Synthesis and Characterization of Novel 2-Alkyl-1,3,4-Oxadiazoles Containing a Phenylazo Group
Abstract
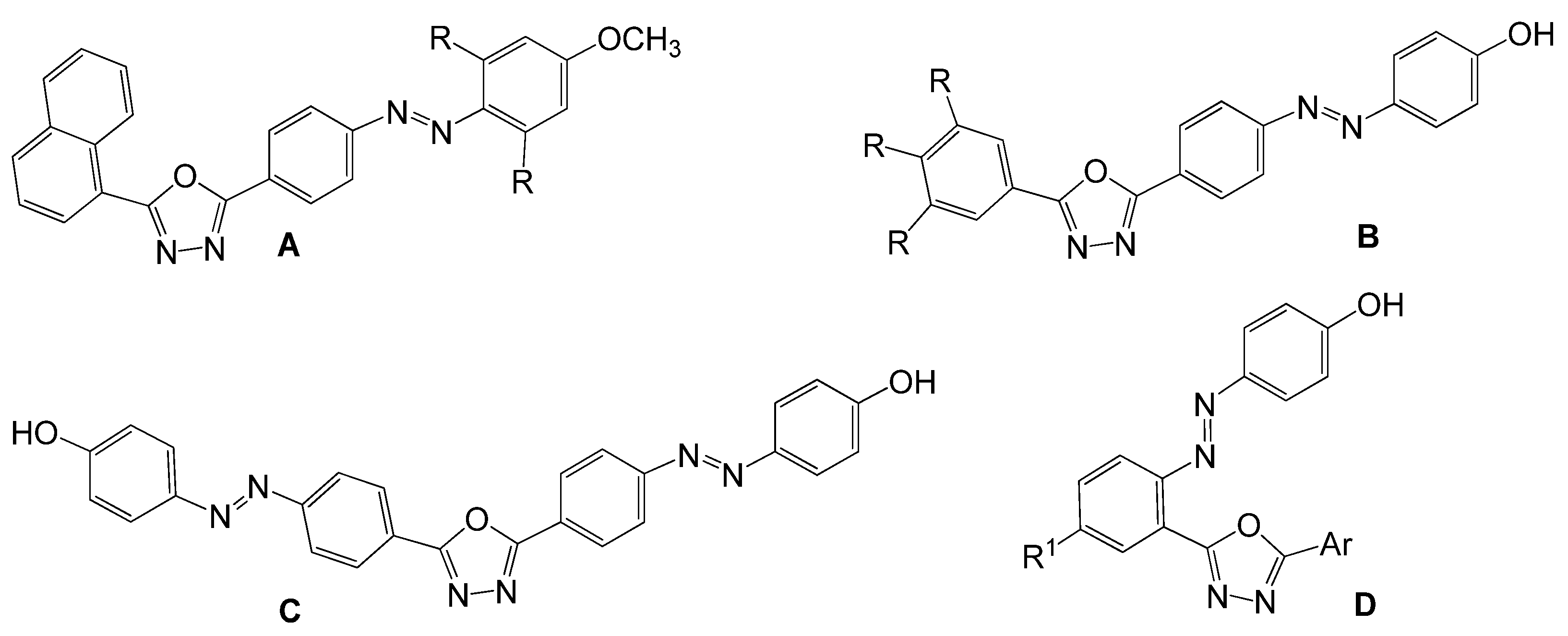
1. Introduction
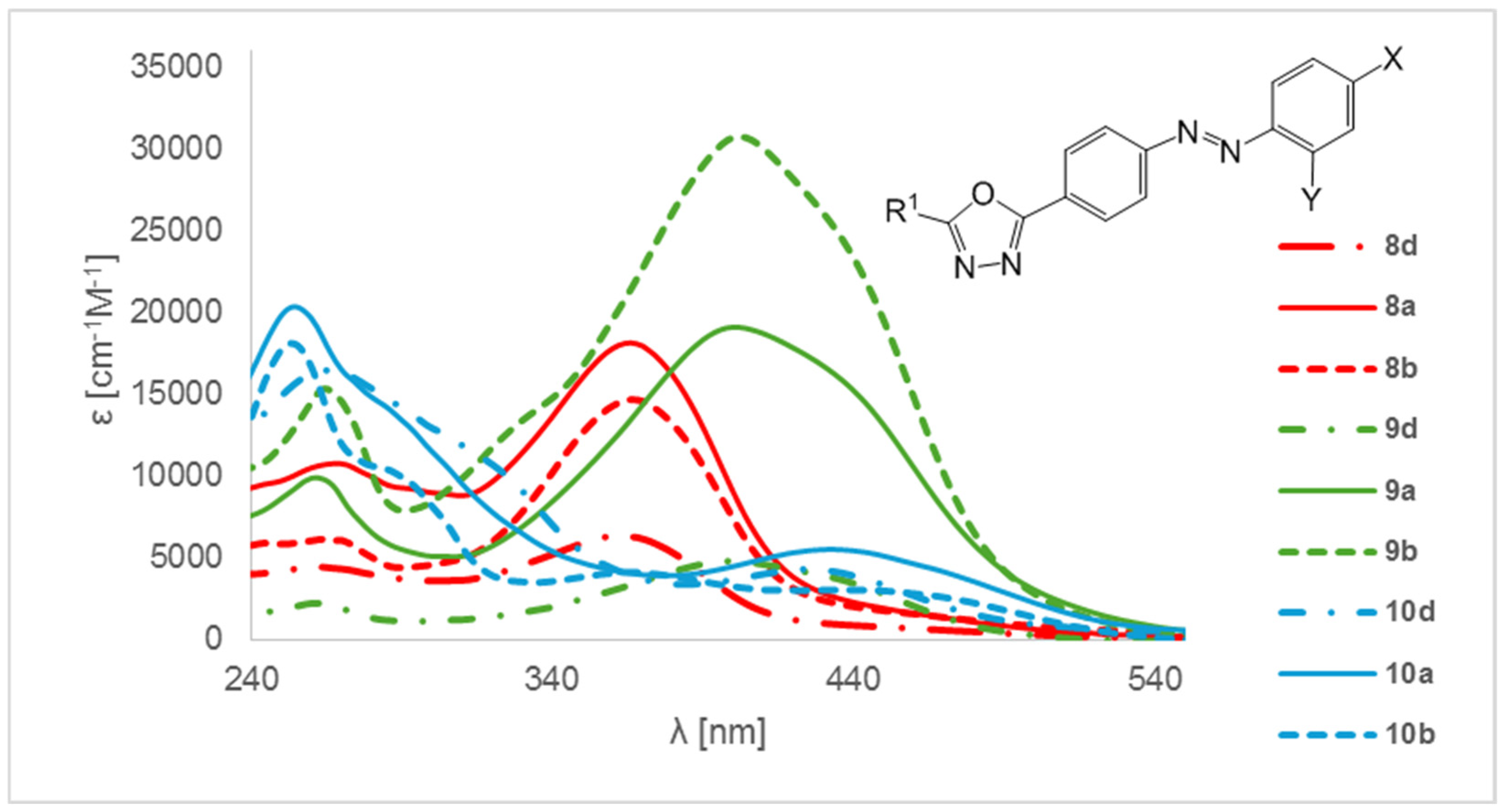
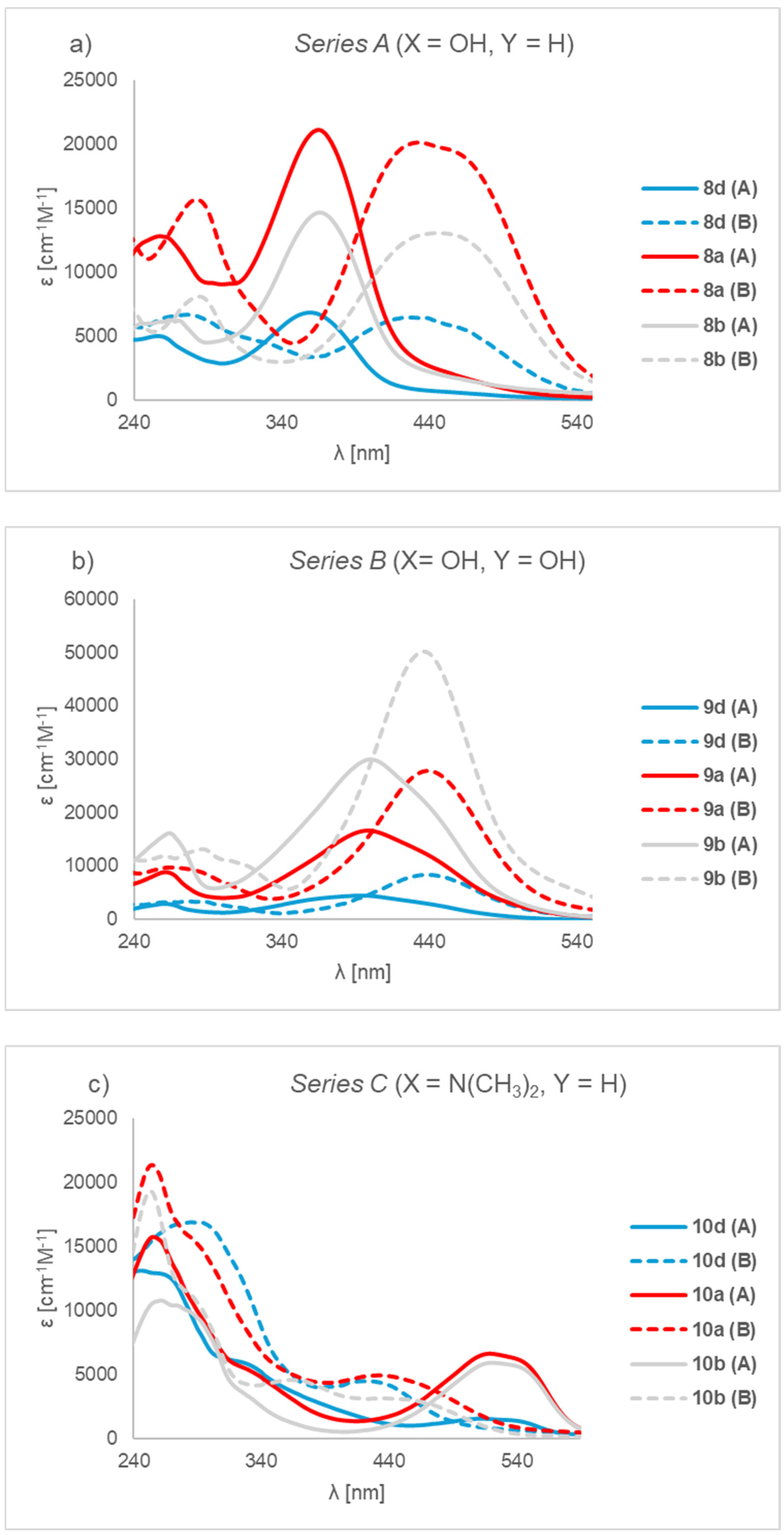
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization
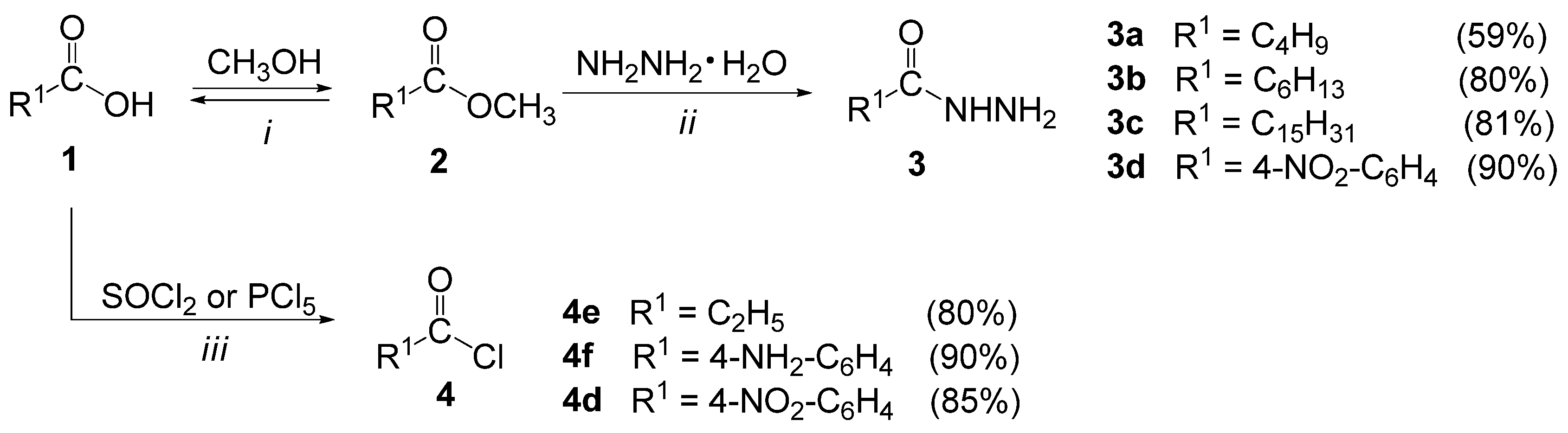
3.2.1. General Procedure for the Synthesis of Methyl Esters (2a–d)
- Methyl valerate (2a). The product was obtained as a colorless liquid (24.40 g, 84%) [55].
- Methyl heptanoate (2b). The product was obtained as a colorless liquid (34.20 g, 95%) [56].
- Methyl palmitate (2c). The product was obtained as a white solid (54.50 g, 85%); mp 29–30 °C (28–30 °C [57]).
- Methyl 4-nitrobenzoate (2d). The product was obtained as yellow solid (39.80 g, 88%); mp 101–102 °C (99–100 °C [58]).
3.2.2. General Procedure for the Synthesis of Acid Hydrazides (3a–d)
- Valeryl hydrazide (3a). The product was obtained as a white solid (13.70 g, 59%); mp 63–64 °C (50.5–51.5 °C [59]).
- Heptanehydrazide (3b). The product was obtained as a white solid (23.00 g, 80%); mp 87–88 °C (82–84 °C [60]).
- Palmitohydrazide (3c). The product was obtained as a white solid (43.80 g, 81%); mp 112–114 °C (112–113 °C [61]).
- 4-Nitrobenzohydrazide (3d). The product was obtained as a yellow solid (30.80 g, 85%); mp 220–221 °C (217–218 °C [62]).
3.2.3. General Procedure for the Synthesis of Acid Chlorides (4d–f)
- Propionyl chloride (4e). The product was obtained as a colorless liquid (14.80 g, 80%); bp 78–80 °C (bp 77–79 °C [63]).
- 4-Aminobenzoyl chloride (4f). The product was obtained as a yellow solid (27.30 g, 90%); bp 168–170 °C/33 mm Hg, mp 35–36 °C (bp 168–170 °C/33 mm Hg, mp 31–39 °C [64]).
- 4-Nitrobenzoyl chloride (4d). The product was obtained as a yellow solid (31.50 g, 85%); bp 175–180 °C/25 mm Hg, mp 71–74 °C (bp 194–196 °C/25 mm Hg, mp 71–73 °C [65]).
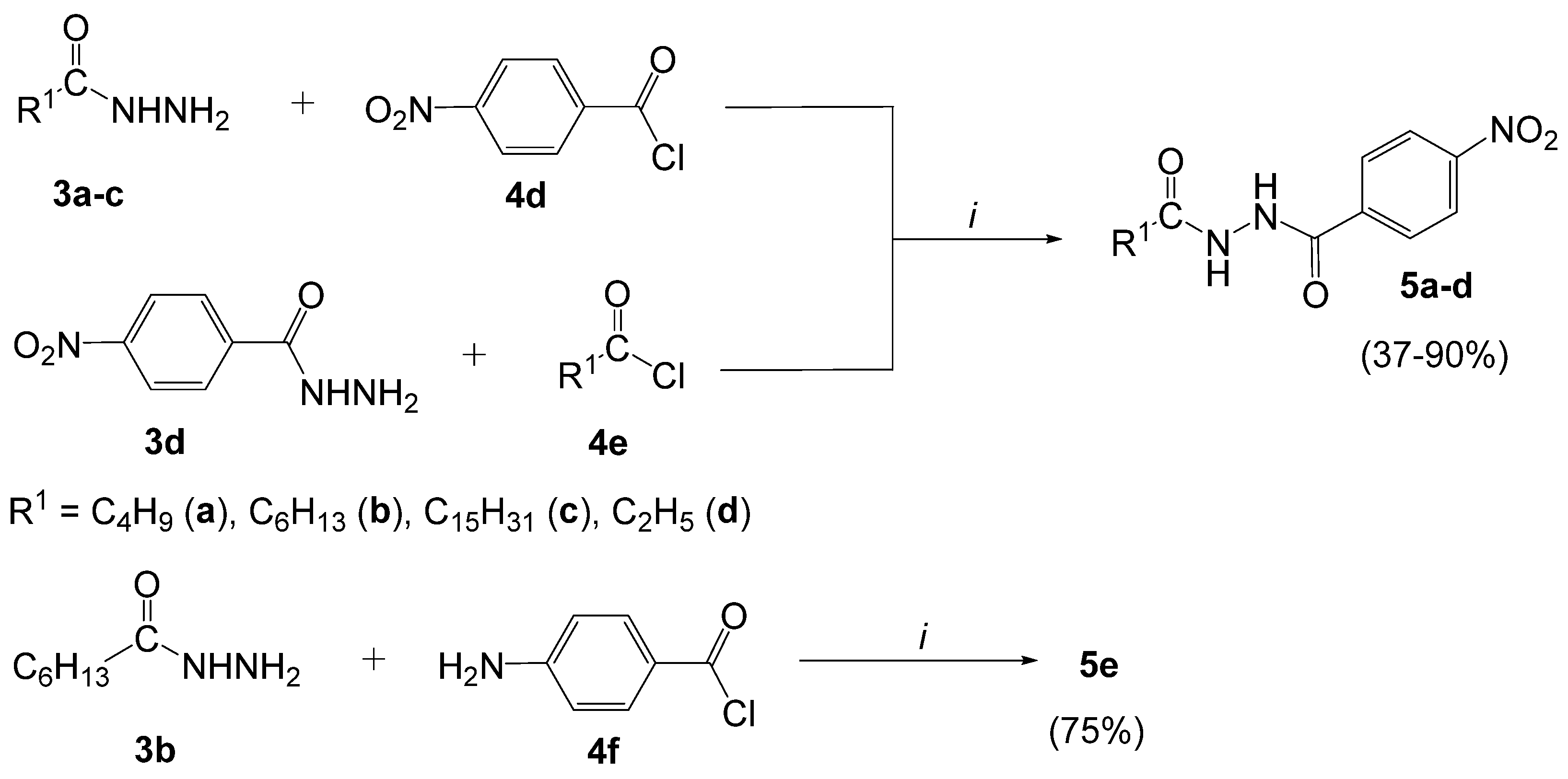
3.2.4. General Procedure for the Synthesis of N,N′-Diacylhydrazines (5a–e)
- 4-Nitro-N′-pentanoylbenzohydrazide (5a). The product was obtained as a white solid (4.70 g, 90%); mp 187–188 °C (188–190 °C [52]). UV-Vis (CH3OH) λmax (logε) 202 (4.19), 262 (4.12) nm.
- N′-Heptanoyl-4-nitrobenzohydrazide (5b). The product was obtained as awhite solid (5.30 g, 88%); mp 181–182 °C. 1H-NMR (400 MHz, DMSO-d6): δ 10.63 (s, 1H, NH), 9.96 (s, 1H, NH), 8.34 (d, 2H, J = 8.8 Hz, Ar), 8.09 (d, 2H, J = 8.8 Hz, Ar), 2.20 (t, 2H, J = 7.2 Hz, CH2), 1.54 (qui, 2H, J = 7.2 Hz, CH2), 1.29−1.34 (m, 6H, CH2), 0.88 (t, 3H, J = 6.8 Hz, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 171.5, 163.9, 149.3, 138.2, 128.9, 123.6, 33.2, 31.0, 28.2, 25.0, 22.0, 13.9; IR (ATR) νmax: 3194 (N-H), 3030, 2960, 2926, 2854, 1609 (N-H), 1592 (N-H), 1509 (N-O), 1349 (C-N), 1212, 867, 847 (N-O), 716 cm−1; HRMS calcd for (C14H19N3O4+H+): 294.1454; found: 294.1458. UV-Vis (CH3OH) λmax (logε) 214 (3.98), 266 (4.09) nm.
- 4-Nitro-N′-hexadecanoylbenzohydrazide (5c). The product was obtained as a white solid (7.10 g, 85%); mp 160–161 °C). IR (ATR) νmax: 3211 (N-H), 2917, 2848, 1609 (N-H), 1592 (N-H), 1574, 1522 (N-O), 1464, 1349 (C-N), 1210, 872, 846 (N-O), 719 cm−1; HRMS calcd for (C23H36N3O4+H+): 420.2862; found: 420.2843.
- N′-Propionyl-4′-nitrobenzohydrazide (5d). The product was obtained as a white solid (1.80 g, 37%); mp 192–193 °C (203–205 °C [52]). UV-Vis (CH3OH) λmax (logε) 262 (4.11) nm.
- 4-Amino-N′-heptanoylbenzohydrazide (5e). The product was obtained as a white solid (3.90 g, 75%); mp 188–189 °C. 1H-NMR (400 MHz, DMSO-d6): δ 9.75 (s, 1H, NH), 9.60 (s, 1H, NH), 7.59 (d, 2H, J = 8.4 Hz, Ar), 6.53 (d, 2H, J = 8.4 Hz, Ar), 5.69 (s, 2H, NH2), 2.14 (t, 2H, J = 7.6 Hz, CH2), 1.51 (qui, 2H, J = 7.6 Hz, CH2), 1.21–1.33 (m, 6H, CH2), 0.87 (t, 3H, J = 6.8 Hz, CH3), 13C NMR (100 MHz, DMSO-d6): δ 171.6, 165.5, 152.1, 129.0, 119.0, 112.5, 33.3, 31.0, 28.2, 25.1, 22.0, 13.4; IR (ATR) νmax: 3463 (N-H), 3355 (N-H), 3209 (N-H), 2924, 1695, 1598 (N-H), 1486, 1307, 1180, 724 cm−1; HRMS calcd for (C14H21N3O2+H+): 264.1712; found: 264.1722. UV-Vis (CH3OH) λmax (logε) 288 (4.38) nm.
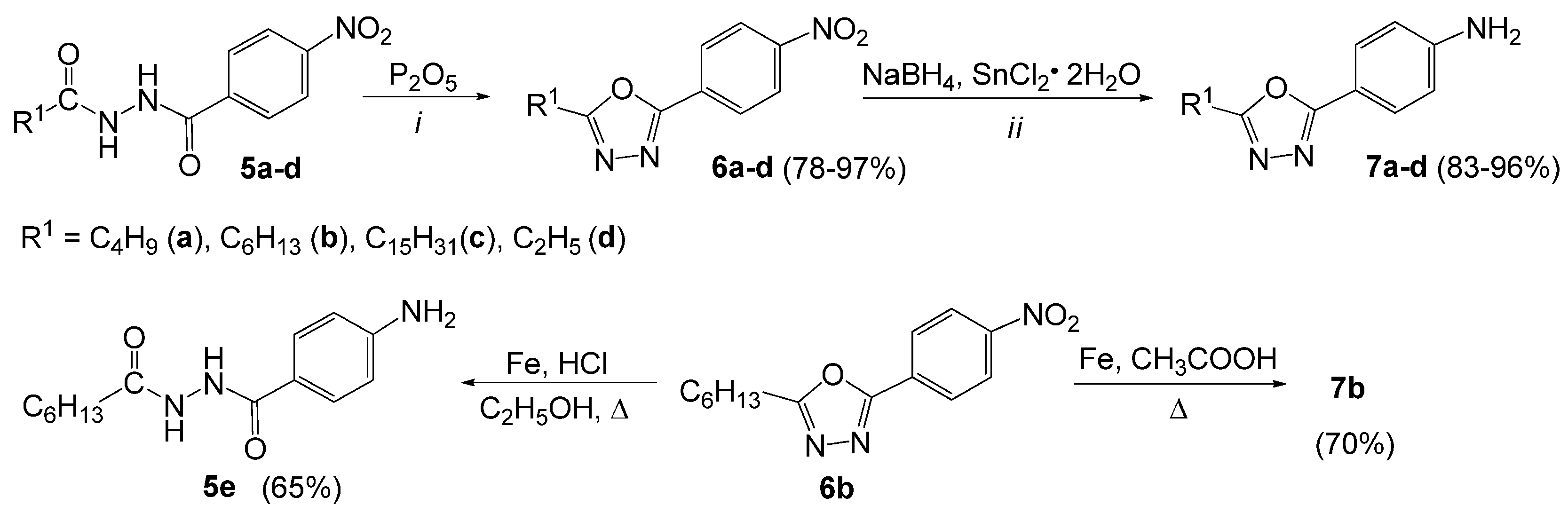
3.2.5. General Procedure for the Synthesis of 2-Alkyl-5-(4-nitrophenyl)-1,3,4-oxadiazoles (6a–d)
- 2-Butyl-5-(4-nitrophenyl)-1,3,4-oxadiazole (6a). The product was obtained as a white solid (1.98 g, 80%); mp 66–67 °C (130–131 °C [66]). 1H-NMR (400 MHz, CDCl3): δ 8.36 (d, 2H, J = 8.8 Hz, Ar), 8.23 (d, 2H, J = 8.8 Hz, Ar), 2.98 (t, 2H, J = 7.6 Hz, CH2), 1.85 (qui, 2H, J = 7.6 Hz, CH2), 1.46 (sextet, 2H, J = 7.2 Hz, CH2), 1.00 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (100 MHz, CDCl3): δ 168.1, 163.0, 149.4, 129.6, 127.6, 124.0, 28.5, 25.2, 22.1, 13.5; IR (ATR) νmax: 3109, 2933, 2872, 1738 (N=C), 1607, 1562, 1517 (N-O), 1348, 1233 (-O-), 1106, 867 (N-O), 714cm−1; HRMS calcd for (C12H13N3O3+H+): 248.1035; found: 248.1007; UV-Vis (CH3OH) λmax (logε) 204 (4.68), 286 (4.40) nm.
- 2-Hexyl-5-(4-nitrophenyl)-1,3,4-oxadiazole (6b). The product was obtained as a white solid (2.26 g, 82%); mp 60–61 °C. 1H-NMR (400 MHz, DMSO-d6): δ, 8.41 (d, 2H, J = 8.8 Hz, Ar), 8.23 (d, 2H, J = 8.8 Hz, Ar), 2.97 (t, 2H, J = 7.6 Hz, CH2), 1.76 (qui, 2H, J = 7.6 Hz, CH2), 1.30−1.38 (m, 6H, CH2), 0.87 (t, 3H, J = 7.6 Hz, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 167.9, 162.5, 149.0, 129.0, 127.7, 124.6, 30.7, 27.9, 25.7, 24.6, 21.9, 13.8; IR (ATR) νmax: 3110, 2916, 2851, 1739 (N=C), 1606, 1567, 1518 (N-O), 1338, 1231 (-O-), 1106, 867 (N-O), 710 cm−1; HRMS calcd for (C14H17N3O3+H+): 276.1348; found: 276.1351; UV-Vis (CH3OH) λmax (logε) 218 (4.02), 288 (4.26) nm.
- 2-Pentadecyl-5-(4-nitrophenyl)-1,3,4-oxadiazole (6c). The product was obtained as a white solid (3.10 g, 78%); mp 89–90 °C). 1H-NMR (400 MHz, CDCl3): δ 8.36 (d, 2H, J = 8.8 Hz, Ar), 8.22 (d, 2H, J = 8.8 Hz, Ar), 2.96 (t, 2H, J = 7.2 Hz, CH2), 1.87 (qui, 2H, J = 7.2 Hz, CH2), 1.25–1.47 (m, 24H, CH2), 0.88 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (100 MHz, CDCl3): δ 168.2, 163.0, 149.4, 129.6, 127.6, 124.3, 31.9, 29.65, 29.64, 29.62, 29.61, 29.6, 29.5, 29.4, 29.3, 29.1, 29.0, 26.5, 25.5, 22.7, 14.1; IR (ATR) νmax: 3120, 2914, 2848, 1738 (N=C), 1607, 1567, 1519 (N-O), 1342, 1218 (-O-), 1107, 868 (N-O), 710 cm−1; HRMS calcd for (C23H35N3O3+H+): 402.2757; found: 402.2755. UV-Vis (CH2Cl2) λmax (logε) 294 (4.25) nm.
- 2-Ethyl-5-(4-nitrophenyl)-1,3,4-oxadiazole (6d). The product was obtained as a white solid (2.10 g, 97%); mp 132–133 °C (131–135 °C [52]). UV-Vis (CH3OH) λmax (logε) 286 (4.47) nm.
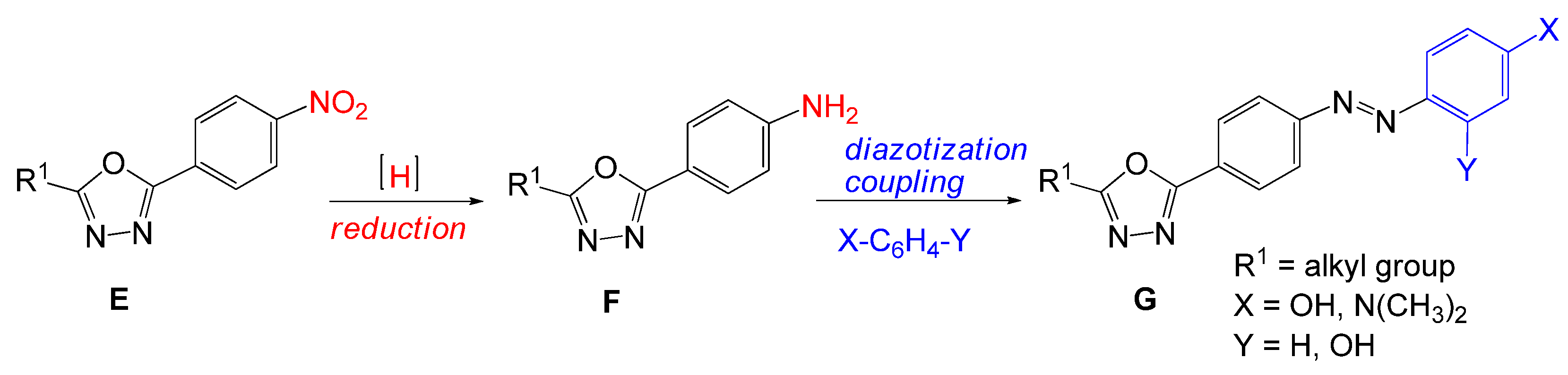
3.2.6. General Procedure for the Synthesis of 4-(5-Alkyl-1,3,4-oxadiazol-2-yl)anilines (7a–d)
- 4-(5-Butyl-1,3,4-oxadiazol-2-yl)aniline (7a). The product was obtained as a brown solid (1.98 g, 91%); mp 103–104 °C (119–122 °C [52]). 1H-NMR (400 MHz, CDCl3): δ 7.80 (d, 2H, J = 8.4 Hz, Ar), 6.71 (d, 2H, J = 8.8 Hz, Ar), 4.06 (s, 2H, NH2), 2.88 (t, 2H, J = 7.6 Hz, CH2), 1.77 (qui, 2H, J = 7.2 Hz, CH2), 1.41 (sextet, 2H, J = 7.2 Hz, CH2), 0.97 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (100 MHz, CDCl3): δ 166.0, 165.0, 149.4, 128.4, 114.6, 112.4, 28.7, 25.1, 22.1, 13.6; IR (ATR) νmax: 3413 (N-H), 3328 (N-H), 3215, 2960, 2960, 2960, 1606 (N-H), 1497, 1315(C-N), 1173 (C-O), 827 cm−1; HRMS calcd for (C12H15N3O+H+): 218.1293; found: 218.1275; UV-Vis (CH3OH) λmax (logε) 202 (4.13), 218 (4.01), 306 (4.36) nm.
- 4-(5-Hexyl-1,3,4-oxadiazol-2-yl)aniline (7b). The product was obtained as a brown solid (2.20 g, 83%); mp 109–110 °C. 1H-NMR (400 MHz, DMSO-d6): δ 7.61 (d, 2H, J = 8.4 Hz, Ar), 6.66 (d, 2H, J = 8.4 Hz, Ar), 5.87 (s, 2H, NH2), 2.85 (t, 2H, J = 7.2 Hz, CH2), 1.70 (qui, 2H, J = 7.2 Hz, CH2), 1.29−1.36 (m, 6H, CH2), 0.86 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 165.6, 164.9, 152.5, 128.0, 114.0, 110.5, 31.2, 28.4, 26.3, 25.0, 22.3, 14.2; IR (ATR) νmax: 3446 (N-H), 3339 (N-H), 3219, 2940, 1738, 1588, 1497, 1319 (C-N), 1176 (C-O), 835 cm−1; HRMS calcd for (C14H19N3O+H+): 246.1606; found: 246.1623. UV-Vis (CH3OH) λmax (logε) 302 (4.43) nm.
- 4-(5-Pentadecyl-1,3,4-oxadiazol-2-yl)aniline (7c). The product was obtained as a yellow solid (3.30 g, 88%); mp 103–104 °C). 1H-NMR (600 MHz, CDCl3): δ 7.81 (d, 2H, J = 8.4 Hz, Ar), 6.72 (d, 2H, J = 8.4 Hz, Ar), 4.04 (s, 2H, NH2), 2.87 (t, 2H, J = 7.8 Hz, CH2), 1.82 (qui, 2H, J = 7.8 Hz, CH2), 1.20−1.42 (m, 24H, CH2), 0.88 (t, 3H, J = 7.8 Hz, CH3); 13C-NMR (151 MHz, CDCl3): δ 166.0, 165.0, 149.4, 128.4, 114.6, 112.5, 31.9, 29.66, 29.65, 29.64, 29.62, 29.61, 29.6, 29.4, 29.3, 29.1, 29.0, 26.7, 25.4, 22.7, 14.1; IR (ATR) νmax: 3337 (N-H), 2916, 2849, 1738, 1607, 1557, 1500, 1378 (C-N), 1178 (C-O), 842 cm−1; HRMS calcd for (C23H37N3O+H+): 372.3015; found: 372.3009. UV-Vis (CH2Cl2) λmax (logε) 292 (4.55) nm.
- 4-(5-Ethyl-1,3,4-oxadiazol-2-yl)aniline (7d). The product was obtained as a yellow solid (1.80 g, 96%); mp 140–141 °C (138–142 °C [52]). UV-Vis (CH3OH) λmax (logε) 302 (4.48) nm.
3.2.7. Reduction of 2-Hexyl-5-(4-nitrophenyl)-1,3,4-oxadiazole (7b) with Iron Filings in Concentrated HCl
3.2.8. Reduction of 2-Hexyl-5-(4-nitrophenyl)-1,3,4-oxadiazole (7b) with Iron Filings in Glacial Acetic Acid
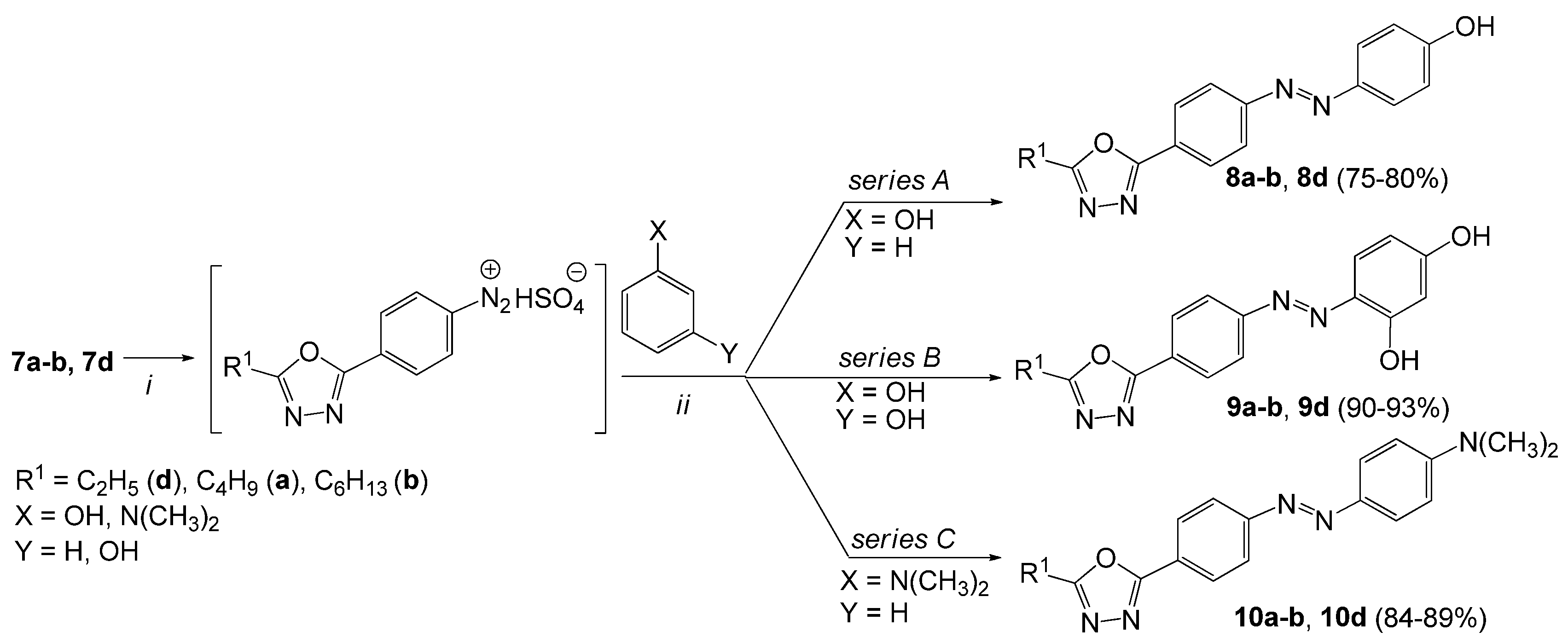
3.2.9. General Procedure for the Synthesis of 4-{[4-(5-Alkyl-1,3,4-oxadiazol-2-yl)phenyl]diazenyl} phenol (8a–b, 8d)
- 4-{[4-(5-Butyl-1,3,4-oxadiazol-2-yl)phenyl]diazenyl}phenol (8a). The product was obtained as a dark brown solid (1.19 g, 80%). It was purified by column chromatography (MeOH:CHCl3, 1:4, v/v); Rf = 0.71; mp 95–98 °C. 1H-NMR (400 MHz, DMSO-d6): δ 10.46 (s, 1H, OH), 8.14 (d, 2H, J = 8.4 Hz, Ar), 7.97 (d, 2H, J = 8.4 Hz, Ar), 7.85 (d, 2H, J = 8.8 Hz, Ar), 6.97 (d, 2H, J = 8.8 Hz, Ar), 2.95 (t, 2H, J = 7.2 Hz, CH2), 1.77 (qui, 2H, J = 7.2 Hz, CH2), 1.41 (sextet, 2H, J = 7.2 Hz, CH2), 0.94 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 167.1, 163.4, 161.6, 153.6, 145.3, 129.3, 127.5, 125.3, 124.6, 116.1, 27.9, 24.3, 21.5, 13.4; IR (ATR) νmax: 3106 (O-H), 2958, 2873, 1586, 1466 (N=N), 1226 (C-OH), 1192, 1138, 849, 746 cm−1; HRMS calcd for (C18H18N4O2+H+): 323.1508; found: 323.1513; UV-Vis (CH3OH) λmax (logε) 268 (4.03), 366 (4.26) nm.
- 4-{[4-(5-Hexyl-1,3,4-oxadiazol-2-yl)phenyl]diazenyl}phenol (8b). The product was obtained as a light brown solid (1.26 g, 78%). It was purified by column chromatography (Benzene:AcOEt, 1:3, v/v); Rf = 0.63; mp 125–126 °C. 1H-NMR (400 MHz, DMSO-d6): δ 10.45 (s, 1H, OH), 8.14 (d, 2H, J = 8.4 Hz, Ar), 7.98 (d, 2H, J = 8.4 Hz, Ar), 7.85 (d, 2H, J = 8.8 Hz, Ar), 6.97 (d, 2H, J = 8.8 Hz, Ar), 2.95 (t, 2H, J = 7.6 Hz, CH2), 1.78 (qui, 2H, J = 7.6 Hz, CH2), 1.30–1.39 (m, 6H, CH2), 0.87 (t, 3H, J = 6.8 Hz, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 167.1, 163.4, 161.6, 153.6, 145.3, 129.3, 127.5, 125.3, 124.6, 116.1, 30.7, 28.0, 25.7, 24.6, 21.9, 13.8; IR (ATR) νmax: 3214 (O-H), 3054, 2924, 2854, 1586, 1464 (N=N), 1220 (C-OH), 1190, 1190, 1138, 850, 749 cm−1; HRMS calcd for (C20H22N4O2+H+): 351.1821; found: 351.1819; UV-Vis (CH3OH) λmax (logε) 264 (3.80), 366 (4.17) nm.
- 4-{[4-(5-Ethyl-1,3,4-oxadiazol-2-yl)phenyl]diazenyl}phenol (8d). The product was obtained as a brown solid (1.01 g, 75%). It was purified by column chromatography (Benzene: AcOEt, 1:3, v/v); Rf = 0,52; mp 185–188 °C. 1H-NMR (400 MHz, DMSO-d6): δ 10.46 (s, 1H, OH), 8.14 (d, 2H, J = 8.4 Hz, Ar), 7.97 (d, 2H, J = 8.4 Hz, Ar), 7.85 (d, 2H, J = 8.8 Hz, Ar), 6.97 (d, 2H, J = 8.8 Hz, Ar), 2.98 (q, 2H, J = 7.6 Hz, CH2), 1.35 (t, 3H, J = 7.6 Hz, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 168.0, 163.4, 161.6, 153.6, 145.3, 129.3, 127.5, 125.3, 122.9, 116.0, 18.4, 10.4; IR (ATR) νmax: 3187 (O-H), 2985, 1683, 1593, 1472 (N=N), 1227 (C-OH), 1099, 844, 755 cm−1; HRMS calcd for (C16H14N4O2+H+): 295.1195; found: 295.1182; UV-Vis (CH3OH) λmax (logε) 262 (3.65), 362 (3.80) nm.
3.2.10. General Procedure for the Synthesis of 4-{[4-(5-Alkyl-1,3,4-oxadiazol-2-yl)phenyl]diazenyl}benzene-1,3-diol (9a–b, 9d)
- 4-{[4-(5-Butyl-1,3,4-oxadiazol-2-yl)phenyl]diazenyl}benzene-1,3-diol (9a). The product was obtained as a brown solid (1.45 g, 93%). It was purified by column chromatography (AcOEt:Hexane, 2:1, v/v); Rf = 0.42; mp 150–154 °C. 1H-NMR (400 MHz, DMSO-d6): δ 12.23 (s, 1H, OH), 10.69 (s, 1H, OH), 8.07 (d, 2H, J = 8.4 Hz, Ar), 7.99 (d, 2H, J = 8.4 Hz, Ar), 7.66 (d, 1H, J = 8.8 Hz, Ar), 6.47 (d, 1H, J = 8.8 Hz, Ar), 6.35 (s, 1H, Ar), 2.91 (t, 2H, J = 7.2 Hz, CH2), 1.73 (qui, 2H, J = 7.2 Hz, CH2), 1.37 (sextet, 2H, J = 7.2 Hz, CH2), 0.90 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 167.5, 164.4, 163.9, 157.8, 152.9, 133.2, 129.5, 128.0, 124.5, 123.0, 110.0, 103.5, 28.3, 24.7, 22.00, 13.9; IR (ATR) νmax: 2956 (O-H), 1601, 1472 (N=N), 1232 (C-OH), 737 cm−1; HRMS calcd for (C18H18N4O3+H+): 339.1457; found: 339.1454; UV-Vis (CH3OH) λmax (logε) 262 (4.00), 400 (4.28) nm.
- 4-{[4-(5-Hexyl-1,3,4-oxadiazol-2-yl)phenyl]diazenyl}benzene-1,3-diol (9b). The product was obtained as a red solid (1.54 g, 91%). It was purified by column chromatography (Benzene: AcOEt, 1:3, v/v); Rf = 0.55; mp 179–180 °C. 1H-NMR (400 MHz, DMSO-d6): δ 12.28 (s, 1H, OH), 10.72 (s, 1H, OH), 8.11 (d, 2H, J = 8.8 Hz, Ar), 8.02 (d, 2H, J = 8.8 Hz, Ar), 7.69 (d, 1H, J = 8.4 Hz, Ar), 6.51 (d, 1H, J = 8.4 Hz, Ar), 6.38 (d, 1H, J = 2.4 Hz, Ar), 2.94 (t, 2H, J = 7.2 Hz, CH2), 1.77 (qui, 2H, J = 7.2 Hz, CH2), 1.29–1.39 (m, 6H, CH2), 0.87 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 167.0, 163.9, 163.4, 157.3, 152.4, 132.8, 129.1, 127.5, 124.1, 122.5, 109.6, 103.0, 30.7, 28.0, 25.7, 24.6, 21.9, 13.8; IR (ATR) νmax: 3094 (O-H), 2951, 2927, 2853, 1591, 1472 (N=N), 1317, 1228 (C-OH), 846, 744 cm−1; HRMS calcd for (C20H22N4O3+H+): 367.1770; found: 367.1747; UV-Vis (CH3OH) λmax (logε) 264 (4.19), 402 (4.49) nm.
- 4-{[4-(5-Ethyl-1,3,4-oxadiazol-2-yl)phenyl]diazenyl}benzene-1,3-diol (9d). The product was obtained as a thick, dark red solid (1.27 g, 90%). It was purified by column chromatography (Hexane:AcOEt, 1:2, v/v); Rf = 0.38; mp 210–214 °C. 1H-NMR (400 MHz, DMSO-d6): δ 12.24 (s, 1H, OH), 10.71 (s, 1H, OH), 8.12 (d, 2H, J = 8.4 Hz, Ar), 8.03 (d, 2H, J = 8.4 Hz, Ar), 7.70 (d, 1H, J = 8.8 Hz, Ar), 6.51 (d, 1H, J = 8.8 Hz, Ar), 6.39 (s, 1H, Ar), 2.94 (q, 2H, J = 7.6 Hz, CH2), 1.35 (t, 3H, J = 7.6 Hz, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 167.4, 164.6, 163.9, 157.3, 152.3, 132.8, 128.95, 127.5, 124.1, 122.5, 109.6, 103.0, 18.4, 10.4; IR (ATR) νmax: 3340 (O-H), 1602, 1477 (N=N), 1242, 1235 (C-OH), 1108, 839 cm−1; HRMS calcd for (C16H14N4O3+H+): 311.1144; found: 311.1145; UV-Vis (CH3OH) λmax (logε) 262 (3.35), 398 (3.68) nm.
3.2.11. General Procedure for the Synthesis of 4-{[4-(5-Alkyl-1,3,4-oxadiazol-2-yl)phenyl]diazenyl}-N,N-dimethylaniline (10a–b, 10d)
- 4-{[4-(5-Butyl-1,3,4-oxadiazol-2-yl)phenyl]diazenyl}-N,N-dimethylaniline (10a). The product was obtained as a dark green solid (1.43 g, 89%). It was purified by column chromatography (Hexane: AcOEt, 7:3, v/v) Rf = 0.22. mp 270–272 °C. 1H-NMR (400 MHz, DMSO-d6): δ 8.10 (d, 2H, J = 7.6 Hz, Ar), 7.92 (d, 2H, J = 6.8 Hz, Ar), 7.72 (d, 2H, J = 6.8 Hz, Ar), 6.85 (d, 2H, J = 7.6 Hz, Ar), 3.04 (s, 6H, CH3), 2.85 (t, 2H, J = 6.4 Hz, CH2), 1.75 (qui, 2H, J = 6.4 Hz, CH2), 1.41 (sextet, 2H, J = 6.4 Hz, CH2), 0.92 (t, 3H, J = 6.4 Hz, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 167.0, 163.1, 154.1, 152.9, 142.3, 128.6, 127.7, 127.4, 125.1, 111.9, 48.4, 28.7, 24.3, 21.5, 13.4; IR (ATR) νmax: 2956 (CH3), 2931, 2871, 1681, 1600, 1507, 1446 (N=N), 1363, 1136 (C-N), 1008, 822, 751, 692 cm−1; HRMS calcd for (C20H23N5O+H+): 350.1981; found: 350.1985; UV-Vis (CH3OH) λmax (logε) 254 (4.31), 432 (3.74) nm.
- 4-{[4-(5-Hexyl-1,3,4-oxadiazol-2-yl)phenyl]diazenyl}-N,N-dimethylaniline (10b). The product was obtained as a dark red liquid (1.46 g, 84%). It was purified by column chromatography (Benzene:AcOEt, 1:3, v/v); Rf = 0.62. 1H-NMR (400 MHz, DMSO-d6): δ 8.00 (d, 2H, J = 8.0 Hz, Ar), 7.59 (d, 2H, J = 7.2 Hz, Ar), 7.31 (d, 2H, J = 7.2 Hz, Ar), 6.70 (d, 2H, J = 8.0 Hz, Ar), 3.32 (s, 6H, CH3), 2.92 (t, 2H, J = 7.2 Hz, CH2), 1.76 (qui, 2H, J = 7.2 Hz, CH2), 1.25–1.36 (m, 6H, CH2), 0.87 (t, 3H, J = 7.6 Hz, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 166.7, 163.2, 154.9, 150.4, 142.7, 128.8, 128.1, 126.3, 125.9, 112.3, 49.3, 30.7, 27.9, 25.7, 24.5, 21.9, 13.8; IR (ATR) νmax: 2927 (CH3), 2857, 1682, 1599, 1495 (N=N), 1362, 1133 (C-N), 1011, 843, 751, 691 cm−1; HRMS calcd for (C22H27N5O+H+): 378.2294; found: 378.2271; UV-Vis (CH3OH) λmax (logε) 254 (4.26), 368 (3.62), 436 (3.49) nm.
- 4-{[4-(5-Ethyl-1,3,4-oxadiazol-2-yl)phenyl]diazenyl}-N,N-dimethylaniline (10d). The product was obtained as a dark red liquid (1.29 g, 87%). It was purified by column chromatography (Benzene:AcOEt, 1:3, v/v); Rf = 0.25. 1H-NMR (400 MHz, DMSO-d6): δ 7.98 (d, 2H, J = 8.8 Hz, Ar), 7.64 (d, 2H, J = 8.4 Hz, Ar), 7.30 (d, 2H, J = 8.4 Hz, Ar), 6.66 (d, 2H, J = 8.8 Hz, Ar), 3.36 (s, 6H, CH3), 2.95 (q, 2H, J = 4.8 Hz, CH2), 1.32 (t, 3H, J = 4.8 Hz, CH3); 13C-NMR (100 MHz, DMSO-d6): δ 167.6, 163.3, 154.3, 151.1, 142.7, 128.1, 127.7, 126.3, 125.7, 111.9, 48.5, 19.7, 10.3; IR (ATR) νmax: 3224, 2974 (CH3), 1647, 1600, 1490 (N=N), 1363, 1170 (C-N), 1012, 820, 704 cm−1; HRMS calcd for (C18H19N5O+H+): 322.1668; found: 322.1622; UV-Vis (CH3OH) λmax (logε) 266 (4.22), 424 (3.63) nm.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhter, M.; Husain, A.; Azad, B.; Ajmal, M. Aroylpropionic Acid Based 2,5-Disubstituted-1,3,4-Oxadiazoles: Synthesis and Their Anti-Inflammatory and Analgesic Activities. Eur. J. Med. Chem. 2009, 44, 2372–2378. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.J.; Choupra, A.; Sharma, R.K.; Jadav, S.S.; Padmaja, P.; Hassan, M.Z.; Al-Tamimi, A.B.S.; Geesi, M.H.; Bakht, M.A. Rationale Design, Synthesis, Cytotoxicity Evaluation, and Molecular Docking Studies of 1,3,4-Oxadiazole Analogues. Anticancer Agents Med. Chem. 2018, 18, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Entesar, O.A.; Khalida, A.T. Synthesis, Characterization and Polymerization of 1,3,4-Oxadiazole Derivatives of Amoxicillin and Evaluation Antibacterial Activities. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 511–522. [Google Scholar] [CrossRef]
- Jasiak, K.; Kudelko, A.; Biernasiuk, A.; Malm, A. Study on Antibacterial and Antifungal Activity of 2-(2-Arylethenyl)-1,3,4-Oxadiazoles. Trends Org. Chem. 2017, 18, 21–28. [Google Scholar]
- Albratty, M.; El-Sharkawy, K.A.; Alhazmi, H.A. Synthesis and Evaluation of Some New 1,3,4-Oxadiazoles Bearing Thiophene, Thiazole, Coumarin, Pyridine and Pyridazine Derivatives as Antiviral Agents. Acta Pharm. 2019, 69, 261–276. [Google Scholar] [CrossRef]
- Buyniski, J.P.; Pircio, A.W.; Schurig, J.E.; Campbell, J.A. Effects of Tiodazosin, Prazosin, Trimazosin and Phentolamine on Blood Pressure, Heart Rate and on Pre- and Postsynaptic α-Adrenergic Receptors in the Rat. Clin. Exp. Hypertens. 1980, 2, 1039–1066. [Google Scholar] [CrossRef]
- Pitasse-Santos, P.; Sueth-Santiago, V.; Lima, M. 1,2,4- and 1,3,4-Oxadiazoles as Scaffolds in the Development of Antiparasitic Agents. J. Braz. Chem. Soc. 2017, 29, 435–456. [Google Scholar] [CrossRef]
- Zou, X.-J.; Lai, L.-H.; Jin, G.-Y.; Zhang, Z.-X. Synthesis, Fungicidal Activity, and 3D-QSAR of Pyridazinone-Substituted 1,3,4-Oxadiazoles and 1,3,4-Thiadiazoles. J. Agric. Food Chem. 2002, 50, 3757–3760. [Google Scholar] [CrossRef]
- Chen, H.; Li, Z.; Han, Y. Synthesis and Fungicidal Activity against Rhizoctonia solani of 2-Alkyl (Alkylthio)-5-Pyrazolyl-1,3,4-Oxadiazoles (Thiadiazoles). J. Agric. Food Chem. 2000, 48, 5312–5315. [Google Scholar] [CrossRef]
- Wong, M.Y.; Krotkus, S.; Copley, G.; Li, W.; Murawski, C.; Hall, D.; Hedley, G.J.; Jaricot, M.; Cordes, D.B.; Slawin, A.M.Z.; et al. Deep-Blue Oxadiazole-Containing Thermally Activated Delayed Fluorescence Emitters for Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 33360–33372. [Google Scholar] [CrossRef]
- Ji Ram, V.; Sethi, A.; Nath, M.; Pratap, R. Five-Membered Heterocycles. In The Chemistry of Heterocycles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–478. [Google Scholar]
- Tamoto, N.; Adachi, C.; Nagai, K. Electroluminescence of 1,3,4-Oxadiazole and Triphenylamine-Containing Molecules as an Emitter in Organic Multilayer Light Emitting Diodes. Chem. Mater. 1997, 9, 1077–1085. [Google Scholar] [CrossRef]
- Schulz, B.; Orgzall, I.; Freydank, A.; Xü, C. Self-Organization of Substituted 1,3,4-Oxadiazoles in the Solid State and at Surfaces. Adv. Colloid Interface Sci. 2005, 116, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Dămăceanu, M.-D.; Rusu, R.-D.; Brumă, M. Polymers Containing 1,3,4-Oxadiazole Rings for Advanced Materials. Mem. Sci. Sect. Rom. Acad. 2011, 34, 105–125. [Google Scholar]
- Katritzky, A.R.; Rogers, J.W.; Witek, R.M.; Vakulenko, A.V.; Mohapatra, P.P.; Steel, P.J.; Damavarapu, R. Synthesis and Characterization of Blowing Agents and Hypergolics. J. Energetic Mater. 2007, 25, 79–109. [Google Scholar] [CrossRef]
- Suwiński, J.; Szczepankiewicz, W. 1,3,4-Oxadiazoles. In Comprehensive Heterocyclic Chemistry III; Elsevier: Amsterdam, The Netherlands, 2008; pp. 397–466. [Google Scholar]
- Bouanis, M.; Tourabi, M.; Nyassi, A.; Zarrouk, A.; Jama, C.; Bentiss, F. Corrosion Inhibition Performance of 2,5-Bis(4-Dimethylaminophenyl)-1,3,4-Oxadiazole for Carbon Steel in HCl Solution: Gravimetric, Electrochemical and XPS Studies. Appl. Surf. Sci. 2016, 389, 952–966. [Google Scholar] [CrossRef]
- Short, F.W.; Long, L.M. Synthesis of 5-aryl-2-oxazolepropionic Acids and Analogs. Antiinflammatory Agents. J. Heterocycl. Chem. 1969, 6, 707–712. [Google Scholar] [CrossRef]
- Jacobsen, N.; Philippides, A. The Hydroxy Derivatives of 2,5-Diphenyl-1,3,4-Oxadiazole. Aust. J. Chem. 1986, 39, 1911. [Google Scholar] [CrossRef]
- Wheelock, C.E.; Nakagawa, Y.; Harada, T.; Oikawa, N.; Akamatsu, M.; Smagghe, G.; Stefanou, D.; Iatrou, K.; Swevers, L. High-Throughput Screening of Ecdysone Agonists Using a Reporter Gene Assay Followed by 3-D QSAR Analysis of the Molting Hormonal Activity. Bioorganic Med. Chem. 2006, 14, 1143–1159. [Google Scholar] [CrossRef]
- Kędzia, A.; Kudelko, A.; Świątkowski, M.; Kruszyński, R. Microwave-Promoted Synthesis of Highly Luminescent s-Tetrazine-1,3,4-Oxadiazole and s-Tetrazine-1,3,4-Thiadiazole Hybrids. Dye. Pigment. 2020, 172, 107865. [Google Scholar] [CrossRef]
- Boström, J.; Hogner, A.; Llinàs, A.; Wellner, E.; Plowright, A.T. Oxadiazoles in Medicinal Chemistry. J. Med. Chem. 2012, 55, 1817–1830. [Google Scholar] [CrossRef]
- Bentiss, F.; Lagrenée, M.; Barbry, D. Rapid Synthesis Of 2,5-Disubstituted 1,3,4-Oxadiazoles Under Microwave Irradiation. Synth. Commun. 2001, 31, 935–938. [Google Scholar] [CrossRef]
- Mazurkiewicz, R.; Grymel, M. ChemInform Abstract: An Efficient Synthesis of 1,3,4-Oxadiazoles from N,N′-Diacylhydrazines Using Ph3P×Br2, Ph3P×CCl4 or Ph3P×CBr4 Adducts as Condensing Agents. ChemInform 1997, 28. [Google Scholar] [CrossRef]
- Jasiak, K.; Kudelko, A. Oxidative Cyclization of N-Aroylhydrazones to 2-(2-Arylethenyl)-1,3,4-Oxadiazoles Using DDQ as an Efficient Oxidant. Tetrahedron Lett. 2015, 56, 5878–5881. [Google Scholar] [CrossRef]
- Dabiri, M.; Salehi, P.; Baghbanzadeh, M.; Bahramnejad, M. A Facile Procedure for the One-Pot Synthesis of Unsymmetrical 2,5-Disubstituted 1,3,4-Oxadiazoles. Tetrahedron Lett. 2006, 47, 6983–6986. [Google Scholar] [CrossRef]
- Rostamizadeh, S.; Housaini, S.A.G. Microwave Assisted Syntheses of 2,5-Disubstituted 1,3,4-Oxadiazoles. Tetrahedron Lett. 2004, 45, 8753–8756. [Google Scholar] [CrossRef]
- Mruthyunjayaswamy, B.H.M.; Shanthaveerappa, B.K. Synthesis and Pharmacological Activity of Malonoyuoxaloyl Hydrazones of 5-Substituted Indole-3-Carboxaldehydes, 3-Terephthaloyl Bis-1-(5′-Substituted-2′-Phenyl Indol-3′-yl)-3-Thioureas and Their Derivatives. Indian J. Heterocycl. Chem. 1998, 8, 31–38. [Google Scholar]
- Milcent, R.; Barbier, G. Oxydation d’hydrazones Par Le Bioxyde de Plomb: Nouvelles Synthèses d’oxadiazoles-1,3,4 et de Dérivés de l’amino-4 Triazol-1,2,4 One-5. J. Heterocycl. Chem. 1983, 20, 77–80. [Google Scholar] [CrossRef]
- Faidallah, H.M.; Sharshira, E.M.; Basaif, S.A.; A-Ba-Oum, A.E.-K. Synthesis and Spectral Characterization of Novel 1,3,4-Oxadiazole and 1,2,4-Triazole Derivatives: Synthesis for Potential Pharmacological Activities. Phosphorus Sulfur Silicon Relat. Elem. 2002, 177, 67–79. [Google Scholar] [CrossRef]
- Werber, G.; Buccheri, F.; Noto, R.; Gentile, M. 1,5-Dipolar Cycloaddition Reactions. Semicarbazone Bromides, 5-alkyl (or Aryl)Amino-1,3,4-oxadiazole-2-carboxylic Acids and Their Esters. J. Heterocycl. Chem. 1977, 14, 1385–1388. [Google Scholar] [CrossRef]
- Jedlovská, E.; Leško, J. A Simple One-Pot Procedure for the Synthesis of 1,3,4-Oxadiazoles. Synth. Commun. 1994, 24, 1879–1885. [Google Scholar] [CrossRef]
- Prabhu, G.; Sureshbabu, V.V. Hypervalent Iodine(V) Mediated Mild and Convenient Synthesis of Substituted 2-Amino-1,3,4-Oxadiazoles. Tetrahedron Lett. 2012, 53, 4232–4234. [Google Scholar] [CrossRef]
- Rajapakse, H.A.; Zhu, H.; Young, M.B.; Mott, B.T. A Mild and Efficient One Pot Synthesis of 1,3,4-Oxadiazoles from Carboxylic Acids and Acyl Hydrazides. Tetrahedron Lett. 2006, 47, 4827–4830. [Google Scholar] [CrossRef]
- Kudelko, A.; Zieliński, W. Microwave-Assisted Synthesis of 2-Styryl-1,3,4-Oxadiazoles from Cinnamic Acid Hydrazide and Triethyl Orthoesters. Tetrahedron Lett. 2012, 53, 76–77. [Google Scholar] [CrossRef]
- Buscemi, S.; Pace, A.; Pibiri, I.; Vivona, N. Competing Ring-Photoisomerization Pathways in the 1,2,4-Oxadiazole Series. An Unprecedented Ring-Degenerate Photoisomerization. J. Org. Chem. 2002, 67, 6253–6255. [Google Scholar] [CrossRef]
- Dupau, P.; Epple, R.; Thomas, A.; Fokin, V.; Sharpless, K.B. Osmium-Catalyzed Dihydroxylation of Olefins in Acidic Media: Old Process, New Tricks. Adv. Synth. Catal. 2002, 344, 421–433. [Google Scholar] [CrossRef]
- Dawson, J.F. Developments in Disperse Dyes. Rev. Prog. Color. Relat. Top. 1978, 9, 25–35. [Google Scholar] [CrossRef]
- Bahtiti, N.H.; Elgammal, W.E.; Ali, A.A.; Belal, A.; Abdullah, O.; Ghoneim, M.M.; Qenawy, M.S.; Abdou, M.M. Novel Monosulfonated Azo Dyes: Design, Synthesis, and Application as Disperse Dyes on Polyester Fabrics. ACS Omega 2024, 9, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Abdou, M.M.; Soliman, A.-G.A.; Kobisy, A.S.; Abu-Rayyan, A.; Al-Omari, M.; Alshwyeh, H.A.; Ragab, A.H.; Al Shareef, H.F.; Ammar, N.S. Preparation and Evaluation of Phenol Formaldehyde-Montmorillonite and Its Utilization in the Adsorption of Lead Ions from Aqueous Solution. ACS Omega 2024, 9, 12015–12026. [Google Scholar] [CrossRef]
- Alshaye, N.A.; Omar, A.Z.; Elhag, M.; Hamed, E.A.; Ahmed, H.A.; Alharbi, N.S.; El-Atawy, M.A.; El-Zawawy, R.O.; El-Rahman, M.A. Synthesis, Characterization, and Dyeing Performance of Novel Bisazo Disperse Dyes: A Combined Spectroscopic and Computational Study. J. Mol. Struct. 2025, 1319, 139582. [Google Scholar] [CrossRef]
- Towns, A.D. Developments in Azo Disperse Dyes Derived from Heterocyclic Diazo Components. Dye. Pigment. 1999, 42, 3–28. [Google Scholar] [CrossRef]
- Langhals, H. Color Chemistry. Synthesis, Properties and Applications of Organic Dyes and Pigments. 3rd Revised Edition. By Heinrich Zollinger. Angew. Chem. Int. Ed. 2004, 43, 5291–5292. [Google Scholar] [CrossRef]
- Dobre, A.F.; Hanganu, A.; Nicolau, I.; Popescu, C.C.; Paun, A.; Mădălan, A.M.; Tablet, C.; Mirea, A.G.; Matache, M. A Synthetic Approach for Oxadiazole-Decorated Azobenzene Photoswitches. Chempluschem 2024, 89, e202300504. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ran, X.; Li, Y.; Li, Q.; Qiu, W.; Guo, L. Photoresponsive Structure Transformation and Emission Enhancement Based on a Tapered Azobenzene Gelator. RSC Adv. 2015, 5, 38283–38289. [Google Scholar] [CrossRef]
- Ran, X.; Wang, H.; Shi, L.; Lou, J.; Liu, B.; Li, M.; Guo, L. Light-Driven Fluorescence Enhancement and Self-Assembled Structural Evolution of an Azobenzene Derivative. J. Mater. Chem. C 2014, 2, 9866–9873. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, S.; Hua, W. The Synthesis and Structure of a New Type of Aromatic Heterocyclic Macrocycle. IV. Synthesis of a 1,3,4-oxadiazole-containing Azomacrocycle. J. Heterocycl. Chem. 1998, 35, 275–277. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, M.; Chen, Y.; Zhang, Y.; Luo, G.; Long, Y.; Yang, W.; Yu, X. Synthesis, Biological Evaluation and Network Pharmacology Based Studies of 1,3,4-Oxadiazole Bearing Azaphenols as Anticancer Agents. Arab. J. Chem. 2024, 17, 105386. [Google Scholar] [CrossRef]
- Kudelko, A.; Wróblowska, M. An Efficient Synthesis of Conjugated 5-Aryl-1,3,4-Oxadiazoles from 3-Heteroarylacrylohydrazides and Acid Chlorides. Tetrahedron Lett. 2014, 55, 3252–3254. [Google Scholar] [CrossRef]
- Kpинoчкин, A.П.; Гyдa, M.P.; Koпчyк, Д.C.; Cлoвecнoвa, H.B.; Koвaлeв, И.C.; Caвчyк, M.И.; Штaйц, Я.K.; Cтapнoвcкaя, E.C.; Зыpянoв, Г.B.; Чyпaхин, O.H. эффeктивный cинтeтичecкий пoдхoд к 1,2,4-тpиaзинaм, имeющим в пoлoжeнии C5 ocтaтки 2-(aминoфeнил)-1,3,4-oкcaдиaзoлoв. Zh. Org. Khim. 2021, 57, 1496–1500. [Google Scholar] [CrossRef]
- Wang, H.; Ryu, J.-T.; Han, Y.S.; Hur, Y.; Park, L.S.; Song, M.; Kwon, Y. Synthesis and Optical Properties of Copolymers Containing Electron Transporting 1,3,4-Oxadiazole Pendant Groups. Mol. Cryst. Liq. Cryst. 2006, 459, 109–389. [Google Scholar] [CrossRef]
- Conole, D.; Beck, T.M.; Jay-Smith, M.; Tingle, M.D.; Eason, C.T.; Brimble, M.A.; Rennison, D. Synthesis and Methemoglobinemia-Inducing Properties of Benzocaine Isosteres Designed as Humane Rodenticides. Bioorg. Med. Chem. 2014, 22, 2220–2235. [Google Scholar] [CrossRef]
- Najare, M.S.; Patil, M.K.; Garbhagudi, M.; Yaseen, M.; Inamdar, S.R.; Khazi, I.A.M. Design, Synthesis and Characterization of π-Conjugated 2,5-Diphenylsubstituted-1,3,4-Oxadiazole-Based D-π-A-π’-D′ Form of Efficient Deep Blue Functional Materials: Photophysical Properties and Fluorescence “Turn-off” Chemsensors Approach. J. Mol. Liq. 2021, 328, 115443. [Google Scholar] [CrossRef]
- Chang, Y.J.; Chou, P.; Lin, S.; Watanabe, M.; Liu, Z.; Lin, J.; Chen, K.; Sun, S.; Liu, C.; Chow, T.J. High-Performance Organic Materials for Dye-Sensitized Solar Cells: Triarylene-Linked Dyads with a 4- Tert -Butylphenylamine Donor. Chem.-Asian J. 2012, 7, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Le, T.V.; Romero, I.; Daugulis, O. “Sandwich” Diimine-Copper Catalyzed Trifluoroethylation and Pentafluoropropylation of Unactivated C(Sp 3)−H Bonds by Carbene Insertion. Chem.-Eur. J. 2023, 29, e202301672. [Google Scholar] [CrossRef] [PubMed]
- Bon, D.J.-Y.D.; Chrenko, D.; Kováč, O.; Ferugová, V.; Lasák, P.; Fuksová, M.; Zálešák, F.; Pospíšil, J. Julia-Kocienski-Like Connective C−C and C=C Bond-Forming Reaction. Adv. Synth. Catal. 2024, 366, 480–487. [Google Scholar] [CrossRef]
- He, F.; Wang, M.; Gao, M.; Zhao, M.; Bai, Y.; Zhao, C. Chemical Composition and Biological Activities of Gerbera anandria. Molecules 2014, 19, 4046–4057. [Google Scholar] [CrossRef]
- Dallinger, D.; Pinho, V.D.; Gutmann, B.; Kappe, C.O. Laboratory-Scale Membrane Reactor for the Generation of Anhydrous Diazomethane. J. Org. Chem. 2016, 81, 5814–5823. [Google Scholar] [CrossRef] [PubMed]
- Maresh, J.; Zhang, J.; Tzeng, Y.-L.; Goodman, N.A.; Lynn, D.G. Rational Design of Inhibitors of VirA–VirG Two-Component Signal Transduction. Bioorganic Med. Chem. Lett. 2007, 17, 3281–3286. [Google Scholar] [CrossRef][Green Version]
- Meguro, K.; Tawada, H.; Miyano, H.; Sato, Y.; Kuwada, Y. Heterocycles. VI. Syntheses of 4H-s-Triazolo [4, 3-a] [1, 4] Benzodiazepines, Novel Tricyclic Psychosedatives. Chem. Pharm. Bull. 1973, 21, 2382–2390. [Google Scholar] [CrossRef][Green Version]
- Agrawal, V.P. Mass Spectra of N ′-isopropylidenealkanohydrazides. J. Mass Spectrom. 1984, 19, 296–297. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S. Synthesis of Acyl Hydrazides and Hydrazones from Activated Amides. Synthesis 2024, 56, 2263–2269. [Google Scholar] [CrossRef]
- Naresh Kumar, R.; Poornachandra, Y.; Nagender, P.; Mallareddy, G.; Ravi Kumar, N.; Ranjithreddy, P.; Ganesh Kumar, C.; Narsaiah, B. Synthesis of Novel Trifluoromethyl Substituted Furo[2,3- b ]Pyridine and Pyrido[3′,2′:4,5]Furo[3,2- d ]Pyrimidine Derivatives as Potential Anticancer Agents. Eur. J. Med. Chem. 2016, 108, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, S.; Hamadi, H.; Kazeminezhad, I. Synthesis of CoFe2O4@Pd/Activated Carbon Nanocomposite as a Recoverable Catalyst for the Reduction of Nitroarenes in Water. J. Solid. State Chem. 2021, 302, 122381. [Google Scholar] [CrossRef]
- Kedzia, A.; Jasiak, K.; Kudelko, A. An Efficient Synthesis of New 2-Aryl-5-Phenylazenyl-1,3,4-Oxadiazole Derivatives from N, N′-Diarylcarbonohydrazides. Synlett 2018, 29, 1745–1748. [Google Scholar] [CrossRef]
- Preetham, R.; Vijaya Kumar, M.S.; Swaroop, T.R.; Kiran, K.R.; Sukrutha, K.P.; Sadashiva, M.P. A Novel and Effective Method for the Synthesis 1,3,4-Oxadiazoles from Carbimidothioates and Benzohydrazides: An Unexpected Cyclization. Synth. Commun. 2023, 53, 568–575. [Google Scholar] [CrossRef]
| Entry | R 1 | Acid Chloride | Product | Yield a [%] |
|---|---|---|---|---|
| 1 | C4H9 | 4-NO2-C6H4-COCl | 5a | 90 |
| 2 | C6H13 | 4-NO2-C6H4-COCl | 5b | 88 |
| 3 | C15H31 | 4-NO2-C6H4-COCl | 5c | 85 |
| 4 | C2H5 | 4-NO2-C6H4-COCl | 5d | 37 |
| 5 | C6H13 | 4-NH2-C6H4-COCl | 5e | 75 |
| Entry | Cyclization Reagent | Solvent | Temperature a [°C] | Time [h] | Yield b [%] |
|---|---|---|---|---|---|
| 1 | POCl3 | toluene | 80 | 8 | 30 |
| 2 | POCl3 | toluene | reflux | 8 | 40 |
| 3 | POCl3 | - | reflux | 24 | 47 |
| 4 | SOCl2 | toluene | 80 | 8 | 55 |
| 5 | SOCl2 | - | reflux | 8 | 70 |
| 6 | SOCl2 | - | reflux | 24 | 78 |
| 7 | P2O5 | - | reflux | 1 | 65 |
| 8 | P2O5 | toluene | reflux | 1 | 70 |
| 9 | P2O5 | toluene | reflux | 1.5 | 80 |
| Entry | R1 | Product 6 | Product 7 |
|---|---|---|---|
| Yield a [%] | Yield b [%] | ||
| 1 | C4H9 (a) | 80 | 91 |
| 2 | C6H13 (b) | 82 | 83 |
| 3 | C15H31 (c) | 78 | 88 |
| 4 | C2H5 (d) | 97 | 96 |
| Entry | Product | R1 | X | Y | Yield a [%] |
|---|---|---|---|---|---|
| 1 | 8a | C4H9 | OH | H | 80 |
| 2 | 8b | C6H13 | OH | H | 78 |
| 3 | 8d | C2H5 | OH | H | 75 |
| 4 | 9a | C4H9 | OH | OH | 93 |
| 5 | 9b | C6H13 | OH | OH | 91 |
| 6 | 9d | C2H5 | OH | OH | 90 |
| 7 | 10a | C4H9 | N(CH3)2 | H | 89 |
| 8 | 10b | C6H13 | N(CH3)2 | H | 84 |
| 9 | 10d | C2H5 | N(CH3)2 | H | 87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górecki, S.; Kudelko, A. Synthesis and Characterization of Novel 2-Alkyl-1,3,4-Oxadiazoles Containing a Phenylazo Group. Molecules 2024, 29, 4316. https://doi.org/10.3390/molecules29184316
Górecki S, Kudelko A. Synthesis and Characterization of Novel 2-Alkyl-1,3,4-Oxadiazoles Containing a Phenylazo Group. Molecules. 2024; 29(18):4316. https://doi.org/10.3390/molecules29184316
Chicago/Turabian StyleGórecki, Sebastian, and Agnieszka Kudelko. 2024. "Synthesis and Characterization of Novel 2-Alkyl-1,3,4-Oxadiazoles Containing a Phenylazo Group" Molecules 29, no. 18: 4316. https://doi.org/10.3390/molecules29184316
APA StyleGórecki, S., & Kudelko, A. (2024). Synthesis and Characterization of Novel 2-Alkyl-1,3,4-Oxadiazoles Containing a Phenylazo Group. Molecules, 29(18), 4316. https://doi.org/10.3390/molecules29184316







