Evaluation of Nitric Oxide-Donating Properties of 11H-indeno[1,2-b]quinoxalin-11-one Oxime (IQ-1) by Electron Paramagnetic Resonance Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
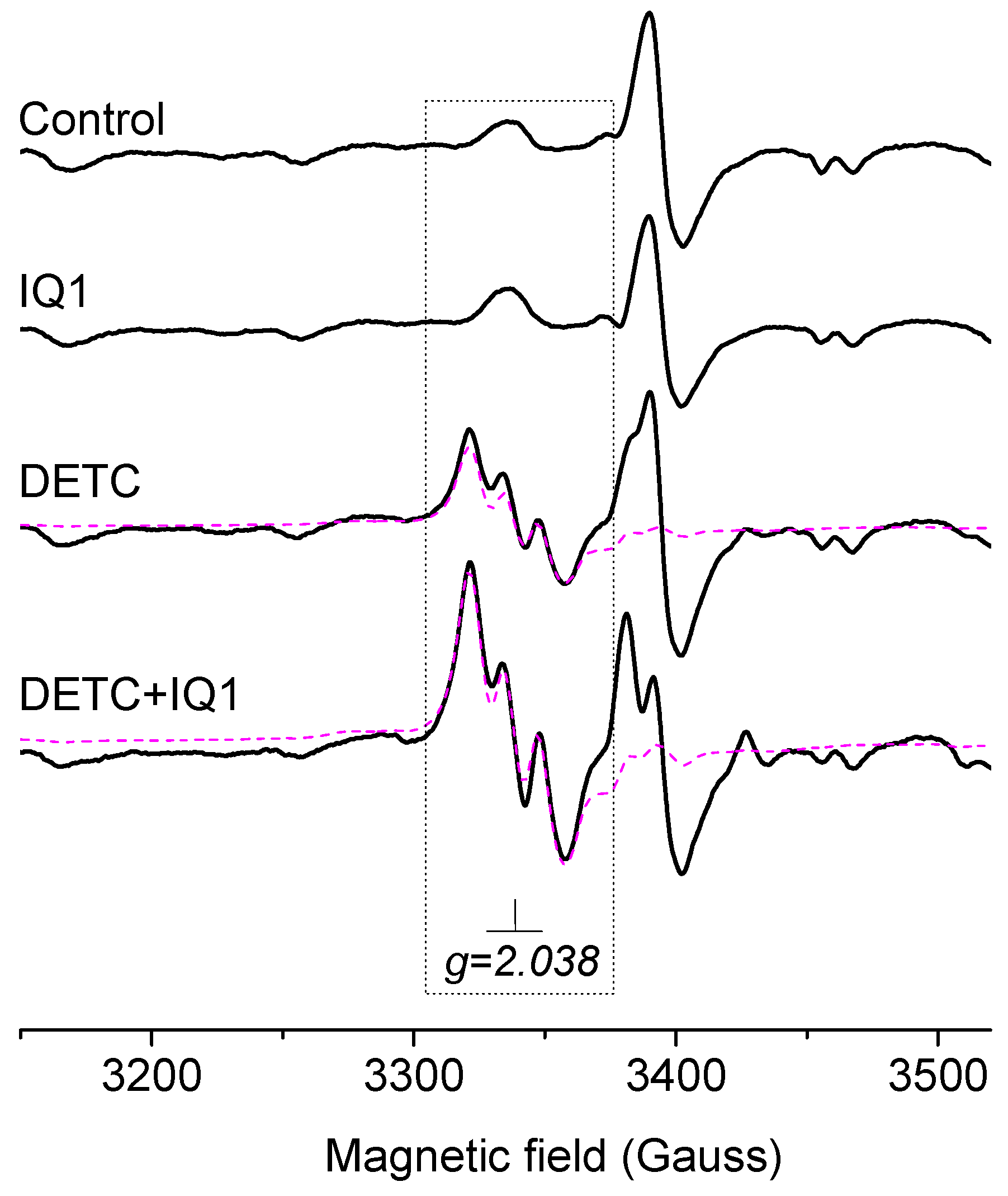
2.1. EPR Signals of Samples from Liver
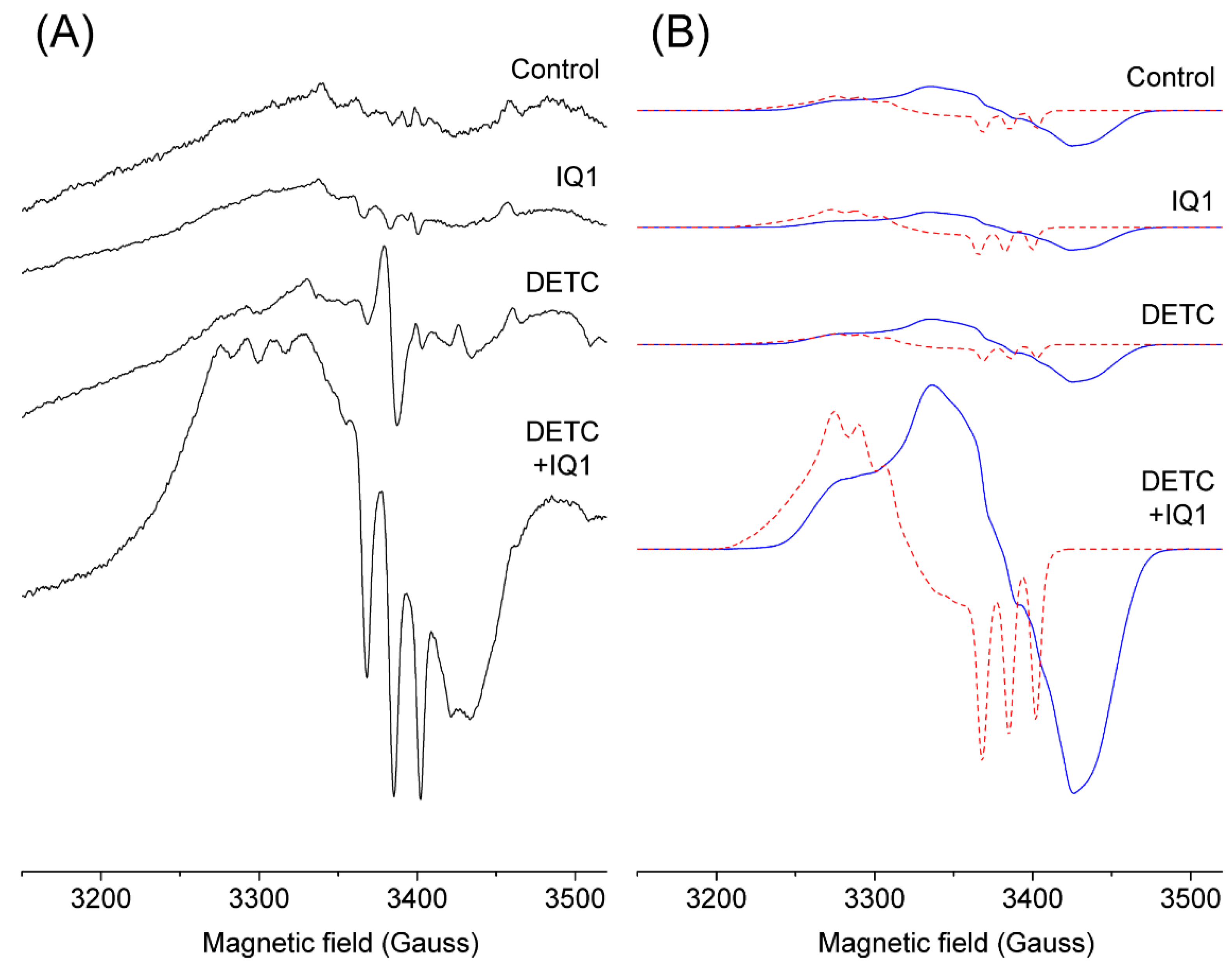
2.2. EPR Signal of Blood Samples
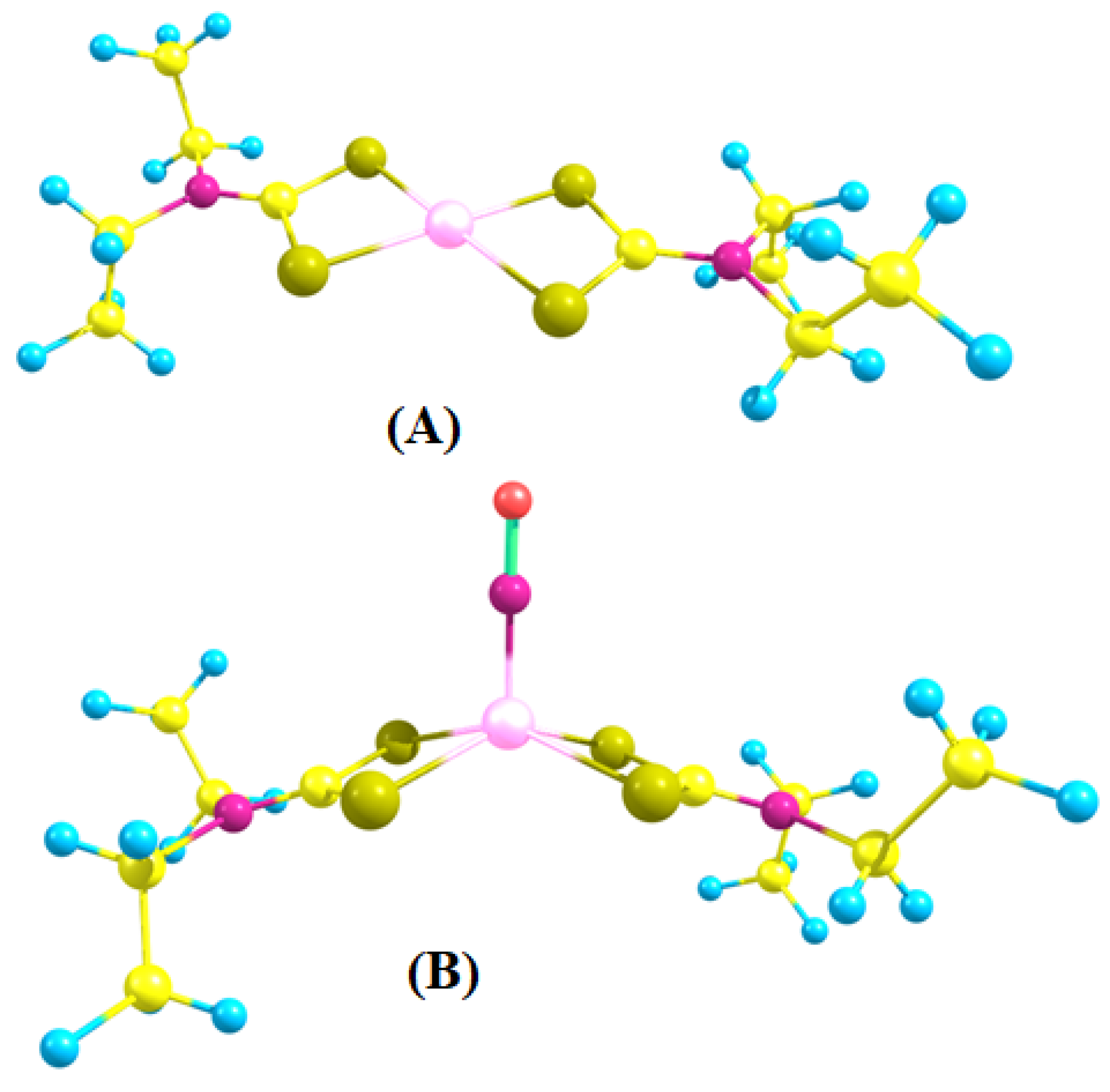
2.3. A DFT Study of NO Complexes and NO Formation from IQ-1
3. Materials and Methods
3.1. Animals
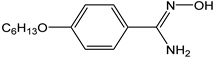
3.2. Chemicals and the Studied Compound
3.3. Experimental Protocol
3.4. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shvedova, M.; Anfinogenova, Y.; Atochina-Vasserman, E.N.; Schepetkin, I.A.; Atochin, D.N. c-Jun N-terminal kinases (JNKs) in myocardial and cerebral ischemia/reperfusion injury. Front. Pharmacol. 2018, 9, 715. [Google Scholar] [CrossRef]
- Köken, T.; İnal, M. The effect of nitric oxide on ischemia-reperfusion injury in rat liver. Clin. Chim. Acta 1999, 288, 55–62. [Google Scholar] [CrossRef]
- Signori, D.; Magliocca, A.; Hayashida, K.; Graw, J.A.; Malhotra, R.; Bellani, G.; Berra, L.; Rezoagli, E. Inhaled nitric oxide: Role in the pathophysiology of cardio-cerebrovascular and respiratory diseases. Intensive Care Med. Exp. 2022, 10, 28. [Google Scholar] [CrossRef]
- Chen, X.M.; Chen, H.S.; Xu, M.J.; Shen, J.G. Targeting reactive nitrogen species: A promising therapeutic strategy for cerebral ischemia-reperfusion injury. Acta Pharmacol. Sin. 2013, 34, 67–77. [Google Scholar] [CrossRef]
- Hu, S.Q.; Ye, J.S.; Zong, Y.Y.; Sun, C.C.; Liu, D.H.; Wu, Y.P.; Song, T.; Zhang, G.Y. S-Nitrosylation of mixed lineage kinase 3 contributes to its activation after cerebral ischemia. J. Biol. Chem. 2012, 287, 2364–2377. [Google Scholar] [CrossRef]
- Iova, O.-M.; Marin, G.-E.; Lazar, I.; Stanescu, I.; Dogaru, G.; Nicula, C.A.; Bulboacă, A.E. Nitric oxide/nitric oxide synthase system in the pathogenesis of neurodegenerative disorders—An overview. Antioxidants 2023, 12, 753. [Google Scholar] [CrossRef]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Gladwin, Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Zhao, F.; Wang, H.; Qu, Y.; Mu, D. Nitric oxide synthase in hypoxic or ischemic brain injury. Rev. Neurosci. 2015, 26, 105–117. [Google Scholar] [CrossRef]
- Yu, H.M.; Xu, J.; Li, C.; Zhou, C.; Zhang, F.; Han, D.; Zhang, G.Y. Coupling between neuronal nitric oxide synthase and glutamate receptor 6-mediated c-Jun N-terminal kinase signaling pathway via S-nitrosylation contributes to ischemia neuronal death. Neuroscience 2008, 155, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Dantas, B.P.V.; Ribeiro, T.P.; Assis, V.L.; Furtado, F.F.; Assis, K.S.; Alves, J.S.; Silva, T.M.S.; Camara, C.A.; França-Silva, M.S.; Veras, R.C.; et al. Vasorelaxation induced by a new naphthoquinone-oxime is mediated by NO-sGC-cGMP pathway. Molecules 2014, 19, 9773–9785. [Google Scholar] [CrossRef] [PubMed]
- Jaros, F.; Straka, T.; Dobesová, Z.; Pintérová, M.; Chalupský, K.; Kuneš, J.; Entlicher, G.; Zicha, J. Vasorelaxant activity of some oxime derivatives. Eur. J. Pharmacol. 2007, 575, 122–126. [Google Scholar] [CrossRef]
- Andronik-Lion, V.; Boucher, J.L.; Delaforge, M.; Henry, Y.; Mansuy, D. Formation of nitric oxide by cytochrome P450-catalyzed oxidation of aromatic amidoximes. Biochem. Biophys. Res. Commun. 1992, 185, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Caro, A.A.; Cederbaum, A.I.; Stoyanovsky, D.A. Oxidation of the ketoxime acetoxime to nitric oxide by oxygen radical-generating systems. Nitric Oxide 2001, 5, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Kirpotina, L.N.; Khlebnikov, A.I.; Hanks, T.S.; Kochetkova, I.; Pascual, D.W.; Jutila, M.A.; Quinn, M.T. Identification and characterization of a novel class of c-Jun N-terminal kinase inhibitors. Mol. Pharmacol. 2012, 81, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Kirpotina, L.N.; Khlebnikov, A.I.; Jutila, M.A.; Quinn, M.T. Anti-inflammatory effects and joint protection in collagen-induced arthritis after treatment with IQ-1S, a selective c-Jun N-terminal kinase inhibitor. J. Pharmacol. Exp. Ther. 2015, 353, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, M.B.; Chernysheva, G.A.; Aliev, O.I.; Smol’iakova, V.I.; Fomina, T.I.; Osipenko, A.N.; Rydchenko, V.S.; Anfinogenova, Y.J.; Khlebnikov, A.I.; Schepetkin, I.A.; et al. Protective effects of a new c-Jun N-terminal kinase inhibitor in the model of global cerebral ischemia in rats. Molecules 2019, 24, 1722. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, M.B.; Chernysheva, G.A.; Smolyakova, V.I.; Aliev, O.I.; Trofimova, E.S.; Sherstoboev, E.Y.; Osipenko, A.N.; Khlebnikov, A.I.; Anfinogenova, Y.J.; Schepetkin, I.A.; et al. Neuroprotective effects of a novel inhibitor of c-Jun N-terminal kinase in the rat model of transient focal cerebral ischemia. Cells 2020, 9, 1860. [Google Scholar] [CrossRef]
- Atochin, D.N.; Schepetkin, I.A.; Khlebnikov, A.I.; Seledtsov, V.I.; Swanson, H.; Quinn, M.T.; Huang, P.L. A novel dual NO-donating oxime and c-Jun N-terminal kinase inhibitor protects against cerebral ischemia-reperfusion injury in mice. Neurosci. Lett. 2016, 618, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Lakeev, A.P.; Frelikh, G.A.; Yanovskaya, E.A.; Kovrizhina, A.R.; Udut, V.V. Quantification of a promising JNK inhibitor and nitrovasodilator IQ-1 and its major metabolite in rat plasma by LC-MS/MS. Bioanalysis 2022, 14, 1423–1441. [Google Scholar] [CrossRef]
- Mikoyan, V.D.; Kubrina, L.N.; Serezhenkov, V.A.; Stukan, R.A.; Vanin, A.F. Complexes of Fe2+ with diethyldithiocarbamate or N-methyl-D-glucamine dithiocarbamate as traps of nitric oxide in animal tissues: Comparative investigations. Biochim. Biophys. Acta-Gen. Subj. 1997, 1336, 225–234. [Google Scholar] [CrossRef]
- Vanin, A.F.; Huisman, A.; van Faassen, E.E. Iron dithiocarbamate as spin trap for nitric oxide detection: Pitfalls and successes. Methods Enzymol. 2002, 359, 27–42. [Google Scholar] [PubMed]
- Hawkins, C.L.; Davies, M.J. Detection and characterisation of radicals in biological materials using EPR methodology. Biochim. Biophys. Acta-Gen. Subj. 2014, 1840, 708–721. [Google Scholar] [CrossRef]
- Vanin, A.F. Dinitrosyl iron complexes with thiol-containing ligands as a “working form” of endogenous nitric oxide. Nitric Oxide 2016, 54, 15–29. [Google Scholar] [CrossRef]
- Jakubowska, M.A.; Pyka, J.; Michalczyk-Wetula, D.; Baczyński, K.; Cieśla, M.; Susz, A.; Ferdek, P.E.; Płonka, B.K.; Fiedor, L.; Płonka, P.M. Electron paramagnetic resonance spectroscopy reveals alterations in the redox state of endogenous copper and iron complexes in photodynamic stress-induced ischemic mouse liver. Redox Biol. 2020, 34, 101566. [Google Scholar] [CrossRef]
- van Faassen, E.E.; Koeners, M.P.; Joles, J.A.; Vanin, A.F. Detection of basal NO production in rat tissues using iron-dithiocarbamate complexes. Nitric Oxide 2008, 18, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Gainutdinov, K.L.; Gavrilova, S.A.; Iyudin, V.S.; Golubeva, A.V.; Davydova, M.P.; Jafarova, G.G.; Andrianov, V.V.; Koshelev, V.B. EPR study of the intensity of the nitric oxide production in rat brain after ischemic stroke. Appl. Magn. Reson. 2011, 40, 267–278. [Google Scholar] [CrossRef]
- Han, T.H.; Hyduke, D.R.; Vaughn, M.W.; Fukuto, J.M.; Liao, J.C. Nitric oxide reaction with red blood cells and hemoglobin under heterogeneous conditions. Proc. Natl. Acad. Sci. USA 2002, 99, 7763–7768. [Google Scholar] [CrossRef]
- Vladimirov, Y.; Borisenko, G.; Boriskina, N.; Kazarinov, K.; Osipov, A. NO-hemoglobin may be a light-sensitive source of nitric oxide both in solution and in red blood cells. J. Photochem. Photobiol. B-Biol. 2000, 59, 115–122. [Google Scholar] [CrossRef]
- Gray, J.P.; Karandrea, S.; Burgos, D.Z.; Jaiswal, A.A.; Heart, E.A. NAD(P)H-dependent quinone oxidoreductase 1 (NQO1) and cytochrome P450 oxidoreductase (CYP450OR) differentially regulate menadione-mediated alterations in redox status, survival and metabolism in pancreatic beta-cells. Toxicol. Lett. 2016, 262, 1–11. [Google Scholar] [CrossRef]
- Vanin, A.; Poltorakov, A. NO spin trapping in biological systems. Front. Biosci. 2009, 14, 4427–4435. [Google Scholar] [CrossRef]
- Jousserandot, A.; Boucher, J.L.; Henry, Y.; Niklaus, B.; Clement, B.; Mansuy, D. Microsomal cytochrome P450 dependent oxidation of N-hydroxyguanidines, amidoximes, and ketoximes: Mechanism of the oxidative cleavage of their C=N(OH) bond with formation of nitrogen oxides. Biochemistry 1998, 37, 17179–17191. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.E. Nitric oxide and iron proteins. Biochim. Biophys. Acta-Bioenerg. 1999, 1411, 290–309. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, D.; Boucher, J.L.; Clement, B. On the mechanism of nitric-oxide formation upon oxidative cleavage of C=N(OH) bonds by NO-synthases and cytochromes P450. Biochimie 1995, 77, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Matveevskaya, V.V.; Pavlov, D.I.; Kovrizhina, A.R.; Sukhikh, T.S.; Sadykov, E.H.; Dorovatovskii, P.V.; Lazarenko, V.A.; Khlebnikov, A.I.; Potapov, A.S. Experimental and computational investigation of the oxime bond stereochemistry in c-Jun N-terminal kinase 3 inhibitors 11H-Indeno[1,2-b]quinoxalin-11-one oxime and tryptanthrin-6-oxime. Pharmaceutics 2023, 15, 1802. [Google Scholar] [CrossRef] [PubMed]
- Pearson, B.D.; Mitsch, R.A.; Cromwell, N.H. Indenoquinolines. III. Derivatives of 11H-indeno[1,2-b]quinoxaline and related indenoquinolines. J. Org. Chem. 1962, 27, 1674–1678. [Google Scholar]
- Ismailova, A.I.; Gnezdilov, O.I.; Obynochny, A.A.; Muranova, L.N.; Andrianov, V.V.; Gainutdinov, K.L.; Nasyrova, A.G.; Nigmatullina, R.R.; Rakhmatullina, F.F.; Zefirov, A.L. ESR study of the nitric oxide production in tissues of animals under an external influence on the functioning of the cardiovascular and nervous systems. Appl. Magn. Reson. 2005, 28, 421–430. [Google Scholar] [CrossRef]
- Andrianov, V.V.; Kulchitsky, V.A.; Yafarova, G.G.; Bazan, L.V.; Bogodvid, T.K.; Deryabina, I.B.; Muranova, L.N.; Silantyeva, D.I.; Arslanov, A.I.; Paveliev, M.N.; et al. Investigation of NO role in neural tissue in brain and spinal cord injury. Molecules 2023, 28, 7359. [Google Scholar] [CrossRef] [PubMed]
- Tseitlin, M.P.; Iyudin, V.S.; Tseitlin, O.A. Advantages of digital phase-sensitive detection for upgrading an obsolete CW EPR spectrometer. Appl. Magn. Reson. 2009, 35, 569–580. [Google Scholar] [CrossRef]
- Szabo, A.; Perutz, M.F. Equilibrium between 6-coordinated and 5-coordinated hemes in nitrosylhemoglobin: Interpretation of electron spin resonance spectra. Biochemistry 1976, 15, 4427–4428. [Google Scholar] [CrossRef]
- Nagai, K.; Hori, H. The influence of quaternary structure on the EPR-spectra of ferric haemoglobin. FEBS Lett. 1978, 93, 275–277. [Google Scholar] [CrossRef][Green Version]
- Piknova, B.; Gladwin, M.T.; Schechter, A.N.; Hogg, N. Electron paramagnetic resonance analysis of nitrosylhemoglobin in humans during NO inhalation. J. Biol. Chem. 2005, 280, 40583–40588. [Google Scholar] [CrossRef]
- Andrianov, V.V.; Kulchitsky, V.A.; Yafarova, G.G.; Zamaro, A.S.; Tokalchik, Y.P.; Bazan, L.V.; Bogodvid, T.K.; Iyudin, V.S.; Pashkevich, S.G.; Dosina, M.O.; et al. Comparative study of the intensity of nitric oxide production and copper content in hippocampus of rats after modeling of hemorrhagic stroke and brain injury. Appl. Magn. Reson. 2021, 52, 1657–1669. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- Hertwig, R.H.; Koch, W. On the parameterization of the local correlation functional. What is Becke-3-LYP? Chem. Phys. Lett. 1997, 268, 345–351. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Rappoport, D.; Furche, F. Property-optimized Gaussian basis sets for molecular response calculations. J. Chem. Phys. 2010, 133, 134105. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Skyner, R.E.; McDonagh, J.L.; Groom, C.R.; van Mourik, T.; Mitchell, J.B.O. A review of methods for the calculation of solution free energies and the modelling of systems in solution. Phys. Chem. Chem. Phys. 2015, 17, 6174–6191. [Google Scholar] [CrossRef]
- Cramer, C.J.; Truhlar, D.G. Density functional theory for transition metals and transition metal chemistry. Phys. Chem. Chem. Phys. 2009, 11, 10757–10816. [Google Scholar] [CrossRef]
- Tirado-Rives, J.; Jorgensen, W.L. Performance of B3LYP density functional methods for a large set of organic molecules. J. Chem. Theory Comput. 2008, 4, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Jensen, F. Basis sets in quantum chemistry. In Reviews in Computational Chemistry; Parrill, A.L., Lipkowitz, K.B., Eds.; Wiley: Hoboken, NJ, USA, 2017; Volume 30, pp. 93–149. [Google Scholar]
- Iikura, H.; Tsuneda, T.; Yanai, T.; Hirao, K. A long-range correction scheme for generalized-gradient-approximation exchange functionals. J. Chem. Phys. 2001, 115, 3540–3544. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
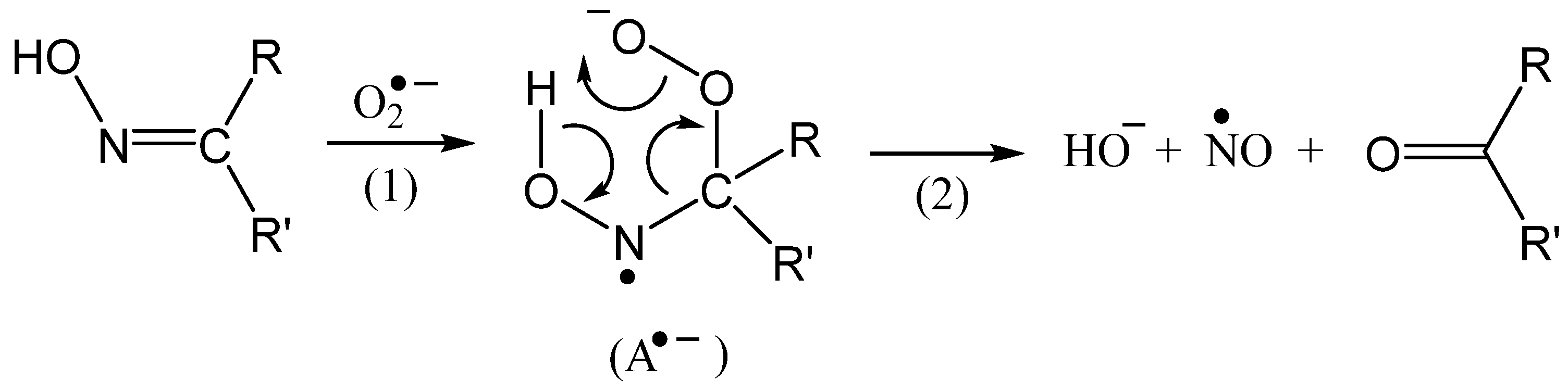
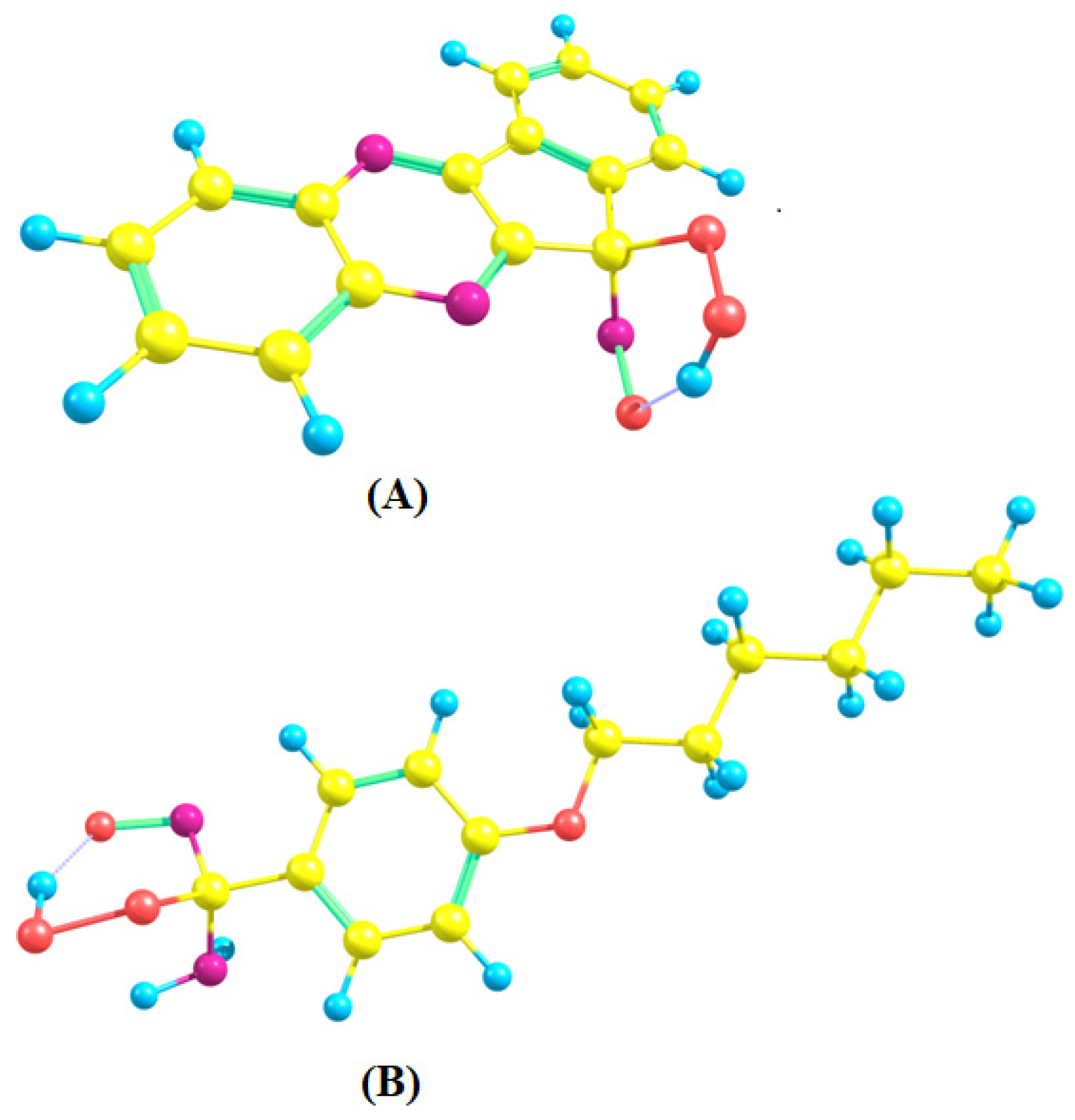
| Substrate | Step 2 | ΔG°298, kcal/mol | |
|---|---|---|---|
| Gas Phase | CPCM Solvation (Water) | ||
| IQ-1 | (1) | −13.06 | 11.66 |
| (2) | −24.85 | −62.95 | |
| (1) + (2) | −37.91 | −51.29 | |
 (AL1) | (1) | −3.64 | 19.36 |
| (2) | −46.51 | −83.18 | |
| (1) + (2) | −50.15 | −63.82 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrianov, V.V.; Schepetkin, I.A.; Bazan, L.V.; Gainutdinov, K.L.; Kovrizhina, A.R.; Atochin, D.N.; Khlebnikov, A.I. Evaluation of Nitric Oxide-Donating Properties of 11H-indeno[1,2-b]quinoxalin-11-one Oxime (IQ-1) by Electron Paramagnetic Resonance Spectroscopy. Molecules 2024, 29, 3820. https://doi.org/10.3390/molecules29163820
Andrianov VV, Schepetkin IA, Bazan LV, Gainutdinov KL, Kovrizhina AR, Atochin DN, Khlebnikov AI. Evaluation of Nitric Oxide-Donating Properties of 11H-indeno[1,2-b]quinoxalin-11-one Oxime (IQ-1) by Electron Paramagnetic Resonance Spectroscopy. Molecules. 2024; 29(16):3820. https://doi.org/10.3390/molecules29163820
Chicago/Turabian StyleAndrianov, Viacheslav V., Igor A. Schepetkin, Leah V. Bazan, Khalil L. Gainutdinov, Anastasia R. Kovrizhina, Dmitriy N. Atochin, and Andrei I. Khlebnikov. 2024. "Evaluation of Nitric Oxide-Donating Properties of 11H-indeno[1,2-b]quinoxalin-11-one Oxime (IQ-1) by Electron Paramagnetic Resonance Spectroscopy" Molecules 29, no. 16: 3820. https://doi.org/10.3390/molecules29163820
APA StyleAndrianov, V. V., Schepetkin, I. A., Bazan, L. V., Gainutdinov, K. L., Kovrizhina, A. R., Atochin, D. N., & Khlebnikov, A. I. (2024). Evaluation of Nitric Oxide-Donating Properties of 11H-indeno[1,2-b]quinoxalin-11-one Oxime (IQ-1) by Electron Paramagnetic Resonance Spectroscopy. Molecules, 29(16), 3820. https://doi.org/10.3390/molecules29163820








