Green Synthesis of Silver Nanoparticles Using Cashew Nutshell Liquid (CNSL): Characterization and Methylene Blue Removal Studies
Abstract
1. Introduction
2. Results
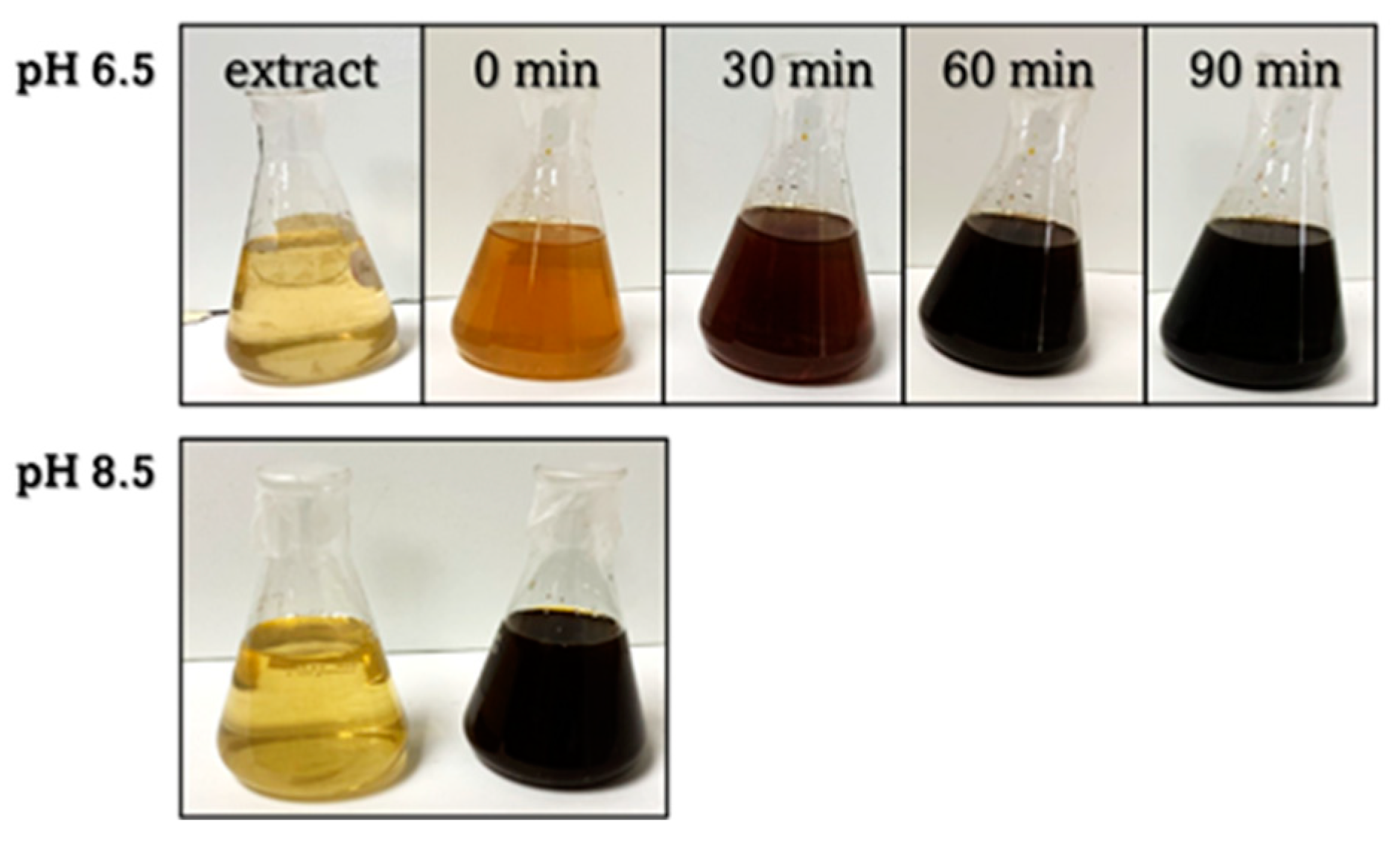
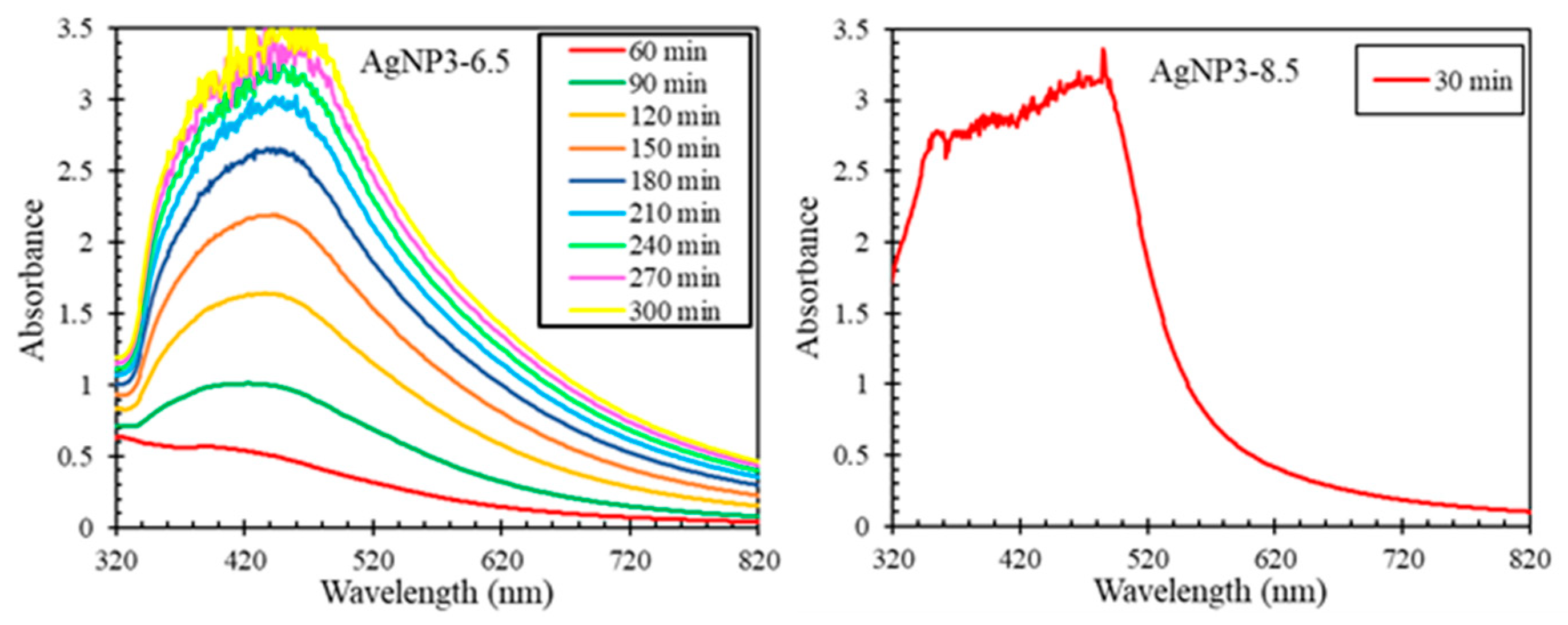
2.1. Formation Rates of AgNPs
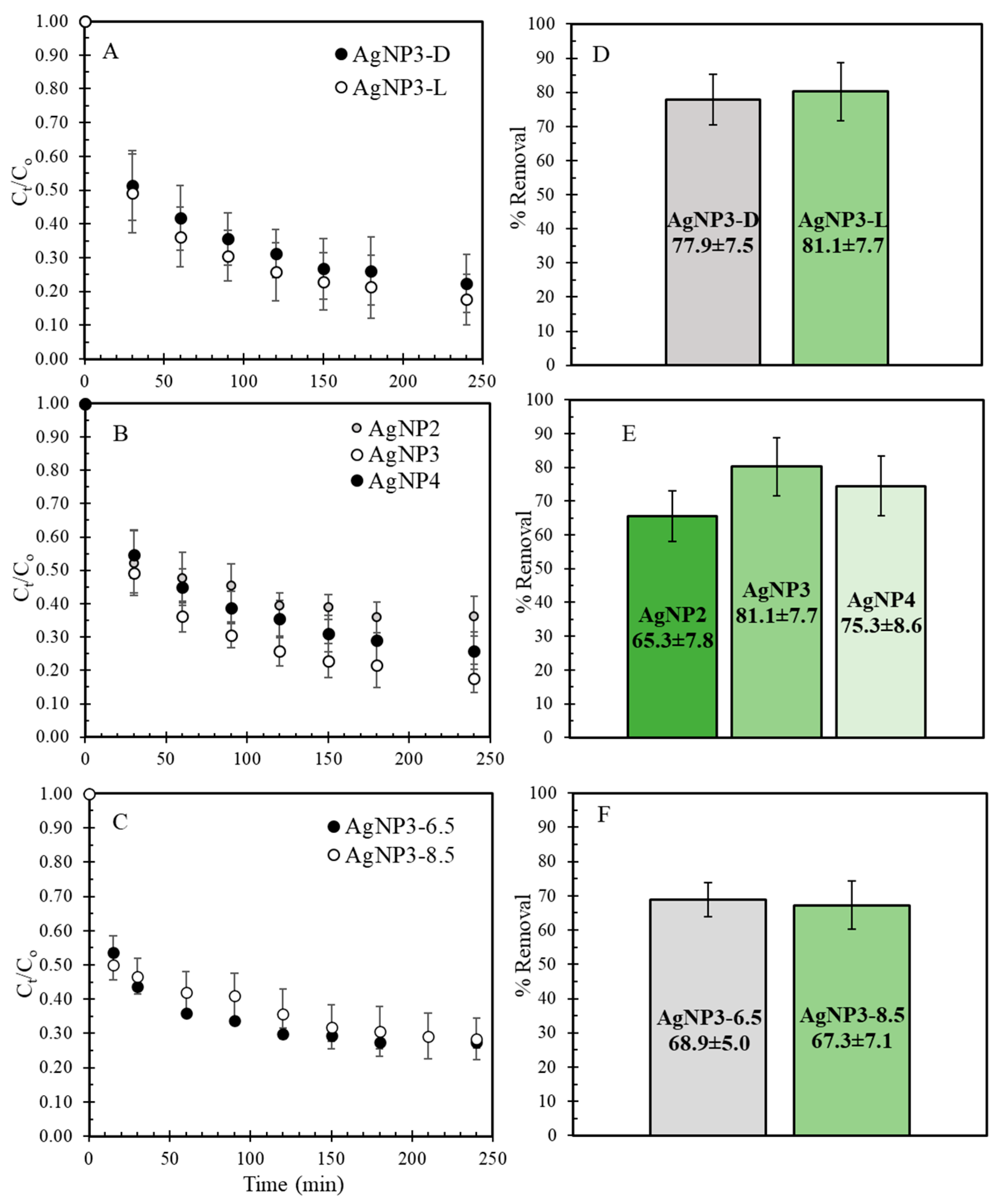
2.2. MB Removal Studies
2.3. XRD Analysis
2.4. XPS Analysis
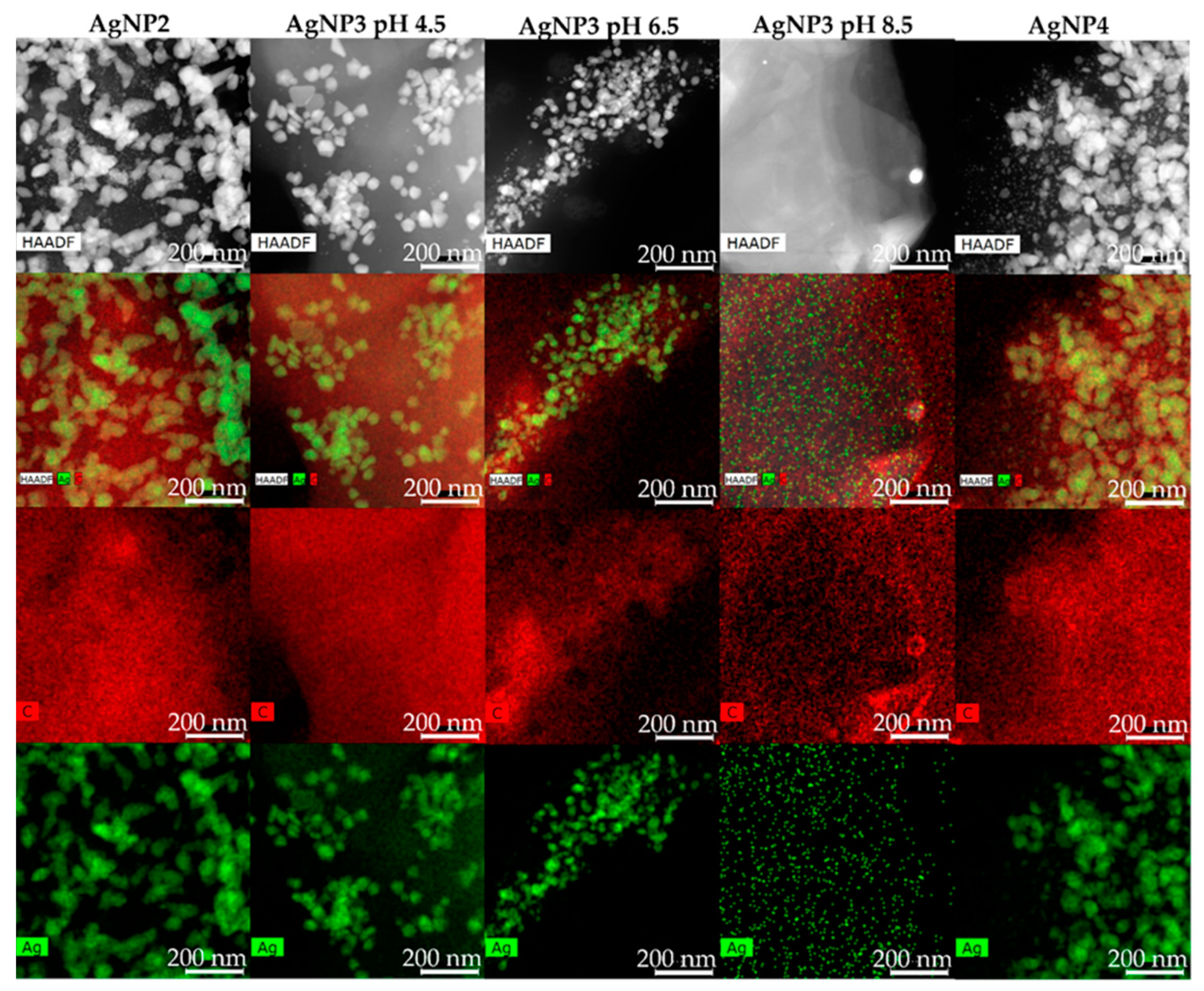
2.5. HRTEM/STEM-EDX Analysis
3. Discussion
4. Materials and Methods
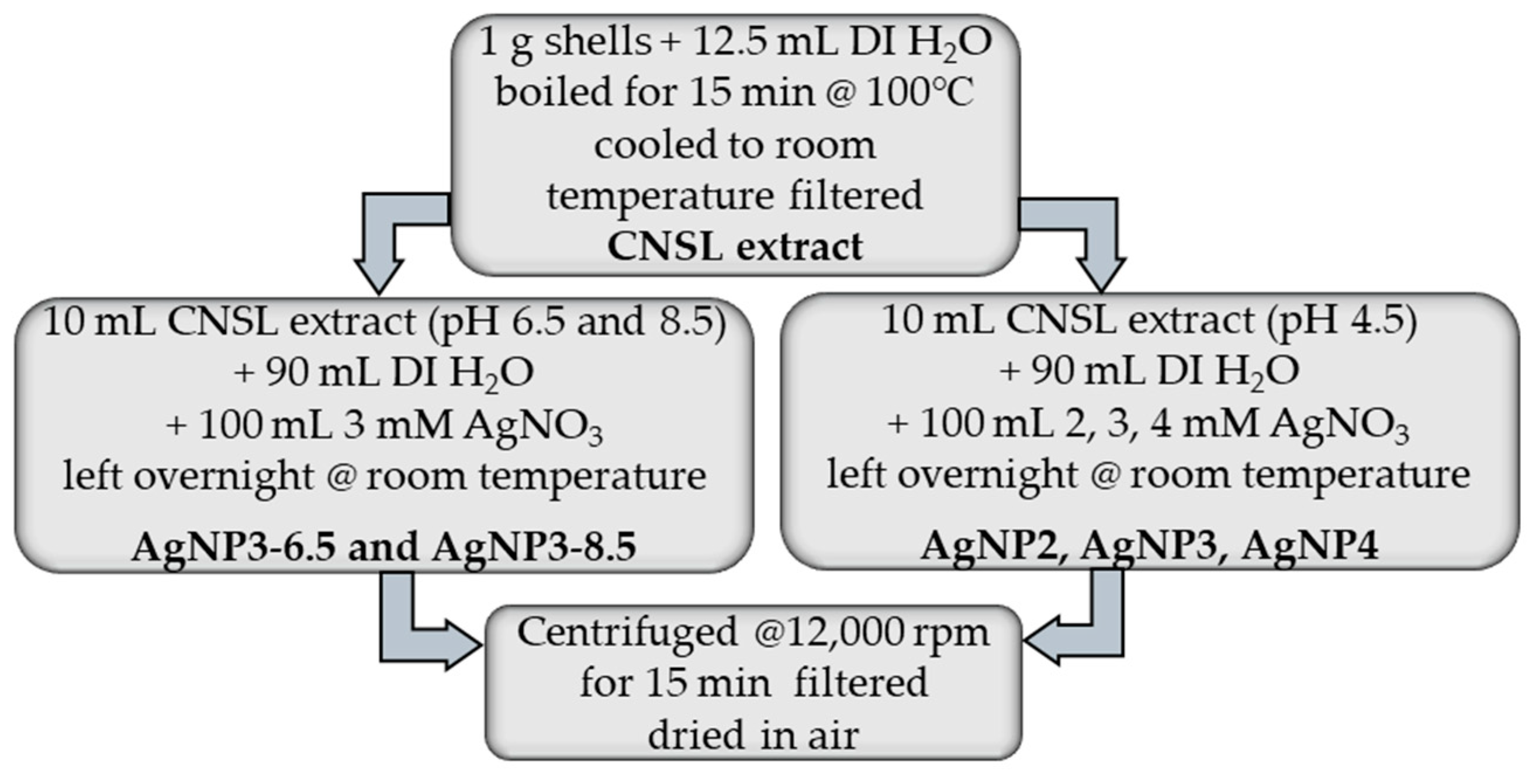
4.1. Preparation of AgNPs
4.2. Dye Removal Studies
4.3. X-ray Photoelectron Spectroscopy (XPS)
4.4. X-ray Diffraction (XRD)
4.5. High-Resolution Transmission Electron Microscopy (HRTEM)/Scanning Transmission Electron Microscopy (STEM)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A Review on Biosynthesis of Silver Nanoparticles and Their Biocidal Properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Sumi, M.B.; Devadiga, A.; Shetty, K.V.; Saidutta, M.B. Solar Photocatalytically Active, Engineered Silver Nanoparticle Synthesis Using Aqueous Extract of Mesocarp of Cocos nucifera (Red Spicata Dwarf). J. Exp. Nanosci. 2017, 12, 14–32. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. JFB 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Liaqat, N.; Jahan, N.; Khalil-Ur-Rahman; Anwar, T.; Qureshi, H. Green Synthesized Silver Nanoparticles: Optimization, Characterization, Antimicrobial Activity, and Cytotoxicity Study by Hemolysis Assay. Front. Chem. 2022, 10, 952006. [Google Scholar] [CrossRef]
- Habeeb Rahuman, H.B.; Dhandapani, R.; Narayanan, S.; Palanivel, V.; Paramasivam, R.; Subbarayalu, R.; Thangavelu, S.; Muthupandian, S. Medicinal Plants Mediated the Green Synthesis of Silver Nanoparticles and Their Biomedical Applications. IET Nanobiotechnol. 2022, 16, 115–144. [Google Scholar] [CrossRef] [PubMed]
- Varghese Alex, K.; Tamil Pavai, P.; Rugmini, R.; Shiva Prasad, M.; Kamakshi, K.; Sekhar, K.C. Green Synthesized Ag Nanoparticles for Bio-Sensing and Photocatalytic Applications. ACS Omega 2020, 5, 13123–13129. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Panda, S.K.; Bastia, A.K.; Mohanta, T.K. Biosynthesis of Silver Nanoparticles from Protium serratum and Investigation of Their Potential Impacts on Food Safety and Control. Front. Microbiol. 2017, 8, 626. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, Applications, Toxicity and Toxicity Mechanisms of Silver Nanoparticles: A Review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Vanaja, M.; Paulkumar, K.; Baburaja, M.; Rajeshkumar, S.; Gnanajobitha, G.; Malarkodi, C.; Sivakavinesan, M.; Annadurai, G. Degradation of Methylene Blue Using Biologically Synthesized Silver Nanoparticles. Bioinorg. Chem. Appl. 2014, 2014, 742346. [Google Scholar] [CrossRef]
- Devi, T.A.; Ananthi, N.; Amaladhas, T.P. Photobiological Synthesis of Noble Metal Nanoparticles Using Hydrocotyle Asiatica and Application as Catalyst for the Photodegradation of Cationic Dyes. J. Nanostruct. Chem. 2016, 6, 75–92. [Google Scholar] [CrossRef]
- Lugaresi, O.; Perales-Rondón, J.V.; Minguzzi, A.; Solla-Gullón, J.; Rondinini, S.; Feliu, J.M.; Sánchez-Sánchez, C.M. Rapid Screening of Silver Nanoparticles for the Catalytic Degradation of Chlorinated Pollutants in Water. Appl. Catal. B Environ. 2015, 163, 554–563. [Google Scholar] [CrossRef]
- Gola, D.; Kriti, A.; Bhatt, N.; Bajpai, M.; Singh, A.; Arya, A.; Chauhan, N.; Srivastava, S.K.; Tyagi, P.K.; Agrawal, Y. Silver Nanoparticles for Enhanced Dye Degradation. Curr. Res. Green Sustain. Chem. 2021, 4, 100132. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Y.; Sun, F.; Chen, J. Research Status and Prospect of Nano Silver (Ag)-Modified Photocatalytic Materials for Degradation of Organic Pollutants. Environ. Sci. Pollut. Res. 2023, 31, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A. Silver Nanoparticles: Synthesis, Characterisation and Biomedical Applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef] [PubMed]
- Chugh, D.; Viswamalya, V.S.; Das, B. Green Synthesis of Silver Nanoparticles with Algae and the Importance of Capping Agents in the Process. J. Genet. Eng. Biotechnol. 2021, 19, 126. [Google Scholar] [CrossRef]
- Khan, Y.; Nasar, M.; Numan, M.; Ullah, I.; Shinwari, Z. Biomimetic Synthesis of Silver Nanoparticles for Breast Cancer Therapeutics and Its Mechanism. Int. J. Nanotechnol. Nanomed. 2018, 3, 1–9. [Google Scholar]
- Talabani, R.F.; Hamad, S.M.; Barzinjy, A.A.; Demir, U. Biosynthesis of Silver Nanoparticles and Their Applications in Harvesting Sunlight for Solar Thermal Generation. Nanomaterials 2021, 11, 2421. [Google Scholar] [CrossRef]
- De Melo, A.P.Z.; De Oliveira Brisola Maciel, M.V.; Sganzerla, W.G.; Da Rosa Almeida, A.; De Armas, R.D.; Machado, M.H.; Da Rosa, C.G.; Nunes, M.R.; Bertoldi, F.C.; Barreto, P.L.M. Antibacterial Activity, Morphology, and Physicochemical Stability of Biosynthesized Silver Nanoparticles Using Thyme (Thymus vulgaris) Essential Oil. Mater. Res. Express 2020, 7, 015087. [Google Scholar] [CrossRef]
- Solís-Sandí, I.; Cordero-Fuentes, S.; Pereira-Reyes, R.; Vega-Baudrit, J.R.; Batista-Menezes, D.; Montes De Oca-Vásquez, G. Optimization of the Biosynthesis of Silver Nanoparticles Using Bacterial Extracts and Their Antimicrobial Potential. Biotechnol. Rep. 2023, 40, e00816. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Iydroose, M.; Lee, S.-M.; Cho, M.; Park, J.-H.; Balachandar, V.; Oh, B.-T. Synthesis of Silver and Gold Nanoparticles Using Cashew Nut Shell Liquid and Its Antibacterial Activity Against Fish Pathogens. Indian J. Microbiol. 2014, 54, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Gaitán-Jiménez, S.-Y.; Restrepo-Sánchez, L.-P.; Parada-Alfonso, F.; Narváez-Cuenca, C.-E. Cashew (Anacardium occidentale) Nut-Shell Liquid as Antioxidant in Bulk Soybean Oil. Molecules 2022, 27, 8733. [Google Scholar] [CrossRef] [PubMed]
- De Lima, S.G.; Feitosa, C.M.; Cito, A.M.G.L.; Moita Neto, J.M.; Lopes, J.A.D.; Leite, A.S.; Brito, M.C.; Dantas, S.M.M.; Melo Cavalcante, A.A.C. Effects of Immature Cashew Nut-Shell Liquid (Anacardium occidentale) against Oxidative Damage in Saccharomyces Cerevisiae and Inhibition of Acetylcholinesterase Activity. Genet. Mol. Res. 2008, 7, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.C.; de Morais, S.M.; Magalhães, D.V.; Batista, W.P.; Vieira, Í.G.P.; Craveiro, A.A.; de Manezes, J.E.S.A.; Carvalho, A.F.U.; de Lima, G.P.G. Antioxidant, Larvicidal and Antiacetylcholinesterase Activities of Cashew Nut Shell Liquid Constituents. Acta Trop. 2011, 117, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Khan, N.; Gul, S.; Khan, S.; Khan, H. Contamination of Water Resources by Food Dyes and Its Removal Technologies. In Water Chemistry; Eyvaz, M., Yüksel, E., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Spagnoli, A.A.; Giannakoudakis, D.A.; Bashkova, S. Adsorption of Methylene Blue on Cashew Nut Shell Based Carbons Activated with Zinc Chloride: The Role of Surface and Structural Parameters. J. Mol. Liq. 2017, 229, 465–471. [Google Scholar] [CrossRef]
- Bello, R.; Rodríguez-Aguado, E.; Smith, V.A.; Grachev, D.; Castellón, E.R.; Bashkova, S. Ni-Doped Ordered Nanoporous Carbon Prepared from Chestnut Wood Tannins for the Removal and Photocatalytic Degradation of Methylene Blue. Nanomaterials 2022, 12, 1625. [Google Scholar] [CrossRef]
- Elbadawy, H.A.; Elhusseiny, A.F.; Hussein, S.M.; Sadik, W.A. Sustainable and Energy-Efficient Photocatalytic Degradation of Textile Dye Assisted by Ecofriendly Synthesized Silver Nanoparticles. Sci. Rep. 2023, 13, 2302. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaban, M.I.; Mahmoud, M.A.; AlHarbi, M.A. Catalytic Degradation of Methylene Blue Using Silver Nanoparticles Synthesized by Honey. Saudi J. Biol. Sci. 2021, 28, 2007–2013. [Google Scholar] [CrossRef]
- Jaast, S.; Grewal, A. Green Synthesis of Silver Nanoparticles, Characterization and Evaluation of Their Photocatalytic Dye Degradation Activity. Curr. Res. Green Sustain. Chem. 2021, 4, 100195. [Google Scholar] [CrossRef]
- Kadam, J.; Dhawal, P.; Barve, S.; Kakodkar, S. Green Synthesis of Silver Nanoparticles Using Cauliflower Waste and Their Multifaceted Applications in Photocatalytic Degradation of Methylene Blue Dye and Hg2+ Biosensing. SN Appl. Sci. 2020, 2, 738. [Google Scholar] [CrossRef]
- Tam, K.T.; Thanh, D.V.; Van, H.T.; Mai, N.T.P.; Hai, C.T.; Phuong, T.M.; Xuan, N.T.; Nguyen, V.-T. Green Synthesis of Silver Nanoparticles Using Extract of Disporopsis longifolia for Photocatalytic Degradation of Methylene Blue. Am. J. Environ. Sci. 2022, 18, 116–124. [Google Scholar] [CrossRef]
- Garg, D.; Sarkar, A.; Chand, P.; Bansal, P.; Gola, D.; Sharma, S.; Khantwal, S.; Surabhi; Mehrotra, R.; Chauhan, N.; et al. Synthesis of Silver Nanoparticles Utilizing Various Biological Systems: Mechanisms and Applications—A Review. Prog. Biomater. 2020, 9, 81–95. [Google Scholar] [CrossRef]
- Kumari, M.; Mishra, A.; Pandey, S.; Singh, S.P.; Chaudhry, V.; Mudiam, M.K.R.; Shukla, S.; Kakkar, P.; Nautiyal, C.S. Physico-Chemical Condition Optimization during Biosynthesis Lead to Development of Improved and Catalytically Efficient Gold Nano Particles. Sci. Rep. 2016, 6, 27575. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M.; Abdeen, A.O. Green Synthesis of Silver Nanoparticles Using Carob Leaf Extract and Its Antibacterial Activity. Int. J. Ind. Chem. 2013, 4, 29. [Google Scholar] [CrossRef]
- Wibowo, A.; Tajalla, G.U.N.; Marsudi, M.A.; Cooper, G.; Asri, L.A.T.W.; Liu, F.; Ardy, H.; Bartolo, P.J.D.S. Green Synthesis of Silver Nanoparticles Using Extract of Cilembu Sweet Potatoes (Ipomoea batatas L Var. Rancing) as Potential Filler for 3D Printed Electroactive and Anti-Infection Scaffolds. Molecules 2021, 26, 2042. [Google Scholar] [CrossRef]
- Anilkumar, P. (Ed.) Cashew Nut Shell Liquid: A Goldfield for Functional Materials, Chaper 1; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Telascrêa, M.; Leão, A.L.; Ferreira, M.Z.; Pupo, H.F.F.; Cherian, B.M.; Narine, S. Use of a Cashew Nut Shell Liquid Resin as a Potential Replacement for Phenolic Resins in the Preparation of Panels—A Review. Mol. Cryst. Liq. Cryst. 2014, 604, 222–232. [Google Scholar] [CrossRef]
- Kishore, S.C.; Perumal, S.; Atchudan, R.; Edison, T.N.J.I.; Sundramoorthy, A.K.; Alagan, M.; Sangaraju, S.; Lee, Y.R. Eco-Friendly Synthesis of Functionalized Carbon Nanodots from Cashew Nut Skin Waste for Bioimaging. Catalysts 2023, 13, 547. [Google Scholar] [CrossRef]
- Rojas, J.V.; Toro-Gonzalez, M.; Molina-Higgins, M.C.; Castano, C.E. Facile Radiolytic Synthesis of Ruthenium Nanoparticles on Graphene Oxide and Carbon Nanotubes. Mater. Sci. Eng. B 2016, 205, 28–35. [Google Scholar] [CrossRef]
- Carmona, E.R.; Benito, N.; Plaza, T.; Recio-Sánchez, G. Green Synthesis of Silver Nanoparticles by Using Leaf Extracts from the Endemic Buddleja globosa Hope. Green Chem. Lett. Rev. 2017, 10, 250–256. [Google Scholar] [CrossRef]
- Ruíz-Baltazar, Á.D.J.; Reyes-López, S.Y.; Mondragón-Sánchez, M.D.L.; Estevez, M.; Hernández-Martinez, A.R.; Pérez, R. Biosynthesis of Ag Nanoparticles Using Cynara cardunculus Leaf Extract: Evaluation of Their Antibacterial and Electrochemical Activity. Results Phys. 2018, 11, 1142–1149. [Google Scholar] [CrossRef]
- Naz, M.; Rafiq, A.; Ikram, M.; Haider, A.; Ahmad, S.O.A.; Haider, J.; Naz, S. Elimination of Dyes by Catalytic Reduction in the Absence of Light: A Review. J. Mater. Sci. 2021, 56, 15572–15608. [Google Scholar] [CrossRef]
- Begum, R.; Najeeb, J.; Sattar, A.; Naseem, K.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Chemical Reduction of Methylene Blue in the Presence of Nanocatalysts: A Critical Review. Rev. Chem. Eng. 2020, 36, 749–770. [Google Scholar] [CrossRef]
- Chandra, A.; Singh, M. Biosynthesis of Amino Acid Functionalized Silver Nanoparticles for Potential Catalytic and Oxygen Sensing Applications. Inorg. Chem. Front. 2018, 5, 233–257. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Zhou, X.; Wang, X.; Liang, K.; Yang, Z.-K.; Shen, C.-C.; Imran, M.; Sahar, S.; Xu, A.-W. Hydrogenation/Oxidation Induced Efficient Reversible Color Switching between Methylene Blue and Leuco-Methylene Blue. RSC Adv. 2017, 7, 30080–30085. [Google Scholar] [CrossRef]
- Kumar, P.; Govindaraju, M.; Senthamilselvi, S.; Premkumar, K. Photocatalytic Degradation of Methyl Orange Dye Using Silver (Ag) Nanoparticles Synthesized from Ulva Lactuca. Colloids Surf. B Biointerfaces 2013, 103, 658–661. [Google Scholar] [CrossRef]
- Sunshine Nut Co. Available online: https://sunshinenuts.com/pages/who-we-are (accessed on 15 August 2024).



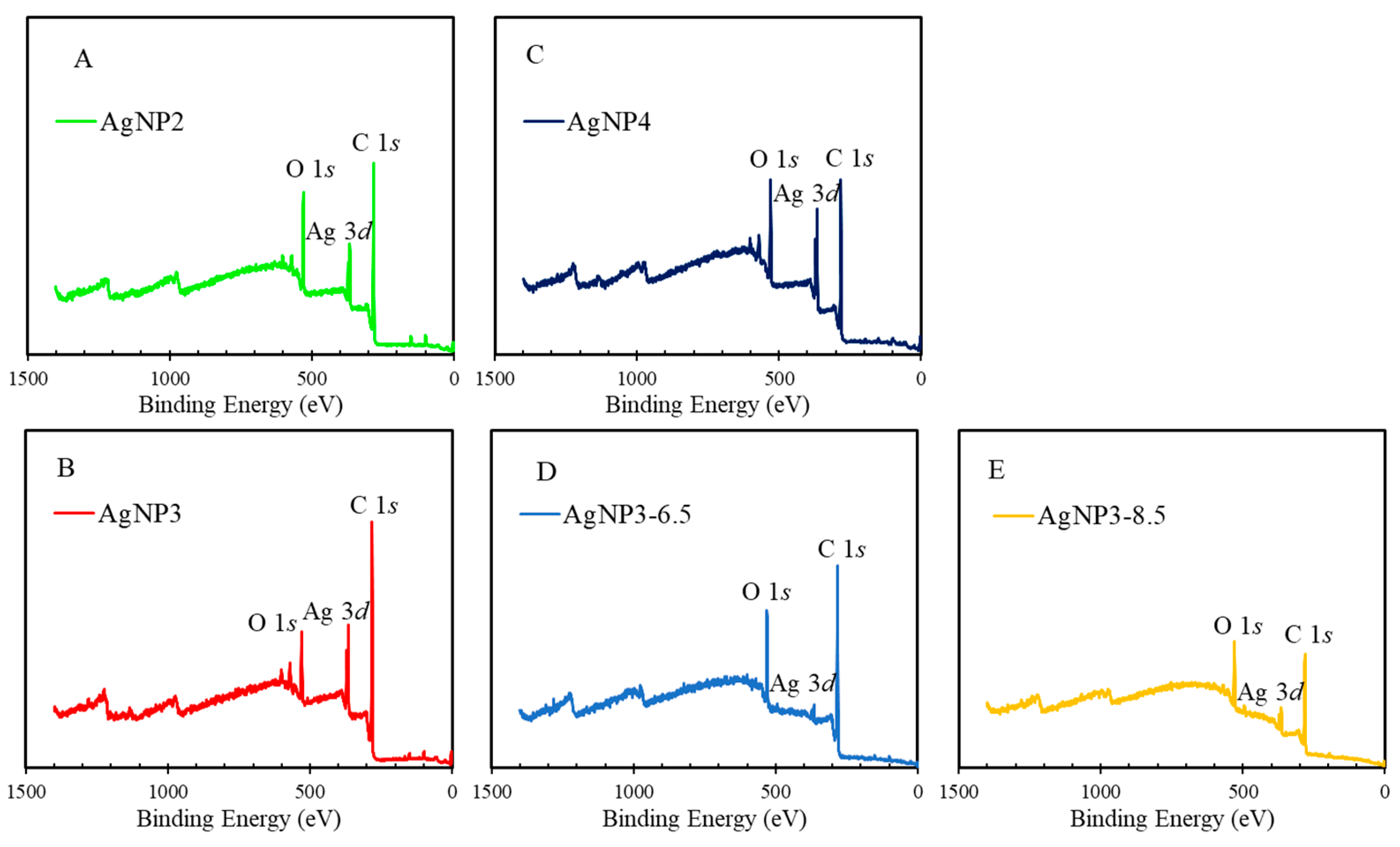



| C 1s (%) | O 1s (%) | Ag 3d (%) | |||
|---|---|---|---|---|---|
| BE, eV | 284.8 C–C | 286.5 C–O | 288.6 O–C=O | 532.6 O–C/O=C | 368.5/374.5 |
| AgNP2 | 86.6 | 10.1 | 2.3 | 19.4 | 1.6 |
| AgNP3 | 91.5 | 8.4 | ND 1 | 12.3 | 1.6 |
| AgNP4 | 78.7 | 16.3 | 5.0 | 20.4 | 2.9 |
| AgNP3-6.5 | 85.5 | 12.4 | 2.1 | 16.9 | 0.4 |
| AgNP3-8.5 | 78.6 | 17.1 | 4.3 | 19.0 | 0.8 |
| Sample | AgNP2 | AgNP3 | AgNP4 | AgNP3-6.5 | AgNP3-8.5 |
|---|---|---|---|---|---|
| Particle size range (nm) | 6.2–25.6 | 0.2–13.2 | 4.6–27.2 | 2.1–17.7 | 3.8–9.8 |
| Plant Extract | Light Source | AgNP Size (nm) | Time (h) | % Removal | Ref. |
|---|---|---|---|---|---|
| Cashew nutshell liquid | Green | 0.2–13.2 | 3 | 81 | This study |
| Dark | 3 | 78 | |||
| Camellia sinensis leaf | Solar | 25–40 | 72 | 95 | [36] |
| Morinda tinctoria leaf | Solar | 79–96 | 72 | 95 | [11] |
| Ulva lactuca seaweed | Solar | 49 | 12 | 85 | [48] |
| Kitchen vegetable waste | Solar | 10–100 | 3 | 88 | [38] |
| Indian screw tree | Solar | 25–45 | 0.5 | 95 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carollo, J.; Ballesteros-Plata, D.; Rodríguez-Aguado, E.; Bashkova, S. Green Synthesis of Silver Nanoparticles Using Cashew Nutshell Liquid (CNSL): Characterization and Methylene Blue Removal Studies. Molecules 2024, 29, 3895. https://doi.org/10.3390/molecules29163895
Carollo J, Ballesteros-Plata D, Rodríguez-Aguado E, Bashkova S. Green Synthesis of Silver Nanoparticles Using Cashew Nutshell Liquid (CNSL): Characterization and Methylene Blue Removal Studies. Molecules. 2024; 29(16):3895. https://doi.org/10.3390/molecules29163895
Chicago/Turabian StyleCarollo, Justyn, Daniel Ballesteros-Plata, Elena Rodríguez-Aguado, and Svetlana Bashkova. 2024. "Green Synthesis of Silver Nanoparticles Using Cashew Nutshell Liquid (CNSL): Characterization and Methylene Blue Removal Studies" Molecules 29, no. 16: 3895. https://doi.org/10.3390/molecules29163895
APA StyleCarollo, J., Ballesteros-Plata, D., Rodríguez-Aguado, E., & Bashkova, S. (2024). Green Synthesis of Silver Nanoparticles Using Cashew Nutshell Liquid (CNSL): Characterization and Methylene Blue Removal Studies. Molecules, 29(16), 3895. https://doi.org/10.3390/molecules29163895







