Abstract
Given that methane (CH4) and nitrogen (N2) have similar properties, achieving high-purity enrichment of CH4 from nitrogen-rich low-grade gas is extremely challenging and is of great significance for sustainable development in energy and the environment. This paper reviews the research progress on carbon-based materials, zeolites, and MOFs as adsorbent materials for CH4/N2 separation. It focuses on the relationship between the composition, pore size, surface chemistry of the adsorbents, CH4/N2 selectivity, and CH4 adsorption capacity. The paper also highlights that controlling pore size and atomic-scale composition and optimizing these features for the best match are key directions for the development of new adsorbents. Additionally, it points out that MOFs, which combine the advantages of carbon-based adsorbents and zeolites, are likely to become the most promising adsorbent materials for efficient CH4/N2 separation.
1. Introduction
Currently, energy resource shortages, environmental pollution, and the intensification of the greenhouse effect are major problems and challenges facing humanity. With the proposal of carbon neutrality and carbon peaking goals, the task of carbon emission reduction has become more urgent [1]. Unconventional natural gas, specifically coalbed methane (CBM), which mainly comprises CH4, is considered a relatively clean fossil fuel as its complete combustion only produces CO2 and H2O. It also serves as an important modern industrial raw material [2]. CH4 is listed as a greenhouse gas in the Kyoto Protocol. It is noteworthy that its ozone-depleting potential is six times that of CO2, and its contribution to the greenhouse effect is 21 times greater, making it a significant driver of the greenhouse effect [3].
With the acceleration of the global development of green and low-carbon energy, the consumption and demand for natural gas continue to increase, signaling the advent of the global natural gas era [4]. It is well known that as the transition from fossil energy to clean energy progresses, conventional natural gas resources can no longer meet the sharply increasing demand for natural gas [5]. Unconventional natural gas will become a strong guarantee for natural gas supply, with unconventional hydrocarbon resources gradually becoming the primary source of energy consumption, with coalbed methane playing a crucial role [6]. In fact, in recent years, approximately 40 billion cubic meters of low-concentration coal mine gas (CH4 ≤ 30%) have been directly vented, resulting not only in resource wastage but also diverging significantly from the post-2020 emission reduction targets set by the United Nations Framework Convention on Climate Change (UNFCCC) at the 21st Conference of the Parties (COP21) held in Paris.
Clearly, CH4 has multiple attributes; it is both an indispensable clean energy source for sustainable development and a carrier of environmental pollution and enhanced greenhouse effects [7]. Therefore, developing CH4 enrichment technologies to utilize CH4 from low-concentration gas is of great significance for maintaining atmospheric balance, mitigating global greenhouse effects, and reducing resource waste [8]. Efficient CH4/N2 separation is a critical step in the enrichment and recovery of low-concentration gas. This paper systematically elucidates the process methods for the adsorption separation of CH4/N2 and the latest advancements in microporous materials used for the adsorption separation of low-concentration gas. It also provides an outlook on future developments, aiming to offer insights into the development of microporous materials for CH4/N2 adsorption separation.
2. Adsorption Separation Technology and Processes
The separation of N2 is a crucial step and technical challenge in the purification and refinement of low-grade gas. Due to the highly similar physicochemical properties of CH4 and N2, as shown in Table 1, CH4 and N2 are typical nonpolar molecules with no dipole moments. Except for the critical temperature, CH4 and N2 have similar kinetic diameters, polarizabilities, and quadrupole moments, with only a certain difference in boiling points, making their effective separation particularly difficult [9,10,11,12,13,14].

Table 1.
Physical and Chemical Properties of CH4 and N2 [15].
Typical purification processes for low-concentration CH4 include the removal of light hydrocarbons (C2+), dehydration, decarbonization, desulfurization, demercurization, and liquefaction-based nitrogen removal, with the latter being the most energy-intensive step in CH4 purification, severely limiting the large-scale application of low-concentration CH4 resources. To date, the purification of low-grade gas mainly employs techniques such as cryogenic distillation [16], membrane separation [17], chemical absorption [18], hydrate technology [19], and adsorption separation [20,21]. Table 2 systematically summarizes the principles and technical advantages and disadvantages of low-grade gas purification methods.

Table 2.
Principles and Technical Analysis of Low-Grade Gas Purification.
Among the various technologies for enriching low-concentration methane, adsorption separation stands out as the most promising candidate for industrial application. Adsorption is generally classified into two types: physical adsorption, also known as Van der Waals adsorption, and chemical adsorption. Physical adsorption occurs as a result of the mutual attraction between the adsorbent and adsorbate, leading to their adherence without the formation of chemical bonds. This process is reversible, allowing the adsorbate to be desorbed from the adsorbent surface under varying conditions. Chemical adsorption, on the other hand, involves the creation or breakage of chemical bonds between the adsorbent and adsorbate through chemical interactions. It results in the formation of new, stronger bonds between the two, often making the process irreversible. Pressure swing adsorption (PSA) exploits the differences in adsorption capacities of various gas molecules by the adsorbent under varying pressures. At elevated pressures, the adsorbent exhibits a strong affinity for certain components (typically impurities) within the gas mixture, whereas at lower pressures, its adsorptive capacity towards these components diminishes. By periodically altering the operating pressure, an alternating process of adsorption and desorption can be achieved, facilitating the separation of gases. The primary models underpinning PSA encompass adsorption equilibrium models, mass transfer kinetics models, mass conservation models, energy conservation models, and momentum conservation principles. To streamline the analysis, several assumptions are typically made, such as the gases adhere to the ideal gas law, radial concentration and temperature gradients are neglected, axial diffusion and axial heat conduction are disregarded, and heat transfer and heat accumulation along the adsorbent bed wall are ignored. Since Skarstrom designed the first pressure swing adsorption (PSA) process, researchers have conducted extensive studies and optimizations. Today, pressure swing adsorption separation processes mainly include steps such as pressurization adsorption, pressure equalization (up/down), purging, and depressurization desorption to improve CH4 enrichment efficiency [22].
Zhang et al. evaluated the enrichment performance of ultra-low concentration gas (CH4 < 5%) using a carbon molecular sieve (CMS-3 KT) as an adsorbent and adopting micro-positive pressure vacuum pressure swing adsorption (~120 kPa) for CH4 enrichment from CH4/O2/N2 mixed gases. At an inlet flow rate of 200 mL/min, optimal enrichment performance was achieved within 0–3 min. The CH4 concentration in the collected product gas was nearly 2.5 times that of the feed gas, with a recovery rate of approximately 80%, and the O2 concentration reduced to about 3%. This process is of great significance for the removal of oxygen and the enrichment of CH4 from oxygen-containing low-concentration coalbed methane [23]. Lu et al. conducted research on the process of enriching low-concentration gas using activated carbon. They determined and calculated the adsorption thermodynamics and kinetics of CH4 and N2 on AC adsorbents through static volumetric isotherm measurement and breakthrough experiments. A mathematical model of the adsorption bed was also established, and a two-stage VPSA process using AC adsorbents for enriching low-concentration CH4 was developed, as shown in Figure 1. This process enriched and purified low-concentration CH4 with an initial concentration of 20% to over 90%, with a CH4 recovery rate of over 98% and an energy consumption of less than 0.504 kW·h·(m3 CH4)−1 [24].
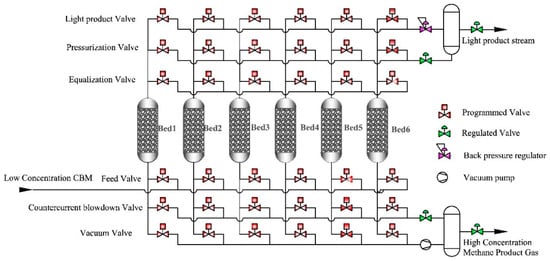
Figure 1.
The schematic diagram of the six-bed VPSA process [24].
To address the limitations of gas mixing at the feed inlet and the discontinuity in the axial concentration distribution in the adsorption column, Guo et al. introduced multiple feed inlets for enrichment and proposed a new dynamic feed dual reflux pressure swing adsorption process (DF-DR-PSA) for enriching low-grade CH4 (2.4 mol.%) and N2 mixtures. They conducted numerical simulations using Norit RB3 activated carbon adsorbent to compare the performance of the traditional DR-PSA process and the dynamic feed DF-DR-PSA process. Under the same energy consumption conditions, the DF-DR-PSA process showed improvements in both CH4 purity (53.5% vs. 47.5%) and recovery rate (81.1% vs. 72.2%) compared to the traditional DR-PSA process, solving the gas mixing issue at the feed inlet and enhancing CH4/N2 separation performance [25]. When the CH4 content in the feed gas is less than 20%, the proportion of recycled gas in the product gas and feed gas may be high, potentially reducing the performance of the VPSA process and increasing the energy consumption for recycling gas transport. To address this issue, Qian et al. proposed an improved integrated VPSA process operating in a simulated moving bed (SMB) mode, using commercial coconut shell activated carbon (AC) as the adsorbent. They applied an eight-column system to enrich CH4 from feed gas with 10–50% CH4 content, as shown in Figure 2, and compared the results of the improved VPSA process with traditional adsorption processes. The results demonstrated that the integrated VPSA process could successfully enrich and purify feed gas with 10% CH4 to produce 99.0% CH4 product gas, even using commercial activated carbon as the adsorbent [26].
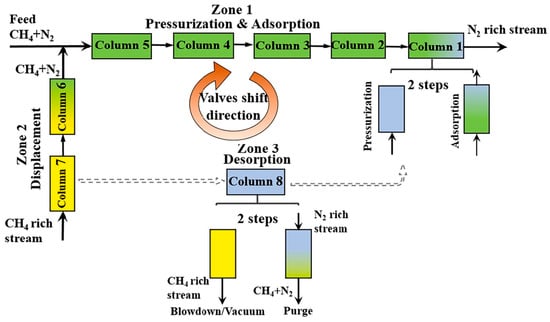
Figure 2.
Schematic flow diagram of the eight-column VPSA process with SMB mode. Different colors represent changes in different state parameters (pressure, gas composition, etc.), and dotted lines represent the transition of the three areas [26].
Currently, there are many scientific issues that need to be addressed in the field of adsorbent materials and adsorption separation processes. Among these, the development and preparation of microporous materials for the efficient separation of CH4/N2 is a particularly prominent problem. Achieving effective control over the pore channels and surface structure of adsorbent materials at the nanoscale and designing high-performance adsorbent materials with multiple coupled separation principles will be an effective solution to overcoming the bottleneck in CH4/N2 efficient adsorption separation technology.
3. Porous Materials for CH4/N2 Adsorption Separation
CH4/N2 adsorption separation is primarily based on the differences in adsorption selectivity between the two gases on solid microporous materials. This selectivity arises from three mechanisms: equilibrium effects, kinetic effects, and steric effects [27]. Taking the equilibrium effect as an example, there are significant differences in the models used to describe the adsorption behavior of different adsorbents under different conditions. Commonly used models to describe adsorption behavior include the Langmuir model, the Freundlich model, and the BET model. The essence of adsorption separation lies in the development of high-performance adsorbents and the design of processes that match these materials. High-performance microporous adsorbent materials are crucial for adsorption separation because they significantly influence the mass transfer process and system efficiency. Excellent adsorption selectivity and adsorption capacity are fundamental requirements for superior adsorbents, along with other factors such as physicochemical stability, regenerability, and environmental friendliness. Currently, the porous materials used for low-concentration gas adsorption separation mainly include carbon-based materials, zeolite molecular sieves, and metal/organic frameworks (MOFs) [28].
3.1. Carbon-Based Adsorbent Materials
Carbon-based materials are widely used in the field of adsorption separation due to their advantages of simple preparation and readily available raw materials. Among these, activated carbon and carbon molecular sieves have good CH4/N2 separation coefficients and CH4 adsorption capacities, making them common carbon-based materials for adsorption separation [29,30,31]. The adsorption performance of some of these carbon-based materials is shown in Table 3.

Table 3.
Adsorption Performance of Carbon-based Materials for CH4/N2.
Researchers have conducted extensive studies on the differences in adsorption performance due to various raw materials used in preparation. In 2015, Gu et al. prepared GAC (C-12) from anthracite using a pre-oxidation/carbonization/steam activation process, achieving a specific surface area of 412.51 m2/g with dominant pore sizes ranging from 5.0 nm to 6.43 nm. The CH4 adsorption capacity was 2.3 mmol/g, and the CH4/N2 selectivity was 3.17 (298 K, 1 MPa) [32]. Adding coal particles to asphalt has also been considered an effective method. In 2016, Arami-Niya et al. prepared AC using petroleum pitch and coal powder as raw materials through a low-pressure foaming method. Figure 3 illustrates the low-pressure foaming process of bubble growth in tar pitch with and without coal powder as an additive. This research group optimized the stability of asphalt foaming and product properties by controlling the ratio of KOH to coal pitch. They produced KOH0.5TP50 with a hierarchical pore structure, an open pore width of approximately 2 mm, well-developed micropores, a BET-specific surface area of 1044 m2/g, and an apparent density of 0.42 g/cm3 [33].
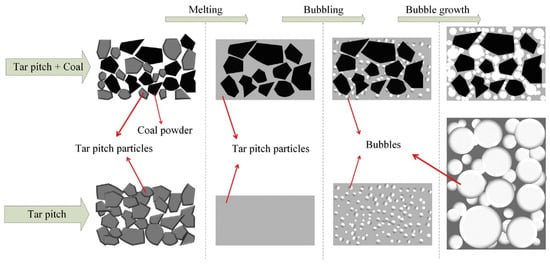
Figure 3.
Illustration of a low-pressure foaming process by bubble growth in tar pitch with and without coal particles as additives [33].
Subsequently, Yuan et al. used low-rank bituminous coal to prepare activated carbon C-12 with a high specific surface area of 3020 m2/g. Under experimental conditions of 6 MPa, its methane adsorption capacity was 13 mmol/g, and the CH4/N2 selectivity was 4.8 [46]. Additionally, coconut shells are often used for the preparation of activated carbon. Qu et al. conducted research on carbon materials prepared from coconut shells and found that the pore structure of carbonized coconut shells was poor. However, after KOH activation, the carbon materials had uniformly distributed regular micropores, higher specific surface area, and reduced polar functional groups on the surface, which facilitated the adsorption of weakly polar CH4 [47]. KOH is a strongly alkaline substance with significant corrosiveness and toxicity, and the preparation and use of carbon materials containing KOH may generate harmful wastewater and waste gases. These two approaches eschewed the highly polluting KOH activator, presenting an economical and green preparation method with broad application prospects for low-cost porous carbon materials. Tang et al. used rice as a carbon source to prepare granular carbon precursors through carbonization, followed by CO2 activation to produce rice-based carbon materials (PRCs) with narrow particle size distribution. Notably, the preparation process of granular PRC avoided the use of binders and KOH, reducing the usage of chemical reagents. The resulting PRC-850 had a BET-specific surface area of 776 m2/g. At 298 K and 100 kPa, its CH4 adsorption capacity and CH4/N2 selectivity reached 1.12 mmol/g and 5.7, respectively [48]. H3PO4-mechanical activation can significantly increase the adsorption volume of activated carbon. Pan et al. used bamboo sawdust as raw material and prepared samples using H3PO4-mechanical activation, with AC-1-400 achieving the largest pore volume (0.8 cm3/g) and the highest specific surface area (1966 m2/g). At 293 K, its CH4 adsorption capacity was 0.87 mmol/g, and the CH4/N2 selectivity was 3.38 [34].
Elemental doping has been proven to be an effective approach for modifying the pore structure of activated carbon, enhancing surface properties (including the specific surface area and micropore volume of the adsorbent material), optimizing the polarity of the adsorbent, and, consequently, improving its adsorption selectivity towards CH4/N2. This methodology offers a viable solution for optimizing the performance of adsorbent materials in targeted applications. In 2017, Yao et al. prepared a pyrolyzed fully Cl-substituted porous covalent triazine framework ClCTF-1-650 (at 650 °C), which exhibited an ultramicropore content of 98% and an N content of 12 at%. Due to its narrow pore size distribution, the N-doped porous carbon material significantly enhanced CH4 adsorption through weak interactions. At 298 K and 1 bar, the CH4 adsorption capacity was as high as 1.47 mmol/g, with a selectivity of 8.1, indicating that high nitrogen doping is an effective means of improving the selective adsorption of methane by microporous activated carbon [35]. Li activated waste wool with KOH and modified it with urea. The urea reacted with O functional groups, resulting in N-enhanced porous N-WAPC. The synthesis schematic is shown in Figure 4. Under experimental conditions of 298 K and 1 bar, the N-WAPC had a selectivity of 7.62 for equimolar CH4/N2 and a CH4 adsorption capacity of 1.01 mmol/g. N-WAPC shows great potential for efficient upgrading of CH4 from mixed gases [36].
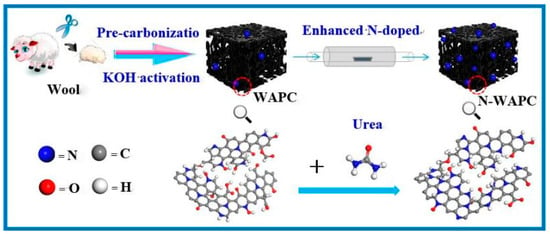
Figure 4.
Schematic illustration of the synthesis of enhanced N-doped porous carbon [36].
Zhang et al. developed a porous carbon material featuring a large surface area of 774.0 m2/g, a high N-doping level of 4.81 atomic percent (at%), and a pore volume of 0.32 cm3/g through a low-temperature one-pot method (involving low-temperature activation and N-doping simultaneously). Experimental results indicate that the utilization of NaNH2 as an activator and the introduction of the N element effectively optimized the pore structure, promoting the formation of abundant porous structures with a significant number of hierarchical pores. This led to an increase in both the specific surface area and the micropore volume. Ultimately, by optimizing the pore-forming agent/carbon ratio and activation temperature, the prepared N-rich microporous carbon (OTSS-2-450) exhibited exceptional CH4/N2 selectivity of 4.9 at 298 K and 1 bar [37]. Wang et al. studied a method for preparing a series of porous carbon microspheres using (polyphosphazene-co-4,4′-sulfonyldiphenol) (PZS) as the raw material and carbonizing it at different temperatures. Among these, PZS-900 (carbonized at 900 °C) demonstrated excellent CH4 capture performance, with a specific surface area of 895.7 m2/g. The pore volume (0.3592 cm3/g) accounted for 76% of the total pore volume (0.4643 cm3/g), significantly enhancing CH4 adsorption performance [49]. Additionally, the hydrophilicity of carbon materials helps improve CH4/N2 separation performance. However, the preparation of N-doped microporous carbon typically requires an excess of corrosive KOH as an activator, limiting its industrial application. Since the process often uses corrosive KOH, Zhang et al. proposed a corrosion-free method to prepare N-doped microporous carbon materials by using potassium citrate as a non-corrosive activator and urea for N-doping. By adjusting the ratio of urea-to-potassium citrate, activation temperature, and time to modify the carbon materials, they found that ACK2N1 exhibited the best adsorption performance. The potassium citrate/urea ratio of 2:1 favored the generation of micropore volumes with pore sizes less than 1 nm and 1–2 nm. The microporous structure, especially ultramicropores (pore size < 1 nm), is beneficial for the adsorption of small molecules such as CH4. Properly increasing the amount of potassium citrate promotes the generation of a narrow spectrum, enhancing CH4 enrichment [38].
The adsorption capacity and selectivity of activated carbon mainly depend on the particle size distribution and surface properties of the activated carbon. Li et al. modified coconut shell-based activated carbon with ammonia water and KOH. They compared the CH4/N2 separation performance of the modified and unmodified coconut shell-based activated carbon through equilibrium and dynamic adsorption experiments. The results showed that after modification with ammonia water, under optimal conditions of 12 h impregnation time and 10% modifier volume concentration, AC NH3·H2O-10%, 12 h at 298 K and 100 kPa had a CH4 adsorption capacity of 1.1 mmol/g and a selectivity of 4.62. This could be due to the introduction of amine and amide groups, which differentiate the adsorption energies of CH4 and N2 on AC. For the ammonia-modified samples, the introduced amine and amide groups greatly favored the selective adsorption of CH4 by differentiating the adsorption of CH4 and N2 on activated carbon. After KOH modification, the reduction of amine and hydroxyl groups weakened the CH4/N2 separation ability. The CH4 adsorption process mainly occurs on the surface of activated carbon, and its adsorption capacity is basically consistent with the volume of activated carbon. This is because of the capacity of surface basic groups; the larger the volume of AC, the more basic group capacity, thus, the stronger the adsorption performance. Increasing the capacity of basic groups through modified activated carbon may have great research value for enhancing CH4 adsorption performance [39]. Pan et al. conducted pressure swing adsorption experiments on low-concentration gas using a series of activated carbons. The activated carbon KCl/AC adsorbent treated with HCl had the largest specific surface area (1865 m2/g) and, at 298 K and 1 bar, the maximum CH4 adsorption capacity (7.89 mL/g), which was a 38.9% increase compared to the original AC (5.68 mL/g). The CH4/N2 selectivity of KCl/AC was 5.33, a 38.4% improvement over AC’s 3.85 [40]. Song et al. studied the chemical activation with phosphoric acid and KOH solutions and the high-temperature treatment in N2 or steam to prepare AC. Among them, AC-PS 800 treated with steam showed a significant increase in adsorption capacity due to the significant increase in micropore area, with pore sizes of 0.45–0.65 nm. Steam treatment of AC-P at 800 °C significantly increased the CH4 adsorption capacity to 6.5 mg/g, about twice that of the commercial sample GH-8 (3.2 mg/g). This confirmed that the combination of acidic chemical activation and high-temperature (i.e., 800 °C) steam treatment is a practical and effective method for enhancing CH4 adsorption performance [50]. Du et al. prepared a novel starch-based ultramicroporous carbon (SC) via an in situ ion activation method. These SCs were derived from starch and 1–6 wt.% acrylic acid, and the resulting materials were suitable for surface cation exchange. After activation, these SCs contained ultramicropores with a narrow pore size distribution of <0.7 nm, with SC-6 having an ultramicropore volume of 0.25 cm3/g, accounting for 78% of the total volume. At 100 kPa and 298 K, the CH4 adsorption capacity reached 1.86 mmol/g [41]. Chen et al. controllably prepared carbon adsorbents with uniform pore width from poly(vinylidene chloride) resin through activation-free pyrolysis. The microporous carbon adsorbents prepared by the activation-free pyrolysis method had a good specific surface area (>1100 m2/g) and a large specific pore volume (>0.37 cm3/g). At room temperature, the CH4 adsorption capacity was as high as 1.57 mmol/g, exceeding most of the best-performing adsorbents reported to date. Moreover, the optimal microporous carbon C-PVDC 700 showed the highest IAST selectivity for CH4 over N2 at 298 K and 100 kPa, with a value of 14.7. The material had numerous micropores distributed between 0.7 and 1.3 nm. Compared to traditional AC, the microporous carbon adsorbent obtained through pyrolysis had a higher proportion of mesopores and macropores. PVDC resin contains many oxygen-containing functional groups and numerous Cl-C-Cl functional groups, which are generally considered beneficial for CH4 adsorption. High selectivity and stability ensure that this material is an ideal adsorbent for LQNG upgrading [42]. Recently, Pereira et al. 3D-printed an integrated structure of Maxsorb-activated carbon using the polymer binder carboxymethyl cellulose (CMC). Based on the pre-established structure, the ink containing the adsorbent and binder was directly written in a layered manner. Using the ExDSL model and considering the equimolar mixture of CH4 and N2 at 303 K and 1 bar, the CH4 adsorption capacity was 1 mol/kg, with a CH4/N2 selectivity of 2.90. The novelty of this study lies in combining 3D printing technology with activated carbon adsorbents, as shown in Figure 5. The potential for CH4 and N2 separation using the Maxsorb-structured activated carbon adsorbent through direct ink writing (DIW) has been demonstrated [51]. Advanced 3D printing technology—direct ink writing (DIW)—is likely to become a valuable method for developing structured adsorbents at the laboratory scale and widely applied in industrial-scale promotion in the future.
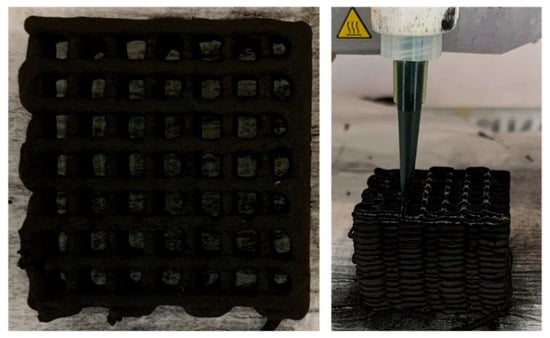
Figure 5.
3D-printed activated carbon monolith [51].
Compared to activated carbon (AC), carbon molecular sieves (CMS) have uniform pore sizes, which utilize the differences in the kinetic molecular diameters of CH4 and N2, leading to different diffusion rates to achieve the separation of mixed gases [52]. The CH4/N2 separation process of carbon molecular sieves mainly includes two mechanisms: kinetic separation and adsorption equilibrium [53]. When the pore diameter of CMS is between the kinetic diameters of the gas molecules, the kinetic separation effect predominates.
In 2004, Youn-Sang Bae studied the adsorption behavior of CH4 and N2 on CMS using volumetric methods and verified the feasibility of separating CH4/N2 mixtures on CMS through kinetic effects [54]. Subsequently, Olga Gorska et al. improved the pore size (mostly 0.8 nm) and specific surface area (1263 ± 50 m2/g) of the carbon molecular sieve DSV61Zn (CMS), which was prepared using “green” resources from willow and ZnCl2 as an activator. The selectivity reached up to 10.2 [55]. Zhang et al. prepared CMS using benzene as the deposition agent through chemical vapor deposition. The average width calculated by the DR equation was 0.47 nm. The maximum adsorption capacity was 1.41 mmol/g, and the selectivity reached up to 4.74, making it suitable for CH4/N2 adsorption separation [43]. Xiong et al. studied the adsorption equilibrium and kinetics of CH4, N2, and O2 on three types of carbon molecular sieves, as well as the corresponding separation performance. The experimental results showed that O2 had the largest diffusion time constant among the three gases, indicating the advantage of kinetic separation based on CMS in CMM deoxygenation and the key role of kinetic separation in CH4 enrichment [56]. Yang et al. modified coal-based carbon molecular sieves (CMS) using hydrocarbon-affinitive organic reagents such as docosane (C24), sodium dodecyl sulfate (SDS), and polyethyleneimine (PEI), followed by low-temperature plasma treatment. The best modification effect was observed for CMS-P-N, with a CH4 saturation adsorption capacity of 6.76 mmol/g and CH4/N2 selectivity of 3.32, indicating that low-temperature plasma treatment could provide a promising method for CH4/N2 adsorption separation [44]. Recently, Fu et al. prepared CMS with different oxidation degrees by combining carbon molecular sieves with KMnO4 oxidation. They studied the effects of microwave irradiation on the pores, functional groups, and high-pressure CH4 adsorption characteristics of the model substances. The results showed that microwave irradiation caused the reorganization of oxygen-containing functional groups in the carbon molecular sieves, blocking the micropores with diameters of 0.40–0.60 nm; meanwhile, the naphthalene and phenanthrene produced by the pyrolysis of macromolecular structures blocked the micropores of 0.70–0.90 nm in the carbon molecular sieves. These structural changes reduced the saturated CH4 adsorption capacity of the oxidized carbon molecular sieves by 2.91–23.28%, indicating that microwave irradiation could promote CH4 desorption. Additionally, the increase in mesopores in the oxidized carbon molecular sieves after microwave irradiation facilitated CH4 diffusion. Microwave irradiation is considered a highly promising technology for improving low-concentration methane enrichment [57]. Compared to AC and CMS, Li et al. reported a simple in situ growth method for preparing carbon nanofibers, as shown in Figure 6, and combined them with an ultramicroporous gas-selective layer for CH4/N2 separation. The thickness of the gas-selective layer could be precisely adjusted within the range of 90–525 nm. The optimal carbon nanofibers exhibited a high CH4/N2 selectivity of 6.8 and a CH4 adsorption capacity of 0.97 mmol/g, with CH4 diffusion kinetics two orders of magnitude faster than commercial activated carbon adsorbents. The high accessibility of adsorption sites and short diffusion paths of the nanofibers facilitated rapid mass transfer and enhanced the dynamic separation of CH4/N2. This study provided a novel approach for designing high-performance CH4/N2 adsorbents through a rational combination of abundant ultramicropores and short diffusion paths [45].
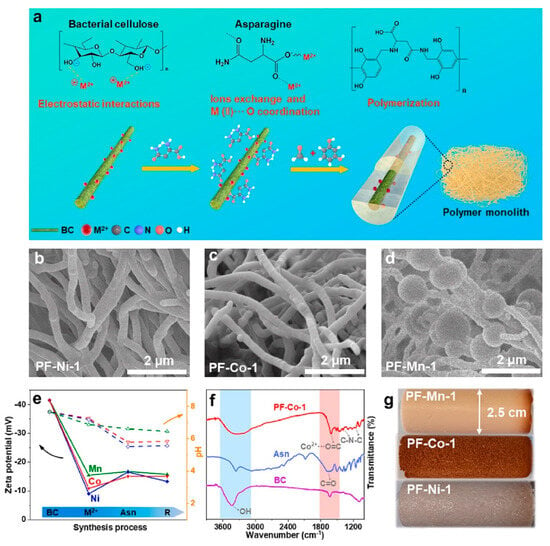
Figure 6.
(a) Schematic of the synthesis strategy for PFs. (b–d) SEM image of PF-Ni-1, PF-Co-1, and PF-Mn-1. (e) The recording of Zeta potential and pH changes during the synthesis process. (f) FT-IR spectra of PF-Co-1, Asn, and BC. (g) Photograph of the as-obtained polymeric aerogels [45].
3.2. Zeolite Molecular Sieves
Zeolite molecular sieves are aluminosilicate crystals with a regular pore structure and excellent adsorption performance. Their basic structure consists of silicon/oxygen and aluminum/oxygen tetrahedra, which are interconnected by oxygen bridges, forming polyhedral cage structures with three-dimensional spaces. They have uniform pore size distribution and can sieve different molecules based on size [58,59]. In the separation of CH4/N2, zeolite molecular sieves can achieve efficient adsorption and separation of CH4 due to their unique pore size and adsorption characteristics. Additionally, synthesizing zeolite molecular sieves with specific pore sizes and structures can further improve the efficiency and selectivity of CH4/N2 separation [60]. Researchers have conducted extensive studies on this. Initial studies on the adsorption performance of conventional zeolite molecular sieves such as 4A, 5A, mordenite, and 13X for CH4/N2 showed that the difference in their equilibrium adsorption capacities was small, making it difficult to achieve efficient CH4/N2 separation [61,62,63,64]. Subsequently, methods such as ion exchange, changing the Si/Al ratio, and composite modification were used to further improve the CH4/N2 separation effect [65]. The adsorption performance of some of these zeolite molecular sieves is shown in Table 4.

Table 4.
Adsorption Performance of Zeolites for CH4/N2.
Controlling the pore size of molecular sieves is an effective method for achieving the kinetic separation of CH4/N2 mixtures on molecular sieves. To address the issue of water content in low-concentration gas, especially when the adsorbent is a hydrophilic material, hydrophobic adsorbents are used to avoid destructive water absorption. Yang et al. developed three hydrophobic microporous high-silica zeolites: DDR (with 8-membered rings), silicalite-1 (with 10-membered rings), and beta (with 12-membered rings). The Si/Al ratios were 230, 1350, and 35, respectively. Experimental results showed that silicalite-1, with the most suitable pores for CH4 adsorption and the highest CH4/N2 selectivity (3.50), was more suitable for low-concentration CH4 enrichment compared to commercially used adsorbents zeolite 5A (2.5) and 13X (1.3) [66]. Kuznicki et al. adjusted the framework of silicate ETS-4 by high-temperature dehydration to modify the effective pore size, allowing gas molecules to enter the crystal interior. This so-called “molecular gate” effect can be used to customize molecular sieves with different adsorption properties, suitable for separating commercially important gas mixtures [73]. B. Majumdar et al. further studied the CH4/N2 adsorption of ETS-4 ion-exchange variants based on the principle that the 8MR channel contracts as the dehydration temperature increases (at the molecular scale). They found that at lower dehydration temperatures, CH4 adsorbed more strongly and diffused more slowly than N2. Gradual dehydration reduced equilibrium capacity and diffusion rates for both gases, affecting the larger CH4 molecules more than the relatively smaller N2 molecules, increasing diffusion coefficients and reducing equilibrium selectivity. Eventually, equilibrium selectivity reversed, leading to a maximum kinetic selectivity value of 205 in samples dehydrated at 400 °C [74]. Shang et al. studied the adsorption and separation performance of CH4 on three CHA-type molecular sieves with different pore diameters: Chabazite-K, SAPO-34, and SSZ-13. Experimental results showed that Chabazite-K had the highest selectivity (5.5). SSZ-13 had the largest pore volume and specific surface area but the lowest selectivity (2.5) and the highest CH4 adsorption capacity, reaching 1.38 mmol/g. With similar Si/Al ratios, the framework’s extra-framework metal cations determined the material’s adsorption capacity and selectivity. Due to the presence of metal cations, the pore size was narrowed, reducing CH4 adsorption capacity, while the increased polarization of CH4 enhanced the interaction between the pores and CH4. SSZ-13, with a larger pore volume, is suitable for recovering CH4 from low-concentration CH4 (CH4 < 20%). Reducing the crystal size of zeolites to achieve enhanced gas adsorption and separation performance has largely been unexplored and underestimated [67]. Compared to micron-sized zeolites, nanoscale zeolites have more adsorption sites and shorter diffusion paths. Yang and his team were the first to successfully prepare nano ZK-5 using β-cyclodextrin as a modulator. The crystal size of ZK-5 was reduced from micron-sized (3 μm) to nanoscale (50–100 nm), and nano ZK-5 exhibited superior specific surface area (370 m2/g) and pore volume (0.22 cm3/g) compared to the micron-sized samples. Compared to micron ZK-5, nano ZK-5 showed a 64% increase in CH4 adsorption capacity. At 298 K, nano ZK-5 showed a CH4 adsorption capacity of 1.34 mmol/g on commercial zeolites [68].
Ion exchange modification of zeolites has also been a popular method for improving the separation of CH4/N2 mixtures in conventional zeolites over the past few decades. As early as 2004, Jayaraman et al. studied the adsorption performance of pure magnesium clinoptilolite for CH4/N2 separation and measured its high-pressure adsorption isotherms. They simulated pressure swing adsorption for pure clinoptilolite, magnesium-based clinoptilolite, and commercial adsorbent ETS-4. The purified clinoptilolite showed a slightly higher recovery rate than ETS-4 but a lower yield, with similar product purity (>95%). Their team also prepared mixed ion-exchange clinoptilolite with Mg2+/Ca2+, K+/Na+ and Mg2+/Na+ and studied the CH4/N2 separation performance of these ion-exchanged clinoptilolites. The results showed that Mg/Na (50/50) mixed ion-exchanged clinoptilolite exhibited good equilibrium and kinetic selectivity at low pressure, superior to pure clinoptilolite [75]. Subsequently, many researchers joined this field of study. Sethia et al. used volumetric gas adsorption to study zeolite-X exchanged with Mg2+, Ca2+, Sr2+, and Ba2+. The results showed that zeolite-X exchanged with Mg2+, Ca2+, Sr2+, and Ba2+ exhibited increased adsorption capacities for CH4 and N2. At 303 K and 1 bar, Sr2+-exchanged zeolite-X showed a CO adsorption capacity of 28.4 molecules per unit cell, while Ca2+-exchanged zeolite-X showed CH4 and N2 adsorption capacities of 18.8 and 13.8 molecules per unit cell, respectively. Ba2+-exchanged zeolite-X showed a CH4/N2 selectivity of 1.78 [76]. D.A. Kennedy et al. performed cation exchange modification of natural clinoptilolite using alkali metal ions, alkaline earth metal ions, transition metal ions, and acid treatment. They evaluated the composition and structural properties of the modified samples using EDS and XRD analysis and compared them with the original clinoptilolite samples. The results showed that cation-exchanged clinoptilolites exhibited a wide range of adsorption characteristics, making them suitable for various gas separation applications via pressure swing adsorption. The high adsorption selectivity of Cs+-exchanged clinoptilolite for CH4 favored CH4/N2 equilibrium separation. Due to pore blocking in Ca2+-exchanged clinoptilolite, CH4 equilibrium capacity and N2/CH4 selectivity were reduced. However, the potential of this material was limited due to increased micropore diffusion resistance. Although Li+- and Ni2+-exchanged clinoptilolites exhibited low CH4/N2 ideal selectivity, they showed high N2/CH4 kinetic selectivity, making them suitable for potential N2/CH4 kinetic separation [77]. Hao et al. ground, concentrated by weight, and treated clinoptilolite with ion exchange using different salt solutions. The modified clinoptilolite powders were then pelletized and used as adsorbents. Under conditions of 0.2 MPa and 298 K, the adsorbents were used for N2/CH4 separation in pressure swing adsorption (PSA). The results showed that the composites had micropores and a large number of mesopores, mainly formed by slit pores from layered stacking. NH4-Cp, Cs-Cp, and Cu-Cp adsorbents showed good equilibrium selectivity for CH4, with selectivities of 2.56, 2.31, and 1.95, respectively. Na-Cp had an N2/CH4 selectivity of 7.25, showing good equilibrium selectivity. Na-Cp adsorbent was suitable for CH4/N2 mixed gas separation, increasing CH4 concentration from 19.7% to 30.72% [78]. In 2019, Dean A. Kennedy et al. studied cation-exchanged clinoptilolite, obtaining modified clinoptilolite through cation exchange modification of natural clinoptilolite. At temperatures between 15 °C and 30 °C, Cs3+- and Fe3+-exchanged clinoptilolite had CH4/N2 selectivities ranging from 2.7 to 5.3 and 3.9 to 7.3, respectively, while typical activated carbon selectivities ranged from 2.1 to 5.5 [79]. Wu et al. developed amine ion-exchanged Y-type molecular sieves for CH4/N2 separation. Through simple ion exchange with tetramethylammonium cation (TMA+) and choline cation (Ch+), the resulting adsorbents significantly increased CH4 adsorption and decreased N2 adsorption. Compared to the original NaY, the CH4/N2 separation performance of the resulting samples greatly improved. At 25 °C and 100 kPa, the CH4/N2 selectivities of TMAY and ChY reached 6.32 and 6.50, respectively [69]. Mahsa Vosoughi et al. synthesized Na-ETS-4 using both Cl-containing and halogen-free methods, and the synthesized adsorbents (Ba-RPZ and Ba-HFZ) were ion-exchanged with Ba2+, as shown in Figure 7. They studied the adsorption equilibrium and kinetics of N2 and CH4 on the two Ba-ETS-4 adsorbents. Static volumetric adsorption was used to determine adsorption data at 30 °C and pressures from 0 to 100 kPa. The results showed that due to the gradual structural contraction with increasing heat treatment temperature, equilibrium capacity and adsorption rates gradually decreased. When the activation temperature increased to 400 °C, equilibrium selectivity and kinetic selectivity decreased due to additional structural deformation. The presence of Cl- in Ba-RPZ added extra steric hindrance, reducing adsorption capacity and gas diffusion coefficients [70].
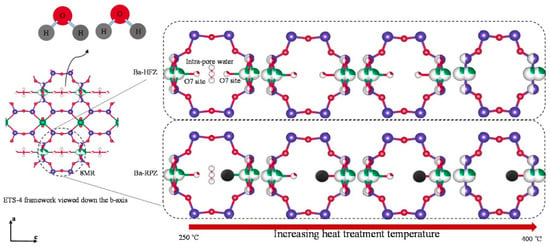
Figure 7.
Schematic of Ba-ETS-4 structural changes during heat treatment process. Titanium atoms are presented in green. Silicon atoms are presented in blue. Oxygen and chlorine are presented in red and black, respectively [70].
In 2023, Mousavi et al. used GCMC simulations to evaluate the CH4 and N2 adsorption capacities and selectivities of 1425 different alkali metal-exchanged zeolites, discovering that some specific zeolite frameworks could achieve equilibrium selectivity for N2. They also studied the effects of alkali metal types (Li+, Na+, K+, Rb+, and Cs+) and Si/Al ratios on the performance of each framework. The results showed that K+ cations exhibited the highest affinity for N2 adsorption, while the smaller Li+ cations had the highest gas absorption. Additionally, a lower Si/Al ratio favored N2/CH4 selectivity [80]. Furthermore, the development of ionic liquid zeolites has provided new ideas for improving the separation of CH4/N2 by zeolites. In 2022, Hu et al. studied the separation of CH4 from N2 using 100 kg of ionic liquid zeolite (ILZ) material in a pressure swing adsorption process. The CH4 concentrations in the feed gas were increased from 5.0% and 16.1% to 11.5% and 34.6%, respectively, with CH4 recovery rates higher than 80% [81]. Researchers have also conducted studies on composite modification of zeolite molecular sieves. In 2020, Tang et al. first synthesized nm/mm-sized (500 nm) K-KFI (Si/Al = 1/4.59) adsorbents using a hydrothermal method and ultrasound-assisted method, achieving a CH4 adsorption capacity of 1.05 mmol/g. The team also treated the zeolite with ultrasound and found that the ultrasound time significantly affected the surface of the K-KFI molecular sieve, reducing its particle size from 1.5 μm to 500 nm, confirming that the ultrasound-assisted method is a fast and effective way to synthesize nm/mm-sized zeolite molecular sieves [71]. In 2021, Zhao et al. reported a new K-ZSM-25 trapdoor molecular sieve material, as shown in Figure 8, where K+ acts as the “gatekeeper” cation. The temperature-dependent oscillation degree of K+ cations regulates the accessibility of the cages, controlling the material’s adsorption capacity. Experimental and theoretical results showed that the small-pore zeolite ZSM-25 provided a unique opportunity for effective N2 adsorption. This material exhibited good N2 capacity, excellent N2/CH4 selectivity (up to 34), outstanding kinetic effects, and regeneration potential at room temperature, making it well-suited for PSA-based industrial separation. The design of this trapdoor material opens new avenues for N2/CH4 separation in various fields, aiming to develop cleaner and more energy-efficient gas processing technologies. Additionally, adjustable pore accessibility offers potential applications for gas storage and molecular encapsulation [82].
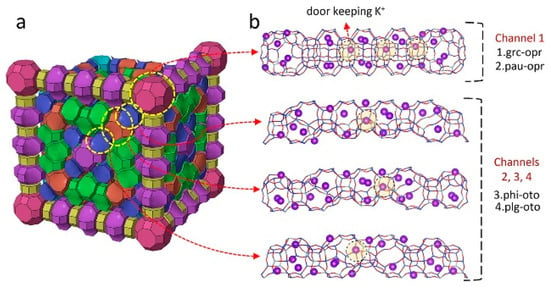
Figure 8.
N2 and CH4 diffusion passes in ZSM-25: (a) 3D view of the ZSM-25 unit cell and (b) four unique channels connected through eight-membered rings as the main routes for gas diffusion consisting of four double-connected cages, namely, (1) grc-opr, (2) pau-opr, (3) phi-oto, and (4) plg-oto. The door-keeping cations are highlighted [82].
In 2021, Yang and colleagues reported a simple and green seed proliferation method to prepare ring-shaped hierarchical K-chabazite molecular sieve nanoclusters with macro-, meso-, and micro-porous structures, as shown in Figure 9. This continuous seed induction method does not require an organic template. By utilizing this unique nanotechnology, the resulting material demonstrated significantly increased CH4 adsorption capacity, gas diffusion rate, and separation productivity compared to commercially available adsorbents. Notably, the raw materials for this adsorbent are easily obtainable, and the synthesis route is environmentally friendly, offering the potential for industrial-scale production [72].
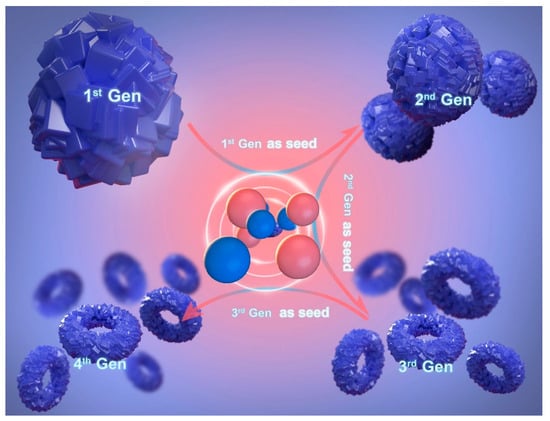
Figure 9.
Synthesis of nanosized K-Chabazite by the seed-passaging route [72].
Recently, in 2024, Ghasemi and colleagues achieved a significant breakthrough by innovatively applying RHO-type zeolite membranes in the field of CH4/N2 separation. They demonstrated that N2 gas molecules can easily pass through the membrane channels, while CH4 molecules, due to their larger molecular diameter, cannot enter the membrane cavities. Their team conducted simulation studies on the N2 separation performance of RHO molecular sieve membranes for CH4/N2 mixtures at 298 K and a pressure difference of up to 30 MPa. The results showed that the RHO molecular sieve membrane exhibited very high permeability and selectivity for N2, surpassing the upper limit defined by Robeson, with a maximum permeability of 2.14 × 105 GPU (gas permeation units). Additionally, they studied the effects of varying the feed gas composition and membrane thickness. The results indicated that permeability increased with decreasing membrane thickness, and the feed gas composition had a significant impact on the separation of CH4 and N2 [83].
3.3. Metal/Organic Frameworks (MOFs)
Metal/organic frameworks (MOFs), also known as porous coordination polymers (PCPs), are a new type of porous material formed by the complexation of inorganic metal centers (metal ions or metal clusters) with organic ligands. Due to their ultra-high specific surface area, ordered pore structure, adjustable pore size, and easily functionalizable framework surface, these materials have been widely applied in gas adsorption and storage, catalysis, bioimaging and sensing, drug delivery, magnetic devices, and nonlinear optics [84]. Recent research has significantly expanded the variety of these materials, mainly including the ZIF series [85], MIL series [86], and UIO series, among others, as shown in Table 5.

Table 5.
Adsorption Performance of Metal/Organic Frameworks (MOFs) for CH4/N2.
Researchers first conducted extensive research based on the difference in polarizability between CH4 and N2, utilizing the difference in equilibrium adsorption capacity exhibited by gases in metal/organic frameworks. In 2012, Möllmer and colleagues found that the CH4/N2 separation factors for [Cu(Me-4py-trz-ia)] and Basolite® A100 were as high as 4.5 and 5.0, respectively. At 0.1 MPa and 298 K, they measured the CH4 adsorption capacities of Basolite A100 and [Cu(Me-4py-trz-ia)] to be 0.71 mmol/g and 1.12 mmol/g, respectively. This study was the first to demonstrate the excellent performance of metal/organic frameworks (MOFs) in the adsorption separation of CH4/N2 [88].
The special microstructural environment within MOFs plays a crucial role in enhancing CH4 adsorption capacity. Li and colleagues reported a novel Cd-1,3,6,8-tetrakis(para-benzoic acid) pyrene framework, ROD-8, which possesses two types of one-dimensional structures (0.65 nm × 1.18 nm and 0.85 × 0.95 nm). The shortest distance between two parallel pyrene core planes in the structure is 0.435 nm. Under conditions of 298 K and 1 bar, the CH4/N2 separation factor reached 9.0, and the CH4 adsorption capacity was 0.77 mmol/g [89]. Liu and colleagues comprehensively characterized MOFs MMA-BPY (M = Co and Ni) and found that they exhibit good framework flexibility. Additionally, M-MA-BPY MOFs showed excellent stability in water and humid air. At 298 K and 1 bar, they demonstrated good CH4 capacities (0.92 and 1.01 mmol/g for Co- and Ni-MA-BPY, respectively) and significant CH4/N2 selectivity (CH4/N2 separation selectivity of 7.2 and 7.4 for Co- and Ni-MA-BPY, respectively) [90]. Subsequently, Feng and colleagues used suspension polymerization and in situ growth techniques to transform the morphology of Al-CDC from three-dimensional (3D) crystals to two-dimensional (2D) nanosheets, forming nanomembranes with higher adsorption efficiency and lower diffusion barriers. Experimental results showed that compared to bulk Al-CDC, its adsorption efficiency increased by 1.73 times, with a separation factor for CH4/N2 (50/50, v/v) mixture of 13.75 and a CH4 adsorption capacity of 1.32 mmol/g [113]. Zhang and colleagues studied an ultramicroporous MOF with nonpolar pore walls, MIL-120Al, which is constructed from infinite rod-like structural units and BTEC ligands. It features a unique aluminum-based three-dimensional open framework with ultramicroporous walls formed by appropriately sized benzene rings, as shown in Figure 10. Benefiting from the kinetic synergistic separation effect, this MOF exhibited excellent separation performance for CH4/N2 mixtures under dynamic conditions, comparable to the previously reported Al-CDC adsorption capacity [91].
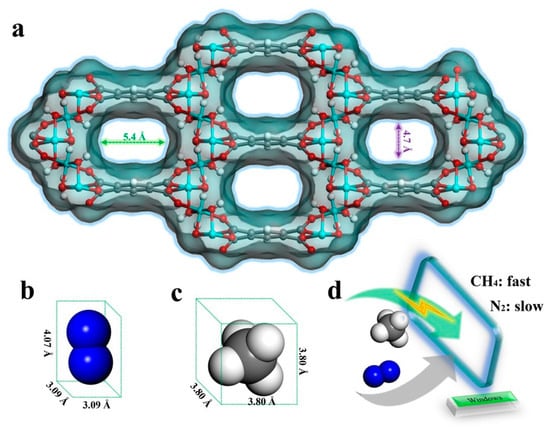
Figure 10.
(a) 3D framework of MIL-120Al; (b,c) molecular size of CH4 and N2; (d) illustration of the different kinetic effects of CH4 and N2 through the window of MIL-120Al. Color code: C, gray; H, white; O, red; Al, cyan; N, blue [91].
Huang and colleagues further explored the potential of Al-MOFs as CH4/N2 separation adsorbents by considering the effects of pore geometry and linker polarity. They synthesized two one-dimensional square Al-MOFs, 10-H and MIL-160, and two corresponding rhombic counterparts, Al-Fum and MIL-53(Al), using two bent ligands with different polarities and two linear ligands. Their research found that Al-Fum exhibited a CH4/N2 separation factor as high as 17.2 at 273 K and 1.0 bar. To verify the accuracy of the experimental results, they used Monte Carlo simulations to model the adsorption density distribution of CH4 and N2 in Al-MOFs, as shown in Figure 11. The results indicated that the adsorption density distribution of CH4 molecules in the channels of Al-MOFs was significantly higher than that of N2 molecules, consistent with the experimental results [92].
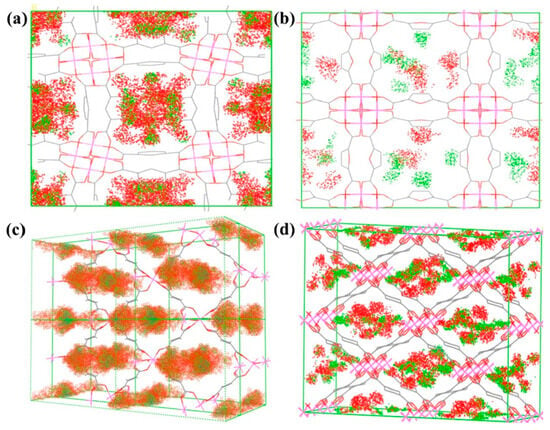
Figure 11.
The simulated distribution of adsorption density on (a) CAU-10-H, (b) MIL-160, (c) Al-Fum, and (d) MIL-53(Al) during the adsorption process (red regions for CH4, green regions for N2) [92].
Considering characteristics such as biocompatibility, cost, toxicity, and natural abundance, Chang and colleagues utilized the properties of calcium metal to prepare a novel calcium-based metal/organic framework material, SBMOF-1 (ligand: 4,4′-SDB), for CH4/N2 adsorption separation. This Ca-MOF, containing low-polarity polyaromatic organic ligands, exhibited a CH4/N2 separation factor as high as 11.5, along with a relatively high CH4 adsorption capacity of approximately 0.92 mmol/g [93]. Additionally, research has found that pore size significantly impacts CH4/N2 adsorption separation. He and colleagues designed UTSA-30 (Ln-MOFs) with three-dimensional channels. By degassing acetone-exchanged UTSA-30 under high vacuum at room temperature, they produced UTSA-30a, which exhibited a CH4 adsorption capacity of 0.6 mmol/g and a CH4/N2 separation factor of up to 5.0 at 298 K and 1 bar [94]. Ma and colleagues successfully designed and synthesized a carboxyl-functionalized PAF material, PAF-26-COOH. Post-metallization of PAF-26-COOH produced a series of PAF-26-COOM derivatives (M = Li, Na, K, Mg). These functionalized materials are ultramicroporous, with DFT results showing pore sizes in the range of 4–6 Å. They exhibited good separation selectivity of 4.2–6.5 under conditions of 298 K and 110 kPa [95].
Hu and colleagues used divalent metal ions to adjust the pore size and polarity of molecular sieves. The results showed that metal formates have different affinities for CH4, exhibiting different CH4 adsorption capacities and CH4/N2 selectivities in the order of Ni > Co > Mg > Mn. Among them, [Ni3(HCOO)6] demonstrated the highest CH4 adsorption capacity (0.81 mmol/g) and CH4/N2 selectivity (6.5) in dynamic adsorption experiments at 0.1 MPa and 298 K. This indicates an optimal synergy between pore contraction and surface properties among the [M3(HCOO)6] frameworks. The adsorption behavior of [M3(HCOO)6] was studied using NH3-TPD, revealing two different adsorption states of NH3 molecules within [M3(HCOO)6], one where gas molecules reside within the pores and another where gas molecules adsorb onto the coordinating metal ions or exposed oxygen-induced adsorption sites. This confirms the important role of metal ions in adjusting pore size and internal surface properties [96]. Building on previous work, Guo and colleagues synthesized [Ni3(HCOO)6] using a novel solvent-free method. At 298 K and 100 kPa, the CH4 adsorption capacity was 0.82 mmol/g (comparable to the previous value), and the CH4/N2 selectivity was 6.18. This method converts the nickel(II) precursor and formic acid into the [Ni3(HCOO)6] framework at mild temperatures, avoiding the use of harmful solvents and providing an environmentally friendly route [114]. Shi and colleagues studied the CH4/N2 adsorption separation performance of three SOD-type ZIF materials (ZIF-8, ZIF-90, and SIM-1 (ZIF-94)) and one RHO-type material (ZIF-93). The results showed that SIM-1 (ZIF-94) exhibited the highest CH4 adsorption capacity (1.51 mmol/g at 298 K and 1 bar). Analysis of the structures and organic ligands of the four ZIFs revealed that the narrow pore size (0.84 nm in SIM-1) plays a key role in CH4 adsorption. IAST calculations indicated that the CH4/N2 separation factor of SIM-1 (ZIF-94) is seven times higher than that of reported ZIFs and most porous materials. The CH4 retention time in SIM-1 is nearly ten times longer (20 min) than in the other three materials. However, SIM-1 cannot currently be scaled up cost-effectively, necessitating the development of greener synthesis methods in the future [97]. Kim and colleagues introduced functional groups (-H, -NH2, -NO2, -Br, and -Br2) into the pores of hydrothermally stable zirconium-based metal/organic frameworks (MOFs), as illustrated by the organic linkers in Figure 12. Experimental and molecular simulation results showed that UiO-66-Br2 exhibited significant adsorption performance, with a CH4 adsorption capacity of 0.72 mmol/g and a CH4/N2 selectivity of 5.06. Simulations and calculations confirmed that introducing bulky functional groups can exploit their high polarizability and reduce pore size, thereby enhancing CH4 adsorption capacity [98].
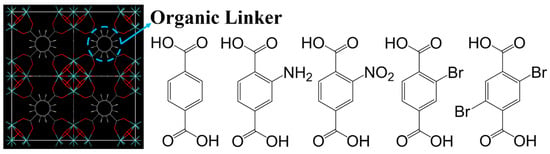
Figure 12.
Crystallographic structure of UiO-66 unit cell and schematic of organic linkers of UiO-66-X materials [98].
Wang and colleagues prepared four nickel-based diamond coordination frameworks (Ni(ina)2, Ni(3-ain)2, Ni(2-ain)2, Ni(pba)2) by introducing functional sites (-NH2) or altering the length of the ligands to fine-tune the pore chemistry and pore size, thereby achieving ultra-high CH4 adsorption and excellent separation performance. Among these, Ni(ina)2 exhibited significant differences in CH4 and N2 adsorption, with a CH4 adsorption capacity of 1.665 mmol/g and a CH4/N2 selectivity of 15.8 at 298 K and 100 kPa. Further GCMC simulations and DFT calculations were conducted to study the mechanisms of selective CH4 capture by Ni(ina)2 and Ni(3-ain)2. The results indicated that Ni(ina)2 and Ni(3-ain)2 provided suitable accommodation spaces for CH4 molecules. However, the ink-bottle-shaped channels with very narrow pore sizes in Ni(2-ain)2 were unsuitable for capturing CH4. Conversely, the larger pore sizes in Ni(pba)2 made it difficult to capture CH4 due to weaker intermolecular interactions [99]. Qadir and colleagues successfully synthesized a microporous Ni-Qc-5 using low-polarity, low-toxicity, and naturally abundant polyaromatic ligands, which exhibited excellent CH4 and N2 separation performance. At room temperature and atmospheric pressure, the CH4 adsorption capacity was 1.3 mmol/g, with a CH4/N2 selectivity of 7.0. The PXRD pattern of the sample obtained using a PANalytical X’pert diffractometer (Malvern, UK) matched well with the simulated pattern derived from crystallographic data, as shown in Figure 13. TGA results indicated that the Ni-Qc-5 MOF sample remained stable below 300 °C, with degradation occurring only above this temperature. Due to its stability and ease of regeneration, this adsorbent has promising applications for capturing coal mine methane at low pressures [100].
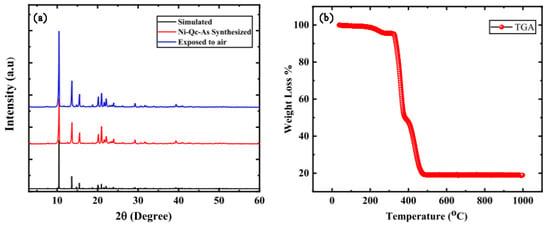
Figure 13.
(a) XRD patterns of experimental and simulated Ni-Qc-5 MOF (b) Thermogravimetric analysis of Ni-Qc-5 MOF [100].
Chang and colleagues were the first to apply the ultramicroporous copper-dicyanoimidazole MOF (NKMOF-8-Me) for CH4/N2 separation. Due to the introduction of aromatic imidazole derivatives, this MOF features regular nonpolar/inert pore surfaces and appropriate pore sizes, resulting in a shorter average distance between CH4 and the pore walls, thereby enhancing CH4 adsorption affinity. The adsorption results indicated that this material exhibits the highest CH4 adsorption capacity (1.76 mmol/g) and a CH4/N2 adsorption selectivity of 9.0 (50:50 v/v). This MOF can be synthesized quickly and easily on a large scale at room temperature and demonstrates high structural stability under various conditions, including exposure to water vapor, boiling water, acid solutions (pH = 1), alkaline solutions (pH = 11), and high temperatures (700 K) [101]. Guo and colleagues reported a stable ultramicroporous Cu(I)-based metal/organic framework (MOF), NKMOF-8-Br, which exhibited an excellent CH4 adsorption capacity (1.84 mmol/g) and high CH4/N2 (50/50, v/v) selectivity of 8.9 at room temperature and atmospheric pressure. The CH4 adsorption capacity surpasses all water-stable MOFs and other types of adsorbents used for CH4/N2 separation [102]. Chen and colleagues reported a Zr-based metal/organic framework (MIP-203-F) featuring a rhombic one-dimensional (1D) dual-pore structure. The presence of side-chain -OH groups effectively divides the structure into two symmetric wall-sharing triangular pores, providing the material with optimal pore size and numerous synergistic polar sites. This promotes efficient CH4 adsorption, taking advantage of its high polarizability, and overcomes the trade-off between CH4 capacity and CH4/N2 selectivity. The resulting MIP-203-F framework structure with 1D channels (Figure 14c) has a pore limiting diameter (PLD) of 3.61 Å and a maximum cavity diameter (LCD) of 5.14 Å, as shown in Figure 14d,e [103].
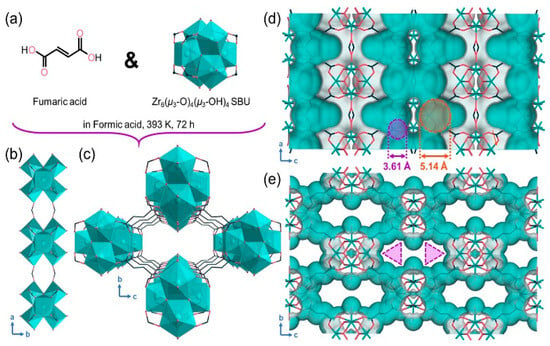
Figure 14.
(a) Chemical structure of fumaric acid ligand and Zr6(μ3-O)4(μ3-OH)4 cluster in MIP-203-F. (b) Formate-linked Zr6-oxo cluster chain along the a-axis. (c) Framework structure of MIP-203-F with the hydroxyl group-divided dual triangular 1D pore. (d) Connolly surface of MIP-203-F with a probe radius of 1.82 A viewed along the b-axis. (e) Van der Waals surface of MIP-203-F viewed along the a-axis [103].
However, Chang and colleagues achieved precise pore size tuning by altering the degassing temperature, inducing the dynamic switching of the initial two-dimensional (2D) framework to prepare Cu-MOF-SCH3. The empty framework and original state can be fully switchable under vacuum and upon exposure to water vapor, demonstrating excellent resistance to water vapor. Notably, this material can be produced on a large scale using supergravity technology, and Cu-MOF-SCH3 exhibits the highest STY (space–time yield) among all 2D MOFs [104]. Studies have shown that the special adsorption sites of metal/organic frameworks can further enhance the CH4/N2 adsorption separation performance. Li and colleagues investigated two isostructural MOFs, [Cu(1,3-BDC)(H2O)]·2H2O and Cu(1,3-BDC)(PY)2, and found that the introduction of the organic ligand pyridine in Cu(1,3-BDC)(PY)2 provided more CH4 adsorption sites, improving the CH4/N2 separation factor to 20.14 at 1 MPa [105]. Li and colleagues were the first to prepare an ultramicroporous [Co3(C4O4)2(OH)2] (C4O42− = squarate) with enhanced negatively charged oxygen binding sites, achieving a maximum CH4/N2 separation factor of 12.5. Due to its high thermal stability and low regeneration cost, this material shows great potential for industrial applications [106]. Niu and colleagues reported a metal framework, ATC-Cu, which has unique relatively adjacent open metal sites that provide very strong binding sites for CH4 at relatively low pressures. ATC-Cu can adsorb 2.90 mmol/g of CH4 at room temperature and atmospheric pressure, and the CH4/N2 selectivity of ATC-Cu, calculated from binary equimolar mixtures, is as high as 9.7 [107]. Lv and colleagues proposed enhancing the CH4 adsorption affinity by controlling the polar sites on the pore walls of aluminum-based metal/organic frameworks (MOFs). Their experiments showed that at 298 K and 1 bar, the CH4/N2 selectivity of CAU-21-BPDC (11.9) was significantly higher than that of CAU-8-BPDC (4.9). CAU-21-BPDC, with four highly symmetric polar sites, exhibited a CH4/N2 selectivity 2.4 times greater than CAU-8-BPDC, which lacks these polar sites. The CH4 adsorption capacity of CAU-8-BPDC was 0.85 mmol/g (298 K, 100 kPa), while that of CAU-21-BPDC was 0.99 mmol/g (298 K, 100 kPa). Additionally, CAU-21-BPDC showed better water stability compared to CAU-8-BPDC, attributed to its lower water vapor adsorption capacity, making it less likely for water vapor to replace the organic ligands in the CAU-21-BPDC framework and disrupt the metal/ligand bonds. This study first confirmed that, besides the strength of metal/ligand bonds, the coordination number of metal ions, the degree of framework interpenetration, and the hydrophobicity of the pore wall surfaces, water vapor adsorption capacity also affects the water stability of MOFs [108]. Zheng and colleagues synthesized a novel 2D layered metal/organic framework, Ni(4-DPDS)2CrO4 (4-DPDS = 4,4′-dipyridyldisulfide), for the first time. This novel 2D MOF exhibited excellent or even better high stability compared to previously reported 3D MOFs. At 273 K and 1 bar, it demonstrated a CH4 adsorption capacity of 0.95 mmol/g and a CH4/N2 selectivity of 7.3. Density functional theory calculations indicated that the energetically favorable binding sites for CH4 molecules were located in the middle of the cavities modified by CrO42− anions. The angled inorganic anions provide polar sites and bring the guest/host interactions very close, thereby enhancing the affinity of Ni(4-DPDS)2CrO4 for CH4 [109]. Considering the high cost and environmental issues of synthesizing metal/organic frameworks (MOFs), Fang and colleagues used fly ash as a raw material. By using sulfuric acid as an extractant and applying direct leaching and roasting processes, they prepared CFAx-FumMOF-y. The CH4/N2 selectivity ranged from 3.98 to 4.65, with CH4 adsorption capacities between 0.844 and 0.895 mmol/g. Among them, CFAs-FumMOF-1 exhibited the highest adsorption selectivity of 4.56. The specific surface area and pore volume of CFAx-FumMOF-y reached 1164.94–1073.08 m2/g and 0.40–0.36 cm3/g, respectively, which are comparable to those of Al-FumMOF (1287.55 m2/g and 0.42 cm3/g) [110]. Recently, Wang and colleagues addressed the challenge of maintaining the high selectivity of porous materials under high pressure by preparing a low-polarity microporous membrane Ni(TMBDC)(DABCO)0.5. Experimental results showed that it achieved a CH4 adsorption capacity of 4.23 mmol/g and a CH4/N2 separation factor of 5.1 at 298 K and 10 bar [111]. Liu and colleagues developed a titanium metal/organic framework adsorbent, ZSTU-1, with dual nano traps. These nano traps enhance CH4 adsorption capacity (1.37 mmol/g) through C-H···O hydrogen bonds and multiple C-H···π interactions, with CH4/N2 selectivity ranging from 21.6 to 12.0. Notably, in a single separation of an equimolar CH4/N2 mixture, the CH4 productivity reached an unprecedented 13.1 L/kg. Furthermore, in the presence of air or water vapor, it far surpassed the CH4 productivity of Co3(C4O4)2(OH)2 (0.29 L/kg) [112].
Researchers have conducted extensive studies on the CH4/N2 adsorption separation performance of MOF materials and have made significant progress. However, some MOFs still suffer from issues such as poor selectivity, low adsorption capacity, high synthesis costs, and poor water stability. These drawbacks greatly affect the adsorption performance and limit practical applications. Given the inherent advantages of MOFs, such as ultra-high specific surface area, adjustable pore size, and easily functionalizable framework surfaces, the continuous search for novel MOF materials with excellent performance is of great importance to meet the demands of large-scale industrial applications in the near future.
4. Conclusions and Perspectives
Developing new technologies for the purification of low-concentration CH4 and enriching unconventional natural gas not only alleviates the shortage of natural gas but also significantly reduces the greenhouse effect and environmental pollution. The main bottleneck in CH4 enrichment is the removal of nitrogen from low-concentration gas. Adsorption separation, as an effective CH4/N2 separation technology, has advantages such as low cost and simple operation, making it commercially promising. The development of high-performance adsorbents is key to continuously improving nitrogen removal efficiency. Research on carbon-based materials, zeolite molecular sieves, and metal/organic frameworks (MOFs) adsorbents for CH4/N2 adsorption has been extensive, with a good understanding of the adsorption behaviors of various adsorbents and the principles for performance optimization. However, there are still areas that need improvement, requiring the exploration of new strategies to enhance the attraction differences between methane and nitrogen with the adsorbents. For example, the surface properties and pore optimization of carbon-based materials, as well as the controllability and reproducibility of heteroatom-doped carbon-based adsorbents, deserve attention and further research. The issue of high surface polarization and diffusion kinetics mutual inhibition in zeolite molecular sieves and how to use metal ions to control the surface potential for directional adjustment, particularly in finely tuning the pore size and surface properties of ultramicropores (<8 μm) at the molecular scale, are also important. MOFs, which combine the advantages of the former two, exhibit unique benefits in CH4/N2 adsorption separation. However, challenges remain in adsorption selectivity, adsorption capacity, operational stability, and commercial application value. Further research and optimization are needed to enhance water stability and control synthesis costs. There is significant room for development in structural modification techniques to purify complex gas mixtures (isomers, gas isotopes). In the future, coupling multiple technical principles or introducing high-tech methods such as 3D printing (DIW) will be directions for researchers to explore continuously.
This paper does not provide any theoretical, equation, or calculation background; it mainly focuses on processes, materials, and properties. The theoretical background and main equations can be found in the following references [115,116,117].
Author Contributions
Conceptualization, P.C. and D.S.; formal analysis, P.C.; investigation, Y.Q. and Y.Y.; data curation, Q.Y.; writing—original draft preparation, D.S.; writing—review and editing, D.S.; visualization, R.Z.; supervision, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Applied Basic Research Program of Liaoning Provincial Science and Technology Department (2023JH2/101300223) and the Innovation Team Project of Liaoning Petrochemical University (LX2024001).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, Y. Promote Carbon Peaking and Carbon Neutrality with the Concept of Global Warming and Carbon Emission Reduction. BCP Bus. Manag. 2023, 39, 439–448. [Google Scholar] [CrossRef]
- Division, E.M. Unconventional Energy Resources: 2017 Review. Nat. Resour. Res. 2018, 28, 1661–1751. [Google Scholar]
- Disclaim Badr, O.; Probert, S.D.; O’Callaghan, P.W. Methane: A greenhouse gas in the Earth’s atmosphere. Appl. Energy 1992, 41, 95–113. [Google Scholar] [CrossRef]
- Chen, J.; Yu, J.; Ai, B.; Song, M.; Hou, W. Determinants of global natural gas consumption and import–export flows. Energy Econ. 2019, 83, 588–602. [Google Scholar] [CrossRef]
- Weijermars, R.; Drijkoningen, G.; Heimovaara, T.J.; Rudolph, E.S.J.; Weltje, G.J.; Wolf, K.H.A.A. Unconventional gas research initiative for clean energy transition in Europe. J. Nat. Gas Sci. Eng. 2011, 3, 402–412. [Google Scholar] [CrossRef]
- Kuyper, J.; Schroeder, H.; Ola Linnér, B. Annual Review of Environment and Resources. Annu. Rev. Environ. Resour. 2018, 43, 343–368. [Google Scholar] [CrossRef]
- Whiting, G.J.; Chanton, J.P. Greenhouse carbon balance of wetlands: Methane emission versus carbon sequestration. Tellus B 2001, 53, 521–528. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Upgrade of Methane from Landfill Gas by Pressure Swing Adsorption. Energy Fuels 2005, 19, 2545–2555. [Google Scholar] [CrossRef]
- Lokhandwala, K.A.; Pinnau, I.; He, Z.; Amo, K.D.; DaCosta, A.; Wijmans, J.G.; Baker, R.W. Membrane separation of nitrogen from natural gas: A case study from membrane synthesis to commercial deployment. J. Membr. Sci. 2010, 346, 270–279. [Google Scholar] [CrossRef]
- Saha, D.; Grappe, H.A.; Chakraborty, A.; Orkoulas, G. Postextraction Separation, On-Board Storage, and Catalytic Conversion of Methane in Natural Gas: A Review. Chem. Rev. 2016, 116, 11436–11499. [Google Scholar] [CrossRef]
- Rufford, T.E.; Smart, S.; Watson, G.; Graham, B.F.; Boxall, J.A.; Costa, J.D.; May, E.F. The removal of CO2 and N2 from natural gas: A review of conventional and emerging process technologies. J. Pet. Sci. Eng. 2012, 94, 123–154. [Google Scholar]
- Yang, R.T. Adsorbents: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Lee, K.; Isley, W.C.; Dzubak, A.L.; Verma, P.; Stoneburner, S.J.; Lin, L.; Howe, J.D.; Bloch, E.D.; Reed, D.A.; Hudson, M.R.; et al. Design of a metal-organic framework with enhanced back bonding for separation of N2 and CH4. J. Am. Chem. Soc. 2014, 136, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal-organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, M.; Farrusseng, D.; Valencia, S.; Aguado, S.; Ravon, U.; Rizzo, C.; Corma, A.; Mirodatos, C. Natural gas treating by selective adsorption: Material science and chemical engineering interplay. Chem. Eng. J. 2009, 155, 553–566. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, J. Experimental Study on Distillation Column Parameters for Liquefaction Device of Low Concentration Coalbed Methane. Processes 2021, 9, 606. [Google Scholar] [CrossRef]
- Janusz-Cygan, A.; Jaschik, J.; Tańczyk, M. Upgrading Biogas from Small Agricultural Sources into Biomethane by Membrane Separation. Membranes 2021, 11, 938. [Google Scholar] [CrossRef]
- Gong, H.; Chen, Z.; Yu, H.; Wu, W.; Weixing, W.; Pang, H.; Du, M. Methane recovery in a combined amine absorption and gas steam boiler as a self-provided system for biogas upgrading. Energy 2018, 157, 744–751. [Google Scholar] [CrossRef]
- Baek, S.; Ahn, Y.; Zhang, J.; Min, J.; Lee, H.; Lee, J.W. Enhanced methane hydrate formation with cyclopentane hydrate seeds. Appl. Energy 2017, 202, 32–41. [Google Scholar] [CrossRef]
- Fakhraei Ghazvini, M.; Vahedi, M.; Najafi Nobar, S.; Sabouri, F. Investigation of the MOF adsorbents and the gas adsorptive separation mechanisms. J. Environ. Chem. Eng. 2020, 9, 104790. [Google Scholar] [CrossRef]
- Fatehi, A.; Loughlin, K.F.; Hassan, M.M. Separation of methane—Nitrogen mixtures by pressure swing adsorption using a carbon molecular sieve. Gas Sep. Purif. 1995, 9, 199–204. [Google Scholar] [CrossRef]
- Delgado, J.A.; Uguina, M.A.; Sotelo, J.L.; Águeda, V.I.; Gómez, P. Numerical simulation of a three-bed PSA cycle for the methane/nitrogen separation with silicalite. Sep. Purif. Technol. 2011, 77, 7–17. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Zhou, F.; Li, X.; Wei, K.; Song, J. Enrichment of Oxygen-Containing Low-Concentration Coalbed Methane with CMS-3KT as the Adsorbent. ACS Omega 2021, 6, 6914–6923. [Google Scholar] [CrossRef]
- Lu, B.; Shen, Y.; Tang, Z.; Zhang, D.; Chen, G. Vacuum pressure swing adsorption process for coalbed methane enrichment. Chin. J. Chem. Eng. 2020, 32, 264–280. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, G.; Chen, K.; Guo, J.; Webley, P.A.; Li, G.K. Capture of dilute methane with a novel dynamic-feed dual-reflux pressure swing adsorption process. AlChE J. 2021, 68, 17390. [Google Scholar] [CrossRef]
- Qian, Z.; Zhou, Y.; Yang, Y.; Li, P. Methane recovery from low-grade unconventional natural gas by the integrated mode of the conventional/improved vacuum pressure swing adsorption processes. Fuel 2023, 331, 125717. [Google Scholar] [CrossRef]
- Anderson, M.W.; Terasaki, O.; Ohsuna, T.; Philippou, A.; Mackay, S.P.; Ferreira, A.; Rocha, J.; Lidin, S. Structure of the microporous titanosilicate ETS-10. Nature 1994, 367, 347–351. [Google Scholar] [CrossRef]
- Nandanwar, S.U.; Corbin, D.R.; Shiflett, M.B. A Review of Porous Adsorbents for the Separation of Nitrogen from Natural Gas. Ind. Eng. Chem. Res. 2020, 59, 13355–13369. [Google Scholar] [CrossRef]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Yi, H.; Li, F.; Ning, P.; Tang, X.; Peng, J.; Li, Y.; Deng, H. Adsorption separation of CO2, CH4, and N2 on microwave activated carbon. Chem. Eng. J. 2013, 215–216, 635–642. [Google Scholar] [CrossRef]
- Rufford, T.E.; Watson, G.; Saleman, T.L.; Hofman, P.S.; Jensen, N.K.; May, E.F. Adsorption Equilibria and Kinetics of Methane + Nitrogen Mixtures on the Activated Carbon Norit RB3. Ind. Eng. Chem. Res. 2013, 52, 14270–14281. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, B.; Qi, Z.; Liu, Z.; Duan, S.; Du, X.; Xian, X. Effects of pore structure of granular activated carbons on CH4 enrichment from CH4/N2 by vacuum pressure swing adsorption. Sep. Purif. Technol. 2015, 146, 213–218. [Google Scholar] [CrossRef]
- Arami-Niya, A.; Rufford, T.E.; Zhu, Z. Activated carbon monoliths with hierarchical pore structure from tar pitch and coal powder for the adsorption of CO2, CH4 and N2. Carbon 2016, 103, 115–124. [Google Scholar] [CrossRef]
- Pan, H.; Zhao, J.; Lin, Q.; Cao, J.X.; Liu, F.; Zheng, B. Preparation and Characterization of Activated Carbons from Bamboo Sawdust and Its Application for CH4 Selectivity Adsorption from a CH4/N2 System. Energy Fuels 2016, 30, 10730–10738. [Google Scholar] [CrossRef]
- Yao, K.X.; Chen, Y.; Lu, Y.; Zhao, Y.; Ding, Y. Ultramicroporous carbon with extremely narrow pore distribution and very high nitrogen doping for efficient methane mixture gases upgrading. Carbon 2017, 122, 258–265. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Wang, B.; Wei, J.; Wang, L.; Shen, M.; Yang, J. Enhanced N-doped Porous Carbon Derived from KOH-Activated Waste Wool: A Promising Material for Selective Adsorption of CO2/CH4 and CH4/N2. Nanomaterials 2019, 9, 266. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Zhang, P.; Wang, J.; Xu, M.; Deng, Q.; Zeng, Z.; Deng, S. Ultra-high surface area and nitrogen-rich porous carbons prepared by a low-temperature activation method with superior gas selective adsorption and outstanding supercapacitance performance. Chem. Eng. J. 2019, 355, 309–319. [Google Scholar] [CrossRef]
- Zhang, L.N.; Dong, Y.; Zhang, D.; Li, W.; Qin, H.; Luo, Z.; Shi, Y.; Lv, Y.; Zhang, C.; Pan, H.; et al. Facile preparation of nitrogen-doped microporous carbon from potassium citrate/urea for effective CH4 separation and uptake. Fuel 2023, 351, 128915. [Google Scholar]
- Li, Z.; Liu, Y.; Zhang, C.Z.; Yang, X.; Ren, J.; Jiang, L. Methane Recovery from Coal Bed Gas Using Modified Activated Carbons: A Combined Method for Assessing the Role of Functional Groups. Energy Fuels 2015, 29, 6858–6865. [Google Scholar] [CrossRef]
- Pan, H.; Yi, Y.; Lin, Q.; Xiang, G.; Zhang, Y.; Liu, F. Effect of Surface Chemistry and Textural Properties of Activated Carbons for CH4 Selective Adsorption through Low-Concentration Coal Bed Methane. J. Chem. Eng. Data 2016, 61, 2120–2127. [Google Scholar] [CrossRef]
- Du, S.; Wu, Y.; Wang, X.; Xia, Q.; Xiao, J.; Zhou, X.; Li, Z. Facile synthesis of ultramicroporous carbon adsorbents with ultra-high CH4 uptake by in situ ionic activation. AlChE J. 2020, 66, 16231. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Z.; Yang, Q.; Yang, Y.; Bao, Z.; Ren, Q. Microporous Carbon Adsorbents Prepared by Activating Reagent-Free Pyrolysis for Upgrading Low-Quality Natural Gas. ACS Sustain. Chem. Eng. 2020, 8, 977–985. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, S.; Li, L.; Wang, P.; Li, X.; Che, Y.; Li, X. Preparation of Carbon Molecular Sieves Used for CH4/N2 Separation. J. Chem. Eng. Data 2018, 63, 1737–1744. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, D.; Meng, Z.; Li, Y. Adsorption separation of CH4/N2 on modified coal-based carbon molecular sieve. Sep. Purif. Technol. 2019, 218, 130–137. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Zhang, M.; Liu, B.; Zheng, Z.; Hao, G.; Lu, A. Carbon Nanofibers with Gas Selective Layer Containing Rich and Accessible Ultramicropores for Methane/Nitrogen Separation. Chem. Eng. J. 2023, 462, 142118. [Google Scholar] [CrossRef]
- Yuan, D.; Zheng, Y.; Li, Q.; Lin, B.; Zhang, G.; Liu, J. Effects of pore structure of prepared coal-based activated carbons on CH4 enrichment from low concentration gas by IAST method. Powder Technol. 2018, 333, 377–384. [Google Scholar] [CrossRef]
- Qu, D.; Yang, Y.; Lu, K.; Yang, L.; Li, P.; Yu, J.; Ribeiro, A.M.; Rodrigues, A.E. Microstructure effect of carbon materials on the low-concentration methane adsorption separation from its mixture with nitrogen. Adsorption 2018, 24, 357–369. [Google Scholar] [CrossRef]
- Tang, R.; Dai, Q.; Liang, W.; Wu, Y.; Zhou, X.; Pan, H.; Li, Z. Synthesis of novel particle rice-based carbon materials and its excellent CH4/N2 adsorption selectivity for methane enrichment from Low-rank natural gas. Chem. Eng. J. 2020, 384, 123388. [Google Scholar] [CrossRef]
- Wang, S.; Wu, P.; Fu, J.; Yang, Q. Heteroatom-doped porous carbon microspheres with ultramicropores for efficient CH4/N2 separation with ultra-high CH4 uptake. Sep. Purif. Technol. 2021, 274, 119121. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, X.; Wang, J.A. Adsorption performance of activated carbon for methane with low concentration at atmospheric pressure. Energy Source Part A 2019, 43, 1337–1347. [Google Scholar] [CrossRef]
- Pereira, A.; Ferreira, A.F.; Rodrigues, A.E.; Ribeiro, A.M.; Regufe, M.J. Study of methane upgrading using an activated carbon 3D-printed adsorbent. J. Environ. Chem. Eng. 2023, 12, 111730. [Google Scholar] [CrossRef]
- Campo, M.C.; Magalhães, F.D.; Mendes, A.M. Comparative study between a CMS membrane and a CMS adsorbent: Part I—Morphology, adsorption equilibrium and kinetics. J. Membr. Sci. 2010, 346, 15–25. [Google Scholar] [CrossRef]
- Yang, Y.; Ribeiro, A.M.; Li, P.; Yu, J.; Rodrigues, A.E. Adsorption Equilibrium and Kinetics of Methane and Nitrogen on Carbon Molecular Sieve. Ind. Eng. Chem. Res. 2014, 53, 16840–16850. [Google Scholar] [CrossRef]
- Bae, Y.; Moon, J.; Ahn, H.W.; Lee, C. Effects of adsorbate properties on adsorption mechanism in a carbon molecular sieve. Korean J. Chem. Eng. 2004, 21, 712–720. [Google Scholar] [CrossRef]
- Gorska, O.; Cyganiuk, A.W.; Olejniczak, A.; Ilnicka, A.; Lukaszewicz, J.P. Salix viminaliswood as a new precursor for manufacturing of carbon molecular sieves for effective methane/nitrogen separation. Open Chem. 2015, 13, 748–755. [Google Scholar] [CrossRef]
- Yang, X.; Li, Z.; Zhang, C.Z.; Wang, H.; Zhang, E.; Xing, Y.; Xiao, P.; Yang, R.T.; Liu, Y.; Webley, P.A. Practical separation performance evaluation of coal mine methane upgrading with carbon molecular sieves. Chem. Eng. J. 2019, 367, 295–303. [Google Scholar] [CrossRef]
- Fu, X.; Tang, X.; Xu, Y.; Zhou, X.; Zhang, D. Microwave irradiation-induced alterations in physicochemical properties and methane adsorption capability of coals: An experimental study using carbon molecular sieve. Chin. J. Chem. Eng. 2024, 68, 165–180. [Google Scholar] [CrossRef]
- Ruthven, D.M. Molecular Sieve Separations. Chem. Ing. Tech. 2011, 83, 44–52. [Google Scholar] [CrossRef]
- Bol, R.; Harkness, D.D. The Use of Zeolite Molecular Sieves for Trapping Low Concentrations of CO2 from Environmental Atmospheres. Radiocarbon 1995, 37, 643–647. [Google Scholar] [CrossRef][Green Version]
- Jensen, N.K.; Rufford, T.E.; Watson, G.; Zhang, D.; Chan, K.I.; May, E.F. Screening Zeolites for Gas Separation Applications Involving Methane, Nitrogen, and Carbon Dioxide. J. Chem. Eng. Data 2012, 57, 106–113. [Google Scholar] [CrossRef]
- Habgood, H.W. The Kinetics of Molecular Sieve Action. Sorption of Nitrogen-Methane Mixtures by Linde Molecular Sieve 4A. Can. J. Chem. 1958, 36, 1384–1397. [Google Scholar]
- Kennedy, D.; Mujcin, M.; Trudeau, E.; Tezel, F.H. Pure and Binary Adsorption Equilibria of Methane and Nitrogen on Activated Carbons, Desiccants, and Zeolites at Different Pressures. J. Chem. Eng. Data 2016, 61, 3163–3176. [Google Scholar] [CrossRef]
- Sethia, G.; Somani, R.S.; Bajaj, H.C. Sorption of Methane and Nitrogen on Cesium Exchanged Zeolite-X: Structure, Cation Position and Adsorption Relationship. Ind. Eng. Chem. Res. 2014, 53, 6807–6814. [Google Scholar] [CrossRef]
- Mofarahi, M.; Bakhtyari, A. Experimental Investigation and Thermodynamic Modeling of CH4/N2 Adsorption on Zeolite 13X. J. Chem. Eng. Data 2015, 60, 683–696. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, X.; Sun, L.; Liu, X. Adsorption separation of carbon dioxide, methane and nitrogen on monoethanol amine modified β-zeolite. J. Nat. Gas Chem. 2009, 18, 167–172. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Wang, W.; Li, L.; Li, J. Adsorption of CO2, CH4, and N2 on 8-, 10-, and 12-Membered Ring Hydrophobic Microporous High-Silica Zeolites: DDR, Silicalite-1, and Beta. Ind. Eng. Chem. Res. 2013, 52, 17856–17864. [Google Scholar] [CrossRef]
- Shang, H.; Li, Y.; Liu, J.; Tang, X.; Yang, J.; Li, J. CH4/N2 separation on methane molecules grade diameter channel molecular sieves with a CHA-type structure. Chin. J. Chem. Eng. 2019, 27, 1044–1049. [Google Scholar] [CrossRef]
- Yang, J.; Tang, X.; Liu, J.; Wang, J.; Shang, H.; Wu, L.; Li, J.; Deng, S. Down-sizing the crystal size of ZK-5 zeolite for its enhanced CH4 adsorption and CH4/N2 separation performances. Chem. Eng. J. 2021, 406, 126599. [Google Scholar] [CrossRef]
- Yaqi, W.; Danhua, Y.; Zeng, S.; Yang, L.; Dong, X.; Zhang, Q.; Xu, Y.P.; Liu, Z. Significant enhancement in CH4/N2 separation with amine-modified zeolite Y. Fuel 2021, 301, 121077. [Google Scholar]
- Vosoughi, M.; Maghsoudi, H. Characterization of size-selective kinetic-based Ba-ETS-4 titanosilicate for nitrogen/methane separation: Chlorine-enhanced steric effects. Sep. Purif. Technol. 2021, 284, 120243. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J.; Shang, H.; Wu, L.; Yang, J. Gas diffusion and adsorption capacity enhancement via ultrasonic pretreatment for hydrothermal synthesis of K-KFI zeolite with nano/micro-scale crystals. Microporous Mesoporous Mater. 2020, 297, 110036. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Liu, P.; Li, L.; Tang, X.; Shang, H.; Li, J.; Chen, B. K-Chabazite Zeolite Nanocrystal Aggregates for Highly Efficient Methane Separation. Angewandte Chem. 2021, 61, 16850. [Google Scholar]
- Kuznicki, S.M.; Bell, V.A.; Nair, S.; Hillhouse, H.W.; Jacubinas, R.M.; Braunbarth, C.M.; Toby, B.H.; Tsapatsis, M. A titanosilicate molecular sieve with adjustable pores for size-selective adsorption of molecules. Nature 2001, 412, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, B.S.; Bhadra, S.J.; Marathe, R.; Farooq, S. Adsorption and Diffusion of Methane and Nitrogen in Barium Exchanged ETS-4. Ind. Eng. Chem. Res. 2011, 50, 3022–3035. [Google Scholar] [CrossRef]
- Jayaraman, A.; Hernández-Maldonado, A.J.; Yang, R.; Chinn, D.; Munson, C.L.; Mohr, D. Clinoptilolites for nitrogen/methane separation. Chem. Eng. Sci. 2004, 59, 2407–2417. [Google Scholar] [CrossRef]
- Sethia, G.; Somani, R.S.; Bajaj, H.C. Adsorption of carbon monoxide, methane and nitrogen on alkaline earth metal ion exchanged zeolite-X: Structure, cation position and adsorption relationship. RSC Adv. 2015, 5, 12773–12781. [Google Scholar] [CrossRef]
- Kennedy, D.; Tezel, F.H. Cation exchange modification of clinoptilolite-Screening analysis for potential equilibrium and kinetic adsorption separations involving methane, nitrogen, and carbon dioxide. Microporous Mesoporous Mater. 2018, 262, 235–250. [Google Scholar] [CrossRef]
- Hao, X.; Hu, H.; Li, Z.; Wu, L.; Liu, X.; Zhang, Y. Adsorption Properties of Modified Clinoptilolite for Methane and Nitrogen. Materials 2018, 10, 2024. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.; Mujcin, M.; Alenko, T.; Tezel, F.H. Pure and mixture adsorption equilibria of methane and nitrogen onto clinoptilolite: Effects of Cs+ and Fe3+ exchanged cations on separation performance. Adsorption 2019, 25, 135–158. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Chen, K.; Yao, J.; Zavabeti, A.; Liu, J.; Li, G.K. Screening of Alkali Metal-Exchanged Zeolites for Nitrogen/Methane Separation. Langmuir 2023, 39, 1277–1287. [Google Scholar] [CrossRef]
- Hu, G.; Xiao, G.; Guo, Y.; Manning, M.; Chen, L.; Yu, L.; Li, K.G.; May, E.F. Separation of methane and nitrogen using ionic liquidic zeolites by pressure vacuum swing adsorption. AlChE J. 2022, 68, 17668. [Google Scholar] [CrossRef]
- Zhao, J.; Mousavi, S.H.; Xiao, G.; Mokarizadeh, A.; Moore, T.; Chen, K.; Gu, Q.; Singh, R.; Zavabeti, A.; Liu, J.; et al. Nitrogen Rejection from Methane via a “Trapdoor” K-ZSM-25 Zeolite. J. Am. Chem. Soc. 2021, 143, 15195–15204. [Google Scholar] [CrossRef]
- Ghasemi, F.; Alizadeh, M.; Azamat, J.; Erfan-Niya, H. Understanding the performance of RHO type zeolite membrane for CH4/N2 separation based on molecular dynamics and deep neural network methods. J. Mol. Graph. Model. 2023, 127, 108673. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.T.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal-Organic Frameworks: Stable Metal-Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- Saha, D.; Bao, Z.; Jia, F.; Deng, S. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A. Environ. Sci. Technol. 2010, 5, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pellitero, J.; Amrouche, H.; Siperstein, F.R.; Pirngruber, G.D.; Nieto-Draghi, C.; Chaplais, G.; Simon-Masseron, A.; Bazer-Bachi, D.; Peralta, D.; Bats, N. Adsorption of CO2, CH4, and N2 on zeolitic imidazolate frameworks: Experiments and simulations. Chemistry 2010, 16, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Munusamy, K.; Sethia, G.; Patil, D.; Somayajulu Rallapalli, P.; Somani, R.S.; Bajaj, H.C. Sorption of carbon dioxide, methane, nitrogen and carbon monoxide on MIL-101(Cr): Volumetric measurements and dynamic adsorption studies. Chem. Eng. J. 2012, 195, 359–368. [Google Scholar] [CrossRef]
- Möllmer, J.; Lange, M.; Möller, A.C.; Patzschke, C.; Stein, K.; Lässig, D.; Lincke, J.; Gläser, R.; Krautscheid, H.; Staudt, R. Pure and mixed gas adsorption of CH4 and N2 on the metal–organic framework Basolite® A100 and a novel copper-based 1,2,4-triazolyl isophthalate MOF. J. Mater. Chem. 2012, 22, 10274–10286. [Google Scholar] [CrossRef]
- Li, R.; Li, M.; Zhou, X.; Ng, S.W.; O’Keeffe, M.; Li, D. ROD-8, a rod MOF with a pyrene-cored tetracarboxylate linker: Framework disorder, derived nets and selective gas adsorption. CrystEngComm 2014, 16, 6291–6295. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Y.; Sun, T.; Guo, Y.; Wei, X.; Zhao, S.; Wang, S. Water Resistant and Flexible MOF Materials for Highly Efficient Separation of Methane from Nitrogen. Ind. Eng. Chem. Res. 2019, 58, 20392–20400. [Google Scholar] [CrossRef]
- Zhang, F.; Shang, H.; Zhai, B.; Li, X.; Zhang, Y.; Wang, X.; Li, J.; Yang, J. Thermodynamic-Kinetic Synergistic Separation of CH4/N2 on A Robust Aluminum-Based Metal-Organic Framework. IChE J. 2022, 69, e18079. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, P.; Liu, J.; Shen, F.; Zhang, Y.; Chai, K.; Ying, Y.; Kang, C.; Zhang, Z.; Ji, H. Enhancing CH4/N2 separation performance within aluminum-based Metal-Organic Frameworks: Influence of the pore structure and linker polarity. Sep. Purif. Technol. 2022, 286, 120446. [Google Scholar] [CrossRef]
- Chang, M.; Ren, J.; Wei, Y.; Yan, T.; Wang, J.; Liu, D.; Chen, J. Discovery of a Scalable Metal–Organic Framework with a Switchable Structure for Efficient CH4/N2 Separation. Chem. Mater. 2023, 35, 4286–4296. [Google Scholar] [CrossRef]
- He, Y.; Xiang, S.; Zhang, Z.; Xiong, S.; Fronczek, F.; Krishna, R.; O’Keeffe, M.; Chen, B. A microporou lanthanide-tricarboxylate framework with the potential for purification of natural gas. Chem. Commun. 2012, 48, 10856–10858. [Google Scholar] [CrossRef]
- Ma, H.; Ren, H.; Zou, X.; Meng, S.; Sun, F.; Zhu, G. Post-metalation of porous aromatic frameworks for highly efficient carbon capture from CO2 + N2 and CH4 + N2 mixtures. Polym. Chem. 2014, 5, 144–152. [Google Scholar] [CrossRef]
- Hu, J.; Sun, T.; Liu, X.; Zhao, S.; Wang, S. Rationally tuning the separation performances of [M3(HCOO)6] frameworks for CH4/N2 mixtures via metal substitution. Microporous Mesoporous Mater. 2016, 225, 456–464. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, J.; Shang, H.; Bai, H.; Zhao, Y.Q.; Yang, J.; Dong, J.; Li, J. Effective CH4 enrichment from N2 by SIM-1 via a strong adsorption potential SOD cage. Sep. Purif. Technol. 2020, 230, 115850. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, S.Y.; Yoon, T.U.; Kim, M.B.; Park, W.; Han, H.H.; Kong, C.; Park, C.; Kim, J.; Bae, Y. Improved methane/nitrogen separation properties of zirconium-based metal–organic framework by incorporating highly polarizable bromine atoms. Chem. Eng. J. 2020, 399, 125717. [Google Scholar] [CrossRef]
- Wang, S.; Shivanna, M.; Yang, Q. Nickel-Based Metal-Organic Frameworks for Coal-Bed Methane Purification with Record CH4/N2 Selectivity. Angew. Chem. 2022, 61, e202201017. [Google Scholar] [CrossRef] [PubMed]
- Qadir, S.; Gu, Y.; Ali, S.; Li, D.; Zhao, S.; Wang, S.; Xu, H.; Wang, S. A thermally stable isoquinoline based ultra-microporous metal-organic framework for CH4 separation from coal mine methane. Chem. Eng. J. 2022, 428, 131136. [Google Scholar] [CrossRef]
- Chang, M.; Wang, F.; Wei, Y.; Yang, Q.; Wang, J.; Liu, D.; Chen, J. Separation of CH4/N2 by an Ultra-Stable Metal-Organic Framework with the Highest Brea. AlChE J. 2022, 68, 17794. [Google Scholar] [CrossRef]
- Guo, P.; Chen, Y.; Chang, M.; Li, Y.; Yang, Q.; Liu, D. A Stable Cu(I)-Based Ultramicroporous NKMOF-8-Br with High CH4 Uptake for Efficient Separation of CH4/N2 Mixtures. J. Chem. Eng. Data 2022, 67, 1654–1662. [Google Scholar] [CrossRef]
- Chen, R.; Li, J.; Zhou, F.; Sheng, B.; Sun, H.; Zheng, F.; Yang, Q.; Zhang, Z.; Ren, Q.; Bao, Z. Zr-Based Metal–Organic Framework with Wall-Shared Dual Ultramicroporous Channels for Effective CH4/N2 Separation. Ind. Eng. Chem. Res. 2023, 62, 13144–13152. [Google Scholar] [CrossRef]
- Chang, M.; Ren, J.; Yang, Q.; Liu, D. A robust calcium-based microporous metal-organic framework for efficient CH4/N2 separation. Chem. Eng. J. 2020, 408, 127294. [Google Scholar] [CrossRef]
- Li, L.; Yang, J.; Li, J.; Chen, Y.; Li, J. Adsorption and molecular simulation of CO2 and CH4 in two-dimensional metal–organic frameworks with the same layered substrate. CrystEngComm 2013, 15, 6782–6789. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Wang, J.; Zhang, Z.; Yang, Q.; Yang, Y.; Ren, Q.; Bao, Z. Highly efficient separation of methane from nitrogen on a squarate-based metal-organic framework. AlChE J. 2018, 64, 3681–3689. [Google Scholar] [CrossRef]
- Niu, Z.; Cui, X.; Pham, T.; Lan, P.C.; Xing, H.; Forrest, K.A.; Wojtas, L.; Space, B.; Ma, S. A Metal-Organic Framework Based Methane Nano-trap for the Capture of Coal-Mine Methane. Angew. Chem. 2019, 58, 1–5. [Google Scholar]
- Lv, D.; Wu, Y.; Chen, J.; Tu, Y.; Yuan, Y.; Wu, H.; Chen, Y.; Liu, B.; Xi, H.; Li, Z.; et al. Improving CH4/N2 selectivity within isomeric Al-based MOFs for the highly selective capture of coal-mine methane. AlChE J. 2020, 66, e16287. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, L.; Chen, R.; Zhang, Z.; Yang, Q.; Yang, Y.; Su, B.; Ren, Q.; Bao, Z. A robust two–dimensional layered metal–organic framework for efficient separation of methane from nitrogen. Sep. Purif. Technol. 2022, 281, 119911. [Google Scholar] [CrossRef]
- Fang, H.; Zhou, C.J.; Xu, S.; Shi, J.; Hu, Y.; Liu, G.; Wu, L. An effective approach for CH4/N2 separation using aluminum-based metal-organic frameworks (MOFs) prepared from coal fly ash. Fuel 2024, 360, 130562. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Yan, J.; Lv, D.; Chen, X.; Xu, F.; Zhou, X.; Li, Z. High-pressure separation performance of Ni(TMBDC)(DABCO)0.5 featured low-polarity channel for CH4/N2 mixture. Sep. Purif. Technol. 2023, 335, 126019. [Google Scholar] [CrossRef]
- Liu, P.; Fan, L.; Li, J.; Wu, Z.; Wang, Y.; Chen, Y.; Gao, J.; Yang, J.; Li, J.; Chen, B.; et al. A Microporous Titanium Metal–Organic Framework with Double Nanotraps for Record CH4/N2 Separation. Chem. Mater. 2024, 36, 2925–2932. [Google Scholar] [CrossRef]
- Feng, W.; Wu, H.; Jin, J.; Liu, D.; Meng, H.; Yun, J.; Mi, J. Transformation of Al-CDC from 3D crystals to 2D nanosheets in macroporous polyacrylates with enhanced CH4/N2 separation efficiency and stability. Chem. Eng. J. 2022, 429, 132285. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, J.; Liu, X.; Sun, T.; Zhao, S.; Wang, S. Scalable solvent-free preparation of [Ni3(HCOO)6] frameworks for highly efficient separation of CH4 from N2. Chem. Eng. J. 2017, 327, 564–572. [Google Scholar] [CrossRef]
- Knaebel, K.S.; Hill, F.B. Pressure swing adsorption: Development of an equilibrium theory for gas separations. Chem. Eng. Sci. 1985, 40, 2351–2360. [Google Scholar] [CrossRef]
- Serbezov, A.; Sotirchos, S.V. Particle-bed model for multicomponent adsorption-based separations: Application to pressure swing adsorption. Chem. Eng. Sci. 1999, 54, 5647–5666. [Google Scholar] [CrossRef]
- Golubyatnikov, O.O.; Akulinin, E.I.; Dvoretsky, S.I.; Dvoretsky, D.S. To the problem of forming the equation system for pressure swing adsorption mathematical model. Chem. Prod. Process Model. 2021, 17, 681–699. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).