Synthesis and Characterization of Copper(II) and Nickel(II) Complexes with 3-(Morpholin-4-yl)propane-2,3-dione 4-Allylthiosemicarbazone Exploring the Antibacterial, Antifungal and Antiradical Properties
Abstract
1. Introduction
2. Results and Discussions
3. Materials and Methods
3.1. Materials
3.2. Synthesis
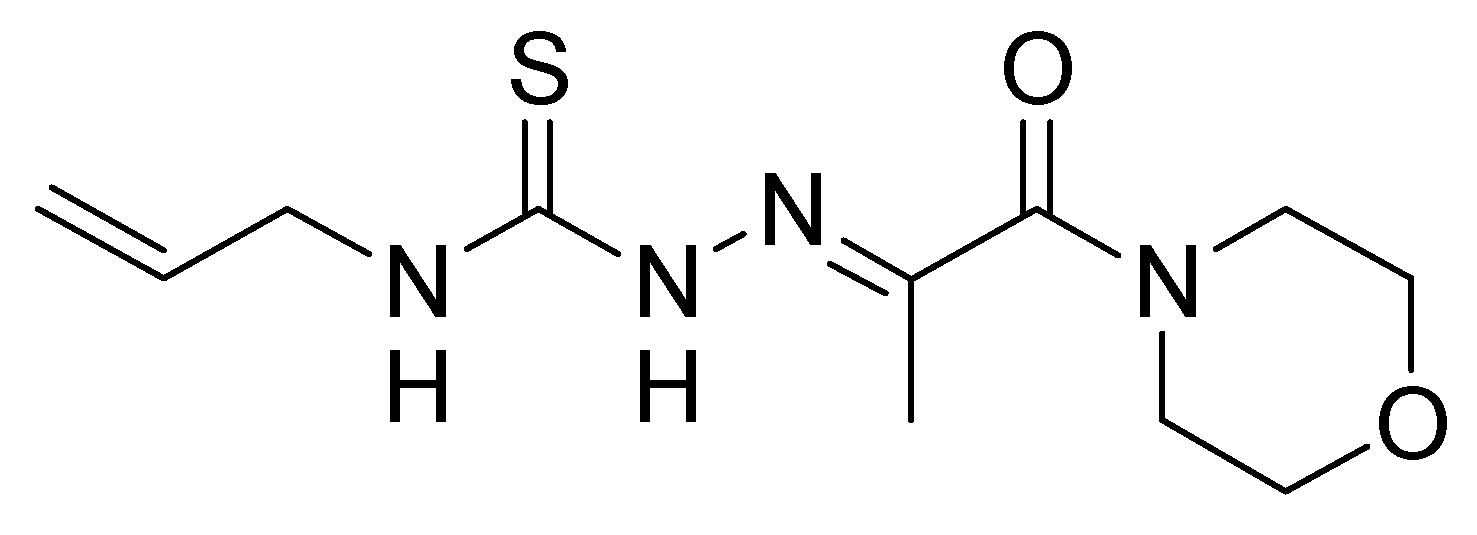
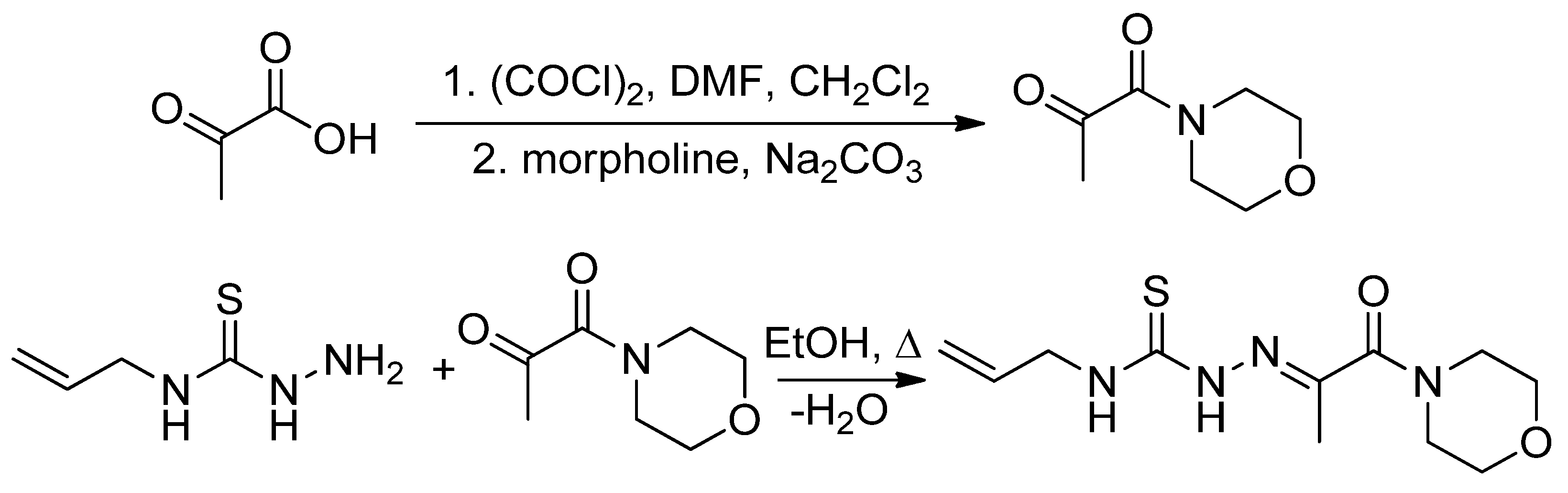
3.2.1. Synthesis of 3-(Morpholin-4-yl)propane-2,3-dione 4-allylthiosemicarbazone
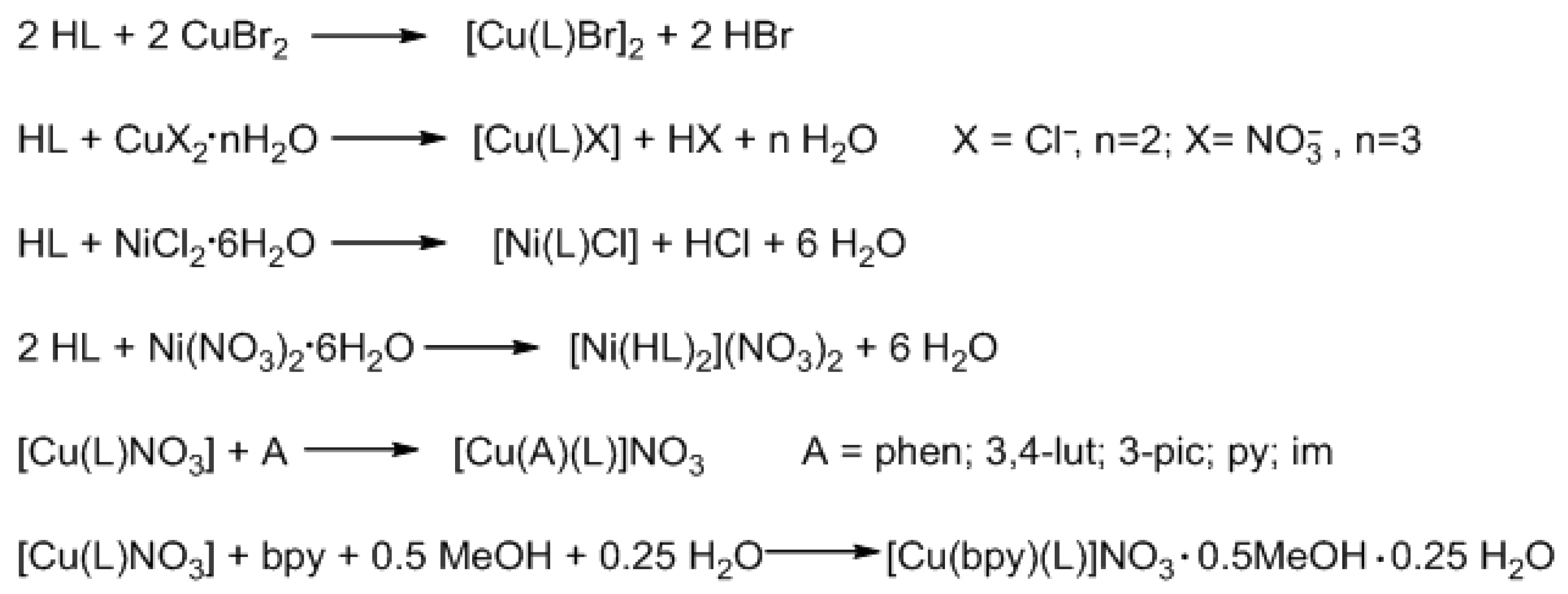
3.2.2. Synthesis of Coordination Compounds
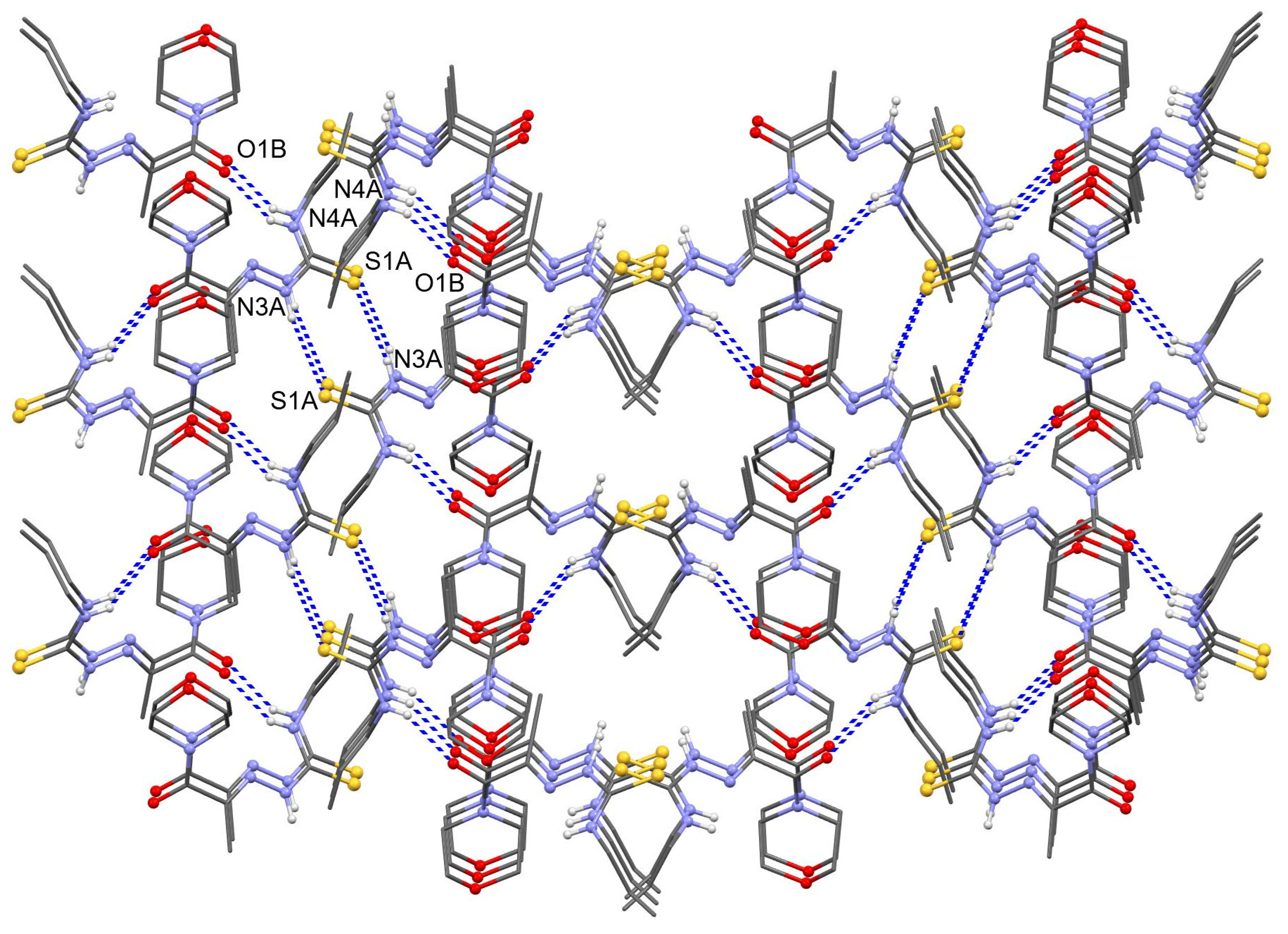
3.3. X-ray Crystallography
3.4. Antibacterial and Antifungal Activity
3.5. Antiradical Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Priyarega, S.; Haribabu, J.; Karvembu, R. Development of thiosemicarbazone-based transition metal complexes as homogeneous catalysts for various organic transformations. Inorganica Chim. Acta 2022, 532, 120742. [Google Scholar] [CrossRef]
- Thamaraichelvan, M.; Sebastian, A.; Ganapathi, M.; Holla, H.; Duraippandi, P.; Narayanan, N.V. Copper thiosemicarbazone modified electrode for hydrazine electrocatalytic oxidation. Results Chem. 2023, 6, 101025. [Google Scholar] [CrossRef]
- Korkmaz, G. A review of recent research on the antimicrobial activities of thiosemicarbazone-based compounds. J. New Results Sci. 2024, 13, 61–83. [Google Scholar] [CrossRef]
- Khan, T.; Zehra, S.; Alvi, A.; Mishra, N.; Lawrence, R.; Joshi, S.; Khan, A.R. Synthesis, Characterization, Computational Studies and Antimicrobial activity evaluation of Mixed ligand-Metal complexes of selected thiosemicarbazones. ChemistrySelect 2024, 9, e202400202. [Google Scholar] [CrossRef]
- Li, T.; Lv, M.; Wen, H.; Du, J.; Wang, Z.; Zhang, S.; Xu, H. Natural products in crop protection: Thiosemicarbazone derivatives of 3-acetyl-N-benzylindoles as antifungal agents and their mechanism of action. Pest Manag. Sci. 2023, 79, 2801–2810. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.A.; Cunha, V.L.; de Souza, J.H.; Martins, C.H.; Franca, E.D.F.; Pivatto, M.; Ellena, J.A.; Faustino, L.A.; Patrocinio, A.O.D.T.; Deflon, V.M.; et al. Zinc (II) complexes bearing N, N, S ligands: Synthesis, crystal structure, spectroscopic analysis, molecular docking and biological investigations about its antifungal activity. J. Inorg. Biochem. 2022, 237, 111995. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.F.; Marques, F.; Pinheiro, T.; Villa de Brito, M.J.; Scalese, G.; Pérez-Díaz, L.; Otero, L.; António, J.P.M.; Gambino, D.; Morais, T.S. Copper (I)-Thiosemicarbazone Complexes with Dual Anticancer and Antiparasitic Activity. ChemMedChem 2023, 18, e202300074. [Google Scholar] [CrossRef]
- Shakya, B.; Yadav, P.N. Thiosemicarbazones as potent anticancer agents and their modes of action. Mini-Rev. Med. Chem. 2020, 20, 638–661. [Google Scholar] [CrossRef] [PubMed]
- Tok, F.; Küçükal, B.; Baltaş, N.; Tatar Yılmaz, G.; Koçyiğit-Kaymakçıoğlu, B. Synthesis of novel thiosemicarbazone derivatives as antidiabetic agent with enzyme kinetic studies and antioxidant activity. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 1284–1294. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeeswari, P.; Thirumurugan, R.; Pavana, R.K. Discovery of new antitubercular oxazolyl thiosemicarbazones. J. Med. Chem. 2006, 49, 3448–3450. [Google Scholar] [CrossRef]
- Hegde, P.L.; Bhat, S.S.; Revankar, V.K.; Shaikh, S.A.; Kumara, K.; Lokanath, N.K. Syntheses, structural characterization and evaluation of the anti-tubercular activity of copper (II) complexes containing 3-methoxysalicylaldehyde-4-methylthiosemicarbazone. J. Mol. Struct. 2022, 1257, 132589. [Google Scholar] [CrossRef]
- Hosseini-Yazdi, S.A.; Mirzaahmadi, A.; Khandar, A.A.; Mahdavi, M.; Rahimian, A.; Eigner, V.; Dušek, M.; Zarrini, G. Copper, nickel and zinc complexes of a new water-soluble thiosemicarbazone ligand: Synthesis, characterization, stability and biological evaluation. J. Mol. Liq. 2017, 248, 658–667. [Google Scholar] [CrossRef]
- Sobiesiak, M.; Cieślak, M.; Krolewska, K.; Kaźmierczak-Barańska, J.; Pasternak, B.; Budzisz, E. Thiosemicarbazone-derived copper (II), cobalt (II) and nickel (II) complexes as potential anticancer agents: Nuclease activity, cytotoxicity and apoptosis studies. New J. Chem. 2016, 40, 9761–9767. [Google Scholar] [CrossRef]
- Aljahdali, M.S. Nickel (II) complexes of novel thiosemicarbazone compounds: Synthesis, characterization, molecular modeling and in vitro antimicrobial activity. Eur. J. Chem. 2013, 4, 434–443. [Google Scholar] [CrossRef]
- Jouad, E.M.; Larcher, G.; Allain, M.; Riou, A.; Bouet, G.M.; Khan, M.A.; Do Thanh, X. Synthesis, structure and biological activity of nickel (II) complexes of 5-methyl 2-furfural thiosemicarbazone. J. Inorg. Biochem. 2001, 86, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.B.; Farh, M.K.; Mayer, P. Synthesis, structural studies and antimicrobial evaluation of nickel (II) complexes of NNS tridentate thiosemicarbazone based ligands. Appl. Organomet. Chem. 2019, 33, e4883. [Google Scholar] [CrossRef]
- West, D.X.; Liberta, A.E.; Padhye, S.B.; Chikate, R.C.; Sonawane, P.B.; Kumbhar, A.S.; Yerande, R.G. Thiosemicarbazone complexes of copper (II): Structural and biological studies. Coord. Chem. Rev. 1993, 123, 49–71. [Google Scholar] [CrossRef]
- Ilies, D.C.; Shova, S.; Radulescu, V.; Pahontu, E.; Rosu, T. Synthesis, characterization, crystal structure and antioxidant activity of Ni(II) and Cu(II) complexes with 2-formilpyridine N(4)-phenylthiosemicarbazone. Polyhedron 2015, 97, 157–166. [Google Scholar] [CrossRef]
- Eğlence-Bakır, S. New nickel (II) complexes containing N2O2 donor thiosemicarbazones: Synthesis, characterization and antioxidant properties. J. Mol. Struct. 2021, 1246, 131121. [Google Scholar] [CrossRef]
- Hosseini-Yazdi, S.A.; Mirzaahmadi, A.; Khandar, A.A.; Eigner, V.; Dušek, M.; Mahdavi, M.; Soltani, S.; Lotfipour, F.; White, J. Reactions of copper (II), nickel (II), and zinc (II) acetates with a new water-soluble 4-phenylthiosemicarbazone Schiff base ligand: Synthesis, characterization, unexpected cyclization, antimicrobial, antioxidant, and anticancer activities. Polyhedron 2017, 124, 156–165. [Google Scholar] [CrossRef]
- Ramachandran, E.; Kalaivani, P.; Prabhakaran, R.; Rath, N.P.; Brinda, S.; Poornima, P.; Padma, V.; Natarajan, K. Synthesis, X-ray crystal structure, DNA binding, antioxidant and cytotoxicity studies of Ni (II) and Pd (II) thiosemicarbazone complexes. Metallomics 2012, 4, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Bal-Demirci, T.; Şahin, M.; Özyürek, M.; Kondakçı, E.; Ülküseven, B. Synthesis, antioxidant activities of the nickel (II), iron (III) and oxovanadium (IV) complexes with N2O2 chelating thiosemicarbazones. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 126, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Graur, V.; Chumakov, Y.; Garbuz, O.; Hureau, C.; Tsapkov, V.; Gulea, A. Synthesis, Structure, and Biologic Activity of Some Copper, Nickel, Cobalt, and Zinc Complexes with 2-Formylpyridine N4-Allylthiosemicarbazone. Bioinorg. Chem. 2022, 2022, 2705332. [Google Scholar] [CrossRef] [PubMed]
- Gulea, A.P.; Graur, V.O.; Diurici, E.C.; Ulchina, I.I.; Bourosh, P.N.; Balan, G.G.; Burduniuc, O.S.; Tsapkov, V.I.; Rudic, V.F. Synthesis, Structure, and Biological Activity of Copper(II), Nickel(II), Cobalt(III), and Iron(III) Coordination Compounds with 2-{2-[(Prop-2-en-1-yl)carbamothioyl]hydrazinylidene}propanoic Acid. Russ. J. Gen. Chem. 2020, 90, 2120–2127. [Google Scholar] [CrossRef]
- Ulchina, I.; Graur, V.; Tsapkov, V.; Chumakov, Y.; Garbuz, O.; Burduniuc, O.; Ceban, E.; Gulea, A. Introducing N-Heteroaromatic Bases into Copper (II) Thiosemicarbazon Complexes: A Way to Change their Biological Activity. ChemistryOpen 2022, 11, e202200208. [Google Scholar] [CrossRef] [PubMed]
- Lukmantara, A.Y.; Kalinowski, D.S.; Kumar, N.; Richardson, D.R. Synthesis and biological evaluation of 2-benzoylpyridine thiosemicarbazones in a dimeric system: Structure–activity relationship studies on their anti-proliferative and iron chelation efficacy. J. Inorg. Biochem. 2014, 141, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Preston III, J.F.; Wei, C.I. Antibacterial mechanism of allyl isothiocyanate. J. Food Prot. 2000, 63, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Behray, M.; Wang, Q.; Wang, W.; Zhou, Z.; Chao, Y.; Bao, Y. Anti-cancer activities of allyl isothiocyanate and its conjugated silicon quantum dots. Sci. Rep. 2018, 8, 1084. [Google Scholar] [CrossRef] [PubMed]
- Tarar, A.; Peng, S.; Cheema, S.; Peng, C.A. Anticancer activity, mechanism, and delivery of allyl isothiocyanate. Bioengineering 2022, 9, 470. [Google Scholar] [CrossRef]
- Huseynova, M.; Medjidov, A.; Taslimi, P.; Aliyeva, M. Synthesis, Characterization, Crystal Structure of the Coordination Polymer Zn(II) with Thiosemicarbazone of Glyoxalic Acid and Their Inhibitory Properties Against Some Metabolic Enzymes. Bioorg. Chem. 2018, 83, 55–62. [Google Scholar] [CrossRef]
- Huseynova, M.; Taslimi, P.; Medjidov, A.; Farzaliyev, V.; Aliyeva, M.; Gondolova; Şahin, O.; Yalçın, B.; Sujayev, A.; Orman, E.B.; et al. Synthesis, Characterization, Crystal Structure, Electrochemical Studies and Biological Evaluation of Metal Complexes with Thiosemicarbazone of Glyoxylic Acid. Polyhedron 2018, 155, 25–33. [Google Scholar] [CrossRef]
- Belicchi Ferrari, M.; Bisceglie, F.; Gasparri Fava, G.; Pelosi, G.; Tarasconi, P.; Albertini, R.; Pinelli, S. Synthesis, characterization and biological activity of two new polymeric copper(II) complexes with α-ketoglutaric acid thiosemicarbazone. J. Inorg. Biochem. 2002, 89, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Miersch, J.; Krauss, G.J.; Grancharov, K.; Bublitz, F.; Spassovska, N.; Golovinsky, E. The effect of pyruvic acid thiosemicarbazone on ornithine carbamoyl transferase of Lycopersicon esculentum Mill. Biol. Plant. 1986, 28, 174–179. [Google Scholar] [CrossRef]
- Cao, R.; Garcia, A.; Castell, E. QSAR of copper(II) complexes with cytotoxic properties. Monatsh. Chem. 1992, 123, 487–491. [Google Scholar] [CrossRef]
- Diaz, A.; Cao, R.; Garcia, A. Characterization and biological properties of a copper(II) complex with pyruvic acid thiosemicarbazone. Monatsh. Chem. 1994, 125, 823–825. [Google Scholar] [CrossRef]
- Graur, I.; Bespalova, T.; Graur, V.; Tsapkov, V.; Garbuz, O.; Melnic, E.; Bourosh, P.; Gulea, A. A new thiosemicarbazone and its 3d metal complexes: Synthetic, structural, and antioxidant studies. J. Chem. Res. 2023, 47. [Google Scholar] [CrossRef]
- Kourounakis, A.P.; Xanthopoulos, D.; Tzara, A. Morpholine as a privileged structure: A review on the medicinal chemistry and pharmacological activity of morpholine containing bioactive molecules. Med. Res. Rev. 2020, 40, 709–752. [Google Scholar] [CrossRef] [PubMed]
- Lenci, E.; Calugi, L.; Trabocchi, A. Occurrence of morpholine in central nervous system drug discovery. ACS Chem. Neurosci. 2021, 12, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Singh, R.K. Morpholine as ubiquitous pharmacophore in medicinal chemistry: Deep insight into the structure-activity relationship (SAR). Bioorg. Chem 2020, 96, 103578. [Google Scholar] [CrossRef]
- Rupak, K.; Vulichi, S.R.; Suman, K.A.P.U.R. Emphasizing morpholine and its derivatives (maid): Typical candidate of pharmaceutical importance. Int. J. Chem. Sci. 2016, 14, 1777–1788. [Google Scholar]
- Asif, M.; Imran, M. A review on chemical and pharmacological interest of morpholine and pyrans derivatives. Fields Crop. Res. 2019, 1, 5–12. [Google Scholar] [CrossRef]
- Ohui, K.; Afanasenko, E.; Bacher, F.; Ting, R.L.X.; Zafar, A.; Blanco-Cabra, N.; Torrents, E.; Dömötör, O.; May, N.; Darvasiova, D.; et al. New water-soluble copper (II) complexes with morpholine–thiosemicarbazone hybrids: Insights into the anticancer and antibacterial mode of action. J. Med. Chem. 2018, 62, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Bacher, F.; Dömötör, O.; Chugunova, A.; Nagy, N.V.; Filipović, L.; Radulović, S.; Enyedy, E.; Arion, V.B. Strong effect of copper (II) coordination on antiproliferative activity of thiosemicarbazone–piperazine and thiosemicarbazone–morpholine hybrids. Dalton Trans. 2015, 44, 9071–9090. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.C.; Booth, B.A.; DeNuzzo, S.M.; Sartorelli, A.C. Potential antitumor agents. 14. 4-substituted 2-formylpyridine thiosemicarbazones. J. Med. Chem. 1976, 19, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Tasleem, M.; Pelletier, J.; Sévigny, J.; Hussain, Z.; Khan, A.; Al-Harrasi, A.; El-kott, A.; Taslimi, P.; Negm, S.; Shafiq, Z.; et al. Synthesis, in vitro, and in silico studies of morpholine-based thiosemicarbazones as ectonucleotide pyrophosphatase/phosphodiesterase-1 and-3 inhibitors. Int. J. Biol. Macromol. 2024, 266, 131068. [Google Scholar] [CrossRef] [PubMed]
- Muralisankar, M.; Haribabu, J.; Bhuvanesh, N.S.; Karvembu, R.; Sreekanth, A. Synthesis, X-ray crystal structure, DNA/protein binding, DNA cleavage and cytotoxicity studies of N(4) substituted thiosemicarbazone based copper (II)/nickel (II) complexes. Inorganica Chim. Acta 2016, 449, 82–95. [Google Scholar] [CrossRef]
- Ibrahim, M.; Farh, A.B.; Plaisier, M.K.; Shalaby, E.M. Synthesis, structural and antimicrobial studies of binary and ternary complexes of a new tridentate thiosemicarbazone. Future Med. Chem. 2018, 10, 2507–2519. [Google Scholar] [CrossRef]
- Youssef, N.S.; Hegab, K.H. Synthesis and Characterization of some Transition Metal Complexes of Thiosemicarbazones Derived from 2-acetylpyrrole and 2-acetylfuran. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2005, 35, 391–399. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, M. Synthesis and characterization of some multi-substituted thiosemicarbazones as the multi-dental ligands of metal ions. Chin. J. Org. Chem. 2001, 21, 681–684. [Google Scholar]
- Perrin, D.D.; Armarego, W.L.; Perrin, D.R. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann/Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Leovac, V.M.; Vojinović, L.S.; Mesaroš-Sečenji, K.F.; Češljević, V.I. Transition metal complexes with thiosemicarbazide-based ligands, part 46: Synthesis and physico-chemical characterization of mixed ligand cobalt (III)-complexes with salicylaldehyde semi-, thiosemi-an. J. Serb. Chem. Soc. 2003, 68, 919–927. [Google Scholar] [CrossRef]
- Mupparapu, N.; Khan, S.; Battula, S.; Kushwaha, M.; Gupta, A.P.; Ahmed, Q.N.; Vishwakarma, R.A. Metal-free oxidative amidation of 2-oxoaldehydes: A facile access to α-ketoamides. Org. Lett. 2014, 16, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- CrysAlis RED, version 1.171.36.32; Oxford Diffraction Ltd.: Abingdon, UK, 2003.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A Found. Adv. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Garbuz, O.; Gudumac, V.; Toderas, I.; Gulea, A. Antioxidant Properties of Synthetic Compounds and Natural Products. Action Mechanisms; Monograph; CEP USM: Chișinău, Moldova, 2023; ISBN 978-9975-62-516-6. [Google Scholar]







| Compound | Staphylococcus aureus ATCC 25923 | Bacillus cereus ATCC 11778 | Acinetobacter baumannii BAA-747 | Escherichia coli ATCC 25922 | Candida albicans ATCC 10231 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MFC | |
| HL | - | - | 62.5 | 62.5 | 250 | 500 | - | - | - | - |
| 1 | 125 | 125 | 62.5 | 62.5 | - | - | - | - | - | - |
| 2 | 250 | 250 | 125 | 125 | 500 | - | - | - | - | - |
| 3 | 62.5 | 125 | 125 | 125 | - | - | - | - | 500 | 500 |
| 5 | 500 | - | 125 | 125 | - | - | - | - | - | - |
| 6 | 31.3 | 31.3 | 15.6 | 15.6 | 125 | 125 | 250 | 250 | 15.6 | 31.3 |
| 7 | 250 | 250 | 250 | 250 | 500 | 500 | - | - | 62.50 | 250 |
| 8 | 125 | 125 | 62.50 | 125 | - | - | - | - | 500 | - |
| 9 | 31.3 | 31.3 | 15.6 | 31.3 | - | - | - | - | 250 | 500 |
| 10 | 31.3 | 125 | 15.6 | 31.3 | - | - | 500 | 500 | - | - |
| 11 | 62.5 | 62.5 | 62.5 | 62.5 | - | - | - | - | 250 | 500 |
| Furacillinum | 9.3 | 9.3 | 4.7 | 4.7 | 18.5 | 37.5 | 4.7 | 9.4 | - | - |
| Nystatine | - | - | - | - | - | - | - | - | 80 | 80 |
| Fluconazole | - | - | - | - | - | - | - | - | 15.6 | 31.3 |
| Compound | HL | 1 | 2 | 3 | 4 | 5 | 6 |
| IC50, μM | 94.4 ± 4.9 | 168.5 ± 5.0 | 128.4 ± 3.5 | 140.3 ± 3.5 | 30.7 ± 0.8 | 19.6 ± 0.1 | 23.0 ± 0.3 |
| Compound | 7 | 8 | 9 | 10 | 11 | Trolox | |
| IC50, μM | 22.3 ± 0.3 | 7.3 ± 0.3 | 6.7 ± 0.2 | 17.1 ± 0.7 | 11.4 ± 0.4 | 33.3 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graur, I.; Graur, V.; Cadin, M.; Garbuz, O.; Bourosh, P.; Melnic, E.; Lozan-Tirsu, C.; Balan, G.; Tsapkov, V.; Fala, V.; et al. Synthesis and Characterization of Copper(II) and Nickel(II) Complexes with 3-(Morpholin-4-yl)propane-2,3-dione 4-Allylthiosemicarbazone Exploring the Antibacterial, Antifungal and Antiradical Properties. Molecules 2024, 29, 3903. https://doi.org/10.3390/molecules29163903
Graur I, Graur V, Cadin M, Garbuz O, Bourosh P, Melnic E, Lozan-Tirsu C, Balan G, Tsapkov V, Fala V, et al. Synthesis and Characterization of Copper(II) and Nickel(II) Complexes with 3-(Morpholin-4-yl)propane-2,3-dione 4-Allylthiosemicarbazone Exploring the Antibacterial, Antifungal and Antiradical Properties. Molecules. 2024; 29(16):3903. https://doi.org/10.3390/molecules29163903
Chicago/Turabian StyleGraur, Ianina, Vasilii Graur, Marina Cadin, Olga Garbuz, Pavlina Bourosh, Elena Melnic, Carolina Lozan-Tirsu, Greta Balan, Victor Tsapkov, Valeriu Fala, and et al. 2024. "Synthesis and Characterization of Copper(II) and Nickel(II) Complexes with 3-(Morpholin-4-yl)propane-2,3-dione 4-Allylthiosemicarbazone Exploring the Antibacterial, Antifungal and Antiradical Properties" Molecules 29, no. 16: 3903. https://doi.org/10.3390/molecules29163903
APA StyleGraur, I., Graur, V., Cadin, M., Garbuz, O., Bourosh, P., Melnic, E., Lozan-Tirsu, C., Balan, G., Tsapkov, V., Fala, V., & Gulea, A. (2024). Synthesis and Characterization of Copper(II) and Nickel(II) Complexes with 3-(Morpholin-4-yl)propane-2,3-dione 4-Allylthiosemicarbazone Exploring the Antibacterial, Antifungal and Antiradical Properties. Molecules, 29(16), 3903. https://doi.org/10.3390/molecules29163903







