Highly Efficient Electrospun Silver Decorated Graphene Oxide Nanocomposites on Poly(vinylidene fluoride) (PVDF@GO-Ag) Hybrid Membrane for Reduction of 4-Nitrophenol
Abstract
1. Introduction
2. Results and Discussions
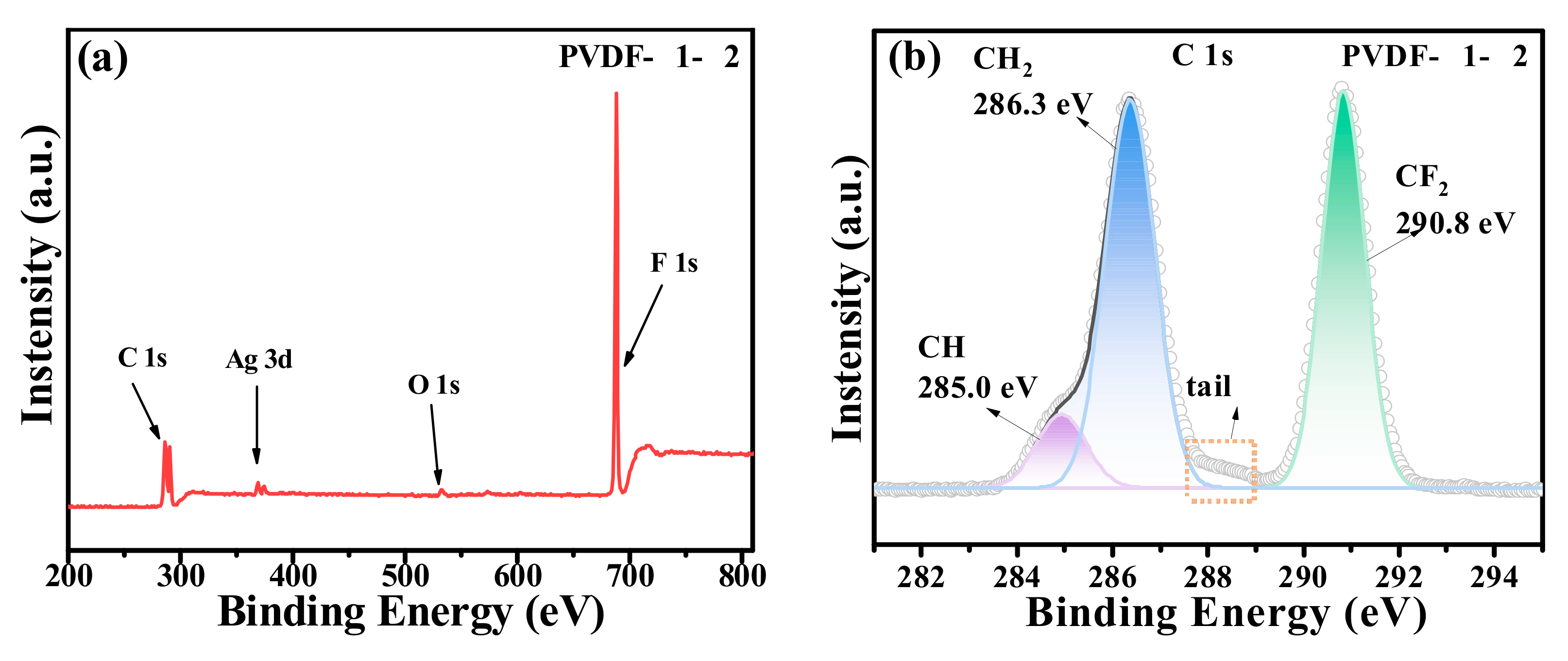
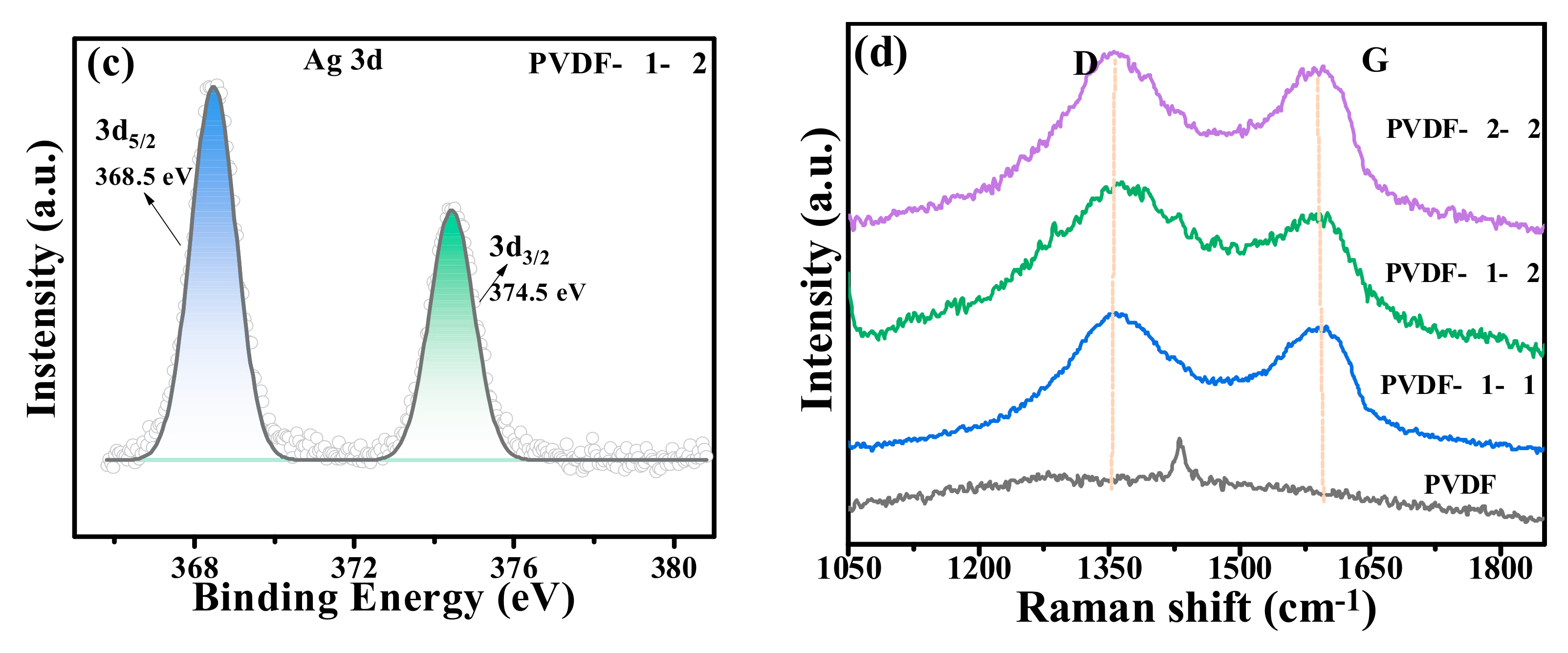
2.1. Characterization of PVDF@GO-Ag Hybrid Membranes
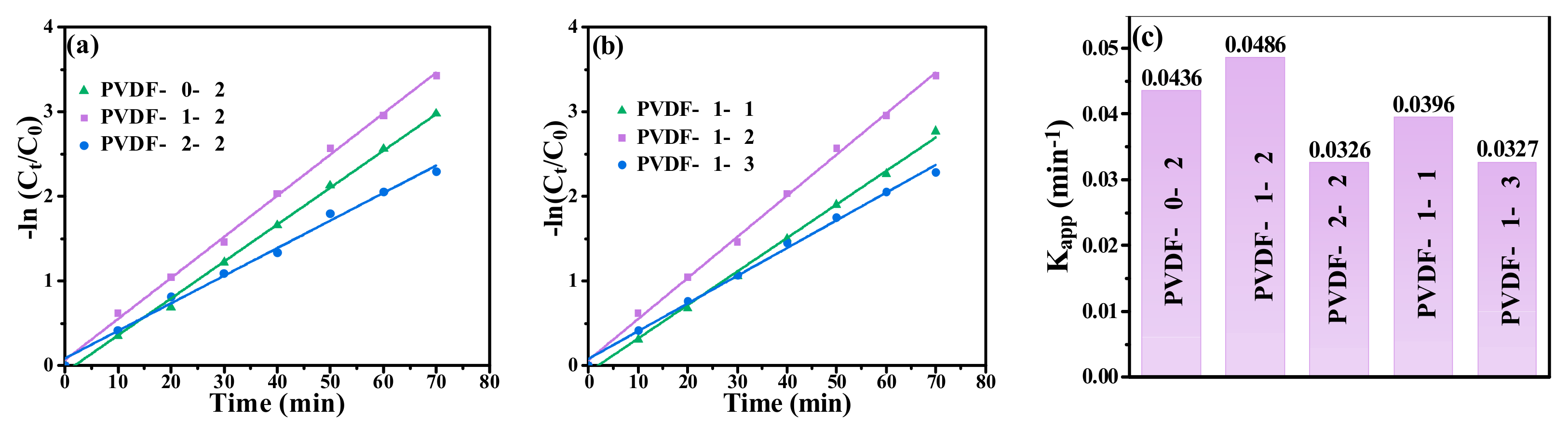
2.2. Catalytic Performance of PVDF@GO-Ag Hybrid Membranes
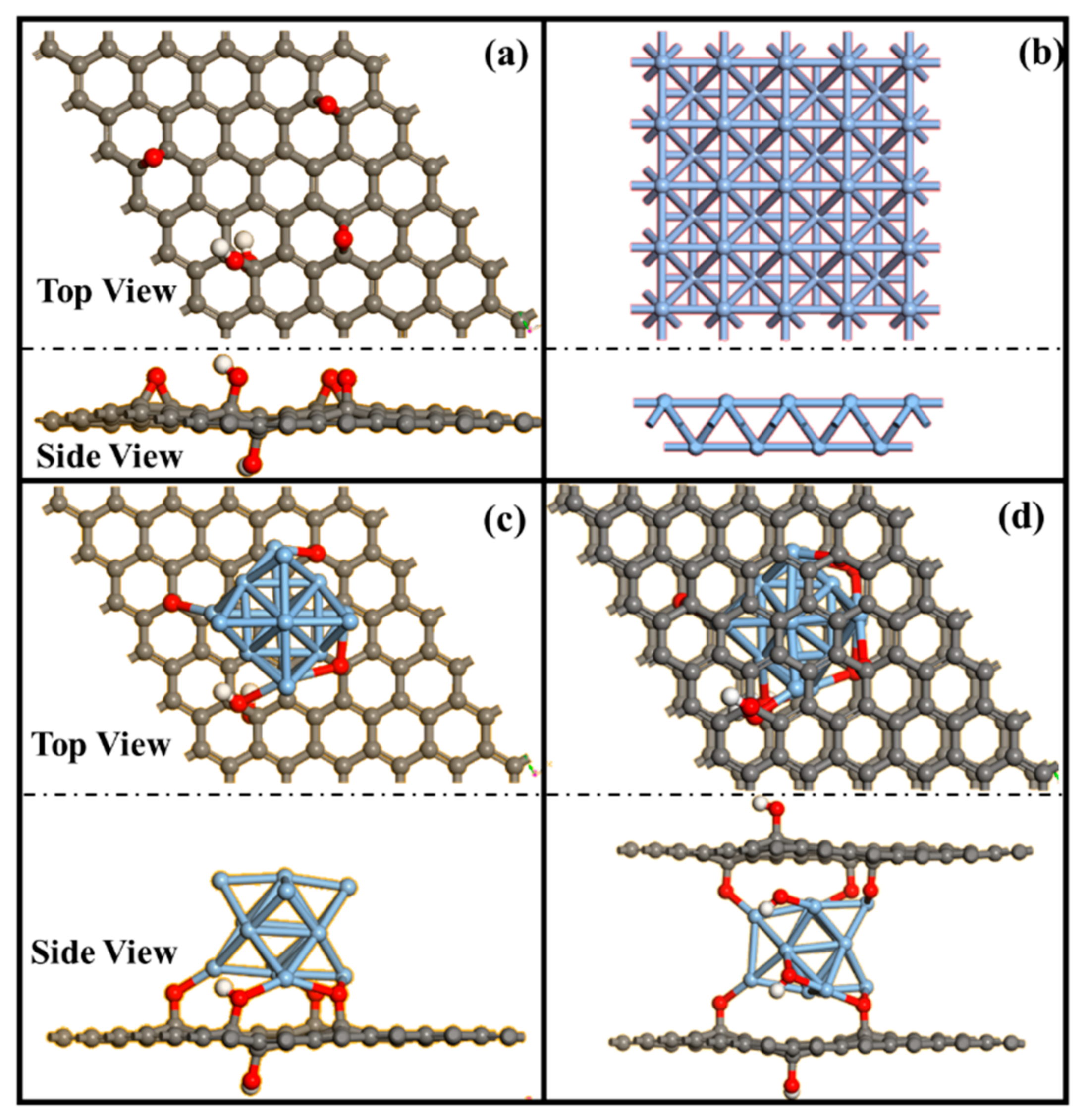
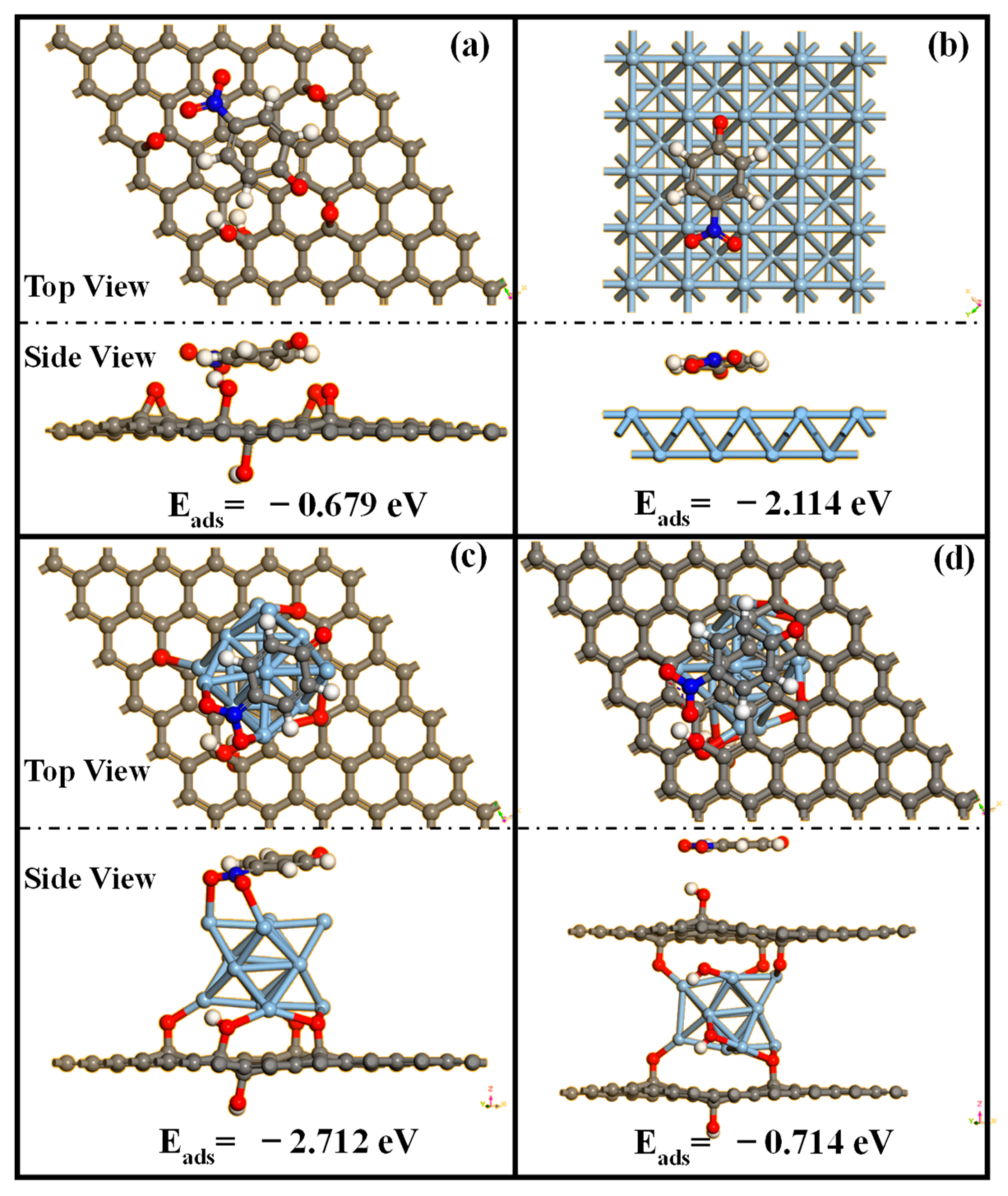
2.3. Investigation of the Effects of Ag and GO Ratios on Catalytic Performance via DFT
2.4. Possible Catalytic Mechanism
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of Graphene Oxide (GO)
3.3. Preparation of Ag-GO Nanocomposites
3.4. Electrospun and Synthesis Mechanism of PVDF@GO-Ag Hybrid Membranes
3.5. Characterizations of PVDF-Based Membranes
3.6. Catalytic Performance Test
3.7. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, A.; Lin, C.; Zhou, H.; Jin, W.; Hu, Y.; Li, D.; Li, Q. Catalytic transformation of 4-nitrophenol into 4-aminophenol over ZnO nanowire array-decorated Cu nanoparticles. Green Chem. Eng. 2024, 5, 205–212. [Google Scholar] [CrossRef]
- Hossain, M.A.; Lee, G.; Jhung, S.H. Covalent-organic framework derived nitrogen-enriched carbon: A remarkable adsorbent to remove 4-nitrophenol from water. Sep. Purif. Technol. 2024, 328, 125068. [Google Scholar] [CrossRef]
- Zhou, Q.; Qin, L.; Yin, Z.; Jiang, H. Facile microwave assisted one-pot solid-state construction of Co-Fe spinel oxide/porous biochar for highly efficient 4-nitrophenol degradation: Effect of chemical blowing and surface vulcanization. Sep. Purif. Technol. 2024, 328, 125033. [Google Scholar] [CrossRef]
- Jacob, B.; Mohan, M.; Dhanyaprabha, K.C.; Thomas, H. Electron transfer enhanced catalytic activity of nitrogen doped reduced graphene oxide supported CuCo2O4 towards the fast reduction of 4-nitrophenol in water. Environ. Res. 2024, 251, 118567. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, O.M.L.; Basheer, A.A.; Khattab, R.A.; Ali, I. Health and environmental effects of persistent organic pollutants. J. Mol. Liq. 2018, 263, 442–453. [Google Scholar] [CrossRef]
- Hu, L.; Liu, X.; Guo, A.; Wu, J.; Wang, Y.; Long, Y.; Fan, G. Cobalt with porous carbon architecture: Towards of 4-nitrophenol degradation and reduction. Sep. Purif. Technol. 2022, 288, 120595. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Y.; Du, F.; Wu, Y.; Zhang, Q.; Jiang, C. Silver nanoparticles/polydopamine coated polyvinyl alcohol sponge as an effective and recyclable catalyst for reduction of 4-nitrophenol. Mater. Chem. Phys. 2019, 225, 42–49. [Google Scholar] [CrossRef]
- Ge, C.; Shu, F.; Guo, X.; Jiao, H.; Shi, D.; Du, C.; Guo, X.; Zhang, Q.; Wu, W.; Jin, Y.; et al. Comparison of Pd nanoparticle-decorated softwood and hardwood activated carbon in catalytic reduction of high-concentrated industrial 4-nitrophenol. Sep. Purif. Technol. 2024, 343, 127149. [Google Scholar] [CrossRef]
- Lv, H.; Gong, Y.; Chen, Z.; Yu, K.; Zhou, Z.; Wei, H.; Zhao, Y.; Bao, M.; Wang, Y. Iron-doped copper-cobalt sulfide nano-box derived from ZIF-67 as a high-efficiency heterogeneous catalyst for hydrogenation of 4-nitrophenol. J. Catal. 2024, 431, 115411. [Google Scholar] [CrossRef]
- Mahto, B.; Barhoi, A.; Ali, H.; Hussain, S. Deciphering the mechanistic insights of 4-nitrophenol reduction catalyzed by a 1D–2D Bi2S3 nanostructured catalyst. Nanoscale 2024, 16, 8060–8073. [Google Scholar] [CrossRef]
- Shi, J.; Ye, R.; Yue, Y.; Liu, J. Insights into interfacial catalysis for aqueous reduction of 4-nitrophenol catalyzed by Ni metal nanoparticles encapsulated within biomass-derived N-doped carbon matrix. Chem. Eng. Sci. 2024, 294, 120122. [Google Scholar] [CrossRef]
- Teimouri, M.; Khosravi-Nejad, F.; Attar, F.; Saboury, A.A.; Kostova, I.; Benelli, G.; Falahati, M. Gold nanoparticles fabrication by plant extracts: Synthesis, characterization, degradation of 4-nitrophenol from industrial wastewater, and insecticidal activity—A review. J. Cleaner Prod. 2018, 184, 740–753. [Google Scholar] [CrossRef]
- Zhai, Z.W.S.; Zhai, B.; Xiao, Z.; An, Q. Preparation and catalytic properties of nano-Au catalytic materials based on the reduction of 4-nitrophenol. Prog. Chem. 2014, 26, 234–247. [Google Scholar] [CrossRef]
- Nemanashi, M.; Meijboom, R. Synthesis and characterization of Cu, Ag and Au dendrimer-encapsulated nanoparticles and their application in the reduction of 4-nitrophenol to 4-aminophenol. J. Colloid Interface Sci. 2013, 389, 260–267. [Google Scholar] [CrossRef]
- Ehsani, A.; Nejatbakhsh, S.; Soodmand, A.M.; Farshchi, M.E.; Aghdasinia, H. High-performance catalytic reduction of 4-nitrophenol to 4-aminophenol using M-BDC (M = Ag, Co, Cr, Mn, and Zr) metal-organic frameworks. Environ. Res. 2023, 227, 115736. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Das, N.C. Advances on catalytic reduction of 4-nitrophenol by nanostructured materials as benchmark reaction. Int. Nano Lett. 2022, 12, 223–242. [Google Scholar] [CrossRef]
- Jacob, J.A.E.; Antony, R.; Ivan Jebakumar, D.S. Synergistic effect of silver nanoparticle-embedded calcite-rich biochar derived from tamarindus indica bark on 4-nitrophenol reduction. Chemosphere 2024, 349, 140765. [Google Scholar] [CrossRef] [PubMed]
- Langer, N.; Kedem, O. Effect of ligands and their removal on the Au nanoparticle-catalyzed reduction of 4-nitrophenol. J. Phys. Chem. C 2022, 126, 13705–13713. [Google Scholar] [CrossRef]
- Brar, K.K.; Magdouli, S.; Othmani, A.; Ghanei, J.; Narisetty, V.; Sindhu, R.; Binod, P.; Pugazhendhi, A.; Awasthi, M.K.; Pandey, A. Green route for recycling of low-cost waste resources for the biosynthesis of nanoparticles (NPs) and nanomaterials (NMs)-A review. Environ. Res. 2022, 207, 112202. [Google Scholar] [CrossRef]
- Alagarasan, J.K.; Shasikala, S.; Rene, E.R.; Bhatt, P.; Thangavelu, P.; Madheswaran, P.; Subramanian, S.; Nguyen, D.D.; Chang, S.W.; Lee, M. Electro-oxidation of heavy metals contaminated water using banana waste-derived activated carbon and Fe3O4 nanocomposites. Environ. Res. 2022, 215, 114293. [Google Scholar] [CrossRef]
- Miculescu, M.; Thakur, V.K.; Miculescu, F.; Voicu, S.I. Graphene-based polymer nanocomposite membranes: A review. Polym. Adv. Technol. 2016, 27, 844–859. [Google Scholar] [CrossRef]
- Shen, T.; Li, Z.; Jiang, Y.; Luo, Z.-G. Synthesis and catalytic performance of graphene oxide-Au nanorings composites. Funct. Mater. Lett. 2019, 12, 1950028. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Q.; Xu, L.; Zhang, S.; Feng, L.; Cheng, L.; Xu, H.; Liu, Z.; Peng, R. Graphene oxide–silver nanocomposite as a highly effective antibacterial agent with species-specific mechanisms. ACS Appl. Mater. Interfaces 2013, 5, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Chen, X.; Wu, Y.; Liu, H. Facile and green synthesis of Au nanorods/graphene oxide nanocomposite with excellent catalytic properties for reduction of 4-nitrophenol. J. Mater. Sci. 2020, 55, 5880–5891. [Google Scholar] [CrossRef]
- Maddinedi, S.B.; Mandal, B.K.; Fazlur-Rahman, N.K. High reduction of 4-nitrophenol using reduced graphene oxide/Ag synthesized with tyrosine. Environ. Chem. Lett. 2017, 15, 467–474. [Google Scholar] [CrossRef]
- Nimita Jebaranjitham, J.; Mageshwari, C.; Saravanan, R.; Mu, N. Fabrication of amine functionalized graphene oxide—AgNPs nanocomposite with improved dispersibility for reduction of 4-nitrophenol. Compos. Part B 2019, 171, 302–309. [Google Scholar] [CrossRef]
- Sahu, D.; Sarkar, N.; Sahoo, G.; Mohapatra, P.; Swain, S.K. Nano silver imprinted graphene oxide as catalyst in reduction of 4-nitrophenol. J. Phys. Org. Chem. 2019, 32, 3971. [Google Scholar] [CrossRef]
- Hong, M.; Wang, B.; Xu, X.; Bin, P.; Zhang, J.; Zhang, Q. Rational design of high-performance continuous flow catalytic membrane reactor based on poly(4-vinylpyridine) brush-anchored Au nanoparticles. J. Membr. Sci. 2022, 662, 121002. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Dong, H.; Meng, M.; Li, C.; Yan, Y.; Chen, J. An overview on membrane strategies for rare earths extraction and separation. Sep. Purif. Technol. 2018, 197, 70–85. [Google Scholar] [CrossRef]
- Yan, X.; Anguille, S.; Bendahan, M.; Moulin, P. Ionic liquids combined with membrane separation processes: A review. Sep. Purif. Technol. 2019, 222, 230–253. [Google Scholar] [CrossRef]
- Shao, D.-D.; Yang, W.-J.; Xiao, H.-F.; Wang, Z.-Y.; Zhou, C.; Cao, X.-L.; Sun, S.-P. Self-cleaning nanofiltration membranes by coordinated regulation of carbon quantum dots and polydopamine. ACS Appl. Mater. Interfaces 2019, 12, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Z.; Li, T.; Ye, J.; Shen, L.; She, Z.; Liu, F. Catalytic PVDF membrane for continuous reduction and separation of p-nitrophenol and methylene blue in emulsified oil solution. Chem. Eng. J. 2018, 334, 579–586. [Google Scholar] [CrossRef]
- Zeng, Z.; Wen, M.; Yu, B.; Ye, G.; Huo, X.; Lu, Y.; Chen, J. Polydopamine induced in-situ formation of metallic nanoparticles in confined microchannels of porous membrane as flexible catalytic reactor. ACS Appl. Mater. Interfaces 2018, 10, 14735–14743. [Google Scholar] [CrossRef]
- Hu, X.; Chen, X.; Giagnorio, M.; Wu, C.; Luo, Y.; Hélix-Nielsen, C.; Yu, P.; Zhang, W. Beaded electrospun polyvinylidene fluoride (PVDF) membranes for membrane distillation (MD). J. Membr. Sci. 2022, 661, 120850. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, R.; Fane, A.G. Engineering superhydrophobic surface on poly(vinylidene fluoride) nanofiber membranes for direct contact membrane distillation. J. Membr. Sci. 2013, 440, 77–87. [Google Scholar] [CrossRef]
- Fan, Y.; Quan, X.; Zhao, H.; Chen, S.; Yu, H.; Zhang, Y.; Zhang, Q. Poly(vinylidene fluoride) hollow-fiber membranes containing silver/graphene oxide dope with excellent filtration performance. J. Appl. Polym. Sci. 2017, 134, 44713. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.; Zeng, X.; Huang, S.; Zhang, H.; Qin, X. Improved desalination properties of hydrophobic GO-incorporated PVDF electrospun nanofibrous composites for vacuum membrane distillation. Sep. Purif. Technol. 2020, 230, 115889. [Google Scholar] [CrossRef]
- Tuichai, W.; Karaphun, A.; Ruttanapun, C. Improved dielectric properties of PVDF polymer composites filled with Ag nanomaterial deposited reduced graphene oxide (rGO) hybrid particles. Mater. Res. Bull. 2022, 145, 111552. [Google Scholar] [CrossRef]
- El Achaby, M.; Arrakhiz, F.Z.; Vaudreuil, S.; Essassi, E.M.; Qaiss, A. Piezoelectric β-polymorph formation and properties enhancement in graphene oxide—PVDF nanocomposite films. Appl. Surf. Sci. 2012, 258, 7668–7677. [Google Scholar] [CrossRef]
- Cho, D.; Chen, S.; Jeong, Y.; Joo, Y.L. Surface hydro-properties of electrospun fiber mats. Fibers Polym. 2015, 16, 1578–1586. [Google Scholar] [CrossRef]
- Geng, C.; Zhao, F.; Wang, Q.; Zheng, S.; Liu, Y.; Niu, H.; Zhang, J.; Dong, H. Anti-biofouling property and anti-leaching investigation of modifier for PVDF ultrafiltration membrane by incorporating antibacterial graphene oxide derivatives. J. Environ. Chem. Eng. 2022, 10, 108558. [Google Scholar] [CrossRef]
- Dong, L.; Liu, X.; Xiong, Z.; Sheng, D.; Zhou, Y.; Lin, C.; Yang, Y. Design of UV-absorbing PVDF membrane via surface-initiated AGET ATRP. Appl. Surf. Sci. 2018, 435, 680–686. [Google Scholar] [CrossRef]
- Shen, X.; Gao, P.; Yang, W.; Ding, Y.; Bao, C.; Wei, Z.; Tian, K. Study on the hydrophobic modification of PVDF membrane by low-temperature plasma etching in combination with grafting in supercritical carbon dioxide. Vacuum 2023, 209, 111782. [Google Scholar] [CrossRef]
- Clark, D.T.; Feast, W.J.; Kilcast, D.; Musgrave, W.K.R. Applications of ESCA to polymer chemistry. III. structures and bonding in homopolymers of ethylene and the fluoroethylenes and determination of the compositions of fluoro copolymers. J. Polym. Sci. Polym. Chem. Ed. 2003, 11, 389–411. [Google Scholar] [CrossRef]
- Ferrari, I.; Motta, A.; Zanoni, R.; Scaramuzzo, F.A.; Amato, F.; Dalchiele, E.A.; Marrani, A.G. Understanding the nature of graphene oxide functional groups by modulation of the electrochemical reduction: A combined experimental and theoretical approach. Carbon 2023, 203, 29–38. [Google Scholar] [CrossRef]
- Kaspar, T.C.; Droubay, T.; Chambers, S.A.; Bagus, P.S. Spectroscopic evidence for Ag(III) in highly oxidized silver films by X-ray photoelectron spectroscopy. J. Phys. Chem. C 2010, 114, 21562–21571. [Google Scholar] [CrossRef]
- Gitipour, A.; Al-Abed, S.R.; Thiel, S.W.; Scheckel, K.G.; Tolaymat, T. Nanosilver as a disinfectant in dental unit waterlines: Assessment of the physicochemical transformations of the AgNPs. Chemosphere 2017, 173, 245–252. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z.; Zhou, J.; Sun, D.; Li, H. The multiple synthesis of 2D layered MXene Ti3C2Tx/Ag/Cu composites with enhanced electrochemical properties. Ceram. Int. 2022, 48, 30524–30535. [Google Scholar] [CrossRef]
- Mei, Y.; Liu, S.; Wu, L.; Zhou, B.; Wang, Z.; Huang, Z.-H.; Zhu, Y.; Wang, M.-X. Kelp derived hierarchically porous carbon aerogels with ultrahigh surface area for high-energy-density supercapacitor in aqueous electrolyte. J. Energy Storage 2024, 77, 109878. [Google Scholar] [CrossRef]
- Qi, M.-Y.; Zhang, Y.; Li, Q.; Wu, L.; Zhou, B.; Wang, Z.; Huang, Z.-H.; Wang, M.-X. Experimental and theoretical insights into metal-free catalytic reduction of nitrophenols over porous nitrogen-doped reduced graphene oxide. Chem. Eng. J. 2023, 474, 145823. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, X.; Ye, X.; Li, Q.; Wang, J.; Wu, L.; Huang, Z.-H.; Wang, M.-X. Activating peroxymonosulfate by high nitrogen-doped biochar from lotus pollen for efficient degradation of organic pollutants from water: Performance, kinetics and mechanism investigation. Sep. Purif. Technol. 2024, 346, 127456. [Google Scholar] [CrossRef]
- Zhang, Q.; Somerville, R.J.; Chen, L.; Yu, Y.; Fei, Z.; Wang, S.; Dyson, P.J.; Min, D. Carbonized wood impregnated with bimetallic nanoparticles as a monolithic continuous-flow microreactor for the reduction of 4-nitrophenol. J. Hazard. Mater. 2023, 443, 130270. [Google Scholar] [CrossRef]
- Saha, S.; Pal, A.; Kundu, S.; Basu, S.; Pal, T. Photochemical green synthesis of calcium-alginate-stabilized Ag and Au nanoparticles and their catalytic application to 4-nitrophenol reduction. Langmuir 2009, 26, 2885–2893. [Google Scholar] [CrossRef]
- Yang, Y.-K.; He, C.-E.; He, W.-J.; Yu, L.-J.; Peng, R.-G.; Xie, X.-L.; Wang, X.-B.; Mai, Y.-W. Reduction of silver nanoparticles onto graphene oxide nanosheets with N,N-dimethylformamide and SERS activities of GO/Ag composites. J. Nanopart. Res. 2011, 13, 5571–5581. [Google Scholar] [CrossRef]
- Patel, A.C.; Li, S.; Wang, C.; Zhang, W.; Wei, Y. Electrospinning of porous silica nanofibers containing silver nanoparticles for catalytic applications. Chem. Mater. 2007, 19, 1231–1238. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, X.; Chen, R.; Ye, D.; Yang, Y.; Liao, Q. Kinetics of light assisted catalytic reduction of 4-NP over Ag/PDA. Chem. Eng. Sci. 2022, 259, 117778. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Xie, J.; Jia, D.; Qin, H.; Liang, Z. Facile solid-state synthesis of Ag/graphene oxide nanocomposites as highly active and stable catalyst for the reduction of 4-nitrophenol. Catal. Commun. 2015, 58, 21–25. [Google Scholar] [CrossRef]
- Xi, J.; Wang, Q.; Duan, X.; Zhang, N.; Yu, J.; Sun, H.; Wang, S. Continuous flow reduction of organic dyes over Pd-Fe alloy based fibrous catalyst in a fixed-bed system. Chem. Eng. Sci. 2021, 231, 116303. [Google Scholar] [CrossRef]
- Shao, W.; Liu, X.; Min, H.; Dong, G.; Feng, Q.; Zuo, S. Preparation, characterization, and antibacterial activity of silver nanoparticle-decorated graphene oxide nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973. [Google Scholar] [CrossRef]
- Tang, S.; Wu, W.; Yu, J. Interfacial interaction of Ag nanoparticles with graphene oxide supports for improving NH3 and NO adsorption: A first-principles study. Phys. Chem. Chem. Phys. 2016, 18, 7797–7807. [Google Scholar] [CrossRef]
- Jabrane, M.; El Hafidi, M.; El Hafidi, M.Y.; Kara, A. Fe-phthalocyanine on Cu(111) and Ag(111): A DFT+vdWs investigation. Surf. Sci. 2022, 716, 121961. [Google Scholar] [CrossRef]
- Anumula, R.; Cui, C.; Yang, M.; Li, J.; Luo, Z. Catalytic oxidation of cyclohexane on small silver clusters supported by graphene oxide. J. Phys. Chem. C 2019, 123, 21504–21512. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Yang, L.-J.; Qing, L.-H.; Lv, X.-M.; Yi, L.-Y.; Li, H.; Chen, Z.-Q. The strong effect of substituents on the carbonyl reduction in graphene oxide: A DFT study. Comput. Theor. Chem. 2015, 1068, 1–7. [Google Scholar] [CrossRef]
- Kong, X.; Chen, Q.; Li, R.; Cheng, K.; Yan, N.; Yu, B. Experimental and theoretical investigations on the negative influence of an applied magnetic field on SERS of Ag nanoparticles. Chem. Commun. 2011, 47, 11237. [Google Scholar] [CrossRef]
- Kong, X.-K.; Chen, Q.-W.; Lun, Z.-Y. Probing the influence of different oxygenated groups on graphene oxide’s catalytic performance. J. Mater. Chem. A 2014, 2, 610–613. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, H.; Chen, C.; Huang, G.; Chen, Q. Insights into the reduction of 4-nitrophenol to 4-aminophenol on catalysts. Chem. Phys. Lett. 2017, 684, 148–152. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhao, Y.H.; Zhou, Y.; Guo, X.L.; Chen, Z.T.; Zhang, W.J.; Zhang, Y.; Chen, J.; Wang, Z.M.; Sun, L.T.; et al. High-efficient catalytic reduction of 4-nitrophenol based on reusable Ag nanoparticles/graphene-loading loofah sponge hybrid. Nanotechnology 2018, 29, 315702. [Google Scholar] [CrossRef] [PubMed]
- Strachan, J.; Barnett, C.; Masters, A.F.; Maschmeyer, T. 4-nitrophenol reduction: Probing the putative mechanism of the model reaction. ACS Catal. 2020, 10, 5516–5521. [Google Scholar] [CrossRef]
- Wang, J.; Pei, X.; Liu, G.; Bai, J.; Ding, Y.; Wang, J.; Liu, F. Gravity-driven catalytic nanofibrous membrane with microsphere and nanofiber coordinated structure for ultrafast continuous reduction of 4-nitrophenol. J. Colloid Interface Sci. 2019, 538, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, Z.; Fu, L.; Zhang, Y.; Yang, H.; Ouyang, J.; Chen, D. Silver nanoparticles assembled on modified sepiolite nanofibers for enhanced catalytic reduction of 4-nitrophenol. Appl. Clay Sci. 2018, 166, 166–173. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza-Santos, I.; Liz-Marza’n, L.M. Formation and stabilization of silver nanoparticles through reduction by N,N-dimethylformamide. Langmuir 1999, 15, 948–951. [Google Scholar] [CrossRef]
- He, L.; Tjong, S.C. Facile synthesis of silver-decorated reduced graphene oxide as a hybrid filler material for electrically conductive polymer composites. RSC Adv. 2015, 5, 15070–15076. [Google Scholar] [CrossRef]
- Liu, H.; Lai, W.; Shi, Y.; Tian, L.; Li, K.; Bian, L.; Xi, Z.; Lin, B. Ag-decorated electrospun polymer/GO fibrous membranes for simultaneous bacterial filtration and termination. J. Membr. Sci. 2024, 695, 122498. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.-E.; Wu, H.-L.; Xu, Z.-L.; Wan, J.-J.; Liu, L.-J.; Xu, S.-J.; Kong, Y.-F.; Wu, Q.; Min, J.; et al. Fabrication of GO-Ag/PVDF/F127 modified membrane IPA coagulation bath for catalytic reduction of 4-nitrophenol. Sep. Purif. Technol. 2020, 235, 116143. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; He, Z.; Jin, L.; Chen, H.; Li, Q.; Wu, L.; Huang, Z.; Wang, M. Highly Efficient Electrospun Silver Decorated Graphene Oxide Nanocomposites on Poly(vinylidene fluoride) (PVDF@GO-Ag) Hybrid Membrane for Reduction of 4-Nitrophenol. Molecules 2024, 29, 3930. https://doi.org/10.3390/molecules29163930
Yang X, He Z, Jin L, Chen H, Li Q, Wu L, Huang Z, Wang M. Highly Efficient Electrospun Silver Decorated Graphene Oxide Nanocomposites on Poly(vinylidene fluoride) (PVDF@GO-Ag) Hybrid Membrane for Reduction of 4-Nitrophenol. Molecules. 2024; 29(16):3930. https://doi.org/10.3390/molecules29163930
Chicago/Turabian StyleYang, Xiaoben, Zhen He, Lei Jin, Huiyang Chen, Qianglin Li, Ling Wu, Zhenghong Huang, and Mingxi Wang. 2024. "Highly Efficient Electrospun Silver Decorated Graphene Oxide Nanocomposites on Poly(vinylidene fluoride) (PVDF@GO-Ag) Hybrid Membrane for Reduction of 4-Nitrophenol" Molecules 29, no. 16: 3930. https://doi.org/10.3390/molecules29163930
APA StyleYang, X., He, Z., Jin, L., Chen, H., Li, Q., Wu, L., Huang, Z., & Wang, M. (2024). Highly Efficient Electrospun Silver Decorated Graphene Oxide Nanocomposites on Poly(vinylidene fluoride) (PVDF@GO-Ag) Hybrid Membrane for Reduction of 4-Nitrophenol. Molecules, 29(16), 3930. https://doi.org/10.3390/molecules29163930








