Abstract
The widespread environmental contamination resulting from the misuse of tetracycline antibiotics (TCs) has garnered significant attention and study by scholars. Photocatalytic technology is one of the environmentally friendly advanced oxidation processes (AOPs) that can effectively solve the problem of residue of TCs in the water environment. This study involved the synthesis of the heterogeneous magnetic photocatalytic material of CoFe2O4/NaBiO3 via the solvothermal method, and it was characterized using different characterization techniques. Then, the photocatalytic system under visible light (Vis) was coupled with peroxymonosulfate (PMS) to explore the performance and mechanism of degradation of tetracycline hydrochloride (TCH) in the wastewater. The characterization results revealed that CoFe2O4/NaBiO3 effectively alleviated the agglomeration phenomenon of CoFe2O4 particles, increased the specific surface area, effectively narrowed the band gap, expanded the visible light absorption spectrum, and inhibited recombination of photogenerated electron–hole pairs. In the Vis+CoFe2O4/NaBiO3+PMS system, CoFe2O4/NaBiO3 effectively activated PMS to produce hydroxyl radicals (·OH) and sulfate radicals (SO4−). Under the conditions of a TCH concentration of 10 mg/L−1, a catalyst concentration of 1 g/L−1 and a PMS concentration of 100 mg/L−1, the degradation efficiency of TCH reached 94% after 100 min illumination. The degradation of TCH was enhanced with the increase in the CoFe2O4/NaBiO3 and PMS dosage. The solution pH and organic matter had a significant impact on TCH degradation. Notably, the TCH degradation efficiency decreased inversely with increasing values of these parameters. The quenching experiments indicated that the free radicals contributing to the Vis+CoFe2O4/NaBiO3+PMS system were ·OH followed by SO4−, hole (h+), and the superoxide radical (O2−). The main mechanism of PMS was based on the cycle of Co3+ and Co2+, as well as Fe3+ and Fe2+. The cyclic tests and characterization by XRD and FT-IR revealed that CoFe2O4/NaBiO3 had good degradation stability. The experimental findings can serve as a reference for the complete removal of antibiotics from wastewater.
1. Introduction
With the development of an intensive and large-scale breeding industry, the use of antibiotics is necessary to enhance farm productivity and the health of livestock and poultry, preventing diseases and promoting growth [1]. Tetracyclines (TCs) are broad-spectrum antibiotics widely adopted in livestock and poultry rearing due to their cost-effectiveness and benefits, such as the prevention and treatment of intestinal infections and growth promotion. However, the metabolic assimilation of TCs in animals is less than 30%, resulting in substantial excretion via feces and urine [2]. Consequently, substandard discharge of farm wastewater leads to the presence of TCs in aquatic environments. It has been reported that the residual TCs in the environment pose a significant threat to human health. Moreover, TCs can directly accumulate in the human body through the food chain, leading to endocrine disorders, joint diseases, central nervous system defects, and other diseases [3]. Furthermore, continuous environmental exposure to sub-therapeutic concentrations of TCs fosters selective pressure, facilitating the proliferation of antibiotic-resistant genes (ARGs) and endangering ecosystem stability and public health [4,5]. Current conventional physical removal techniques, such as adsorption and membrane filtration, are inadequate for the complete elimination of TCs, achieving merely a phase transfer rather than a true removal [6,7]. Similarly, traditional biological treatment technologies are also unable to effectively remove TCs, such as activated sludge, which mainly removes them by adsorption rather than biodegradation [8,9]. A study found that there are a large number of ARGs stored in the sludge produced by activated sludge and biofilm processes [10]. Therefore, it is urgent to find a method that can remove TCs from water quickly, safely, and completely.
In recent years, advanced oxidation processes (AOPs) based on free radicals with high redox potential have been recognized as an effective and promising method for removing environmental risks caused by antibiotics [11,12]. Among them, photocatalytic oxidation technology using abundant solar energy has attracted wide attention due to its great potential in solving the current environmental pollution problems as well as energy shortage [13]. Bismuth-based semiconductor photocatalytic materials such as bismuth molybdate (Bi2MoO6) [14], bismuth tungstate (BiVO4) [15], and sodium bismuthate (NaBiO3) [16] have become the research hotspots of photocatalysts due to their unique layered structures, suitable forbidden bandwidths, tunable morphology, high photonicity, and excellent electron transport efficiency [17]. Generally, Bi5+-containing oxides have a more vital ability to absorb visible light than Bi3+-containing oxides. NaBiO3 belongs to perovskite-type complex oxide (ABO3), which is one of the most common Bi5+-containing compounds [18] and has strong oxidation and photocatalytic abilities [19]. However, NaBiO3 photocatalysts have limitations such as high electron–hole pair recombination rate [20] and inability to be recycled, which limit their application in practice. Various strategies, including metal doping and the creation of heterojunctions, have been implemented to enhance the photocatalytic efficiency of NaBiO3. Wu [21] adeptly anchored nano-flower-like NaBiO3 onto g-C3N4 using a straightforward hydrothermal method, resulting in the formation of a direct Z-scheme g-C3N4/NaBiO3 heterojunction. This structure exhibited remarkable degradation capabilities for Tetracycline (TC) and notable stability. The internal electric field within the heterojunction promotes effective charge separation, while also providing a high redox potential for the photogenerated carriers. This facilitates the generation of additional reactive oxygen species (·O2− and ·OH), leading to rapid TC degradation. A study found that the introduction of Fe3+ ions significantly reduces the recombination rate of photoinduced electron–hole pairs in NaBiO3, markedly improving its photocatalytic performance. Following 100 min of exposure, the photocatalytic degradation rate constant for TCH with Fe3+-doped NaBiO3 was found to be double that of the Vis+NaBiO3 system [22]. Wu [23] synthesized a NaBiO3/Poly (N-methylaniline) (PNMA) composite through a one-pot method, resulting in flake-like NaBiO3/PNMA photocatalysts. These composites, formed via in situ polymerization, exhibit strong interfacial effects that induce oxygen vacancy generation and an upward shift in the energy band structure. Consequently, these modifications significantly boost charge separation and light absorption efficiencies, showcasing superior catalytic activity in the oxidative degradation of TC. In order to solve the problem of the difficult recovery of NaBiO3, magnetic nanoparticles can be selected as carriers to obtain efficient and recyclable bismuth-based materials for water pollution treatment.
Cobalt ferrite (CoFe2O4), a typical spinel-type ferrite, has gained recognition as a potent catalyst for the degradation of organic pollutants in recent years. Its excellent catalytic efficiency is attributed to the synergistic effects of cobalt (Co) and iron (Fe), serving as active sites. Moreover, the integration with visible light irradiation enhances its catalytic capabilities [24]. Additionally, CoFe2O4 not only catalyzes peroxymonosulfate (PMS) synergistically under light irradiation, but also demonstrates outstanding electrocatalytic performance. This is characterized by high electronic conductivity, facilitation of charge carrier transfer, and reduction in electronic overpotential [25]. Most notably, CoFe2O4 features excellent magnetic recyclability [26], underscoring its potential for repeated use in catalytic processes. Utilizing CoFe2O4 in the synthesis of magnetic composite photocatalysts presents a commendable strategy. With a band gap ranging from 1 to 3 eV and inherent ferromagnetism, CoFe2O4 facilitates swift separation from the liquid–solid system under an external magnetic field. This property has led to its widespread application in photocatalytic oxidation technologies [27]. Al-Musawi [28] demonstrated that loading CoFe2O4 onto multi-walled carbon nanotubes reduced the band gap from 3.2 eV to 2.1 eV while simultaneously enhancing the light absorption rate. Lin [29] prepared CoFe2O4-modified BiOCl hierarchical microspheres, which, under mild light irradiation, exhibited piezoelectric photocatalytic reaction rates for TCH, bisphenol A, and phenol significantly higher than those observed in standard photocatalysis. Jing [30] developed a visible-light-driven magnetic CoFe2O4/Ag/Ag3VO4 photocatalyst via a hydrothermal method, which effectively degraded methyl orange and TC and eradicated Escherichia coli, maintaining stable degradation ability and crystal structure. A highly efficient S-scheme heterojunction CoFe2O4/NiFe2O4 was developed by integrating CoFe2O4 and NiFe2O4 nanosheets [31]. The 5%-CoFe2O4/NiFe2O4 demonstrated remarkable photocatalytic degradation capabilities for TC, achieving a removal rate of 76.1% within 60 min. This enhancement in photocatalytic performance is primarily attributed to the formation of the S-scheme heterojunction, which significantly increases the capacity for visible light absorption and facilitates charge separation. Gogoi [32] reported that a catalyst composed of CoFe2O4 nanoparticles and g-C3N4 nanosheets (CF-gC3N4) displayed exceptional catalytic efficiency in degrading various widely used antibiotics. The catalyst achieved complete degradation of TCH under simulated sunlight irradiation within 12 min, and under microwave irradiation, the degradation was accomplished in just 1 min. The catalyst can be easily separated using magnetic methods, exhibits a catalytic efficiency of up to 91%, and maintains good stability. Furthermore, CoFe2O4 can has been successfully combined with various materials like TiO2 [33], ZnO [34], graphene [35], g-C3N4 [36], and BiVO4 [37], enhancing photocatalytic activity to varying degrees. Specifically, CoFe2O4 nanoparticles on loaded NaBiO3 surfaces not only boost the photocatalytic performance of composite catalysts, but also contribute to preventing the direct or indirect emission of pollutants, thereby mitigating primary and secondary environmental pollution.
Achieving efficient degradation with a singular photocatalytic technology proves challenging. However, merging two distinct AOPs circumvents their individual limitations while leveraging their synergistic effect to further augment pollutant removal efficiency. SO4−·-based persulfate AOPs (SR-AOPs) have recently emerged as a focal point in organic wastewater treatment in recent years, offering advantages over ·OH and SO4−·, including a higher redox potential (2.5–3.1 V) [38], longer half-life (30–40 μs) [39], broader pH stability, and enhanced robustness. CoFe2O4 has been widely used to activate persulfate (PS) for catalytic oxidation degradation of various pollutants [40,41]. Moreover, visible light activation of PS presents a quicker, more reliable method. For instance, Li [40] used nano-CoFe2O4-activated PMS to degrade atrazine (ATZ), achieving over 99% removal within 30 min. Song [41] explored the Triphenyl phosphate (TPhP) degradation via CoFe2O4-activated PMS, demonstrating a 99.5% removal efficiency under ambient conditions, underscoring the efficacy of the CoFe2O4 -activated PMS process for TPhP degradation. Thus, the photocatalytic technology coupled with persulfate technology using CoFe2O4/NaBiO3 as the catalyst can significantly enhance removal efficiency and reaction speed.
This study introduces CoFe2O4/NaBiO3 heterogeneous magnetic photocatalysts, synthesized via the solvothermal method. CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3 were characterized by various analytical techniques. These catalysts, coupled with PMS, formed Vis+CoFe2O4/NaBiO3+PMS systems, providing a synergistic reaction pathway for TCH removal. The effects of reaction conditions and fulvic acid (FA) coexistence on TCH degradation were investigated, and the main free radical species promoting TCH degradation in a collaborative system were determined. Subsequent cyclic experiments evaluated the composite photocatalyst’s stability and reusability. The magnetic nature of CoFe2O4/NaBiO3 significantly eases catalyst recycling challenges, paving the way for future research on the development of bismuth-based materials with excellent performance and novel solutions for persistent organic pollution eradication.
2. Results and Discussion
2.1. Characterization of Photocatalysts
Scanning electron microscopy (SEM) images of CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3 are presented in Figure 1a–d, elucidating the morphological characteristics of the synthesized samples. The commercially available NaBiO3 (Figure 1a) exhibits a layered structure composed of nanosheets varying in shape and size. This expansive and smooth surface area of NaBiO3 provides an optimal substrate for forming composite with other materials. The CoFe2O4 surface is characterized by uniformly shaped spherical nanoparticles, albeit with some degree of agglomeration, which may obscure active sites and consequently dampen its catalytic efficacy (Figure 1b). This agglomeration is postulated to stem from the inherent strong magnetic properties of CoFe2O4. A comparative analysis of Figure 1a–d reveals the successful deposition of CoFe2O4 nanoparticles onto layered NaBiO3 flakes. This arrangement allows NaBiO3 to effectively disperse the CoFe2O4 particles, mitigating their natural tendency to agglomerate, thereby unveiling active sites on CoFe2O4/NaBiO3 and enhancing its photocatalytic efficiency.
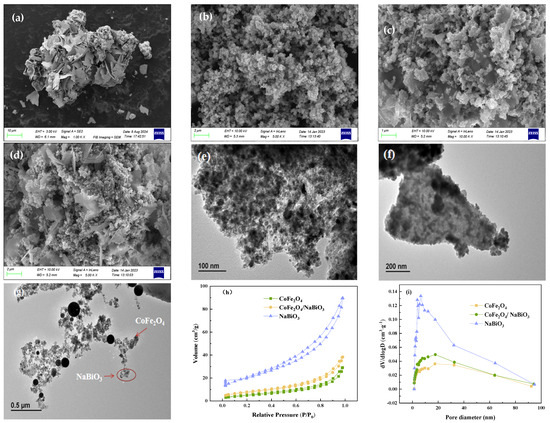
Figure 1.
SEM and TEM images, adsorption–desorption curves, and pore size distributions of CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3. (a) NaBiO3 at 10 μm; (b) CoFe2O4 at 2 μm; (c,d) CoFe2O4/NaBiO3 at 1 μm and 2 μm, respectively. TEM images of samples: (e) CoFe2O4; (f) NaBiO3; (g) CoFe2O4/NaBiO3. (h) Adsorption–desorption curve of samples. (i) Pore size distribution of samples.
To further corroborate the successful integration of CoFe2O4 nanoparticles with NaBiO3, transmission electron microscope (TEM) images of CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3 are provided in Figure 1e–g. The TEM image of pure CoFe2O4 (Figure 1e) reveals relatively uniform nanoparticles with an average size of about 20 nm, prone to aggregation due to magnetic properties. The NaBiO3 (Figure 1f) is predominantly sheet-like, with a thickness ranging from 10 to 15 nm and a length from 200 to 600 nm. Its overall structure is thin, featuring uneven thickness and a coarse surface, conducive to CoFe2O4 loading. The TEM image of the CoFe2O4/NaBiO3 composite (Figure 1g) distinctly shows the NaBiO3 surface adorned with small CoFe2O4 nanoparticles, highlighting a unique microstructure that optimizes the interface between NaBiO3 and CoFe2O4. This configuration significantly improves the separation efficiency of photogenerated electrons and holes, thereby augmenting the photocatalytic performance of the composite [30].
Brunauer–Emmett–Teller (BET) analyses were conducted to determine the specific surface area and pore size distribution of CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3. As shown in Figure 1h, all catalysts exhibited type IV isotherms with H3 hysteresis loops, indicative of mesoporous structures and a high adsorption capacity at elevated P/P0 ratios, likely due to slit pores formed by particle aggregation. Among them, NaBiO3 exhibited the highest adsorption capacity, succeeded by CoFe2O4/NaBiO3, with CoFe2O4 showing the least. This suggests that the presence of CoFe2O4 incorporation obstructs numerous pores within NaBiO3. The specific surface areas for NaBiO3, CoFe2O4, and CoFe2O4/NaBiO3 were measured at 72.14 m2·g−1, 17.38 m2·g−1, and 26.94 m2·g−1 respectively, indicating that NaBiO3 serves as an effective medium for dispersing CoFe2O4 particles and amplifying the specific surface area to expose a greater number of active sites. As shown in Figure 1i, the pore size distribution of NaBiO3 predominantly ranges between 5 and 10 nm, whereas for CoFe2O4 and CoFe2O4/NaBiO3, it is chiefly between 10 and 30 nm.
Figure 2 shows the X-ray photoelectron spectroscopy (XPS) spectra for CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3 composite. Analysis of Figure 2a reveals the detection of elements such as Co, Fe, C, O, and Bi within the CoFe2O4/NaBiO3 composite. Figure 2b–e display high-resolution XPS spectra for the elements Co, Fe, C, and Bi, respectively. The high-resolution XPS spectra of the CoFe2O4 (Figure 2b) identifies four prominent peaks corresponding to Fe 2p, with the peaks at 710.5 eV (and a satellite peak at 716.7 eV) and 723.6 eV (with a satellite peak at 733.2 eV) attributed to Fe 2p3/2 and Fe 2p1/2, respectively. The peak at 710.5 eV indicates the presence of Fe2+, and the binding energy of 723.6 eV aligns with the characteristic value of Fe3+ [42]. Upon forming the CoFe2O4 and NaBiO3 composite, these characteristic peaks shift to 710.9, 718.2, 724.1, and 732.8 eV, respectively, indicating a transformation in the chemical environment of CoFe2O4/NaBiO3 and confirming a strong interaction between CoFe2O4 and NaBiO3. Figure 2c illustrates three distinct peaks of Co 2p, centered at 780.4 eV (with a satellite peak at 785.3 eV) and 795.6 eV, corresponding to the orbital peaks of Co 2p3/2 and Co 2p1/2, respectively, primarily associated with Co2+ [43]. The slight shift in these orbital peaks towards higher binding energies in the composite suggests a decrease in electron density, indicative of a robust integration of the elements. The detection of Co 2p and Fe 2p peaks confirms the presence of spinel CoFe2O4 within the composite [44]. The C1s XPS spectra for both CoFe2O4 and CoFe2O4/NaBiO3 are differentiated into three peaks of 284.6 eV, 286.8 eV, and 288.7 eV (Figure 2d), corresponding to C-C, C=O and C-O, respectively [45]. In Figure 2e, the Bi element is precisely identified, with Bi 4f exhibiting two peaks at binding energies of 158.6 and 163.8 eV, characteristic of Bi 4f7/2 and Bi 4f5/2, respectively, and indicative of Bi5+ [46]. For the composite, these peaks shift to 159.3 and 164.6 eV, respectively, aligning with the properties of Bi3+ [47], thereby evidencing a valence state change of the Bi element on the material’s surface and confirming the presence of Bi2O3 as detected in XRD analyses. This shift and the elemental migration observed in the composite compared to the pure substances suggest that CoFe2O4 and NaBiO3 form a heterojunction upon combination, facilitating the transfer of photogenerated electrons.
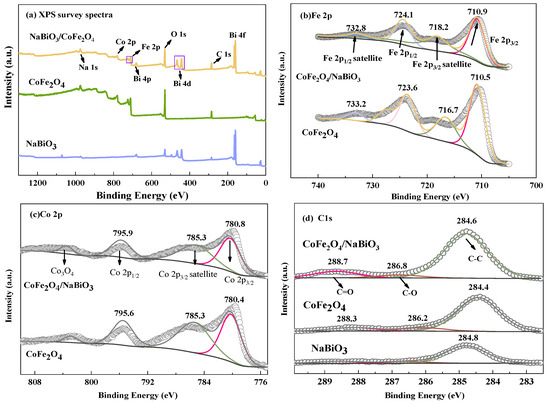
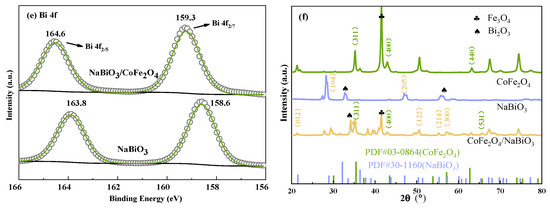
Figure 2.
XPS spectra and XRD patterns of CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3. (a) XPS survey spectra; (b) Fe 2p orbital diagram; (c) Co 2p orbital diagram; (d) C 1s orbital diagram; (e) Bi 4f orbital diagram; (f) XRD pattern.
To further elucidate the phase and crystal structure of CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3, XRD analysis was conducted (Figure 2f). For CoFe2O4 nanoparticles, peaks were observed at 2θ values of 35.451°, 43.472°, and 62.726°, corresponding to the (311), (400), and (440) crystal planes of spinel CoFe2O4 (PDF#03-0864) alongside the detected Fe3O4 peaks. For pure NaBiO3 nanosheets, characteristic peaks were observed at 2θ values of 28.927° and 47.123°, consistent with the crystal planes (104) and (205) of NaBiO3 (PDF#30–1160), with Bi2O3 peaks also identified. Within the CoFe2O4/NaBiO3 composite catalyst, NaBiO3 characteristic peaks were observed at 2θ values of 21.498°, 51.407°, 55.367°, and 57.322°, corresponding to (012), (122), (214), and (300), respectively. Additionally, CoFe2O4 peaks corresponding to the (311), (400), and (531) crystal faces were observed at 2θ values of 34.451°, 43.472°, and 65.701°, respectively. Fe3O4 and Bi2O3 were also observed in the composite. XRD results indicate that the magnetic photocatalytic material CoFe2O4/NaBiO3 was successfully prepared.
The Fourier transform infrared spectroscopy (FT-IR) spectra of CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3 are depicted in Figure 3a. The spectra reveal a broad absorption peak between 3300 and 4450 cm−1 alongside absorption peaks within the 1400–1649 cm−1 range, likely attributable to the stretching vibration of the O-H group in water molecules adsorbed on the catalyst surface [48,49]. For NaBiO3, an absorption peak at 590 cm−1 is mainly due to the vibration of Bi-O bonds [50]. An observable peak at 1048 cm−1 might represent the stretching vibration of C-O in the metal–organic chelate, resulting from the chelation of ethylene glycol and metal ions during the synthesis of CoFe2O4. The peak range of 1380−1390 cm−1 could be associated with the formation of-C-O-H in the in-plane band, a result of the interaction between CoFe2O4 and NaBiO3. Additionally, the absorption peak at 575 cm−1 is likely indicative of Fe-O and Co-O bond vibrations within CoFe2O4. The comparative analysis of peaks between CoFe2O4/NaBiO3 and CoFe2O4 further confirms the successful synthesis of the CoFe2O4/NaBiO3 composite.
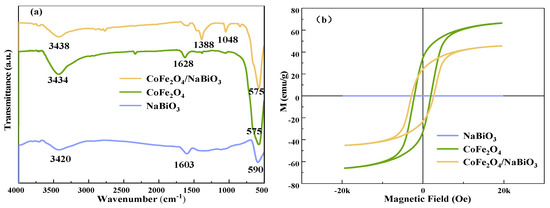
Figure 3.
FT-IR and VSM spectra of CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3. (a) FT-IR spectra; (b) VSM spectra.
The vibrating sample magnetometer (VSM) profiles for NaBiO3, CoFe2O4, and CoFe2O4/NaBiO3, presented in Figure 3b, demonstrate that NaBiO3 exhibits negligible saturation magnetization strength, in contrast to CoFe2O4 and CoFe2O4/NaBiO3, which display pronounced hysteresis line trends. At a magnetic field strength of 20 k, the saturation magnetization reaches 66.4 emu·g−1 for CoFe2O4 and 45.5 emu·g−1 for CoFe2O4/NaBiO3. The higher saturation magnetization and coercivity of CoFe2O4 compared to CoFe2O4/NaBiO3 indicates robust magnetism in both catalysts. However, the magnetism of CoFe2O4/NaBiO3 is slightly reduced upon the incorporation of NaBiO3, a factor that does not impede the magnetic recovery of the composite photocatalysts.
The ultraviolet-visible diffuse reflectance spectroscopy (UV-Vis DRS) and photoluminescence spectroscopy (PL) spectra of CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3 are showcased in Figure 4. Analysis of Figure 4a reveals that both CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3 are capable of both ultraviolet and visible light, with CoFe2O4/NaBiO3 exhibiting enhanced absorption intensity, particularly favorable for visible light absorption. The threshold wavelengths for CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3 are 822, 875, and 869 nm, respectively, indicating absorption in the infrared spectrum for all mentioned catalysts. The bandwidths of CoFe2O4 and CoFe2O4/NaBiO3 were evaluated according to the Kubelka–Munk formula [51]: αhυ = A (hυ − Eg)n/2, where α is the absorption coefficient; h is Planck constant; υ is the frequency of incident light; and A is a constant. When the semiconductor is a direct transition semiconductor, n is 1; when the semiconductor is an indirect transition semiconductor, n is 4. The calculation results are shown in Figure 4b. The bandwidths of CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3 are 1.1, 1.3, and 1.08 eV, respectively, proving that the combination of CoFe2O4 and NaBiO3 semiconductors can effectively reduce the forbidden bandwidth and, at the same time, enhance the absorption of visible light, thus improving the degradation performance of CoFe2O4/NaBiO3. Figure 4c shows the PL spectra of CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3, reflecting the dynamics of photogenerated electron–hole pair separation and recombination at an excitation wavelength of 340 nm. NaBiO3 exhibits a strong emission peak at 450 nm, whereas CoFe2O4 and CoFe2O4/NaBiO3 show significant emission peaks near 410 nm. Notably, CoFe2O4/NaBiO3 substantially diminishes the intensity of the emission band, suggesting that the integration of CoFe2O4 and NaBiO3 effectively hinders the rapid recombination of electron–hole pairs, thereby augmenting the photocatalytic performance.
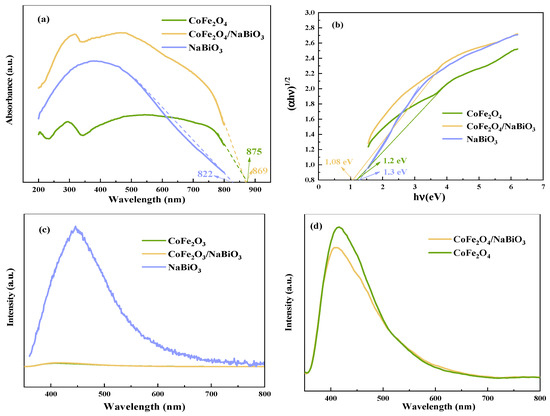
Figure 4.
UV-Vis DRS and PL spectra of CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3. (a) UV-Vis DRS spectra; (b) the forbidden bandwidth diagram; (c) PL spectra; (d) local amplification of PL spectra.
2.2. Properties of Photocatalytic Degradation of TCH in Different Systems
To assess the photocatalytic performance of CoFe2O4, NaBiO3 and CoFe2O4/NaBiO3, various reaction systems were established for conducting TCH photocatalytic degradation experiments. The outcomes of these experiments are illustrated in Figure 5. Initially, an adsorption experiment under dark conditions was conducted with a TCH concentration of 10 mg/L−1. After 20 min, CoFe2O4 exhibited a TCH removal rate of merely 18.7%, whereas NaBiO3 achieved a removal efficiency of up to 45%. The CoFe2O4/NaBiO3 composite demonstrated a removal efficiency of 28.4%, indicating enhanced adsorption capacity compared to CoFe2O4 alone. This improvement is likely attributable to an increased specific surface area. However, the introduction of CoFe2O4 resulted in a slight reduction in adsorption performance compared to NaBiO3 alone, possibly due to CoFe2O4 occupying some of the narrow channels within the NaBiO3 nanosheets, thereby impacting its adsorption capabilities. Therefore, it can be inferred that it is difficult to achieve rapid and complete removal of TCH through the adsorption reaction relying solely on CoFe2O4, NaBiO3, and CoFe2O4/NaBiO3. Subsequent to the adsorption phase, a 500 W xenon lamp was activated to initiate the photocatalytic oxidation process. The results revealed that, after 120 min of illumination, TCH removal rates under single illumination were less than 5%. In contrast, the degradation efficiency for the Vis+CoFe2O4, the Vis+NaBiO3, and the Vis+CoFe2O4/NaBiO3 system reached 40.2%, 75%, and 52.2%, respectively. This indicates that CoFe2O4 possesses inherent photocatalytic activity, as evidenced by a 22.5% increase in TCH removal rate compared to adsorption alone. Moreover, the Vis+CoFe2O4/NaBiO3 system exhibited an enhanced photocatalytic performance compared to CoFe2O4 alone. A pseudo-first-order kinetic model was employed to calculate the photodegradation reaction kinetics, employing the formula ln (C0/Ct) = kt, where C0 and Ct represent the initial and time-dependent TCH concentration, respectively, and k is the kinetic constant [52]. The reaction rate for the Vis+CoFe2O4/NaBiO3 system increased from 0.0031 min−1 to 0.004 min−1, aligning with the observed efficiency improvements. The improvement of degradation efficiency and rate are attributed to increased effective light absorption and the reduced recombination of photobiogenic carriers.
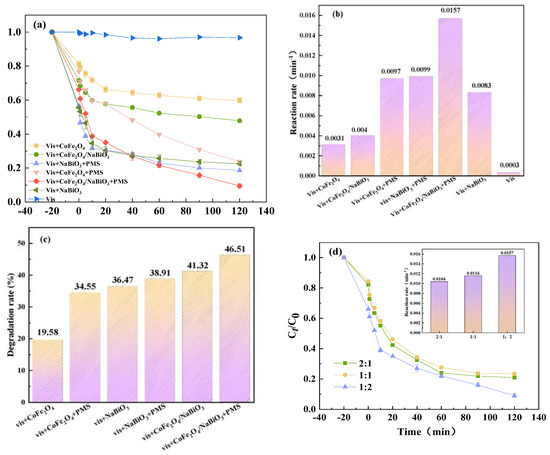
Figure 5.
The degradation efficiency on TCH and the corresponding degradation rate constants. (a,b) The removal efficiency and reaction rate of different reaction systems; (c) degradation rates of TOC in different systems; (d,e) effects of different CoFe2O4 and NaBiO3 composite ratios on TCH removal efficiency and reaction rate. Conditions: [pH] unregulated, [TCH] = 10 mg/L−1, [PMS] = 100 mg/L−1, [catalyst] = 0.5 g/L−1.
Introducing PMS as an electron acceptor to the photocatalytic system was expected to further improve the degradation efficiency. After incorporating PMS, the TCH removal efficiency in the Vis+CoFe2O4 system surged by approximately 36%, reaching 76.2%, with the reaction rate escalating from 0.0031 min−1 to 0.0097 min−1. This indicates that the visible light in conjunction with CoFe2O4 effectively activate PMS, generating highly oxidation species and enhancing TCH degradation. In the Vis+CoFe2O4NaBiO3+PMS system, TCH removal soared to 61% after only 10 min of illumination and reached 90.5% after 2 h, marking a 38% increase in final degradation efficiency compared to the Vis+CoFe2O4/NaBiO3 system without PMS. The reaction rate also increased from 0.004 min−1 to 0.0157 min−1, demonstrating significant improvements in the synergistic interaction between photocatalysis and PMS activation. This synergy is primarily due to PMS’s ability to capture photoinduced electrons and mitigate the recombination of photocatalyst carriers, thereby facilitating the generation of reactive oxygen species (ROS) and enhancing TCH degradation [53].
Additionally, the mineralization rate of organic matter is a critical metric for evaluating catalytic performance. The total organic carbon (TOC) analyzer (TOC, enviro TOC, Elementar, Germany) was employed to analyze the mineralization degree of the aforementioned systems on TCH (Figure 5c). This finding is in congruence with Figure 5a. The outcomes manifested that, subsequent to 120 min of illumination, the TOC removal rate of the Vis+CoFe2O4 system was 19.58%, and the rate of the Vis+CoFe2O4/NaBiO3 system incrementally escalated to 27.15%, yet still remained inferior to that of the Vis+NaBiO3 system (36.47%). This could potentially be attributed to the introduction of CoFe2O4, which diminishes the specific surface area of NaBiO3, thereby impinging upon its photocatalytic performance. In contrast to the solitary photocatalytic oxidation systems, the photocatalytically coupled PMS exhibits a higher TOC removal rate, particularly in the Vis+CoFe2O4/NaBiO3+PMS system (46.51%), signifying that upon the addition of PMS, more active substances for degrading TOC are engendered within the system, giving rise to a higher removal rate. This further substantiates the preponderance of photocatalytically coupled PMS systems in TCH degradation and mineralization.
The catalytic efficiency of CoFe2O4/NaBiO3 composite catalysts across various ratios was evaluated to identify the formulation with the highest efficacy. The results are depicted in Figure 5d. Analysis of the figure reveals that the catalytic system comprising Vis+CoFe2O4/NaBiO3+PMS demonstrated the lowest TCH removal rate of 76.8% when the NaBiO3 to CoFe2O4 ratio is 1:1. Conversely, when the ratio was adjusted to 2:1, the degradation rate increased to 90.5% after 120 min, with the corresponding quasi-first-order kinetic rate constant k reaching a peak value of 0.0157 min−1. These findings suggest that enhancing the CoFe2O4 content within the composite catalyst leads to improved catalytic performance. This improvement can be attributed to the increase in active sites resulting from a higher CoFe2O4 loading ratio, which facilitates the photocatalytic activation of PMS, generating a greater number of active species. Furthermore, NaBiO3 aids in the transfer process of carriers to the surface, culminating in the most effective TCH removal rate.
In practical applications, the effectiveness of photocatalysts significantly depends on their stability. Cyclic experiments were carried out to study the stability of CoFe2O4/NaBiO3. Leveraging the magnetic properties of the photocatalyst facilitated its easy recycling. It was found that the CoFe2O4/NaBiO3 composite exhibited a recovery rate of 90.3%, effectively addressing the challenge of recovering NaBiO3 due to its strong magnetic properties. The durability of the recovered CoFe2O4/NaBiO3 was assessed through degradation tests conducted over five cycles, with the results depicted in Figure 6a,b. After five cycles, the degradation efficiency of TCH in the reaction system of Vis+CoFe2O4/NaBiO3+PMS decreased from 90.5% to 73.5%, and the reaction rate decreased from 0.0157 min−1 to 0.0097 min−1. Concurrently, the adsorption removal rate of CoFe2O4/NaBiO3 for TCH experienced a decline of approximately 13% after five cycles (Figure 6a). This suggests that multiple cycles significantly undermined the adsorption performance of the composite catalyst. The presence of more macropores in CoFe2O4/NaBiO3 was beneficial for the entry and adsorption of TCH molecules at active site. However, upon light activation, the active species produced through photocatalysis and PMS activation encountered challenges in accessing the catalyst’s interior, leading to the occupation of the active site and a subsequent reduction in adsorption performance. Consequently, both the adsorption efficiency and the catalytic performance of CoFe2O4/NaBiO3 witnessed a decline, which can be attributed to losses incurred during the catalyst’s magnetic recovery process and the adsorption of intermediate degradation products on the catalyst surface. These factors contribute to a reduction in the catalyst’s specific surface area and reactive active sites, ultimately hindering the degradation of organic matter [54]. XRD and FT-IR analyses were carried out on CoFe2O4/NaBiO3 before and after usage, and the results are shown in Figure 6c,d. The analyses revealed no emergence of new characteristic peaks following photocatalysis, with only a slight reduction in existing peaks observed. Therefore, it can be concluded that the synthesized CoFe2O4/NaBiO3 is a reusable and stable high-performance photocatalyst under visible light.
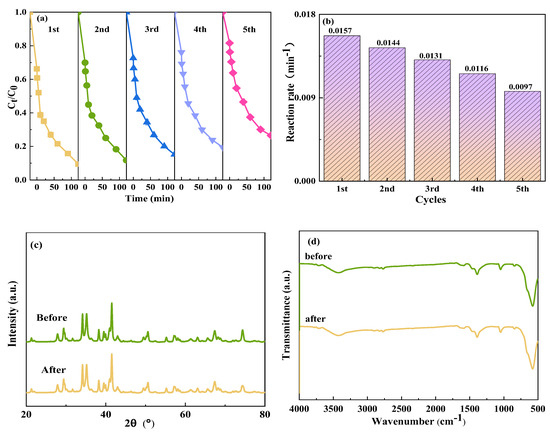
Figure 6.
Stability of CoFe2O4/NaBiO3 and XRD and FT-IR spectra before and after photocatalytic reaction. (a,b) The TCH degradation efficiency and rate in the cyclic experiments; (c,d) XRD and FT-IR spectra before and after cycling. Conditions: [pH] unregulated, [TCH] = 10 mg/L−1, [PMS] = 100 mg/L−1, [catalyst] = 0.5 g/L−1.
2.3. Effect of Experimental Conditions on the Degradation of TCH in the Reaction System
2.3.1. Effect of Catalyst and Oxidant Concentration
The degradation efficiency of TCH in the Vis+CoFe2O4/NaBiO3+PMS system increased with the increase in the catalyst concentration (Figure 7). When the added concentration of CoFe2O4/NaBiO3 was increased from 0.25 g·L−1 to 0.5 g·L−1, the adsorption removal efficiency of TCH increased by about 20% after 20 min of dark reaction. After photocatalytic degradation, the degradation efficiency greatly improved from 72.6% to 90.5%, and the reaction rate improved from 0.0096 min−1 to 0.0157 min−1. When the catalyst addition concentration was increased from 0.5 g·L−1 to 1 g·L−1, the degradation efficiency was only increased from 90.5% to 94%, and the reaction rate from 0.0157 min−1 to 0.0189 min−1. Although the degradation efficiency was improved, the increase was slight. The possible reason is that when the concentration of CoFe2O4 is higher, although it can provide more active sites, it also increases the turbidity of the reaction solution to a certain extent, which affects the reaction of CoFe2O4/NaBiO3 with light. Moreover, CoFe2O4 formed Fe2+ during the activated PMS process and reacted with the generated SO4−· and ·OH to consume the active species. Therefore, when the dosage of CoFe2O4 increases, more Fe2+ is produced. With a fixed dosage of PMS, the generated Fe2+ consumes more free radicals, which in turn affects the removal of TCH.
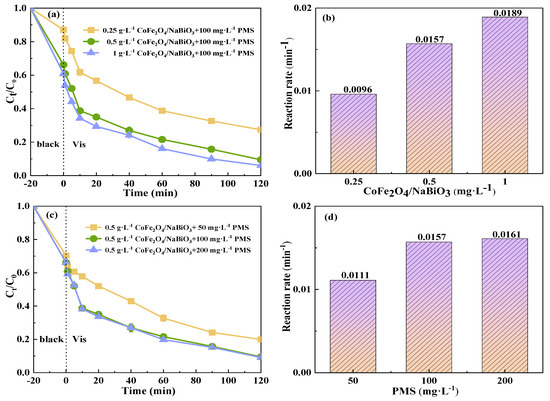
Figure 7.
The effects of CoFe2O4/NaBiO3 and PMS concentrations on the degradation of TCH in the Vis+CoFe2O4/NaBiO3+PMS system. (a,b) The effects of CoFe2O4/NaBiO3 concentration on TCH removal efficiency and rate. Conditions: [pH] unregulated, [TCH] = 10 mg/L−1, [PMS] = 100 mg/L−1, [catalyst] = 0.25, 0.5 and 1.0 g/L−1. (c,d) The effects of PMS concentration on TCH removal efficiency and rate. Conditions: [pH] unregulated, [TCH] = 10 mg/L−1, [catalyst] = 0.5 g/L−1, [PMS] = 50, 100, 200 mg/L−1.
In the Vis+CoFe2O4/NaBiO3+PMS system, as the source of SO4−·, the concentration of PMS will directly affect the degradation efficiency of TCH. As depicted in Figure 7c,d, with the concentration of PMS in the system increasing, the degradation efficiency and reaction rate of TCH were correspondingly enhanced. Additionally, prior research has similarly documented a positive correlation between the degradation rate of pollutants and the concentration of PS [55]. When the PMS concentration was 50 mg/L−1, the TCH degradation efficiency and reaction rate after 120 min of light exposure were 80% and 0.0111 min−1, respectively. With the concentration rising to 100 mg/L−1, both the TCH degradation efficiency and reaction rate significantly increased, reaching 90.5% and 0.0157 min−1. The observed increase in TCH degradation rate may be attributed to the activation of more sulfate ions through the addition of additional PMS, leading to the formation of SO4−·(Equation (1)). However, as the concentration of PMS increased to 200 mg/L−1, the observed increase in both the TCH degradation rate and the reaction rate diminished, with values of 91% and 0.0161 min−1, respectively, which could be attributed to the fact that the concentration of PMS was too high when the concentration of PMS was added to 200 mg/L−1. Large amounts of SO4−·and OH produced by PMS were quenched with the excess PMS within a short time to generate SO5−·(Equations (2) and (3)), which had weakened oxidation capacity, resulting in a minor increase in TCH degradation efficiency and rate and a lower SO4− utilization rate [56].
2HSO5− + e+ → SO4−·+ SO42− + H+
SO4−·+ HSO5− → SO5−·+SO42− + H+
OH + HSO5− → SO5−·+ H2O
2.3.2. Effect of TCH Concentration
Figure 8 shows the effect of the initial TCH concentration on its degradation by the Vis+CoFe2O4/NaBiO3+PMS system. As the initial concentration of TCH was elevated from 5 mg/L−1 to 50 mg/L−1, the degradation efficiency of TCH by the system declined from 93% to 42%, representing a decrease of approximately 49%. Concurrently, the reaction rate decreased from 0.0189 min−1 to 0.0035 min−1. This decrease can be attributed to the high initial concentration of pollutants, which likely hampered the entry of photons into the photocatalytic reaction as well as the generation of reactive radicals [57,58,59]. In addition, given that the production of active species by CoFe2O4/NaBiO3 remained constant at specific concentrations, an increase in TCH resulted in a corresponding rise in degradation intermediates. The intermediates compete with TCH molecules, further impeding the reaction rate.
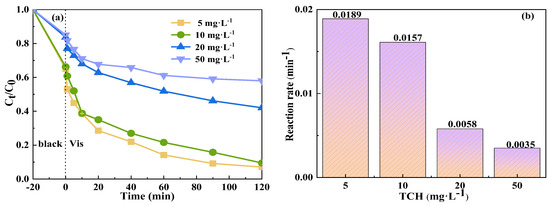
Figure 8.
Effect of initial TCH concentration on the degradation of TCH in the Vis+CoFe2O4/NaBiO3+PMS system. (a) Degradation efficiency; (b) reaction rate. Conditions: [pH] unregulated, [TCH] = 5, 10, 20, 50 mg/L−1, [PMS] = 100 mg/L−1, [catalyst] = 0.5 g/L−1.
2.3.3. Effect of Initial pH
The removal efficiency of TCH showed a trend of initial increase followed by a subsequent decrease with varying pH levels, as shown in Figure 9. Specifically, as the pH value increased from 3 to 7, the degradation efficiency of TCH improved from 81.9% to 89.2%, and the reaction rate also rose from 0.0125 min−1 to 0.0151 min−1. However, upon increasing the pH from 7 to 9, the degradation efficiency remained constant at 89.2%, yet the reaction rate slightly decreased to 0.0147 min−1. Further elevation of the pH from 9 to 11, which represents an extremely alkaline condition, resulted in a significant reduction in degradation efficiency to 73.8%, with the reaction rate also falling to 0.0087 min−1. These observations indicate that the degradation efficiency and rate are optimal under neutral pH conditions. In contrast, under extremely acidic conditions, the performance is superior to that under alkaline conditions. This disparity is due to the reaction of OH− with SO4−· in alkaline solutions to produce ·OH, which becomes the predominant active species [60]. The redox potential of ·OH (1.8–2.7 V) was significantly lower than that of SO4−·(2.5–3.1 V), implying a reduced oxidation ability of OH [61] and, consequently, a diminished degradation efficiency of TCH. Conversely, under acidic conditions, PMS mainly exists in the form of H2SO5, and the catalyst is less effective in activating PMS to produce SO4−·, thus affecting the degradation efficiency of TCH. Based on these findings, it is evident that the reaction system exhibits a broad adaptability to pH variations, enabling a higher TCH removal efficiency without the need for pH adjustment in practical applications.
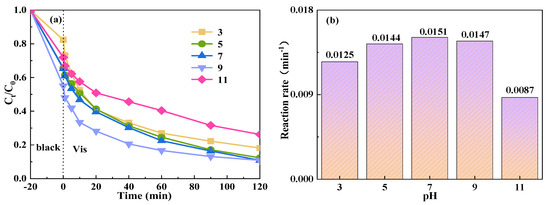
Figure 9.
Effect of initial pH value on the degradation of TCH in the Vis+CoFe2O4/NaBiO3+PMS system. (a) The TCH degradation efficiency diagram. (b) The reaction rate diagram. Conditions: [pH] = 3, 5, 7, 9, 11; [TCH] = 10 mg/L−1, [PMS] = 100 mg/L−1, [catalyst] = 0.5 g/L−1.
2.3.4. Effects of NOM
The composition of wastewater from actual livestock farming is notably complex, containing a significant amount of natural organic matter (NOM), which can affect the oxidation reaction of free radicals. NOM is known to quench free radicals and revert free radical intermediates back into their parent compounds [55]. Consequently, to simulate real-world aquaculture wastewater and examine the influence of organic acids on the degradation of TCH by a photocatalytic system coupled with PMS, FA was introduced. As shown in Figure 10, the addition of FA exerted a discernible inhibitory effect on the catalytic degradation of TCH in the Vis+CoFe2O4/NaBiO3+PMS system. This inhibitory effect became more pronounced with increasing concentrations of FA. Specifically, in the absence of FA (0 mg/L−1), the degradation efficiency of TCH reached 90.5%, with a reaction rate of 0.0157 min−1. However, both the degradation efficiency and reaction rate decreased gradually with higher FA concentrations. At an FA concentration of 20 mg/L−1, the degradation efficiency plummeted to 35.6%, and the reaction rate decreased to 0.0037 min−1. This phenomenon can be attributed to the carboxyl and hydroxyl functional groups in FA, which act as free radical scavengers, competing with TCH for active species and thereby impeding TCH degradation [62]. Furthermore, FA can occupy the reaction sites on CoFe2O4 [63], thereby reducing TCH degradation.
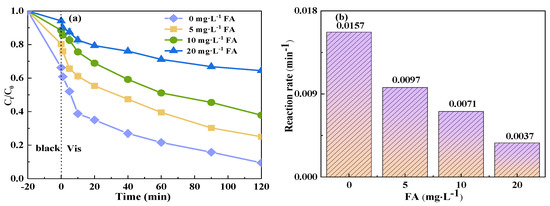
Figure 10.
Effect of NOM addition on the degradation of TCH in the Vis+CoFe2O4/NaBiO3+PMS system. (a) Degradation efficiency; (b) reaction rate. Conditions: [pH] unregulated, [TCH] = 10 mg/L−1, [PMS] = 100 mg/L−1, [catalyst] = 0.5 g/L−1, [FA] = 0, 5, 10, 20 mg/L−1.
2.4. Photocatalytic Mechanism of Vis+CoFe2O4/NaBiO3+PMS System
To elucidate the primary free radicals generated within the system and clarify the degradation mechanism, various chemical scavengers were introduced into the Vis+CoFe2O4/NaBiO3+PMS system. The findings, presented in Figure 11, indicate that the addition of tertbutyl alcohol (TBA) and ethanol (EtOH) significantly reduced the degradation efficiency from 90.5% to 41% and 48.4%, respectively, and the reaction rate from 0.0157 min−1 to 0.0028 min−1 and 0.0036 min−1. This suggests that ·OH and SO4−· are the main active species responsible for TCH degradation in this system. The addition of sodium oxalate (SO) and p-benzoquinone (BQ) resulted in a slight decrease in degradation efficiency by about 11% (79.6%) and 6% (84.8%), respectively, with reaction rates of 0.0114 min−1 and 0.0133 min−1, indicating that small amounts of h+ and ·O2− were also produced during the degradation process. The production of ·OH might stem from the photo-Fenton process. The photogenerated electron–hole pair reacts in situ with dissolved oxygen or H2O to generate H2O2 (Equations (4) and (5)) [64]. It, along with Fe3+/Fe2+, Co3+/Co2+, constitutes the ideal conditions for the photo-Fenton, leading to the production of ·OH (Equations (6) and (7)) [65]. The formation path of free radicals primarily occurs through interaction of Co2+ on the catalyst surface with H2O molecules adsorbed on the catalyst, forming CoOH+, which further reacts with PMS to form SO4−· (Equations (8) and (9)) [66]. SO4−·can directly degrade the target pollutants and react with H2O or OH− to form ·OH (Equations (10) and (11)) [67]. The synergistic effect of CoFe2O4 and NaBiO3 is beneficial to the activation of PMS and promotes the circulation of Co2+/Co3+ and Fe3+/Fe2+ valence states to form a dynamic equilibrium (Equations (12)–(16)). Comparative PL tests revealed that CoFe2O4/NaBiO3 broadens the response to visible light and inhibits the recombination of photogenerated electron–hole pairs, facilitating the generation of h+, which acts to degrade pollutants, ultimately mineralizing TCH into CO2 and H2O.
2h+ + 2H2O → H2O2 + 2H+
2H+ + O2+2e− → H2O2
Fe2+ + H2O2 → Fe3+ +·OH + OH−
Co2+ + H2O2 → Co3+ +·OH + OH−
Co2+ + H2O ↔ CoOH+ + H+
CoOH+ + HSO5− → CoO+ + SO4−·+ H2O
SO4−·+ H2O → SO42−·+·OH+H+
SO4−·+ OH− → SO42−·+·OH
Co2+ + HSO5− → Co3+ + SO4−·+ OH−
Co3+ + HSO5− → Co2+ + SO5−·+ H+
Fe3+ + HSO5− → Fe2+ + SO5−·+ H+
Fe2+ + HSO5− → Fe3+ + SO4−·+ OH−
Fe3+ + Co2+ → Fe2+ + Co3+
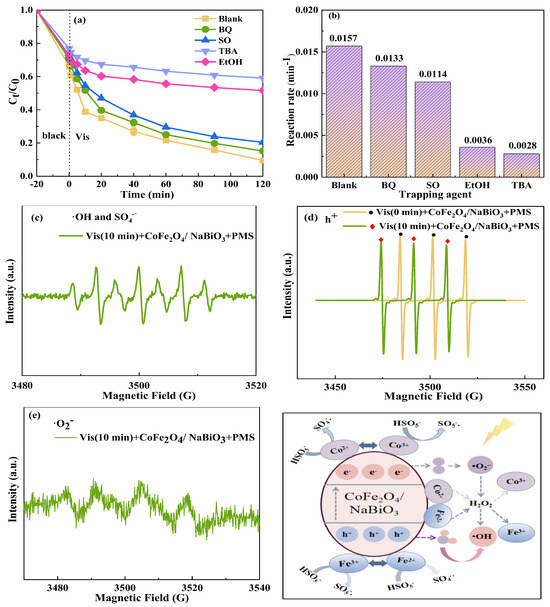
Figure 11.
(a) The photocatalytic degradation efficiency of TCH with different scavengers. (b) Reaction rate; (c) DMPO-·OH and DMPO-SO4−·; (d) TEMPO-h+; (e) DMPO-·O2−. (f) Schematic diagram of degradation mechanism. Conditions: [pH] unregulated, [TCH] = 10 mg/L−1, [PMS] = 100 mg/L−1, [catalyst] = 0.5 g/L−1, [FA] = 0, 5, 10, 20 mg/L−1.
Electron paramagnetic resonance (EPR) tests further confirmed the active species produced by the Vis+CoFe2O4/NaBiO3+PMS system during TCH degradation (Figure 11c–f). The EPR spectrum showed DMP-OH and DMPO-SO4−· signal peaks (Figure 11c), attributed to the oxidation of the trapping agent rather than direct signals from OH and SO4−·, possibly due to the generation of high-valence iron–oxygen species during the redox degradation of pollutants. DMPO was directly oxidized to form a DMPO-X peak shape [68]. The free radical capture test corroborated that ·OH and SO4−· are produced in the system and play a significant role in pollutant removal. The detection of holes and superoxide radicals under illuminated conditions further supports the system’s capacity to generate these species for pollutant degradation. Figure 11d shows the signal peak of the holes. It was found that the signal intensity generated under no-light conditions was the electronic signal contained in the capturing agent TEMPO. When the signal peak intensity is weakened under light conditions, it proves that the system can generate holes. Figure 11e shows the comparison of DMPO-·O2− signal peaks of the system. After illumination for 10 min, the system detected a six-fold signal peak of DMPO-O2−, indicating that ·O2− can be generated in the system during illumination and used for pollutant degradation.
Therefore, through the free radical capture test and EPR test, it was determined that the primary active species in the Vis+CoFe2O4/NaBiO3+PMS system are mainly ·OH > SO4−·> h+ > O2−.
3. Materials and Methods
3.1. Reagents
TCH and sodium bismuth dihydrate (NaBiO3·2H2O) were purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China).; iron nitrate heptahydrate (Fe(NO3)3·9H2O) and cobalt nitrate hexahydrate (Co(NO3)2·6H2O) were purchased from Beijing Minda Technology Co. (Beijing, China); citric acid (C6H8O4), ethylene glycol ((CH2OH)2), ethanol (C2H5OH), sodium oxalate (Na2C2O4), p-benzoquinone (C6H4O2), and tert-butyl alcohol (C4H10O) were purchased from Tianjin Xinbote Chemical Co., Ltd. (Tianjin, China); and potassium monopersulfate (KHSO5) was purchased from Shanghai McLean Biochemical Technology Co. (Shanghai, China).
3.2. Preparation of CoFe2O4/NaBiO3
CoFe2O4 was synthesized through the sol–gel method as follows: Firstly, a stoichiometric ratio of 1:2:3 for Fe(NO3)3·9H2O, Co(NO3)2·6H2O, and citric acid was accurately weighed and dissolved in 20 mL of deionized water. This mixture was then stirred at 60 °C for 40 min. Subsequently, 23 mL of ethylene glycol was added to the solution to elevate the temperature to 90 °C. The mixture was continuously stirred until a gel-like resin polymer was formed. This gel was then transferred to a crucible with a lid and placed in a muffle furnace where it underwent calcination at 500 °C for 4 h. Upon cooling, the resultant product was CoFe2O4.
The preparation of CoFe2O4/NaBiO3 was carried out using solvothermal method: The procedure began with weighing the molar ratio of NaBiO3 and CoFe2O4 at 1:2. The measured CoFe2O4 was then dispersed into 100 mL of methanol and subjected to ultrasonically dispersed for 20 min to achieve a homogeneous solution. Following this, NaBiO3 was introduced into the solution. After stirring for 40 min, the resultant suspension was transferred into a 100 mL Teflon-sealed autoclave and reacted at 180 °C for 10 h. Once the reaction vessel had naturally cooled to room temperature, the obtained products were filtered and subsequently washed 2–3 times using deionized water and anhydrous ethanol, respectively. The final step involved drying the washed products at 60 °C for 12 h in the oven. The dried product was designated as CoFe2O4/NaBiO3.
3.3. Characterization of the Catalysts
The surface morphology of CoFe2O4 and CoFe2O4/NaBiO3 was observed by scanning electron microscopy (SEM; ZEISS Gemini 300, Karl Zeiss, Oberkochen, Germany), with the operating voltage of the morphology at 3 kV. Prior to analysis, all samples underwent a gold sputtering process for 45 s to enhance image clarity. The specific surface area and pore structure characteristics of the catalysts were determined using an Automatic Specific Surface Area and Aperture Distribution Analyzer (BET; AUTOSORB IQ, Quantachrome, Boynton Beac, FL, USA). This analysis involved plotting nitrogen adsorption–desorption isothermal and pore size distribution curves. The chemical composition of CoFe2O4/NaBiO3 was further investigated through X-ray photoelectron spectroscopy (XPS; Thermo Scientific K-Alpha XPS, Thermo Fisher, Waltham, MA, USA). The phase structure of the samples was characterized by X-ray diffraction (XRD; BrukerAXS D8 Advance, Bruker, Karlsruhe, Germany) utilizing a cobalt target over a scanning range of 20–80°and a scanning speed of 2°·min−1. An infrared spectrometer (FT-IR; Thermo Scientific Nicolet iS20, Thermo Scientific, Waltham, MA, USA), with a testing wavenumber range of range of 500–4000 cm−1, was used to analyze the chemical bonds and functional groups present in the samples. The UV-Vis diffuse reflectance spectra of the samples were measured by a UV-Vis diffuse reflectance spectrometer (UV-Vis DRS; UV-3600i Plus, Shimadzu, Kyoto, Japan) in the wavelength of 200–800 nm. Lastly, the magnetic properties of the catalysts were characterized by a hysteresis loop test (LakeShore 7404, LakeShore, Ouachita Parish, LA, USA).
3.4. Photocatalytic Degradation Experiments
To evaluate the catalytic performance of the catalysts, TCH was degraded at its natural pH under visible light irradiation. An initial step involved dispersing 100 mg of photocatalyst in 200 mL of TCH solution (10 mg/L−1). The mixture was then stirred in darkness for 20 min using a magnetic rotor stirrer to ensure uniform dispersion, followed by exposure to a 500w xenon lamp (DY500G, Guangzhou Xingtron Electronics Co., Ltd., Guangzhou, China) to simulate visible light degradation. Samples of approximately 5 mL were extracted at predetermined intervals and filtered through a 0.45 μm pore-sized filter membrane, and the absorbance was measured at 356 nm using an ultraviolet spectrophotometer to determine the TCH concentration based on standard calibration curves. The identification of free radicals in the Vis+CoFe2O4/NaBiO3+PMS system was conducted by introducing various quenchers. Investigations into TCH degradation efficiency included varying parameters such as catalyst dosage, PMS concentration, pH levels, initial TCH concentration, and organic matter content. Following adsorption and degradation experiments, the spent catalyst was collected, rinsed with deionized water, and dried under vacuum at 60 °C to assess its stability for subsequent experimental procedures.
4. Conclusions
The findings of this study demonstrate that utilizing NaBiO3 as a carrier for the synthesis of CoFe2O4/NaBiO3 effectively addresses the issue of CoFe2O4 agglomeration and concurrently addresses the quandary that NaBiO3 is arduous to retrieve. This approach notably enhances the photocatalytic properties of the material by increasing its narrowing the band gap, broadening the absorption of visible light, and significantly reducing the recombination of photogenerated electron–hole pairs, thereby augmenting its photocatalytic efficiency. The coupled Vis+CoFe2O/NaBiO3+PMS system exhibited a high capability for degrading TCH, achieving a degradation efficiency of 94% after 100 min of illumination under specific conditions: a TCH concentration of 10 mg/L−1, a catalyst concentration of 1 g·L−1, and a PMS concentration of 100 mg/L−1. It was observed that the alkaline environment inhibited the oxidative degradation of TCH, with the highest degradation efficiency occurring under neutral conditions. In addition, the presence of NOM was found to hinder the degradation process. The predominant free radicals contributing to the reaction system were identified as ·OH and SO4−·. The CoFe2O4/NaBiO3 composite material not only possesses excellent magnetic properties, facilitating easy recovery, but also displays commendable stability, making it a viable option for the treatment of actual wastewater. The primary mechanism underlying the activation of PMS by Vis+CoFe2O4/NaBiO3 involves the valence cycling of Co3+ and Co2+, as well as between Fe3+ and Fe2+.
Author Contributions
J.Z.: investigation, writing—original draft, writing—review and editing. X.B. and Y.Y.: preparation of catalysts and photocatalytic experiments. S.Z. and C.L.: data processing. W.H.: writing—review and editing. X.L.: formal analysis, methodology. F.L.: project administration, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Bingtuan Science and Technology Program (2021DB019; 2022CB001-01), the President’s foundation of Tarim University (TDZKCX202404; TDZKSS202203), and the earmarked fund for XJARS (XJARS-06).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon request.
Acknowledgments
The authors thank their institutions for providing the necessary research facilities for this work. Thanks to the Analytical Test Center of Tarim University for its help in the process of photocatalytic characterization analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Luo, T.; Feng, H.; Tang, L.; Lu, Y.; Tang, W.; Chen, S.; Yu, J.; Xie, Q.; Ouyang, X.; Chen, Z. Efficient degradation of tetracycline by heterogeneous electro-Fenton process using Cu-doped Fe@Fe2O3: Mechanism and degradation pathway. Chem. Eng. J. 2020, 382, 122970. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, L.; Chen, X.; Meng, S.; Xie, Y.; Sheng, M.; Cao, G. The role of nitrification inhibitors on the removal of antibiotics in livestock wastewater by aerobic biodegradation. Sci. Total Environ. 2022, 806, 150309. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, G.; Liu, Y.; Lu, S.; Qin, P.; Guo, X.; Bi, B.; Wang, L.; Xi, B.; Wu, F.; et al. Occurrence and fate of antibiotics and antibiotic resistance genes in typical urban water of Beijing, China. Environ. Pollut. 2019, 246, 163–173. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Sun, H.; Zhao, L.; Liu, Y. Fate of tetracycline in enhanced biological nutrient removal process. Chemosphere 2018, 193, 998–1003. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Ke, Y.; Chen, C.; Xie, S. A comprehensive review on biodegradation of tetracyclines: Current research progress and prospect. Sci. Total Environ. 2022, 814, 152852. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Wang, P.; Wang, Z.; Zuo, C.; Chen, W.; Ao, T. Facile fabrication of N-doped hierarchical porous carbons derived from soft-templated ZIF-8 for enhanced adsorptive removal of tetracycline hydrochloride from water. J. Hazard. Mater. 2022, 423, 127103. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Huo, S.; Xin, Y.; Gao, M.; Wang, Y.; Liu, W.; Zhang, C.; Ma, X. Heterogeneous photo-electro-Fenton degradation of tetracycline through nitrogen/oxygen self-doped porous biochar supported CuFeO2 multifunctional cathode catalyst under visible light. Appl. Catal. B Environ. 2022, 312, 121442. [Google Scholar] [CrossRef]
- De Godos, I.; Muñoz, R.; Guieysse, B. Tetracycline removal during wastewater treatment in high-rate algal ponds. J. Hazard. Mater. 2012, 229–230, 446–449. [Google Scholar] [CrossRef]
- de Souza, D.I.; Dottein, E.M.; Giacobbo, A.; Rodrigues, M.A.S.; de Pinho, M.N.; Bernardes, A.M. Nanofiltration for the removal of norfloxacin from pharmaceutical effluent. J. Environ. Chem. Eng. 2018, 6, 6147–6153. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Ye, C.; Lin, W.; Zhang, M.; Chen, L.; Li, J.; Yu, X. Biofilm processes in treating mariculture wastewater may be a reservoir of antibiotic resistance genes. Mar. Pollut. Bull. 2017, 118, 289–296. [Google Scholar] [CrossRef]
- Dargahi, A.; Hasani, K.; Mokhtari, A.; Vosoughi, M.; Moradi, M.; Vaziri, Y. Highly effective degradation of 2,4-Dichlorophenoxyacetic acid herbicide in a three dimensional sono-electro-Fenton (3D/SEF) system using powder activated carbon (PAC)/Fe3O4 as magnetic particle electrode. J. Environ. Chem. Eng. 2021, 9, 105889. [Google Scholar] [CrossRef]
- Nasseh, N.; Arghavan, F.S.; Rodriguez-Couto, S.; Panahi, A.H.; Esmati, M.; A-Musawi, T.J. Preparation of activated carbon@ZnO composite and its application as a novel catalyst in catalytic ozonation process for metronidazole degradation. Adv. Powder Technol. 2020, 31, 875–885. [Google Scholar] [CrossRef]
- Dong, C.; Yang, Y.; Hu, X.; Cho, Y.; Jang, G.; Ao, Y.; Wang, L.; Shen, J.; Park, J.H.; Zhang, K. Self-cycled photo-Fenton-like system based on an artificial leaf with a solar-to-H2O2 conversion efficiency of 1.46%. Nat. Commun. 2022, 13, 4982. [Google Scholar] [CrossRef]
- He, Q.; Chen, Z.; Liu, G.; Zhang, Y.; Cai, F.; Lü, J. Phase controlled bismuth molybdates with enhanced photocatalytic degradation of tetracycline under visible irradiation. Inorg. Chem. Commun. 2019, 108, 107522. [Google Scholar] [CrossRef]
- Zhu, P.; Luo, D.; Liu, M.; Duan, M.; Lin, J.; Wu, X. Flower-globular BiOI/BiVO4/g-C3N4 with a dual Z-scheme heterojunction for highly efficient degradation of antibiotics under visible light. Sep. Purif. Technol. 2022, 297, 121503. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhang, J.; Li, W.; Zhou, P.; Pan, Z.; Lai, B. Nonradical induced degradation of bisphenol AF by NaBiO3 coupled peroxymonosulfate process: Performance and mechanism. Sep. Purif. Technol. 2022, 285, 120356. [Google Scholar] [CrossRef]
- Ma, M.; Chen, Y.; Tong, Z.; Liu, Y.; Ma, Y.; Wang, R.; Bi, Y.; Liao, Z. Research progress of magnetic bismuth-based materials in photocatalysis: A review. J. Alloys Compd. 2021, 886, 161096. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Kohtani, S.; Hiro, J.; Yamamoto, N.; Kudo, A.; Tokumura, K.; Nakagaki, R. Adsorptive and photocatalytic properties of Ag-loaded BiVO4 on the degradation of 4-n-alkylphenols under visible light irradiation. Catal. Commun. 2005, 6, 185–189. [Google Scholar] [CrossRef]
- Nobuhiro, K.; Ayumi, N.; Akira, M.; Takahiro, T.; Masaki, A.; Hajime, Y.; Eisuke, M.; Chikako, M.; Yoshihiro, K. Hydrothermal synthesis and crystal structure of a new lithium copper bismuth oxide, LiCuBiO4. J. Solid State Chem. 2017, 245, 30–33. [Google Scholar] [CrossRef]
- Wu, Y.; 1 Zhao, X.; Huang, S.; Li, Y.; Zhang, X.; Zeng, G.; Niu, L.; Ling, Y.; Zhang, Y. Facile construction of 2D g-C3N4 supported nanoflower-like NaBiO3 with direct Z-scheme heterojunctions and insight into its photocatalytic degradation of tetracycline. J. Hazard. Mater. 2021, 414, 125547. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Li, F.; Zhang, J.; Zhong, M.; Yang, Y.; Khan, S. Photocatalytic degradation of tetracycline antibiotics in swine wastewater using Fe3+-loaded NaBiO3 coupled with sodium persulfate. Catal. Commun. 2023, 174, 106579. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, H. One-pot preparation of NaBiO3/PNMA composite: Surface properties and photocatalytic performance. Appl. Surf. Sci. 2021, 544, 148910. [Google Scholar] [CrossRef]
- Long, X.; Feng, C.; Ding, D.; Chen, N.; Yang, S.; Chen, H.; Wang, X.; Chen, R. Oxygen vacancies-enriched CoFe2O4 for peroxymonosulfate activation: The reactivity between radical-nonradical coupling way and bisphenol A. J. Hazard. Mater. 2021, 418, 126357. [Google Scholar] [CrossRef]
- Pang, B.; Lin, S.; Shi, Y.; Wang, Y.; Chen, Y.; Ma, S.; Feng, J.; Zhang, C.; Yu, L.; Dong, L. Synthesis of CoFe2O4/graphene composite as a novel counter electrode for high performance dye-sensitized solar cells. Electrochim. Acta 2019, 297, 70–76. [Google Scholar] [CrossRef]
- Annie Vinosha, A.; Manikandan, A.; Preetha, A.C.; Dinesh, A.; Slimani, Y.; Almessiere, M.A.; Baykal, A.; Xavier, B.; Nirmala, G.F. Review on recent Advances of synthesis, magnetic properties, and water treatment applications of Cobalt Ferrite nanoparticles and nanocomposites. J. Supercond. Nov. Magn. 2021, 34, 995–1018. [Google Scholar] [CrossRef]
- Kumar, A.; Chandel, M.; Sharma, A.; Thakur, M.; Kumar, A.; Pathania, D.; Singh, L. Robust visible light active PANI/LaFeO3/CoFe2O4 ternary heterojunction for the photo-degradation and mineralization of pharmaceutical effluent: Clozapine. J. Environ. Chem. Eng. 2021, 9, 106159. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; McKay, G.; Rajiv, P.; Mengelizadeh, N.; Balarak, D. Efficient sonophotocatalytic degradation of acid blue 113 dye using a hybrid nanocomposite of CoFe2O4 nanoparticles loaded on multi-walled carbon nanotubes. J. Photochem. Photobiol. A Chem. 2022, 424, 113617. [Google Scholar] [CrossRef]
- Lin, E.; Huang, R.; Wu, J.; Kang, Z.; Ke, K.; Qin, N.; Bao, D. Recyclable CoFe2O4 modified BiOCl hierarchical microspheres utilizing photo, photothermal and mechanical energy for organic pollutant degradation. Nano Energy 2021, 89, 106403. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Y.; Huang, S.; Xie, M.; He, M.; Xu, H.; Li, H.; Zhang, Q. Novel magnetic CoFe2O4/Ag/Ag3VO4 composites: Highly efficient visible light photocatalytic and antibacterial activity. Appl. Catal. B Environ. 2016, 199, 11–22. [Google Scholar] [CrossRef]
- Song, J.; Zhang, J.; Zada, A.; Ma, Y.; Qi, K. CoFe2O4/NiFe2O4 S-scheme composite for photocatalytic decomposition of antibiotic contaminants. Ceram. Int. 2023, 49, 12327–12333. [Google Scholar] [CrossRef]
- Gogoi, D.; Das, M.R.; Ghosh, N.N. 2-D g-C3N4 supported CoFe2O4 nanoparticles as an efficient S-scheme catalyst for various antibiotic degradation. Appl. Surf. Sci. 2023, 619, 156753. [Google Scholar] [CrossRef]
- Maria Magdalane, C.; Maria Assuntha Priyadharsini, G.; Kaviyarasu, K.; Irudaya Jothi, A.; Gnanamani Simiyon, G. Synthesis and characterization of TiO2 doped cobalt ferrite nanoparticles via microwave method: Investigation of photocatalytic performance of congo red degradation dye. Surf. Interfaces 2021, 25, 101296. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Pugazhenthiran, N.; Mangalaraja, R.V.; Asiri, A.M.; Anandan, S. ZnO supported CoFe2O4 nanophotocatalysts for the mineralization of Direct Blue 71 in aqueous environments. J. Hazard. Mater. 2013, 252–253, 171–179. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, H.; Sun, X.; Wang, X. Combination of cobalt ferrite and graphene: High- performance and recyclable visible-light photocatalysis. Appl. Catal. B Environ. 2012, 111, 280–287. [Google Scholar] [CrossRef]
- Huang, S.; Xu, Y.; Xie, M.; Xu, H.; He, M.; Xia, J.; Huang, L.; Li, H. Synthesis of magnetic CoFe2O4/g-C3N4 composite and its enhancement of photocatalytic ability under visible-light. Colloids Surf. A Physicochem. Eng. Asp. 2015, 478, 71–80. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Jing, J.; Mu, J.; Wang, R.; Du, C.; Su, Y. Photoelectrocatalytic peroxymonosulfate activation over CoFe2O4-BiVO4 photoanode for environmental purification: Unveiling of multi-active sites, interfacial engineering and degradation pathways. J. Colloid Interface Sci. 2023, 644, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, X.; Jiang, J.; Ma, J.; Liu, G.; Cao, Y.; Liu, W.; Li, J.; Pang, S.; Kong, X.; et al. Degradation of sulfamethoxazoleby UV, UV/H2O2 and UV/persulfate (PDS): Formation of oxidation products and effect of bicarbonate. Water Res. 2017, 118, 196–207. [Google Scholar] [CrossRef]
- Feng, M.; Qu, R.; Zhang, X.; Sun, P.; Sui, Y.; Wang, L.; Wang, Z. Degradation of flumequine in aqueous solution by persulfate activated with common methods and Polyhydroquinone-coated magnetite/multi-walled carbon nanotubes catalysts. Water Res. 2015, 85, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Xu, M.J.; Yao, G.; Lai, B. Enhancement of the degradation of atrazine through CoFe2O4 activated peroxymonosulfate (PMS) process: Kinetic, degradation intermediates, and toxicity evaluation. Chem. Eng. J. 2018, 348, 1012–1024. [Google Scholar] [CrossRef]
- Song, Q.; Feng, Y.; Wang, Z.; Liu, G.; Lv, W. Degradation of triphenyl phosphate (TPhP) by CoFe2O4-activated peroxymonosulfate oxidation process: Kinetics, pathways, and mechanisms. Sci. Total Environ. 2019, 681, 331–338. [Google Scholar] [CrossRef]
- Sin, J.; Lam, S.; Zeng, H.; Lin, H.; Li, H.; Huang, L.; Tham, K.; Mohamed, A.R. Enhanced synchronous photocatalytic 4-chlorophenol degradation and Cr(VI) reduction by novel magnetic separable visible-light-driven Z-scheme CoFe2O4/P-doped BiOBr heterojunction nanocomposites. Environ. Res. 2022, 212, 113394. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gou, G.; Zhao, H.; Liu, C.; Li, N.; Li, L.; Tan, B.; Lai, B. Efficient peroxymonosulfate activation by CoFe2O4-CeO2 composite: Performance and catalytic mechanism. Chem. Eng. J. 2022, 435, 134840. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, K.; Jia, Y.; Zhu, G.; Wang, Q. A novel magnetically separable CoFe2O4-BiO2−x heterojunction with enhanced photocatalytic activity under near-infrared light irradiation. Mater. Lett. 2021, 303, 130497. [Google Scholar] [CrossRef]
- Zhou, L.; Ji, L.; Ma, P.; Shao, Y.; Zhang, H.; Gao, W.; Li, Y. Development of carbon nanotubes/CoFe2O4 magnetic hybrid material for removal of tetrabromobisphenol A and Pb(II). J. Hazard. Mater. 2014, 265, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yang, F.; Zhu, L.; Wang, N.; Tang, H. Bi3+ self doped NaBiO3 nanosheets: Facile controlled synthesis and enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2015, 164, 151–158. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Wei, S.; Wu, Y.; Zheng, Y.; Yuan, F.; Hou, J. Study on photocatalytic degradation of amoxicillin in wastewater by Bi2WO6/nano-ZnO. Opt. Mater. 2022, 123, 111835. [Google Scholar] [CrossRef]
- Kasinathan, M.; Thiripuranthagan, S.; Sivakumar, A.; Ranganathan, S.; Vembuli, T.; Kumaravel, S.; Erusappan, E. Fabrication of novel Bi2MoO6/N-rGO catalyst for the efficient photocatalytic degradation of harmful dyes. Mater. Res. Bull. 2020, 125, 110782. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Song, P.; Xu, R.; Wang, H. Facile synthesis of Bi2MoO6/ZnSnO3 heterojunction with enhanced visible light. Appl. Surf. Sci. 2018, 430, 561–570. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Feizpoor, S.; Seifzadeh, D.; Ghosh, S. Improving visible-light-induced photocatalytic ability of TiO2 through coupling with Bi3O4Cl and carbon dot nanoparticles. Sep. Purif. Technol. 2020, 238, 116404. [Google Scholar] [CrossRef]
- Zhao, G.; Ding, J.; Zhou, F.; Chen, X.; Wei, L.; Gao, Q.; Wang, K.; Zhao, Q. Construction of a visible-light-driven magnetic dual Z-scheme BiVO4/g-C3N4/NiFe2O4 photocatalyst for effective removal of ofloxacin: Mechanisms and degradation pathway. Chem. Eng. J. 2021, 405, 126704. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, X.; Nie, C.; Jiang, Z.; Xie, Z.; Lin, C. Shape controlled growth of gold nanoparticles by a solution synthesis. Chem. Commun. 2005, 33, 4181–4183. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Tang, Y.; Wang, M.; Jin, C.; Liu, J.; Li, S.; Li, Z.; Zhu, J. The enhanced peroxymonosulfate-assisted photocatalytic degradation of tetracycline under visible light by g-C3N4/Na-BiVO4 heterojunction catalyst and its mechanism. J. Environ. Chem. Eng. 2021, 9, 105524. [Google Scholar] [CrossRef]
- Ding, D.; Yang, S.; Chen, L.; Cai, T. Degradation of norfloxacin by CoFe alloy nanoparticles encapsulated in nitrogen doped graphitic carbon (CoFe@N-GC) activated peroxymonosulfate. Chem. Eng. J. 2020, 392, 123725. [Google Scholar] [CrossRef]
- Fan, Y.; Ji, Y.; Kong, D.; Lu, J.; Zhou, Q. Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process. J. Hazard. Mater. 2015, 300, 39–47. [Google Scholar] [CrossRef]
- Yang, Q.; Choi, H.; Chen, Y.; Dionysiou, D.D. Heterogeneous activation of peroxymonosulfate by supported cobalt catalysts for the degradation of 2,4-dichlorophenol in water: The effect of support, cobalt precursor, and UV radiation. Appl. Catal. B Environ. 2008, 77, 300–307. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Lv, X.; Zhang, Y.; Guo, G. Photocatalytic organic pollutants degradation in metal–organic frameworks. Energy Environ. Sci. 2014, 9, 2831–2867. [Google Scholar] [CrossRef]
- Gong, J.; Wang, B.; Zeng, G.; Yang, C.; Niu, C.; Niu, Q.; Zhou, W.; Liang, Y. Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J. Hazard. Mater. 2009, 16, 1517–1522. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Wu, H.; Zhang, C.; Dai, J.; Liang, J.; Yu, J.; Ren, X.; Yi, H.; Cheng, M.; et al. Biological technologies for the remediation of co-contaminatedsoil. Crit. Rev. Biotechnol. 2017, 37, 1062–1076. [Google Scholar] [CrossRef]
- Nie, W.; Mao, Q.; Ding, Y.; Hu, Y.; Tang, H. Highly efficient catalysis of chalcopyrite with surface bonded ferrous species for activation of peroxymonosulfate toward degradation of bisphenol A: A mechanism study. J. Hazard. Mater. 2019, 364, 59–68. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Zhou, Y.; Fang, L.; Shao, Y. Degradation of atenolol by UV/peroxymonosulfate: Kinetics, effect of operational, parameters and mechanism. Chemosphere 2013, 93, 2717–2724. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Song, Q.; Lv, W.; Liu, G. Degradation of ketoprofen by sulfate radical-based advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Chemosphere 2017, 189, 643–651. [Google Scholar] [CrossRef]
- Guan, Y.; Ma, J.; Ren, Y.; Liu, Y.; Xiao, J.; Lin, L.; Zhang, C. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res. 2013, 47, 5431–5438. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Zhang, Y.; Wang, Y.; Sun, S.; Wu, W.; Wu, Z. Nanostructured semiconductor supported iron catalysts for heterogeneous photo-Fenton oxidation: A review. J. Mater. Chem. A 2020, 8, 15513–15546. [Google Scholar] [CrossRef]
- Barrocas, B.T.; Osawa, R.; Conceição Oliveira, M.; Monteiro, O.C. Enhancing Removal of Pollutants by Combining Photocatalysis and Photo-Fenton Using Co, Fe-Doped Titanate Nanowires. Materials 2023, 16, 2051. [Google Scholar] [CrossRef]
- Chen, L.; Ding, D.; Liu, C.; Cai, H.; Qu, Y.; Yang, S.; Gao, Y.; Cai, T. Degradation of norfloxacin by CoFe2O4-GO composite coupled with peroxymonosulfate: A comparative study and mechanistic consideration. Chem. Eng. J. 2018, 334, 273–284. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Chen, S.; Hu, J.; Lu, L.; Wu, L.; Liang, Z.; Tang, J.; Hou, H.; Liang, S.; Yang, J. Iron porphyrin-TiO2 modulated peroxymonosulfate activation for efficient degradation of 2,4,6-trichlorophenol with high-valent iron-oxo species. Chemosphere 2022, 309, 136744. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).