Recent Advances in Carborane-Based Crystalline Porous Materials
Abstract
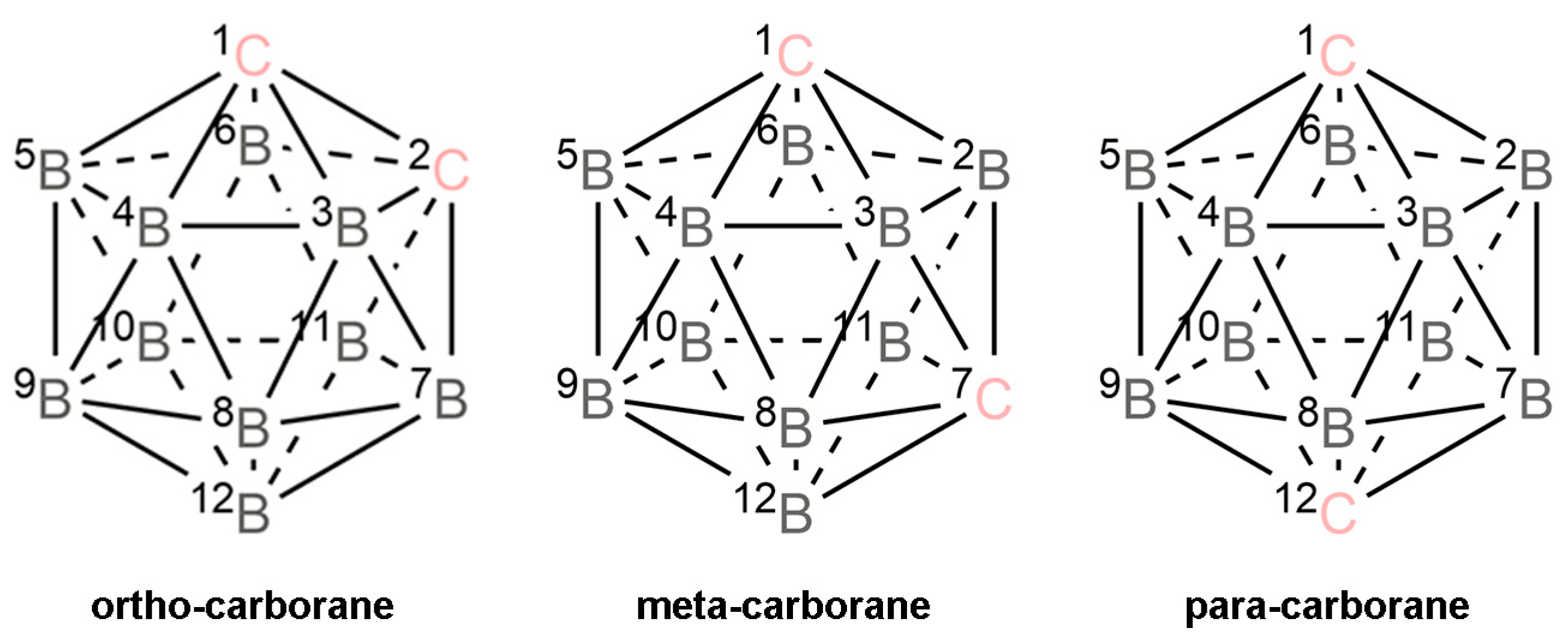
1. Introduction
2. Carborane-Based Metal–Organic Frameworks
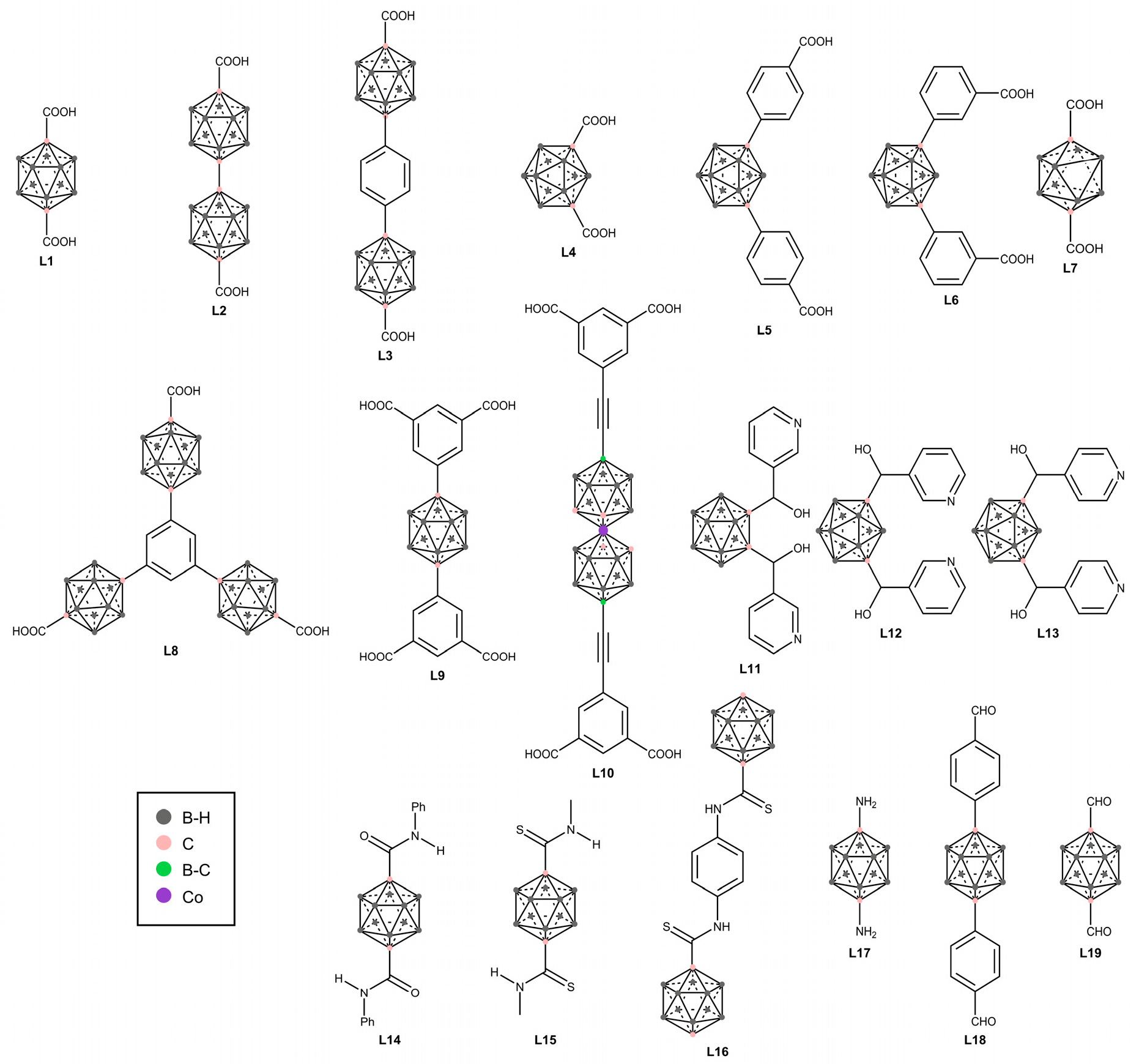
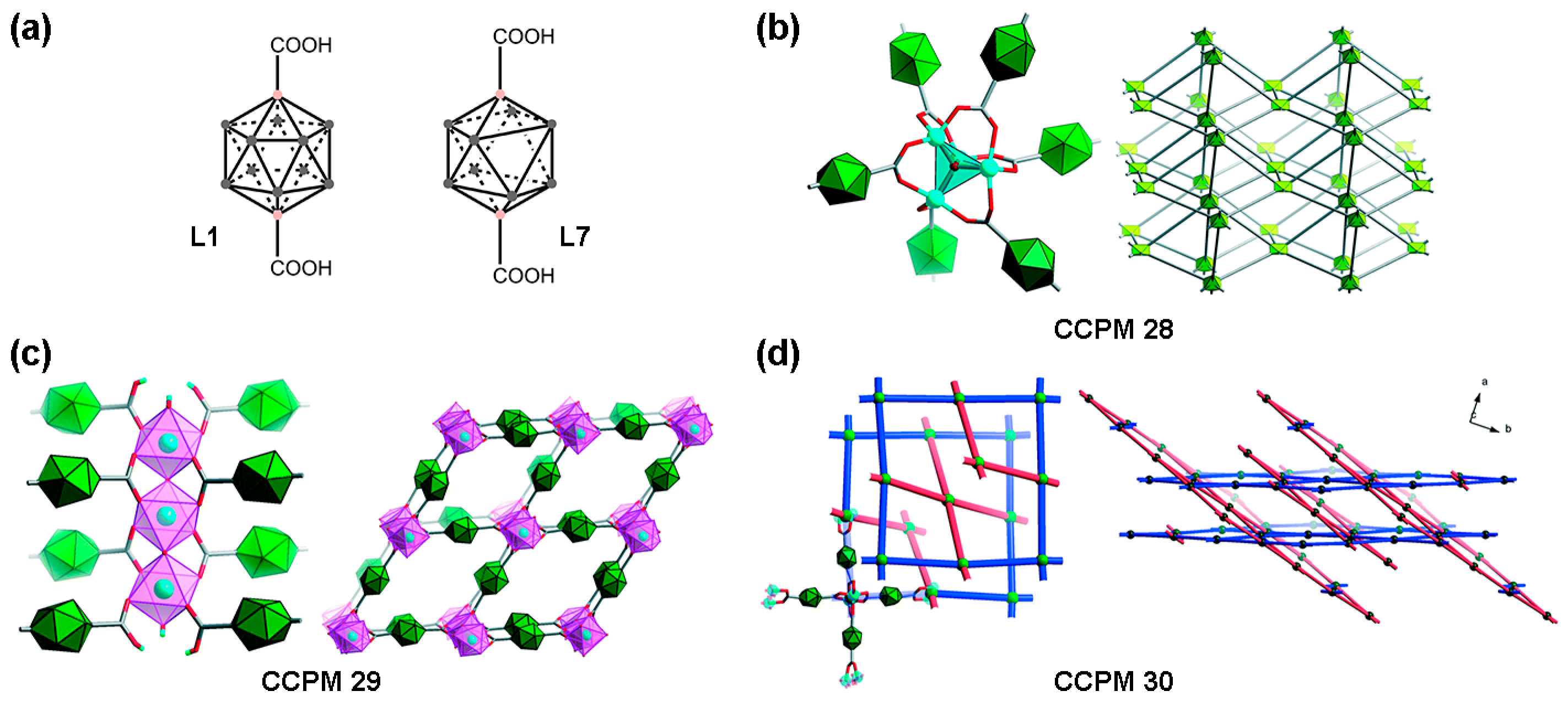
2.1. Carboxylate-Based Carborane MOFs
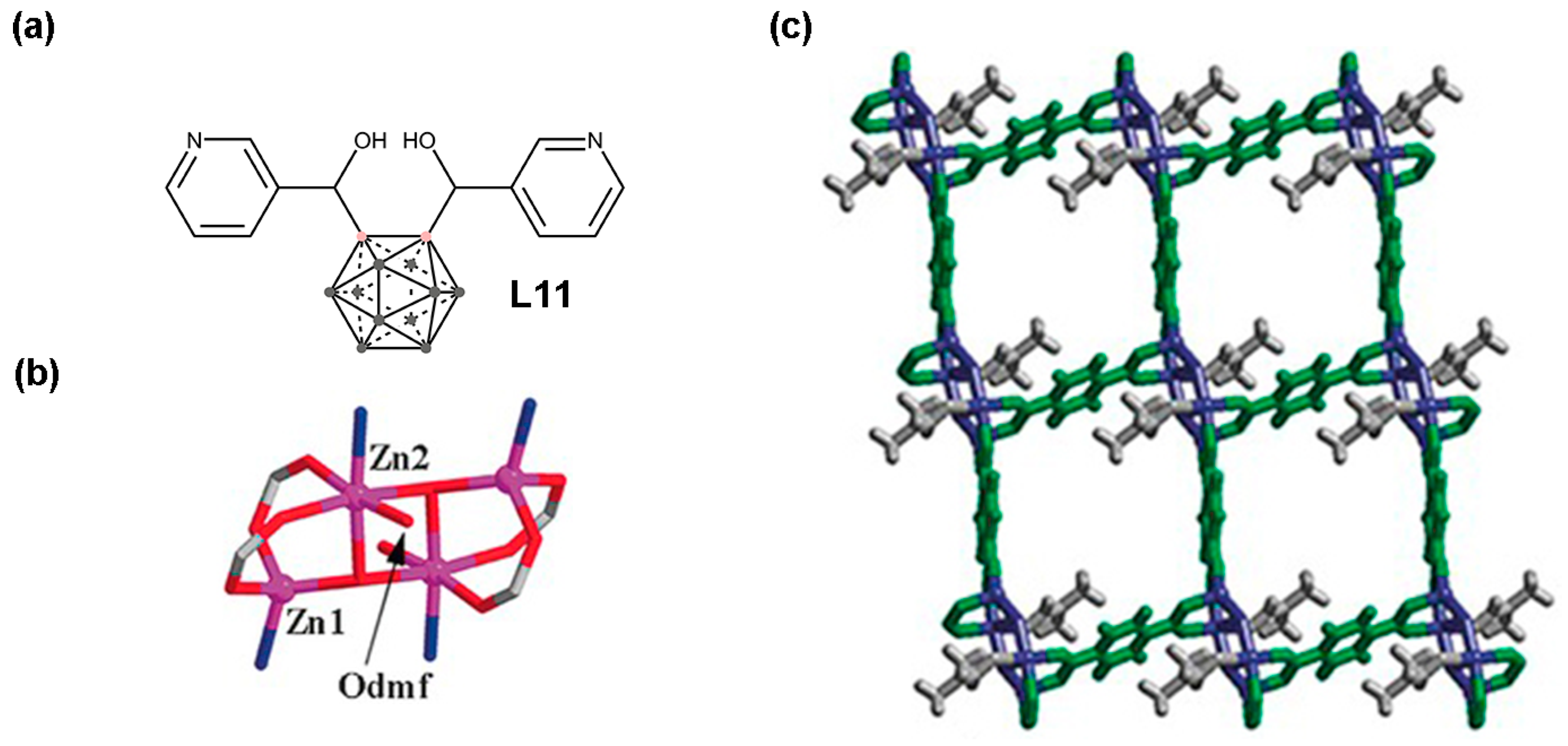
2.2. Pyridine-Based Carborane MOFs
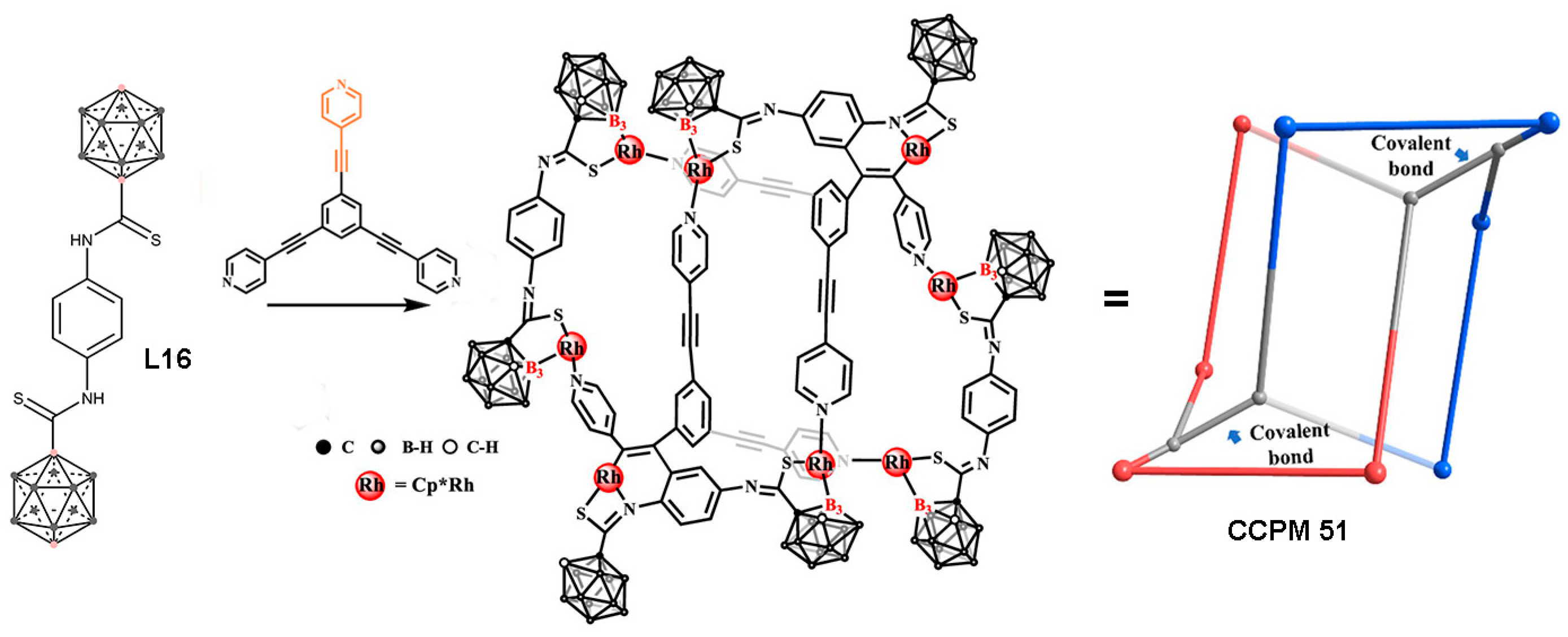
3. Carborane-Based Metal–Organic Cages
3.1. Steric-Effect-Directed B–H Bond Activation of Carboranes
3.2. “Cage Walking”
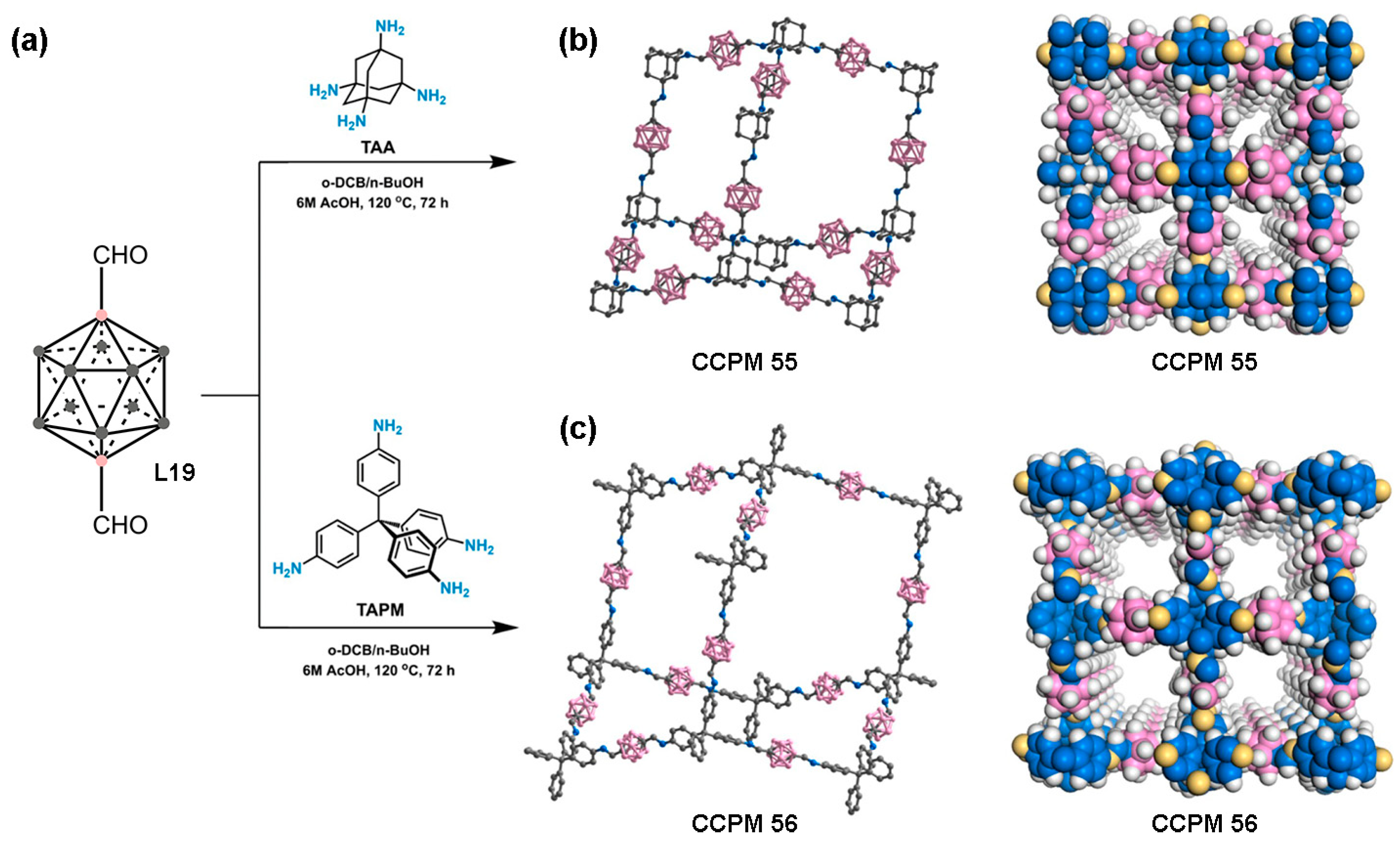
4. Carborane-Based Covalent Organic Frameworks
4.1. Host–Guest Interactions
4.2. Acting as Building Blocks
4.2.1. Two-Dimensional COFs
4.2.2. Three-Dimensional COFs
4.3. Post-Synthetic Modification
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Sayed, E.-S.M.; Yuan, Y.D.; Zhao, D.; Yuan, D. Zirconium Metal–Organic Cages: Synthesis and Applications. Acc. Chem. Res. 2022, 55, 1546–1560. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Xiao, W.; Cheng, M.; Liu, Y.; Wang, J.; Zhang, G.; Wei, Z.; Li, L.; Du, L.; Wang, G.; Liu, H. Functional Metal/Carbon Composites Derived from Metal–Organic Frameworks: Insight into Structures, Properties, Performances, and Mechanisms. ACS Catal. 2023, 13, 1759–1790. [Google Scholar] [CrossRef]
- Yang, L.; Qian, S.; Wang, X.; Cui, X.; Chen, B.; Xing, H. Energy-efficient separation alternatives: Metal–organic frameworks and membranes for hydrocarbon separation. Chem. Soc. Rev. 2020, 49, 5359–5406. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, L.; Yaghi, O.M. Three Future Directions for Metal–Organic Frameworks. Chem. Mater. 2023, 35, 5711–5712. [Google Scholar] [CrossRef]
- Lin, H.; Xiao, Z.; Le, K.N.; Yan, T.h.; Cai, P.; Yang, Y.; Day, G.S.; Drake, H.F.; Xie, H.; Bose, R.; et al. Assembling Phenothiazine into a Porous Coordination Cage to Improve Its Photocatalytic Efficiency for Organic Transformations. Angew. Chem. Int. Ed. 2022, 61, e202214055. [Google Scholar] [CrossRef]
- Gosselin, A.J.; Rowland, C.A.; Bloch, E.D. Permanently Microporous Metal–Organic Polyhedra. Chem. Rev. 2020, 120, 8987–9014. [Google Scholar] [CrossRef]
- Fang, Y.; Murase, T.; Sato, S.; Fujita, M. Noncovalent Tailoring of the Binding Pocket of Self-Assembled Cages by Remote Bulky Ancillary Groups. J. Am. Chem. Soc. 2013, 135, 613–615. [Google Scholar] [CrossRef]
- Vardhan, H.; Yusubov, M.; Verpoort, F. Self-assembled metal–organic polyhedra: An overview of various applications. Coord. Chem. Rev. 2016, 306, 171–194. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.-G.; Su, C.-Y.; Wang, W. Construction of Covalent Organic Framework for Catalysis: Pd/COF-LZU1 in Suzuki–Miyaura Coupling Reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef]
- Tan, K.T.; Ghosh, S.; Wang, Z.; Wen, F.; Rodríguez-San-Miguel, D.; Feng, J.; Huang, N.; Wang, W.; Zamora, F.; Feng, X.; et al. Covalent organic frameworks. Nat. Rev. Methods Primers 2023, 3, 1. [Google Scholar] [CrossRef]
- Huang, N.; Zhai, L.; Coupry, D.E.; Addicoat, M.A.; Okushita, K.; Nishimura, K.; Heine, T.; Jiang, D. Multiple-component covalent organic frameworks. Nat. Commun. 2016, 7, 12325. [Google Scholar] [CrossRef]
- King, R.B. Trivalent Polyhedra as Duals of Borane Deltahedra: From Molecular Endohedral Germanium Clusters to the Smallest Fullerenes. Molecules 2023, 28, 496. [Google Scholar] [CrossRef] [PubMed]
- Avdeeva, V.V.; Nikiforova, S.E.; Malinina, E.A.; Sivaev, I.B.; Kuznetsov, N.T. Composites and Materials Prepared from Boron Cluster Anions and Carboranes. Materials 2023, 16, 6099. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Qiu, Z.; Xie, Z. Iridium-catalysed regioselective borylation of carboranes via direct B–H activation. Nat. Commun. 2017, 8, 14827. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, J.; Zhao, D.; Sun, F.; Tian, S.; Tu, D.; Lu, C.; Yan, H. Site-Selective Functionalization of Carboranes at the Electron-Rich Boron Vertex: Photocatalytic B−C Coupling via a Carboranyl Cage Radical. Angew. Chem. Int. Ed. 2022, 61, e202205672. [Google Scholar] [CrossRef]
- Farha, O.K.; Spokoyny, A.M.; Mulfort, K.L.; Hawthorne, M.F.; Mirkin, C.A.; Hupp, J.T. Synthesis and Hydrogen Sorption Properties of Carborane Based Metal−Organic Framework Materials. J. Am. Chem. Soc. 2007, 129, 12680–12681. [Google Scholar] [CrossRef]
- Bae, Y.-S.; Farha, O.K.; Spokoyny, A.M.; Mirkin, C.A.; Hupp, J.T.; Snurr, R.Q. Carborane-based metal–organic frameworks as highly selective sorbents for CO2 over methane. Chem. Commun. 2008, 35, 4135–4137. [Google Scholar] [CrossRef]
- Farha, O.K.; Spokoyny, A.M.; Mulfort, K.L.; Galli, S.; Hupp, J.T.; Mirkin, C.A. Gas-Sorption Properties of Cobalt(II)–Carborane-Based Coordination Polymers as a Function of Morphology. Small 2009, 5, 1727–1731. [Google Scholar] [CrossRef]
- Bae, Y.-S.; Spokoyny, A.M.; Farha, O.K.; Snurr, R.Q.; Hupp, J.T.; Mirkin, C.A. Separation of gas mixtures using Co(ii) carborane-based porous coordination polymers. Chem. Commun. 2010, 46, 3478. [Google Scholar] [CrossRef] [PubMed]
- Spokoyny, A.M.; Farha, O.K.; Mulfort, K.L.; Hupp, J.T.; Mirkin, C.A. Porosity tuning of carborane-based metal–organic frameworks (MOFs) via coordination chemistry and ligand design. Inorg. Chim. Acta 2010, 364, 266–271. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Wang, L.; Cui, X.; Xing, H. Pillar iodination in functional boron cage hybrid supramolecular frameworks for high performance separation of light hydrocarbons. J. Mater. Chem. A 2019, 7, 27560–27566. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Wang, L.; Duttwyler, S.; Xing, H. A Microporous Metal-Organic Framework Supramolecularly Assembled from a Cu(II) Dodecaborate Cluster Complex for Selective Gas Separation. Angew. Chem. 2019, 58, 8145–8150. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Krishna, R.; Wang, L.; Yang, L.; Cui, X.; Duttwyler, S.; Xing, H. Rational Design of Microporous MOFs with Anionic Boron Cluster Functionality and Cooperative Dihydrogen Binding Sites for Highly Selective Capture of Acetylene. Angew. Chem. Int. Ed. 2020, 59, 17664–17669. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Hu, J.; Duttwyler, S.; Cui, X.; Xing, H. Solvent-dependent supramolecular self-assembly of boron cage pillared metal–organic frameworks for selective gas separation. CrystEngComm 2020, 22, 2649–2655. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Duttwyler, S.; Zhang, Y. Efficient adsorption separation of methane from CO2 and C2–C3 hydrocarbons in a microporous closo-dodecaborate [B12H12]2- pillared metal–organic framework. J. Solid State Chem. 2021, 299, 122167. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Zhang, Y.; Xu, N.; Krishna, R.; Hu, J.; Jiang, Y.; He, Y.; Xing, H. Interpenetration Symmetry Control Within Ultramicroporous Robust Boron Cluster Hybrid MOFs for Benchmark Purification of Acetylene from Carbon Dioxide. Angew. Chem. Int. Ed. 2021, 60, 22865–22870. [Google Scholar] [CrossRef]
- Sun, W.; Hu, J.; Duttwyler, S.; Wang, L.; Krishna, R.; Zhang, Y. Highly selective gas separation by two isostructural boron cluster pillared MOFs. Sep. Purif. Technol. 2022, 283, 120220. [Google Scholar] [CrossRef]
- Sun, W.; Jin, Y.; Wu, Y.; Lou, W.; Yuan, Y.; Duttwyler, S.; Wang, L.; Zhang, Y. A new boron cluster anion pillared metal organic framework with ligand inclusion and its selective acetylene capture properties. Inorg. Chem. Front. 2022, 9, 5140–5147. [Google Scholar] [CrossRef]
- Wang, L.; Wu, S.; Hu, J.; Jiang, Y.; Li, J.; Hu, Y.; Han, Y.; Ben, T.; Chen, B.; Zhang, Y. A novel hydrophobic carborane-hybrid microporous material for reversed C2H6 adsorption and efficient C2H4/C2H6 separation under humid conditions. Chem. Sci. 2024, 15, 5653–5659. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.L.; Lin, Y.J.; Yu, W.B.; Jin, G.X. Porous Frameworks Based on Carborane–Ln2(CO2)6: Architecture Influenced by Lanthanide Contraction and Selective CO2 Capture. ChemPlusChem 2012, 77, 141–147. [Google Scholar] [CrossRef]
- Huang, S.-L.; Weng, L.-H.; Jin, G.-X. Bottom-up synthesis of coordination polymers based on carborane backbones and Cu2(CO2)4 paddle-wheel: Ligand metathesis with metallotecons. Dalton Trans. 2012, 41, 11657. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Q.; Kong, T. Tetraethylenepentamine-modified MCM-41/silica gel with hierarchical mesoporous structure for CO2 capture. Chem. Eng. J. 2015, 273, 472–480. [Google Scholar] [CrossRef]
- Gan, L.; Chidambaram, A.; Fonquernie, P.G.; Light, M.E.; Choquesillo-Lazarte, D.; Huang, H.; Solano, E.; Fraile, J.; Viñas, C.; Teixidor, F.; et al. A Highly Water-Stable meta-Carborane-Based Copper Metal–Organic Framework for Efficient High-Temperature Butanol Separation. J. Am. Chem. Soc. 2020, 142, 8299–8311. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Andres-Garcia, E.; Mínguez Espallargas, G.; Planas, J.G. Adsorptive Separation of CO2 by a Hydrophobic Carborane-Based Metal–Organic Framework under Humid Conditions. ACS Appl. Mater. Interfaces 2023, 15, 5309–5316. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, W.; Song, M.; Wang, F.; Hu, X.; Guo, Q.; Liu, Y. Polyetheramine improves the CO2 adsorption behavior of tetraethylenepentamine-functionalized sorbents. Chem. Eng. J. 2019, 364, 475–484. [Google Scholar] [CrossRef]
- Li, Z.; Choquesillo-Lazarte, D.; Fraile, J.; Viñas, C.; Teixidor, F.; Planas, J.G. Rational design of carborane-based Cu2-paddle wheel coordination polymers for increased hydrolytic stability. Dalton Trans. 2022, 51, 1137–1143. [Google Scholar] [CrossRef]
- Gan, L.; Fonquernie, P.G.; Light, M.E.; Norjmaa, G.; Ujaque, G.; Choquesillo-Lazarte, D.; Fraile, J.; Teixidor, F.; Viñas, C.; Planas, J.G. A Reversible Phase Transition of 2D Coordination Layers by B–H∙∙∙Cu(II) Interactions in a Coordination Polymer. Molecules 2019, 24, 3204. [Google Scholar] [CrossRef]
- Li, Z.; Núñez, R.; Light, M.E.; Ruiz, E.; Teixidor, F.; Viñas, C.; Ruiz-Molina, D.; Roscini, C.; Planas, J.G. Water-Stable Carborane-Based Eu3+/Tb3+ Metal–Organic Frameworks for Tunable Time-Dependent Emission Color and Their Application in Anticounterfeiting Bar-Coding. Chem. Mater. 2022, 34, 4795–4808. [Google Scholar] [CrossRef]
- Boldog, I.; Bereciartua, P.J.; Bulánek, R.; Kučeráková, M.; Tomandlová, M.; Dušek, M.; Macháček, J.; De Vos, D.; Baše, T. 10-Vertex closo-carborane: A unique ligand platform for porous coordination polymers. CrystEngComm 2016, 18, 2036–2040. [Google Scholar] [CrossRef]
- Boldog, I.; Dušek, M.; Jelínek, T.; Švec, P.; Ramos, F.S.d.O.; Růžička, A.; Bulánek, R. Porous 10- and 12-vertex (bi)-p-dicarba-closo-boranedicarboxylates of cobalt and their gas adsorptive properties. Microporous Mesoporous Mater. 2018, 271, 284–294. [Google Scholar] [CrossRef]
- Idrees, K.B.; Kirlikovali, K.O.; Setter, C.; Xie, H.; Brand, H.; Lal, B.; Sha, F.; Smoljan, C.S.; Wang, X.; Islamoglu, T.; et al. Robust Carborane-Based Metal–Organic Frameworks for Hexane Separation. J. Am. Chem. Soc. 2023, 145, 23433–23441. [Google Scholar] [CrossRef]
- Clingerman, D.J.; Morris, W.; Mondloch, J.E.; Kennedy, R.D.; Sarjeant, A.A.; Stern, C.; Hupp, J.T.; Farha, O.K.; Mirkin, C.A. Stabilization of a highly porous metal–organic framework utilizing a carborane-based linker. Chem. Commun. 2015, 51, 6521–6523. [Google Scholar] [CrossRef]
- Kennedy, R.D.; Krungleviciute, V.; Clingerman, D.J.; Mondloch, J.E.; Peng, Y.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Hupp, J.T.; Yildirim, T.; et al. Carborane-Based Metal–Organic Framework with High Methane and Hydrogen Storage Capacities. Chem. Mater. 2013, 25, 3539–3543. [Google Scholar] [CrossRef]
- Kennedy, R.D.; Clingerman, D.J.; Morris, W.; Wilmer, C.E.; Sarjeant, A.A.; Stern, C.L.; O’Keeffe, M.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K.; et al. Metallacarborane-Based Metal–Organic Framework with a Complex Topology. Cryst. Growth Des. 2014, 14, 1324–1330. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.-Y.; Day, G.S.; Ryder, M.R.; Zhou, H.-C. Destruction of Metal–Organic Frameworks: Positive and Negative Aspects of Stability and Lability. Chem. Rev. 2020, 120, 13087–13133. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H.-L. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef]
- Di Salvo, F.; Paterakis, C.; Tsang, M.Y.; García, Y.; Viñas, C.; Teixidor, F.; Giner Planas, J.; Light, M.E.; Hursthouse, M.B.; Choquesillo-Lazarte, D. Synthesis and Crystallographic Studies of Disubstituted Carboranyl Alcohol Derivatives: Prevailing Chiral Recognition? Cryst. Growth Des. 2013, 13, 1473–1484. [Google Scholar] [CrossRef]
- Rodríguez-Hermida, S.; Tsang, M.Y.; Vignatti, C.; Stylianou, K.C.; Guillerm, V.; Pérez-Carvajal, J.; Teixidor, F.; Viñas, C.; Choquesillo-Lazarte, D.; Verdugo-Escamilla, C.; et al. Switchable Surface Hydrophobicity–Hydrophilicity of a Metal–Organic Framework. Angew. Chem., Int. Ed. 2016, 55, 16049–16053. [Google Scholar] [CrossRef]
- Tan, F.; López-Periago, A.; Light, M.E.; Cirera, J.; Ruiz, E.; Borrás, A.; Teixidor, F.; Viñas, C.; Domingo, C.; Planas, J.G. An Unprecedented Stimuli-Controlled Single-Crystal Reversible Phase Transition of a Metal–Organic Framework and Its Application to a Novel Method of Guest Encapsulation. Adv. Mater. 2018, 30, 1800726. [Google Scholar] [CrossRef]
- Tsang, M.Y.; Rodríguez-Hermida, S.; Stylianou, K.C.; Tan, F.; Negi, D.; Teixidor, F.; Viñas, C.; Choquesillo-Lazarte, D.; Verdugo-Escamilla, C.; Guerrero, M.; et al. Carborane Bis-pyridylalcohols as Linkers for Coordination Polymers: Synthesis, Crystal Structures, and Guest-Framework Dependent Mechanical Properties. Cryst. Growth Des. 2017, 17, 846–857. [Google Scholar] [CrossRef]
- Li, Z.; Fraile, J.; Viñas, C.; Teixidor, F.; Planas, J.G. Post-synthetic modification of a highly flexible 3D soft porous metal–organic framework by incorporating conducting polypyrrole: Enhanced MOF stability and capacitance as an electrode material. Chem. Commun. 2021, 57, 2523–2526. [Google Scholar] [CrossRef]
- Cui, P.-F.; Liu, X.-R.; Jin, G.-X. Supramolecular Architectures Bearing Half-Sandwich Iridium- or Rhodium-Based Carboranes: Design, Synthesis, and Applications. J. Am. Chem. Soc. 2023, 145, 19440–19457. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.-F.; Liu, X.-R.; Guo, S.-T.; Lin, Y.-J.; Jin, G.-X. Steric-Effects-Directed B–H Bond Activation of para-Carboranes. J. Am. Chem. Soc. 2021, 143, 5099–5105. [Google Scholar] [CrossRef]
- Liu, X.-R.; Cui, P.-F.; Guo, S.-T.; Lin, Y.-J.; Jin, G.-X. “Cage Walking” Synthetic Strategy for Unusual Unsymmetrical Supramolecular Cages. J. Am. Chem. Soc. 2023, 145, 8569–8575. [Google Scholar] [CrossRef]
- Cui, P.-F.; Liu, X.-R.; Lin, Y.-J.; Li, Z.-H.; Jin, G.-X. Highly Selective Separation of Benzene and Cyclohexane in a Spatially Confined Carborane Metallacage. J. Am. Chem. Soc. 2022, 144, 6558–6565. [Google Scholar] [CrossRef]
- Cui, P.-F.; Lin, Y.-J.; Li, Z.-H.; Jin, G.-X. Dihydrogen Bond Interaction Induced Separation of Hexane Isomers by Self-Assembled Carborane Metallacycles. J. Am. Chem. Soc. 2020, 142, 8532–8538. [Google Scholar] [CrossRef]
- Fujii, S.; Masuno, H.; Taoda, Y.; Kano, A.; Wongmayura, A.; Nakabayashi, M.; Ito, N.; Shimizu, M.; Kawachi, E.; Hirano, T.; et al. Boron Cluster-based Development of Potent Nonsecosteroidal Vitamin D Receptor Ligands: Direct Observation of Hydrophobic Interaction between Protein Surface and Carborane. J. Am. Chem. Soc. 2011, 133, 20933–20941. [Google Scholar] [CrossRef]
- Shi, Y.; Fu, Q.; Li, J.; Liu, H.; Zhang, Z.; Liu, T.; Liu, Z. Covalent Organic Polymer as a Carborane Carrier for Imaging-Facilitated Boron Neutron Capture Therapy. ACS Appl. Mater. Interfaces 2020, 12, 55564–55573. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, Z.; Fu, Q.; Shen, X.; Zhang, Z.; Sun, W.; Wang, J.; Sun, J.; Zhang, Z.; Liu, T.; et al. Localized nuclear reaction breaks boron drug capsules loaded with immune adjuvants for cancer immunotherapy. Nat. Commun. 2023, 14, 1884. [Google Scholar] [CrossRef]
- Li, M.; Yu, J.; Xue, Y.; Wang, K.; Wang, Q.; Xie, Z.; Wang, L.; Yang, Y.; Wu, J.; Qiu, X.; et al. Preparation of Carborane-Tailored Covalent Organic Frameworks by a Postsynthetic Modification Strategy as a Barrier to Polysulfide in Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2023, 15, 2922–2932. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wang, P.; Jiang, D. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 16068. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, J.; Qiu, X.; Li, M.; He, G.; Wang, Q.; Xie, Z.; Li, X.; Yu, H. Amphiphilic Carborane-Based Covalent Organic Frameworks as Efficient Polysulfide Nano-Trappers for Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2021, 13, 60373–60383. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cui, Q.; Chen, H.; Huang, N. Carborane-Based Three-Dimensional Covalent Organic Frameworks. J. Am. Chem. Soc. 2023, 145, 24202–24209. [Google Scholar] [CrossRef]
- Segura, J.L.; Royuela, S.; Mar Ramos, M. Post-synthetic modification of covalent organic frameworks. Chem. Soc. Rev. 2019, 48, 3903–3945. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Lin, X.; Huang, J.; Zhang, L. Recent Advances in Carborane-Based Crystalline Porous Materials. Molecules 2024, 29, 3916. https://doi.org/10.3390/molecules29163916
Meng Y, Lin X, Huang J, Zhang L. Recent Advances in Carborane-Based Crystalline Porous Materials. Molecules. 2024; 29(16):3916. https://doi.org/10.3390/molecules29163916
Chicago/Turabian StyleMeng, Yuxuan, Xi Lin, Jinyi Huang, and Liangliang Zhang. 2024. "Recent Advances in Carborane-Based Crystalline Porous Materials" Molecules 29, no. 16: 3916. https://doi.org/10.3390/molecules29163916
APA StyleMeng, Y., Lin, X., Huang, J., & Zhang, L. (2024). Recent Advances in Carborane-Based Crystalline Porous Materials. Molecules, 29(16), 3916. https://doi.org/10.3390/molecules29163916





