Ionic Liquids toward Enhanced Carotenoid Extraction from Bacterial Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microorganism and Culture Conditions
2.3. Carotenoid Extraction Using Ionic Liquids
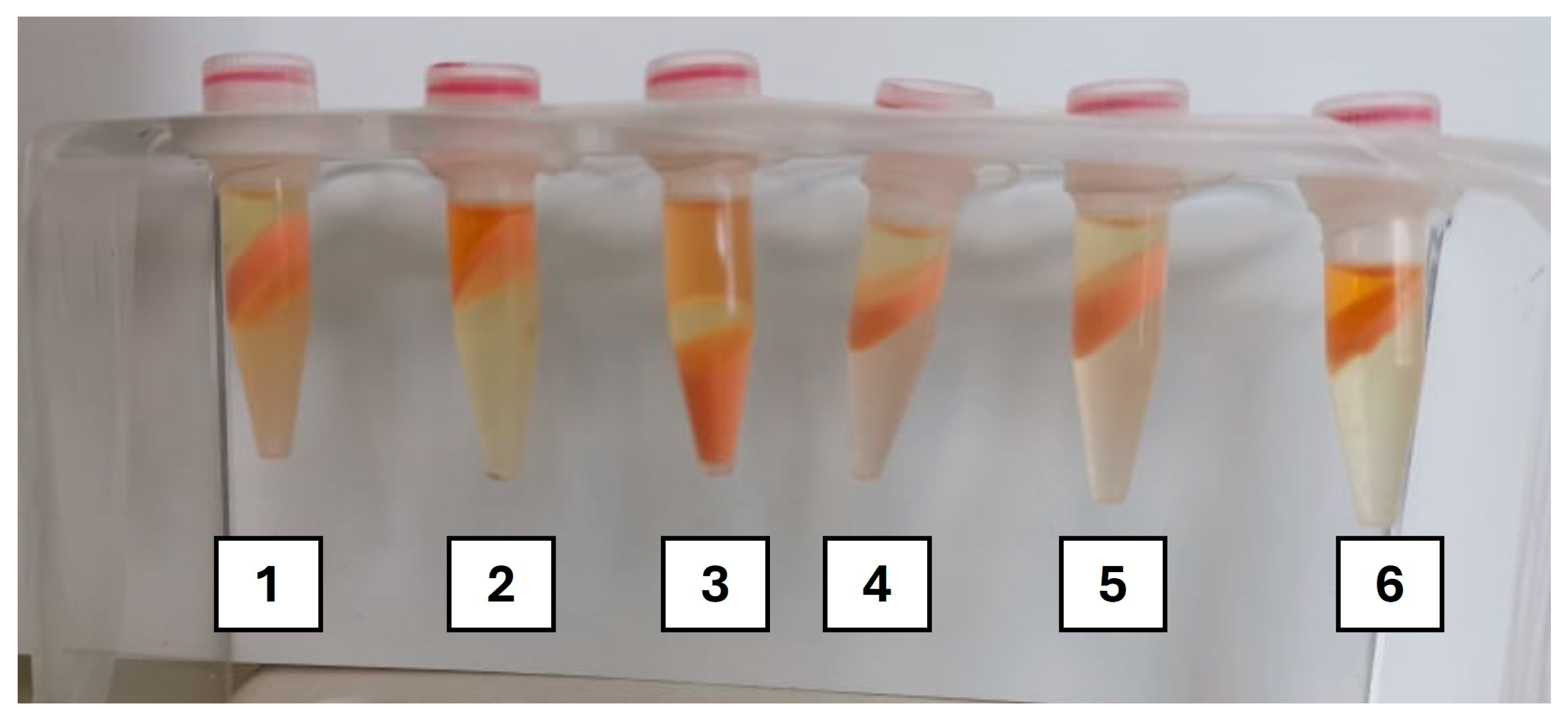
2.3.1. Initial Screening
2.3.2. Experimental Design Methodology
- -
- #ED1 had a wider range to broadly understand how each of the factors influenced the response. Thus, the volume of IL#18 (X1) ranged between 25 µL and 1000 µL, and the volume of EAc (X2) ranged between 250 µL and 2000 µL.
- -
- #ED2 was based on the results of #ED1, with the goal of improving extraction efficiency. Therefore, the volume of IL#18 (X1) ranged between 0 µL and 50 µL, and the volume of EAc (X2) ranged between 250 µL and 1125 µL.
2.4. Validation of the Extraction Procedure with IL#18 and EAc
3. Results and Discussion
3.1. Extraction of Carotenoids Using ILs
3.1.1. Screening Assays
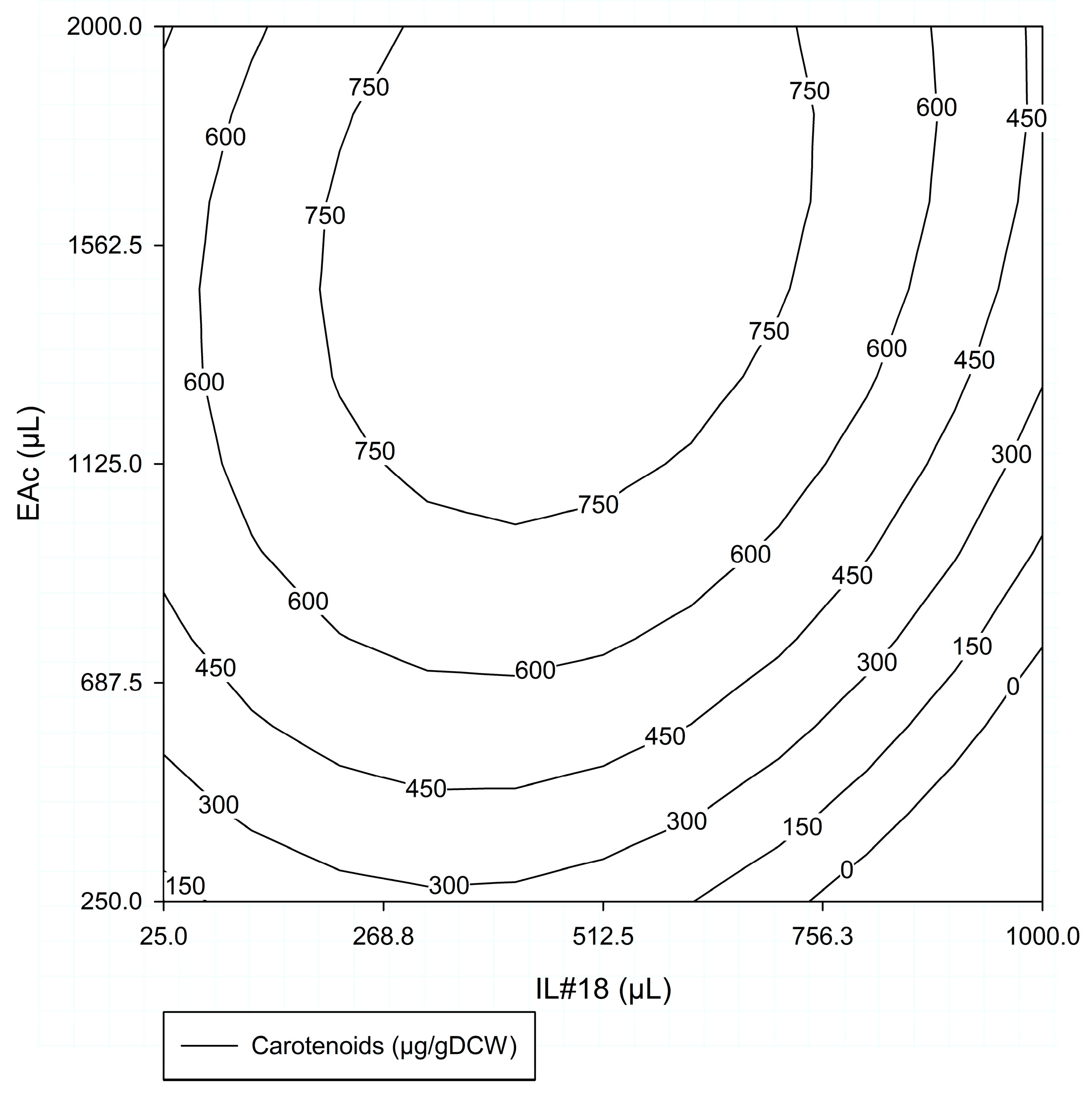
3.1.2. Experimental Design (ED) for Carotenoid Extraction Optimization
#ED1—Response Surface
- Analysis for #ED1 Factors
#ED2—Response Surface
- Analysis for #ED2 Factors
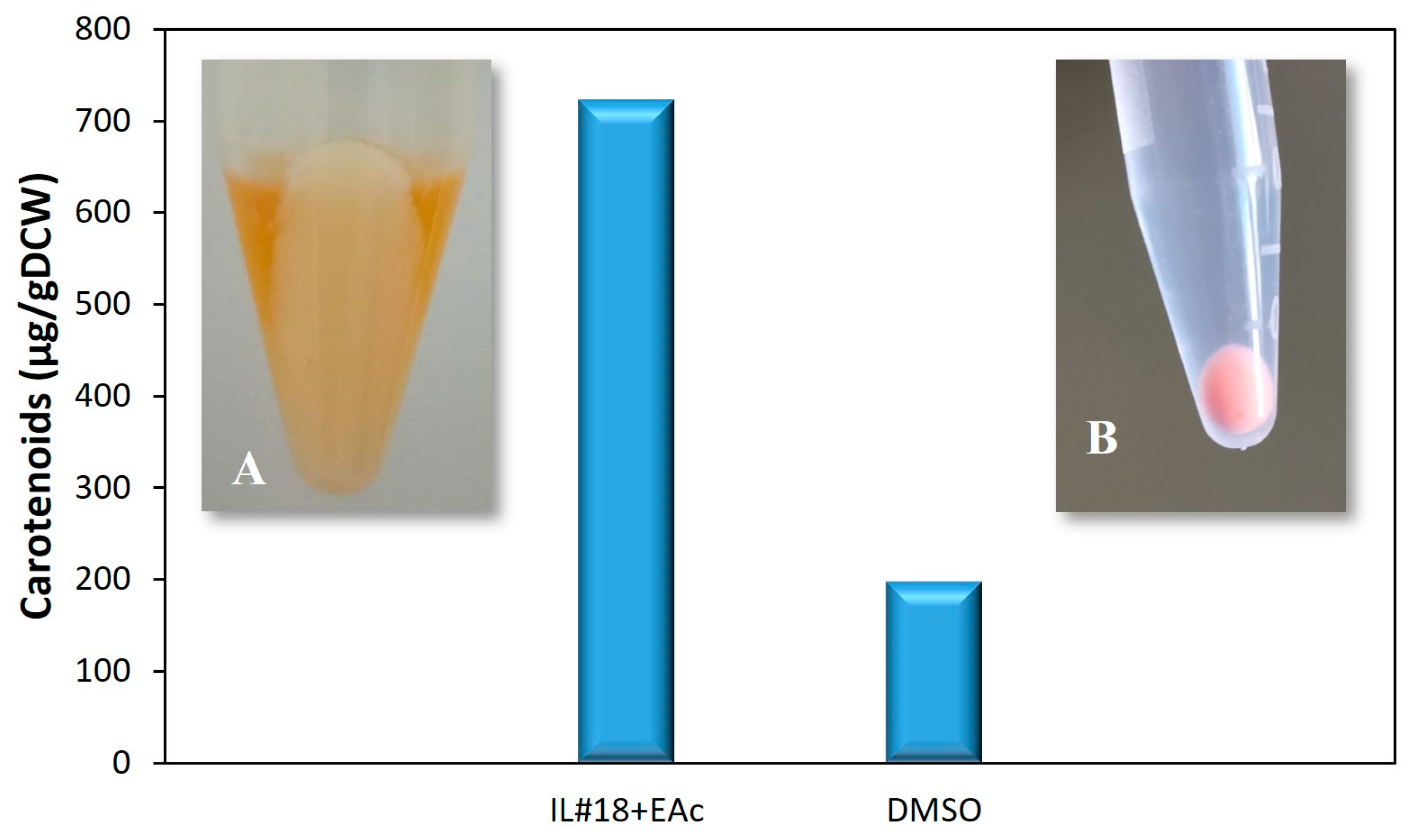
3.2. Validation of the Extraction Procedure with IL#18 and EAc
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ardila-Leal, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. A brief history of colour, the environmental impact of synthetic dyes and removal by using laccases. Molecules 2021, 26, 3813. [Google Scholar] [CrossRef]
- Calegari-Santos, R.; Diogo, R.A.; Fontana, J.D.; Bonfim, T.M.B. Carotenoid production by halophilic archaea under different culture conditions. Curr. Microbiol. 2016, 72, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C.-W. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- More, V. Carotenoids Market—Market Forecast Till 2032 2024 [MRFR/F-B & N/0752-HCR Report, 115 Pages], April 2014. Available online: https://www.marketresearchfuture.com/reports/carotenoids-market-1260 (accessed on 28 June 2024).
- Berman, J.; Zorrilla-López, U.; Farré, G.; Zhu, C.; Sandmann, G.; Twyman, R.M.; Capell, T.; Christou, P. Nutritionally important carotenoids as consumer products. Phytochem. Rev. 2015, 14, 727–743. [Google Scholar] [CrossRef]
- Kirti, K.; Amita, S.; Priti, S.; Kumar, A.M.; Jyoti, S. Colorful world of microbes: Carotenoids and their applications. Adv. Biol. 2014, 2024, 837891. [Google Scholar] [CrossRef]
- Esteban, R.; Moran, J.F.; Becerril, J.M.; García-Plazaola, J.I. Versatility of carotenoids: An integrated view on diversity, evolution, functional roles and environmental interactions. Environ. Exp. Bot. 2015, 119, 63–75. [Google Scholar] [CrossRef]
- Goswami, G.; Chaudhuri, S.; Dutta, D. Effect of pH and temperature on pigment production from an isolated bacterium. Chem. Eng. Trans. 2010, 20, 127–132. [Google Scholar]
- Ramachandran, H.; Iqbal, M.A.; Amirul, A.-A. Identification and characterization of the yellow pigment synthesized by Cupriavidus sp. USMAHM13. Appl. Biochem. Biotechnol. 2014, 174, 461–470. [Google Scholar] [CrossRef]
- Joshi, K.; Kumar, P.; Kataria, R. Microbial carotenoid production and their potential applications as antioxidants: A current update. Process Biochem. 2023, 128, 190–205. [Google Scholar] [CrossRef]
- López, G.-D.; Álvarez-Rivera, G.; Carazzone, C.; Ibáñez, E.; Leidy, C.; Cifuentes, A. Bacterial carotenoids: Extraction, characterization, and applications. Crit. Rev. Anal. Chem. 2023, 53, 1239–1262. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, W.; Din, G.; Rafiq, M.; Iqbal, A.; Khan, S.; Zada, S.; Ali, B.; Kang, S. Pigment production by cold-adapted bacteria and fungi: Colorful tale of cryosphere with wide range applications. Extremophiles 2020, 24, 447–473. [Google Scholar] [CrossRef]
- Ram, S.; Mitra, M.; Shaha, F.; Tirkey, S.R.; Mishra, S. Bacteria as an alternate biofactory for carotenoid production: A review of its applications, opportunities and challenges. J. Funct. Foods 2020, 67, 103867. [Google Scholar] [CrossRef]
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021, 337, 125398. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.; Surmann, P.; Vallentin, G.; Fuhrmann, H. A small-scale method for quantitation of carotenoids in bacteria and yeasts. J. Microbiol. Methods 2007, 70, 142–149. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Castellino, M.; Renna, M.; Santamaria, P.; Caponio, F. Setup of an extraction method for the analysis of carotenoids in microgreens. Foods 2020, 9, 459. [Google Scholar] [CrossRef]
- Orr, V.C.A.; Plechkova, N.V.; Seddon, K.R.; Rehmann, L. Disruption and wet extraction of the microalgae Chlorella vulgaris using room-temperature ionic liquids. ACS Sustain. Chem. Eng. 2016, 4, 591–600. [Google Scholar] [CrossRef]
- Silva, T.P.; Paixão, S.M.; Tavares, J.; Gil, C.V.; Torres, C.A.V.; Freitas, F.; Alves, L. A New biosurfactant/bioemulsifier from Gordonia alkanivorans strain 1B: Production and characterization. Processes 2022, 10, 845. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Roca, M. Recent developments in the analysis of carotenoids by mass spectrometry. In Progress in Carotenoid Research; Chapter 2; Zepka, L.Q., Jacob-Lopes, E., De Rosso, V.V., Eds.; IntechOpen: London, UK, 2018; pp. 17–44. [Google Scholar] [CrossRef]
- Chen, J.; Xie, F.; Li, X.; Chen, L. Ionic liquids for the preparation of biopolymer materials for drug/gene delivery: A review. Green Chem. 2018, 20, 4169–4200. [Google Scholar] [CrossRef]
- Bogel-Lukasik, R.; Bogel-Lukasik, E. Ionic liquids. In An Introduction to Green Chemistry Methods; Chapter 5; Luque, R., Colmenares, J.C., Eds.; Future Science Publishing: London, UK, 2013; pp. 70–83. [Google Scholar]
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddleston, J.G.; Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Controlling the aqueous miscibility of ionic liquids: Aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J. Am. Chem. Soc. 2003, 125, 6632–6633. [Google Scholar] [CrossRef]
- Eppink, M.H.M.; Ventura, S.P.M.; Coutinho, J.A.P.; Wijffels, R.H. Multiproduct microalgae biorefineries mediated by ionic liquids. Trends Biotechnol. 2021, 39, 1131–1143. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P. Use of ionic liquids in chitin biorefinery: A systematic review. Front. Bioeng. Biotechnol. 2020, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.S.; Moniruzzaman, M.; Mahmood, H.; Sivapragasam, M.; Bustam, M.A. Extraction of β-carotene from organic phase using ammonium based ionic liquids aqueous solution. J. Mol. Liq. 2017, 227, 15–20. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid-mediated extraction and separation processes for bioactive compounds: Past, present and future trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Papapostolou, H.; Kachrimanidou, V.; Alexandri, M.; Plessas, S.; Papadaki, A.; Kopsahelis, N. Natural carotenoids: Recent advances on separation from microbial biomass and methods of analysis. Antioxidants 2023, 12, 1030. [Google Scholar] [CrossRef]
- Silva, T.P.; Paixão, S.M.; Alves, L. Ability of Gordonia alkanivorans strain 1B for high added value carotenoids production. RSC Adv. 2016, 6, 58055–58063. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Paixão, S.M.; Silva, T.P.; Roseiro, J.C.; Alves, L. Influence of culture conditions towards optimal carotenoid production by Gordonia alkanivorans strain 1B. Bioprocess Biosyst. Eng. 2018, 41, 143–155. [Google Scholar] [CrossRef]
- Silva, T.P.; Paixão, S.M.; Fernandes, A.S.; Roseiro, J.C.; Alves, L. New insights on carotenoid production by Gordonia alkanivorans strain 1B. In Carotenoids—New Perspectives and Application; Chapter 2; Martinez-Espinosa, R.M., Ed.; Part of Book Series: Physiology; IntechOpen: London, UK, 2022; Volume 16, pp. 11–35. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Carvalho, V.; Ebinuma, S.; Gonzalez-Miquel, M.; Coutinho, J.A.P.; Fernando, J.; Pereira, B. Protic ionic liquids as cell disrupting agents for the recovery of intracellular carotenoids from yeast Rhodotorula glutinis CCT-2186. ACS Sustain. Chem. Eng. 2019, 7, 16765–16776. [Google Scholar] [CrossRef]
- Capela, E.V.; Coutinho, J.A.P.; Freire, M.G. Application of ionic liquids in separation and fractionation processes. In Green Chemistry and Chemical Engineering. Encyclopedia of Sustainability Science and Technology Series; Han, B., Wu, T., Eds.; Springer: New York, NY, USA, 2019; pp. 637–665. [Google Scholar] [CrossRef]
- Ola, P.D.; Matsumoto, M. Metal Extraction with Ionic Liquids-Based Aqueous Two-Phase System. In Recent Advances in Ionic Liquids; Chapter 8; Rahman, M.M., Ed.; IntechOpen: London, UK, 2018; pp. 145–158. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, S.; Ma, P. Use of ionic liquids as ‘green’ solvents for extractions. J. Chem. Technol. Biotechnol. 2005, 80, 1089–1096. [Google Scholar] [CrossRef]
- Alves, L.; Salgueiro, R.; Rodrigues, C.; Mesquita, E.; Matos, J.; Gírio, F.M. Desulfurization of dibenzothiophene, benzothiophene, and other thiophene analogs by a newly isolated bacterium, Gordonia alkanivorans strain 1B. Appl. Biochem. Biotechnol. 2005, 120, 199–208. [Google Scholar] [CrossRef]
- Silva, T.P.; Paixão, S.M.; Alves, L. A new impetus for biodesulfurization: Bypassing sulfate inhibition in biocatalyst production. Green Chem. 2023, 25, 6416–6431. [Google Scholar] [CrossRef]
- Silva, T.P.; Paixão, S.M.; Tavares, J.; Paradela, F.; Crujeira, T.; Roseiro, J.C.; Alves, L. Streamlining the biodesulfurization process: Development of an integrated continuous system prototype using Gordonia alkanivorans strain 1B. RSC Adv. 2024, 14, 725–742. [Google Scholar] [CrossRef]
- Alves, L.; Paixão, S.M. Fructophilic behaviour of Gordonia alkanivorans strain 1B during dibenzothiophene desulfurization process. New Biotechnol. 2014, 31, 73–79. [Google Scholar] [CrossRef]
- Ruiz, C.A.S.; Kwaijtaal, J.; Peinado, O.C.; Van Den Berg, C.; Wijffels, R.H.; Eppink, M.H. Multistep fractionation of microalgal biomolecules using selective aqueous two-phase systems. ACS Sustain. Chem. Eng. 2020, 8, 2441–2452. [Google Scholar] [CrossRef]
- Nobre, B.; Marcelo, F.; Passos, R.; Beirão, L.; Palavra, A.; Gouveia, L.; Mendes, R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006, 223, 787–790. [Google Scholar] [CrossRef]
- Doehlert, D.H. Uniform Shell Designs. J. R. Stat. Soc. Ser. C (Appl. Stat.) 1970, 19, 231–239. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2014, 114, 9651–9653. [Google Scholar] [CrossRef]
- Sharaff, A.; Rangarajan, R. Effect of cation alkyl chain length on the extractive desulfurization performance of ionic liquids. Ind. Eng. Chem. Res. 2011, 50, 12449–12454. [Google Scholar]
- Tran, P.H.L.; Tran, T.T.D.; Park, J.B.; Lee, B.J. Controlled release systems containing solid dispersions: Strategies and mechanisms. Pharm. Res. 2016, 33, 235–246. [Google Scholar] [CrossRef]
- Zeng, C.X.; Yu, D.; Luo, J.; Zhou, Z. Ionic liquid-based microwave-assisted extraction of essential oils from Citrus reticulata Blanco peel. Sep. Purif. Technol. 2015, 153, 195–203. [Google Scholar]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Han, D.; Armstrong, D.W. Ionic liquids in separations. Acc. Chem. Res. 2007, 40, 1079–1086. [Google Scholar] [CrossRef]
- Zheng, S.; Nie, Y.; Zhang, S.; Zhang, X.; Wang, L. Highly efficient dissolution of wool keratin by dimethylphosphate ionic liquids. ACS Sustain. Chem. Eng. 2015, 3, 2925–2932. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Blanco, P.; Gascón, I.; Blanco, D. Influence of Ionic Liquid Structure on the Extraction of Bioactive Compounds. ACS Sustain. Chem. Eng. 2021, 9, 1320–1330. [Google Scholar]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Kontro, I.; Svedström, K.; Duša, F.; Ahvenainen, P.; Ruokonen, S.K.; Witos, J.; Wiedmer, S.K. Effects of phosphonium-based ionic liquids on phospholipid membranes studied by small-angle X-ray scattering. Chem. Phys. Lipids 2016, 201, 59–66. [Google Scholar] [CrossRef]
- Cheng, W.; Xian, F.; Zhou, Z.; Hu, K.; Gao, J. Solubility and stability of carotenoids in ammonium-and phosphonium-based ionic liquids: Effect of solvent nature, temperature and water. Molecules 2023, 28, 3618. [Google Scholar] [CrossRef] [PubMed]
- Mussagy, C.U.; Santos-Ebinuma, V.C.; Pereira, J.F.B. Microbial carotenoids extraction using aqueous solutions of cholinium- based ionic liquids. In Proceedings of the XXII National Bioprocesses Symposium (SINAFERM) XIII Enzymatic Hydrolysis of Biomass Symposium (SHEB), Uberlandia, Minas Gerais, Brazil, 28–31 July 2019; Available online: https://proceedings.science/sinaferm/sinaferm-sheb-2019/papers/microbial-carotenoids-extraction-using-aqueous-solutions-of-cholinium-based-ioni?lang=en (accessed on 2 August 2024), ISSN 2447-2816.
- Choi, S.-A.; Oh, Y.-K.; Lee, J.; Sim, S.J.; Hong, M.E.; Park, J.-Y.; Kim, M.-S.; Kim, S.W.; Lee, J.-S. High-efficiency cell disruption and astaxanthin recovery from Haematococcus pluvialis cyst cells using room-temperature imidazolium-based ionic liquid/water mixtures. Bioresour. Technol. 2019, 274, 120–126. [Google Scholar] [CrossRef]
- Basar, A.O.; Prieto, C.; Durand, E.; Villeneuve, P.; Sasmazel, H.T.; Lagaron, J. Encapsulation of β-Carotene by Emulsion Electrospraying Using Deep Eutectic Solvents. Molecules 2020, 25, 981. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Hucke, H.U.; Ramos, N.F.; Ribeiro, H.F.; Alves, M.B.; Mustafa, A.; Pereira, J.F.B.; Farias, F.O. Tailor-made solvents for microbial carotenoids recovery. Appl. Microbiol. Biotechnol. 2024, 108, 234. [Google Scholar] [CrossRef]
- Safi, C.; Ursu, A.V.; Laroche, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Aqueous extraction of proteins from microalgae: Effect of different cell disruption methods. Algal Res. 2014, 3, 61–65. [Google Scholar] [CrossRef]
- Paz, A.V.C.; Rigano, F.; Cafarella, C.; Tropea, A.; Mondello, L.; Galan, J.P.M.; Mussagy, C.U. Process intensification of ultrasound assisted deep eutectic solvent-based extraction of astaxanthin-rich extract derived from the non-conventional bacterium Paracoccus carotinifaciens. Sep. Purif. Technol. 2024, 339, 126674. [Google Scholar]
- Alves, L.; Paixão, S.M.; Pacheco, R.; Ferreira, A.F.; Silva, C.M. Biodesulphurization of Fossil Fuels: Energy, Emissions and Cost Analysis. RSC Adv. 2015, 5, 34047–34057. [Google Scholar] [CrossRef]
- Paixão, S.M.; Silva, T.P.; Arez, B.F.; Alves, L. Advances in the Reduction of the Costs Inherent to Fossil Fuels Biodesulfurization Towards its Potential Industrial Application. In Nanocomposites for the Desulfurization of Fuels; Chapter 7; Saleh, T., Ed.; IGI Global: Hershey, PA, USA, 2020; pp. 235–283. [Google Scholar] [CrossRef]



| No. (#) | Ionic Liquids | Brand |
|---|---|---|
| 1 | 1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | Solchemar |
| 2 | 1,3-Dimethylimidazolium dimethyl phosphate | Io-li-tec |
| 3 | Trihexyl(tetradecyl)phosphonium bis(trifluoromethylsulfonyl)imide | Solchemar |
| 4 | 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | Io-li-tec |
| 5 | 1-Hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | Io-li-tec |
| 6 | 1-Ethyl-3-Methylimidazolium hydrogensulfate | Solchemar |
| 7 | Triisobutyl(methyl)phosphonium tosylate | Io-li-tec |
| 8 | 1-Ethyl-3-Methylimidazolium thiocyanate | Io-li-tec |
| 9 | 1-Butyl-3-Methylimidazolium thiocyanate | Io-li-tec |
| 10 | 1-Butyl-3-Methylimidazolium tetracholoroferrate (III) | Io-li-tec |
| 11 | 1-Butyl-3-Methylimidazolium dicyanamide | Io-li-tec |
| 12 | 1-Butyl-3-Methylimidazolium trifluoromethanesulfonate | Io-li-tec |
| 13 | 1-Ethyl-3-Methylimidazolium dihydrogen phosphate | Solchemar |
| 14 | 1-Ethyl-3-methylimidazolium trifluoromethanesulfonate | Solchemar |
| 15 | 1-Ethyl-3-Methylimidazolium methanesulfonate | Solchemar |
| 16 | Didecyl-dimethylammonium nitrate | Io-li-tec |
| 17 | 2-hydroxyethylammonium formate | Io-li-tec |
| 18 | Tributyl(ethyl)phosphonium diethyl phosphate | Solchemar |
| 19 | 1-Methylimidazolium bis(trifluoromethylsulfonyl)imide | Io-li-tec |
| Test (#) | Factors | Response | ||
|---|---|---|---|---|
| IL#18 Volume (25–1000 µL) | EAc Volume (250–2000 µL) | Total Carotenoids (µg/gDCW) | ||
| 1 | 512.5 | 1125 | 839.1 | |
| 2 | 512.5 | 1125 | 725.3 | |
| 3 | 1000 | 1125 | 210.4 | |
| 4 | 1000 | 1125 | 210.4 | |
| 5 | 25 | 1125 | 558.1 | |
| 6 | 25 | 1125 | 527.8 | |
| 7 | 756.25 | 1882.75 | 717.2 | |
| 8 | 756.25 | 1882.75 | 808.1 | |
| 9 | 268.75 | 367.25 | 350.9 | |
| 10 | 268.75 | 367.25 | 333.8 | |
| 11 | 756.25 | 367.25 | 114.3 | |
| 12 | 756.25 | 367.25 | 117.9 | |
| 13 | 268.75 | 1882.75 | 666.7 | |
| 14 | 268.75 | 1882.75 | 828.3 | |
| Model | Response: Total Carotenoids |
|---|---|
| Model parameters | |
| β0 | 782.3 |
| β1 | −146.05 |
| β2 | 303.61 |
| β12 | 139.38 |
| β11 | −405.61 |
| β22 | −251.68 |
| Model validation (Fischer test) | |
| Effectiveness of the parameters | 51.01 |
| Significance level (α) F (5,8) | 0.001 |
| Lack of fit | 1.4 |
| Significance level (α) F (1,7) | <0.05 |
| R2 | 0.97 |
| Test (#) | Factors | Response | ||
|---|---|---|---|---|
| IL#18 Volume (0–50 µL) | EAc Volume (250–1125 µL) | Total Carotenoids (µg/gDCW) | ||
| 1 | 25 | 687.5 | 464.9 | |
| 2 | 25 | 687.5 | 451.2 | |
| 3 | 50 | 687.5 | 487.6 | |
| 4 | 50 | 687.5 | 555.0 | |
| 5 | 0 | 687.5 | 315.7 | |
| 6 | 0 | 687.5 | 304.5 | |
| 7 | 37.5 | 1066.375 | 724.6 | |
| 8 | 37.5 | 1066.375 | 770.4 | |
| 9 | 12.5 | 308.625 | 15.4 | |
| 10 | 12.5 | 308.625 | 15.4 | |
| 11 | 37.5 | 308.625 | 16.7 | |
| 12 | 37.5 | 308.625 | 16.7 | |
| 13 | 12.5 | 1066.375 | 697.7 | |
| 14 | 12.5 | 1066.375 | 694.3 | |
| Model | Response: Total Carotenoids |
|---|---|
| Model parameters | |
| β0 | 458.11 |
| β1 | 79.21 |
| β2 | 407.44 |
| β12 | 28.98 |
| β11 | −42.41 |
| β22 | −104.85 |
| Model validation (Fischer test) | |
| Effectiveness of the parameters | 141.96 |
| Significance level (α) F (5,8) | 0.001 |
| Lack of fit | 16.79 |
| Significance level (α) F (1,7) | 0.01 |
| R2 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, T.P.; Alves, L.; Salgado, F.; Roseiro, J.C.; Łukasik, R.M.; Paixão, S.M. Ionic Liquids toward Enhanced Carotenoid Extraction from Bacterial Biomass. Molecules 2024, 29, 4132. https://doi.org/10.3390/molecules29174132
Silva TP, Alves L, Salgado F, Roseiro JC, Łukasik RM, Paixão SM. Ionic Liquids toward Enhanced Carotenoid Extraction from Bacterial Biomass. Molecules. 2024; 29(17):4132. https://doi.org/10.3390/molecules29174132
Chicago/Turabian StyleSilva, Tiago P., Luís Alves, Francisco Salgado, José C. Roseiro, Rafał M. Łukasik, and Susana M. Paixão. 2024. "Ionic Liquids toward Enhanced Carotenoid Extraction from Bacterial Biomass" Molecules 29, no. 17: 4132. https://doi.org/10.3390/molecules29174132
APA StyleSilva, T. P., Alves, L., Salgado, F., Roseiro, J. C., Łukasik, R. M., & Paixão, S. M. (2024). Ionic Liquids toward Enhanced Carotenoid Extraction from Bacterial Biomass. Molecules, 29(17), 4132. https://doi.org/10.3390/molecules29174132







