An Investigation of Novel Series of 2-Thioxo-1,3-dithiol-carboxamides as Potential Antispasmodic Agents: Design, Synthesis via Coupling Reactions, Density Functional Theory Calculations, and Molecular Docking
Abstract
1. Introduction
2. Results and Discussion
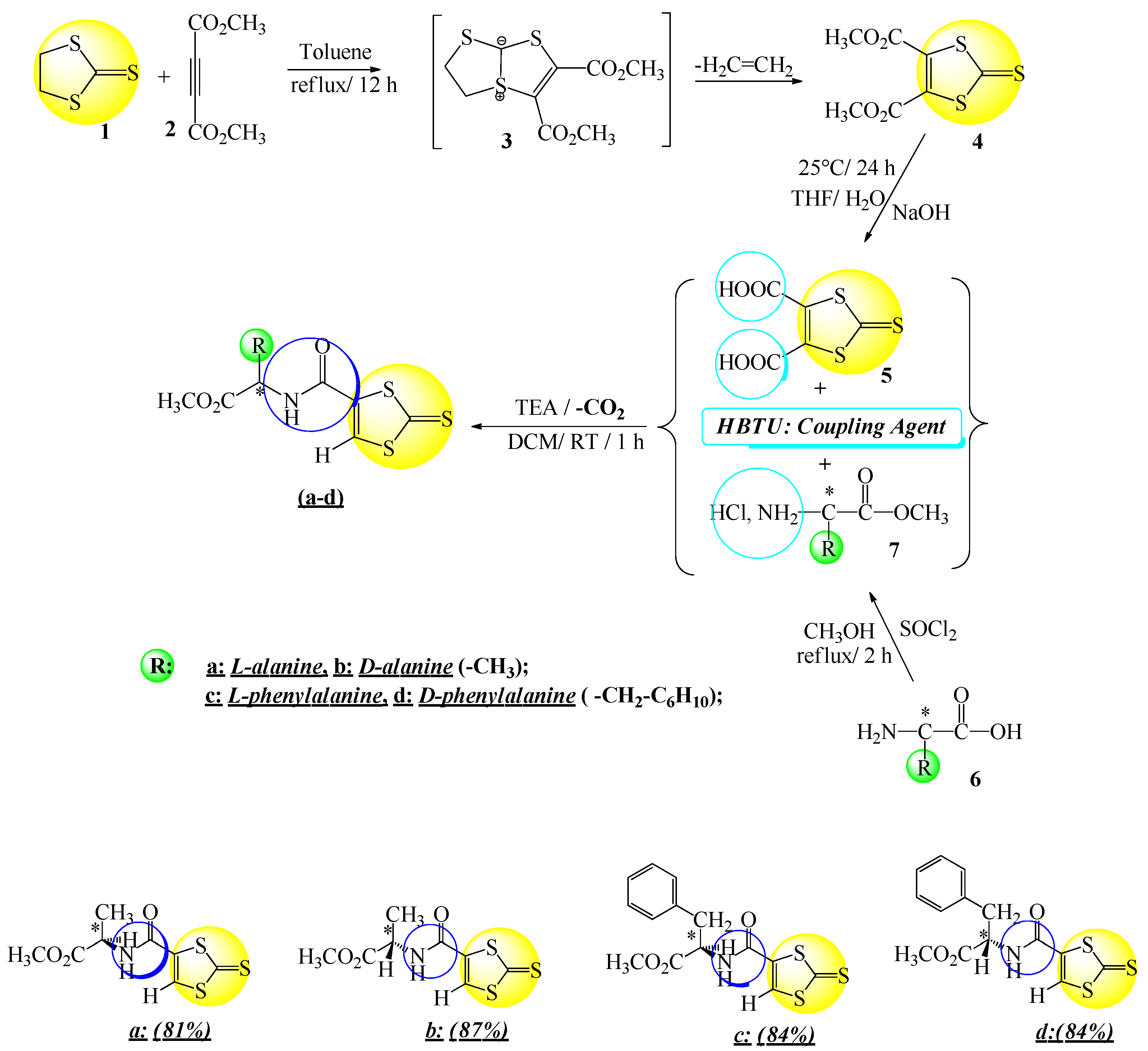
2.1. Synthesis of 2-Thioxo-1,3-dithiol-carboxamides (TDTCAs)
2.2. Plausible Mechanism of the Coupling Reaction by HBTU
2.3. Structural and Electronic Properties of the Synthesized Compounds
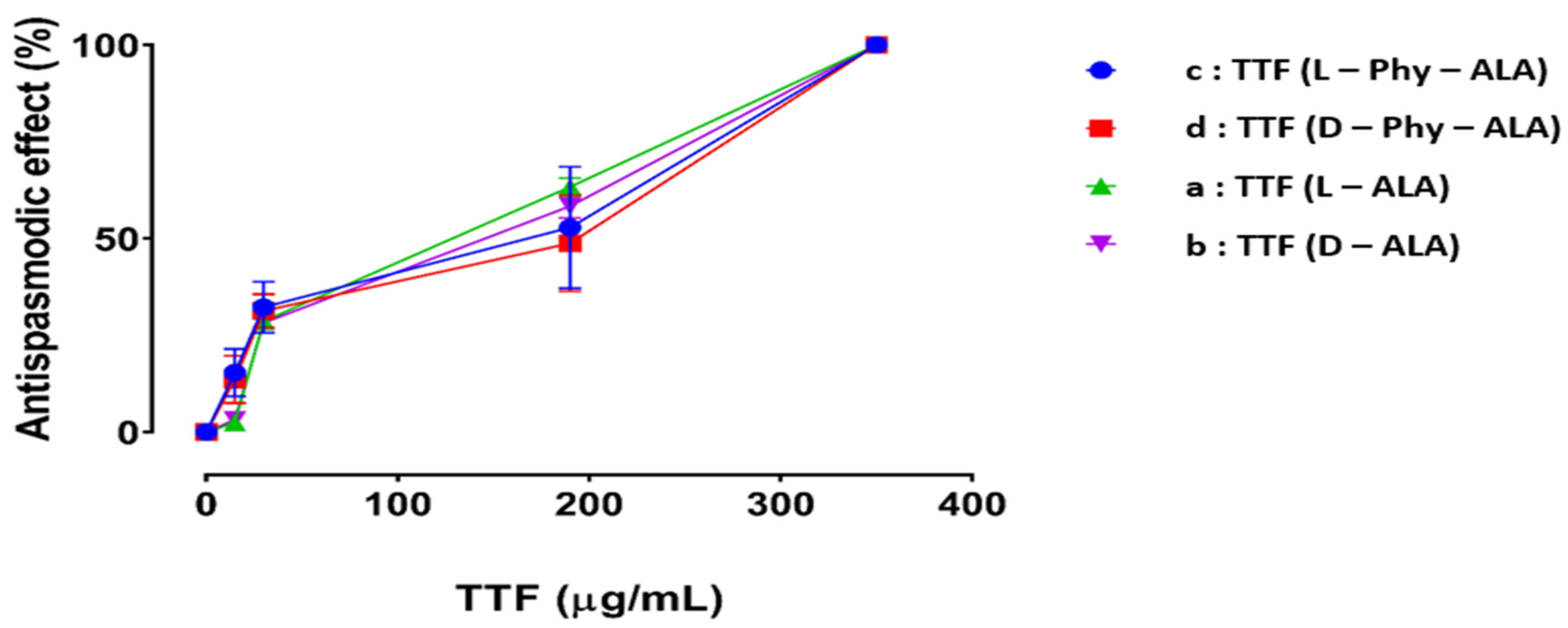
2.4. Antispasmodic Activity
Effect of 2-Thioxo-1,3-dithiol Derivatives Containing Carboxamides Compounds (TDTCAs) on ACh Induced Contractions of Rabbit Jejunum
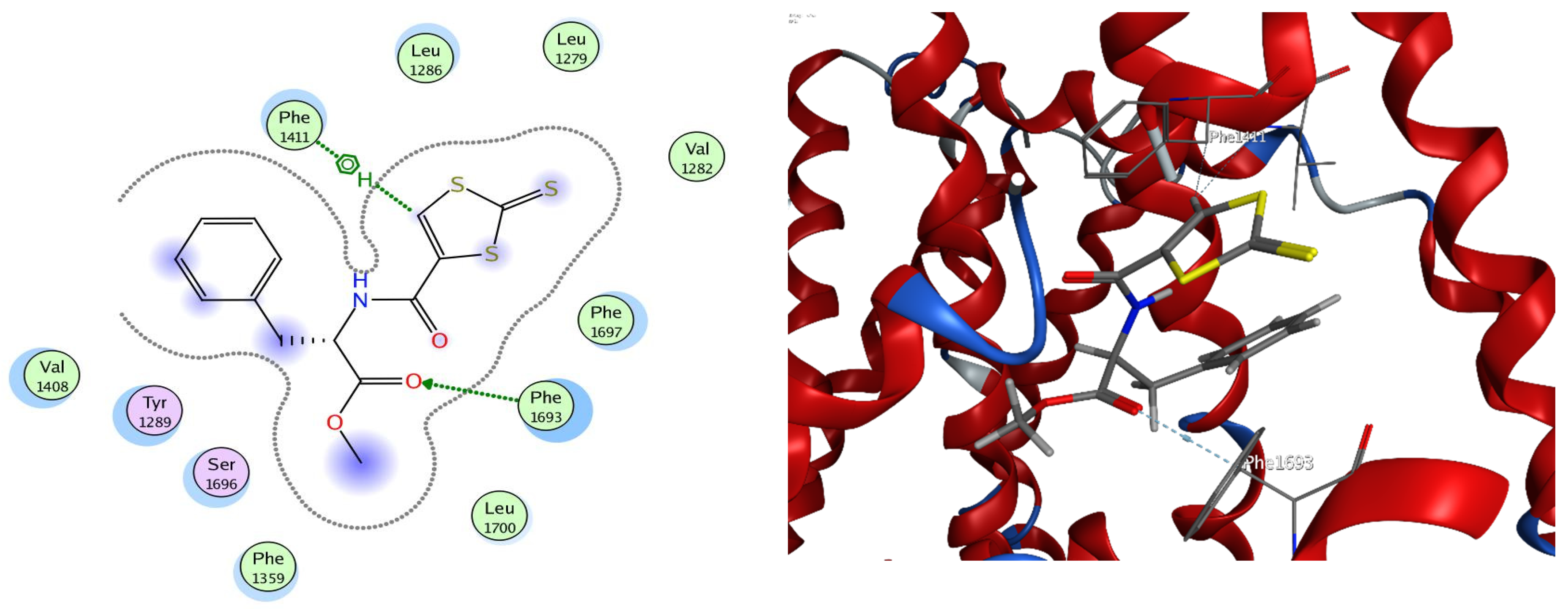
2.5. Molecular Docking Studies
3. Experimental
3.1. General Methods
3.2. Synthesis of 2-Thioxo-1,3-dithiol-4,5-dicarboxylic Acid Dimethyl Ester (4)
3.3. Synthesis of 2-Thioxo-1,3-dithiol-4,5-dicarboxylic Acid (5)
3.4. Procedure for the Preparation of Carboxylic Acids Groups Derived from Amino Acids (7)
3.5. Procedure for the Preparation of 2-Thioxo-1,3-dithiol-carboxamides (a–d)
3.6. Computational Method
3.7. Antispasmodic Activity
3.7.1. Animals
3.7.2. Tissue Preparation
3.7.3. Experimental Protocol
3.8. Molecular Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willey, C.; Iwao, M.; Castle, R.N.; Lee, M.L. Determination of sulfur heterocycles in coal liquids and shale oils. Anal. Chem. 1981, 53, 400–407. [Google Scholar] [CrossRef]
- Laxmikeshav, K.; Kumari, P.; Shankaraiah, N. Expedition of sulfur-containing heterocyclic derivatives as cytotoxic agents in medicinal chemistry: A decade update. Med. Res. Rev. 2022, 42, 513–575. [Google Scholar] [CrossRef]
- Gharge, S.; Alegaon, S.G. Recent Studies of Nitrogen and Sulfur Containing Heterocyclic Analogues as Novel Antidiabetic Agents: A review. Chem. Biodivers. 2024, 21, e202301738. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Heterocyclic Compounds: Importance in Anticancer Drug Discovery. Anti-Cancer Agents Med. Chem. 2022, 22, 3196–3207. [Google Scholar] [CrossRef]
- Micucci, M.; Viale, M.; Chiarini, A.; Spinelli, D.; Frosini, M.; Tavani, C.; Budriesi, R. Aryl-4-nitrobenzothiochromans S, S-dioxide: From calcium-channel modulators properties to multidrug-resistance reverting activity. Molecules 2020, 25, 1056. [Google Scholar] [CrossRef]
- Rudolph, J.; Theis, H.; Hanke, R.; Endermann, R.; Johannsen, L.; Geschke, F. seco-Cyclothialidines: New concise synthesis, inhibitory activity toward bacterial and human DNA topoisomerases, and antibacterial properties. J. Med. Chem. 2001, 44, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Shi, Y.X.; Ma, Y.; Zhang, C.Y.; Dong, W.L.; Pan, L.; Wang, B.L.; Li, B.J.; Li, Z.M. Synthesis, antifungal activities and 3D-QSAR study of N-(5-substituted-1,3,4-thiadiazol-2-yl)cyclopropanecarboxamides. Eur. J. Med. Chem. 2009, 44, 2782–2786. [Google Scholar] [CrossRef] [PubMed]
- Diedhiou, D.; Faye, M.; Candy, L.; Vandenbossche, V.; Raynaud, C.; Sock, O.; Rigal, L. Chemical Composition of the Essential Oil of Neem (Azadirachta indica A. Juss.) Seeds Harvested in Senegal. Indian J. Sci. Technol. 2023, 16, 118–122. [Google Scholar] [CrossRef]
- Ansari, M.I.; Khan, M.M.; Saquib, M.; Khatoon, S.; Hussain, M.K. Dithiolethiones: A privileged pharmacophore for anticancer therapy and chemoprevention. Future Med. Chem. 2018, 10, 1241–1260. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.H.M.; Junior, H.C.; Hollauer, E.; Comerlato, N.M.; Ferreira, G.B. An experimental and theoretical study of the electronic spectra of tetraethylammonium [tris(1,3-dithiole-2-thione-4,5-dithiolato)M] and tetraethylammonium [tris(1,3-dithiole-2-one-4,5-dithiolato)M], for M = Sn(IV) and Sb(V). J. Mol. Struct. 2021, 1223, 129219. [Google Scholar] [CrossRef]
- Khan, T.; McDouall, J.J.; McInnes, E.J.; Skabara, P.J.; Frère, P.; Coles, S.J.; Hursthouse, M.B. A combined substituent and supramolecular approach for improving the electron donor properties of 1,3-dithiole-2-thione derivatives. J. Mater. Chem. 2003, 13, 2490–2498. [Google Scholar] [CrossRef]
- Bigoli, F.; Deplano, P.; Mercuri, M.L.; Pellinghelli, M.A.; Trogu, E.F. Synthetic, Structural and spectroscopic studies of the donating properties of sulfur-2-rich molecules towards I2: X-ray structure of 1,3-Dithiole-2-thione Diiodine. Phosphorus Sulfur Silicon Relat. Elem. 1992, 72, 65–72. [Google Scholar] [CrossRef]
- Hansen, T.K.; Becher, J. Applications of the 1,3-Dithiole Unit in a Post-TTF Era. Adv. Mater. 1993, 5, 288–292. [Google Scholar] [CrossRef]
- da Cruz, A.G.B.; Wardell, J.L.; Rocco, A.M. Hybrid organic–inorganic materials based on polypyrrole and 1,3-dithiole-2-thione-4,5-dithiolate (DMIT) containing dianions. J. Mater. Sci. 2008, 43, 5823–5836. [Google Scholar] [CrossRef]
- Feng, L.; Li, W.; Liu, Y.; Jiang, W.D.; Kuang, S.Y.; Wu, P.; Jiang, J.; Tang, L.; Tang, W.N.; Zhang, Y.-A.; et al. Protective role of phenylalanine on the ROS-induced oxidative damage, apoptosis and tight junction damage via Nrf2, TOR and NF-κB signalling molecules in the gill of fish. Fish Shellfish Immunol. 2017, 60, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Feng, C.L. Metal-Ion-Mediated Supramolecular Chirality of L-Phenylalanine Based Hydrogels. Angew. Chem. 2018, 130, 5757–5761. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Chapter 15—Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). Recent Adv. Nat. Prod. Anal. 2020, 505–567. [Google Scholar]
- Sharma, A.; Mehta, V.; Rani, S.; Noda, M.; Sugiyama, M.; Chander, H.; Kaur, B. Biomedical applications of L-alanine produced by Pediococcus acidilactici BD16 (alaD+). Appl. Microbiol. Biotechnol. 2022, 106, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Neiland, O.Y.; Valters, R.A.; Pukitis, G.G.; Tilika, V.Z.; Edzhinya, A.S. Conversions of 2-thioxo-1,3-dithiol-4,5-dicarboxylic acid dimethyl ester—The path to a new heterocyclic system, (4H,6H)-1,3-dithiolo[4,5-d]pyrimidine-2,5,7-trione and 5,6-dimercaptouracil derivatives. Chem. Heterocycl. Compd. 1992, 28, 1079–1083. [Google Scholar] [CrossRef]
- Moussaoui, O.; El Hadrami, E.M.; Touimi, G.B.; Bennani, B.; Tama, A.B.; Rodi, Y.K.; Chakroune, S. Synthesis of a new series of quinoline-carboxamides based on methylated aminoesters: NMR characterization and antimicrobial activity. Mediterr. J. Chem. 2019, 9, 326–336. [Google Scholar] [CrossRef]
- Moussaoui, O.; Bhadane, R.; Sghyar, R.; El Hadrami, E.M.; El Amrani, S.; Tama, A.B.; Rodi, Y.K.; Chakroune, S.; Salo-Ahen, O.M.H. Novel Amino Acid Derivatives of Quinolines as Potential Antibacterial and Fluorophore Agents. Sci. Pharm. 2020, 88, 57. [Google Scholar] [CrossRef]
- Moussaoui, O.; Bhadane, R.; Sghyar, R.; Ilaš, J.; El Hadrami, E.M.; Chakroune, S.; Salo-Ahen, O.M.H. Design, Synthesis, in vitro and in silico Characterization of 2-Quinolone-L-alaninate-1,2,3-triazoles as Antimicrobial Agents. ChemMedChem 2022, 17, e202100714. [Google Scholar] [CrossRef] [PubMed]
- Moussaoui, O.; Byadi, S.; Hachim, M.E.; Sghyar, R.; Bahsis, L.; Moslova, K.; Aboulmouhajir, A.; Rodi, Y.K.; Podlipnik, C.; El Hadrami, E.M.; et al. Selective synthesis of novel quinolones-amino esters as potential antibacterial and antifungal agents: Experimental, mechanistic study, docking and molecular dynamic simulations. J. Mol. Struct. 2021, 1241, 130652. [Google Scholar] [CrossRef]
- Vrettos, E.I.; Sayyad, N.; Mavrogiannaki, E.M.; Stylos, E.; Kostagianni, A.D.; Papas, S.; Mavromoustakos, T.; Tzakos, A.G. Unveiling and tackling guanidinium peptide coupling reagent side reactions towards the development of peptide-drug conjugates. RSC Adv. 2017, 7, 50519–50526. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. The fundamental nature and role of the electrostatic potential in atoms and molecules. Theor. Chem. Acc. 2002, 108, 134–142. [Google Scholar] [CrossRef]
- RCSB Protein Data Bank. Available online: https://www.rcsb.org/pdb/welcome.do (accessed on 1 April 2024).
- Lahyaoui, M.; El-Idrissi, H.; Saffaj, T.; Ihssane, B.; Saffaj, N.; Mamouni, R.; Rodi, Y.K. QSAR modeling, molecular docking and molecular dynamic simulation of phosphorus-substituted quinoline derivatives as topoisomerase I inhibitors. Arab. J. Chem. 2023, 16, 104783. [Google Scholar] [CrossRef]
- Lahyaoui, M.; Diane, A.; El-Idrissi, H.; Saffaj, T.; Rodi, Y.K.; Ihssane, B. QSAR modeling and molecular docking studies of 2-oxo-1, 2-dihydroquinoline-4-carboxylic acid derivatives as pglycoprotein inhibitors for combating cancer multidrug resistance. Heliyon 2023, 9, e13020. [Google Scholar] [CrossRef] [PubMed]
- Sghyar, R.; Sert, Y.; El Ibrahimi, B.; Moussaoui, O.; Hadrami, E.M.E.L.; Ben-Tama, A.; Mague, J.T.; Talbaoui, A.; Sebbar, N.K.; Essassi, E.M. New tetrazoles compounds incorporating galactose moiety: Synthesis, crystal structure, spectroscopic characterization, Hirshfeld surface analysis, molecular docking studies, DFT calculations and anti-corrosion property anticipation. J. Mol. Struct. 2022, 1247, 131300. [Google Scholar] [CrossRef]
- Sghyar, R.; Basavarajaiah, S.M.; Chda, A.; Moussaoui, O.; El Hadrami, E.M.; Ben-Tama, A.; Aarab, L.; Mague, J.T.; Prashantha, K.; Javeed, M.; et al. Design, synthesis, biological evaluation on immune cell proliferation, crystal structures, spectroscopic characterizations, DFT calculations, ADME analysis, and molecular docking studies with COX of novel tetrazole-Galactopyranosyl analogues. J. Mol. Struct. 2023, 1287, 135695. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009; Revision A, 1. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J. Chem. Phys. 1992, 96, 2155–2160. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed]
- NIH National Institutes of Health. Guide for the Care and Use of Laboratory Animals; (NIH Publication No. 85-23, Revised in 1985); NIH Publication: Bethesda, MD, USA, 1985; ISBN 9780309154000. [Google Scholar]



| Compounds | M3 (kcal/mol) | Cav1.2 (kcal/mol) | Beta-2 Adrenergic Receptor (kcal/mol) | Alpha Adrenergic Receptor (kcal/mol) |
|---|---|---|---|---|
| a | −3.96 | −3.86 | --- | −5.02 |
| b | −3.93 | −4.24 | --- | −5.01 |
| c | −4.86 | −6.09 | −4.05 | −6.27 |
| d | −5.32 | −5.88 | −4.56 | −6.47 |
| Compounds | S (kcal/mol) | Rmsd_Refine (kcal/mol) | E_Conf (kcal/mol) | E_Place (kcal/mol) | E_Score1 (kcal/mol) | E_Refine (kcal/mol) | E_Score2 (kcal/mol) |
|---|---|---|---|---|---|---|---|
| a | −5.02 | 4.08 | 3.36 | −57.11 | −7.01 | −16.86 | −5.02 |
| b | −5.01 | 4.08 | 3.36 | −57.11 | −7.01 | −16.86 | −5.01 |
| c | −6.27 | 2.04 | 29.20 | −81.21 | −8.21 | −24.54 | −6.27 |
| −6.19 | 1.94 | 26.80 | −74.38 | −8.24 | −21.08 | −6.19 | |
| −6.16 | 1.88 | 47.78 | −79.36 | −8.25 | −15.37 | −6.16 | |
| −6.15 | 1.68 | 32.46 | −74.36 | −7.96 | −22.11 | −6.15 | |
| −6.14 | 2.18 | 26.92 | −60.80 | −8.02 | −21.96 | −6.14 | |
| d | −6.47 | 1.36 | 33.08 | −53.93 | −7.79 | −22.45 | −6.47 |
| −6.27 | 1.34 | 50.12 | −59.75 | −7.72 | −14.22 | −6.27 | |
| −6.25 | 2.07 | 31.87 | −54.93 | −8.03 | −21.80 | −6.25 | |
| −6.13 | 1.45 | 24.94 | −71.34 | −7.98 | −24.25 | −6.13 | |
| −6.09 | 2.29 | 26.56 | −72.24 | −8.44 | −22.74 | −6.09 |
| Compounds | Ligand | Receptor | Interaction | Distance (Å) | E (kcal/mol) |
|---|---|---|---|---|---|
| c | S5 | SD MET 1293 | H-donor | 4.4 | −0.4 |
| 6-ring | 6-ring PHE 1411 | pi-pi | 3.89 | 0 | |
| d | O29 | CB PHE 1693 | H-acceptor | 3.03 | −0.9 |
| C2 | 6-ring PHE 1411 | H-pi | 3.65 | −0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sghyar, R.; Lahyaoui, M.; Aflak, N.; Moussaoui, O.; Chda, A.; Bencheikh, R.; El Hadrami, E.M.; Sebbar, N.K.; Alanazi, A.S.; Hefnawy, M. An Investigation of Novel Series of 2-Thioxo-1,3-dithiol-carboxamides as Potential Antispasmodic Agents: Design, Synthesis via Coupling Reactions, Density Functional Theory Calculations, and Molecular Docking. Molecules 2024, 29, 3855. https://doi.org/10.3390/molecules29163855
Sghyar R, Lahyaoui M, Aflak N, Moussaoui O, Chda A, Bencheikh R, El Hadrami EM, Sebbar NK, Alanazi AS, Hefnawy M. An Investigation of Novel Series of 2-Thioxo-1,3-dithiol-carboxamides as Potential Antispasmodic Agents: Design, Synthesis via Coupling Reactions, Density Functional Theory Calculations, and Molecular Docking. Molecules. 2024; 29(16):3855. https://doi.org/10.3390/molecules29163855
Chicago/Turabian StyleSghyar, Riham, Mouad Lahyaoui, Noura Aflak, Oussama Moussaoui, Alae Chda, Rachid Bencheikh, El Mestafa El Hadrami, Nada Kheira Sebbar, Ashwag S. Alanazi, and Mohamed Hefnawy. 2024. "An Investigation of Novel Series of 2-Thioxo-1,3-dithiol-carboxamides as Potential Antispasmodic Agents: Design, Synthesis via Coupling Reactions, Density Functional Theory Calculations, and Molecular Docking" Molecules 29, no. 16: 3855. https://doi.org/10.3390/molecules29163855
APA StyleSghyar, R., Lahyaoui, M., Aflak, N., Moussaoui, O., Chda, A., Bencheikh, R., El Hadrami, E. M., Sebbar, N. K., Alanazi, A. S., & Hefnawy, M. (2024). An Investigation of Novel Series of 2-Thioxo-1,3-dithiol-carboxamides as Potential Antispasmodic Agents: Design, Synthesis via Coupling Reactions, Density Functional Theory Calculations, and Molecular Docking. Molecules, 29(16), 3855. https://doi.org/10.3390/molecules29163855







