Abstract
This work presents the results of studying dilute aqueous solutions of commercial Ln(NO3)3 · xH2O salts with Ln = Ce-Lu using X-ray diffraction (XRD), IR spectroscopy, X-ray absorption spectroscopy (XAS: EXAFS/XANES), and pH measurements. As a reference point, XRD and XAS measurements for characterized Ln(NO3)3 · xH2O microcrystalline powder samples were performed. The local structure of Ln-nitrate complexes in 20 mM Ln(NO3)3 · xH2O aqueous solution was studied under total external reflection conditions and EXAFS geometry was applied to obtain high-quality EXAFS data for solutions with low concentrations of Ln3+ ions. Results obtained by EXAFS spectroscopy showed significant contraction of the first coordination sphere during the dissolution process for metal ions located in the middle of the lanthanide series. It was established that in Ln(NO3)3 · xH2O solutions with Ln = Ce, Sm, Gd, Yb (c = 134, 100, 50 and 20 mM) there are coordinated and, to a greater extent, non-coordinated nitrate groups with bidentate and predominantly monodentate bonds with Ln ions, the number of which increases upon transition from cerium to ytterbium. For the first time, the antibacterial and antifungal activity of Ln(NO3)3 · xH2O Ln = Ce, Sm, Gd, Tb, Yb solutions with different concentrations and pH was presented. Cross-relationships between the concentration of solutions and antimicrobial activity with the type of Ln = Ce, Sm, Gd, Tb, Yb were established, as well as the absence of biocidal properties of solutions with a concentration of 20 mM, except for Ln = Yb. The important role of experimental conditions in obtaining and interpreting the results was noted.
1. Introduction
Traditionally, the coordination chemistry of lanthanides has been a matter of exceptional interest for wide ranging industrial applications, primarily for development of effective methods for separation and purification of rare-earth elements [1]. The efficiency of the extraction process is greatly dependent on solvation properties of lanthanide ions, such as complexation with extractants, solubility of salts, dissociation/association of ion pairs, etc. The optimization of industrial technologies for extraction of rare-earth elements, as well as elaboration of novel “green” methods for their recycling and recovering, requires deeper insight into molecular mechanisms regulating metal–ligand interaction in aqueous/organic media. Ln(NO3)3 · xH2O salts are excellent precursors for production of ultra-high purity compounds, and certain catalyst and nanoscale materials [2]. In addition, Ln(NO3)3 · xH2O is used in the growth of large-sized polyfunctional crystals doped with Ln3+ ions from aqueous solutions [3].
According to the World Health Organization (WHO), infectious diseases are spreading faster and emerging more quickly than ever before. Due to the increasing incidents related to new and reemerging infectious diseases, the discovery and development of new antimicrobial compounds with diverse structures and action mechanism is urgently needed. The antibacterial effects of lanthanides (Ln) have been studied since the 19th century [4] and have been employed since then with more or less success in the treatment of various diseases. Recently much interest in the aqueous chemistry of lanthanides has been stimulated by the growing implications of these elements in medicine and biotechnologies [5]. Cerium nitrate solutions are commonly recognized as the topical agent of choice for the treatment of burn wounds [6,7] and exhibit superior antibacterial activity [8]. In particular, Ce(NO3)3 · 6H2O salt solutions (~50 mM) are used as an antiseptic agent for the treatment of indwelling medical devices (implants, catheters) [7].
The antimicrobial properties of Ln nitrate solutions are presented, practically, only in one work [8] for Ce3+(NO3)3 · 6H2O (c = 133 mM, pH = 4.2), which suggests a connection between the growth inhibition zone (D, mm) of S. aureus, E. coli, and P. aeruginosa bacteria with the formal charge of Ce3+ and with the solution concentration. The value of D, mm of bacteria in the presence of Ce3+(NO3)3 ·6H2O solution, is much higher than that on the penicillin antibiotic with a wide spectrum of action [8]. It is not excluded that the absence of interaction of Ce3+ ions in an aqueous solution of Ce3+(NO3)3 ·6H2O salt [8] and Yb3+ ions in Yb3+(NO3)3 ·xH2O solution (c = 0.1 mM, pH = 6.5) [9] with Langmuir phospholipid monolayers of phosphatidylethanolamine (DPPE) and phosphatidylglycerol (DPPG) is associated with damage to the protein, rather than the phospholipid component of the bacterial membrane and/or with the “penetration” of Ce3+ and Yb3+ ions into the cell and disruption of its water–ion balance. Antifungal properties of Ln(NO3)3 ·xH2O solutions are not available in the literature. In general, lanthanide compounds are now considered to be a potential alternative to antibiotics in antimicrobial and antifungal therapy [5].
Investigations into the hydration behavior of lanthanides in diluted solutions are of special interest for biological sciences, when low Ln3+ concentrations are used to reduce possible cytotoxic effects. It is also important to bear in mind that the coordination environment of Ln3+ ions is strongly dependent on lanthanide salt concentration and can differ significantly in dilute aqueous solutions compared to more concentrated ones [10]. Thus, increasing attention in lanthanide biochemistry has been paid to experimental techniques that provide atomic-level information on the solution structure of Ln3+ ions under diluted conditions [11]. On the other hand, while maintaining the functional properties of dilute solutions, a decrease in the content of Ln3+ ions is ensured when drugs are taken orally.
The techniques for studying the nature (size and/or structure) of the Ln shell in solutions can be classified as direct or indirect methods [12]. The direct methods include X-ray [13,14] and neutron diffraction [15], X-ray absorption spectroscopy (EXAFS) [16,17] FT-IR [18,19] and Raman spectroscopy [19,20], and nuclear magnetic resonance (NMR) [21]. The indirect methods involve compressibility, NMR exchange, and adsorption spectroscopy measurements [21]. In addition, ab initio quantum mechanical calculations [22] and molecular dynamics (MD) are applied using the classical or mixed quantum/classical interaction potential, which are combined with UV-visible spectroscopy [23] or X-ray absorption spectroscopy (XAS), or a combination of MD simulations and XAS spectroscopy [24]. Experimental, highly sensitive microcalorimetry is used to calculate the thermodynamic characteristics of weak interactions in solutions [25] together with luminescence emission spectra and the lifetime of Ln [26]. X-ray absorption spectroscopy has been proven to be exceptionally informative in investigating the coordination environment of Ln3+ ions in aqueous solutions [11,16,24,27,28,29,30,31]. Most X-ray absorption spectroscopy studies reported in the literature are focused on the solutions of Ln3+ chloride salts. Their results cannot be transferred to solutions of other salts, nor the approaches used.
The purpose of this work is to identify the structural features of dilute aqueous solutions of lanthanide nitrates and to establish the role of the concentration and pH of the medium in the implementation of antimicrobial properties.
To characterize aqueous solutions of Ln(NO3)3 · xH2O (Ln = Ce-Lu), modern informative methods of X-ray diffraction (XRD), infrared spectroscopy (FT-IR), and X-ray absorption spectroscopy (XAS) were chosen. In the present study we used the main advantage of X-ray absorption spectroscopy under total external reflection (TER), namely that the extremely low intensity of background scattering and accordingly high signal-to-noise ratio allows for the detection of week spectroscopic signals from samples with small amounts of absorbing atoms. X-ray absorption spectroscopy measurements under TER was performed for 20 mM Ln(NO3)3 · xH2O aqueous solutions across the lanthanide series from Ce to Lu. In addition, an X-ray standing wave (XRSW) technique was applied to examine the adsorption behavior of Ln3+ ions at the air/liquid interface.
2. Results and Discussion
The local structure of Ln3+ nitrate complexes in salt and dilute aqueous solution was systematically studied across the lanthanide series from Ce to Lu.
2.1. X-ray Standing Wave Studies
Additional XRSW measurements were carried out to examine the adsorption behavior of Ln3+ ions at the solution surface, in particular the possible enrichment of Ln3+ ions at the air/liquid interface. In XRSW experiments, the intensity of characteristic fluorescence exited by the incident X-ray beam is recorded as a function of the incident angle θ. The key idea of the XRSW method is as follows: the angular dependence of fluorescence yield is highly sensitive to the position of atoms in the direction normal to the sample surface. Thus, XRSW measurements offer an opportunity to locate the atoms directly from the analysis of the corresponding fluorescence curve [32].
Experimental XRSW data, obtained for studied Ln3+ aqueous solutions, exhibited essentially similar behavior. As a typical example, Figure 1 shows angular dependence of Ce L3-fluorescence from Ce(NO3)3 · 6H2O salt solution.
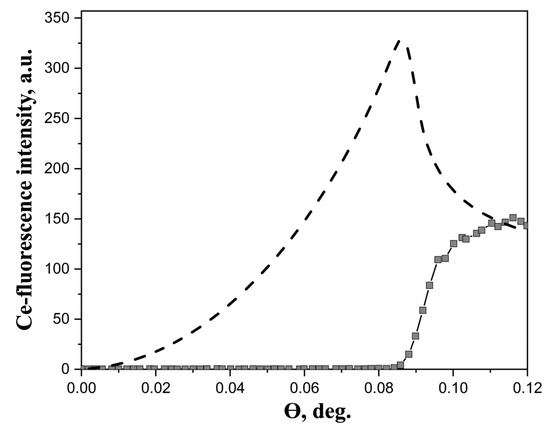
Figure 1.
Experimental angular dependence of Ce-fluorescence yield from 20 mM aqueous solution of Ce(NO3)3 · 6H2O salts (squares). The dashed line represents the calculated angular dependence of the film, in which metal ions are distributed in a layer 10 Å thick at the air/liquid interface. The energy of the incident beam was 13.6 keV.
Generally, two factors should be kept in mind when analyzing XRSW data collected under TER. The first factor is the dramatic changes in electric field intensity above the reflecting surface, as the incidence angle is scanned within the TER region. As a direct result of these changes, large modulations arise in angular dependence of fluorescence yield from atoms located in the near-surface region; calculated angular dependence, presented in Figure 1, is a perfect illustration of this characteristic feature. The calculations have been performed using the recursion formalism developed by Parratt [33]. As can be seen in Figure 1, angular dependence of fluorescence yield for near-surface distribution of atoms drastically increases from zero at θ = 0° and reaches the maximum value in the vicinity of the critical angle θC.
In marked contrast, the angular dependence of fluorescence yield from the atoms, which are present in the bulk liquid subphase, exhibits different behavior. In this case, the most important factor is the changes in the penetration depth of the electric field into the subphase. As the incident angle increases above the critical angle θC, the penetration depth abruptly rises from several nanometers (for θ < θC) to several hundreds of microns (for θ > θC), which in turn results in the sharp increase in fluorescence signal. It can be seen from Figure 1 that the Ce fluorescence curve recorded in our experiments corresponds to this type of angular dependence. These observations evidence that no enhancement of Ln3+ concentration in the near-surface region occurs in the dilute aqueous solutions of Ln(NO3)3 · xH2O salts and the distribution of Ln3+ ions can be considered as homogeneous.
2.2. X-ray Absorption Spectroscopy Studies
As a reference point in studying Ln(NO3)3 · xH2O aqueous solutions (c = 20 mM), we performed X-ray absorption spectroscopy measurements also for Ln(NO3)3 · xH2O microcrystalline powder samples (Table S1). For appropriate fitting of the first intense peak in a Fourier-transformed spectrum, one should take into account two coordination shells around the metal ion: oxygen atoms (in crystalline Ln(NO3)3 · xH2O: CNLn = 4OH2O + 6ONO3 except for CNCe = 11–5OH2O + 6ONO3, CNYb(Lu) = 9–3OH2O + 6ONO3; Ln bond is monodentate with water oxygen and bidentate with oxygen of nitrate groups), nitrogen atoms (in crystalline Ln(NO3)3 · LH2O coordination number is 3: CNLn = 3NNO3) [34,35,36,37,38,39,40,41,42,43,44]. The results of such fitting are presented in Table S1 and in Figure S1.
These fits are performed with fixed coordination numbers. Such fitting makes it possible to evaluate the capabilities of the EXAFS method for determining structural parameters. The observation results presented in Table S1 demonstrate that only the first coordination shell radius could be determined with appropriate precision.
This analysis was then applied to the experimental EXAFS data for solutions. However, in the case of solutions, coordination numbers for the oxygen and nitrogen coordination shells were considered as free parameters, and the Debye–Waller parameter for the nitrogen coordination shell was fixed to reduce the number of independent parameters during fitting. The results are also presented in Table S1 and in Figure S2. In the case of solutions, the only parameter which could be determined from EXAFS fitting with appropriate precision is an interatomic distance Ln-O for the first coordination shell. Figure 2 contains the Ln-O bond distances (R, Å) over the Ln series.
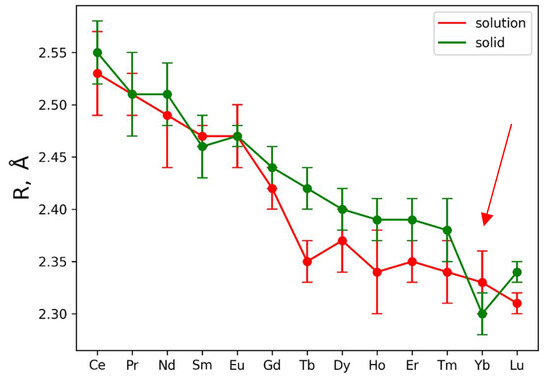
Figure 2.
Dependence of Ln-O distance (R, Å) from central atom type. Red and green curves are used to plot data for solutions and solids, respectively. The red arrow indicates a “break” in the interatomic distances at Yb.
The plotted error bars associated with each data point are based on EXAFS fitting uncertainty. It is clearly seen from the presented Ln-O distances that Ln-O for Ln = Tb, Dy, Ho, Er, Tm ions demonstrate significant contraction of the oxygen shell in solutions (Figure 2). Such observation is also in agreement with measured XANES spectra, which are plotted in Figure S5. Vertical lines in Figure S5 show the position of the second maximum in XANES spectra; shifting these lines towards higher energies indicates shrinking of the first coordination shell [45].
Concluding the results obtained by the examination of XANES spectra and the fitting of the EXAFS ones, contraction of the first coordination shell for the metals Ln = Tb, Dy, Ho, Er, Tm is observed. Stability of the nitrate complexes could not be determined by a simple EXAFS fitting procedure.
It should be noted that the nitrogen coordination number in aquatic solutions has been a subject of disagreement. Questions such as whether an eventual coordination change occurs within each series or whether inner or outer sphere complexation occurs for specific ligands have not been completely resolved. Thus, according to [25], the “light” lanthanides in aqueous solutions are nine-coordinated, whereas the “heavy” ones are eight-coordinated. According to [46], La-Nd has larger coordination numbers than Tb-Lu, and in the region between them (Ln = Sm-Gd) there are transitional structures or a mixture of structures. The authors of [47] believe that the coordination numbers change in the Nd-Dy region. It is interesting to note that the authors of the review [48] do not exclude fractional coordination (between 8 and 9) Ln = Gd-Ho in Ln(NO3) solutions. According to [49], the exact coordination numbers of rare-earth ions in the nitrate complexes of solutions are unknown. The latter statement seems to be correct, since CNLn in aqueous solutions depends on the concentration: in highly concentrated solutions, coordination with fewer solvent molecules can be realized than in dilute ones, and in significantly dilute solutions, even more so due to the shortage of water molecules.
Analysis of Figure 2 and Table S1 data allows for the distinguishing of two Ln(NO3)3 · xH2O regions: Ln = Ce-Eu (region 1) where the Ln-O interatomic distances in solid and liquid phases are almost identical, and Ln = Gd-Lu (region 2) where the interatomic distances in solid phases are larger compared to liquid ones (excluding experimental errors; the trend was analyzed) with a clear separation of the Ln-O distances with Ln = Tb and Lu (taking into account experimental errors) and a “break” in the interatomic distances at Yb (indicated by the red arrow in Figure 2). Moreover, it is not excluded that in region 1 of Ln(NO3)3 · xH2O solutions, both bidentate and monodentate bonds of Ln with nitrate groups are realized, and in region 2 only monodentate bonds occur.
According to Raman spectroscopy data [50], in a wide range of Ce(NO3)3 concentrations in aqueous solution, nitrate ions are bound to Ce both monodentately and bidentately, which is consistent with our data (Table S1, Figure 2). Complexation of Nd3+ and Eu3+ ions with the given nitrate group was studied by spectrophotometry and microcalorimetry [26]. The authors are of the opinion that both inner-sphere and outer-sphere nitrate complexes of Ln3+ ions exist in solutions. Based on thermodynamic and spectroscopic data, it is assumed that the weak complex of Nd3+ with nitrate in solution forms an inner sphere, and the nature of complex formation increases with an increase in temperature. In addition, it is possible that nitrate binds Eu3+ and possibly also Nd3+ bidentately in aqueous solutions.
Bonal S. et al. [25] used microcalorimetry to determine the stability constant, Gibbs energy, enthalpies, and entropies to analyze the very weak complexation of Ln = La-Lu nitrate anion in dilute aqueous solutions of Ln(NO3)3 · xH2O at room temperature (298.15 K), with an analysis of changes in the thermodynamic properties of (LnNO3)2+ across the lanthanide series. With a decrease in the ionic radius of Ln3+, it is more difficult for the nitrate anion with a slightly larger size in the NO3 group than a water molecule to “penetrate” into the inner sphere of the Ln3+ cation. Therefore, for Tm-Lu, the inner sphere consists exclusively of water molecules, which was confirmed by Raman measurements performed on aqueous solutions of rare-earth nitrates in the liquid state at room temperature and in the glassy state at liquid nitrogen temperature [49]. Moreover, as the cation becomes smaller, the preference for monodentate nitrate binding increases, due to avoided repulsions in the first coordination sphere. These literature data do not contradict our analysis of EXAFS results (Table S1, Figure 2).
Dobler et al. [22] used a quantum chemical study to show that an increase in the number of water molecules in the first coordination sphere of Ln3 promotes monodentate coordination of nitrate. The change from bidentate to monodentate coordination is also observed before the salt dissociation; i.e., in an aqueous solution, nitrates on the way to dissociation pass from bidentate to monodentate coordination. The thermodynamic explanation for this process is interesting. In solution, the enthalpy (ΔH) and entropy (ΔS) energy components are antagonists. Bidentate binding may be promoted by entropy, which reduces the number of “frozen” water molecules coordinated with Ln3+ ions. On the other hand, monodentate bonding may be preferable from an enthalpy point of view, since up to six hydrogen bonds can be formed with the oxygens of the three monodentate nitrates (ONO3) instead of three with the bidentate nitrate groups. If we compare the course of interatomic distances (Table S1, Figure 2) according to EXAFS data with the curves ΔS of complexes (LnNO3)2+ and ΔH from Ln [22], then we can detect similarities for Ln = Ce-Eu with the ΔS curve. This may be an indirect confirmation of the presence of a certain amount of nitrate groups bidentately bound to Ln = Ce-Eu.
The authors of [23] used MD with explicit polarization and UV-visible spectroscopy to study solutions of Nd3+ and Dy3+ nitrates from a “highly diluted” solution to experimental saturation. It has been established that the bidentate mode is somewhat more stable for Nd3+ than for Dy3+; at the end of the lanthanide series, the ratio between the bidentate and monodentate conformations decreases, while only the monodentate mode is present in Lu.
Moreover, in solution, hydrated nitrate complexes can exhibit an equilibrium between several polyhydrate forms involving different types of nitrate binding modes [51].
Analysis of the limited literature data on the study of dilute solutions of Ln(NO3)3 · xH2O salts confirms the different structural behavior of Ln3+ ions in them, and different results are observed depending on the calculation methods and experimental conditions. It should be noted that experimental concentrations are typically far from standard and cannot be correctly extrapolated to infinite dilution to obtain a thermodynamic equilibrium constant that is valid only for a particular medium and concentration range (ionic strength).
2.3. X-ray Diffraction
According to the structural analysis of Ln(NO3)3 · xH2O salts [34,35,36,37,38,39,40,41,42,43,44], with an increase in the Ln atomic number, the total content of water molecules decreases: x= 6 for Ce-Sm, x = 6 and 5 for Eu-Tb, x = 5 for Dy-Yb (except for Tm with x = 6 and 5), and x = 4 and 3 for Lu. In the inner sphere (in square brackets) of coordination compounds of the form [Ln(O2NO3)3(OH2)n] · (x − n)H2O, the number of water molecules also decreases along the Ln series (n = 5 for Ce, n = 4 for Pr-Yb, n = 3 for Lu) connected monodentantly to Ln. In the outer sphere (outside square brackets), the number of water molecules (x − n) for all Ln except Ce, and the content of (NO3)1− groups bidentantly coordinated with Ln ions remains constant (3 NO3).
It should be noted that in the Ln(NO3)3 · 6H2O structures, starting from Gd, the length of one Ln-ONO3 bond is greatly increased compared to others (bond asymmetry: Δ = 0.202 Å). The bond asymmetry increases in the structure with Tb (Δ = 0.220 Å) and is at its maximum (Δ = 0.523 Å) in the structure with Tm [35]. For example, in the Tm(NO3)3 · 6H2O [35], Tm ions are bound to six oxygen ions from three nitrate groups (Tm has two bonds with oxygen–bidentate coordination): an asymmetric coordination with one shorter (2.4039 (17)Å – 2.4677 (17)Å) bond and one longer Tm—O distance (2.5034 (18) Å, 2.5252 (18)Å, 2.991 (2)Å each) (Δ, Å value is the difference between the largest short distance and the largest long distance). In accordance with the fact that the corresponding Tm—O distance (2.991 Å) is even larger than the distance between the Tm atom and the central N atoms of the two remaining anions, the coordination arrangement of Tm should be described as CN Tm = 8 (3OH2O + 2ONO3 + 2ONO3 + 1ONO3) for the inner sphere and CN Tm = 9 + 1 (instead of CN Tm = 10) taking into account the outer sphere [35]. This asymmetric bonding seems to be associated with a steric effect of the coordinating water molecules. The increasing asymmetry in the binding mode of one nitrate group is related to the decrease in the ionic radius of the Ln.
An analysis of the interatomic distances given in the literature showed that for the same Ln, for which x = 5 and x = 6 in the Ln(NO3)3 · xH2O composition (Ln = Eu-Tb, Tm), with an increase in molecules of crystallization water, the average Ln-OH2O distance decreases (ΔLn-O(H2O)~0.032 Å is the difference between the average interatomic distances Ln-OH2O in Ln(NO3)3 · xH2O structures with x = 5 and x = 6), and Ln-ONO3 (ΔLn-O(NO3) is the difference between the average interatomic distances Ln-ONO3 in Ln(NO3)3 · xH2O structures with x = 5 and x = 6), on the contrary, increases, reaching a maximum value (ΔLn-O(NO3) =0.058 Å) for Tm [35]. In this case, the average Ln-O interatomic distance in the first coordination sphere increases upon transition from x = 5 to x = 6 since ΔLn-O(NO3) > ΔLn-O(H2O). For Lu(NO3)3 · xH2O in the transition from x = 3 to x = 4, the value of ΔLn-O(NO3) =0.004 Å is very small, which contributes to an increase in Ln-O (R,Å) in the Lu(NO3)3 · 3H2O structure compared to Lu(NO3)3 · 4H2O (Table S2, Figure 3).
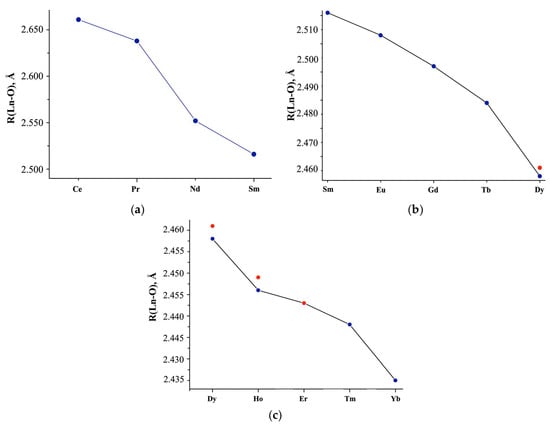
Figure 3.
(a) Average Ln-O interatomic distances (R, Å) in the structures of Ln(NO3)3 · xH2O salts, according to literature data highlighting the (b) Sm-Dy, (c) Dy-Yb regions. Red dots: interatomic distances calculated from phase analysis of the commercial samples we studied.
The X-ray diffraction study of commercial Ln(NO3)3 · xH2O samples [52] indicates single-phase Ln(NO3)3 · 6H2O with Ce (structure 1; x = 6), Pr-Tb (structure 2; x = 6), Tm (structure 3; x = 5), two-phase with Dy-Er (structure 2 + structure 3), Yb (structure 3 + unknown structure 4), and uncertainty with Lu (unknown structure 5 or non-single-phase sample). Although the crystal structures of 1–3 differ from each other (the closest are structures 2 and 3), and the structures of 4 and 5 are unknown, their main structural fragments are the same.
Table S2 and Figure 3a,b show the average interatomic distances Ln-O (R, Å) without separation into Ln-OH2O and Ln-ONO3, calculated based on the Ln coordination in the inner sphere, taking into account the quantitative analysis of non-single-phase Ln(NO3)3 · xH2O with Ln = Dy, Ho, Er (Figure 3a–c; red dots). The structural parameters are consistent with the data given in [22] for Ln(NO3)3 · xH2O with Ln = Ce-Sm (x = 6; CN = 11 for Ce, CN = 10 for Nd-Sm), but differ for Eu (x = 6 according to our data, x = 5 according to [22]) and interatomic Lu-O distances (Figure 3a, blue double dots), which we either calculated or took from structural data [42].
X-ray diffraction patterns of Ln(NO3)3 · xH2O solutions (Figure 4) are represented by two pronounced halos with interplanar distances in the intervals d = ~7.16–~6.34 Å (first peak; 2θ~13°) and d = 3.423–3.119 Å (second peak; 2θ~28°) and diffuse peak with a maximum at d~2.15 Å (third peak; 2θ~42°).
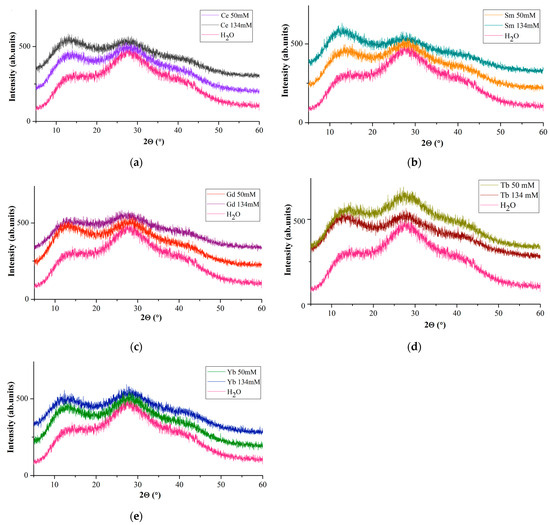
Figure 4.
Diffraction patterns of water and solutions with c = 50 mM and c = 134 mM: Ln(NO3)3 · xH2O with (a) Ln = Ce, (b) Sm, (c) Gd, (d) Tb, (e) Yb.
A comparison of diffuse reflections of Ln(NO3)3 · xH2O solutions with Ln = Ce, Sm, Gd, Tb, Yb (Figure 4) with similar reflections of a dilute aqueous solution of lanthanum nitrate [53] shows their similarity. The main diffuse peaks for water (Figure 4, first and second peaks) occur at 14.17 and 27.92°(2θ) (d = 6.24 and 3.193 Å), along with a very weak and diffuse peak at ~39.85°(2θ) (d~2.26 Å) (Figure 4, third peak), and are present in the diffraction patterns of all solutions. It occupies an intermediate position between van der Waals molecules and molecular formations with a covalent chemical bond, and the components of the cluster retain their specific individuality.
If we take the intensities of the first (I(1)rel) and second (I(2)rel) diffuse reflections for water as a reference, then on the diffraction patterns of Ln(NO3)3 · xH2O solutions at c = 134 mM I(1)rel > I(2)rel for Ln = Ce, Sm (for Sm to a greater extent); for Gd, the intensities of the first and second diffuse reflections are most pronounced at c = 50 mM; for Yb, at c = 50 and 134 mM, the values of I(1)rel and I(2)rel are almost the same (Figure 4). The diffraction patterns of Tb(NO3)3 · xH2O solutions (Figure 4d) are strikingly different from the diffraction patterns of Ln(NO3)3 · xH2O solutions with Ln = Ce, Sm, Gd, Yb (Figure 4a–c): I(2)rel > I(1)rel at c= 50 mM; I(1)rel is more pronounced than I(2)rel at c = 134 mM (Figure 4d). Different diffraction patterns are characteristic of Ln(NO3)3 · xH2O solutions (Ln = Ce, Sm, Gd, Tb, Yb) of commercial samples that we studied and may indicate different hydrolysis processes depending on the Ln type.
When comparing diffraction patterns of aqueous solutions [48,53,54] and Ln(NO3)3 · xH2O salts [34,36,37,42], the position of the first diffuse peak generally coincides with the region of the most intense Bragg reflections in the range of 2θ~12÷~15°, in particular, 10 with d~6.7 Å, caused by Ln ions. These values are in the regions of increased electron density observed in the radial distribution functions of aqueous solutions.
Modern ideas about the structure of lanthanide nitrate hydrates in the solid phase and aqueous solutions are considered in the review [55], where, based on quantum-chemical calculations and experimental data, for Ln = Pr-Yb, five types of structural isomers were established for the [Ln(NO3)3(H2O)4] complexes, with different mutual arrangements of nitrato- and aqualigands, and for Ln = Lu, two types of isomers were found for the [Ln(NO3)3(H2O)3] complexes. Let us pay attention to the difference in Lu-O interatomic distances in the salt and Lu(NO3)3 solution according to EXAFS data (Figure 2).
In dilute aqueous solutions (c < 10 mM), Ln3+ ions are in the structure in the form of aqua ions [Ln(H2O)n]3+, and with an increase in the concentration of the salt solution, as well as with an increase in the concentration of nitrate anions in the solution, water molecules in the internal coordination sphere of Ln3+ cations are replaced by bidentate nitrate anions. In our case, solutions with c = 20, 50, 100, 134 mM were considered, which, in accordance with the results of work [55], does not exclude the presence of bidentate nitrate anions, at least for Ln = Ce-Eu (region 1) in Figure 2 (EXAFS data).
2.4. FT-IR Spectroscopy Data
Figure 5a shows the IR spectra of solutions of commercial Ln(NO3)3 · xH2O salts (c = 20 mM and 50 mM) with Ln = Ce, Sm, Gd, Yb, and the bandwidth correspondence presented in Table S3.
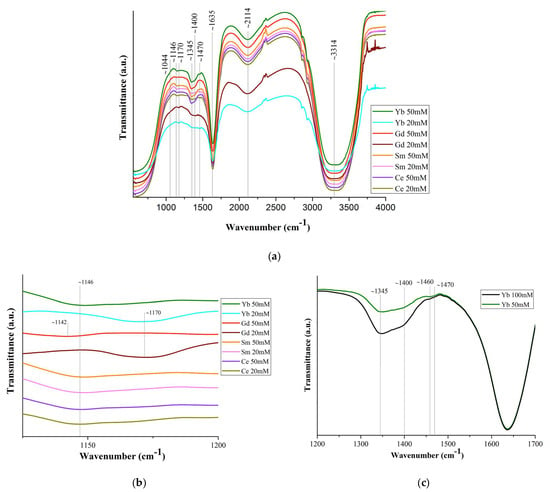
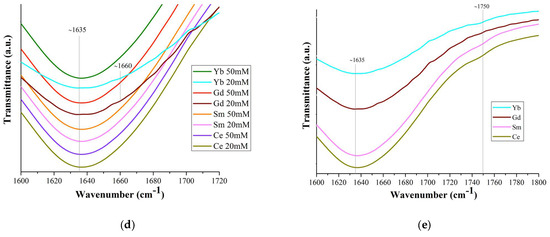
Figure 5.
(a) FT-IR spectra of Ln(NO3)3 · xH2O solutions (Ln = Ce, Sm, Gd, Yb); (c) part of the spectrum (1200–1500 cm−1) of Yb(NO3)3 · xH2O solution (c = 50 mM and 100 mM); ((b,d,e) for c = 20 mM) spectrum sections.
The band shift ~1044 cm−1 is associated with the bond covalency degree of nitrate ions with Ln ones [56], which increases along the Ln series. Based upon this shift in the 1050 cm−1 region, the following series may be set up for decreasing covalency of the metal–nitrate bond: Gd > Ce [57]. This should lead to a general tendency for interatomic distances and coordination numbers to decrease due to a decrease in the size of Ln, which is confirmed by the EXAFS data in Figure 2. A very weak band at 1146 cm−1 (Figure 5b) may correspond to bending vibrations of the hydronium ion δ (H3O+), which can be present in a solution with pH < 7 [58].
The splitting of the ~1400 cm−1 band into two (~1345 and ~1400 cm−1) (Figure 5c) indicates an asymmetric vibration of the uncoordinated hydrated nitrate ion. The difference (Δυ~125 cm−1) between the bands ~1345 and ~1470 cm−1 indicates partial coordination of the nitrate ions to Ln [57]. With an increase in the concentration of the Ln(NO3)3 · xH2O solutions (from 20 mM to 100 mM), the ~1470 cm−1 band becomes more clearly defined (Figure 5c). This means an increase in the number of bound nitrate ions and their gradual dominance over water molecules in competition to enter the inner coordination sphere. The band at 1470 cm−1 is quite wide and, in accordance with [17], apparently results from the superposition of bands of monodentate and bidentate coordinated nitrate ions [59,60].
The strong band at ~1635 cm−1 is the result of the superposition of water scissoring bending and N=O stretching: the vibrational peak for N=O stretching of bidentate coordinated nitrate ions usually appears at 1630–1788 cm−1 (Figure 5d). All bands in the 1625–1524 cm−1 region characterize isolated N=O bonds (consistent with the bidentate structure of nitrates) and the bands below 1520 cm−1 are coupled vibrations, consistent with monodentate (but not only) nitrate structures [61].
The weak band in the region of ~1750 cm−1 (Figure 5a,e) corresponds to the combination band of the symmetrical stretch and in-plane bending of the nitrate ion. With an increase in the Ln atomic number, the position of this band shifts (Figure 5a,e) to the long-wavelength region (1745 cm−1 for Ce, 1750 cm−1 for Sm, 1752 cm−1 for Yb; it was not possible to detect it for Gd), due to a weakening of the O–N bond in the nitrate ligand owing to an increase in electron transfer to the Ln3+ metal ion with an increase in the charge density.
A wide weak band at ~2114 cm−1 (Figure 5a) corresponds to the composite vibration of water molecules: bending vibration, together with stretching and intermolecular vibration due to the rotation of the water molecule. The band at 2130 cm−1 corresponds to the combination of oscillation ν2 + νL: deformation together with libration. The libration oscillations of the water molecule are intermolecular and are associated with the molecule rotation [62]. The broad strong band ~3314 cm−1 corresponds to the stretching of free, hydrogen-bonded and coordinated water molecules. The band is greatly broadened, which indicates the implementation of strong hydrogen bonding in Ln(NO3)3 · xH2O solutions (Figure 5a).
The wide intensive band at 2700–3700 cm−1 corresponds to three oscillations of the water molecule: asymmetric stretching oscillation (3490 cm−1), symmetric stretching oscillation (3280 cm−1), and the overtone of the bending oscillation (3250 cm−1). The intensity of the maximum of the bending band, on the contrary, decreases with a reduction of the temperature in the same range [63].
A wide intense band below ~800 cm−1, responsible for stretching vibrations of water molecules, overlaps the bands of bending vibrations of nitrate ions lying in this spectrum region (Figure 5a).
The position and intensity of the transmission bands corresponding to vibrations of nitrate ions in these spectra are very close for all samples, and the intensity of the bands increases with an increase in the concentration of solutions. It should be noted that from the IR spectra of Ln(NO3)3 · xH2O solutions with low-intensity, overlapping, or even partially absent transmission bands, it is quite difficult to identify nitrate ions coordinated monodentately by Ln3+ ions. However, judging by the spectra, part (from ~10% to ~50%) of the nitrate ions is coordinated by Ln3+ ions mono- or bidentately (mainly monodentately), and the remaining nitrate groups are uncoordinated.
The IR spectra of Ln(NO3)3 · xH2O salts contain transmission bands both observed for solutions (marked with * in Table S3 and in the text) and those belonging only to the salts. Thus, bidentately coordinated nitrate ions correspond to intense bands at ~1460 cm−1* and 1280 cm−1, medium-intensity bands at ~1660 cm−1* (in solutions, this band is present for Gd and Yb) (Figure 5d), and 1044 cm−1*. The difference between the band values at 1280 and 1044 cm−1 (Δ = 236 cm−1) indicates bidentate chelating of nitrate ions [61].
Therefore, the results of IR spectroscopy of commercial Ln(NO3)3 · xH2O salts with Ln = Ce, Sm, Gd, Yb confirm the structural analysis data known from the literature on the implementation of only bidentately coordinated nitrate groups in them. As for Ln(NO3)3 · xH2O solutions, according to IR spectroscopy, in dilute solutions of 20 mM there are Ln3+ ions coordinated by nitrate groups, but in very small quantities, bidentantly and monodentantly (mainly) linked with Ln3+ ions. The number of monodentate nitrate groups increases from Ce to Yb: the ratio of the intensity of the band (shoulder) at 1750 cm−1 to the intensity of the water bending vibration band at 1635 cm−1 increases from Ce to Yb (Figure 5e).
It should be noted that the peculiarity of the diffraction patterns of Ln(NO3)3 · xH2O solutions (Ln = Ce, Sm, Gd, Yb) with c = 50 and 134 mM (Figure 4) is also preserved in the IR spectra of solutions with c = 20 and 50 mM (Figure 5b,d) and is consistent with the EXAFS data: an increase in the number of monodentate nitrate groups in the Ln3+ series with an increase in the degree of bond covalence.
The hydrolysis of Ln3+ cations is gaining increasing importance due to the growing interest in the biological aspects of their complexes [64,65,66]. In aqueous solutions of Ln(NO3)3 · xH2O salts, hydrolysis occurs—a chemical reaction between salt ions with H+ and OH− ions of water. Ln(NO3)3 · xH2O salts are formed by the weak base of the multivalent Ln3+ ion and the strong HNO3 acid [67]; therefore, hydrolysis occurs through cations (pH < 7), which bind to the water anion in a stepwise manner (the general stages of hydrolysis of Ln3+ nitrates are presented by analogy with Fe3+ nitrate [68] due to the fact that there are no works in the literature on the systematization of the hydrolysis of rare-earth element nitrates):
- Stage I:
Ln(NO3)3 + HOH ↔ Ln(OH)(NO3)2 + HNO3 (I.1)
Ln3+ + 3NO3− + HOH ↔ Ln(OH)2+ + 2NO3− + H+ + NO3− (I.2)
Ln3+ + HOH ↔ Ln(OH)2+ + H+ (I.3)
- Stage II:
Ln(OH)(NO3)2 + HOH ↔ Ln(OH)2NO3 + HNO3 (II.1)
Ln(OH)2+ + 2NO3− + HOH ↔ Ln(OH)2+ + NO3− + H+ + NO3− (II.2)
Ln(OH)2+ + HOH ↔ Ln(OH)2+ + H+ (II.3)
- Stage III:
Ln(OH)2NO3 + HOH ↔ Ln(OH)3 + HNO3 (III.1)
Ln(OH)2+ + NO3− + HOH ↔ Ln(OH)3 + H+ + NO3− (III.2)
Ln(OH)2+ + HOH ↔ Ln(OH)3 + H+ (III.3)
According to [69], in the formation of Dy(OH)3, the final product of stage III of dysprosium nitrate hydrolysis (III.3) is preceded in a very narrow pH range~6.9–7.1 by basic dysprosium salts, in particular, Dy(NO3)(OH)2. The authors of [70] believe that the lanthanides are quite sensitive towards the water content of the medium and the aqueous ions Ln are hydrolyzed in water according to the equation [Ln + (H2O)n]3+ + H2O ↔ [Ln(OH)(H2O)n−1]2+ + H3O+. The authors then neglected the molecules of hydration water and presented the number, nature of the species in solution, and their hydrolysis constants for Ln3+ = Ce, Pr, Nd, Eu, Sm at c = 0.1 mol/dm3 (100 mM) and at 25 °C, which can be described as Ln3+ + HOH ↔ Ln(OH)2+ + H+ (I.3).
Hydrolysis at stage II and, in particular, at stage III practically does not occur at room temperature, at which we conducted the experiment. This is also facilitated by ions that are formed during hydrolysis at stage I, which suppress hydrolysis at stage II, shifting the equilibrium of reactions to the left. In accordance with Le Chatelier’s principle, when diluting solutions (decreasing the concentration of hydrogen ions), the degree of hydrolysis increases. The tendency towards hydrolysis increases with an increase in atomic number and a decrease in ionic radius of Ln [70]: when moving from “light” to “heavy” Ln, hydrolysis increases.
The degree of hydrolysis can be influenced by the composition of the substances involved in hydrolysis and the concentration of hydrolysis products, as well as the process temperature. The structure of Ln(NO3)3 in solutions depends on the degree of hydrolysis of solutions: the composition of the outer and inner spheres (the presence and content of nitrate ions and water molecules) and the density of ligands (the same groups).
For solutions of Ln(NO3)3 · xH2O salts (t = 25 °C; Ln = Ce, Sm, Tb, Gd, Yb), pH < 7, but the pH values are different (Figure 6).
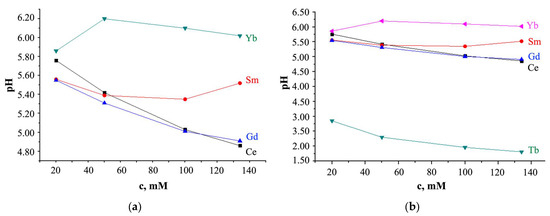
Figure 6.
Relationship between the pH value and the concentration of Ln(NO3)3 · xH2O solution with (a) x = 6 for Ln = Ce, Sm, Gd and x is unknown for Yb; (b) x = 6 for Ln = Ce, Sm, Tb, Gd and x is unknown for Yb (pH measurement error is ±0.03).
For Ln = Ce and Gd, with increasing solution concentration, the pH value decreases (Figure 6); for Ln = Sm, pH values first decrease from pH = 5.52 (c = 134 mM) to 5.35 (c = 100 mM), and then increase from pH = 5.39 (c = 50 mM) to 5.56 (c = 20 mM); at c > 20 mM, turbidity of the solution was observed; the pH value of a solution with Yb increases with an increase in its concentration with a maximum value of pH = 6.20 at c = 50 mM.
As can be seen from Figure 6, the Tb(NO3)3 · xH2O salts solution is distinguished from other solutions by lower pH values, which smoothly decrease with increasing solution concentration. This behavior of the Ln(NO3)3 · xH2O solution with Ln = Tb, which differs from the behavior of solutions with Ln = Ce, Sm, Gd, Yb, is consistent with the EXAFS data (Figure 2) and with X-ray data (Figure 4d), which also stands out among other solutions. Different hydrolysis products, more or less complex, are formed depending on the type of Ln and anion, the solution concentration, and the pH value. So, according to [71], the Ln(OH)(NO3)3− (in particular, Ln = La, Lu) anion complexes contain the Ln3+ ions, and the LnO(NO3)3− (in particular, Ln = Ce) anion complexes contain the Ln4+ ions; for Ln = Pr and Tb it is proposed that the oxidation states are intermediate between Ln3+ and Ln4+. In solutions of Ln chlorides, Ln(H2O)9]3+ complexes with Ln = La, Pr, Nd [72], and Ln(H2O)8]3+ with Ln = Tb-Lu [73] were found, and in solutions of Tb sulfates, Tb(H2O)6]3+ complex was discovered [74]. The fewer water molecules in the immediate environment of Ln ions, the smaller the average Ln-O interatomic distance, the smaller the size of the Ln ion, the higher the hydrolysis rate, and the lower the pH [75]. Thus, it is not excluded that the smaller interatomic distance Tb-O in the nitrate solution (Figure 2) is due to the smaller number of water molecules in the inner sphere of the complex with terbium compared to other Ln, which contributes to a decrease in pH.
It is necessary to pay attention to the obvious increase in pH with increasing temperature from 21 °C (the temperature at which the XAS solution experiments were performed) to 25 °C (the temperature at which IR spectra were obtained and the pH of the solutions was determined), which should be accompanied by an increase in hydrolysis. In the case of a salt formed with a weak base and a strong acid (our case), the Ln3+ cation undergoes hydrolysis, and the reaction is accompanied by the formation of H3O+ ions (their presence was not excluded when analyzing the IR spectra of solutions) (Table S3, Figure 5). An increase in the H3O+ content in the solution leads to a decrease in the concentration of OH− ions.
To establish the influence of the type of Ln, concentration and pH on the antimicrobial activity of Ln(NO3)3 · xH2O solutions, solutions with Ln = Ce, Sm, Gd, Tb, Yb from the beginning, middle, and end of the RE series, were selected. Figure 7 shows the relationship between the growth inhibition zone value (D, mm value) and the solution concentration (Figure 7a) and pH (Figure 7b), respectively.
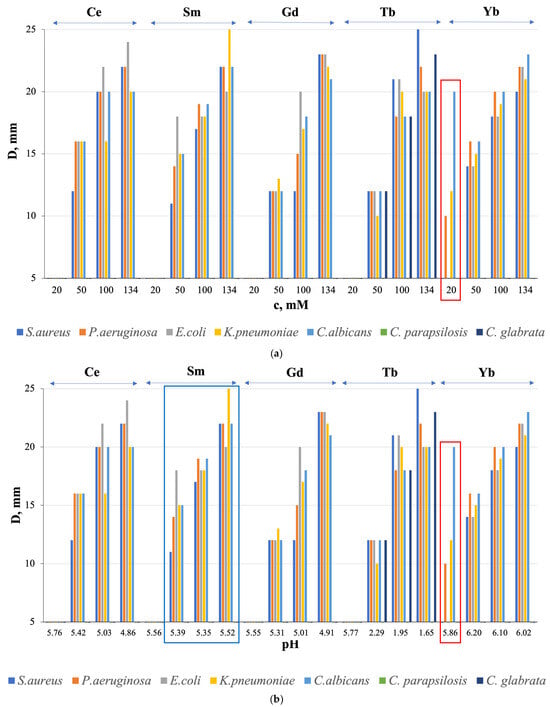
Figure 7.
Relationship between the growth inhibition zone (D, mm) and (a) concentration (c, mM) and (b) pH of Ln(NO3)3 · xH2O solutions (Ln = Ce, Sm, Gd, Tb, Yb). Convergence of results based on three independent measurements; D ± 0.02 mm.
All solutions with Ln = Ce, Sm, Gd, Tb, Yb showed average antibacterial activity at c = 50, 100, and 134 mM, except for Ln = Gd and Tb with c = 50 mM with low activity against all microorganisms, and Ln = Sm with c = 134 mM with high activity against K. pneumoniae (Figure 7a). Antifungal properties against the fungi C. albicans and C. glabrata were also found, with medium activity in all solutions with c = 50, 100, and 134 mM and with high activity for Ln = Tb with c= 100 and 134 mM (Figure 7a). Moreover, antimicrobial activity is absent for solutions with a concentration of c = 20 mM, except for a solution with Ln = Yb (Figure 7a, red rectangle). At the same time, the sensitivity of the fungus C. albicans to a solution with c = 20 mM is higher than to a solution with c = 50 mM. We detected the observed “homeopathic” effect for the first time for these objects.
The microorganism growth inhibition zone (D, mm value) has a general tendency to increase with increasing solution concentration (Figure 7a) with the best results at c = 134 mM for Ln = Sm and Tb, respectively, in relation to the bacterium K. pneumoniae and the fungi C. albicans and C. glabrata (Figure 7b).
HNO3 solutions do not exhibit AMA (except for HNO3 with pH = 1.98 and with D = 18 mm in relation to S. aureus), which indicates a connection between biocidal properties and Ln3+ ions, and not with NO31− ions, and the absence of a connection with the pH (hydrogen index) of HNO3. As for the relationship between the value of D, mm and the pH of Ln(NO3)3 · xH2O solutions, the general tendency of increasing D, mm with decreasing pH was found for Ln = Ce, Gd, Tb (the maximum value of the growth inhibition zone of the fungus C. glabrata was achieved at pH = 1.65), and Yb (in the pH range from 6.20 to 6.02) (Figure 7b); for Ln = Sm, the opposite effect was obtained (Figure 7b) with the maximum growth inhibition zone of K. pneumoniae bacteria at pH = 5.52, and for Ln = Yb at pH = 5.86 the values of D, mm are less than for pH = 6.02 (Figure 7b). It follows that, in general, there is no relationship between AMA and solution pH.
Judging by the obtained results, the antimicrobial activity of the studied Ln(NO3)3 · xH2O solutions is influenced by the phase purity of the samples and the nature of Ln, which determines the composition and structure of nitrates and, in some cases, the pH of the solution, which in turn is associated with its concentration and vice versa. So, from the entire Ln series studied, a solution with Ln = Yb (non-single-phase sample) (Figure 2, red arrow) and with Ln = Tb (Figure 2) stands out. On the other hand, according to [5], Ln have similar ionic radii to calcium but, due to their higher charge (Ln3+), Ln ions have a high affinity for Ca2+ sites on biological molecules, and therefore Ln3+ are able to block calcium channels. Thus, even though the Ln3+ ions themselves cannot cross cell membranes, they can act by blocking the exterior face of the calcium channel. It is possible that the absence of direct quantitative or semi-quantitative correlations with individual characteristics of Ln(NO3)3 · xH2O solutions is due to the different composition and structure of bacteria and fungi, as well as individual bacteria, making these relationships less pronounced.
Compounds with rare-earth ions are biocompatible, relatively cheap, and are included as additives in anti-inflammatory, regenerating, analgesic, wound-healing, and antimicrobial ointments and preparations, such as EPLAN, which contains lanthanum nitrate [76]. First obtained data on the antibactericidal and antifungicidal (unique results) activity of solutions of commercial Ln(NO3)3 · xH2O salts with Ln = Ce, Sm, Gd, Tb, Yb, including the manifestation of biocidal properties of Yb(NO3)3·xH2O solution with c = 20 mM, make it possible to consider these objects as potential antimicrobial drugs, expanding the range of known materials.
One should not forget about bacteria and fungi, the composition and structure of which must be taken into account when considering the antimicrobial process, which is beyond the scope of this work.
3. Materials and Methods
The nitrate salts Ln(NO3)3 · xH2O with 99.9% Ln= Ce(x = 6), Pr, Nd(x = 6), Sm(x = 6), Eu, Gd, Tb(x = 6), Dy(x = 5), Ho(x = 5), Er, Tm, Yb, Lu were purchased from LANHIT LTD (Russia) and used as received. We studied 20 (in most measurements), 50, 100, and 134 mM Ln(NO3)3 · xH2O aqueous solutions (separate measurements were performed for solutions with Ln = Ce, Sm, Gd, Yb) with water pH 6.2. All solutions were prepared using ultrapure water (Milli-Q Advantage A10 Water Purification System, Millipore, France).
3.1. X-ray Studies
X-ray experiments have been carried out at the LANGMUIR beamline (Kurchatov Center for Synchrotron Radiation, Russia) [77]. The Langmuir trough was filed with water vapor-saturated helium to decrease X-ray scattering. Aqueous solutions of Ln(NO3)3 · xH2O salts at a concentration of 20 mM were filled in the Langmuir trough mounted on the diffractometer. All measurements were performed at room temperature, t = 21 °C.
3.1.1. X-ray Absorption Spectroscopy Measurements at Liquid Surface
Ln(NO3)3 aqueous solutions were measured in fluorescence mode under TER geometry. The channel-cut monochromator Si (111) with spectral width of ~2 eV was used to perform energy scan over the range of 400 eV. Ln3+ L3-edge absorption spectra were collected at the fixed incidence angle of 0.9 × θC (θC is the critical angle of TER for water). The energy dispersive Vortex EX detector was mounted above the water subphase at an angle of 90°. To obtain high-quality EXAFS spectra, we averaged several energy scans recorded in the multipass mode of data acquisition. The reproducibility of the energy position of the monochromator was determined to be within 0.18 eV.
3.1.2. X-ray Absorption Spectroscopy Measurements of Powder Samples
Ln(NO3)3 · xH2O microcrystalline samples were studied at the Structural Materials Science beamline (Kurchatov Center for Synchrotron Radiation, Moscow, Russia) [78]. EXAFS spectra at the lanthanide L3-edge were collected in transmission mode using two ionization chambers filled with N2/Ar mixtures.
3.1.3. XRSW Measurements
The fluorescence intensity was recorded by an energy dispersive Vortex EX detector (Hitachi, Japan) mounted above the water subphase at an angle of 90°. The characteristic fluorescence spectra were collected for each angle of incidence in the angular range corresponding to the TER region.
3.2. EXAFS Data Analysis
The extraction of the experimental fine-structure χ(k) from the atomic background function was performed using conventional procedures described elsewhere [79,80]. EXAFS data analysis was carried out in the single-scattering approximation based on the equation, which describes EXAFS as a sum of the contributions from different coordination shells with radii Ri and coordination numbers Ni:
where is a many-body reduction factor, that accounts for amplitude damping due to the multielectron effect; and are the backscattering amplitude and phase shift for photoelectron scattered by neighbor atoms; λ(k) is the mean-free-path of the photoelectron; is Debye–Waller factor, which represents the mean square displacement of an atom from equilibrium position. Backscattering amplitude and phase shift were calculated using the ab initio code FEFF8.5L [81,82]. The photoelectron inelastic losses were accounted for within the one-plasmon approximation using the complex exchange-correlation Hedin−Lundqvist potential [83]. FEEF calculations were performed for atomic clusters, which include the nearest environment of lanthanide ions: 4 H2O molecules and 3 NO3 ligands. Such calculations allow performing EXAFS spectra fitting using paths associated with H2O and NO3 molecules. Fourier transformation is calculated for χ(k) × k2 using k-range = 2–9 Å−1; k2-weighted EXAFS data were fitted in space of their Fourier transforms |FT(χ(k) × k2)|. The value of factor was fixed at 0.9. All data processing and quantitative analysis of EXAFS spectra were performed using Larch 0.9.80 software package [84].
EXAFS spectra obtained in our experiments for Ce and Pr exhibited distinct signs of multi-electron excitation, which is a well-known feature of the electronic structure of these ions [85]. Thus, in analysis of the EXAFS spectra for Ce and Pr we used the ATHENA program from the IFFEFIT package [86] to exclude the influence of multi-electron excitation effect.
3.3. X-ray Diffraction Experiments
X-ray diffraction experiments with Ln(NO3)3 · xH2O aqueous solutions of the beginning (Ce and Sm), middle (Gd) and end (Yb), ions of the Ln series with concentrations of c = 50 and 134 mM were tested using an Al container and difractometer PowDiX 600 (ADANI, Minsk, Belarus) equipped with a MYTHEN2 R 1D (Dectris) detector at room temperature (t = 21 °C), using monochromatic CuKα1 radiation (λ = 1.5406 Å) with a Ni-filter 0.02 mm thick, on a diffracted beam; θ-2θ, 2θ ± 0.01°), and continuous shooting in the range of 5–60° (30 kV, 10 mA).
3.4. FT-IR Spectroscopy
Fourier-transform infrared spectra of Ln(NO3)3 · xH2O (Ln = Ce, Sm, Gd, Yb) solutions (c = 20 and 50 for Ln = Ce, Sm, Gd, Yb; c = 100 for Ln = Yb) were collected in a FSM-2201 (Infraspec, Saint Petersburg, Russia) spectrometer in the 500–4000 cm−1 range. An average of 15 scans per sample with a resolution of 1 cm−1 formed each spectrum. Aqueous solutions were analyzed using an ZnSe Attenuated Total Reflectance (ATR) accessory. A Fourier-transform infrared spectrometer WQF-530A (BFRL, Beijing, China) was used to study Ln(NO3)3 · xH2O (Ln = Ce, Sm, Gd, Yb) salts: wavenumber range 500–4000 cm−1, spectral resolution 0.85 cm−1, number of scans 256.
3.5. The Antimicrobial Activity
The study of antimicrobial activity (AMA) of Ln(NO3)3 · xH2O (Ln = Ce, Sm, Gd, Tb, Yb) solutions (c = 20, 50, 100, 134 mM) was carried out by the disk diffusion method for antimicrobial activity against bacteria (S. aureus, E. coli, P. aeruginosa, K. pneumoniae) and fungi (C. albicans, C. glabrata, C. parapsilosis). The test bacterium was seeded as a “lawn” in Petri dishes on Mueller–Hinton agar. A thin-walled cylinder (6–8 mm in diameter) was used to make holes on the agar surface, into which test samples were added in the powder form with the addition of 2–3 drops of NaCl physiological solution (0.9 wt.%). Next, Petri dishes were placed in a thermostat at 37 °C for 24 h. The sensitivity of microorganisms to the objects under study was assessed by the bacterial growth inhibition zone (D, mm) around the hole (including the hole diameter) using a ruler [87]. The sensitivity degree of microorganisms to the studied samples depends on the D, mm value: the larger it is, the higher the sensitivity. According to the classification presented in [88], there are four groups of growth inhibition zone diameters of microorganisms in the presence of antimicrobial agents: D < 10 mm indicates a lack of sensitivity, D = 11–15 mm indicates low sensitivity, D = 15–25 mm denotes an average sensitivity, and D > 25 mm represents high sensitivity to the drug. Microbiological studies were carried out in a second-class microbiological protection box, equipped with a UV lamp and laminar air flow.
4. Conclusions
Based on the analysis of the results of studying commercial Ln(NO3)3 · xH2O salts (X-ray diffraction, IR spectroscopy, XAS) and their diluted solutions (XAS), as well as solutions with Ln = Ce, Sm, Gd, Yb (X-ray diffraction, pH measurement, IR spectroscopy) together with literature data, it was shown that in solution, a few nitrate anions can be coordinated bidentantly and monodentantly depending on the type of Ln, and also be uncoordinated. Water molecules are chemically bonded to Ln ions via oxygen and, in addition, form multiple hydrogen bonds with water molecules and nitrate groups.
For the first time, X-ray absorption spectroscopy under total external reflection geometry has been used to study the local environment of lanthanide ions in dilute aqueous solutions (20 mM Ln(NO3)3 · xH2O). The applied experimental technique allowed for high-quality EXAFS data to be collected for all elements from Ce to Lu (except for Pm). All solutions were measured under identical experimental conditions, all EXAFS spectra were processed using the same software package and modeling procedures, which provided significant advantages in comparative studies of lanthanide coordination. A comparison of Ln-O interatomic distances for salts and their solutions, determined by the EXAFS method, showed that for a number of lanthanides from the middle of the lanthanide series, the oxygen coordination sphere contracts in solutions, which may be due to partial or complete dissociation of the nitrate complex in water.
The obtained data for Ln(NO3)3 · xH2O salts with specific compositions and structures, and their solutions, showed the need to characterize the initial samples synthesized by researchers or commercially available. Only salts and their solutions being identical in composition and structure makes it possible to correctly analyze the conducted research. Otherwise, the discrepancy between the results of an experimental study regarding “the same objects” or calculations within the experiment may lead to misunderstandings.
The revealed biocidal properties of solutions of commercial Ln(NO3)3 · xH2O) salts with Ln = Ce, Sm, Gd, Tb, Yb make it possible to continue studying the antimicrobial activity of both Ln(NO3)3 · xH2O salts and solutions with their characterization by different methods for the entire Ln series. This will allow to establish correlations between the characteristics of drugs with antimicrobial properties and the composition and structure of microorganisms to identify optimal combinations for creating new medical objects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29174023/s1, Table S1: structural parameters derived from EXAFS fitting procedure; Table S2: average Ln-O interatomic distances in the first coordination sphere in the structures of Ln(NO3)3 · xH2O salts; Table S3: assignment of the bands in the IR spectra of the Ln(NO3)3 · xH2O aqueous solutions; Figure S1: Fourier-transformed EXAFS spectra for Ln(NO3)3 · xH2O salts. Experimental spectra are shown using black color, and red color is used to plot fitted curved; Figure S2: Fourier-transformed EXAFS spectra for Ln(NO3)3 · xH2O aqueous solutions. Experimental spectra are shown using black color, and red color is used to plot fitted curved; Figure S3: the experimental and calculated EXAFS-signals χ(k)*k2 for Ln(NO3)3 · xH2O aqueous solutions. Experimental spectra are shown using black color, and red color is used to plot fitted curved; Figure S4: the experimental and calculated EXAFS-signals χ(k)*k2 for Ln(NO3)3 · xH2O crystalline powders. Experimental spectra are shown using black color, and red color is used to plot fitted curved; Figure S5: Ln LIII XANES spectra for Ln(NO3)3 · xH2O salts (black color) and aqueous solutions (red color). Vertical lines are used to mark the position of the second maximum.
Author Contributions
Conceptualization, G.K.; methodology, G.K.; validation, G.K. and A.T.; formal analysis, G.K. and E.D.; investigation, G.K., A.T., A.R. and A.D.; data curation, G.K. and E.D.; writing—original draft preparation, G.K. and A.T.; writing—review and editing, G.K.; visualization, E.D.; supervision, G.K.; funding acquisition, G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation (grant no. FSFZ-2024-0026).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ansari, S.A.; Mohapatra, P.K. A review on solid phase extraction of actinides and lanthanides with amide based extractants. J. Chromatogr. 2017, 1499, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; You, H.; Jia, G.; Zheng, Y.; Huang, Y.; Song, Y.; Yang, M.; Zhang, L.; Zhang, H. Hierarchically Nanostructured Coordination Polymer: Facile and Rapid Fabrication and Tunable Morphologies. Cryst. Growth Des. 2010, 10, 790–797. [Google Scholar] [CrossRef]
- Nazarov, M.; Young, N. New Generation of Terbium and Europium Activated Phosphors; Pan Stanford Publishing: Boca Raton, FL, USA, 2011. [Google Scholar]
- Drossbach, G.P. Ueber den Einfluss der Elemente der Cer-und Zircongruppe auf das Wachstum von Bakterien. Zentralbl Bakteriol Parasitenk Infekt. Abt. 1897, 1, 57–58. [Google Scholar]
- Cota, I.; Marturano, V.; Tylkowski, B. Ln complexes as double faced agents: Study of antibacterial and antifungal activity. Coordin. Chem. Rev. 2019, 396, 49–71. [Google Scholar] [CrossRef]
- Garner, J.P.; Heppell, P.S.J. Cerium nitrate in the management of burns. Burns 2005, 31, 539–547. [Google Scholar] [CrossRef]
- Silva-Dias, A.; Miranda, I.M.; Branco, J.; Cobrado, L.; Monteiro-Soares, M.; Pina-Vaz, C.; Rodrigues, A.G. In vitro antifungal activity and in vivo antibiofilm activity of cerium nitrate against Candida species. J. Antimicrob. Chemother. 2015, 70, 1083–1093. [Google Scholar] [CrossRef]
- Gainanova, A.A.; Kuz’micheva, G.M.; Terekhova, R.P.; Pashkin, I.I.; Trigub, A.L.; Malysheva, N.E.; Svetogorov, R.D.; Alimguzina, A.R.; Koroleva, A.V. New antimicrobial objects with cerium ions in the composition of salts, solutions, composite systems based on Ce 3+(NO3)3 × 6H2O. New J. Chem. 2022, 46, 19271. [Google Scholar] [CrossRef]
- Kuz’micheva, G.M.; Timaeva, O.I.; Novikova, N.N.; Yakunin, S.N.; Rogachev, A.V.; Svetogorov, R.D.; Pashkin, I.I.; Terekhova, R.P. Antimicrobial Activity of Composite Hydrogels in the Poly(N-vinylpyrrolidone)–RE(NO3)3·xH2O (RE Are Rare-Earth Ions) System. Crystallogr. Rep. 2020, 65, 922–932. [Google Scholar] [CrossRef]
- Beuchat, C.; Hagberg, D.; Spezia, R.; Gagliardi, L. Hydration of Lanthanide Chloride Salts: A Quantum Chemical and Classical Molecular Dynamics Simulation Study. J. Phys. Chem. B 2010, 114, 15590–15597. [Google Scholar] [CrossRef]
- Allen, P.G.; Bucher, J.J.; Shuh, D.K.; Edelstein, N.M.; Craig, I. Coordination Chemistry of Trivalent Lanthanide and Actinide Ions in Dilute and Concentrated Chloride Solutions. Inorg. Chem. 2000, 39, 595–601. [Google Scholar] [CrossRef]
- Rizkalla, E.N.; Choppin, G.R. Lanthanides and actinides hydration and hydrolysis. In Handbook on the Physics and Chemistry of Rare Earths; Gschneidner, K.A., Jr., Eying, L., Choppin, G.R., Lander, G.H., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1994; Volume 18, pp. 529–558. [Google Scholar]
- Yokoyama, H.; Johansson, G. Structures of Nitrate Complexes of Erbium in Aqueous Solutions. Acta Chem. Scand. 1990, 4, 567–573. [Google Scholar] [CrossRef][Green Version]
- Smirnov, P.R.; Grechin, O.V.; Trostin, V.N. Concentration Dependence of the Structure of Aqueous Solutions of Lutetium Nitrate According to X-ray Diffraction. Russ. J. Phys. Chem. A 2014, 88, 250–253. [Google Scholar] [CrossRef]
- Butcher, T.A.; Formon, G.J.M.; Dunne, P.; Hermans, T.M.; Ott, F.; Noirez, L.; Coey, J.M.D. Neutron imaging of liquid-liquid systems containing paramagnetic salt solutions. Appl. Phys. Lett. 2020, 116, 022405. [Google Scholar] [CrossRef]
- Yaita, T.; Narita, H.; Suzuki, S.; Tachimori, S. Structural study of lanthanides(III) in aqueous nitrate and chloride solutions by EXAFS. J. Radioanal. Nucl. Chem. 1999, 239, 371–375. [Google Scholar] [CrossRef]
- Ohta, A.; Kagi, H.; Tsuno, H.; Nomura, M.; Kawabe, I. Influence of multi-electron excitation on EXAFS spectroscopy of trivalent rare-earth ions and elucidation of change in hydration number through the series. Am. Mineral. 2008, 93, 1384–1392. [Google Scholar] [CrossRef]
- Nelson, D.L.; Irish, D.E. Interactions in Lanthanide Systems. I. A Raman and Infrared Study of Aqueous Gadolinium Nitrate. J. Chem. Phys. 1971, 54, 4479–4489. [Google Scholar] [CrossRef]
- Onghena, B.; Papagni, E.; Rezende Souza, E.; Banerjee, D.; Binnemans, K.; Hoogerstraete, T. Speciation of lanthanide ions in the organic phase after extraction from nitrate media by basic extractants. RSC Adv. 2018, 8, 32044–32054. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, W.W.; Irmer, G. On the Hydration of the Rare Earth Ions in Aqueous Solution. J. Solut. Chem. 2020, 49, 316–331. [Google Scholar] [CrossRef]
- Fratiello, A.; Kubo-Anderson, V.; Azimi, S.; Flores, T.; Marinez, E.; Matejka, D.; Perrigan, R.; Vigil, M. A Hydrogen-I, Nitrogen-15, and Chlorine-35 NMR Coordination Study of Lu(CIO4)3 and Lu(NO3)3 in Aqueous Solvent Mixtures. J. Solut. Chem. 1990, 19, 811–829. [Google Scholar] [CrossRef]
- Dobler, M.; Guilbaud, P.; Dedieub, A.; Wipff, G. Interaction of trivalent lanthanide cations with nitrate anions: A quantum chemical investigation of monodentate/bidentate binding modes. New J. Chem. 2001, 25, 1458–1465. [Google Scholar] [CrossRef]
- Duvail, M.; Ruas, A.; Venault, L.; Moisy, P.; Guilbaud, P. Molecular Dynamics Studies of Concentrated Binary Aqueous Solutions of Lanthanide Salts: Structures and Exchange Dynamics. Inorg. Chem. 2010, 49, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Migliorati, V.; Serva, A.; Sessa, F.; Lapi, A.; D’Angelo, P. Influence of Counterions on the Hydration Structure of Lanthanide Ions in Dilute Aqueous Solutions. J. Phys. Chem. B 2018, 122, 2779–2791. [Google Scholar] [CrossRef]
- Bonal, C.; Morel, J.-P.; Morel-Desrosiers, N. Interactions between lanthanide cations and nitrate anions in water Part 2. Microcalorimetric determination of the Gibbs energies, enthalpies and entropies of complexation of Y3‘ and trivalent lanthanide cations. J. Chem. Soc. Faraday Trans. 1998, 94, 1431–1436. [Google Scholar] [CrossRef]
- Rao, L.; Tian, G. Complexation of Lanthanides with Nitrate at Variable Temperatures: Thermodynamics and Coordination Modes. Inorg. Chem. 2009, 48, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Bera, M.K.; Luo, G.; Schlossman, M.L.; Soderholm, L.; Lee, S.; Antonio, M.R. Erbium(III) Coordination at the Surface of an Aqueous Electrolyte. J. Phys. Chem. B 2015, 119, 8734–8745. [Google Scholar] [CrossRef]
- Shiery, R.C.; Fulton, J.L.; Balasubramanian, M.; Nguyen, M.-T.; Lu, J.-B.; Li, J.; Rousseau, R.; Glezakou, V.-A.; Cantu, D.C. Coordination Sphere of Lanthanide Aqua Ions Resolved with Ab Initio Molecular Dynamics and X-ray Absorption Spectroscopy. Inorg. Chem. 2021, 60, 3117–3130. [Google Scholar] [CrossRef]
- Persson, I.; D’Angelo, P.; De Panfilis, S.; Sandström, M.; Erikssonet, L. Hydration of Lanthanoid (III) Ions in Aqueous Solution and Crystalline Hydrates Studied by EXAFS Spectroscopy and Crystallography: The Myth of the “Gadolinium Break”. Chem. Eur. J. 2008, 14, 3056–3066. [Google Scholar] [CrossRef] [PubMed]
- Solera, J.A.; García, J.; Proietti, M.G. Multielectron excitations at the L edges in rare-earth ionic aqueous solutions. Phys. Rev. B 1995, 51, 2678. [Google Scholar] [CrossRef]
- Plakhova, T.; Romanchuk, A.; Yakunin, S.; Dumas, T.; Demir, S.; Wang, S.; Minasian, S.; Shuh, D.; Tyliszczak, T.; Shiryaev, A.; et al. Solubility of Nanocrystalline Cerium Dioxide: Experimental Data and Thermodynamic Modeling. J. Phys. Chem. C 2016, 120, 22615–22626. [Google Scholar] [CrossRef]
- Zegenhagen, J.; Kazimirov, A. X-ray Standing Wave Technique, Principles and Applications; World Scientific Publishing: Singapore, 2013; 556p. [Google Scholar]
- Parratt, L.G. Surface Studies of Solids by Total Reflection of X-rays. Phys. Rev. 1954, 95, 359–369. [Google Scholar] [CrossRef]
- Milinski, N.; Ribar, B.; Sataric, M. Pentaaquatrinitratocerium(III) monohydrate, Ce(H2O)5(NO3)3·H2O. Cryst. Struct. Commun. 1980, 9, 473–477. [Google Scholar]
- Klein, W. Crystal structures of the penta- and hexahydrate of thulium nitrate. Acta Cryst. 2020, E76, 1863–1867. [Google Scholar] [CrossRef] [PubMed]
- Taha, Z.A.; Ajlouni, A.; Hijazi, A.K.; Kühn, F.E.; Herdtweck, E. Redetermination of [Gd(NO3)3(H2O)4]·2H2O. Acta Cryst. 2012, E68, i56–i57. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, R.; Sasaki, M.; Satoh, S.; Isoda, H.; Kino, Y.; Shiozaki, Y. Report on Temperature Dependence of Crystal Structure for Samarium Nitrate Having Metastable Phenomena. J. Phys. Soc. Jpn. 2000, 69, 3297–3303. [Google Scholar] [CrossRef]
- Decadt, R.; Van Der Voort, P.; Van Driessche, I.; Van Deun, R.; Van Hecke, K. Redetermination of [Pr(NO3)3(H2O)4]_2H2O. Acta Cryst. 2012, E68, i59–i60. [Google Scholar]
- Stumpf, T.; Bolte, M. Tetraaquatrinitratoeuropium (III) dehydrate. Acta Cryst. 2001, E57, i10–i11. [Google Scholar]
- Moret, E.; Bunzli, J.-C.G.; Schenk, K.J. Structural and luminescence study of europium and terbium nitrate hexahydrates. Inorg. Chim. Acta 1990, 178, 83–88. [Google Scholar] [CrossRef]
- Rogers, D.J.; Taylor, N.J.; Toogood, G.E. Tetraaquatrinitratoneodymium(III) dihydrate, [Nd(NO3)3(H2O)4].2H2O. Acta Cryst. 1983, C39, 939–941. [Google Scholar] [CrossRef]
- Junk, P.C.; Kepert, D.L.; Skelton, B.W.; White, A.H. Structural Systematics of Rare Earth Complexes. XIII. (“Maximally”) Hydrated (Heavy) Rare Earth Nitrates. Aust. J. Chem. 1999, 52, 497–505. [Google Scholar]
- Rincke, C.; Schmidt, H.; Voigt, W. Rebuttal of the Existence of Solid Rare Earth Bicarbonates and the Crystal Structure of Holmium Nitrate Pentahydrate. Z. Für Anorg. Und Allg. Chem. 2017, 643, 437–442. [Google Scholar] [CrossRef]
- Klein, W. Crystal structure of tetraaqua-tris(nitrato-κ2O,O′) erbium(III) monohydrate, Er(NO3)3·5H2O, H10ErN3O14. Z. Fur. Krist. New Cryst. Struct. 2022, 237, 265–266. [Google Scholar]
- Natoli, C.R. Distance Dependence of Continuum and Bound State of Excitonic Resonances in X-ray Absorption Near Edge Structure (XANES). In EXAFS and Near Edge Structure III. Springer Proceedings in Physics; Hodgson, K.O., Hedman, B., Penner-Hahn, J.E., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 2, pp. 38–42. [Google Scholar]
- Rizkalla, E.N.; Choppin, G.R. Hydration and hydrolysis of lanthanides. In Handbook on the Physics and Chemistry of Rare; Gschneidner, K.A., Eyring, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 15, pp. 393–443. [Google Scholar]
- Rizkalla, E.N.; Choppin, G.R. Hydration of lanthanides and actinides in solution. J. Alloys Compd. 1992, 180, 325–336. [Google Scholar] [CrossRef]
- Smirnov, P.R.; Trostin, V.N. Structural Parameters of the Immediate Environment of Ions in Aqueous Solutions of Inorganic Electrolytes; Ivanovo Publishing House: Ivanovo, Russia, 2011; 400p. (In Russian) [Google Scholar]
- Kanno, H.; Hiraishi, J. Raman study of aqueous rare earth nitrate solutions in liquid and glassy states. J. Phys. Chem. 1984, 88, 2787–2792. [Google Scholar] [CrossRef]
- Choppin, G.R.; Strazik, W.F. Complexes of Trivalent Lanthanide and Actinide Ions. I. Outer-Sphere Ion Pairs. Inorg. Chem. 1965, 4, 1250–1254. [Google Scholar]
- Klimov, V.D.; Chudinov, E.G. Application of Infrared Spectroscopy to Study Bonds in Complexes of Rare Earth Nitrates with Alkylammonium Nitrates; Order-of-Lenin I. V. Kurchatov Institute of Atomic Energy: Moscow, Russia, 1974. (In Russian) [Google Scholar]
- Setyaeva, E.A.; Glushko, A.A.; Kuzmicheva, G.M.; Neznanov, A.A.; Terekhova, R.P.; Svetogorov, R.D. Application of information technologies for the selection of salts and solutions RE(NO3)3·xH2O (RE=La-Lu, Y, Sc) with optimal structural characteristics and biocidal and neutron studies. In Book of Reports of the Kurchatov Forum of Synchrotron and Neutron Research; NRC “Kurchatov Institute”: Moscow, Russia, 2023. (In Russian) [Google Scholar]
- Grechin, O.V.; Smirnov, P.R.; Trostin, V.N. X-Ray Diffraction Study of Aqueous solutions of Lanthanum Chloride and Nitrate. Chem. Chem. Technol. 2013, 56, 15–20. (In Russian) [Google Scholar]
- Smirnov, P.R.; Grechin, O.V.; Vashurin, A.S. Ion Coordination in Aqueous Lanthanum Chloride and Lanthanum Nitrate Solutions as Probed by X-Ray Diffraction. Russ. J. Inorg. Chem. 2022, 67, 382–387. [Google Scholar] [CrossRef]
- Yatsenko, A.V.; Gloriozov, I.P.; Zhokhova, N.I.; Paseshnichenko, K.A.; Aslanov, L.A.; Ustynyuk, Y.A. Structure of lanthanide nitrates in solution and in the solid state: DFT modelling of hydration effects. J. Mol. Liq. 2021, 323, 115005. [Google Scholar] [CrossRef]
- Rojas-Mena, A.; López-González, H.; Rojas-Hernández, A. Preparation and Characterization of Holmium-Beta-Cyclodextrin Complex. Adv. Mater. Phys. Chem. 2015, 5, 87–94. [Google Scholar] [CrossRef][Green Version]
- Vratny, F. Infrared Spectra of Metal Nitrates. Appl. Spectrosc. 1959, 13, 59–70. [Google Scholar] [CrossRef]
- Falk, M.; Giguere, P.A. Infrared spectrum of the H3O+ ion in aqueous solutions. Can. J. Chem. 1957, 35, 1195–1204. [Google Scholar] [CrossRef]
- Sriramula, B.S.; Katreddi, H.R. Rare Earth Nitrate Complexes with an ONO Schiff Base Ligand: Spectral, Thermal, Luminescence and Biological Studies. Iran. J. Chem. Chem. Eng. 2017, 36, 101–109. [Google Scholar]
- Sidyakin, P.V.; Karpov, V.L.; Egorov, B.N.; Egorova, Z.S. Radiation transformations in epoxy oligomers based on epichlorohydrin and n,n’-dioxydiphenylpropane. Polym. Sci. Ser. A 1971, 8, 2195–2206. [Google Scholar]
- Mihaylov, M.Y.; Zdravkova, V.R.; Ivanova, E.Z.; Aleksandrov, H.A.; Petko, P.S.; Vayssilov, G.N.; Hadjiivanov, K.I. Infrared Spectra of Surface Nitrates: Revision of the Current Opinions Based on the Case Study of Ceria. J. Catal. 2021, 394, 245–258. [Google Scholar] [CrossRef]
- Khakhalin, A.V.; Koroleva, A.V. Investigation of the temperature dependence for the spectra of supercooled water in the middle infrared. Mosc. Univ. Phys. Bull. 2014, 1, 66–69. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Jana, S. Spectroscopic Characterization of Disodium Hydrogen Orthophosphate and Sodium Nitrate after Biofield Treatment. J. Chromatogr. Sep. Tech. 2015, 6, 2–5. [Google Scholar] [CrossRef]
- Alberts, A.S.; Brighton, S.W.; Kempf, P.; Louw, W.K.; Beek, A.V.; Kritzenger, V.; Westerink, H.P.; Vanrensburg, A.J. Samarium-153-EDTMP for Palliation of Ankylosing Spondylitis, Paget’s Disease and Rheumatoid Arthritis. J. Nucl. Med. 1995, 36, 1417–1420. [Google Scholar] [PubMed]
- de Witt, G.C.; May, P.M.; Webb, J.; Hefter, G. Biospeciation, by potentiometry and computer simulation, of Sm-EDTMP, a bone tumor palliative agent. Biometals 1996, 9, 351–361. [Google Scholar] [CrossRef]
- de Witt, G.C.; May, P.M.; Webb, J.; Hefter, G. Potentiometric and computer studies of yttrium-EDTMP. Inorg. Chim. Acta. 1998, 275–276, 37–42. [Google Scholar] [CrossRef]
- Hydrolysis. Available online: https://skysmart.ru/articles/chemistry/gidroliz (accessed on 22 October 2022).
- Chemer. Available online: https://chemer.ru/services/hydrolysis/salts/Fe(NO3)3 (accessed on 29 July 2024).
- Devyatov, F.V.; Rubanov, A.V. Hydrolytic properties of nitrates Ni(II), Cu(II), Dy(III). Proc. Kazan Univ. Nat. Sci. Ser. 2006, 148, 92–101. (In Russian) [Google Scholar]
- Bentouhami, E.; Bouet, G.M.; Meullemeestre, J.; Verling, F.; Khan, M.A. Physicochemical study of the hydrolysis of Rare-Earth elements (III) and thorium (IV). Comptes Rendus. Chimie. 2004, 7, 537–545. [Google Scholar] [CrossRef]
- Lucena, A.F.; Lourenço, C.; Michelini, M.C.; Rutkowski, P.X.; Carretas, J.M.; Zorz, N.; Berthon, L.; Dias, A.; Oliveira, M.C.; Gibson, J.K.; et al. Synthesis and hydrolysis of gas-phase lanthanide and actinide oxide nitrate complexes: A correspondence to trivalent metal ion redox potentials and ionization energies. Phys. Chem. Chem. Phys. 2015, 17, 9942–9950. [Google Scholar] [CrossRef]
- Habenschuss, A.; Spedding, F.H. The coordination (hydration) of rare earth ions in aqueous chloride solutions from x-ray diffraction. II. LaCl3, PrCl3, and NdCl3). J. Chem. Phys. 1979, 70, 3758–3763. [Google Scholar] [CrossRef]
- Habenschuss, A.; Spedding, F.H. The coordination (hydration) of rare earth ions in aqueous chloride solutions from x ray diffraction. I.TbCl3, DyCl3, ErCl3, TmCl3,and LuCl3. J. Chem. Phys. 1979, 70, 2797–2806. [Google Scholar] [CrossRef]
- Poluektov, N.S.; Kononenko, L.I.; Efryushina, N.P.; Beltyukova, S.V. Spectrophotometric and Luminescent Methods for Determining Lanthanides; Naukova Dumka: Kiev, Russia, 1989; p. 256. [Google Scholar]
- Esquivel-Castro, T.A.; Martínez-Luévanos, A.; Estrada-Flores, S.; Cano-Salazar, L.F. Porous Materials for Applications in Energy and Environment. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Martínez, L.M.T., Kharisov, B.I., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–19. [Google Scholar]
- Blatun, L.A.; Mitish, V.A.; Terekhova, R.P.; Grishina, I.A.; Alekseev, A.A.; Kirienko, A.I.; Bogdanets, L.I.; Titkova, Y.S.; Novozhilov, A.A.; Smirnov, S.V.; et al. EPLAN (ointment, solution) is a new medication for the topical treatment of skin and soft tissue infections at multidisciplinary hospital. Wounds Wound Infect. Prof. B.M. Kostyuchenok J. 2014, 1, 13–21. (In Russian) [Google Scholar] [CrossRef]
- Yakunin, S.N.; Novikova, N.N.; Rogachev, A.V.; Trigub, A.L.; Kuzmicheva, G.M.; Stepina, N.D.; Rozenberg, O.A.; Yurieva, E.A.; Kovalchuk, M.V. Spectral-Selective X-Ray Studies at the “Langmuir” Beamline of the Kurchatov Synchrotron Radiation Source. Crystallogr. Rep. 2022, 67, 799–812. [Google Scholar] [CrossRef]
- Chernyshov, A.A.; Veligzhanin, A.A.; Zubavichus, Y.V. Structural materials science end-station at the Kurchatov synchrotron radiation source: Recent instrumentation up-grades and experimental results. Nucl. Instr. Methods Phys. Res. 2009, A603, 95–98. [Google Scholar] [CrossRef]
- Bunker, G. Introduction to XAFS: A Practical Guide to X-ray Absorption Fine Structure Spectroscopy; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Aksenov, V.L.; Koval’chuk, M.V.; Kuz’min, A.Y.; Purans, Y.; Tyutyunnikov, S.I. Development of methods of EXAFS spectroscopy on synchrotron radiation beams: Review. Crystallogr. Rep. 2006, 51, 908–935. [Google Scholar] [CrossRef]
- Ankudinov, A.L.; Ravel, B.; Rehr, J.J.; Conradson, S.D. RealSpace Multiple-Scattering Calculation and Interpretation of X-RayAbsorption Near-Edge Structure. Phys. Rev. B 1998, 58, 7565. [Google Scholar] [CrossRef]
- Rehr, J.J.; Albers, R.C. Theoretical Approaches to X-Ray Absorption Fine Structure. Rev. Mod. Phys. 2000, 72, 621. [Google Scholar] [CrossRef]
- Hedin, L.; Lundqvist, B.I. Explicit Local Exchange-Correlation Potentials. J. Phys. C Solid State Phys. 1971, 4, 2064. [Google Scholar] [CrossRef]
- Newville, M.L. An Analysis Package for XAFS and Related Spectroscopies. J. Phys. Conf. Ser. 2013, 430, 012007. [Google Scholar] [CrossRef]
- Fonda, E.; Andreatta, D.; Colavita, P.E.; Vlaica, G. EXAFS analysis of the L3 edge of Ce in CeO2: Effects of multi-electron excitations and final-state mixed valence. J. Synchrotron Rad. 1999, 6, 34–42. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, B.M.; Biryukova, S.V.; Tamm, T.I. Methodical Recommendations for Experimental (Preclinical) Study of Drugs for Local Treatment of Purulent Wounds; USSR Ministry of Health: Moscow, Russia, 1989; 145p. [Google Scholar]
- Blatun, L.A. Wounds and wound infections. Prof. B.M. Kostyuchenok J. 2015, 2, 36–44. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).