photocatalytic Activity of (Ga1−xZnx)(N1−xOx) Solid Solution for Overall Water Splitting under Visible Light. J. Catal. 2008, 254, 198–204. [Google Scholar] [CrossRef]
- Reece, S.Y.; Hamel, J.A.; Sung, K.; Jarvi, T.D.; Esswein, A.J.; Pijpers, J.J.H.; Nocera, D.G. Wireless Solar Water Splitting Using Silicon-Based Semiconductors and Earth-Abundant Catalysts. Science 2011, 334, 645–648. [Google Scholar] [CrossRef]
- Picher, M.; Mazzucco, S.; Blankenship, S.; Holland, G.; Sharma, R. Optical Spectroscopy Integrated with Environmental Scanning Transmission Electron Microscope: A Comprehensive In Situ Characterization Platform. Microsc. Microanal. 2014, 20, 1748–1749. [Google Scholar] [CrossRef][Green Version]
- Miller, B.K.; Crozier, P.A. System for In Situ UV-Visible Illumination of Environmental Transmission Electron Microscopy Samples. Microsc. Microanal. 2013, 19, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Miller, B.K.; Crozier, P.A. Atomic Level In Situ Observation of Surface Amorphization in Anatase Nanocrystals During Light Irradiation in Water Vapor. Nano Lett. 2013, 13, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Jiang, Y.; Liao, H.-G.; Roeffaers, M.B.J.; Lai, F.; Huang, H.; Tang, Z. Visualizing Light-Induced Dynamic Structural Transformations of Au Clusters-Based Photocatalyst via in Situ TEM. Nano Res. 2021, 14, 2805–2809. [Google Scholar] [CrossRef]
- Yu, S.; Jiang, Y.; Sun, Y.; Gao, F.; Zou, W.; Liao, H.; Dong, L. Real Time Imaging of Photocatalytic Active Site Formation during H2 Evolution by In-Situ TEM. Appl. Catal. B Environ. 2021, 284, 119743. [Google Scholar] [CrossRef]
- Cavalca, F.; Laursen, A.B.; Wagner, J.B.; Damsgaard, C.D.; Chorkendorff, I.; Hansen, T.W. Light-Induced Reduction of Cuprous Oxide in an Environmental Transmission Electron Microscope. ChemCatChem 2013, 5, 2667–2672. [Google Scholar] [CrossRef]
- Vadai, M.; Angell, D.K.; Hayee, F.; Sytwu, K.; Dionne, J.A. In-Situ Observation of Plasmon-Controlled Photocatalytic Dehydrogenation of Individual Palladium Nanoparticles. Nat. Commun. 2018, 9, 4658. [Google Scholar] [CrossRef]
- Sytwu, K.; Vadai, M.; Hayee, F.; Angell, D.K.; Dai, A.; Dixon, J.; Dionne, J.A. Driving Energetically Unfavorable Dehydrogenation Dynamics with Plasmonics. Science 2021, 371, 280–283. [Google Scholar] [CrossRef]
- Kim, M.; Ham, S.-Y.; Cheng, D.; Wynn, T.A.; Jung, H.S.; Meng, Y.S. Advanced Characterization Techniques for Overcoming Challenges of Perovskite Solar Cell Materials. Adv. Energy Mater. 2021, 11, 2001753. [Google Scholar] [CrossRef]
- Gong, X.; Gnanasekaran, K.; Chen, Z.; Robison, L.; Wasson, M.C.; Bentz, K.C.; Cohen, S.M.; Farha, O.K.; Gianneschi, N.C. Insights into the Structure and Dynamics of Metal–Organic Frameworks via Transmission Electron Microscopy. J. Am. Chem. Soc. 2020, 142, 17224–17235. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Pei, A.; Yan, K.; Sun, Y.; Wu, C.-L.; Joubert, L.-M.; Chin, R.; Koh, A.L.; Yu, Y.; et al. Atomic Structure of Sensitive Battery Materials and Interfaces Revealed by Cryo–Electron Microscopy. Science 2017, 358, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Noh, H.; Gianneschi, N.C.; Farha, O.K. Interrogating Kinetic versus Thermodynamic Topologies of Metal–Organic Frameworks via Combined Transmission Electron Microscopy and X-Ray Diffraction Analysis. J. Am. Chem. Soc. 2019, 141, 6146–6151. [Google Scholar] [CrossRef] [PubMed]
- Wiktor, C.; Turner, S.; Zacher, D.; Fischer, R.A.; Tendeloo, G.V. Imaging of Intact MOF-5 Nanocrystals by Advanced TEM at Liquid Nitrogen Temperature. Microporous Mesoporous Mater. 2012, 162, 131–135. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Alvarado, J.; Wang, S.; Sina, M.; Lu, B.; Bouwer, J.; Xu, W.; Xiao, J.; Zhang, J.-G.; et al. New Insights on the Structure of Electrochemically Deposited Lithium Metal and Its Solid Electrolyte Interphases via Cryogenic TEM. Nano Lett. 2017, 17, 7606–7612. [Google Scholar] [CrossRef]
- Zhao, Y.-C.; Zhou, W.-K.; Zhou, X.; Liu, K.-H.; Yu, D.-P.; Zhao, Q. Quantification of Light-Enhanced Ionic Transport in Lead Iodide Perovskite Thin Films and Its Solar Cell Applications. Light Sci. Appl. 2017, 6, e16243. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Li, Y.; Huang, W.; Zhang, Z.; Chen, G.; Wang, H.; Wu, G.-H.; Rolston, N.; Vila, R.; et al. Unravelling Degradation Mechanisms and Atomic Structure of Organic-Inorganic Halide Perovskites by Cryo-EM. Joule 2019, 3, 2854–2866. [Google Scholar] [CrossRef]
- Lee, S.; Seong, J.G.; Jo, Y.; Hwang, S.-J.; Gwak, G.; Park, Y.; Kim, Y.C.; Lim, K.H.; Park, H.-Y.; Jang, J.H.; et al. Self-Assembled Network Polymer Electrolyte Membranes for Application in Fuel Cells at 250 °C. Nat. Energy 2024, 9, 849–861. [Google Scholar] [CrossRef]
- Yoshida, K.; Nozaki, T.; Hirayama, T.; Tanaka, N. In Situ High-Resolution Transmission Electron Microscopy of Photocatalytic Reactions by Excited Electrons in Ionic Liquid. J. Electron Microsc. 2007, 56, 177–180. [Google Scholar] [CrossRef]
- Ohno, Y.; Taishi, T.; Yonenaga, I. In Situ Analysis of Optoelectronic Properties of Dislocations in ZnO in TEM Observations. Phys. Status Solidi (A) 2009, 206, 1904–1911. [Google Scholar] [CrossRef]
- Ohno, Y. Development of an Apparatus for In-Situ Near-Field Photoexcitation in a Transmission Electron Microscope. Appl. Phys. Express 2012, 5, 125204. [Google Scholar] [CrossRef]
- He, B.; Zhang, Y.; Liu, X.; Chen, L. In-Situ Transmission Electron Microscope Techniques for Heterogeneous Catalysis. ChemCatChem 2020, 12, 1853–1872. [Google Scholar] [CrossRef]
- Montgomery, E.; Leonhardt, D.; Roehling, J. Ultrafast Transmission Electron Microscopy: Techniques and Applications. Microsc. Today 2021, 29, 46–54. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, C.; Moore, T.M.; Magel, G.A.; Garfinkel, D.A.; Camden, J.P.; Stanford, M.G.; Duscher, G.; Rack, P.D. Exploring Photothermal Pathways via in Situ Laser Heating in the Transmission Electron Microscope: Recrystallization, Grain Growth, Phase Separation, and Dewetting in Ag0.5Ni0.5 Thin Films. Microsc. Microanal. 2018, 24, 647–656. [Google Scholar] [CrossRef]
- Dahan, R.; Gorlach, A.; Haeusler, U.; Karnieli, A.; Eyal, O.; Yousefi, P.; Segev, M.; Arie, A.; Eisenstein, G.; Hommelhoff, P.; et al. Imprinting the Quantum Statistics of Photons on Free Electrons. Science 2021, 373, eabj7128. [Google Scholar] [CrossRef]
- Vanacore, G.M.; Madan, I.; Carbone, F. Spatio-Temporal Shaping of a Free-Electron Wave Function via Coherent Light–Electron Interaction. Riv. Nuovo Cim. 2020, 43, 567–597. [Google Scholar] [CrossRef]
- Houben, L.; Luysberg, M.; Brammer, T. Illumination Effects in Holographic Imaging of the Electrostatic Potential of Defects and pn Junctions in Transmission Electron Microscopy. Phys. Rev. B 2004, 70, 165313. [Google Scholar] [CrossRef]
- de Jonge, N.; Peckys, D.B. Live Cell Electron Microscopy Is Probably Impossible. ACS Nano 2016, 10, 9061–9063. [Google Scholar] [CrossRef]
- Zheng, Q.; Feng, T.; Hachtel, J.A.; Ishikawa, R.; Cheng, Y.; Daemen, L.; Xing, J.; Idrobo, J.C.; Yan, J.; Shibata, N.; et al. Direct Visualization of Anionic Electrons in an Electride Reveals Inhomogeneities. Sci. Adv. 2021, 7, eabe6819. [Google Scholar] [CrossRef]
- Zachman, M.J.; Fung, V.; Polo-Garzon, F.; Cao, S.; Moon, J.; Huang, Z.; Jiang, D.; Wu, Z.; Chi, M. Measuring and Directing Charge Transfer in Heterogenous Catalysts. Nat. Commun. 2022, 13, 3253. [Google Scholar] [CrossRef]
- Yan, X.; Gadre, C.A.; Aoki, T.; Pan, X. Probing Molecular Vibrations by Monochromated Electron Microscopy. Trends Chem. 2022, 4, 76–90. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramasundaram, S.; Jeevanandham, S.; Vijay, N.; Divya, S.; Jerome, P.; Oh, T.H. Unraveling the Dynamic Properties of New-Age Energy Materials Chemistry Using Advanced In Situ Transmission Electron Microscopy. Molecules 2024, 29, 4411. https://doi.org/10.3390/molecules29184411
Ramasundaram S, Jeevanandham S, Vijay N, Divya S, Jerome P, Oh TH. Unraveling the Dynamic Properties of New-Age Energy Materials Chemistry Using Advanced In Situ Transmission Electron Microscopy. Molecules. 2024; 29(18):4411. https://doi.org/10.3390/molecules29184411
Chicago/Turabian StyleRamasundaram, Subramaniyan, Sampathkumar Jeevanandham, Natarajan Vijay, Sivasubramani Divya, Peter Jerome, and Tae Hwan Oh. 2024. "Unraveling the Dynamic Properties of New-Age Energy Materials Chemistry Using Advanced In Situ Transmission Electron Microscopy" Molecules 29, no. 18: 4411. https://doi.org/10.3390/molecules29184411
APA StyleRamasundaram, S., Jeevanandham, S., Vijay, N., Divya, S., Jerome, P., & Oh, T. H. (2024). Unraveling the Dynamic Properties of New-Age Energy Materials Chemistry Using Advanced In Situ Transmission Electron Microscopy. Molecules, 29(18), 4411. https://doi.org/10.3390/molecules29184411








