Synthesis and Properties of Dibenzo-Fused Naphtho[2,3-b:6,7-b′]disilole and Naphtho[2,3-b:6,7-b′]diphosphole
Abstract
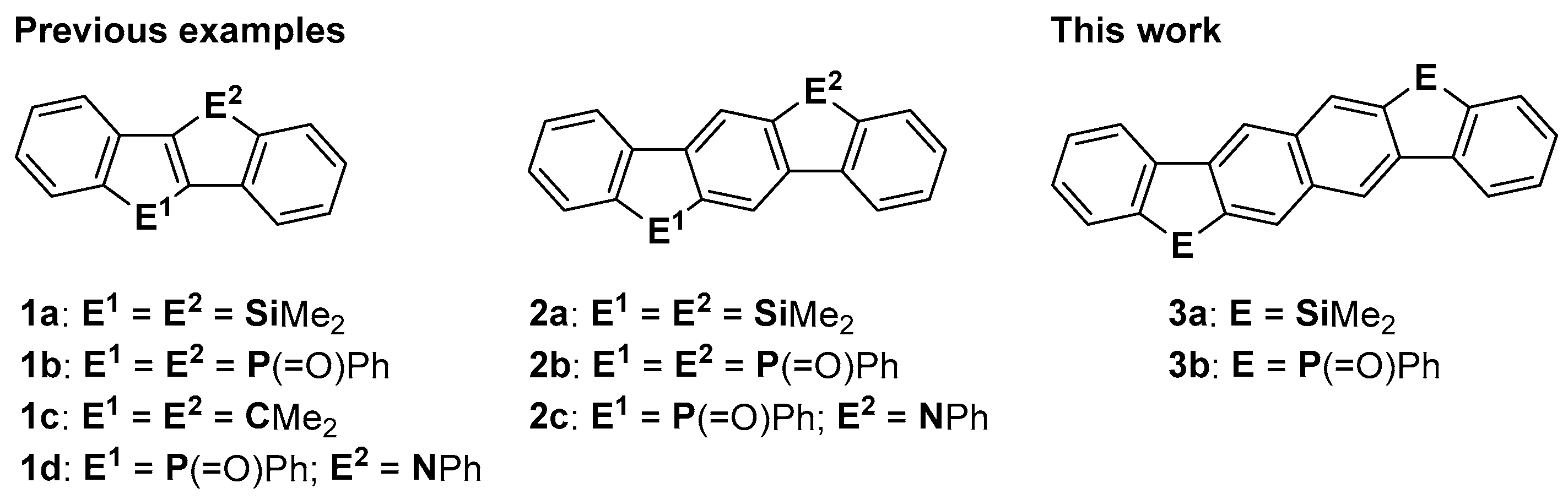
1. Introduction
2. Results
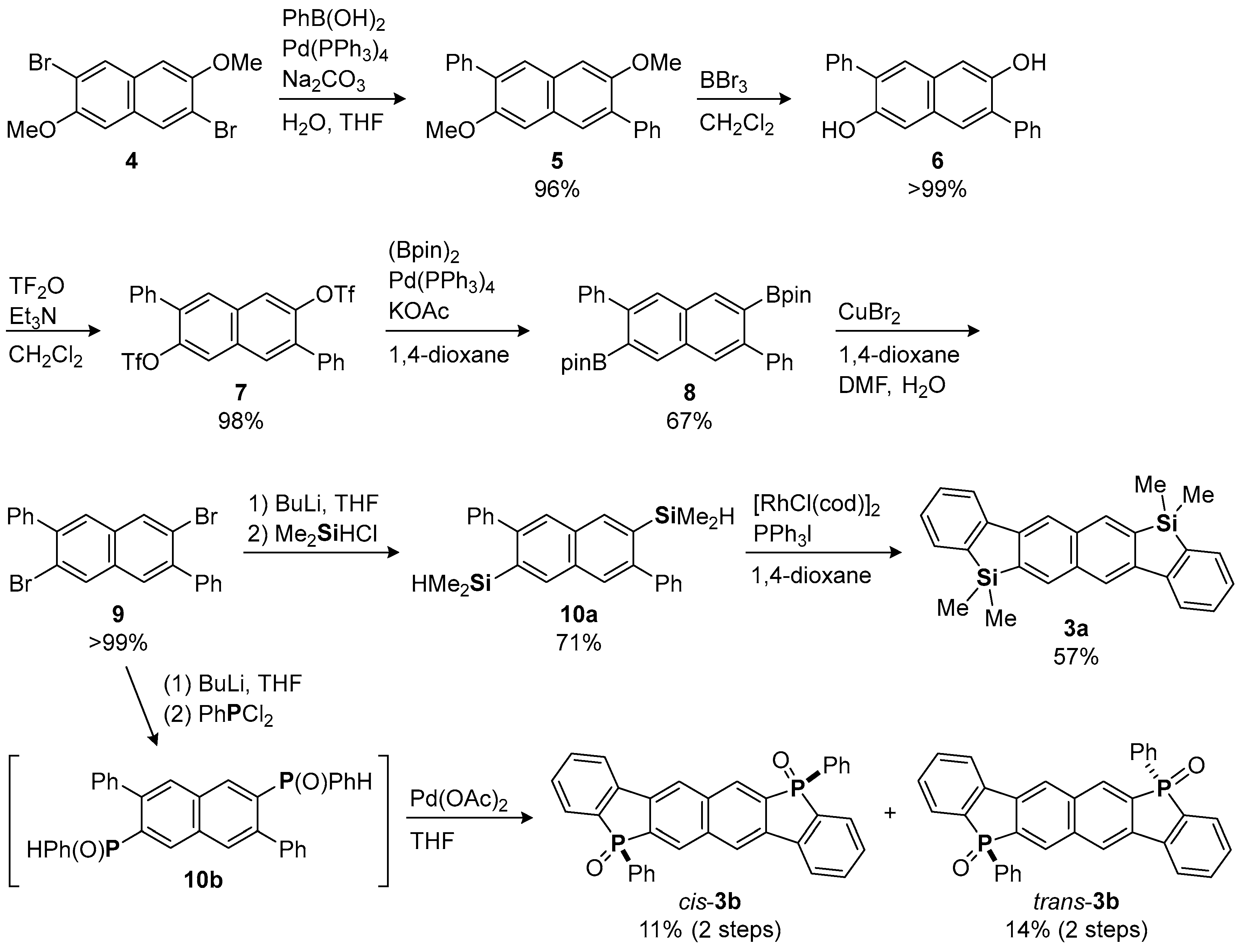
2.1. Synthesis and X-ray Crystallography
2.2. Photophysical Properties
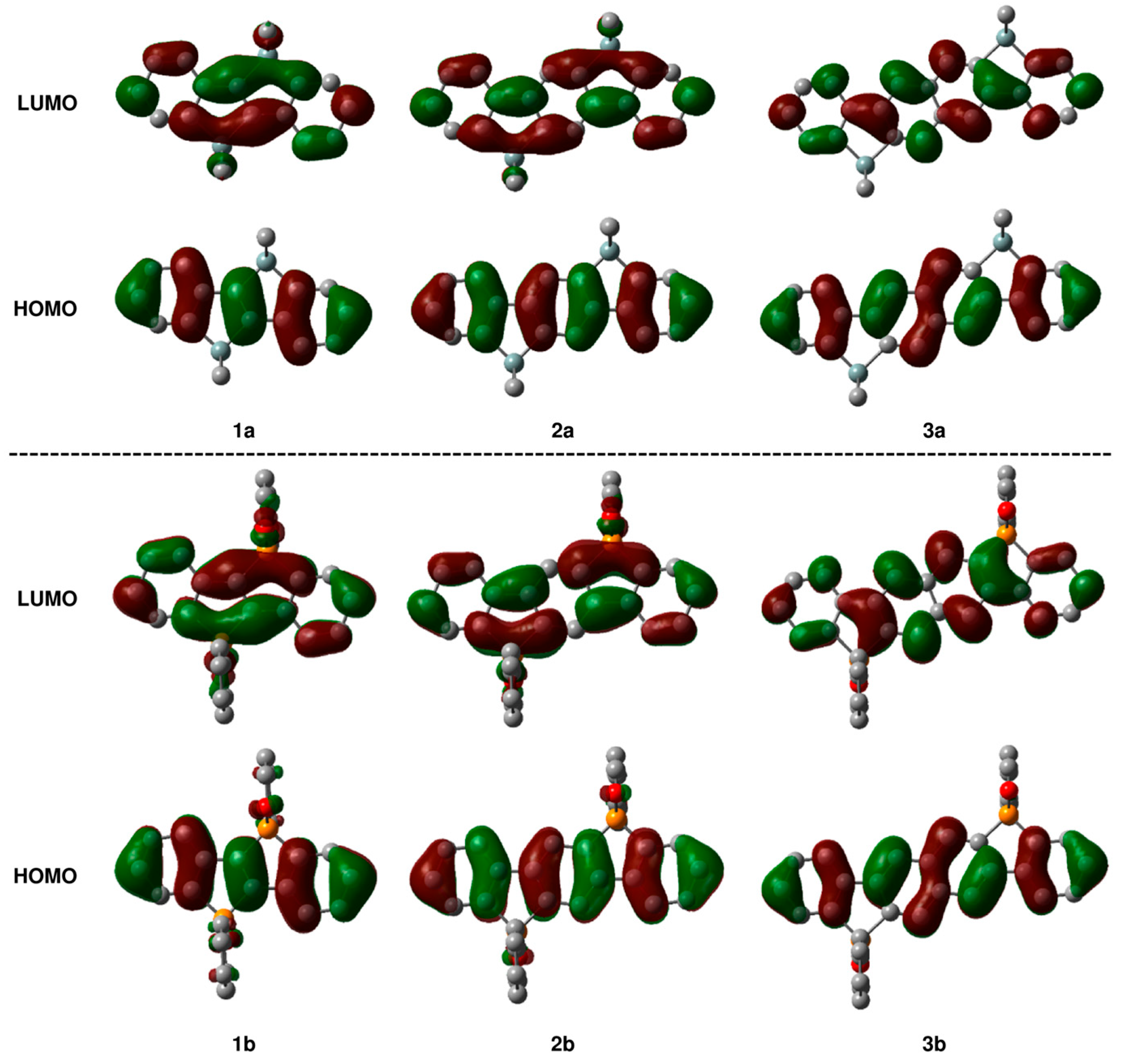
2.3. Theoretical Study on Photophysical Properties
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis
3.3. X-ray Crystallography
3.4. Computational Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Mao, Z.; Xie, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Xu, J.; Chi, Z.; Aldred, M.P. Recent advances in organic thermally activated delayed fluorescence materials. Chem. Soc. Rev. 2017, 46, 915–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Duan, L. Polycyclic Aromatic Hydrocarbon Derivatives toward Ideal Electron-Transporting Materials for Organic Light-Emitting Diodes. J. Phys. Chem. Lett. 2019, 10, 2528–2537. [Google Scholar] [CrossRef] [PubMed]
- Aumaitre, C.; Morin, J.F. Polycyclic Aromatic Hydrocarbons as Potential Building Blocks for Organic Solar Cells. Chem. Rec. 2019, 19, 1142–1154. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Q. Recent progress in non-fullerene small molecule acceptors in organic solar cells (OSCs). J. Mater. Chem. C 2017, 5, 1275–1302. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Chow, P.C.Y.; Jiang, K.; Zhang, J.; Zhu, Z.; Zhang, J.; Huang, F.; Yan, H. Nonfullerene Acceptor Molecules for Bulk Heterojunction Organic Solar Cells. Chem. Rev. 2018, 118, 3447–3507. [Google Scholar] [CrossRef]
- Wang, C.; Dong, H.; Hu, W.; Liu, Y.; Zhu, D. Semiconducting pi-conjugated systems in field-effect transistors: A material odyssey of organic electronics. Chem. Rev. 2012, 112, 2208–2267. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Y.; Zhu, D. Pi-conjugated molecules with fused rings for organic field-effect transistors: Design, synthesis and applications. Chem. Soc. Rev. 2010, 39, 1489–1502. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Y.; Wang, Z. Heteroarenes as high performance organic semiconductors. Chem. Soc. Rev. 2013, 42, 6113–6127. [Google Scholar] [CrossRef]
- Cai, Z.; Awais, M.A.; Zhang, N.; Yu, L. Exploration of Syntheses and Functions of Higher Ladder-type π-Conjugated Heteroacenes. Chem 2018, 4, 2538–2570. [Google Scholar] [CrossRef]
- Skalik, J.; Koprowski, M.; Rozycka-Sokolowska, E.; Balczewski, P. The hetero-Friedel-Crafts-Bradsher Cyclizations with Formation of Ring Carbon-Heteroatom (P, S) Bonds, Leading to Organic Functional Materials. Materials 2020, 13, 4751. [Google Scholar] [CrossRef]
- Matano, Y.; Imahori, H. Design and synthesis of phosphole-based pi systems for novel organic materials. Org. Biomol. Chem. 2009, 7, 1258–1271. [Google Scholar] [CrossRef] [PubMed]
- Stolar, M.; Baumgartner, T. Phosphorus-containing materials for organic electronics. Chem. Asian J. 2014, 9, 1212–1225. [Google Scholar] [CrossRef] [PubMed]
- Hibner-Kulicka, P.; Joule, J.A.; Skalik, J.; Bałczewski, P. Recent studies of the synthesis, functionalization, optoelectronic properties and applications of dibenzophospholes. RSC Adv. 2017, 7, 9194–9236. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Fukazawa, A.; Taki, M. Phosphole P—Oxide—Containing π─Electron Materials: Synthesis and Applications in Fluorescence Imaging. J. Synth. Org. Chem. Jpn. 2017, 75, 1179–1187. [Google Scholar] [CrossRef]
- Ohshita, J. Group 14 metalloles condensed with heteroaromatic systems. Org. Photonics Photovolt. 2016, 4, 52–59. [Google Scholar] [CrossRef]
- Biagiotti, G.; Perini, I.; Richichi, B.; Cicchi, S. Novel Synthetic Approach to Heteroatom Doped Polycyclic Aromatic Hydrocarbons: Optimizing the Bottom-Up Approach to Atomically Precise Doped Nanographenes. Molecules 2021, 26, 6306. [Google Scholar] [CrossRef]
- Itami, K.; Ito, H.; Kawahara, K.P. Heteroatom-Embedding Annulative π-Extension (Hetero-APEX) Reactions: An Overview. Synthesis 2023, 56, 1335–1354. [Google Scholar]
- Delouche, T.; Hissler, M.; Bouit, P.-A. Polycyclic aromatic hydrocarbons containing heavy group 14 elements: From synthetic challenges to optoelectronic devices. Coord. Chem. Rev. 2022, 464, 214553. [Google Scholar] [CrossRef]
- Wu, J.; Wu, S.; Geng, Y.; Yang, G.; Muhammad, S.; Jin, J.; Liao, Y.; Su, Z. Theoretical study on dithieno [3,2-b:2’,3’-d]phosphole derivatives: High-efficiency blue-emitting materials with ambipolar semiconductor behavior. Theor. Chem. Acc. 2010, 127, 419–427. [Google Scholar] [CrossRef]
- Gou, G.; Zhang, Z.; Yuan, B.; Fan, T.; Li, L. Synthesis, photophysical properties and optical stabilities of dimethylthio modified dibenzosiloles: High quantum yield emitters. Dyes Pigm. 2021, 194, 109642. [Google Scholar] [CrossRef]
- Chan, K.L.; McKiernan, M.J.; Towns, C.R.; Holmes, A.B. Poly(2,7-dibenzosilole): A Blue Light Emitting Polymer. J. Am. Chem. Soc. 2005, 127, 7662–7663. [Google Scholar] [CrossRef]
- Dauth, A.; Love, J.A. Synthesis and reactivity of 2-azametallacyclobutanes. Dalton Trans. 2012, 41, 7782–7791. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.; Asok, N.; Beland, V.A.; Gaffen, J.R.; LeBlanc, J.; Baumgartner, T. Development of the P-Arylation of Dithieno [3,2-b : 2’,3’-d]phosphole with Aryl Iodonium Salts. Chempluschem 2023, 88, e202300133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, S.; Zhang, Z.; Yan, X. Synthesis and Photophysical Properties of Silole-Fused Cycloparaphenylenes. J. Org. Chem. 2024, 89, 681–686. [Google Scholar] [CrossRef]
- Uematsu, K.; Hayasaka, C.; Takase, K.; Noguchi, K.; Nakano, K. Transformation of Thia [7]helicene to Aza [7]helicenes and [7]Helicene-like Compounds via Aromatic Metamorphosis. Molecules 2022, 27, 606. [Google Scholar] [CrossRef]
- Kudoh, Y.; Fujii, K.; Kimura, Y.; Minoura, M.; Matano, Y. Synthesis and Optical Properties of 1,2,5,10-Tetraphenylanthra [2,3-b]phosphole Derivatives. J. Org. Chem. 2022, 87, 10493–10500. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Sun, Y.; Zhong, D.; Feng, Z.; Yang, X.; Su, B.; Sun, Y.; Zhou, G.; Jiao, B.; et al. Blue emitters with various electron-donors attached to the 9-phenyl-9-phosphafluorene oxide (PhFIOP) moiety and their thermally activated delayed fluorescence (TADF) behavior. Mater. Chem. Front. 2023, 7, 1841–1854. [Google Scholar] [CrossRef]
- Wang, J.; Taki, M.; Ohba, Y.; Arita, M.; Yamaguchi, S. Fluorescence Lifetime Imaging of Lipid Heterogeneity in the Inner Mitochondrial Membrane with a Super-photostable Environment-Sensitive Probe. Angew. Chem. Int. Ed. 2024, 63, e202404328. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Xu, C.; Tamao, K. Bis-Silicon-Bridged Stilbene Homologues Synthesized by New Intramolecular Reductive Double Cyclization. J. Am. Chem. Soc. 2003, 125, 13662–13663. [Google Scholar] [CrossRef]
- Fukazawa, A.; Hara, M.; Okamoto, T.; Son, E.C.; Xu, C.H.; Tamao, K.; Yamaguchi, S. Bis-phosphoryl-bridged stilbenes synthesized by an intramolecular cascade cyclization. Org. Lett. 2008, 10, 913–916. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Tamao, K. Theoretical Study of the Electronic Structure of 2,2′-Bisilole in Comparison with 1,1′-Bi-1,3-cyclopentadiene: σ*–π* Conjugation and a Low-Lying LUMO as the Origin of the Unusual Optical Properties of 3,3′,4,4′-Tetraphenyl-2,2′-bisilole. Bull. Chem. Soc. Jpn. 1996, 69, 2327–2334. [Google Scholar] [CrossRef]
- Anthony, J.E. Functionalized Acenes and Heteroacenes for Organic Electronics. Chem. Rev. 2006, 106, 5028–5048. [Google Scholar] [CrossRef]
- Müller, M.; Ahrens, L.; Brosius, V.; Freudenberg, J.; Bunz, U.H.F. Unusual stabilization of larger acenes and heteroacenes. J. Mater. Chem. C 2019, 7, 14011–14034. [Google Scholar] [CrossRef]
- Dorel, R.; Echavarren, A.M. Strategies for the Synthesis of Higher Acenes. Eur. J. Org. Chem. 2017, 2017, 601129. [Google Scholar] [CrossRef]
- Hanifi, D.; Pun, A.; Liu, Y. Synthesis and properties of bisphosphole-bridged ladder oligophenylenes. Chem. Asian J. 2012, 7, 2615–2620. [Google Scholar] [CrossRef]
- Li, L.; Xiang, J.; Xu, C. Synthesis of Novel Ladder Bis-Silicon-Bridged p-Terphenyls. Org. Lett. 2007, 9, 4877–4879. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, D.; Chen, Y.H.; Lei, A.; Knochel, P. Preparation of Polyfunctional Biaryl Derivatives by Cyclolanthanation of 2-Bromobiaryls and Heterocyclic Analogues Using nBu(2) LaCl⋅4 LiCl. Angew. Chem. Int. Ed. 2019, 58, 15631–15635. [Google Scholar] [CrossRef]
- Ureshino, T.; Yoshida, T.; Kuninobu, Y.; Takai, K. Rhodium-Catalyzed Synthesis of Silafluorene Derivatives via Cleavage of Silicon−Hydrogen and Carbon−Hydrogen Bonds. J. Am. Chem. Soc. 2010, 132, 14324–14326. [Google Scholar] [CrossRef]
- Shimizu, M.; Mochida, K.; Katoh, M.; Hiyama, T. Photophysical properties of heteroaromatic ring-fused (di)benzosiloles. Sci. China Mater. 2011, 54, 1937–1947. [Google Scholar] [CrossRef]
- Kuninobu, Y.; Yoshida, T.; Takai, K. Palladium-Catalyzed Synthesis of Dibenzophosphole Oxides via Intramolecular Dehydrogenative Cyclization. J. Org. Chem. 2011, 76, 7370–7376. [Google Scholar] [CrossRef]
- Furukawa, S.; Kobayashi, J.; Kawashima, T. Application of the sila-Friedel–Crafts reaction to the synthesis of π-extended silole derivatives and their properties. Dalton Trans. 2010, 39, 9329–9336. [Google Scholar] [CrossRef] [PubMed]
- Truong, M.A.; Morishita, S.; Noguchi, K.; Nakano, K. The Synthesis and Properties of Ladder-Type pi-Conjugated Compounds with Pyrrole and Phosphole Rings. Molecules 2023, 29, 38. [Google Scholar] [CrossRef] [PubMed]
- Higashino, T.; Ishida, K.; Sakurai, T.; Seki, S.; Konishi, T.; Kamada, K.; Kamada, K.; Imahori, H. Pluripotent Features of Doubly Thiophene-Fused Benzodiphospholes as Organic Functional Materials. Chem. Eur. J. 2019, 25, 6425–6438. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Higashino, T.; Wada, Y.; Kaji, H.; Saeki, A.; Imahori, H. Thiophene-Fused Naphthodiphospholes: Modulation of the Structural and Electronic Properties of Polycyclic Aromatics by Precise Fusion of Heteroles. Chempluschem 2021, 86, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Murai, M.; Okada, R.; Asako, S.; Takai, K. Rhodium-Catalyzed Silylative and Germylative Cyclization with Dehydrogenation Leading to 9-Sila- and 9-Germafluorenes: A Combined Experimental and Computational Mechanistic Study. Chem. Eur. J. 2017, 23, 10861–10870. [Google Scholar] [CrossRef]
- Hayasaka, C.; Nagano, S.; Nakano, K. Synthesis of π-extended oxacenes and their application to organic field-effect transistors. Org. Electron. 2022, 100, 106335. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: A suite of computer programs for crystal structure solution of proteins. J. Appl. Crystallogr. 2007, 40, 609–613. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Bruhn, T.; Schaumloffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef]


| λabs (nm) | ε (×104·M·cm−1) | λem (nm) | Φ | |
|---|---|---|---|---|
| 3a a | 347 | 4.67 | 387 | 0.08 (0.30) g |
| cis-3b b | 345, 402 | 5.27, 0.11 | 412 | 0.11 (0.03) g |
| trans-3b b | 345, 402 | 4.63, 0.12 | 412 | 0.11 (0.08) g |
| 1a c | 360 | 1.20 | 426 | 0.58 |
| trans-1b d | 395 | 0.69 | 480 | 0.98 |
| 2a e | 322 | 1.78 | 370 | 0.45 |
| trans-2b f | 330, 383 | ~3.0, 0.28 | 420 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morishita, S.; Hayasaka, C.; Noguchi, K.; Nakano, K. Synthesis and Properties of Dibenzo-Fused Naphtho[2,3-b:6,7-b′]disilole and Naphtho[2,3-b:6,7-b′]diphosphole. Molecules 2024, 29, 4313. https://doi.org/10.3390/molecules29184313
Morishita S, Hayasaka C, Noguchi K, Nakano K. Synthesis and Properties of Dibenzo-Fused Naphtho[2,3-b:6,7-b′]disilole and Naphtho[2,3-b:6,7-b′]diphosphole. Molecules. 2024; 29(18):4313. https://doi.org/10.3390/molecules29184313
Chicago/Turabian StyleMorishita, Suzuho, Chikara Hayasaka, Keiichi Noguchi, and Koji Nakano. 2024. "Synthesis and Properties of Dibenzo-Fused Naphtho[2,3-b:6,7-b′]disilole and Naphtho[2,3-b:6,7-b′]diphosphole" Molecules 29, no. 18: 4313. https://doi.org/10.3390/molecules29184313
APA StyleMorishita, S., Hayasaka, C., Noguchi, K., & Nakano, K. (2024). Synthesis and Properties of Dibenzo-Fused Naphtho[2,3-b:6,7-b′]disilole and Naphtho[2,3-b:6,7-b′]diphosphole. Molecules, 29(18), 4313. https://doi.org/10.3390/molecules29184313







