Synthesis and Antiallergic Activity of Dicoumarin Derivatives
Abstract
1. Introduction
2. Results and Discussion
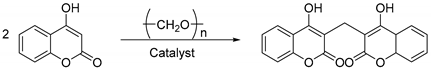
2.1. Synthesis of Dicoumarin Derivatives
2.1.1. Selection of the Optimal Catalyst
2.1.2. Selection of the Optimal Solvent
2.1.3. Selection of the Optimal Reaction Temperature
2.1.4. Production of Dicoumarin Derivatives from Aldehyde Substrates Other than Paraformaldehyde
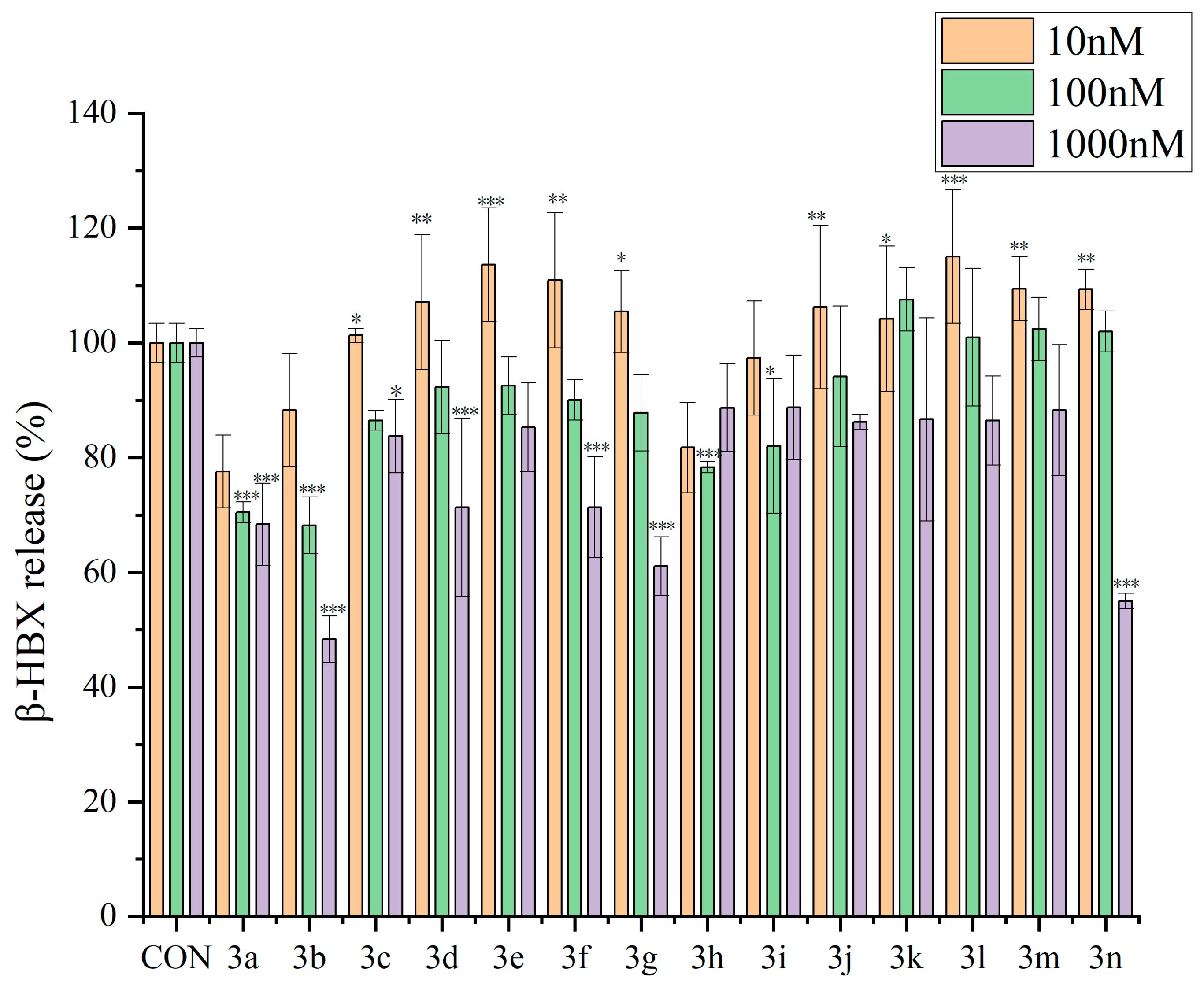
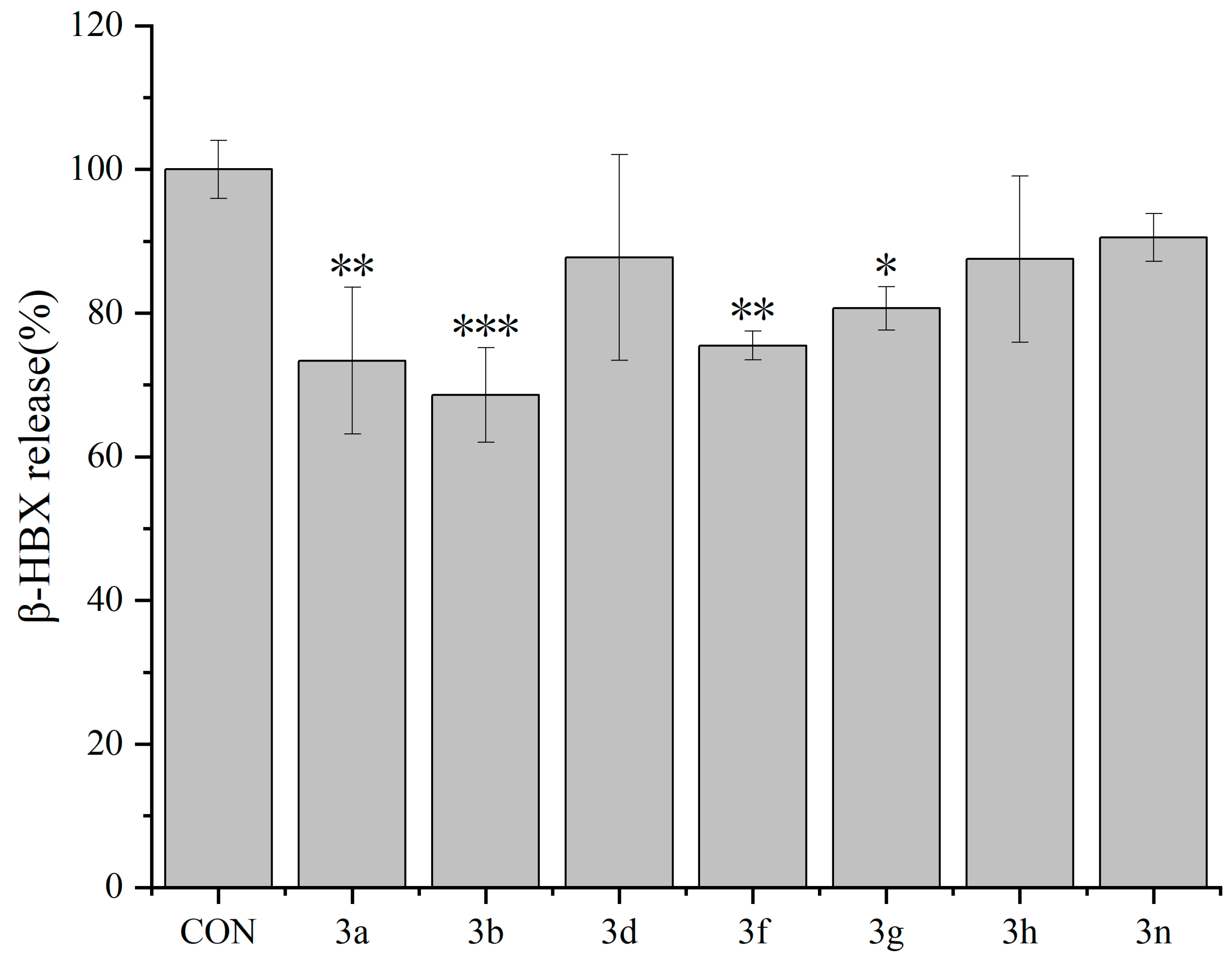
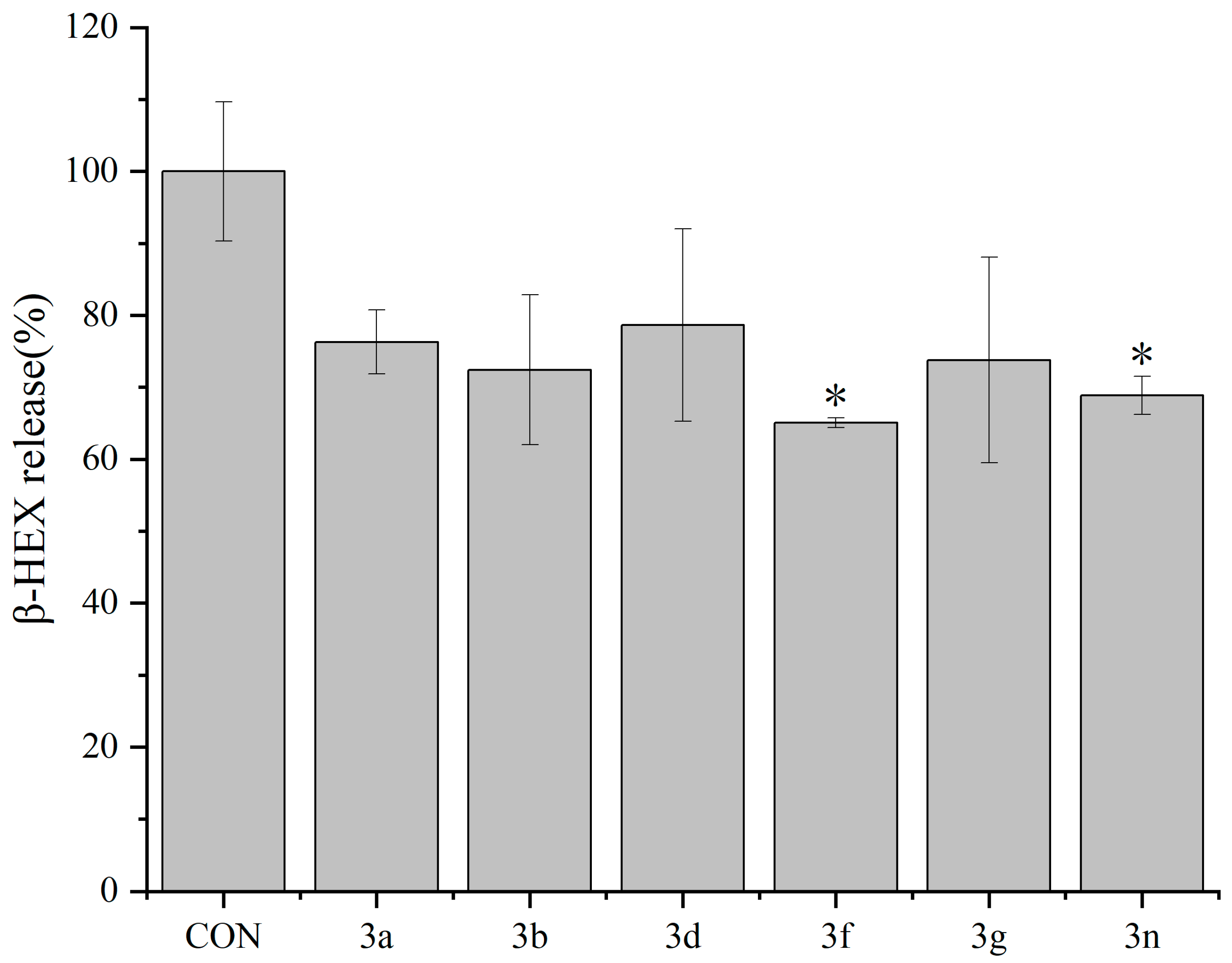
2.2. Antiallergic Activity of Dicoumarin Derivatives
2.2.1. Inhibitory Effects of Dicoumarin Derivatives on the Degranulation of RBL-2H3 Cells
2.2.2. Inhibitory Effects of Dicoumarin Derivatives on the Degranulation of mBMMCs
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Synthesis and Characterization of the Compounds
3.2.1. General Approach to the Preparation of Compounds 3a–3n
3.2.2. 3,3′-Methylenebis(4-hydroxy-2H-chromen-2-one) (3a)
3.2.3. 3,3′-(Phenylmethylene)bis(4-hydroxy-2H-chromen-2-one) (3b)
3.2.4. 3,3′-((2-Bromo-5-hydroxyphenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) (3c)
3.2.5. 3,3′-(2-Methylpropane-1,1-diyl)bis(4-hydroxy-2H-chromen-2-one) (3d)
3.2.6. 3,3′-((3-Hydroxyphenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) (3e)
3.2.7. 3,3′-(Furan-2-ylmethylene)bis(4-hydroxy-2H-chromen-2-one) (3f)
3.2.8. 3,3′-(3-Phenylpropane-1,1-diyl)bis(4-hydroxy-2H-chromen-2-one) (3g)
3.2.9. 3,3′-(Pyridin-4-ylmethylene)bis(4-hydroxy-2H-chromen-2-one) (3h)
3.2.10. 3,3′-((3-Methylthiophen-2-yl)methylene)bis(4-hydroxy-2H-chromen-2-one) (3i)
3.2.11. 3,3′-((3,4-Dimethoxyphenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) (3j)
3.2.12. 3,3′-((4-Methoxyphenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) (3k)
3.2.13. 3,3′-((4-Hydroxy-3-methoxyphenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) (3l)
3.2.14. 3,3′-((4-Hydroxyphenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) (3m)
3.2.15. 3,3′-(Ethane-1,1-diyl)bis(4-hydroxy-2H-chromen-2-one) (3n)
3.3. Compounds Anti-Allergy Activity Assay
3.3.1. RBL-2H3 Cell Culture
3.3.2. Isolation, Culture, and Identification of Mouse Bone Marrow-Derived Mucosal Mast Cells
3.3.3. β-Hexosaminidase Release Experiment in RBL-2H3 Cells
3.3.4. β-Hexosaminidase Release Experiment in mBMMCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, S.-W.; Sun, X.; Han, J.-H.; Kim, T.-Y.; Koppula, S.; Kang, T.-B.; Hwang, J.-K.; Lee, K.-H. Fermentation-mediated enhancement of ginseng’s anti-allergic activity against IgE-mediated passive cutaneous anaphylaxis in vivo and in vitro. J. Microbiol. Biotechnol. 2018, 28, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ. 2020, 13, 100080. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yi, X.; Hajavi, J. New and old adjuvants in allergen-specific immunotherapy: With a focus on nanoparticles. J. Cell. Physiol. 2020, 236, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.P.; Dahl, R. Comparison of intranasal corticosteroids and antihistamines in allergic rhinitis: A review of randomized, controlled trials. Am. J. Respir. Med. 2003, 2, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Sindher, S.B.; Long, A.; Chin, A.R.; Hy, A.; Sampath, V.; Nadeau, K.C.; Chinthrajah, R.S. Food allergy, mechanisms, diagnosis and treatment: Innovation through a multi-targeted approach. Allergy 2022, 77, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Dhiman, N.K. Natural Anti-inflammatory and Anti-allergy Agents: Herbs and Botanical Ingredients. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2022, 21, 90–114. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, Y.; Kumar, K. Structural and clinical impact of anti-allergy agents: An overview. Bioorg. Chem. 2020, 94, 103351. [Google Scholar] [CrossRef] [PubMed]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Ren, Q.-C.; Gao, C.; Xu, Z.; Feng, L.-S.; Liu, M.-L.; Wu, X.; Zhao, F. Bis-coumarin Derivatives and Their Biological Activities. Curr. Top. Med. Chem. 2018, 18, 101–113. [Google Scholar] [CrossRef]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef]
- Silva, V.L.M.; Silva-Reis, R.; Moreira-Pais, A.; Ferreira, T.; Oliveira, P.A.; Ferreira, R.; Cardoso, S.M.; Sharifi-Rad, J.; Butnariu, M.; Costea, M.A.; et al. Dicoumarol: From chemistry to antitumor benefits. Chin. Med. 2022, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Sangshetti, J.N.; Kokare, N.D.; Shinde, D.B. Water mediated efficient one-pot synthesis of bis-(4-hydroxycoumarin)methanes. Green Chem. Lett. Rev. 2009, 2, 233–235. [Google Scholar] [CrossRef]
- Abbasi, F.; Azizi, N.; Abdoli-senejani, M. Highly efficient synthesis of dicoumarols and xanthene derivatives in presence of Brønsted–Lewis acidic ionic liquids catalyst. J. Iran. Chem. Soc. 2017, 14, 2097–2103. [Google Scholar] [CrossRef]
- Mehrabi, H.; Abusaidi, H. Synthesis of biscoumarin and 3,4-dihydropyrano[c]chromene derivatives catalysed by sodium dodecyl sulfate (SDS) in neat water. J. Iran. Chem. Soc. 2010, 7, 890–894. [Google Scholar] [CrossRef]
- Karimi-Jaberi, Z.; Nazarifar, M.R.; Pooladian, B. Tris(hydrogensulfato) boron as a solid heterogeneous catalyst for the rapid synthesis of α,α′-benzylidene bis(4-hydroxycoumarin) derivatives. Lett. Chin. Chem. Lett. 2012, 23, 781–784. [Google Scholar] [CrossRef]
- Zhu, A.; Wang, M.; Li, L.; Wang, J. Tetramethylguanidium-based ionic liquids as efficient and reusable catalysts for the synthesis of biscoumarin at room temperature. RSC Adv. 2015, 5, 73974–73979. [Google Scholar] [CrossRef]
- Khan, K.M.; Iqbal, S.; Lodhi, M.A.; Maharvi, G.M.; Ullah, Z.; Choudhary, M.I.; Rahman, A.U.; Perveen, S.J.B.; Chemistry, M. Biscoumarin: New class of urease inhibitors; economical synthesis and activity. Bioorg. Med. Chem. 2004, 12, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Simijonović, D.; Vlachou, E.-E.; Petrović, Z.D.; Hadjipavlou-Litina, D.J.; Litinas, Κ.E.; Stanković, N.; Mihović, N.; Mladenović, M.P. Dicoumarol derivatives: Green synthesis and molecular modelling studies of their anti-LOX activity. Bioorg. Chem. 2018, 80, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Zare-Akbari, L.E.H.M. A novel nanomagnetic solid acid catalyst for the synthesis of new functionalized bis-coumarin derivatives under microwave irradiations in green conditions. Appl. Organomet. Chem. 2020, 34, e5649. [Google Scholar] [CrossRef]
- Kurume, A.; Kamata, Y.; Yamashita, M.; Wang, Q.; Matsuda, H.; Yoshikawa, M.; Kawasaki, I.; Ohta, S. Synthesis of 3-substituted isocoumarins and their inhibitory effects on degranulation of RBL-2H3 cells induced by antigen. Chem. Pharm. Bull. 2008, 56, 1264–1269. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.; Zhang, Y.; Gao, Y.; Liu, H.; Liu, G.M. Coumarin alleviates ovalbumin-induced food anaphylaxis in a mouse model by affecting mast cell function. Food Funct. 2019, 10, 6767–6778. [Google Scholar] [CrossRef] [PubMed]
- Kupwade, R.V.; Pandit, K.S.; Desai, U.V.; Kulkarni, M.A.; Wadgaonkar, P.P. Diethylamine-catalyzed environmentally benign synthesis of 1-oxo-hexahydroxanthenes and bis-coumarins at ambient temperature. Res. Chem. Intermed. 2016, 42, 6313–6325. [Google Scholar] [CrossRef]
- Xu, J.; Ai, J.; Liu, S.; Peng, X.; Yu, L.; Geng, M.; Nan, F. Design and synthesis of 3,3′-biscoumarin-based c-Met inhibitors. Org. Biomol. Chem. 2014, 12, 3721–3734. [Google Scholar] [CrossRef] [PubMed]
- Montagut-Romans, A.; Boulven, M.; Lemaire, M.; Popowycz, F. Efficient C-3 reductive alkylation of 4-hydroxycoumarin by dehydrogenative oxidation of benzylic alcohols through ruthenium catalysis. New J. Chem. 2014, 38, 1794–1801. [Google Scholar] [CrossRef]
- Sullivan, W.R.; Huebner, C.F.; Stahmann, M.A. Studies on 4-Hydroxycoumarins. II. The Condensation of Aldehydes with 4-Hydroxycoumarins1. J. Am. Chem. Soc. 1943, 65, 2288–2291. [Google Scholar] [CrossRef]
- Hayati, P.; Eghbali, K.; Rezaei, R. Magnetic nanoparticles tris(hydrogensulfato) boron as an efficient heterogeneous acid catalyst for the synthesis of α,ά-benzylidene bis(4-hydroxycoumarin) derivatives under solvent-free condition. Res. Chem. Intermed. 2019, 45, 5067–5089. [Google Scholar] [CrossRef]
- Seddighi, M.; Shirini, F.; Mamaghani, M.J.R.A. Sulfonated rice husk ash (RHA-SO3H) as a highly efficient and reusable catalyst for the synthesis of some bis-heterocyclic compounds. RSC Adv. 2013, 3, 24046–24053. [Google Scholar] [CrossRef]
- Khan, K.M.; Iqbal, S.; Lodhi, M.A.; Maharvi, G.M.; Perveen, S.; Choudhary, M.I.; Atta-ur-Rahman Chohan, Z.H.; Supuran, C.T. Synthesis and Urease Enzyme Inhibitory Effects of Some Dicoumarols. J. Enzym. Inhib. Med. Chem. 2004, 19, 367–371. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patil, S.K.; Awale, D.V.; Vadiyar, M.M.; Patil, S.A.; Bhise, S.C. Simple protic ionic liquid [Et3NH][HSO4] as a proficient catalyst for facile synthesis of biscoumarins. Res. Chem. Intermed. 2017, 43, 5365–5376. [Google Scholar] [CrossRef]
- Siddiqui, Z.N.; Farooq, F. Zn(Proline)2: A novel catalyst for the synthesis of dicoumarols. Catal. Sci. Technol. 2011, 1, 810. [Google Scholar] [CrossRef]
- Boroujeni, K.P.; Ghasemi, P.; Rafienia, Z. Synthesis of biscoumarin derivatives using poly(4-vinylpyridine)-supported dual acidic ionic liquid as a heterogeneous catalyst. Monatshefte Chem. 2014, 145, 1023–1026. [Google Scholar] [CrossRef]
- Bavandi, H.; Habibi, Z.; Yousefi, M. Porcine pancreas lipase as a green catalyst for synthesis of bis-4-hydroxy coumarins. Bioorg. Chem. 2020, 103, 104139. [Google Scholar] [CrossRef] [PubMed]
 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Catalyst Dosage/eq. | Time/h | Yield/% |
| 1 | H2SO4 | 2 | 12 | 6 |
| 2 | Triethylamine | 2 | 12 | 25 |
| 3 | Pyrrolidine | 2 | 3 | 60 |
| 4 | Diethylamine | 1 | 3 | 87 |
| 5 | Diethylamine | 2 | 3 | 95 |
| 6 | Diethylamine | 3 | 3 | 90 |
| Entry | Solvent | Yield/% |
| 1 | Anhydrous ethanol | 95 |
| 2 | 95% ethanol | 80 |
| 3 | MeOH | 80 |
| 4 | CH3CN | 45 |
| 5 | CH2Cl2 | 50 |
| 6 | DMSO | 75 |
| Entry | Temperature/°C | Yield/% |
| 1 | room temperature | 9.9 |
| 2 | 50 | 57 |
| 3 | 80 | 95 |
| 4 | 90 | 95 |
 | ||||||
|---|---|---|---|---|---|---|
| R = -H, -C6H5, -C6H4BrO, -C3H7, -C6H5O, -C4H3O, -C8H9, -C5H4N, -C5H5S, -C8H9O2, -C7H7O, -C7H7O2, -C6H5O, or -CH3. | ||||||
| Entry | Aldehyde | Product | Time/h | Yield/% | M.p. (°C) | |
| Obs. | Lit. | |||||
| 1 | HCHO | 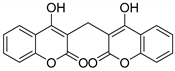 3a | 3 | 95 | 260–262 | 264–265 [23] |
| 2 | 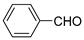 |  3b | 4 | 95 | 143–145 | 231–232 [24] |
| 3 | 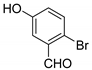 | 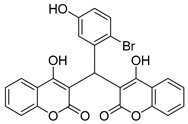 3c | 5 | 93 | 160–161 | - |
| 4 | 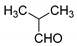 | 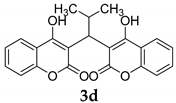 3d | 5 | 95 | 196–198 | 199–200 [25] |
| 5 |  | 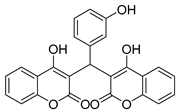 3e | 3.5 | 87 | 198–200 | 210.5 [17] |
| 6 | 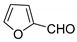 | 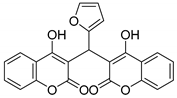 3f | 4 | 91 | 220–222 | 194–196 [26] |
| 7 | 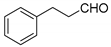 | 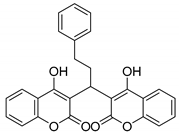 3g | 1 | 86 | 195–197 | 197–198 [27] |
| 8 |  | 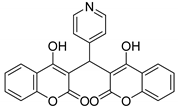 3h | 2 | 73 | 231–234 | 218 [28] |
| 9 | 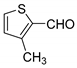 |  3i | 3 | 88 | 130–132 | 134–136 [26] |
| 10 | 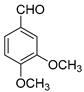 | 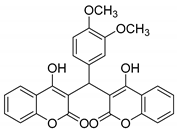 3j | 4 | 85 | 266–268 | 262–264 [29] |
| 11 | 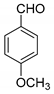 | 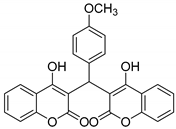 3k | 7 | 93 | 243–244 | 240–242 [29] |
| 12 | 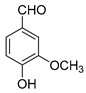 |  3l | 5 | 72 | 249–251 | 254–259 [30] |
| 13 | 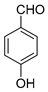 |  3m | 20 | 81 | 220–223 | 220–222 [23] |
| 14 | CH3CHO |  3n | 5.5 | 87 | 173–175 | 176–178 [25] |
| Entry | Catalyst | Reaction Conditions | Time (h) | Yield/(%) | References |
| 1 | SDS (20 mol%) | H2O, 60 °C | 2.3 | 90 | [17] |
| 2 | [P4VPy-BuSO3H]HSO4 | Toluene, 90 °C | 0.8 | 93 | [31] |
| 3 | Lipase (PPL) | EtOH, 45 °C | 6 | 85 | [32] |
| 4 | Et3N | EtOH, RT | 24 | 90 | [23] |
| 5 | piperidine | EtOH, RT | 4 | 92 | [17] |
| 7 | Diethylamine (2 eq.) | EtOH, reflux | 3 | 95 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, X.; Zhou, D. Synthesis and Antiallergic Activity of Dicoumarin Derivatives. Molecules 2024, 29, 3799. https://doi.org/10.3390/molecules29163799
Zhang Y, Wang X, Zhou D. Synthesis and Antiallergic Activity of Dicoumarin Derivatives. Molecules. 2024; 29(16):3799. https://doi.org/10.3390/molecules29163799
Chicago/Turabian StyleZhang, Yuying, Xiaoyu Wang, and Dejun Zhou. 2024. "Synthesis and Antiallergic Activity of Dicoumarin Derivatives" Molecules 29, no. 16: 3799. https://doi.org/10.3390/molecules29163799
APA StyleZhang, Y., Wang, X., & Zhou, D. (2024). Synthesis and Antiallergic Activity of Dicoumarin Derivatives. Molecules, 29(16), 3799. https://doi.org/10.3390/molecules29163799






