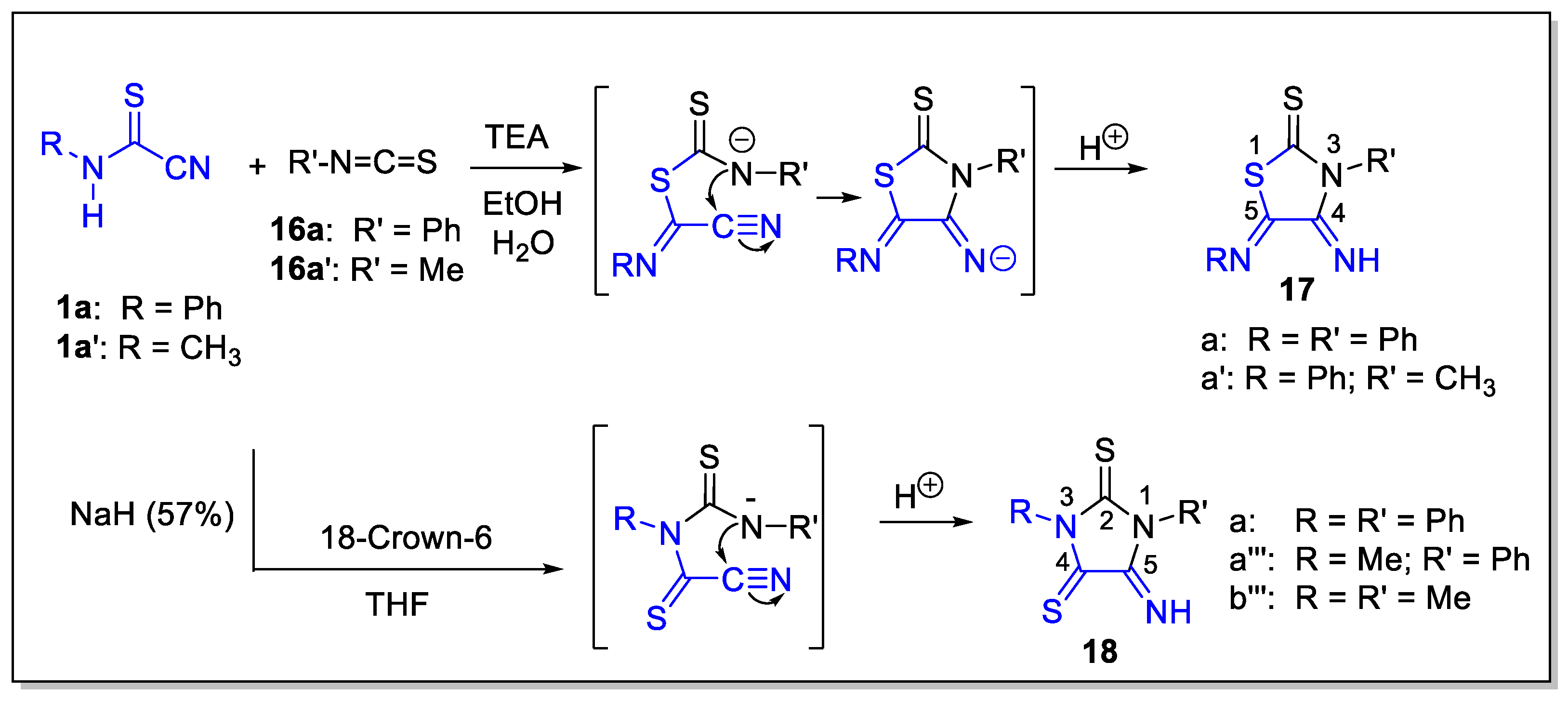
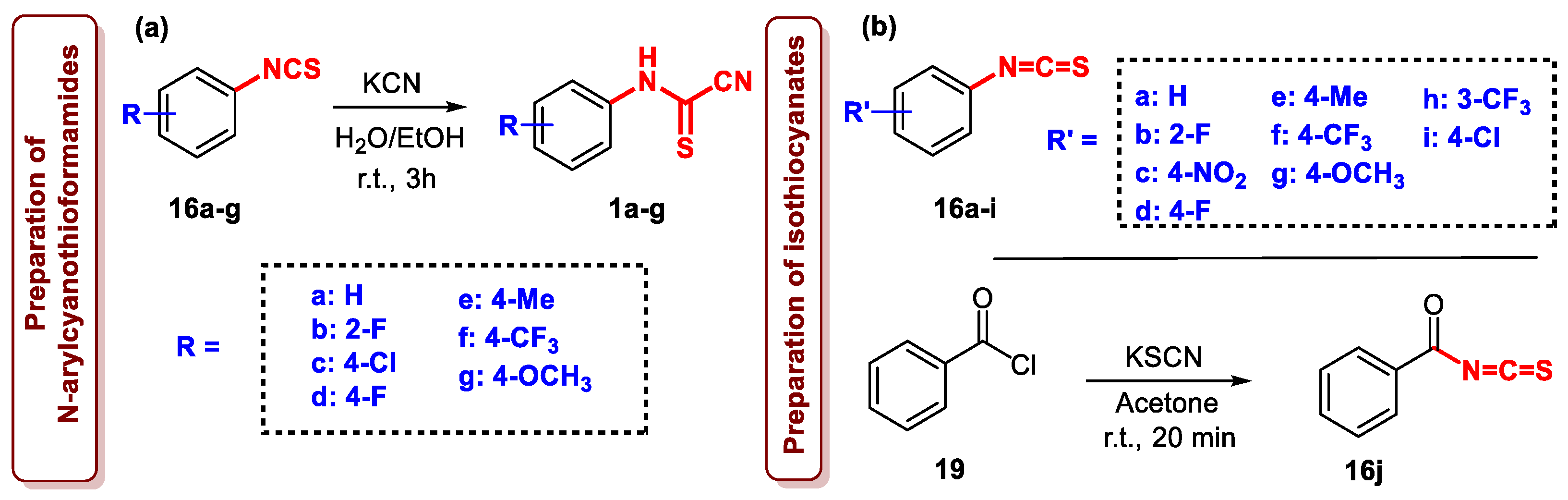
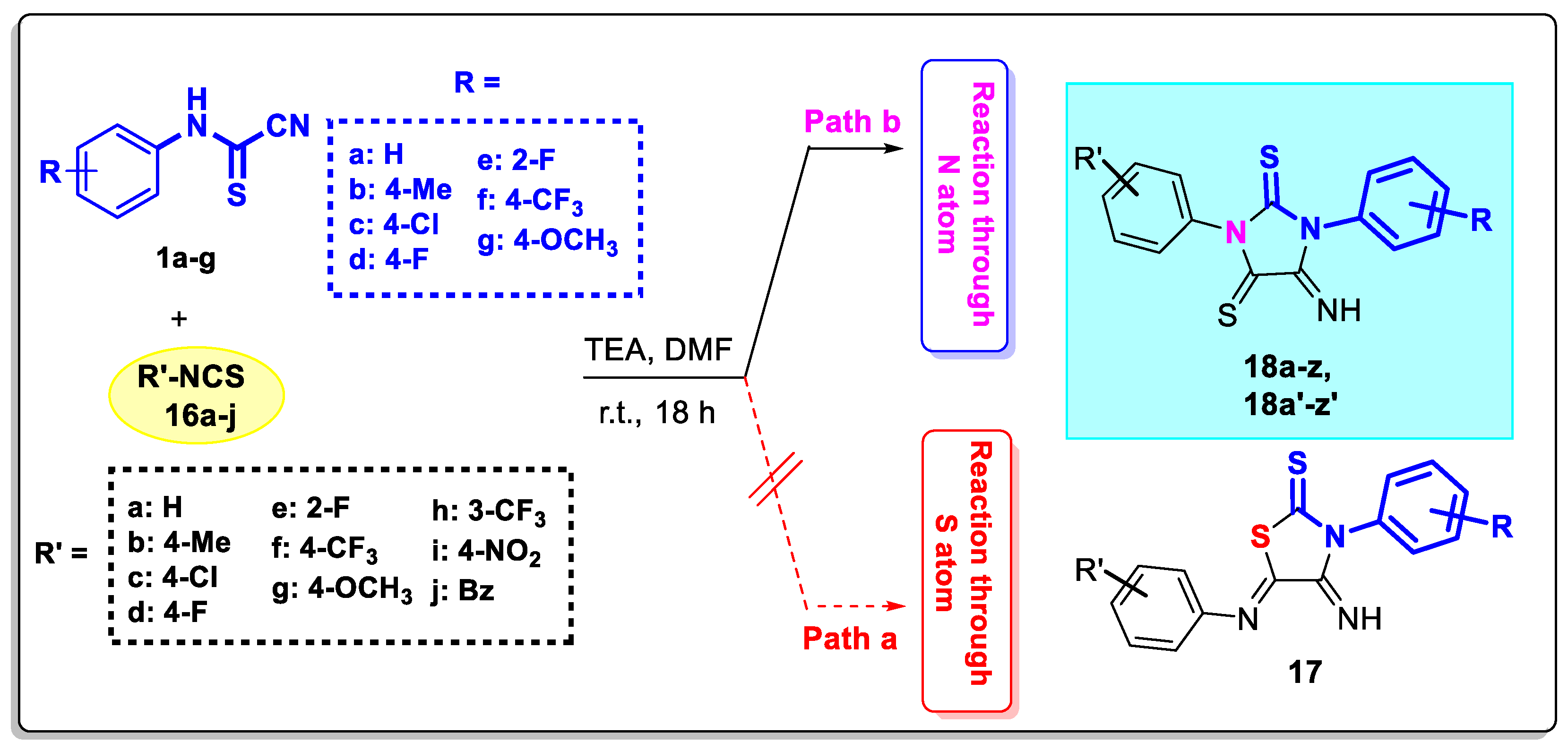
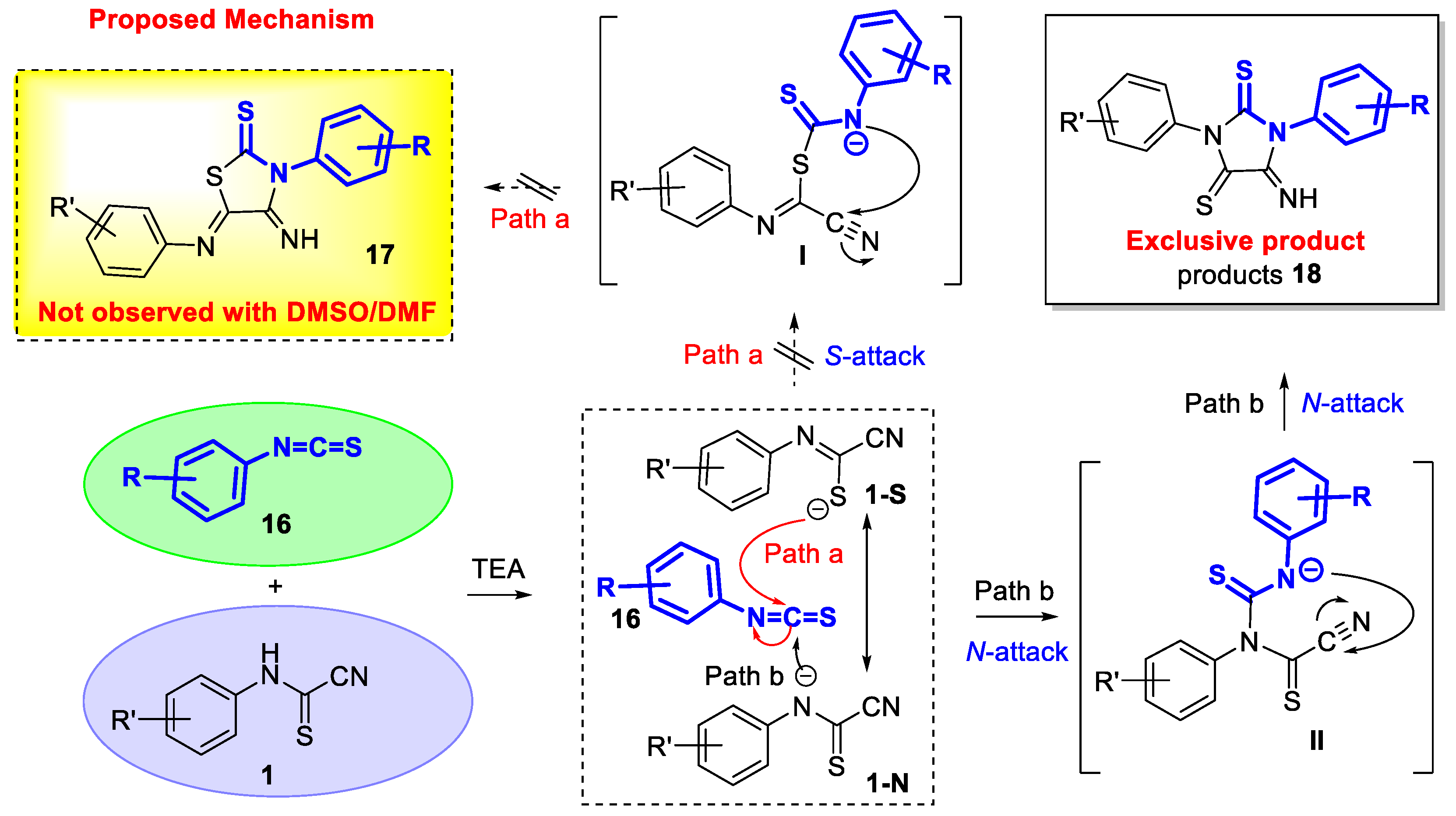
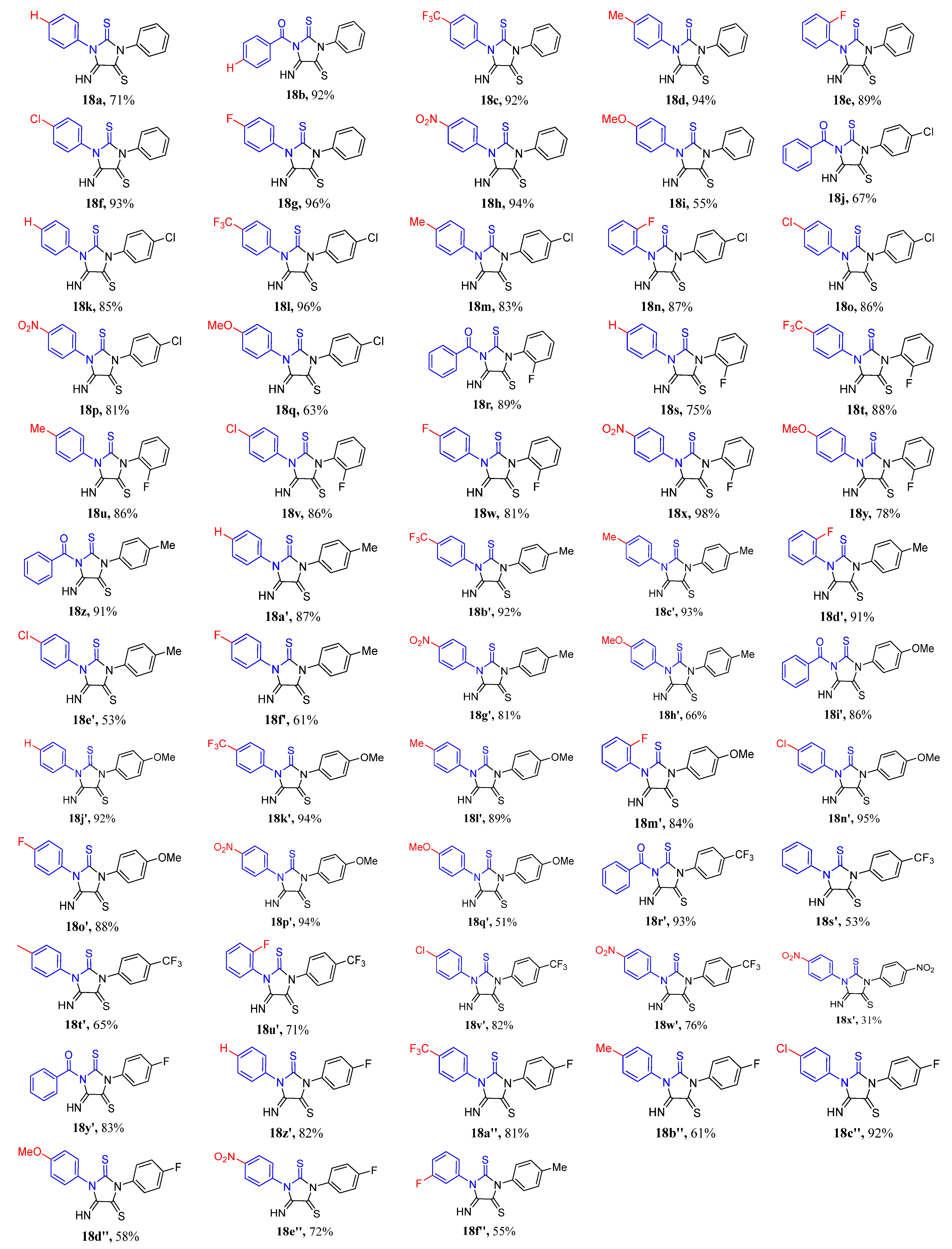
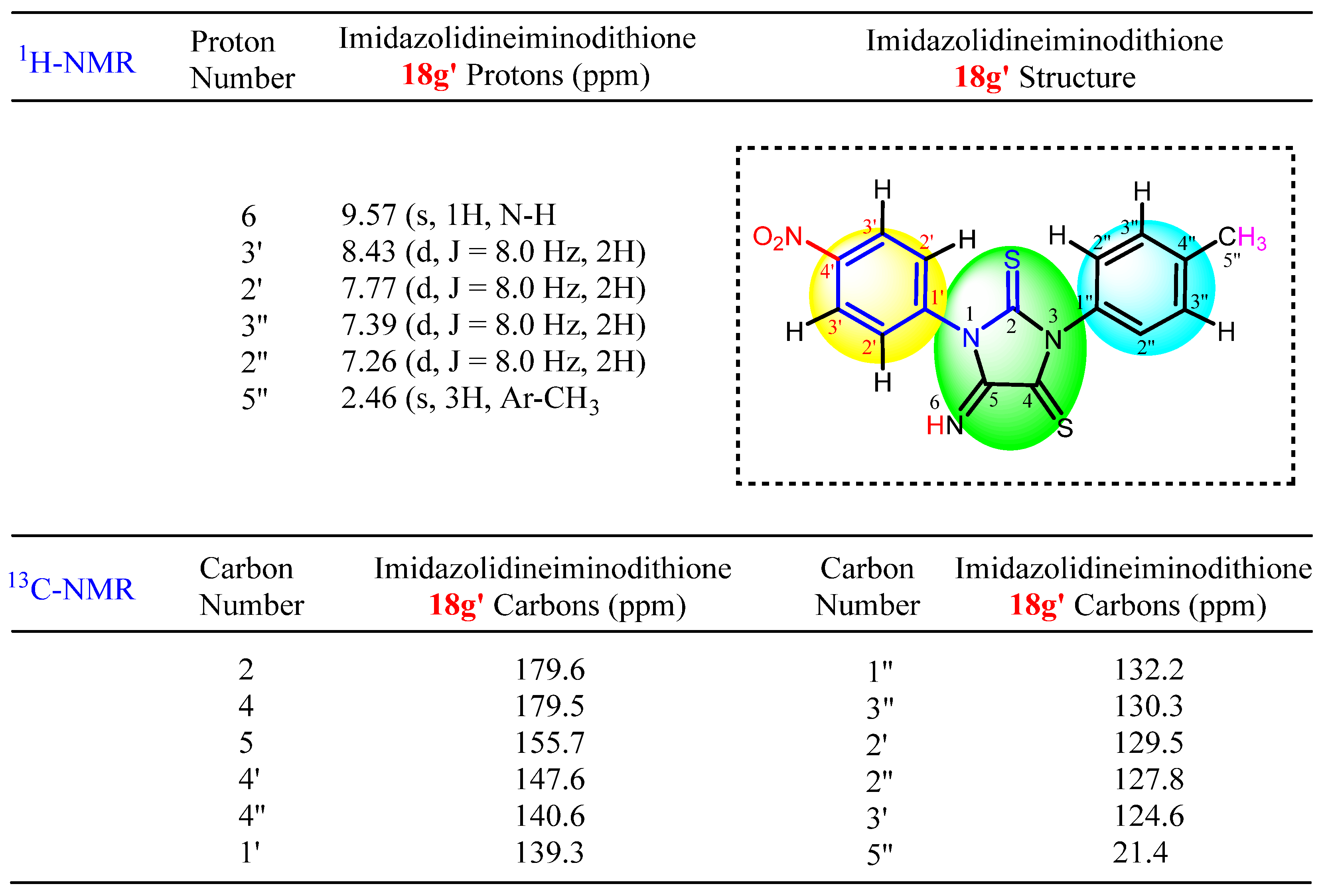
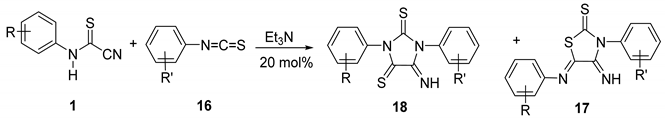
3.2. Experimental Data
5-imino-1,3-diphenylimidazolidine-2,4-dithione (18a).
Brown solid (71% yield); IR (KBr) 3232 (NH), 1652 (C=N), 1593, 1494 (C=S), 1415, 1391, 1287, 1159, 1065 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.50 (s, 1H, NH), 7.62–7.48 (m, 8H, Ar-H), 7.42–7.37 (m, 2H, Ar-H) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.4 (C=S), 180.1 (C=S), 156.5 (C=NH), 135.9 (N(1)-Cq), 134.7 (N(3)-Cq), 129.6 (CH), 129.3 (2xCH), 129.1 (2xCH), 129.0 (CH), 128.8 (2xCH), 128.7 (2xCH) ppm; 1H NMR (DMSO-d6, 400 MHz) δ 9.88 (s, 1H, NH), 7.62–7.42 (m, 10H, Ar-H) ppm; 13C NMR (DMSO-d6, 100 MHz) δ 181.2 (C=S), 181.1 (C=S), 156.3 (C=NH), 135.2 (N(1)-Cq), 133.9 (N(3)-Cq), 130.0 (CH), 129.6 (CH), 129.5 (2xCH), 129.4 (2xCH), 128.3 (2xCH), 128.2 (2xCH) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H12N3S2: 298.0473; found: 298.0465.
(5-imino-3-phenyl-2,4-dithioxoimidazolidin-1-yl)(phenyl)methanone (18b).
Brown solid (92% yield); IR (KBr) 3240 (NH), 1668 (C=O), 1597 (C=N), 1496 (C=S), 1384, 1314, 1292, 1249, 1128, 1052 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.58 (s, 1H, NH), 8.04 (d, J = 8.0 Hz, 2H, Ar-H), 7.73 (t, J = 8.0 Hz, 1H, Ar-H), 7.62–7.53 (m, 5H, Ar-H), 7.37 (d, J = 8.0 Hz, 2H, Ar-H) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.6 (C=S), 178.1 (C=S), 167.0 (C=O), 155.0 (C=NH), 135.6 (CH), 134.3 (O=C-Cq), 131.2 (2xCH), 131.1 (N(3)-Cq), 130.2 (CH), 129.6 (2xCH), 129.2 (2xCH), 128.2 (2xCH) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H12N3OS2: 326.0422; found: 326.0433.
5-imino-3-phenyl-1-(4-(trifluoromethyl)phenyl)imidazolidine-2,4-dithione (18c).
Dark brown solid (92% yield); IR (KBr) 3227 (NH), 1659 (C=N), 1612, 1497 (C=S), 1416, 1386, 1320, 1281, 1185, 1125, 1070 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.55 (s, 1H, NH), 7.84 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.69 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.63–7.53 (m, 3H, Ar-H), 7.42–7.36 (m, 2H, Ar-H) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.9 (C=S), 179.7 (C=S), 156.0 (C=NH), 136.9 (N(1)-Cq), 135.0 (N(3)-Cq), 131.3 (q, J = 33.0 Hz, C-CF3), 130.2 (CH), 129.6 (2xCH), 128.9 (2xCH), 128.2 (2xCH), 126.6 (q, J = 4.0 Hz, 2xCH), 123.5 (q, J = 271.0 Hz, CF3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H11N3F3S2: 366.0347; found: 366.0358.
5-imino-3-phenyl-1-(p-tolyl)imidazolidine-2,4-dithione (18d).
Brown solid (94% yield); IR (KBr) 3209 (NH), 1654 (C=N), 1592, 1515 (C=S), 1497, 1415, 1390, 1275, 1129, 1071 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.48 (s, 1H, NH), 7.61–7.53 (m, 3H, Ar-H), 7.42–7.35 (m, 6H, Ar-H), 2.43 (s, CH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.5 (C=S), 180.1 (C=S), 156.5 (C=NH), 139.7 (CH3-C), 135.2 (N(3)-Cq), 131.2 (N(1)-Cq), 130.1 (2xCH), 129.9 (CH), 129.4 (2xCH), 128.2 (2xCH), 127.8 (2xCH), 21.3 (CH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H14N3S2: 312.0629; found: 312.0635.
1-(2-fluorophenyl)-5-imino-3-phenylimidazolidine-2,4-dithione (18e).
Reddish brown solid (89% yield); IR (KBr) 3247 (NH), 1659 (C=N), 1594, 1509 (C=S), 1497, 1460, 1412, 1390, 1305, 1165, 1066 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 10.09 (s, 1H, NH), 7.69–7.39 (m, 9H, Ar-H) ppm; 13C NMR (DMSO-d6, 100 MHz) δ 180.8 (C=S), 180.5 (C=S), 157.6 (d, J = 250.0 Hz, C-F), 155.1 (C=NH), 135.6 (N(3)-Cq), 131.8 (d, J = 8.0 Hz, CH), 131.3 (2xCH), 129.8 (CH), 129.4 (2xCH), 128.6 (CH), 125.2 (d, J = 4.0 Hz, CH), 121.0 (d, J = 12.0 Hz, N(1)-Cq), 116.6 (d, J = 19.0 Hz, CH) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H11N3FS2: 316.0378; found: 316.0371.
1-(4-chlorophenyl)-5-imino-3-phenylimidazolidine-2,4-dithione (18f).
Light brown solid (93% yield); IR (KBr) 3234 (NH), 1656 (C=N), 1593, 1493 (C=S), 1413, 1382, 1289, 1258, 1159, 1066 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.51 (s, 1H, NH), 7.62–7.55 (m, 3H, Ar-H), 7.53 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.46 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.40–7.36 (m, 2H, Ar-H) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.1 (C=S), 179.9 (C=S), 156.2 (C=NH), 135.4 (C-Cl), 135.1 (N(1)-Cq), 132.3 (N(3)-Cq), 130.1 (CH), 129.7 (2xCHp-chlorophenyl), 129.6 (2xCHp-chlorophenyl), 129.5 (2xCH), 128.2 (2xCH) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H11N3ClS2: 332.0083; found: 332.0074.
1-(4-fluorophenyl)-5-imino-3-phenylimidazolidine-2,4-dithione (18g).
Brown solid (96% yield); IR (KBr) 3233 (NH), 1657 (C=N), 1511 (C=S), 1417, 1385, 1287, 1252, 1067 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.50 (s, 1H, NH), 7.62–7.54 (m, 3H, Ar-Hphenyl), 7.52–7.45 (m, 2H, Ar-Hphenyl), 7.42–7.35 (m, 2H, Ar-Hphenyl), 7.30–7.22 (m, 2H, Ar-Hphenyl) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.3 (C=S), 180.0 (C=S), 162.7 (d, J = 248.0 Hz, C-F), 156.4 (C=NH), 135.1 (N(3)-Cq), 130.2 (CHphenyl), 130.1 (d, J = 8.0 Hz, 2xCHp-fluorophenyl), 129.7 (d, J = 3.0 Hz, N(1)-Cq), 129.5 (2xCHphenyl), 128.2 (2xCHphenyl), 116.6 (d, J = 23.0 Hz, 2xCHp-fluorophenyl) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H11FN3S2: 316.0378; found: 316.0388.
5-imino-1-(4-nitrophenyl)-3-phenylimidazolidine-2,4-dithione (18h).
Dark green solid (94% yield); IR (KBr) 3236 (NH), 1658 (C=N), 1593, 1529 (C=S), 1382, 1349, 1301, 1162, 1066 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.58 (s, 1H, NH), 8.43 (d, J = 8.0 Hz, 2H, Ar-Hp-nitrophenyl), 7.78 (d, J = 8.0 Hz, 2H, Ar-Hp-nitrophenyl), 7.63–7.54 (m, 3H, Ar-Hph), 7.40–7.35 (m, 2H, Ar-Hph) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.5 (C=S), 179.4 (C=S), 155.7 (C=NH), 147.6 (NO2-Cq), 139.2 (N(1)-Cq), 124.9 (N(3)-Cq), 130.2 (CH), 129.6 (2xCHp-nitrophenyl), 129.5 (2xCHph), 128.2 (2xCHph), 124.6 (2xCHp-nitrophenyl) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H11N4O2S2: 343.0323; found: 343.0329.
5-imino-1-(4-methoxyphenyl)-3-phenylimidazolidine-2,4-dithione (18i).
Brown solid (55% yield); IR (KBr) 3215 (NH), 1656 (C=N), 1591, 1514 (C=S), 1391, 1293, 1247, 1126, 1073 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.46 (s, 1H, NH), 7.59–7.53 (m, 3H, Ar-Hph), 7.40 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 7.39–7.36 (m, 2H, Ar-Hph), 7.06 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 3.85 (s, 3H, OCH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.5 (C=S), 180.1 (C=S), 159.9 (OCH3-Cq), 156.5 (C=NH), 135.1 (N(3)-Cq), 129.8 (CHph), 129.3 (2xCHph), 129.2 (2xCHp-methoxyphenyl), 128.1 (2xCHph), 126.1 (N(1)-Cq), 114.4 (2xCHp-methoxyphenyl), 55.3 (OCH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H14N3OS2: 328.0578; found: 328.0583.
(3-(4-chlorophenyl)-5-imino-2,4-dithioxoimidazolidin-1-yl)(phenyl)methanone (18j).
Light brown solid (67% yield); IR (KBr) 3227 (NH), 1726 (C=O), 1662 (C=N), 1599, 1494 (C=S), 1399, 1288, 1185, 1015 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.59 (s, 1H, NH), 8.02 (d, J = 8.0 Hz, 2H, Ar-H), 7.72 (t, J = 8.0 Hz, 1H, Ar-H), 7.58–7.51 (m, 4H, Ar-H), 7.32 (d, J = 8.0 Hz, 2H, Ar-H) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.4 (C=S), 177.7 (C=S), 166.9 (C=O), 154.9 (C=NH), 136.3 (C-Cl), 135.7 (CH), 132.6 (N(3)-Cq), 131.2 (2xCH), 131.1 (N(1)-Cq), 130.0 (2xCH), 129.7 (2xCH), 129.2 (2xCH), ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H11ClN3OS2: 360.0032; found: 360.0025.
3-(4-chlorophenyl)-5-imino-1-phenylimidazolidine-2,4-dithione (18k).
Light brown solid (85% yield); IR (KBr) 3242 (NH), 1651 (C=N), 1595, 1492 (C=S), 1416, 1391, 1289, 1158, 1122, 1067 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.51 (s, 1H, NH), 7.64–7.45 (m, 7H, Ar-H), 7.35 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.0 (C=S), 179.9 (C=S), 156.4 (C=NH), 136.1 (C-Cl), 133.8 (N(1)-Cq), 133.5 (N(3)-Cq), 129.8 (2xCHp-chlorophenyl), 129.7 (2xCHp-chlorophenyl), 129.6 (CH), 129.5 (2xCH), 128.2 (2xCH) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H11N3ClS2: 332.0083; found: 332.0071.
3-(4-chlorophenyl)-5-imino-1-(4-(trifluoromethyl)phenyl)imidazolidine-2,4-dithione (18l).
Dark green solid (96% yield); IR (KBr) 3226 (NH), 1660 (C=N), 1612, 1493 (C=S), 1410, 1383, 1319, 1286, 1185, 1127, 1070 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.56 (s, 1H, NH), 7.84 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.67 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.56 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.34 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.5 (C=S), 179.4 (C=S), 155.9 (C=NH), 136.8 (C-Cl), 136.2 (N(1)-Cq), 133.3 (N(3)-Cq), 131.4 (q, J = 32.0 Hz, C-CF3), 129.9 (2xCHp-chlorophenyl), 129.6 (2xCHp-chlorophenyl), 128.8 (2xCHp-(trifluoromethyl)phenyl), 126.6 (q, J = 4.0 Hz, 2xCH p-(trifluoromethyl)phenyl), 123.5 (q, J = 274.0 Hz, CF3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H10ClN3F3S2: 399.9957; found: 399.9948.
3-(4-chlorophenyl)-5-imino-1-(p-tolyl)imidazolidine-2,4-dithione (18m).
Light brown solid (83% yield); IR (KBr) 3245 (NH), 1654 (C=N), 1513 (C=S), 1491, 1417, 1390, 1287, 1156, 1123, 1064 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.51 (s, 1H, NH), 7.54 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.41–7.36 (m, 4H, Ar-Hp-tolyl), 7.35 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 2.44 (s, 3H, CH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.2 (C=S), 180.0 (C=S), 156.5 (C=NH), 139.8 (Cq-Me), 136.0 (C-Cl), 133.5 (N3-Cq), 131.1 (N1-Cq), 130.2 (2xCHp-tolyl), 129.8 (2xCHp-chlorophenyl), 129.7 (2xCHp-chlorophenyl), 127.8 (2xCHp-tolyl), 21.3 (CH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H13N3ClS2: 346.0239; found: 346.0248.
3-(4-chlorophenyl)-1-(2-fluorophenyl)-5-iminoimidazolidine-2,4-dithione (18n).
Light brown solid (87% yield); IR (KBr) 3243 (NH), 1660 (C=N), 1591, 1508 (C=S), 1492, 1413, 1387, 1301, 1163, 1162, 1065 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.50 (s, 1H, NH), 7.55 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.53–7.46 (m, 2H, Ar-H), 7.36 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.34–7.28 (m, 2H, Ar-H) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.9 (C=S), 179.4 (C=S), 157.8 (d, J = 252.0 Hz, C-F), 155.5 (C=NH), 136.1 (C-Cl), 133.4 (N(3)-Cq), 131.9 (d, J = 8.0 Hz, CH), 130.3 (CH), 129.8 (2xCH), 129.7 (2xCH), 125.0 (d, J = 4.0 Hz, CH), 121.5 (d, J = 12.0 Hz, N(1)-Cq), 117.1 (d, J = 19.0 Hz, CH) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H10N3FClS2: 349.9989; found: 349.9978.
1,3-bis(4-chlorophenyl)-5-iminoimidazolidine-2,4-dithione (18o).
Light brown solid (86% yield); IR (KBr) 3233 (NH), 1652 (C=N), 1493 (C=S), 1395, 1293 1161, 1091 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.52 (s, 1H, NH), 7.55 (d, J = 8.0 Hz, 2H, Ar-H), 7.54 (d, J = 8.0 Hz, 2H, Ar-H), 7.44 (d, J = 8.0 Hz, 2H, Ar-H), 7.33 (d, J = 8.0 Hz, 2H, Ar-H) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.7 (C=S), 179.6 (C=S), 156.1 (C=NH), 136.2 (C-Cl), 135.6 (C-Cl), 133.4 (N-Cq), 132.2 (N-Cq), 129.9 (2xCH), 129.8 (2xCH), 129.7 (2xCH), 129.5 (2xCH) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H10N3Cl2S2: 365.9693; found: 365.9698.
3-(4-chlorophenyl)-5-imino-1-(4-nitrophenyl)imidazolidine-2,4-dithione (18p).
Green solid (81% yield); IR (KBr) 3230 (NH), 1661 (C=N), 1595, 1522 (C=S), 1492, 1407, 1347, 1286, 1089, 856 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 10.12 (s, 1H, NH), 8.45 (d, J = 8.0 Hz, 2H, Ar-Hp-nitrophenyl), 7.84 (d, J = 8.0 Hz, 2H, Ar-Hp-nitrophenyl), 7.68 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.51 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl) ppm; 13C NMR (DMSO-d6, 100 MHz) δ 180.9 (C=S), 180.5 (C=S), 155.6 (C=NH), 147.3 (NO2-Cq), 140.1 (N(1)-Cq), 134.5 (C-Cl), 134.4 (N(3)-Cq), 130.6 (2xCHp-chlorophenyl), 130.4 (2xCHp-nitrophenyl), 129.6 (2xCHp-chlorophenyl), 124.5 (2xCHp-nitrophenyl) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H10ClN4O2S2: 376.9934; found: 376.9945.
3-(4-chlorophenyl)-5-imino-1-(4-methoxyphenyl)imidazolidine-2,4-dithione (18q).
Brown solid (63% yield); IR (KBr) 3217 (NH), 1654 (C=N), 1514 (C=S), 1493 (C=S), 1384, 1284, 1250 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.49 (s, 1H, NH), 7.54 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.39 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 7.34 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.06 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 3.86 (s, 3H, OCH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.3 (C=S), 180.1 (C=S), 160.2 (OCH3-Cq), 156.6 (C=NH), 136.0 (C-Cl), 133.6 (N(3)-Cq), 129.8 (2xCHp-chlorophenyl), 129.7 (2xCHp-chlorophenyl), 129.3 (2xCHp-methoxyphenyl), 126.3 (N(1)-Cq), 114.8 (2xCHp-methoxyphenyl), 55.5 (OCH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H13N3ClOS2: 360.0189; found: 362.0176.
(3-(2-fluorophenyl)-5-imino-2,4-dithioxoimidazolidin-1-yl)(phenyl)methanone (18r).
Light brown solid (89% yield); IR (KBr) 3235 (NH), 1715 (C=O), 1667 (C=N), 1596, 1503 (C=S), 1385, 1310, 1190, 1025 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.60 (s, 1H, NH), 8.02 (d, J = 8.0 Hz, 2H, Ar-H), 7.72 (t, J = 8.0 Hz, 1H, Ar-H), 7.60–7.52 (m, 3H, Ar-H), 7.43–7.27 (m, 3H, Ar-H) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.1 (C=S), 177.1 (C=S), 166.8 (C=O), 157.3 (d, J = 253.0 Hz, C-F), 154.9 (C=NH), 135.6 (CH), 132.5 (d, J = 8.0 Hz, CH), 131.2 (2xCH), 131.0 (Cq), 130.2 (CH), 129.2 (2xCH), 125.0 (d, J = 4.0 Hz, CH), 122.0 (d, J = 13.0 Hz, N(3)-Cq), 117.1 (d, J = 18.0 Hz, CH) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H11N3FOS2: 344.0328; found: 344.0341.
3-(2-fluorophenyl)-5-imino-1-phenylimidazolidine-2,4-dithione (18s).
Red orange solid (75% yield); IR (KBr) 3223 (NH), 1658 (C=N), 1593, 1499 (C=S), 1414, 1389, 1302, 1227, 1127, 1067 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.52 (s, 1H, NH), 7.62–7.49 (m, 6H, Ar-H), 7.46–7.29 (m, 3H, Ar-H) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.7 (C=S), 179.4 (C=S), 157.4 (d, J = 253.0 Hz, C-F), 156.4 (C=NH), 133.9 (N(1)-Cq), 132.2 (d, J = 8.0 Hz, CH), 130.2 (CH), 129.6 (CH), 129.5 (2xCH), 128.2 (2xCH), 124.9 (d, J = 4.0 Hz, CH), 122.8 (d, J = 13.0 Hz, N(3)-Cq), 117.0 (d, J = 18.0 Hz, CH) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H11N3FS2: 316.0378; found: 316.0369.
3-(2-fluorophenyl)-5-imino-1-(4-(trifluoromethyl)phenyl)imidazolidine-2,4-dithione (18t).
Light brown solid (88% yield); IR (KBr) 3230 (NH), 1666 (C=N), 1596, 1505 (C=S), 1416, 1386, 1294, 1165, 1068 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.56 (s, 1H, NH), 7.84 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.70 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.62–7.54 (m, 1H, Ar-H2-fluorophenyl), 7.45–7.29 (m, 3H, Ar-H2-fluorophenyl) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.2 (C=S), 178.9 (C=S), 157.4 (d, J = 253.0 Hz, C-F), 155.9 (C=NH), 136.8 (N(1)-Cq), 132.4 (d, J = 8.0 Hz, CH2-fluorophenyl), 131.4 (q, J = 33.0 Hz, C-CF3), 130.2 (CH2-fluorophenyl), 128.8 (2xCHp-(trifluoromethyl)phenyl), 126.6 (q, J = 4.0 Hz, 2xCH p-(trifluoromethyl)phenyl), 125.0 (d, J = 4.0 Hz, CH2-fluorophenyl), 123.5 (q, J = 271.0 Hz, CF3), 122.7 (d, J = 13.0 Hz, N(3)-Cq), 117.1 (d, J = 19.0 Hz, CH2-fluorophenyl) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H10N3F4S2: 384.0252; found: 384.0257.
3-(2-fluorophenyl)-5-imino-1-(p-tolyl)imidazolidine-2,4-dithione (18u).
Burgundy solid (86% yield); IR (KBr) 3234 (NH), 1655 (C=N), 1593, 1512 (C=S), 1416, 1387, 1300, 1127, 1064 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.49 (s, 1H, NH), 7.59–7.52 (m, 1H, Ar-H), 7.44–7.28 (m, 7H, Ar-H), 2.43 (s, 3H, CH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.8 (C=S), 179.5 (C=S), 157.4 (d, J = 253.0 Hz, C-F), 156.5 (C=NH), 139.8 (Cq-CH3), 132.2 (d, J = 8.0 Hz, CH), 131.1 (N(1)-Cq), 130.2 (2xCH), 130.1 (CH), 127.9 (2xCH), 124.9 (d, J = 4.0 Hz, CH), 122.9 (d, J = 13.0 Hz, N(3)-Cq), 117.0 (d, J = 19.0 Hz, CH), 21.3 (CH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H13N3FS2: 330.0535; found: 330.0543.
1-(4-chlorophenyl)-3-(2-fluorophenyl)-5-iminoimidazolidine-2,4-dithione (18v).
Light brown solid (86% yield); IR (KBr) 3242 (NH), 1655 (C=N), 1594, 1494 (C=S), 1415, 1387, 1302, 1232, 1164, 1128, 1066 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.52 (s, 1H, NH), 7.54 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.60–7.51 (m, 1H, Ar-H), 7.47 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.44–7.27 (m, 3H, Ar-H) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.4 (C=S), 179.1 (C=S), 157.4 (d, J = 253.0 Hz, C-F), 156.1 (C=NH), 135.5 (C-Cl), 132.3 (d, J = 8.0 Hz, CH), 132.2 (N(1)-Cq), 130.2 (CH), 129.7 (2xCH), 129.6 (2xCH), 125.0 (d, J = 3.0 Hz, CH), 122.7 (d, J = 13.0 Hz, N(3)-Cq), 117.1 (d, J = 19.0 Hz, CH) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H10N3FClS2: 349.9989; found: 349.9980.
3-(2-fluorophenyl)-1-(4-fluorophenyl)-5-iminoimidazolidine-2,4-dithione (18w).
Green solid (81% yield); IR (KBr) 3233 (NH), 1657 (C=N), 1511 (C=S) cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.52 (s, 1H, NH), 7.61–7.45 (m, 3H, Ar-H), 7.44–7.21 (m, 5H, Ar-H) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.5 (C=S), 179.4 (C=S), 162.7 (d, J = 249.0 Hz, C-F), 157.4 (d, J = 252.0 Hz, C-F), 156.3 (C=NH), 132.3 (d, J = 8.0 Hz, 2xCHp-fluorophenyl), 130.2 (d, J = 3.0 Hz, CHm-fluorophenyl), 130.1 (CHm-fluorophenyl), 129.7 (d, J = 3.0 Hz, N(1)-Cq), 125.0 (d, J = 8.0 Hz, CHm-fluorophenyl), 122.8 (d, J = 13.0 Hz, N(3)-Cq), 117.0 (d, J = 19.0 Hz, CHm-fluorophenyl), 116.6 (d, J = 23.0 Hz, 2xCHp-fluorophenyl) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H10F2N3S2: 334.0284; found: 334.0293.
3-(2-fluorophenyl)-5-imino-1-(4-nitrophenyl)imidazolidine-2,4-dithione (18x).
Brown solid (98% yield); IR (KBr) 3227 (NH), 1663 (C=N), 1596, 1521 (C=S), 1387, 1344, 1309, 1129, 1068 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 9.60 (s, 1H, NH), 8.43 (d, J = 8.0 Hz, 2H, Ar-Hp-nitrophenyl), 7.78 (d, J = 8.0 Hz, 2H, Ar-Hp-nitrophenyl), 7.61–7.55 (m, 1H, Ar-H2-fluorophenyl), 7.44–7.30 (m, 3H, Ar-H2-fluorophenyl) ppm; 13C NMR (CDCl3, 100 MHz) δ 178.9 (C=S), 178.5 (C=S), 157.4 (d, J = 253.0 Hz, C-F), 155.6 (C=NH), 147.6 (NO2-Cq), 139.1 (N(1)-Cq), 132.5 (d, J = 8.0 Hz, CH), 130.1 (CH), 129.5 (2xCHp-nitrophenyl), 125.1 (d, J = 4.0 Hz, CH), 124.6 (2xCHp-nitrophenyl), 122.5 (d, J = 13.0 Hz, N(3)-Cq), 117.1 (d, J = 18.0 Hz, CH) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H10FN4O2S2: 361.0229; found: 361.0234.
3-(2-fluorophenyl)-5-imino-1-(4-methoxyphenyl)imidazolidine-2,4-dithione (18y).
Brown solid (78% yield); IR (KBr) 3222 (NH), 1659 (C=N), 1605, 1514 (C=S), 1392, 1299, 1247, 1024 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.49 (s, 1H, NH), 7.59–7.52 (m, 1H, Ar-H), 7.44–7.27 (m, 5H, Ar-H), 7.07 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 3.86 (s, 3H, OCH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.8 (C=S), 179.7 (C=S), 160.1 (OCH3-Cq), 157.4 (d, J = 253.0 Hz, C-F), 156.8 (C=NH), 132.2 (d, J = 8.0 Hz, CH), 130.2 (CH), 129.3 (2xCHp-methoxyphenyl), 126.3 (N(1)-Cq), 124.9 (d, J = 4.0 Hz, CH), 122.9 (d, J = 13.0 Hz, N(3)-Cq), 117.0 (d, J = 19.0 Hz, CH), 114.8 (2xCHp-methoxyphenyl), 55.5 (OCH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H13N3FOS2: 346.0484; found: 346.0495.
(5-imino-2,4-dithioxo-3-(p-tolyl)imidazolidin-1-yl)(phenyl)methanone (18z).
Dark brown solid (91% yield); IR (KBr) 3228 (NH), 1728 (C=O), 1660 (C=N), 1599, 1513 (C=S), 1398, 1290, 1187, 1025 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.57 (s, 1H, NH), 8.03 (d, J = 8.0 Hz, 2H, Ar-H), 7.72 (t, J = 8.0 Hz, 1H, Ar-H), 7.56 (t, J = 8.0 Hz, 2H, Ar-H), 7.38 (d, J = 8.0 Hz, 2H, Ar-H), 7.24 (d, J = 8.0 Hz, 2H, Ar-H), 2.45 (s, 3H, CH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.6 (C=S), 178.2 (C=S), 167.0 (C=O), 155.0 (C=NH), 140.5 (CH3-Cq), 135.6 (CH), 131.6 (N(3)-Cq and Bz-Cq), 131.2 (2xCH), 130.3 (2xCHp-tolyl), 129.2 (2xCH), 127.8 (2xCHp-tolyl), 21.4 (CH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C17H14N3OS2: 340.0578; found: 340.0589.
5-imino-1-phenyl-3-(p-tolyl)imidazolidine-2,4-dithione (18a′).
Dark brown solid (87% yield); IR (KBr) 3213 (NH), 1655 (C=N), 1592, 1512 (C=S), 1496, 1414, 1393, 1293, 1124, 1073 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.49 (s, 1H, NH), 7.61–7.54 (m, 2H, Ar-H), 7.54–7.47 (m, 3H, Ar-H), 7.39 (d, J = 8.0 Hz, 2H, Ar-H), 7.28 (d, J = 8.0 Hz, 2H, Ar-H), 2.46 (s, CH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.6 (C=S), 180.3 (C=S), 156.6 (C=NH), 140.4 (CH3-C), 134.1 (N(1)-Cq), 132.7 (N(3)-Cq), 130.3 (2xCH), 129.6 (CH), 129.5 (2xCH), 128.3 (2xCH), 128.0 (2xCH), 21.5 (CH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H14N3S2: 312.0629; found: 312.0637.
5-imino-3-(p-tolyl)-1-(4-(trifluoromethyl)phenyl)imidazolidine-2,4-dithione (18b′).
Light brown solid (92% yield); IR (KBr) 3233 (NH), 1656 (C=N), 1613, 1590, 1513 (C=S), 1417, 1390, 1322, 1277, 1126, 1071 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.53 (s, 1H, NH), 7.84 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.68 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.39 (d, J = 8.0 Hz, 2H, Ar-Hp-tolyl), 7.27 (d, J = 8.0 Hz, 2H, Ar-Hp-tolyl), 2.46 (s, 3H, CH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.0 (C=S), 179.8 (C=S), 156.1 (C=NH), 140.5 (CH3-Cq), 137.0 (N(1)-Cq), 132.4 (N(3)-Cq), 130.5 (q, J = 33.0 Hz, C-CF3), 130.3 (2xCHp-tolyl), 128.9 (2xCHp-(trifluoromethyl)phenyl), 127.9 (2xCHp-tolyl), 126.6 (q, J = 4.0 Hz, 2xCHp-(trifluoromethyl)phenyl), 123.5 (q, J = 271.0 Hz, CF3), 21.4 (CH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C17H13N3F3S2: 380.0503; found: 380.0511.
5-imino-1,3-di-p-tolylimidazolidine-2,4-dithione (18c′).
Brown solid (93% yield); IR (KBr) 3225 (NH), 1650 (C=N), 1512 (C=S), 1413, 1387, 1290, 1274, 1148, 1069 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.48 (s, 1H, NH), 7.38 (d, J = 8.0 Hz, 2H, Ar-H), 7.37–7.36 (m, 4H, Ar-H), 7.27 (d, J = 8.0 Hz, 2H, Ar-H), 2.45 (s, CH3), 2.43 (s, CH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.6 (C=S), 180.2 (C=S), 156.6 (C=NH), 140.2 (CH3-C), 139.7 (CH3-C), 132.6 (N(3)-Cq), 131.3 (N(1)-Cq), 130.2 (2xCH), 130.1 (2xCH), 127.9 (2xCH), 127.8 (2xCH), 21.4 (CH3), 21.3 (CH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C17H16N3S2: 326.0786; found: 326.0775.
1-(2-fluorophenyl)-5-imino-3-(p-tolyl)imidazolidine-2,4-dithione (18d′).
Brown solid (91% yield); IR (KBr) 3241 (NH), 1660 (C=N), 1592, 1506 (C=S), 1412, 1386, 1301, 1162, 1067cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.48 (s, 1H, NH), 7.57–7.48 (m, 2H, Ar-H), 7.39 (d, J = 8.0 Hz, 2H, Ar-Hp-(chlorophenyl), 7.36–7.31 (m, 2H, Ar-H), 7.29 (d, J = 8.0 Hz, 2H, Ar-Hp-(chlorophenyl), 2.46 (s, 3H, CH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.2 (C=S), 179.9 (C=S), 157.9 (d, J = 253.0 Hz, C-F), 155.7 (C=NH), 140.4 (Cq-CH3), 132.5 (N(3)-Cq), 131.8 (d, J = 8.0 Hz, CH), 130.4 (CH), 130.2 (2xCH), 127.9 (2xCH), 125.0 (d, J = 4.0 Hz, CH), 121.7 (d, J = 13.0 Hz, N(1)-Cq), 117.1 (d, J = 19.0 Hz, CH), 21.4 (CH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H13N3FS2: 330.0535; found: 330.0524.
1-(4-chlorophenyl)-5-imino-3-(p-tolyl)imidazolidine-2,4-dithione (18e′).
Brown solid (91% yield); IR (KBr) 3225 (NH), 1654 (C=N), 1586, 1512 (C=S), 1495, 1413, 1383, 1333, 1271, 1123, 1067 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.49 (s, 1H, NH), 7.54 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.45 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.37 (d, J = 8.0 Hz, 2H, Ar-Hp-tolyl), 7.25 (d, J = 8.0 Hz, 2H, Ar-Hp-tolyl), 2.45 (s, 3H, CH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.3 (C=S), 180.0 (C=S), 156.2 (C=NH), 140.4 (Cq-Me), 135.4 (C-Cl), 132.5 (N1-Cq), 132.4 (N3-Cq), 130.3 (2xCHp-tolyl), 129.7 (2xCHp-chlorophenyl), 129.6 (2xCHp-chlorophenyl), 127.9 (2xCHp-tolyl), 21.4 (CH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H13N3ClS2: 346.0239; found: 346.0244.
1-(4-fluorophenyl)-5-imino-3-(p-tolyl)imidazolidine-2,4-dithione (18f′).
Light brown solid (61% yield); IR (KBr) 3234 (NH), 1656 (C=N), 1510 (C=S), 1383, 1295, 1149, 1067 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.50 (s, 1H, NH), 7.52–7.45 (m, 2H, Ar-H), 7.39 (d, J = 8.0 Hz, 2H, Ar-Htolyl), 7.30–7.21 (m, 4H, Ar-H), 2.46 (s, 3H, Ar- CH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.5 (C=S), 180.0 (C=S), 162.6 (d, J = 248.0 Hz, C-F), 156.4 (C=NH), 140.3 (Cq-Me), 132.5 (N(3)-Cq), 130.2 (2xCHp-tolyl), 130.2 (d, J = 9.0 Hz, 2xCHp-fluorophenyl), 129.8 (d, J = 3.0 Hz, N(1)-Cq), 127.8 (2xCHp-tolyl), 116.5 (d, J = 23.0 Hz, 2xCHp-fluorophenyl), 21.4 (CH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H13FN3S2: 330.0535; found: 330.0523.
5-imino-1-(4-nitrophenyl)-3-(p-tolyl)imidazolidine-2,4-dithione (18g′).
Brown solid (81% yield); IR (KBr) 3229 (NH), 1661 (C=N), 1595, 1522 (C=S), 1382, 1348, 1298, 1157, 1072 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.57 (s, 1H, NH), 8.43 (d, J = 8.0 Hz, 2H, Ar-Hp-nitrophenyl), 7.77 (d, J = 8.0 Hz, 2H, Ar-Hp-nitrophenyl), 7.39 (d, J = 8.0 Hz, 2H, Ar-Hp-tolyl), 7.26 (d, J = 8.0 Hz, 2H, Ar-Hp-tolyl), 2.46 (s, 3H, CH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.6 (C=S), 179.5 (C=S), 155.7 (C=NH), 147.6 (NO2-Cq), 140.6 (CH3-Cq), 139.3 (N(1)-Cq), 132.2 (N(3)-Cq), 130.3 (2xCHp-tolyl), 129.5 (2xCHp-nitrophenyl), 127.8 (2xCHp-tolyl), 124.6 (2xCHp-nitrophenyl), 21.4 (CH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H13N4O2S2: 357.0480; found: 357.0471.
5-imino-1-(4-methoxyphenyl)-3-(p-tolyl)imidazolidine-2,4-dithione (18h′).
Brown solid (66% yield); IR (KBr) 3228 (NH), 1655 (C=N), 1608, 1513 (C=S), 1388, 1291, 1250, 1150, 1029 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.46 (s, 1H, NH), 7.40 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 7.38 (d, J = 8.0 Hz, 2H, Ar-Hp-tolyl), 7.27 (d, J = 8.0 Hz, 2H, Ar-Hp-tolyl), 7.06 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 3.86 (s, 3H, OCH3), 2.45 (s, 3H, ArCH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.8 (C=S), 180.2 (C=S), 160.0 (OCH3-Cq), 156.7(C=NH), 140.2 (C-Me), 132.5 (N(3)-Cq), 130.1 (2xCHp-tolyl), 129.3 (2xCHp-methoxyphenyl), 127.8 (2xCHp-tolyl), 126.3 (N(1)-Cq), 114.7 (2xCHp-methoxyphenyl), 55.4 (OCH3), 21.4 (ArCH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C17H16N3OS2: 342.0735; found: 342.0727.
(5-imino-3-(4-methoxyphenyl)-2,4-dithioxoimidazolidin-1-yl)(phenyl)methanone (18i′).
Brown solid (86% yield); IR (KBr) 3225 (NH), 1726 (C=O), 1661 (C=N), 1599, 1514 (C=S), 1402, 1290, 1168, 1027 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.56 (s, 1H, NH), 8.02 (d, J = 8.0 Hz, 2H, Ar-H), 7.72 (t, J = 8.0 Hz, 1H, Ar-H), 7.55 (d, J = 8.0 Hz, 2H, Ar-H), 7.28 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 7.06 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 3.87 (s, 3H, OCH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.9 (C=S), 178.3 (C=S), 167.1 (C=O), 160.5 (OCH3-Cq), 155.0 (C=NH), 135.6 (CH), 131.2 (2xCH), 130.4 (O=C-Cq), 129.3 (2xCHp-methoxyphenyl), 129.2 (2xCH), 126.6 (N(3)-Cq), 114.8 (2xCHp-methoxyphenyl), 55.5 (OCH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C17H14N3O2S2: 356.0527; found: 356.0539.
5-imino-3-(4-methoxyphenyl)-1-phenylimidazolidine-2,4-dithione (18j′).
Brown solid (92% yield); IR (KBr) 3214 (NH), 1653 (C=N), 1606, 1590, 1513 (C=S), 1499, 1393, 1302, 1280, 1248, 1129, 1078 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.49 (s, 1H, NH), 7.61–7.54 (m, 2H, Ar-Hph), 7.53–7.48 (m, 3H, Ar-Hph), 7.31 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 7.07 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 3.87 (s, 3H, OCH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.7 (C=S), 180.3 (C=S), 160.3 (OCH3-Cq), 156.5 (C=NH), 134.0 (N(1)-Cq), 129.5 (CHph), 129.5 (2xCHp-methoxyphenyl), 129.3 (2xCHph), 128.2 (2xCHph), 127.6 (N(3)-Cq), 114.7 (2xCHp-methoxyphenyl), 55.4 (OCH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H14N3OS2: 328.0578; found: 328.0583.
5-imino-3-(4-methoxyphenyl)-1-(4-(trifluoromethyl)phenyl)imidazolidine-2,4-dithione (18k′).
Light brown solid (94% yield); IR (KBr) 3247 (NH), 1659 (C=N), 1612, 1590, 1512 (C=S), 1414, 1387, 1328, 1277, 1255, 1172, 1074 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.53 (s, 1H, NH), 7.83 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.67 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.30 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 7.07 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 3.88 (s, 3H, OCH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.2 (C=S), 179.9 (C=S), 160.5 (OCH3-Cq), 156.1 (C=NH), 137.1 (N(1)-Cq), 131.3 (q, J = 33.0 Hz, C-CF3), 129.3 (2xCHp-methoxyphenyl), 128.9 (2xCHp-(trifluoromethyl)phenyl), 127.4 (N(3)-Cq), 126.6 (q, J = 4.0 Hz, 2xCHp-(trifluoromethyl)phenyl), 123.5 (q, J = 271.0 Hz, CF3), 114.5 (2xCHp-methoxyphenyl), 55.5 (OCH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C17H13N3F3OS2: 396.0452; found: 396.0461.
5-imino-3-(4-methoxyphenyl)-1-(p-tolyl)imidazolidine-2,4-dithione (18l′).
Reddish brown solid (89% yield); IR (KBr) 3235 (NH), 1652 (C=N), 1589, 1510 (C=S), 1390, 1289, 1247, 1156, 1068 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.48 (s, 1H, NH), 7.40–7.35 (m, 4H, Ar-Hp-tolyl), 7.31 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 7.07 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 3.87 (s, 3H, OCH3), 2.43 (s, 3H, ArCH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.9 (C=S), 180.4 (C=S), 160.4 (OCH3-Cq), 156.6 (C=NH), 139.7 (C-Me), 131.4 (N(1)-Cq), 130.2 (2xCHp-tolyl), 129.4 (2xCHp-methoxyphenyl), 127.9 (2xCHp-tolyl), 127.7 (N(3)-Cq), 114.7 (2xCHp-methoxyphenyl), 55.5 (OCH3), 21.4 (ArCH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C17H16N3OS2: 342.0735; found: 342.0723.
1-(2-fluorophenyl)-5-imino-3-(4-methoxyphenyl)imidazolidine-2,4-dithione (18m′).
Reddish brown solid (84% yield); IR (KBr) 3245 (NH), 1656 (C=N), 1607, 1590, 1514 (C=S), 1412, 1389, 1310, 1253, 1161, 1067 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.47 (s, 1H, NH), 7.57–7.47 (m, 2H, Ar-H), 7.38–7.28 (m, 4H, Ar-H), 7.07 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 3.87 (s, 3H, OCH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.3 (C=S), 180.1 (C=S), 160.4 (OCH3-Cq), 157.8 (d, J = 252.0 Hz, C-F), 155.7 (C=NH), 131.8 (d, J = 7.0 Hz, CH), 130.4 (CH), 129.3 (2xCHp-methoxyphenyl), 127.5 (N(3)-Cq), 125.0 (d, J = 4.0 Hz, CH), 121.7 (d, J = 13.0 Hz, N(1)-Cq), 117.0 (d, J = 19.0 Hz, CH), 114.7 (2xCHp-methoxyphenyl), 55.5 (OCH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H13N3FOS2: 346.0484; found: 346.0474.
1-(4-chlorophenyl)-5-imino-3-(4-methoxyphenyl)imidazolidine-2,4-dithione (18n′).
Brown solid (95% yield); IR (KBr) 3205 (NH), 1655 (C=N), 1590, 1513 (C=S), 1496, 1382, 1280, 1252, 1148, 1069 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.49 (s, 1H, NH), 7.53 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.45 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.29 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 7.06 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 3.87 (s, 3H, OCH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.4 (C=S), 180.1 (C=S), 160.4 (OCH3-Cq), 156.2 (C=NH), 135.4 (C-Cl), 132.3 (N(1)-Cq), 129.7 (2xCHp-chlorophenyl), 129.6 (2xCHp-chlorophenyl), 129.3 (2xCHp-methoxyphenyl), 127.5 (N(3)-Cq), 114.7 (2xCHp-methoxyphenyl), 55.5 (OCH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H13N3ClOS2: 360.0189; found: 362.0174.
1-(4-fluorophenyl)-5-imino-3-(4-methoxyphenyl)imidazolidine-2,4-dithione (18o′).
Brown solid (88% yield); IR (KBr) 3234 (NH), 1655 (C=N), 1607, 1514 (C=S), 1387, 1284, 1153, 1068 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.49 (s, 1H, NH), 7.51–7.43 (m, 2H, Ar-H), 7.32–7.22 (m, 4H, Ar-H), 7.07 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 3.87 (s, 3H, OMe) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.7 (C=S), 180.2 (C=S), 162.7 (d, J = 249.0 Hz, C-F), 160.4 (C-O), 156.4 (C=NH), 130.2 (d, J = 9.0 Hz, 2xCHp-fluorophenyl), 129.9 (d, J = 3.0 Hz, N(1)-Cq), 129.3 (2xCHp-methoxyphenyl), 127.6 (N(3)-Cq), 116.6 (d, J = 23.0 Hz, 2xCHp-fluorophenyl), 114.7 (2xCHp-methoxyphenyl), 55.5 (OCH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H13FN3OS2: 346.0484; found: 346.0491.
5-imino-3-(4-methoxyphenyl)-1-(4-nitrophenyl)imidazolidine-2,4-dithione (18p′).
Brown solid (94% yield); IR (KBr) 3237 (NH), 1659 (C=N), 1595, 1523 (C=S), 1391, 1349, 1296, 1157, 1069 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.57 (s, 1H, NH), 8.42 (d, J = 8.0 Hz, 2H, Ar-Hp-nitrophenyl), 7.77 (d, J = 8.0 Hz, 2H, Ar-Hp-nitrophenyl), 7.29 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 7.07 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 3.88 (s, 3H, OCH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.8 (C=S), 179.7 (C=S), 160.5 (OCH3-Cq), 155.7 (C=NH), 147.6 (NO2-Cq), 139.3 (N(1)-Cq), 129.5 (2xCHp-nitrophenyl), 129.3 (2xCHp-methoxyphenyl), 127.3 (N(3)-Cq), 124.6 (2xCHp-nitrophenyl), 114.8 (2xCHp-methoxyphenyl), 55.5 (OCH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H13N4O3S2: 373.0429; found: 373.0434.
5-imino-1,3-bis(4-methoxyphenyl)imidazolidine-2,4-dithione (18q′).
Brown solid (51% yield); IR (KBr) 3225 (NH), 1652 (C=N), 1608, 1513 (C=S), 1391, 1285, 1254, 1151, 1025 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.46 (s, 1H, NH), 7.40 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 7.30 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 7.08–7.03 (m, 4H, Ar-Hp-methoxyphenyl), 3.87 (s, 3H, OCH3), 3.86 (s, 3H, OCH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.9 (C=S), 180.3 (C=S), 160.2 (OCH3-Cq), 159.9 (OCH3-Cq), 156.6 (C=NH), 129.3 (2xCHp-chlorophenyl), 129.2 (2xCHp-chlorophenyl), 127.6 (N(1)-Cq), 126.3 (N(3)-Cq), 114.6 (2xCHp-methoxyphenyl), 114.5 (2xCHp-methoxyphenyl), 55.4 (OCH3), 55.3 (OCH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C17H16N3O2S2: 358.0684; found: 358.0694.
(5-imino-2,4-dithioxo-3-(4-(trifluoromethyl)phenyl)imidazolidin-1-yl)(phenyl)methanone (18r′).
Brown solid (93% yield); IR (KBr) 3272 (NH), 1633 (C=N), 1605 (C=N), 1507, cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.61 (s, 1H, NH), 8.03 (d, J = 8.0 Hz, 2H, Ar-HBz), 7.85 (d, J = 8.0 Hz, 2H, Ar-Htrifluoromethyl)phenyl), 7.74 (t, J = 8.0 Hz, 1H, Ar-HBz), 7.58 (d, J = 8.0 Hz, 2H, Ar-Htrifluoromethyl)phenyl), 7.53 (d, J = 8.0 Hz, 2H, Ar-HBz) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.2 (C=S), 177.4 (C=S), 166.9 (C=O), 154.8 (C=NH), 137.2 (N(3)-Cq), 135.8 (CH), 132.2 (q, J = 33.0 Hz, C-CF3), 131.2 (2xCH), 131.0 (O=C-Cq), 129.3 (2xCH), 129.31 (2xCH), 126.8 (q, J = 3.0 Hz, 2xCH), 123.4 (q, J = 271.0 Hz, CF3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C17H11F3N3OS2: 394.0296; found: 394.0285.
5-imino-1-phenyl-3-(4-(trifluoromethyl)phenyl)imidazolidine-2,4-dithione (18s′).
Brownish oil (53% yield); IR (KBr) 3469 (NH), 1613 (C=N), 1397, 1325, 1170, 1065 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.52 (s, 1H, NH), 7.85 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.61–7.46 (m, 7H, Ar-H) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.8 (C=S), 179.7 (C=S), 156.3 (C=NH), 138.0 (N(3)-Cq), 133.7 (N(1)-Cq), 132.0 (q, J = 32.0 Hz, C-CF3), 129.7 (CH), 129.6 (2xCH), 129.1 (2xCH), 128.2 (2xCH), 126.7 (q, J = 3.0 Hz, 2xCH), 123.4 (q, J = 272.0 Hz, CF3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H11N3F3S2: 366.0347; found: 366.0356.
5-imino-1-(p-tolyl)-3-(4-(trifluoromethyl)phenyl)imidazolidine-2,4-dithione (18t′).
Brownish oil (65% yield); IR (KBr) 3273 (NH), 1658 (C=N), 1613 (C=N), 1514 (C=S), 1393, 1325, 1169, 1055 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.49 (s, 1H, NH), 7.84 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.56 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.39–7.30 (m, 4H, Ar-Hp-tolyl), 2.41 (s, 3H, CH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.9 (C=S), 179.8 (C=S), 156.5 (C=NH), 140.1 (CH3-Cq), 138.0 (N(3)-Cq), 131.9 (q, J = 33.0 Hz, C-CF3), 131.0 (N(1)-Cq), 130.3 (2xCHp-tolyl), 129.1 (2xCHp-(trifluoromethyl)phenyl), 127.9 (2xCHp-tolyl), 126.6 (q, J = 4.0 Hz, 2xCHp-(trifluoromethyl)phenyl), 123.4 (q, J = 271.0 Hz, CF3), 21.3 (CH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C17H13N3F3S2: 380.0503; found: 380.0509.
1-(2-fluorophenyl)-5-imino-3-(4-(trifluoromethyl)phenyl)imidazolidine-2,4-dithione (18u′).
Brownish oil (71% yield); IR (KBr) 3427 (NH), 1678 (C=N), 1614, 1507, 1393, 1326, 1170, 1065 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.52 (s, 1H, NH), 7.85 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.57 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.87–7.79 (m, 1H, Ar-H2-fluorophenyl), 7.38–7.27 (m, 3H, Ar-H2-fluorophenyl) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.8 (C=S), 179.1 (C=S), 157.8 (d, J = 252.0 Hz, C-F), 155.5 (C=NH), 137.7 (N(1)-Cq), 132.0 (d, J = 8.0 Hz, CH2-fluorophenyl), 132.0 (q, J = 33.0 Hz, C-CF3), 130.3 (CH2-fluorophenyl), 129.1 (2xCHp-(trifluoromethyl)phenyl), 126.7 (q, J = 4.0 Hz, 2xCH p-(trifluoromethyl)phenyl), 125.0 (d, J = 4.0 Hz, CH2-fluorophenyl), 123.4 (q, J = 271.0 Hz, CF3), 121.4 (d, J = 13.0 Hz, N(3)-Cq), 117.1 (d, J = 19.0 Hz, CH2-fluorophenyl) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H10N3F4S2: 384.0252; found: 384.0243.
1-(4-chlorophenyl)-5-imino-3-(4-(trifluoromethyl)phenyl)imidazolidine-2,4-dithione (18v′).
Brownish oil (82% yield); IR (KBr) 3437 (NH), 1659 (C=N), 1493, 1387, 1324, 1168, 1065 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.53 (s, 1H, NH), 7.85 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.55 (d, J = 8.0 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.52 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl), 7.44 (d, J = 8.0 Hz, 2H, Ar-Hp-chlorophenyl) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.6 (C=S), 179.4 (C=S), 156.0 (C=NH), 137.9 (C-Cl), 135.7 (N(3)-Cq), 132.0 (N(1)-Cq), 132.0 (q, J = 33.0 Hz, C-CF3), 129.8 (2xCHp-chlorophenyl), 129.6 (2xCHp-chlorophenyl), 129.1 (2xCHp-(trifluoromethyl)phenyl), 126.7 (q, J = 4.0 Hz, 2xCH p-(trifluoromethyl)phenyl), 123.4 (q, J = 271.0 Hz, CF3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H10ClN3F3S2: 399.9957; found: 399.9952.
5-imino-1-(4-nitrophenyl)-3-(4-(trifluoromethyl)phenyl)imidazolidine-2,4-dithione (18w′).
Brown solid (76% yield); IR (KBr) 3232 (NH), 1662 (C=N), 1595, 1521 (C=S), 1388, 1326, 1171, 1065 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.63 (s, 1H, NH), 8.43 (d, J = 8.0 Hz, 2H, Ar-Hp-nitrophenyl), 7.86 (d, J = 8.0 Hz, 2H, Ar-Htrifluoromethyl)phenyl), 7.77 (d, J = 8.0 Hz, 2H, Ar-Hp-nitrophenyl), 7.55 (d, J = 8.0 Hz, 2H, Ar-Htrifluoromethyl)phenyl) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.1 (C=S), 178.9 (C=S), 155.5 (C=NH), 147.7 (NO2-Cq), 139.0 (N(1)-Cq), 137.8 (N(3)-Cq), 132.2 (q, J = 33.0 Hz, C-CF3), 129.5 (2xCHp-nitrophenyl), 129.1 (2xCH), 126.8 (q, J = 4.0 Hz, 2xCH), 124.6 (2xCHp-nitrophenyl), 123.4 (q, J = 272.0 Hz, CF3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H10F3N4O2S2: 411.0197; found: 411.0188.
5-imino-1,3-bis(4-nitrophenyl)imidazolidine-2,4-dithione (18x′).
Dark green solid (31% yield); IR (KBr) 3435 (NH), 1660 (C=N), 1594, 1522 (C=S), 1495, 1387, 1348, 1287 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 10.22 (s, 1H, NH), 8.47 (d, J = 8.0 Hz, 2H, Ar-H), 8.46 (d, J = 8.0 Hz, 2H, Ar-H), 7.85 (d, J = 8.0 Hz, 2H, Ar-H), 7.81 (d, J = 8.0 Hz, 2H, Ar-H), ppm; 13C NMR (DMSO-d6, 100 MHz) δ 180.7 (C=S), 180.1 (C=S), 155.4 (C=NH), 148.0 (NO2-Cq), 147.3 (NO2-Cq), 141.0 (N(1)-Cq), 139.9 (N(3)-Cq), 130.5 (2xCH), 130.3 (2xCH), 129.6 (2xCH), 124.7 (2xCH), 124.4 (2xCH) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H10N5O4S2: 388.0174; found: 388.0163.
(3-(4-fluorophenyl)-5-imino-2,4-dithioxoimidazolidin-1-yl)(phenyl)methanone (18y′).
Burgundy solid (83% yield); IR (KBr) 3226 (NH), 1726 (C=O), 1661 (C=N), 1600 (C=N), 1512 (C=S), 1400, 1296, 1239, 1050 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.58 (s, 1H, NH), 8.02 (d, J = 8.0 Hz, 2H, Ar-H), 7.73 (t, J = 8.0 Hz, 1H, Ar-H), 7.56 (t, J = 8.0 Hz, 2H, Ar-H), 7.38–7.33 (m, 2H, Ar-Hp-fluorophenyl), 7.30–7.23 (m, 2H, Ar-Hp-fluorophenyl) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.6 (C=S), 177.9 (C=S), 167.0 (C=O), 163.1 (d, J = 250.0 Hz, C-F), 154.9 (C=NH), 135.7 (CH), 131.2 (2xCH), 131.1 (Ph-Cq), 130.3 (d, J = 9.0 Hz, 2xCHp-fluorophenyl), 130.0 (d, J = 3.0 Hz, N(3)-Cq), 129.2 (2xCH), 116.8 (d, J = 23.0 Hz, 2xCHp-fluorophenyl) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H11FN3OS2: 344.0328; found: 344.0338.
3-(4-fluorophenyl)-5-imino-1-phenylimidazolidine-2,4-dithione (18z′).
Brown solid (82% yield); IR (KBr) 3243 (NH), 1654 (C=N), 1595, 1509 (C=S), 1417, 1394, 1286, 1230, 1159, 1068 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.52 (s, 1H, NH), 7.61–7.47 (m, 5H, Ar-Hphenyl), 7.41–7.36 (m, 2H, Ar-Hp-fluorophenyl), 7.29–7.22 (m, 2H, Ar-Hp-fluorophenyl) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.3 (C=S), 180.1 (C=S), 163.0 (d, J = 249.0 Hz, C-F), 156.4 (C=NH), 133.9 (N(1)-Cq), 131.0 (d, J = 4.0 Hz, N(3)-Cq), 130.3 (d, J = 9.0 Hz, 2xCHp-fluorophenyl), 129.6 (CH), 129.5 (2xCH), 128.2 (2xCH), 116.7 (d, J = 23.0 Hz, 2xCHp-fluorophenyl) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H11FN3OS2: 316.0378; found: 316.0393.
3-(4-fluorophenyl)-5-imino-1-(4-(trifluoromethyl)phenyl)imidazolidine-2,4-dithione (18a″)
Brown solid (81% yield); IR (KBr) 3225 (NH), 1661 (C=N), 1611, 1511 (C=S), 1416, 1325, 1234, 1172, 1064 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.55 (s, 1H, NH), 7.84 (d, J = 8.0 Hz, 2H, Ar-Htrifluoromethyl)phenyl), 7.67 (d, J = 8.0 Hz, 2H, Ar-Htrifluoromethyl)phenyl), 7.40–7.35 (m, 2H, Ar-Hp-fluorophenyl), 7.30–7.24 (m, 2H, Ar-Hp-fluorophenyl) ppm; 13C NMR (CDCl3, 100 MHz) δ 179.8 (C=S), 179.9 (C=S), 163.1 (d, J = 250.0 Hz, C-F), 155.9 (C=NH), 136.9 (N(1)-Cq), 131.4 (q, J = 33.0 Hz, C-CF3), 130.8 (d, J = 3.0 Hz, N(3)-Cq), 130.3 (d, J = 9.0 Hz, 2xCHp-fluorophenyl), 128.8 (2xCHtrifluoromethyl)phenyl), 126.6 (q, J = 3.0 Hz, 2xCHtrifluoromethyl)phenyl), 123.5 (q, J = 271.0 Hz, CF3), 116.8 (d, J = 23.0 Hz, 2xCHp-fluorophenyl) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H10F4N3S2: 384.0252; found: 384.0261.
3-(4-fluorophenyl)-5-imino-1-(p-tolyl)imidazolidine-2,4-dithione (18b″)
Brown solid (61% yield); IR (KBr) 3220 (NH), 1654 (C=N), 1598, 1510 (C=S), 1390, 1300, 1154, 1067 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.50 (s, 1H, NH), 7.42–7.34 (m, 6H, Ar-H), 7.29–7.22 (m, 2H, Ar-Hp-fluorophenyl) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.4 (C=S), 180.2 (C=S), 162.9 (d, J = 249.0 Hz, C-F), 156.5 (C=NH), 139.8 (Cq-Me), 131.2 (N(1)-Cq), 131.0 (d, J = 4.0 Hz, N(3)-Cq), 130.3 (d, J = 9.0 Hz, 2xCHp-fluorophenyl), 130.2 (2xCHp-tolyl), 127.8 (2xCHp-tolyl), 116.7 (d, J = 23.0 Hz, 2xCHp-fluorophenyl), 21.3 (CH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H13FN3S2: 330.0535; found: 330.0529.
1-(4-chlorophenyl)-3-(4-fluorophenyl)-5-iminoimidazolidine-2,4-dithione (18c″)
Brown solid (92% yield); IR (KBr) 3230 (NH), 1655 (C=N), 1598, 1510 (C=S), 1494 (C=S), 1389, 1303, 1156, 1066 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.52 (s, 1H, NH), 7.54 (d, J = 8.0 Hz, 2xCHp-chlorophenyl), 7.45 (d, J = 8.0 Hz, 2xCHp-chlorophenyl), 7.39–7.33 (m, 2H, Ar-Hp-fluorophenyl), 7.29–7.22 (m, 2H, Ar-Hp-fluorophenyl) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.0 (C=S), 179.9 (C=S), 163.0 (d, J = 250.0 Hz, C-F), 156.1 (C=NH), 135.5 (C-Cl), 132.2 (N(1)-Cq), 130.9 (d, J = 4.0 Hz, N(3)-Cq), 130.3 (d, J = 9.0 Hz, 2xCHp-fluorophenyl), 129.8 (2xCHp-chlorophenyl), 129.6 (2xCHp-chlorophenyl), 116.7 (d, J = 23.0 Hz, 2xCHp-fluorophenyl) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H10FClN3S2: 349.9989; found: 349.9978.
3-(4-fluorophenyl)-5-imino-1-(4-methoxyphenyl)imidazolidine-2,4-dithione (18d″)
Red brown solid (58% yield); IR (KBr) 3229 (NH), 1654 (C=N), 1607, 15101 (C=S), 1388, 1303, 1155, 1068 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.49 (s, 1H, NH), 7.421–7.35 (m, 4H, Ar-H), 7.28–7.22 (m, 2H, Ar-Hp-fluorophenyl), 7.06 (d, J = 8.0 Hz, 2H, Ar-Hp-methoxyphenyl), 3.86 (s, 3H, OMe) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.6 (C=S), 180.3 (C=S), 162.9 (d, J = 250.0 Hz, C-F), 160.1 (C-O), 156.6 (C=NH), 131.0 (d, J = 4.0 Hz, N(3)-Cq), 130.3 (d, J = 9.0 Hz, 2xCHp-fluorophenyl), 129.3 (2xCHp-methoxyphenyl), 126.3 (N(1)-Cq), 116.7 (d, J = 23.0 Hz, 2xCHp-fluorophenyl), 114.8 (2xCHp-methoxyphenyl), 55.5 (CH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H13FN3OS2: 346.0484; found: 346.0495.
3-(4-fluorophenyl)-5-imino-1-(4-nitrophenyl)imidazolidine-2,4-dithione (18e″)
Green solid (72% yield); IR (KBr) 3231 (NH), 1660 (C=N), 1596, 1522 (C=S), 1418, 1386, 1285, 1073 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.60 (s, 1H, NH), 8.43 (d, J = 8.0 Hz, 2H, Ar-Hp-nitrophenyl), 7.77 (d, J = 8.0 Hz, 2H, Ar-Hp-nitrophenyl), 7.40–7.34 (m, 2H, Ar-Hp-fluorophenyl), 7.31–7.24 (m, 2H, Ar-Hp-fluorophenyl), ppm; 13C NMR (CDCl3, 100 MHz) δ 179.4 (C=S), 179.3 (C=S), 163.1 (d, J = 250.0 Hz, C-F), 155.6 (C=NH), 147.6 (NO2-Cq), 139.2 (N(1)-Cq), 130.6 (d, J = 3.0 Hz, N(3)-Cq), 130.2 (d, J = 9.0 Hz, 2xCHp-fluorophenyl), 129.5 (2xCHp-nitrophenyl), 124.6 (2xCHp-nitrophenyl), 116.8 (d, J = 23.0 Hz, 2xCHp-fluorophenyl) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C15H10FN4O2S2: 361.0229; found: 361.0238.
1-(3-fluorophenyl)-5-imino-3-(p-tolyl)imidazolidine-2,4-dithione (18e″)
Brown solid (55% yield); IR (KBr) 3232 (NH), 1657 (C=N), 1599, 1511 (C=S), 1495 (C=S) cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.51 (s, 1H, NH), 7.57–7.51 (m, 1H, Ar-H), 7.41–7.18 (m, 7H, Ar-Hm-fluorophenyl), 2.46 (s, 3H, Ar- CH3) ppm; 13C NMR (CDCl3, 100 MHz) δ 180.2 (C=S), 179.9 (C=S), 162.7 (d, J = 248.0 Hz, C-F), 156.2 (C=NH), 140.4 (Cq-Me), 135.1 (d, J = 10.0 Hz, N(1)-Cq), 132.5 (N(3)-Cq), 130.6 (d, J = 9.0 Hz, CHm-fluorophenyl), 130.3 (2xCHp-tolyl), 127.9 (2xCHp-tolyl), 124.2 (d, J = 9.0 Hz, CHm-fluorophenyl), 116.7 (d, J = 21.0 Hz, CHm-fluorophenyl), 116.1 (d, J = 24.0 Hz, CHm-fluorophenyl), 21.4 (CH3) ppm; HRMS (ESI+): m/z [M + H]+ calcd for C16H13FN3S2: 330.0535; found: 330.0529.