Olea europaea L. Leaves as a Source of Anti-Glycation Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of OPA-EXTs
2.2. Characterization of TTP70 Extract
2.3. The Anti-Glycation Role of Olea europea L. Leaf Extracts
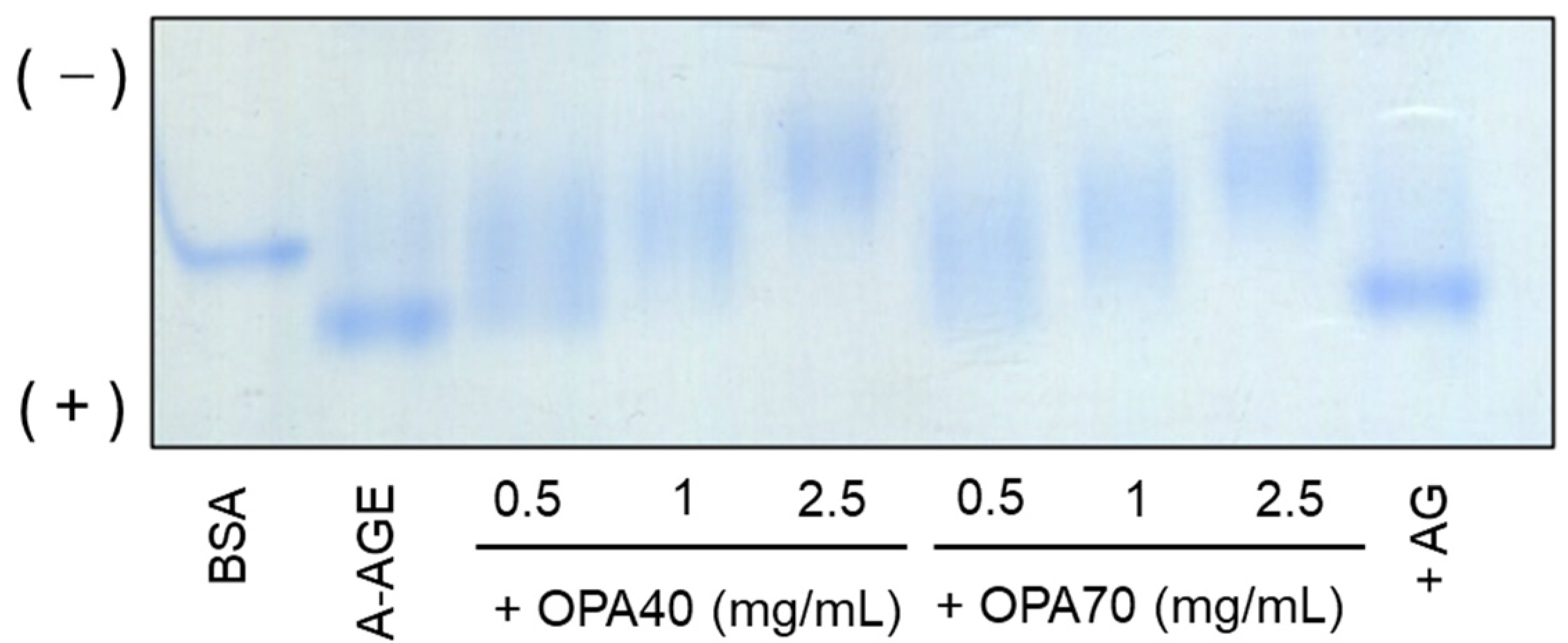
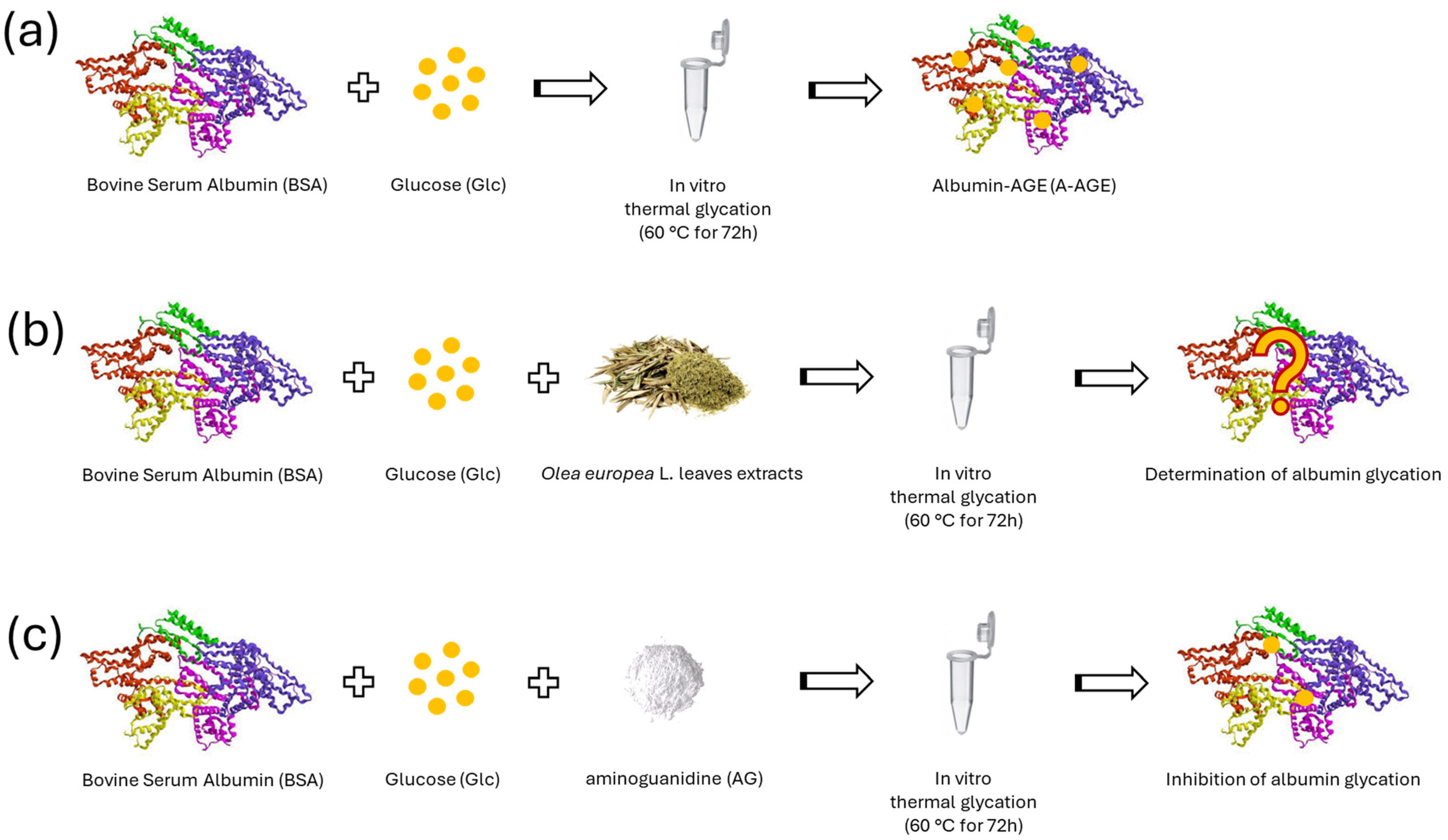
2.3.1. The Inhibitory Effect of OPA-EXTs in the In Vitro Albumin–Glycation System (A-AGE)
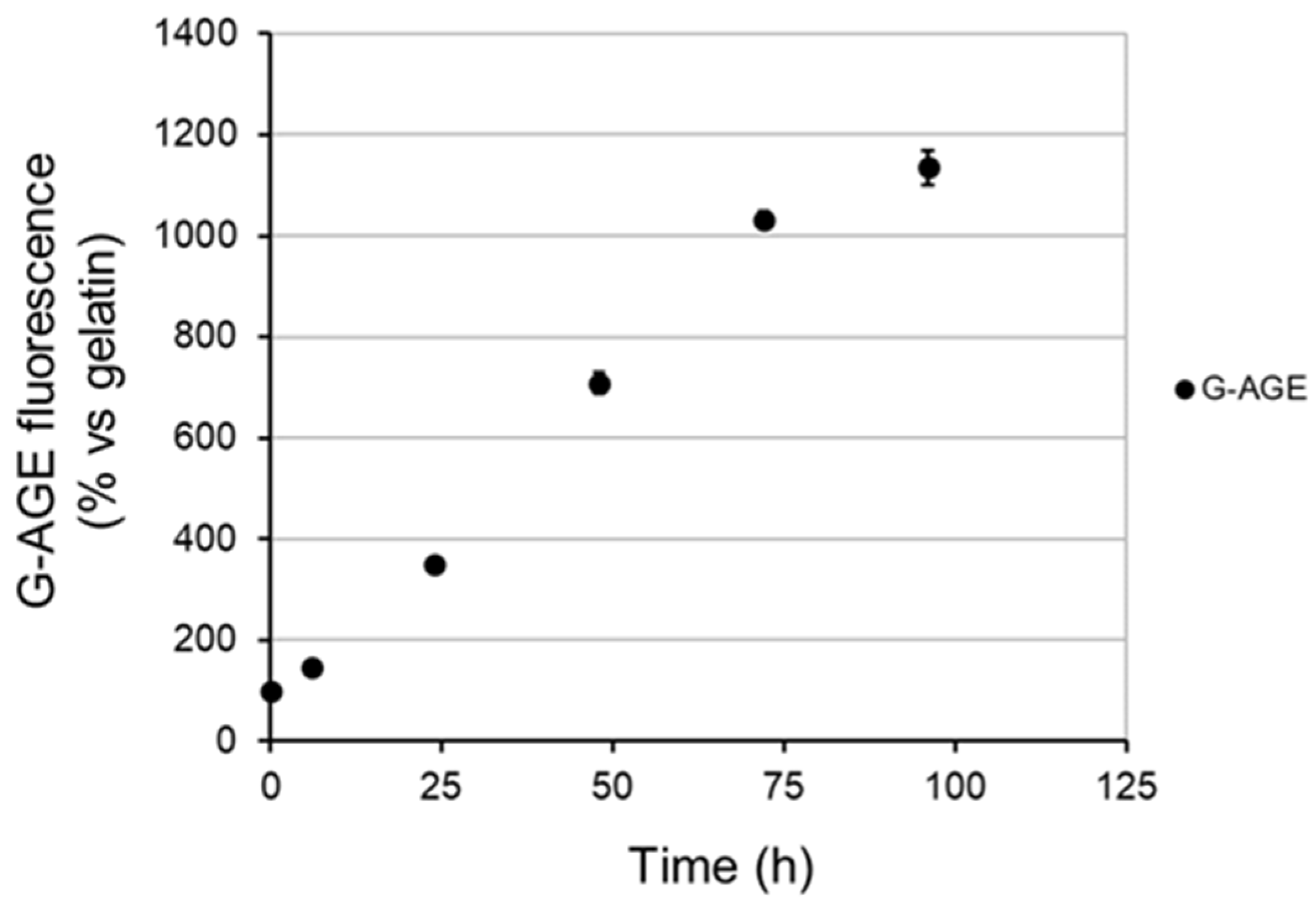
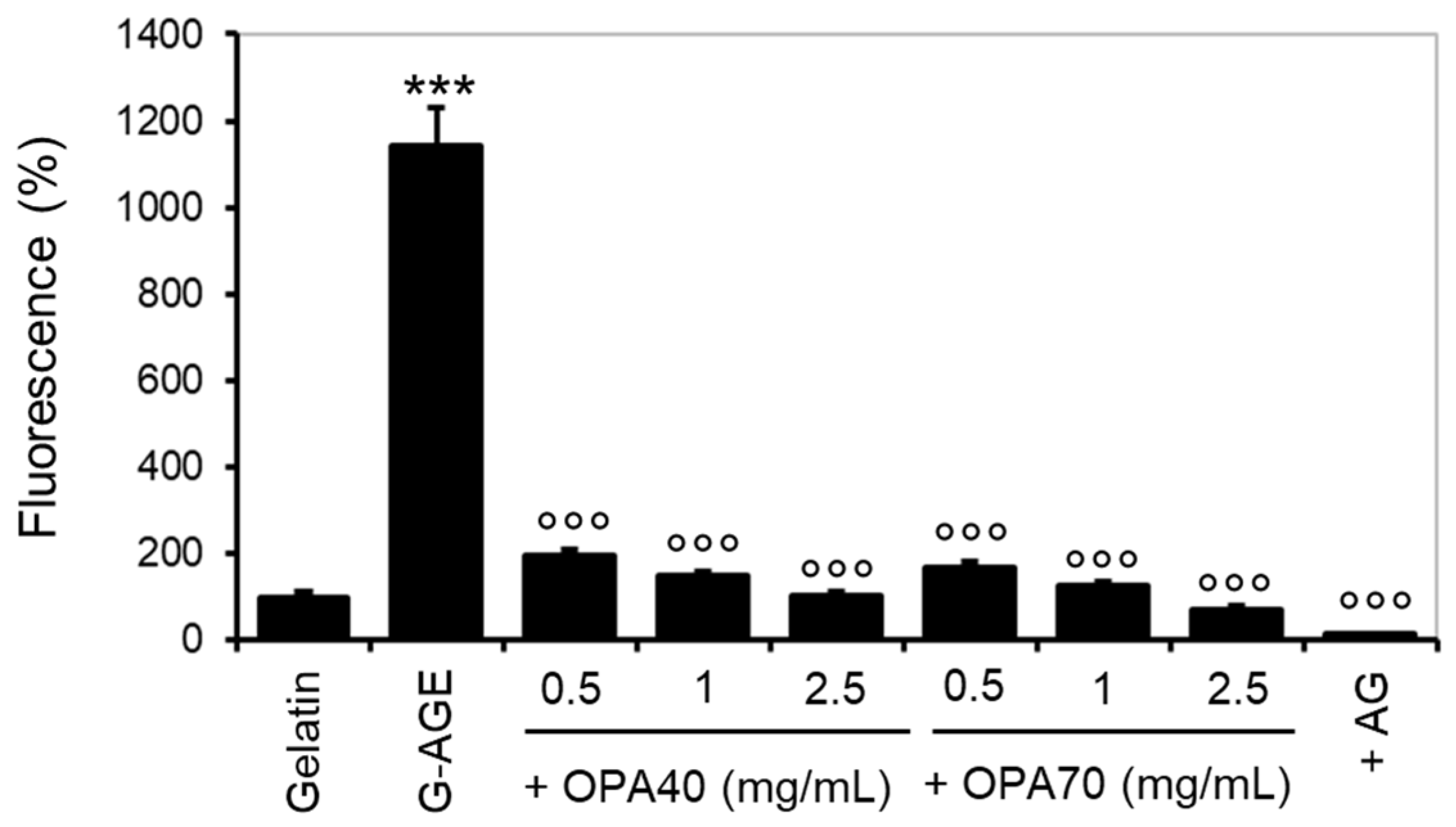
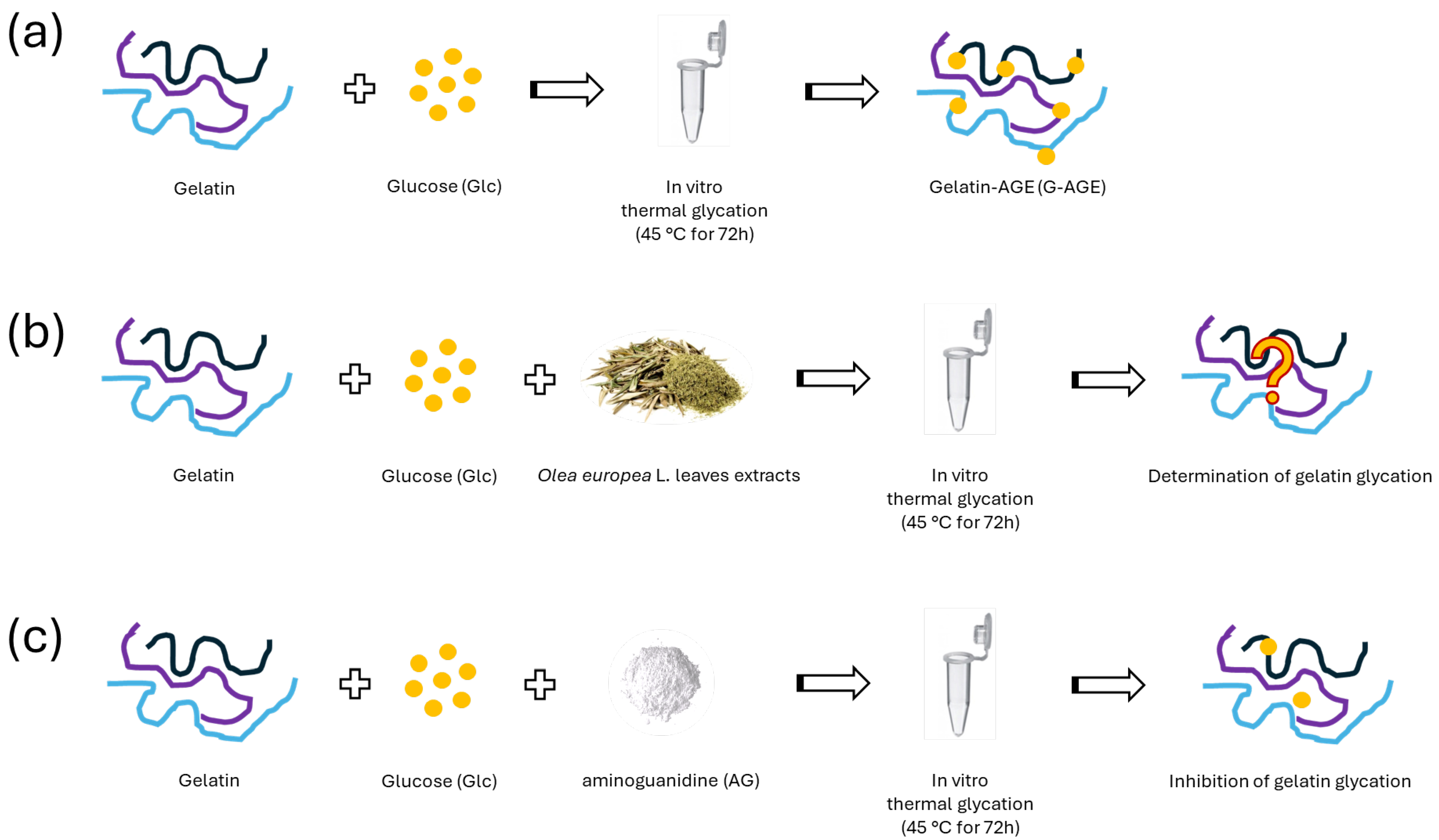
2.3.2. The Inhibitory Effect of OPA-EXTs in the In Vitro Gelatin–Glycation System (G-AGE)
2.3.3. The Inhibitory Effect of TTP70 Compared with OA in the In Vitro Albumin Glycation System (A-AGE)
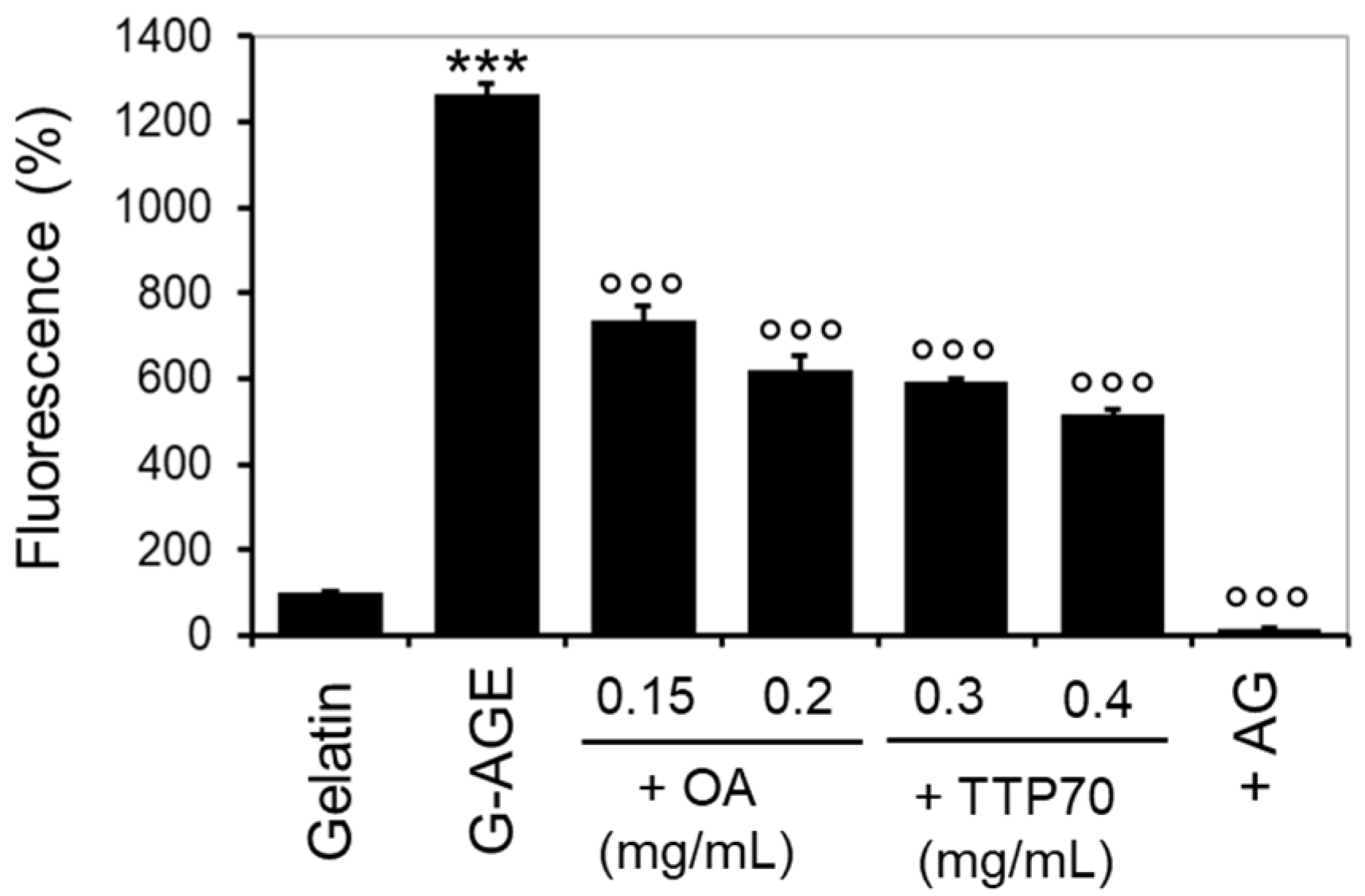
2.3.4. The Inhibitory Effect of TTP70 Compared with OA in the In Vitro Gelatin Glycation System (G-AGE)
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Olea europaea L. Extracts (EXTs)
3.2.1. TTP70 Preparation
3.2.2. OPA40 and OPA70 Preparation
3.3. HPLC-DAD Analysis
3.4. In Vitro AGE Formation by Albumin–Glucose System (A-AGE)
3.5. In Vitro AGE Formation by Gelatin–Glucose System (G-AGE)
3.6. Native Polyacrylamide Gel Electrophoresis (N-PAGE)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.S.; Jacob-Dolan, J.W.; Pham, V.T.T.; Sjoblom, N.M.; Scheck, R.A. The chemical language of protein glycation. Nat. Chem. Biol. 2024, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Zgutka, K.; Tkacz, M.; Tomasiak, P.; Tarnowski, M. A Role for Advanced Glycation End Products in Molecular Ageing. Int. J. Mol. Sci. 2023, 24, 9881. [Google Scholar] [CrossRef]
- Pal, R.; Bhadada, S.K. AGEs accumulation with vascular complications, glycemic control and metabolic syndrome: A narrative review. Bone 2023, 176, 116884. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Schröter, D.; Schreiner, M.; Grune, T. Dietary advanced glycation end products and their relevance for human health. Ageing Res. Rev. 2018, 47, 55–66. [Google Scholar] [CrossRef]
- Garay-Sevilla, M.E.; Rojas, A.; Portero-Otin, M.; Uribarri, J. Dietary AGEs as Exogenous Boosters of Inflammation. Nutrients 2021, 13, 2802. [Google Scholar] [CrossRef]
- Van der Lugt, T.; Weseler, A.R.; Gebbink, W.A.; Vrolijk, M.F.; Opperhuizen, A.; Bast, A. Dietary Advanced Glycation Endproducts Induce an Inflammatory Response in Human Macrophages in Vitro. Nutrients 2018, 10, 1868. [Google Scholar] [CrossRef]
- Davis, K.E.; Prasad, C.; Vijayagopal, P.; Juma, S.; Imrhan, V. Advanced Glycation End Products, Inflammation, and Chronic Metabolic Diseases: Links in a Chain? Crit. Rev. Food Sci. Nutr. 2014, 56, 989–998. [Google Scholar] [CrossRef]
- Uceda, A.B.; Mariño, L.; Casasnovas, R.; Adrover, M. An overview on glycation: Molecular mechanisms, impact on proteins, pathogenesis, and inhibition. Biophys. Rev. 2024, 16, 189–218. [Google Scholar] [CrossRef] [PubMed]
- Bansode, S.; Bashtanova, U.; Li, R.; Clark, J.; Müller, K.H.; Puszkarska, A.; Goldberga, I.; Chetwood, H.H.; Reid, D.G.; Colwell, L.J.; et al. Glycation changes molecular organization and charge distribution in type I collagen fibrils. Sci. Rep. 2020, 10, 3397. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Aryal, P.; Darkwah, E.K. Advanced Glycation End Products in Health and Disease. Microorganisms 2022, 10, 1848. [Google Scholar] [CrossRef] [PubMed]
- Sourris, K.C.; Watson, A.; Jandeleit-Dahm, K. Inhibitors of Advanced Glycation End Product (AGE) Formation and Accumulation. Handb. Exp. Pharmacol. 2021, 264, 395–423. [Google Scholar] [CrossRef]
- Reddy, V.P.; Beyaz, A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov. Today 2006, 11, 646–654. [Google Scholar] [CrossRef]
- Velichkova, S.; Foubert, K.; Pieters, L. Natural Products as a Source of Inspiration for Novel Inhibitors of Advanced Glycation Endproducts (AGEs) Formation. Planta Medica 2021, 87, 780–801. [Google Scholar] [CrossRef]
- Guo, Z.; Jia, X.; Zheng, Z.; Lu, X.; Zheng, Y.; Zheng, B.; Xiao, J. Chemical composition and nutritional function of olive (Olea europaea L.): A review. Phytochem. Rev. 2018, 17, 1091–1110. [Google Scholar] [CrossRef]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef]
- Markhali, F.S.; Teixeira, J.A.; Rocha, C.M.R. Olive Tree Leaves—A Source of Valuable Active Compounds. Processes 2020, 8, 1177. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Gegechkori, V.; Mohammed, E.U.R.; Ku, H.; Morton, D.W. Isolation of Bioactive Pentacyclic Triterpenoid Acids from Olive Tree Leaves with Flash Chromatography. Appl. Sci. 2022, 12, 996. [Google Scholar] [CrossRef]
- Stiti, N.; Hartmann, M.A. Nonsterol Triterpenoids as Major Constituents of Olea europaea. J. Lipids 2012, 476595. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Pérez-Jiménez, A.; García-Salguero, L.; Mokhtari, K.; Reyes-Zurita, F.J.; Peragón-Sánchez, J.; Lupiáñez, J.A. Nutraceutical Role of Polyphenols and Triterpenes Present in the Extracts of Fruits and Leaves of Olea europaea as Antioxidants, Anti-Infectives and Anticancer Agents on Healthy Growth. Molecules 2022, 27, 2341. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quesada, C.; López-Biedma, A.; Warleta, F.; Campos, M.; Beltrán, G.; Gaforio, J.J. Bioactive properties of the main triterpenes found in olives, virgin olive oil, and leaves of Olea europaea. J. Agric. Food Chem. 2013, 61, 12173–12182. [Google Scholar] [CrossRef]
- Guinda, A.; Rada, M.; Delgado, T.; Gutiérrez-Adánez, P.; Castellano, J.M. Pentacyclic triterpenoids from olive fruit and leaf. J. Agric. Food Chem. 2010, 58, 9685–9691. [Google Scholar] [CrossRef]
- Wasim, M.; Bergonzi, M.C. Unlocking the Potential of Oleanolic Acid: Integrating Pharmacological Insights and Advancements in Delivery Systems. Pharmaceutics 2024, 16, 692. [Google Scholar] [CrossRef]
- Luo, Q.; Wei, Y.; Lv, X.; Chen, W.; Yang, D.; Tuo, Q. The Effect and Mechanism of Oleanolic Acid in the Treatment of Metabolic Syndrome and Related Cardiovascular Diseases. Molecules 2024, 29, 758. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Leri, M.; Vasarri, M.; Barletta, E.; Schiavone, N.; Bergonzi, M.C.; Bucciantini, M.; Degl’Innocenti, D. The Protective Role of Oleuropein Aglycone against Pesticide-Induced Toxicity in a Human Keratinocytes Cell Model. Int. J. Mol. Sci. 2023, 24, 14553. [Google Scholar] [CrossRef]
- Topuz, S.; Bayram, M. Oleuropein Extraction from Leaves of Three Olive Varieties (Olea europaea L.): Antioxidant and Antimicrobial Properties of Purified Oleuropein and Oleuropein Extracts. J. Food Process. Preserv. 2022, 46, e15697. [Google Scholar] [CrossRef]
- Souilem, S.; El-Abbassi, A.; Kiai, H.; Hafidi, A.; Sayadi, S.; Galanakis, C.M. Chapter 1—Olive oil production sector: Environmental effects and sustainability challenges. In Olive Mill Waste: Recent Advances for Sustainable Management; Galanakis, C.M., Waste, O.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–28. [Google Scholar] [CrossRef]
- Vasarri, M.; Barletta, E.; Ramazzotti, M.; Degl’Innocenti, D. In vitro anti-glycation activity of the marine plant Posidonia oceanica (L.) Delile. J. Ethnopharmacol. 2020, 259, 112960. [Google Scholar] [CrossRef] [PubMed]
- Vasarri, M.; Barletta, E.; Vinci, S.; Ramazzotti, M.; Francesconi, A.; Manetti, F.; Degl’Innocenti, D. Annona cherimola Miller Fruit as a Promising Candidate against Diabetic Complications: An In Vitro Study and Preliminary Clinical Results. Foods 2020, 9, 1350. [Google Scholar] [CrossRef] [PubMed]
- Rabilloud, T. Solubilisation of proteins for electrophoretic analyses. Electrophoresis 1996, 17, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive). Evid. Based Complement. Alternat. Med. 2015, 2015, 541591. [Google Scholar] [CrossRef]
- Paul, R.G.; Bailey, A.J. Glycation of collagen: The basis of its central role in the late complications of ageing and diabetes. Int. J. Biochem. Cell Biol. 1996, 28, 1297–1310. [Google Scholar] [CrossRef]
- Pringle, N.A.; van de Venter, M.; Koekemoer, T.C. Gelatin as a convenient surrogate protein to model the in vitro effects of advanced glycation end-product formation. Exp. Dermatol. 2018, 27, 1309–1311. [Google Scholar] [CrossRef]
- Bondarenko, L.B. Diabetes and Collagen: Interrelations. Avicenna J. Med. Biochem. 2019, 7, 64–71. [Google Scholar] [CrossRef]
- Rada, M.; Ruiz-Gutiérrez, V.; Guinda, Á. Determination of triterpenic acids in human serum by high-performance liquid chromatography: Triterpenoid interaction with serum protein. J. Agric. Food Chem. 2011, 59, 2308–2313. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Xu, J.; Li, J.; Cheng, G.; Cai, J.; Dang, J.; Yu, M.; Wang, W.; Duan, W.; et al. Inhibitory Effects of Myriocin on Non-Enzymatic Glycation of Bovine Serum Albumin. Molecules 2022, 27, 6995. [Google Scholar] [CrossRef]
- De Stefani, C.; Vasarri, M.; Salvatici, M.C.; Grifoni, L.; Quintela, J.C.; Bilia, A.R.; Degl’Innocenti, D.; Bergonzi, M.C. Microemulsions Enhance the In Vitro Antioxidant Activity of Oleanolic Acid in RAW 264.7 Cells. Pharmaceutics 2022, 14, 2232. [Google Scholar] [CrossRef]
- Vasarri, M.; Degl’Innocenti, D.; Albonetti, L.; Bilia, A.R.; Bergonzi, M.C. Pentacyclic Triterpenes from Olive Leaves Formulated in Microemulsion: Characterization and Role in De Novo Lipogenesis in HepG2 Cells. Int. J. Mol. Sci. 2023, 24, 12113. [Google Scholar] [CrossRef] [PubMed]
- García-Estévez, I.; Alcalde-Eon, C.; Escribano-Bailón, M.T. Flavanol Quantification of Grapes via Multiple Reaction Monitoring Mass Spectrometry. Application to Differentiation among Clones of Vitis vinifera L. cv. Rufete Grapes. Agric. Food Chem. 2017, 65, 6359–6368. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Piné, R.; Lozano-Sánchez, J.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-ESI-QTOF-MS as a powerful analytical tool for characterising phenolic compounds in olive-leaf extracts. Phytochem. Anal. 2013, 24, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Nicolì, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellis, L. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea europaea L. Cultivar Leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef]
- Laguerre, M.; Lόpez Giraldo, L.J.; Piombo, G.; Figueroa-Espinoza, M.C.; Pina, M.; Benaissa, M.; Combe, A.; Rossignol Castera, A.; Lecomte, J.; Villeneuve, P. Characterization of Olive-Leaf Phenolics by ESI-MS and Evaluation of their Antioxidant Capacities by the CAT Assay. J. Am. Oil Chem. Soc. 2009, 86, 1215–1225. [Google Scholar] [CrossRef]
- Bergonzi, M.C.; De Stefani, C.; Vasarri, M.; Ivanova Stojcheva, E.; Ramos-Pineda, A.M.; Baldi, F.; Bilia, A.R.; Degl’Innocenti, D. Encapsulation of Olive Leaf Polyphenol-Rich Extract in Polymeric Micelles to Improve Its Intestinal Permeability. Nanomaterials 2023, 13, 3147. [Google Scholar] [CrossRef]
- Séro, L.; Sanguinet, L.; Blanchard, P.; Dang, B.T.; Morel, S.; Richomme, P.; Séraphin, D.; Derbré, S. Tuning a 96-Well Microtiter Plate Fluorescence-Based Assay to Identify AGE Inhibitors in Crude Plant Extracts. Molecules 2013, 18, 14320–14339. [Google Scholar] [CrossRef]


| Compound | OPA40 | OPA70 |
|---|---|---|
| Oleuropein | 41.67 | 66.59 |
| Hydroxytyrosol | 0.16 | 0.31 |
| Verbascoside | 1.18 | 1.13 |
| Oleuroside | 4.39 | 5.85 |
| Luteolin | 0.02 | 0.04 |
| Luteolin-7-O-glucoside | 1.05 | 0.69 |
| Luteolin-4-O-glucoside | 0.46 | 0.44 |
| Luteolin diglucoside | 0.06 | 0 |
| Apigenin-7-O-glucoside | 0.17 | 0.27 |
| Apigenin-7-O-rutinoside | 0.13 | 0 |
| 7-epilogalin | 0.30 | 0 |
| Elenolic acid glucoside | 0.04 | 0 |
| Total | 49.63 | 75.32 |
| Compound | %w/w |
|---|---|
| Oleanolic acid | 36.73 |
| Maslinic acid | 14.41 |
| Uesolic acid | 8.63 |
| Erythrodiol | 0.97 |
| Uvaol | 4.61 |
| Total | 65.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasarri, M.; Bergonzi, M.C.; Ivanova Stojcheva, E.; Bilia, A.R.; Degl’Innocenti, D. Olea europaea L. Leaves as a Source of Anti-Glycation Compounds. Molecules 2024, 29, 4368. https://doi.org/10.3390/molecules29184368
Vasarri M, Bergonzi MC, Ivanova Stojcheva E, Bilia AR, Degl’Innocenti D. Olea europaea L. Leaves as a Source of Anti-Glycation Compounds. Molecules. 2024; 29(18):4368. https://doi.org/10.3390/molecules29184368
Chicago/Turabian StyleVasarri, Marzia, Maria Camilla Bergonzi, Emilija Ivanova Stojcheva, Anna Rita Bilia, and Donatella Degl’Innocenti. 2024. "Olea europaea L. Leaves as a Source of Anti-Glycation Compounds" Molecules 29, no. 18: 4368. https://doi.org/10.3390/molecules29184368
APA StyleVasarri, M., Bergonzi, M. C., Ivanova Stojcheva, E., Bilia, A. R., & Degl’Innocenti, D. (2024). Olea europaea L. Leaves as a Source of Anti-Glycation Compounds. Molecules, 29(18), 4368. https://doi.org/10.3390/molecules29184368








