Computational Exploration of the Mechanism of Action of a Sorafenib-Containing Ruthenium Complex as an Anticancer Agent for Photoactivated Chemotherapy
Abstract
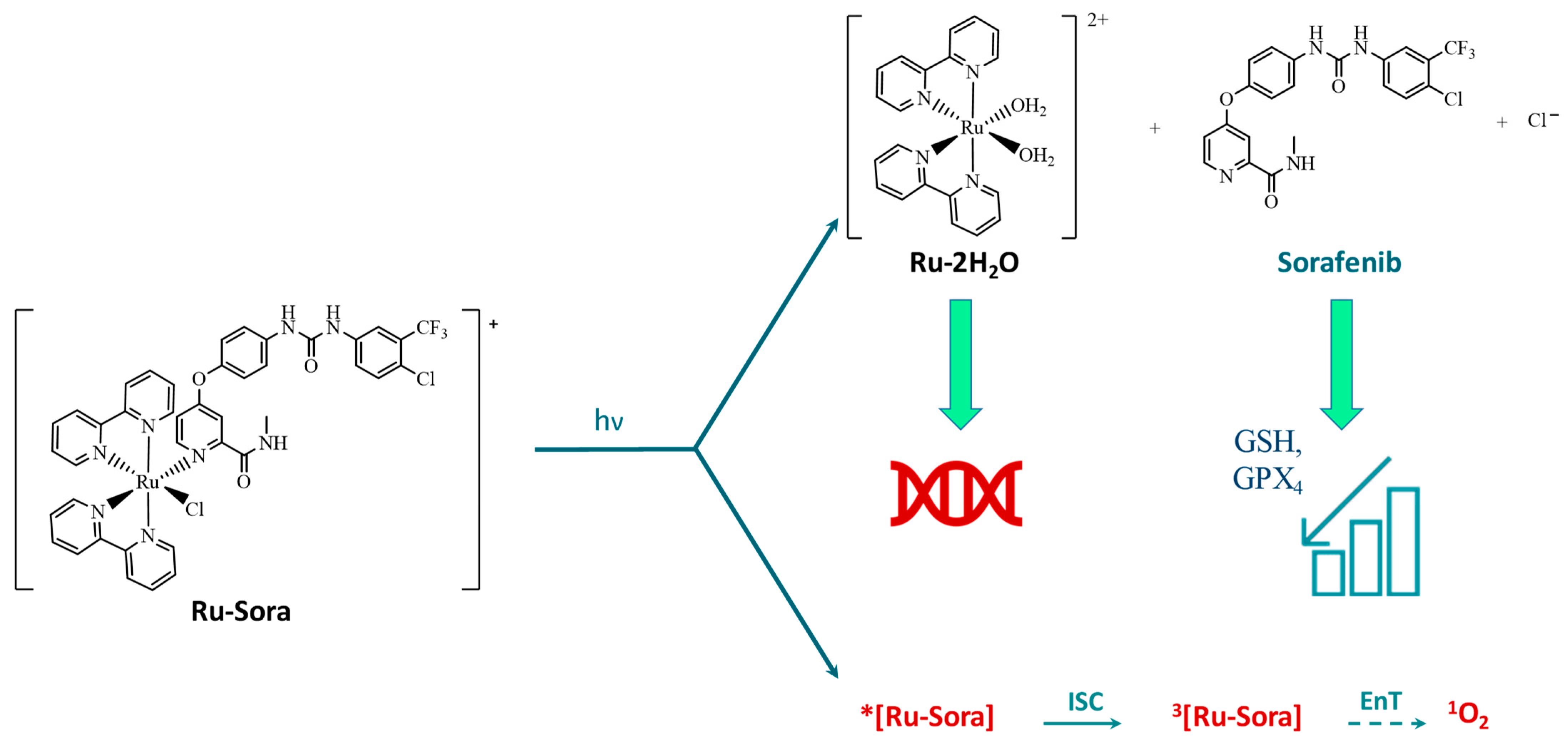
1. Introduction
2. Results and Discussion
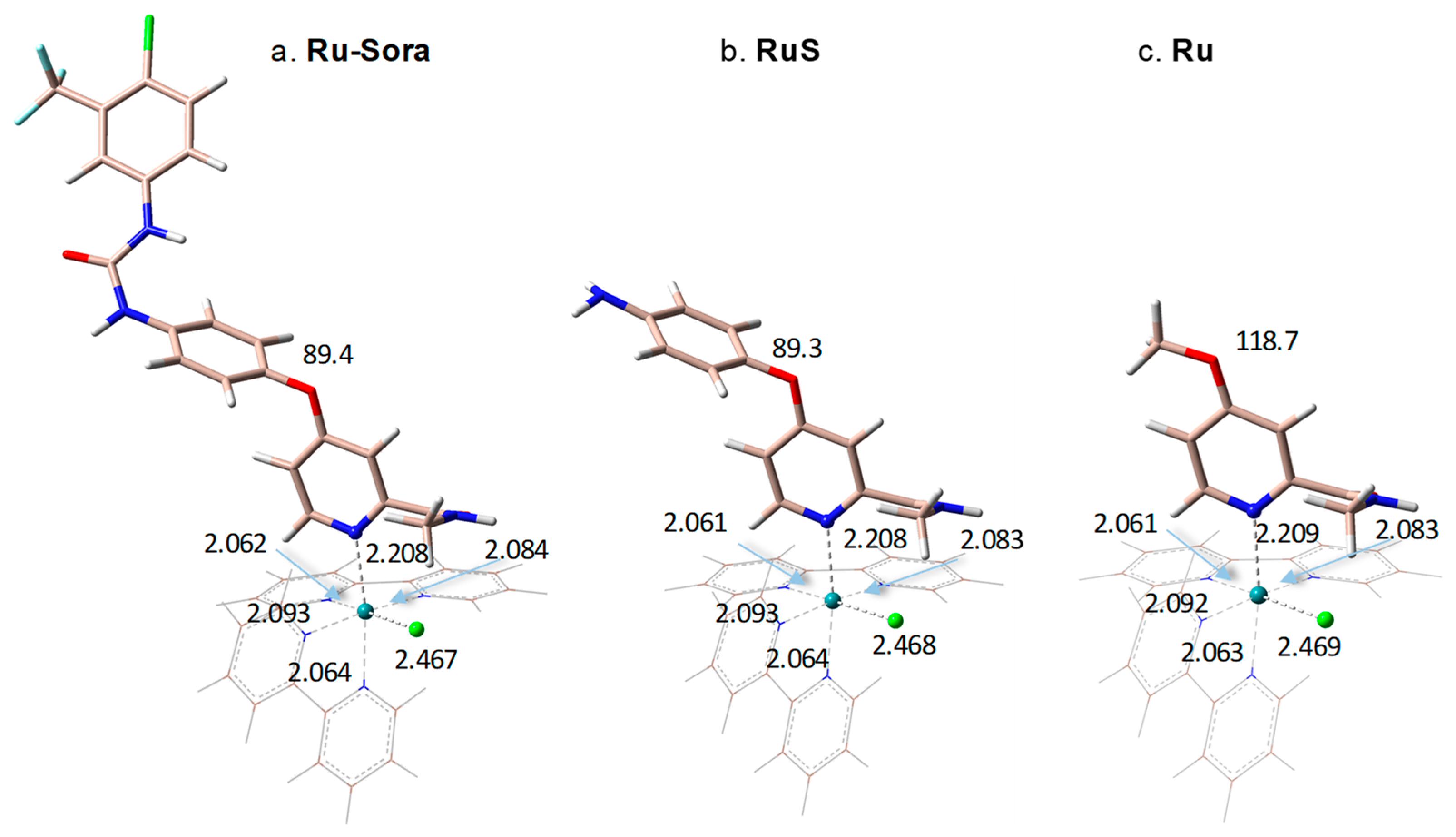
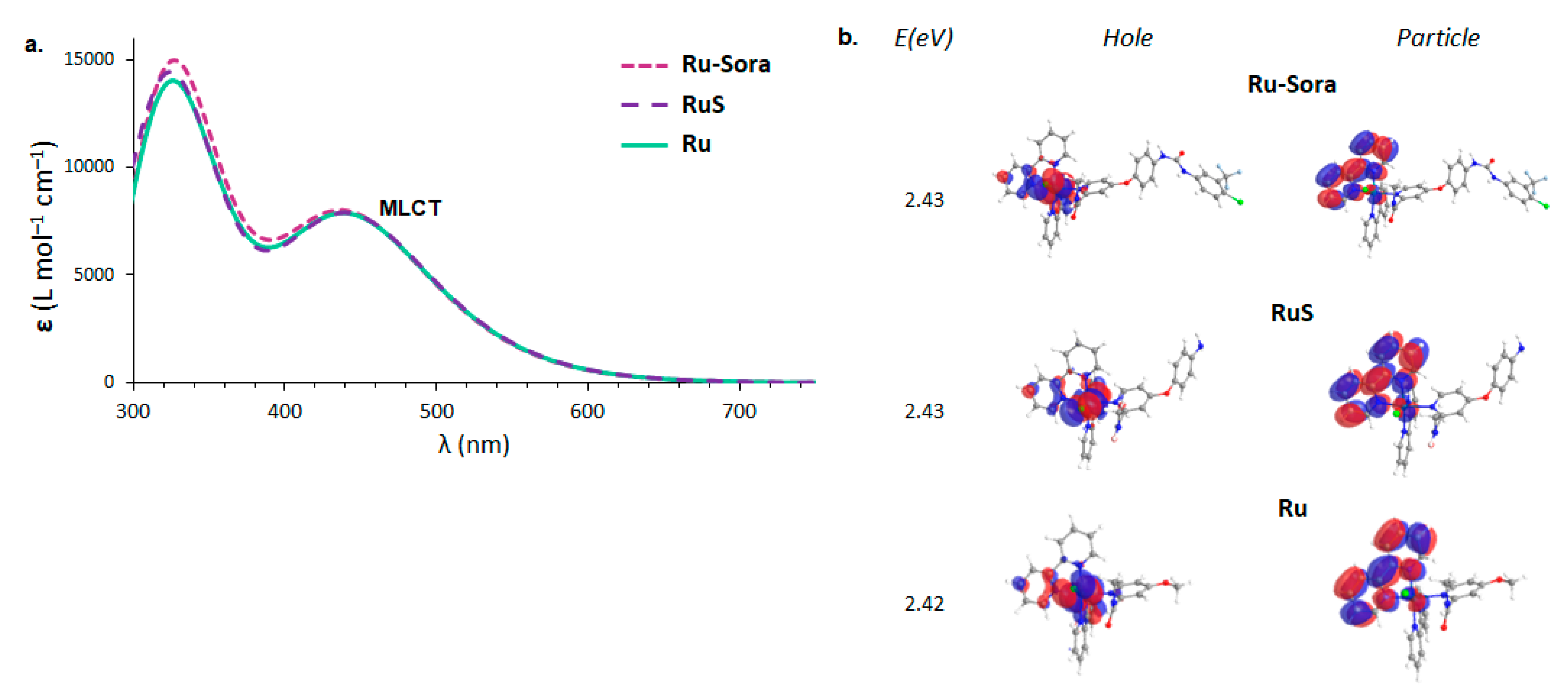
2.1. Structural and Electronic Properties
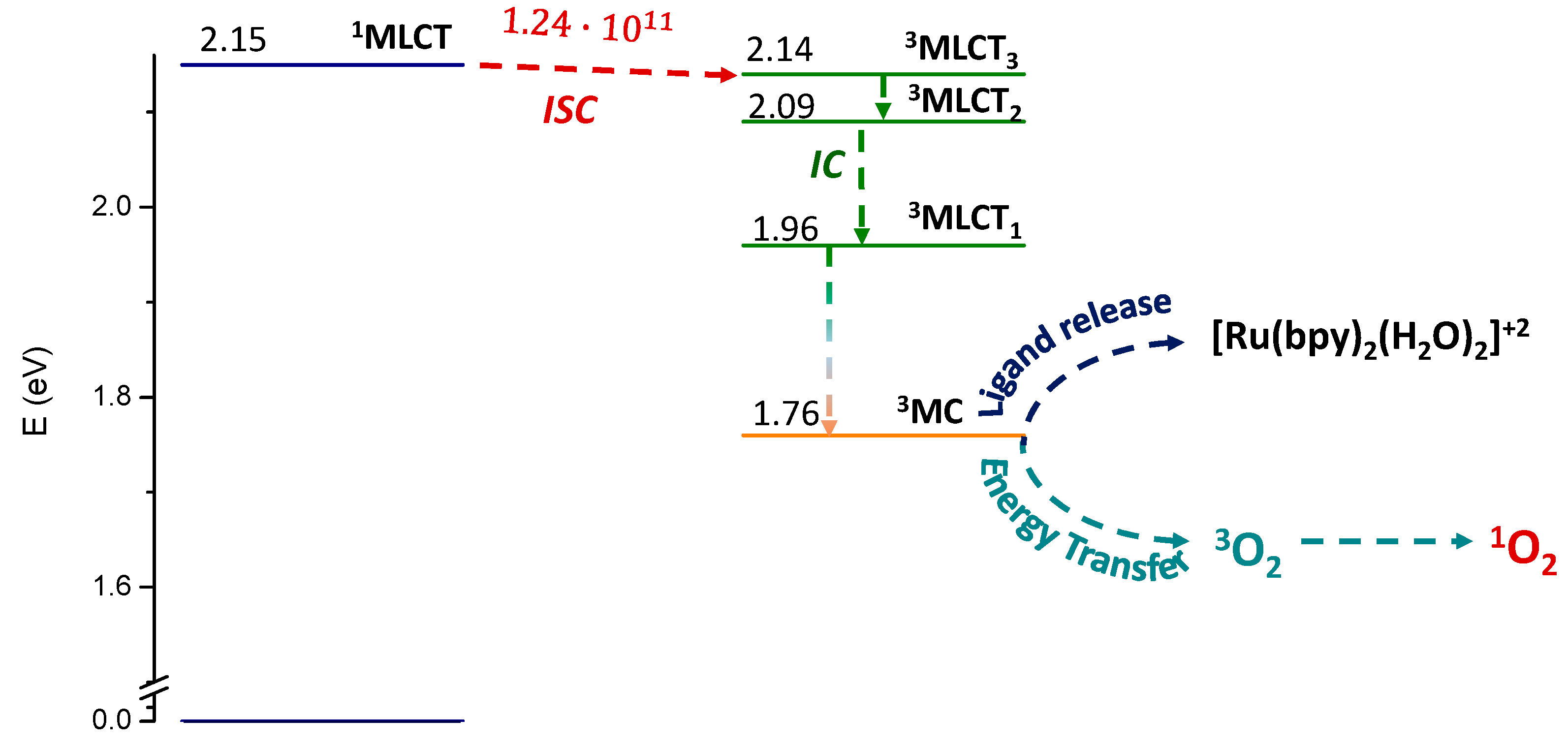
2.2. Photodynamic Processes
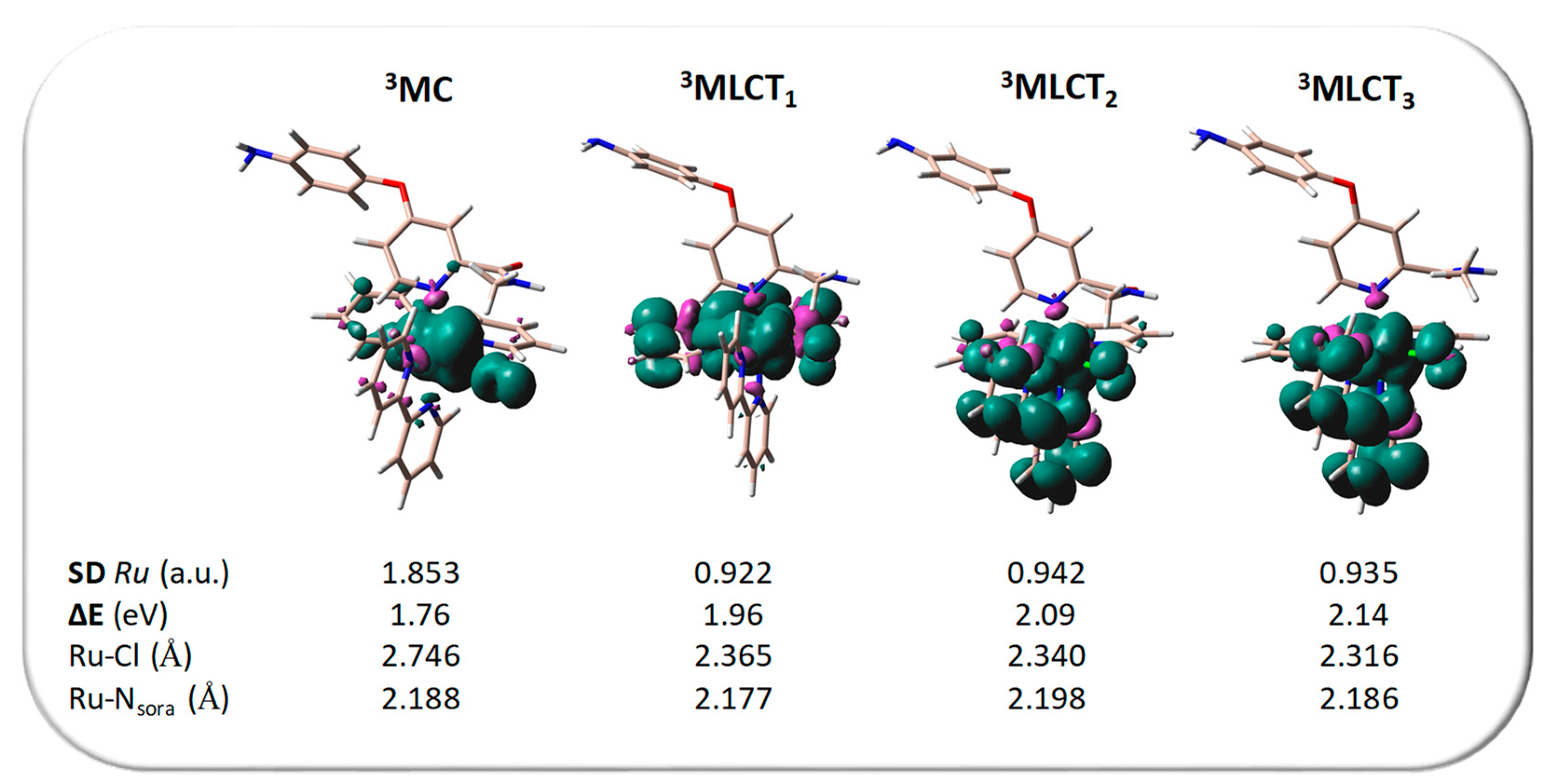
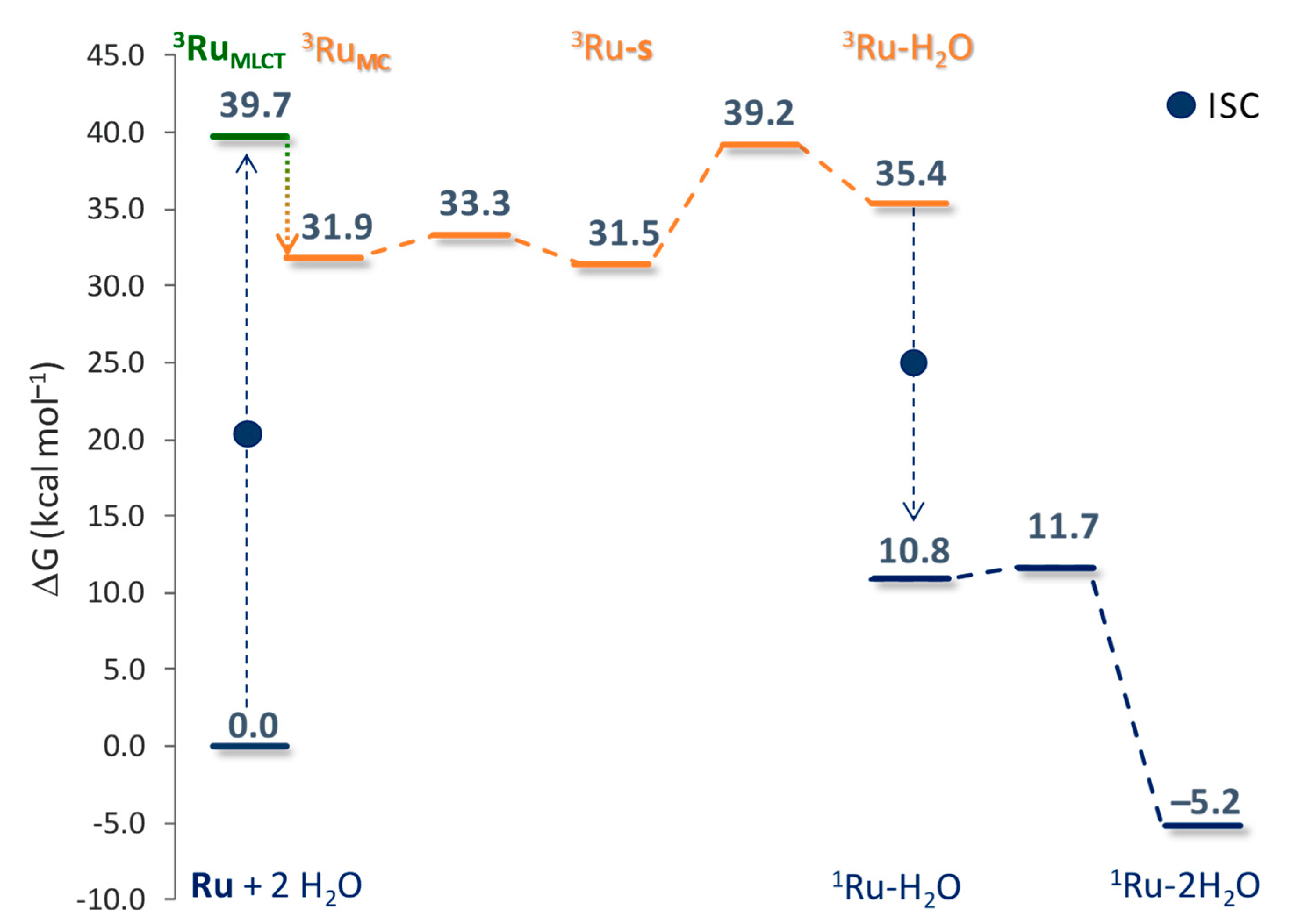
2.3. Sorafenib Release Mechanism
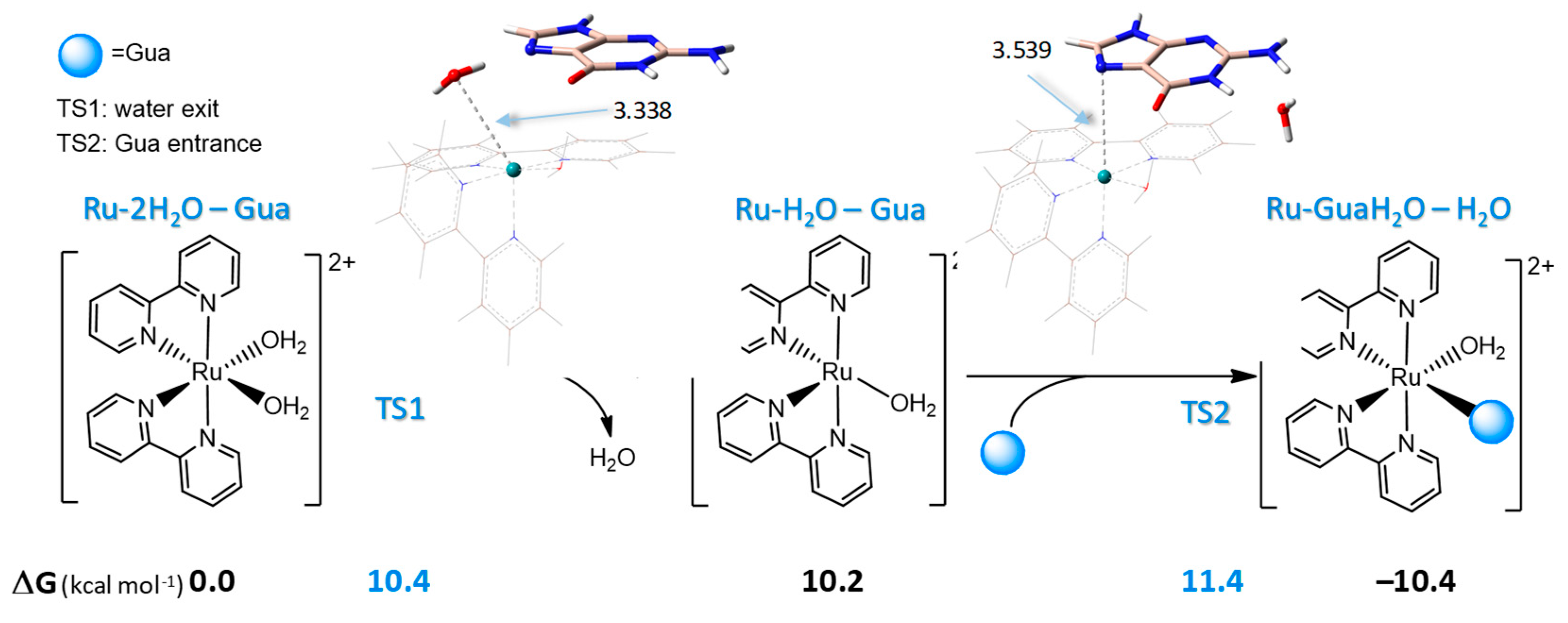
2.4. Interaction of Ru-2H2O with DNA
3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA A Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Barretta, P.; Scoditti, S.; Belletto, D.; Ponte, F.; Vigna, V.; Mazzone, G.; Sicilia, E. Ruthenium Complexes Bearing Nile Red Chromophore and One of Its Derivative: Theoretical Evaluation of PDT-Related Properties. J. Comput. Chem. 2024, 45, 2034–2041. [Google Scholar] [CrossRef]
- Ponte, F.; Scopelliti, D.M.; Sanna, N.; Sicilia, E.; Mazzone, G. How Computations Can Assist the Rational Design of Drugs for Photodynamic Therapy: Photosensitizing Activity Assessment of a Ru(II)-BODIPY Assembly. Molecules 2022, 27, 5635. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Cao, B.; Zhong, M.; Liu, M.; Xiong, X.; Zou, T. Organogold(III) Complexes Display Conditional Photoactivities: Evolving From Photodynamic into Photoactivated Chemotherapy in Response to O2 Consumption for Robust Cancer Therapy. Angew. Chem. Int. Ed. 2022, 61, e202212689. [Google Scholar] [CrossRef] [PubMed]
- Hakkennes, M.L.A.; Meijer, M.S.; Menzel, J.P.; Goetz, A.-C.; Van Duijn, R.; Siegler, M.A.; Buda, F.; Bonnet, S. Ligand Rigidity Steers the Selectivity and Efficiency of the Photosubstitution Reaction of Strained Ruthenium Polypyridyl Complexes. J. Am. Chem. Soc. 2023, 145, 13420–13434. [Google Scholar] [CrossRef]
- Toupin, N.; Steinke, S.J.; Nadella, S.; Li, A.; Rohrabaugh, T.N., Jr.; Samuels, E.R.; Turro, C.; Sevrioukova, I.F.; Kodanko, J.J. Photosensitive Ru(II) Complexes as Inhibitors of the Major Human Drug Metabolizing Enzyme CYP3A4. J. Am. Chem. Soc. 2021, 143, 9191–9205. [Google Scholar] [CrossRef]
- Huang, C.; Liang, C.; Sadhukhan, T.; Banerjee, S.; Fan, Z.; Li, T.; Zhu, Z.; Zhang, P.; Raghavachari, K.; Huang, H. In-Vitro and In-Vivo Photocatalytic Cancer Therapy with Biocompatible Iridium(III) Photocatalysts. Angew. Chem. Int. Ed. 2021, 60, 9474–9479. [Google Scholar] [CrossRef]
- Farrer, N.J.; Salassa, L.; Sadler, P.J. Photoactivated Chemotherapy (PACT): The Potential of Excited-State d-Block Metals in Medicine. Dalton Trans. 2009, 10690–10701. [Google Scholar] [CrossRef]
- Schatzschneider, U. Photoactivated Biological Activity of Transition-Metal Complexes. Eur. J. Inorg. Chem. 2010, 2010, 1451–1467. [Google Scholar] [CrossRef]
- Farrer, N.J.; Woods, J.A.; Salassa, L.; Zhao, Y.; Robinson, K.S.; Clarkson, G.; Mackay, F.S.; Sadler, P.J. A Potent Trans-Diimine Platinum Anticancer Complex Photoactivated by Visible Light. Angew. Chem. Int. Ed. 2010, 49, 8905–8908. [Google Scholar] [CrossRef] [PubMed]
- Imberti, C.; Zhang, P.; Huang, H.; Sadler, P.J. New Designs for Phototherapeutic Transition Metal Complexes. Angew. Chem. Int. Ed. 2020, 59, 61–73. [Google Scholar] [CrossRef]
- Shi, H.; Carter, O.W.L.; Ponte, F.; Imberti, C.; Gomez-Gonzalez, M.A.; Cacho-Nerin, F.; Quinn, P.D.; Parker, J.E.; Sicilia, E.; Huang, H.; et al. A Photodynamic and Photochemotherapeutic Platinum-Iridium Charge-Transfer Conjugate for Anticancer Therapy. Angew. Chem. Int. Ed. 2024, 63, e202400476. [Google Scholar] [CrossRef]
- Steinke, S.J.; Gupta, S.; Piechota, E.J.; Moore, C.E.; Kodanko, J.J.; Turro, C. Photocytotoxicity and Photoinduced Phosphine Ligand Exchange in a Ru(II) Polypyridyl Complex. Chem. Sci. 2022, 13, 1933–1945. [Google Scholar] [CrossRef]
- Denison, M.; Garcia, S.P.; Ullrich, A.; Podgorski, I.; Gibson, H.; Turro, C.; Kodanko, J.J. Ruthenium-Cathepsin Inhibitor Conjugates for Green Light-Activated Photodynamic Therapy and Photochemotherapy. Inorg. Chem. 2024, 63, 7973–7983. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Xiao, C.; Li, Z.; Yang, X. Engineering Nanomedicine for Glutathione Depletion-Augmented Cancer Therapy. Chem. Soc. Rev. 2021, 50, 6013–6041. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Yang, M.; Dong, X. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv. Mater. 2019, 31, 1904197. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Lai, Y.; Lu, N.; Luo, S.; Wang, H.; Zhang, P. A Photoactivated Sorafenib-Ruthenium(II) Prodrug for Resistant Hepatocellular Carcinoma Therapy through Ferroptosis and Purine Metabolism Disruption. J. Med. Chem. 2022, 65, 13041–13051. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, P.S.; Bhattarai, H.K. Singlet Oxygen, Photodynamic Therapy, and Mechanisms of Cancer Cell Death. J. Oncol. 2022, 2022, 7211485. [Google Scholar] [CrossRef]
- Österman, T.; Persson, P. Excited State Potential Energy Surfaces of Bistridentate RuII Complexes—A TD-DFT Study. Chem. Phys. 2012, 407, 76–82. [Google Scholar] [CrossRef]
- Alcover-Fortuny, G.; Wu, J.; Caballol, R.; de Graaf, C. Quantum Chemical Study of the Interligand Electron Transfer in Ru Polypyridyl Complexes. J. Phys. Chem. A 2018, 122, 1114–1123. [Google Scholar] [CrossRef]
- Li, Y.; Fan, X.-W.; Wang, J.; Kong, C.-P.; Chen, J.; Wang, S.-P.; Li, H.-C.; Bai, F.-Q.; Zhang, H.-X. Comparative Study on the Photophysical Properties between Carbene-Based Fe (II) and Ru (II) Complexes. Appl. Organomet. Chem. 2020, 34, e5821. [Google Scholar] [CrossRef]
- Butera, V.; Mazzone, G.; Detz, H. Dinuclear Ruthenium(II)-Pyrrolide Complexes Linked by Different Organic Units as PDT Photosensitizers: Computational Study of the Linker Influence on the Photophysical Properties. ChemPhotoChem 2022, 6, e202200094. [Google Scholar] [CrossRef]
- Escudero, D.; González, L. RASPT2/RASSCF vs. Range-Separated/Hybrid DFT Methods: Assessing the Excited States of a Ru(II)Bipyridyl Complex. J. Chem. Theory Comput. 2012, 8, 203–213. [Google Scholar] [CrossRef]
- Elias, M.G.; Mehanna, S.; Elias, E.; Khnayzer, R.S.; Daher, C.F. A Photoactivatable Chemotherapeutic Ru(II) Complex Bearing Bathocuproine Ligand Efficiently Induces Cell Death in Human Malignant Melanoma Cells through a Multi-Mechanistic Pathway. Chem.-Biol. Interact. 2021, 348, 109644. [Google Scholar] [CrossRef]
- Spiegel, M.; Adamo, C. Tuning the Photophysical Properties of Ru(II) Photosensitizers for PDT by Protonation and Metallation: A DFT Study. J. Phys. Chem. A 2023, 127, 3625–3635. [Google Scholar] [CrossRef]
- Plasser, F. TheoDORE: A Toolbox for a Detailed and Automated Analysis of Electronic Excited State Computations. J. Chem. Phys. 2020, 152, 084108. [Google Scholar] [CrossRef]
- Zeng, C.; Li, Y.; Zheng, H.; Ren, M.; Wu, W.; Chen, Z. Nature of Ultrafast Dynamics in the Lowest-Lying Singlet Excited State of [Ru(Bpy)3]2+. Phys. Chem. Chem. Phys. 2024, 26, 6524–6531. [Google Scholar] [CrossRef] [PubMed]
- Dabbish, E.; Mazzone, G.; Russo, N.; Sicilia, E. Mechanism of Action of the Curcumin Cis-Diammineplatinum(II) Complex as a Photocytotoxic Agent. Inorg. Chem. Front. 2020, 7, 2759–2769. [Google Scholar] [CrossRef]
- Simone, B.C.D.; Mazzone, G.; Russo, N.; Sicilia, E.; Toscano, M. Excitation Energies, Singlet–Triplet Energy Gaps, Spin–Orbit Matrix Elements and Heavy Atom Effects in BOIMPYs as Possible Photosensitizers for Photodynamic Therapy: A Computational Investigation. Phys. Chem. Chem. Phys. 2018, 20, 2656–2661. [Google Scholar] [CrossRef] [PubMed]
- Hemnani, T.; Parihar, M.S. Reactive oxygen species and oxidative dna damage. Indian J. Physiol. Pharmacol. 1998, 42, 440–452. [Google Scholar] [PubMed]
- Belletto, D.; Ponte, F.; Mazzone, G.; Sicilia, E. A Detailed Density Functional Theory Exploration of the Photodissociation Mechanism of Ruthenium Complexes for Photoactivated Chemotherapy. Dalton Trans. 2024, 53, 8243–8253. [Google Scholar] [CrossRef] [PubMed]
- Kayanuma, M. Photosubstitution Reaction of a Bidentate Ligand in a Ru(II) Complex in Aqueous Solution. Comput. Theor. Chem. 2022, 1213, 113745. [Google Scholar] [CrossRef]
- Soupart, A.; Dixon, I.M.; Alary, F.; Heully, J.-L. DFT Rationalization of the Room-Temperature Luminescence Properties of Ru(Bpy)32+ and Ru(Tpy)22+: 3MLCT–3MC Minimum Energy Path from NEB Calculations and Emission Spectra from VRES Calculations. Theor. Chem. Acc. 2018, 137, 37. [Google Scholar] [CrossRef]
- Yin, C.-W.; Tsai, M.-K.; Chen, Y.J. Low-Temperature Observation of the Excited-State Decay of Ruthenium-(Mono-2,2′:6′,2″-Terpyridine) Ions with Innocent Ligands: DFT Modeling of an 3MLCT–3MC Intersystem Crossing Pathway. ACS Omega 2023, 8, 11623–11633. [Google Scholar] [CrossRef]
- Nisbett, K.; Tu, Y.-J.; Turro, C.; Kodanko, J.J.; Schlegel, H.B. DFT Investigation of Ligand Photodissociation in [RuII(Tpy)(Bpy)(Py)]2+ and [RuII(Tpy)(Me2bpy)(Py)]2+ Complexes. Inorg. Chem. 2018, 57, 231–240. [Google Scholar] [CrossRef]
- Busemann, A.; Flaspohler, I.; Zhou, X.-Q.; Schmidt, C.; Goetzfried, S.K.; van Rixel, V.H.S.; Ott, I.; Siegler, M.A.; Bonnet, S. Ruthenium-Based PACT Agents Based on Bisquinoline Chelates: Synthesis, Photochemistry, and Cytotoxicity. J. Biol. Inorg. Chem. 2021, 26, 667–674. [Google Scholar] [CrossRef]
- Betanzos-Lara, S.; Habtemariam, A.; Clarkson, G.J.; Sadler, P.J. Organometallic Cis-Dichlorido Ruthenium(II) Ammine Complexes. Eur. J. Inorg. Chem. 2011, 2011, 3257–3264. [Google Scholar] [CrossRef]
- Chen, Q.; Cuello-Garibo, J.-A.; Bretin, L.; Zhang, L.; Ramu, V.; Aydar, Y.; Batsiun, Y.; Bronkhorst, S.; Husiev, Y.; Beztsinna, N.; et al. Photosubstitution in a Trisheteroleptic Ruthenium Complex Inhibits Conjunctival Melanoma Growth in a Zebrafish Orthotopic Xenograft Model. Chem. Sci. 2022, 13, 6899–6919. [Google Scholar] [CrossRef] [PubMed]
- Zamora, A.; Denning, C.A.; Heidary, D.K.; Wachter, E.; Nease, L.A.; Ruiz, J.; Glazer, E.C. Ruthenium-Containing P450 Inhibitors for Dual Enzyme Inhibition and DNA Damage. Dalton Trans. 2017, 46, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Bešker, N.; Coletti, C.; Marrone, A.; Re, N. Binding of Antitumor Ruthenium Complexes to DNA and Proteins: A Theoretical Approach. J. Phys. Chem. B 2007, 111, 9955–9964. [Google Scholar] [CrossRef] [PubMed]
- Ponte, F.; Belletto, D.; Leonetti, R.; Sanna, N.; Scoditti, S.; Mazzone, G.; Sicilia, E. DFT Computational Analysis of the Mechanism of Action of Ru(II) Polypyridyl Complexes as Photoactivated Chemotherapy Agents: From Photoinduced Ligand Solvolysis to DNA Binding. Inorg. Chem. 2024. submitted. [Google Scholar]
- Barretta, P.; Ponte, F.; Scoditti, S.; Vigna, V.; Mazzone, G.; Sicilia, E. Computational Analysis of the Behavior of BODIPY Decorated Monofunctional Platinum(II) Complexes in the Dark and under Light Irradiation. J. Phys. Chem. A 2022, 126, 7159–7167. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, G.; Scoditti, S.; Caligiuri, R.; Ricciardi, L.; Sicilia, E.; Lupo, M.G.; Rimoldi, I.; Godbert, N.; La Deda, M.; Ionescu, A.; et al. Cytotoxicity of Alizarine versus Tetrabromocathecol Cyclometalated Pt(II) Theranostic Agents: A Combined Experimental and Computational Investigation. Inorg. Chem. 2022, 61, 7188–7200. [Google Scholar] [CrossRef] [PubMed]
- Belletto, D.; Ponte, F.; Sanna, N.; Scoditti, S.; Sicilia, E. G-Quadruplex DNA Selective Targeting for Anticancer Therapy: A Computational Study of a Novel PtII Monofunctional Complex Activated by Adaptive Binding. Dalton Trans. 2023, 52, 13517–13527. [Google Scholar] [CrossRef] [PubMed]
- Šebesta, F.; Burda, J.V. Study on Electronic Properties, Thermodynamic and Kinetic Parameters of the Selected Platinum(II) Derivatives Interacting with Guanine. J. Inorg. Biochem. 2017, 172, 100–109. [Google Scholar] [CrossRef]
- Alberto, M.E.; Butera, V.; Russo, N. Which One among the Pt-Containing Anticancer Drugs More Easily Forms Monoadducts with G and A DNA Bases? A Comparative Study among Oxaliplatin, Nedaplatin, and Carboplatin. Inorg. Chem. 2011, 50, 6965–6971. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; GaussView 5.0.; Wallingford, E.U.A., Ed.; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted Ab Initio Pseudopotentials for the Rare Earth Elements. J. Chem. Phys. 1989, 90, 1730–1734. [Google Scholar] [CrossRef]
- Butera, V.; Detz, H. Hydrogenation of CO2 to Methanol by the Diphosphine–Ruthenium(II) Cationic Complex: A DFT Investigation to Shed Light on the Decisive Role of Carboxylic Acids as Promoters. Catal. Sci. Technol. 2021, 11, 3556–3567. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Hirata, S.; Head-Gordon, M. Time-Dependent Density Functional Theory within the Tamm–Dancoff Approximation. Chem. Phys. Lett. 1999, 314, 291–299. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange–Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Account. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. A New Local Density Functional for Main-Group Thermochemistry, Transition Metal Bonding, Thermochemical Kinetics, and Noncovalent Interactions. J. Chem. Phys. 2006, 125, 194101. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof Exchange-Correlation Functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System, Version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Barretta, P.; Ponte, F.; Scoditti, S.; Mazzone, G. Computational Assessment of Novel Ruthenium Phenoxazine-Based Complexes as Photosensitizers in Photodynamic Therapy. Eur. J. Inorg. Chem. 2024, e202400309. [Google Scholar] [CrossRef]
- Pantazis, D.A.; Chen, X.-Y.; Landis, C.R.; Neese, F. All-Electron Scalar Relativistic Basis Sets for Third-Row Transition Metal Atoms. J. Chem. Theory Comput. 2008, 4, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Fortino, M.; Collini, E.; Bloino, J.; Pedone, A. Unraveling the Internal Conversion Process within the Q-Bands of a Chlorophyll-like-System through Surface-Hopping Molecular Dynamics Simulations. J. Chem. Phys. 2021, 154, 094110. [Google Scholar] [CrossRef]
- Fortino, M.; Cozza, C.; Bonomi, M.; Pietropaolo, A. Multi-Replica Biased Sampling for Photoswitchable π-Conjugated Polymers. J. Chem. Phys. 2021, 154, 174108. [Google Scholar] [CrossRef]


| [Ru]+ | 3[Ru]+ | O2 | |
|---|---|---|---|
| VEA a | −2.49 | −4.52 | −2.09 |
| VIP a | 5.17 | 3.13 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barretta, P.; Ponte, F.; Escudero, D.; Mazzone, G. Computational Exploration of the Mechanism of Action of a Sorafenib-Containing Ruthenium Complex as an Anticancer Agent for Photoactivated Chemotherapy. Molecules 2024, 29, 4298. https://doi.org/10.3390/molecules29184298
Barretta P, Ponte F, Escudero D, Mazzone G. Computational Exploration of the Mechanism of Action of a Sorafenib-Containing Ruthenium Complex as an Anticancer Agent for Photoactivated Chemotherapy. Molecules. 2024; 29(18):4298. https://doi.org/10.3390/molecules29184298
Chicago/Turabian StyleBarretta, Pierraffaele, Fortuna Ponte, Daniel Escudero, and Gloria Mazzone. 2024. "Computational Exploration of the Mechanism of Action of a Sorafenib-Containing Ruthenium Complex as an Anticancer Agent for Photoactivated Chemotherapy" Molecules 29, no. 18: 4298. https://doi.org/10.3390/molecules29184298
APA StyleBarretta, P., Ponte, F., Escudero, D., & Mazzone, G. (2024). Computational Exploration of the Mechanism of Action of a Sorafenib-Containing Ruthenium Complex as an Anticancer Agent for Photoactivated Chemotherapy. Molecules, 29(18), 4298. https://doi.org/10.3390/molecules29184298





