Fungal Extracellular Vesicle Proteins with Potential in Biological Interaction
Abstract
1. Introduction
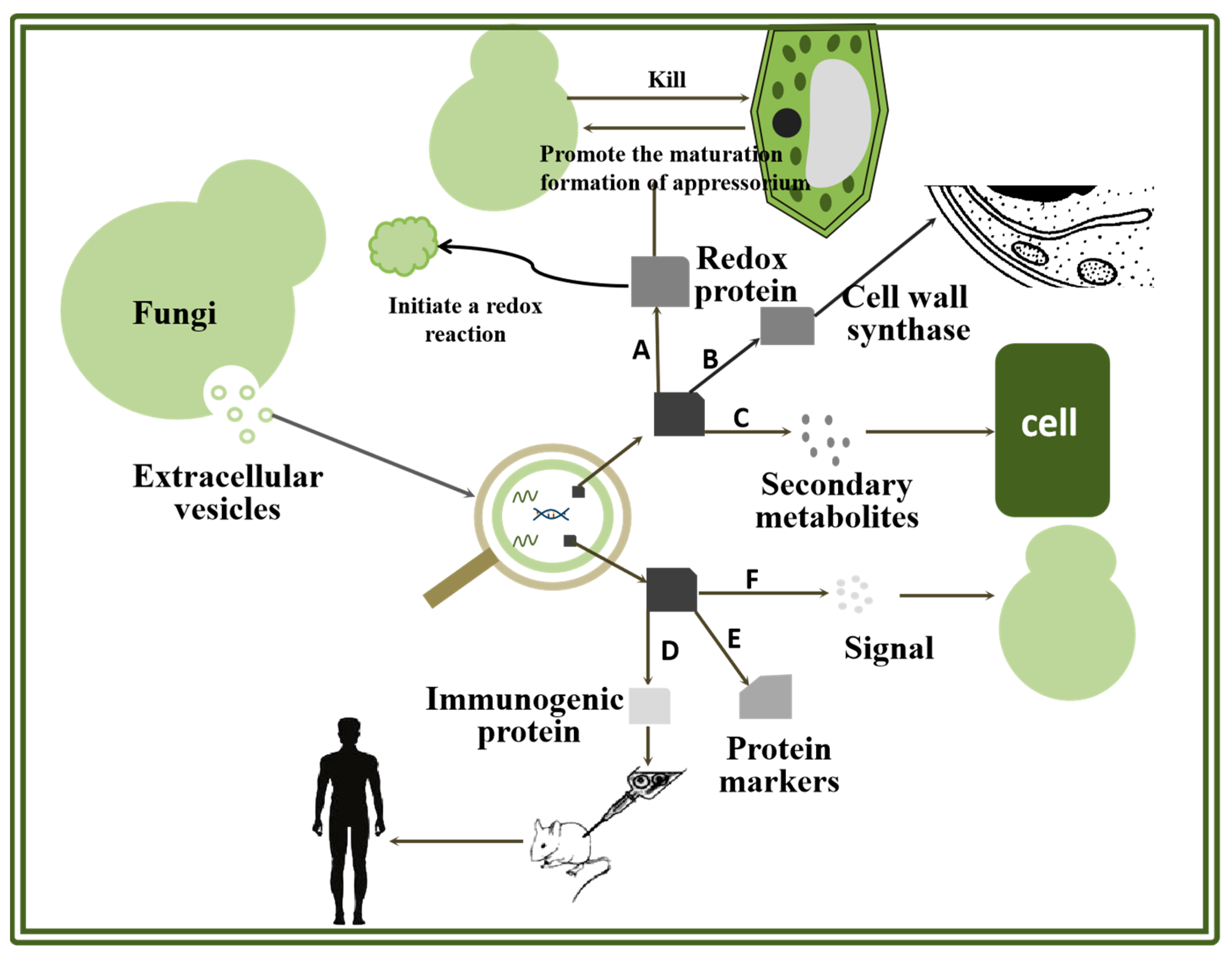
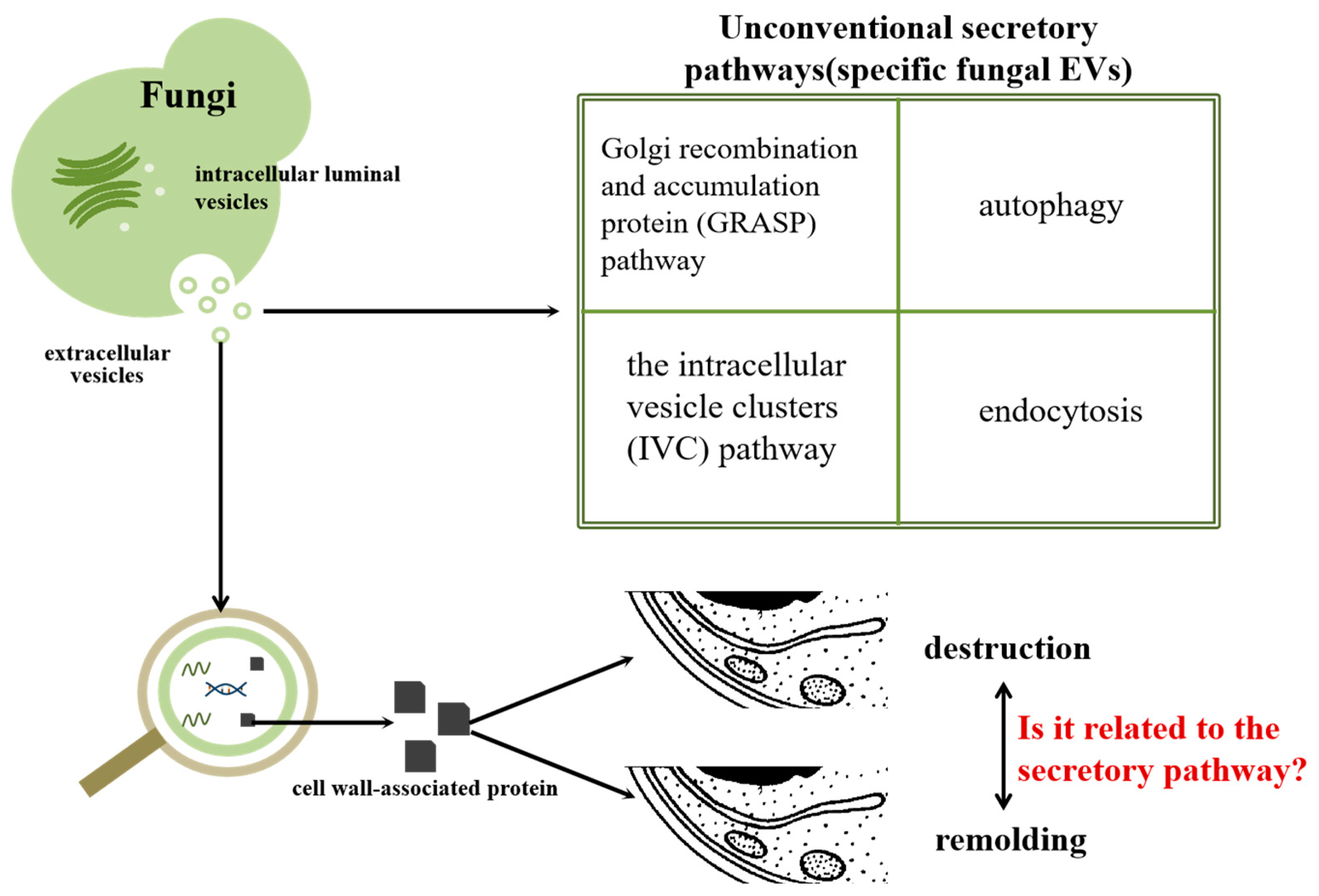
2. The Different Proteins of EVs in Fungi
2.1. Role of Proteins in Cell Wall Synthesis
2.2. Role of Protein in Virulence Transmission
2.3. Role of Protein in Transmitting Information
2.4. Role of Protein in Oxidation Reaction
2.5. Role of Protein in the Study of Markers
2.5.1. Sur 7 Family
2.5.2. Other Marker Proteins
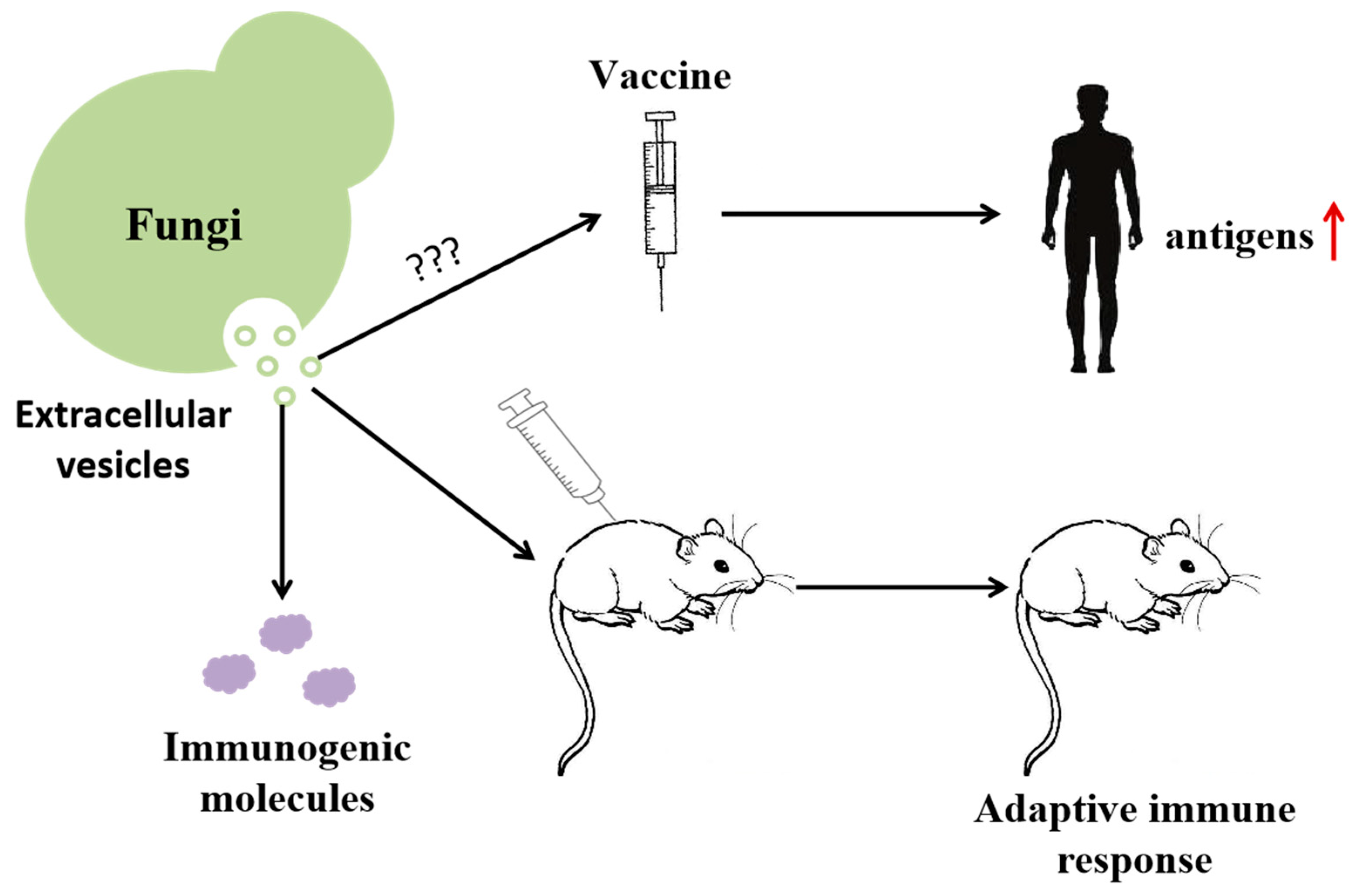
2.6. Role of Protein in Vaccine Research and Development
2.6.1. Candida albicans
2.6.2. Cryptococcus
3. Summary and Prospect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karkowska-Kuleta, J.; Kulig, K.; Karnas, E.; Zuba-Surma, E.; Woznicka, O.; Pyza, E.; Kuleta, P.; Osyczka, A.; Rapala-Kozik, M.; Kozik, A. Characteristics of extracellular vesicles released by the pathogenic Yeast-Like fungi Candida glabrata, Candida parapsilosis and Candida tropicalis. Cells 2020, 9, 1722. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, J.; Rodrigues, M.L.; Janbon, G. Extracellular vesicles in fungi: Past, present, and future perspectives. Front. Cell. Infect. Microbiol. 2020, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Casadevall, A. A two-way road: Novel roles for fungal extracellular vesicles. Mol. Microbiol. 2018, 110, 11–15. [Google Scholar] [CrossRef]
- Echeverría-Bugueño, M.; Espinosa-Lemunao, R.; Irgang, R.; Avendaño-Herrera, R. Identification and characterization of outer membrane vesicles from the fish pathogen Vibrio ordalii. J. Fish Dis. 2020, 43, 621–629. [Google Scholar] [CrossRef]
- Lai, Y.; Jiang, B.; Hou, F.; Huang, X.; Ling, B.; Lu, H.; Zhong, T.; Huang, J. The emerging role of extracellular vesicles in fungi: A double-edged sword. Front. Microbiol. 2023, 14, 1216895. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular vesicles: Unique intercellular delivery vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef]
- Van, N.G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar]
- Regente, M.; Pinedo, M.; San Clemente, H.; Balliau, T.; Jamet, E.; de la Canal, L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef] [PubMed]
- Liebana-Jordan, M.; Brotons, B.; Falcon-Perez, J.M.; Gonzalez, E. Extracellular vesicles in the fungi kingdom. Int. J. Mol. Med. 2021, 22, 7221. [Google Scholar] [CrossRef]
- Arvizu-Rubio, V.J.; García-Carnero, L.C.; Mora-Montes, H.M. Moonlighting proteins in medically relevant fungi. PeerJ 2022, 10, e14001. [Google Scholar] [CrossRef]
- Yin, H.; Xie, J.; Xing, S.; Lu, X.; Yu, Y.; Ren, Y.; Tao, J.; He, G.; Zhang, L.; Yuan, X.; et al. Machine learning-based analysis identifies and validates serum exosomal proteomic signatures for the diagnosis of colorectal cancer. Cell Rep. Med. 2024, 5, 101689. [Google Scholar] [CrossRef]
- Garrett, N.R.; Pink, R.C.; Lawson, C. Contribution of extracellular particles isolated from Morus sp. (Mulberry) fruit to their reported protective health benefits: An in vitro study. Int. J. Mol. Sci. 2024, 25, 6177. [Google Scholar] [CrossRef]
- Piibor, J.; Waldmann, A.; Prasadani, M.; Kavak, A.; Andronowska, A.; Klein, C.; Kodithuwakku, S.; Fazeli, A. Investigation of Uterine Fluid Extracellular Vesicles’ Proteomic Profiles Provides Novel Diagnostic Biomarkers of Bovine Endometritis. Biomolecules 2024, 14, 626. [Google Scholar] [CrossRef]
- Honorato, L.; de Araujo, J.F.D.; Ellis, C.C.; Piffer, A.C.; Pereira, Y.; Frases, S.; de Sousa Araújo, G.R.; Pontes, B.; Mendes, M.T.; Pereira, M.D.; et al. Extracellular vesicles regulate biofilm formation and Yeast-to-Hypha differentiation in Candida albicans. mBio 2022, 13, e0030122. [Google Scholar] [CrossRef]
- Bitencourt, T.A.; Hatanaka, O.; Pessoni, A.M.; Freitas, M.S.; Trentin, G.; Santos, P.; Rossi, A.; Martinez-Rossi, N.M.; Alves, L.L.; Casadevall, A.; et al. Fungal extracellular vesicles are involved in intraspecies intracellular communication. Microb. Biotechnol. 2022, 13, e0327221. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.J.; Staton, C.C.; Oliver, J.T.; Bouffard, J.; Kazmirchuk, T.D.D.; Magi, M.; Brett, C.L. Thermotolerance in S. cerevisiae as a model to study extracellular vesicle biology. J. Extracell. Vesicles 2024, 13, e12431. [Google Scholar] [CrossRef]
- Kulig, K.; Rapala-Kozik, M.; Karkowska-Kuleta, J. Extracellular vesicle production: A bidirectional effect in the interplay between host and Candida fungi. Curr. Res. Microb. Sci. 2024, 7, 100255. [Google Scholar] [CrossRef]
- Duan, H.; Meng, F.; Liu, X.; Qi, P.; Peng, X.; Li, C.; Wang, Q.; Zhao, G.; Lin, J. Extracellular vesicles from Candida albicans modulate immune cells function and play a protective role in fungal keratitis. Microb. Pathog. 2024, 189, 106606. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Li, X.; Gao, H.; Liu, Y.; Liu, Y.; Zheng, J.; Zhu, J.; Zhao, C.; Shi, Y.; Lu, J.; et al. Biyang floral mushroom-derived exosome-like nanovesicles: Characterization, absorption stability and ionizing radiation protection. Food Funct. 2024, 15, 6900–6913. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.; Caballero, Z.; De León, L.F.; Spadafora, C. Extracellular vesicles could carry an evolutionary footprint in interkingdom communication. Front. Cell. Infect. Microbiol. 2020, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Zarnowski, R.; Noll, A.; Chevrette, M.G.; Sanchez, H.; Jones, R.; Anhalt, H.; Fossen, J.; Jaromin, A.; Currie, C.; Nett, J.E.; et al. Coordination of fungal biofilm development by extracellular vesicle cargo. Nat. Commun. 2021, 12, 6235. [Google Scholar] [CrossRef]
- Wang, T.; Li, L.; Hong, W. SNARE proteins in membrane trafficking. Traffic 2017, 18, 767–775. [Google Scholar] [CrossRef]
- Huisman, R.; Hontelez, J.; Bisseling, T.; Limpens, E. SNARE complexity in Arbuscular mycorrhizal symbiosis. Front. Plant Sci. 2020, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Nakayasu, E.S.; Oliveira, D.L.; Nimrichter, L.; Nosanchuk, J.D.; Almeida, I.C.; Casadevall, A. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell 2008, 7, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.C.; Nakayasu, E.S.; Matsuo, A.L.; Sobreira, T.J.; Longo, L.V.; Ganiko, L.; Almeida, I.C.; Puccia, R. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: Comparative analysis with other pathogenic fungi. J. Proteome Resarch 2012, 11, 1676–1685. [Google Scholar] [CrossRef]
- Oliveira, D.L.; Nakayasu, E.S.; Joffe, L.S.; Guimarães, A.J.; Sobreira, T.J.; Nosanchuk, J.D.; Cordero, R.J.; Frases, S.; Casadevall, A.; Almeida, I.C.; et al. Characterization of yeast extracellular vesicles: Evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. Public Libr. Sci. One 2010, 5, e11113. [Google Scholar] [CrossRef] [PubMed]
- Octaviano, C.E.; Abrantes, N.E.; Puccia, R. Extracellular vesicles from Paracoccidioides brasiliensis can induce the expression of fungal virulence traits In Vitro and enhance infection in mice. Front. Cell. Infect. Microbiol. 2022, 12, 834653. [Google Scholar] [CrossRef]
- Muñoz, E.L.; Fuentes, F.B.; Felmer, R.N.; Yeste, M.; Arias, M.E. Extracellular vesicles in mammalian reproduction: A review. Zygote 2022, 30, 440–463. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.H.; Zhu, J.; Wang, Y.C.; Wu, J.; Liu, J.R.; Guo, H.D. Effects of exosomal miRNAs in the diagnosis and treatment of Alzheimer’s disease. Mech. Ageing Dev. 2021, 200, 111593. [Google Scholar] [CrossRef] [PubMed]
- Vacchi, E.; Burrello, J.; Di Silvestre, D.; Burrello, A.; Bolis, S.; Mauri, P.; Vassalli, G.; Cereda, C.W.; Farina, C.; Barile, L.; et al. Immune profiling of plasma-derived extracellular vesicles identifies Parkinson disease. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e866. [Google Scholar] [CrossRef]
- Kholafazad Kordasht, H.; Hasanzadeh, M. Biomedical analysis of exosomes using biosensing methods: Recent progress. Anal. Methods 2020, 12, 2795–2811. [Google Scholar] [CrossRef] [PubMed]
- Burrello, J.; Bianco, G.; Burrello, A.; Manno, C.; Maulucci, F.; Pileggi, M.; Nannoni, S.; Michel, P.; Bolis, S.; Melli, G.; et al. Extracellular vesicle surface markers as a diagnostic tool in transient ischemic attacks. Stroke 2021, 52, 3335–3347. [Google Scholar] [CrossRef]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the international society for extracellular vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Bleackley, M.R.; Dawson, C.S.; Anderson, M.A. Fungal extracellular vesicles with a focus on proteomic analysis. Proteomics 2019, 19, 1800232. [Google Scholar] [CrossRef] [PubMed]
- Dawson, C.S.; Garcia-Ceron, D.; Rajapaksha, H.; Faou, P.; Bleackley, M.R.; Anderson, M.A. Protein markers for Candida albicans EVs include claudin-like Sur7 family proteins. J. Extracell. Vesicles 2020, 9, 1750810. [Google Scholar] [CrossRef]
- Garcia-Ceron, D.; Dawson, C.S.; Faou, P.; Bleackley, M.R.; Anderson, M.A. Size-exclusion chromatography allows the isolation of EVs from the filamentous fungal plant pathogen Fusarium oxysporum f. sp. vasinfectum (Fov). Proteomics 2021, 21, e2000240. [Google Scholar] [CrossRef]
- Rizzo, J.; Wong, S.S.W.; Gazi, A.D.; Moyrand, F.; Chaze, T.; Commere, P.H.; Novault, S.; Matondo, M.; Péhau-Arnaudet, G.; Reis, F.C.G.; et al. Cryptococcus extracellular vesicles properties and their use as vaccine platforms. J. Extracell. Vesicles 2021, 10, e12129. [Google Scholar] [CrossRef]
- Curto, M.Á.; Butassi, E.; Ribas, J.C.; Svetaz, L.A.; Cortés, J.C.G. Natural products targeting the synthesis of β(1,3)-D-glucan and chitin of the fungal cell wall. Existing drugs and recent findings. Phytomedicine 2021, 88, 153556. [Google Scholar] [CrossRef]
- Sutton, J.A.F.; Carnell, O.T.; Lafage, L.; Gray, J.; Biboy, J.; Gibson, J.F.; Pollitt, E.J.G.; Tazoll, S.C.; Turnbull, W.; Hajdamowicz, N.H.; et al. Staphylococcus aureus cell wall structure and dynamics during host-pathogen interaction. PLoS Pathog. 2021, 17, e1009468. [Google Scholar] [CrossRef]
- Plaza, V.; Silva-Moreno, E.; Castillo, L. Breakpoint: Cell wall and glycoproteins and their crucial role in the phytopathogenic fungi infection. Curr. Protein Pept. Sci. 2020, 21, 227–244. [Google Scholar] [CrossRef]
- Sobral, R.; Tomasz, A. The staphylococcal cell wall. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef]
- De Vallée, A.; Dupuy, J.W.; Moriscot, C. Extracellular vesicles of the plant pathogen botrytis cinerea. J. Fungi 2023, 9, 495. [Google Scholar] [CrossRef]
- Silva, G.R.; de Pina Cavalcanti, F.; Melo, R.M.; Cintra, E.; Lima, E.M.; Hamann, P.R.V.; do Vale, L.H.F.; Ulhoa, C.J.; Almeida, F.; Noronha, E.F. Extracellular vesicles from the mycoparasitic fungus Trichoderma harzianum. Antonie Leeuwenhoek 2024, 117, 64. [Google Scholar] [CrossRef] [PubMed]
- Rutter, B.D.; Chu, T.T.; Dallery, J.F.; Zajt, K.K.; O’Connell, R.J.; Innes, R.W. The development of extracellular vesicle markers for the fungal phytopathogen Colletotrichum higginsianum. J. Extracell. Vesicles 2022, 11, e12216. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, J.; Chaze, T.; Miranda, K.; Roberson, R.W.; Gorgette, O.; Nimrichter, L.; Matondo, M.; Latgé, J.P.; Beauvais, A.; Rodrigues, M.L. Characterization of extracellular vesicles produced by Aspergillus fumigatus protoplasts. Msphere 2020, 5, e00476-20. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, R.; Hernáez, M.L.; Redondo, E.; Calvo, G.; Radau, S.; Pardo, M.; Gil, C.; Monteoliva, L. Candida albicans hyphal extracellular vesicles are different from yeast ones, carrying an active proteasome complex and showing a different role in host immune response. Microbiol. Spectr. 2022, 10, e00698-22. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bona, A.; Amador-García, A.; Gil, C.; Monteoliva, L. The external face of Candida albicans: A proteomic view of the cell surface and the extracellular environment. J. Proteom. 2018, 180, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Valdez, A.F.; de Souza, T.N.; Bonilla, J.J.A. Traversing the cell wall: The chitinolytic activity of Histoplasma capsulatum extracellular vesicles facilitates their release. J. Fungi 2023, 9, 1052. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Bleackley, M.; Chisanga, D.; Gangoda, L.; Fonseka, P.; Liem, M.; Kalra, H.; Al Saffar, H.; Keerthikumar, S.; Ang, C.S.; et al. Extracellular vesicles secreted by Saccharomyces cerevisiae are involved in cell wall remodelling. Commun. Biol. 2019, 2, 305. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, K.; Hu, H.; Xing, X.; Huang, X.; Gao, H. Extracellular vesicles: Their functions in plant-pathogen interactions. Mol. Polecular Plant Pathol. 2022, 23, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Jurick, W.M.; Peng, H.; Beard, H.S.; Garrett, W.M.; Lichtner, F.J.; Luciano-Rosario, D.; Macarisin, O.; Liu, Y.; Peter, K.A.; Gaskins, V.L.; et al. Blistering1 modulates penicillium expansum virulence via vesicle-mediated protein secretion. Mol. Cell. Proteom. 2020, 19, 344–361. [Google Scholar] [CrossRef]
- Vallejo, M.C.; Matsuo, A.L.; Ganiko, L.; Medeiros, L.C.; Miranda, K.; Silva, L.S.; Freymüller-Haapalainen, E.; Sinigaglia-Coimbra, R.; Almeida, I.C.; Puccia, R. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic α-Galactosyl epitopes. Eukaryot. Cell 2011, 10, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; Alves, A.; Andolfi, A. Secondary metabolites of lasiodiplodia theobromae: Distribution, chemical diversity, bioactivity, and implications of their occurrence. Toxins 2020, 12, 457. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Andolfi, A. Phytopathogenic fungi and toxicity. Toxins 2021, 13, 689. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Macías-Rubalcava, M.L.; Garrido-Santos, M.Y. Phytotoxic compounds from endophytic fungi. Appl. Microbiol. Biotechnol. 2022, 106, 931–950. [Google Scholar] [CrossRef]
- Ikeda, M.A.K.; de Almeida, J.R.F.; Jannuzzi, G.P.; Cronemberger-Andrade, A.; Torrecilhas, A.C.T.; Moretti, N.S.; da Cunha, J.P.C.; de Almeida, S.R.; Ferreira, K.S. Extracellular vesicles from Sporothrix brasiliensis are an important virulence factor that induce an increase in fungal burden in experimental sporotrichosis. Front. Microbiol. 2018, 9, 286. [Google Scholar] [CrossRef]
- Garcia-Ceron, D.; Lowe, R.G.T.; McKenna, J.A.; Brain, L.M.; Dawson, C.S.; Clark, B.; Berkowitz, O.; Faou, P.; Whelan, J.; Bleackley, M.R.; et al. Extracellular vesicles from Fusarium graminearum contain protein effectors expressed during infection of corn. J. Fungi 2021, 7, 977. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.D.; Sievwright, I.K.; Auld, G.C.; Moore, N.R.; Gow, N.A.; Booth, N.A. Candida albicans binds human plasminogen: Identification of eight plasminogen-binding proteins. Mol. Microbiol. 2003, 47, 1637–1651. [Google Scholar] [CrossRef] [PubMed]
- Konečná, K.; Klimentová, J.; Benada, O.; Němečková, I.; Janďourek, O.; Jílek, P.; Vejsová, M. A comparative analysis of protein virulence factors released via extracellular vesicles in two Candida albicans strains cultivated in a nutrient-limited medium. Microb. Ecol. 2019, 136, 103666. [Google Scholar] [CrossRef]
- Bielska, E.; Sisquella, M.A.; Aldeieg, M.; Birch, C.; O’Donoghue, E.J.; May, R.C. Pathogen-derived extracellular vesicles mediate virulence in the fatal human pathogen Cryptococcus gattii. Nat. Commun. 2018, 9, 1556. [Google Scholar] [CrossRef] [PubMed]
- Castelli, R.F.; Pereira, A.; Honorato, L.; Valdez, A.; de Oliveira, H.C.; Bazioli, J.M.; Garcia, A.W.A.; Klimeck, T.D.F.; Reis, F.C.G.; Camillo-Andrade, A.C.; et al. Corrected and republished from: “Extracellular Vesicle Formation in Cryptococcus deuterogattii Impacts Fungal Virulence”. Infect. Immun. 2024, 92, e0003724. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Regev-Rudzki, N. Biogenesis of extracellular vesicles from the pathogen perspective: Transkingdom strategies for delivering messages. Curr. Opin. Cell Biol. 2024, 88, 102366. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Ye, X.; Chen, X.; Zhou, Y.; Cheng, L.; Zhou, X.; Ren, B. The two-component signal transduction system and its regulation in Candida albicans. Virulence 2021, 12, 1884–1899. [Google Scholar] [CrossRef]
- Iraci, N.; Leonardi, T.; Gessler, F.; Vega, B.; Pluchino, S. Focus on extracellular vesicles: Physiological role and signalling properties of extracellular membrane vesicles. Int. J. Mol. Med. 2016, 17, 171. [Google Scholar] [CrossRef]
- Zingl, F.G.; Kohl, P.; Cakar, F.; Leitner, D.R.; Mitterer, F.; Bonnington, K.E.; Rechberger, G.N.; Kuehn, M.J.; Guan, Z.; Reidl, J.; et al. Outer membrane vesiculation facilitates surface exchange and in vivo adaptation of Vibrio cholerae. Cell Host Microbe 2020, 27, 225–237.e8. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Liu, S.; Shen, H.; Deng, G.; Zeng, S. Extracellular vesicles promote the formation of Pre-Metastasis niche in gastric cancer. Front. Immunol. 2022, 13, 813015. [Google Scholar] [CrossRef]
- Vu, L.T.; Gong, J.; Pham, T.T.; Kim, Y.; Le, M.T.N. MicroRNA exchange via extracellular vesicles in cancer. Cell Prolif. 2020, 53, e12877. [Google Scholar] [CrossRef]
- Munich, S.; Sobo-Vujanovic, A.; Buchser, W.J.; Beer-Stolz, D.; Vujanovic, N.L. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology 2012, 1, 1074–1083. [Google Scholar] [CrossRef]
- Johansson, H.J.; Vallhov, H.; Holm, T.; Gehrmann, U.; Andersson, A.; Johansson, C.; Blom, H.; Carroni, M.; Lehtiö, J.; Scheynius, A. Extracellular nanovesicles released from the commensal yeast Malassezia sympodialis are enriched in allergens and interact with cells in human skin. Sci. Rep. 2018, 8, 9182. [Google Scholar] [CrossRef]
- Tan, Z.L.; Li, J.F.; Luo, H.M.; Liu, Y.Y.; Jin, Y. Plant extracellular vesicles: A novel bioactive nanoparticle for tumor therapy. Front. Pharmacol. 2022, 13, 1006299. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.M.; Espadas-Moreno, J.; Luque-Garcia, J.L.; Casadevall, A. Interaction of Cryptococcus neoformans extracellular vesicles with the cell wall. Eukaryot. Cell 2014, 13, 1484–1493. [Google Scholar] [CrossRef]
- Bleackley, M.R.; Samuel, M.; Garcia-Ceron, D.; McKenna, J.A.; Lowe, R.G.T.; Pathan, M.; Zhao, K.; Ang, C.S.; Mathivanan, S.; Anderson, M.A. Extracellular vesicles from the cotton pathogen Fusarium oxysporum f. sp. vasinfectum induce a Phytotoxic response in plants. Front. Plant Sci. 2022, 10, 1610. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Juvvadi, P.R.; Soderblom, E.J.; Moseley, M.A.; Steinbach, W.J. Hsp70 and the cochaperone stiA (Hop) orchestrate Hsp90-Mediated caspofungin tolerance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2015, 59, 4727–4733. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Camejo, D.; Guzmán-Cedeño, Á.; Moreno, A. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Q.; Feng, Y.; Dong, Y.; Zhang, Z.; Wang, Y.; Liu, W. Responsive mechanism of Hemerocallis citrina baroni to complex saline-alkali stress revealed by photosynthetic characteristics and antioxidant regulation. Plant Cell Rep. 2024, 43, 176. [Google Scholar] [CrossRef]
- Hatami, M.; Ghorbanpour, M. Metal and metal oxide nanoparticles-induced reactive oxygen species: Phytotoxicity and detoxification mechanisms in plant cell. Plant Physiol. Biochem. 2024, 213, 108847. [Google Scholar] [CrossRef]
- Stavrou, A.; Ortiz, A. Extracellular Vesicles: A novel tool in nanomedicine and cancer treatment. Cancers 2022, 14, 4450. [Google Scholar] [CrossRef]
- Ho, J.; Chaiswing, L.; St. Clair, D.K. Extracellular vesicles and cancer therapy: Insights into the role of oxidative stress. Antioxidants 2022, 11, 1194. [Google Scholar] [CrossRef]
- Chiaradia, E.; Tancini, B.; Emiliani, C.; Delo, F.; Pellegrino, R.M.; Tognoloni, A.; Urbanelli, L.; Buratta, S. Extracellular vesicles under oxidative stress conditions: Biological properties and physiological roles. Cells 2021, 10, 1763. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhi, X.; Wang, Y.; Wang, Y. Genome-wide survey and expression analysis of NIN-like Protein (NLP) genes reveals its potential roles in the response to nitrate signaling in tomato. BMC Plant Biol. 2021, 21, 347. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Hou, X.; Li, Y.; Zhang, J.; Bai, W.; Qian, H.; Sun, Z. Extracellular vesicles: Opening up a new perspective for the diagnosis and treatment of mitochondrial dysfunction. J. Nanobiotechnol. 2024, 22, 487. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z. A double-edged sword: Reactive oxygen species (ROS) during the rice blast fungus and host interaction. Febs J. 2022, 289, 5505–5515. [Google Scholar] [CrossRef]
- Kulig, K.; Bednaruk, K.; Rudolphi-Szydło, E.; Barbasz, A.; Wronowska, E.; Barczyk-Woznicka, O.; Karnas, E.; Pyza, E.; Zuba-Surma, E.; Rapala-Kozik, M.; et al. Stress conditions affect the immunomodulatory potential of Candida albicans extracellular vesicles and their impact on cytokine release by THP-1 human macrophages. Int. J. Mol. Sci. 2023, 24, 17179. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, Z.; Liu, Y.; Liao, B.; Zong, Y.; Shi, Y.; Liao, M.; Wang, J.; Zhou, X.; Cheng, L.; et al. Extracellular vesicles of Candida albicans regulate its own growth through the L-arginine/nitric oxide pathway. Appl. Microbiol. Biotechnol. 2023, 107, 355–367. [Google Scholar] [CrossRef]
- Chen, Q.; Che, C.; Yang, S.; Ding, P.; Si, M.; Yang, G. Anti-inflammatory effects of extracellular vesicles from Morchella on LPS-stimulated RAW264.7 cells via the ROS-mediated p38 MAPK signaling pathway. Mol. Cell. Biochem. 2023, 478, 317–327. [Google Scholar] [CrossRef]
- Lanze, C.E.; Konopka, J.B. Sur7 mediates a novel pathway for PIP regulation in C. albicans that promotes stress resistance and cell wall morphogenesis. Mol. Biol. Cell 2024, 35, ar99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.B.; Qiu, T.T.; Guan, Y.; Huang, Z.H.; Ye, X.Y. Analyses of transcriptomics and metabolomics reveal pathway of vacuolar Sur7 contributed to biocontrol potential of entomopathogenic Beauveria bassiana. J. Invertebr. Pathol. 2021, 181, 107564. [Google Scholar] [CrossRef]
- Sakata, K.T.; Hashii, K.; Yoshizawa, K.; Tahara, Y.O.; Yae, K.; Tsuda, R.; Tanaka, N.; Maeda, T.; Miyata, M.; Tabuchi, M. Coordinated regulation of TORC2 signaling by MCC/eisosome-associated proteins, Pil1 and tetraspan membrane proteins during the stress response. Mol. Microbiol. 2022, 117, 1227–1244. [Google Scholar] [CrossRef]
- Atanasova, L.; Gruber, S.; Lichius, A.; Radebner, T.; Abendstein, L.; Münsterkötter, M.; Stralis-Pavese, N.; Łabaj, P.P.; Kreil, D.P.; Zeilinger, S. The Gpr1-regulated Sur7 family protein Sfp2 is required for hyphal growth and cell wall stability in the mycoparasite Trichoderma atroviride. Sci. Rep. 2018, 8, 12064. [Google Scholar] [CrossRef]
- Hill, E.H.; Solomon, P.S. Extracellular vesicles from the apoplastic fungal wheat pathogen Zymoseptoria tritici. Fungal Biol. Biotechnol. 2020, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Lanze, C.E.; Zhou, S.; Konopka, J.B. The Sur7 cytoplasmic C terminus regulates morphogenesis and stress responses in Candida albicans. Mol. Microbiol. 2021, 116, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Rehan, F.; Zhang, M.; Fang, J.; Greish, K. Therapeutic of nanomedicine: Recent developments and future perspectives. Molecules 2024, 29, 2073. [Google Scholar] [CrossRef] [PubMed]
- Kulig, K.; Karnas, E.; Woznicka, O.; Kuleta, P.; Zuba-Surma, E.; Pyza, E.; Osyczka, A.; Kozik, A.; Rapala-Kozik, M.; Karkowska-Kuleta, J. Insight into the properties and immunoregulatory effect of extracellular vesicles produced by Candida glabrata, Candida parapsilosis, and Candida tropicalis Biofilms. Front. Cell. Infect. Microbiol. 2022, 12, 879237. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National prevalence of fungal Diseases-Estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Witchley, J.N.; Penumetcha, P.; Abon, N.V.; Woolford, C.A.; Mitchell, A.P.; Noble, S.M. Candida albicans morphogenesis programs control the balance between gut commensalism and invasive infection. Cell Host Microbe 2019, 25, 432–443.e6. [Google Scholar] [CrossRef]
- Kumamoto, C.A.; Gresnigt, M.S.; Hube, B. The gut, the bad and the harmless: Candida albicans as a commensal and opportunistic pathogen in the intestine. Curr. Opin. Microbiol. 2020, 56, 7–15. [Google Scholar] [CrossRef]
- Pérez, J.C. Candida albicans dwelling in the mammalian gut. Curr. Opin. Microbiol. 2019, 52, 41–46. [Google Scholar] [CrossRef]
- Pérez, J.C. The interplay between gut bacteria and the yeast Candida albicans. Gut Microbes 2021, 13, 979877. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans biofilms and human disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- Oliveira, B.T.M.; Dourado, T.M.H.; Santos, P.W.S. Extracellular Vesicles from Candida haemulonii var. vulnera Modulate Macrophage Oxidative Burst. J. Fungi 2023, 9, 562. [Google Scholar]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence 2022, 13, 89–121. [Google Scholar] [CrossRef]
- Gushiken, A.C.; Saharia, K.K.; Baddley, J.W. Cryptococcosis. Infectious Disease Clinics of North America 2021, 35, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Matsumoto, T.; Kimura, U. Cutaneous cryptococcosis. J. Med. Mycol. 2019, 60, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Biscotto, I.; Barreto, M.M.; Rodrigues, R.S.; Marchiori, E. Vertebral cryptococcosis: An uncommon cause of a paravertebral mass. Rev. Soc. Bras. Med. Trop. 2022, 53, e20190353. [Google Scholar] [CrossRef]
- Johnson, M.M.; Gajurel, K. Disseminated cryptococcosis with cutaneous manifestation. Transpl. Infect. Dis. 2021, 23, e13412. [Google Scholar] [CrossRef]
- Worrall, D.M.; Lerner, D.K.; Naunheim, M.R.; Woo, P. Laryngeal Cryptococcosis: An evolving Rare Clinical Entity. Ann. Otol. Rhinol. Laryngol. 2019, 128, 472–479. [Google Scholar] [CrossRef]
- Espinosa Saltarén., L.; Andrade Pérez, R. Renal cryptococcosis. N. Engl. J. Med. 2020, 383, 2371. [Google Scholar] [CrossRef]
- Setianingrum, F.; Rautemaa-Richardson, R.; Denning, D.W. Pulmonary cryptococcosis: A review of pathobiology and clinical aspects. Med. Mycol. 2019, 57, 133–150. [Google Scholar] [CrossRef]
- Oppenheimer, A.R.; Valente, N.Y.S.; Silva, D.H.M. Cutaneous cryptococcosis simulating pyoderma gangrenosum. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200120. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhao, Y.; Zhou, Y.; Dai, S.; Zhu, N.; Meng, Q.; Fan, S.; Zhao, W.; Yuan, X. Fungal Extracellular Vesicle Proteins with Potential in Biological Interaction. Molecules 2024, 29, 4012. https://doi.org/10.3390/molecules29174012
Xu J, Zhao Y, Zhou Y, Dai S, Zhu N, Meng Q, Fan S, Zhao W, Yuan X. Fungal Extracellular Vesicle Proteins with Potential in Biological Interaction. Molecules. 2024; 29(17):4012. https://doi.org/10.3390/molecules29174012
Chicago/Turabian StyleXu, Jingyan, Yujin Zhao, Yanguang Zhou, Shijie Dai, Na Zhu, Qingling Meng, Sen Fan, Weichun Zhao, and Xiaofeng Yuan. 2024. "Fungal Extracellular Vesicle Proteins with Potential in Biological Interaction" Molecules 29, no. 17: 4012. https://doi.org/10.3390/molecules29174012
APA StyleXu, J., Zhao, Y., Zhou, Y., Dai, S., Zhu, N., Meng, Q., Fan, S., Zhao, W., & Yuan, X. (2024). Fungal Extracellular Vesicle Proteins with Potential in Biological Interaction. Molecules, 29(17), 4012. https://doi.org/10.3390/molecules29174012




