Chain Extension of Piperazine in Ethanol: Synthesis of 2-(4-(2-(Phenylthio)ethyl)piperazinyl)acetonitriles and ACAT-1 Inhibitors
Abstract
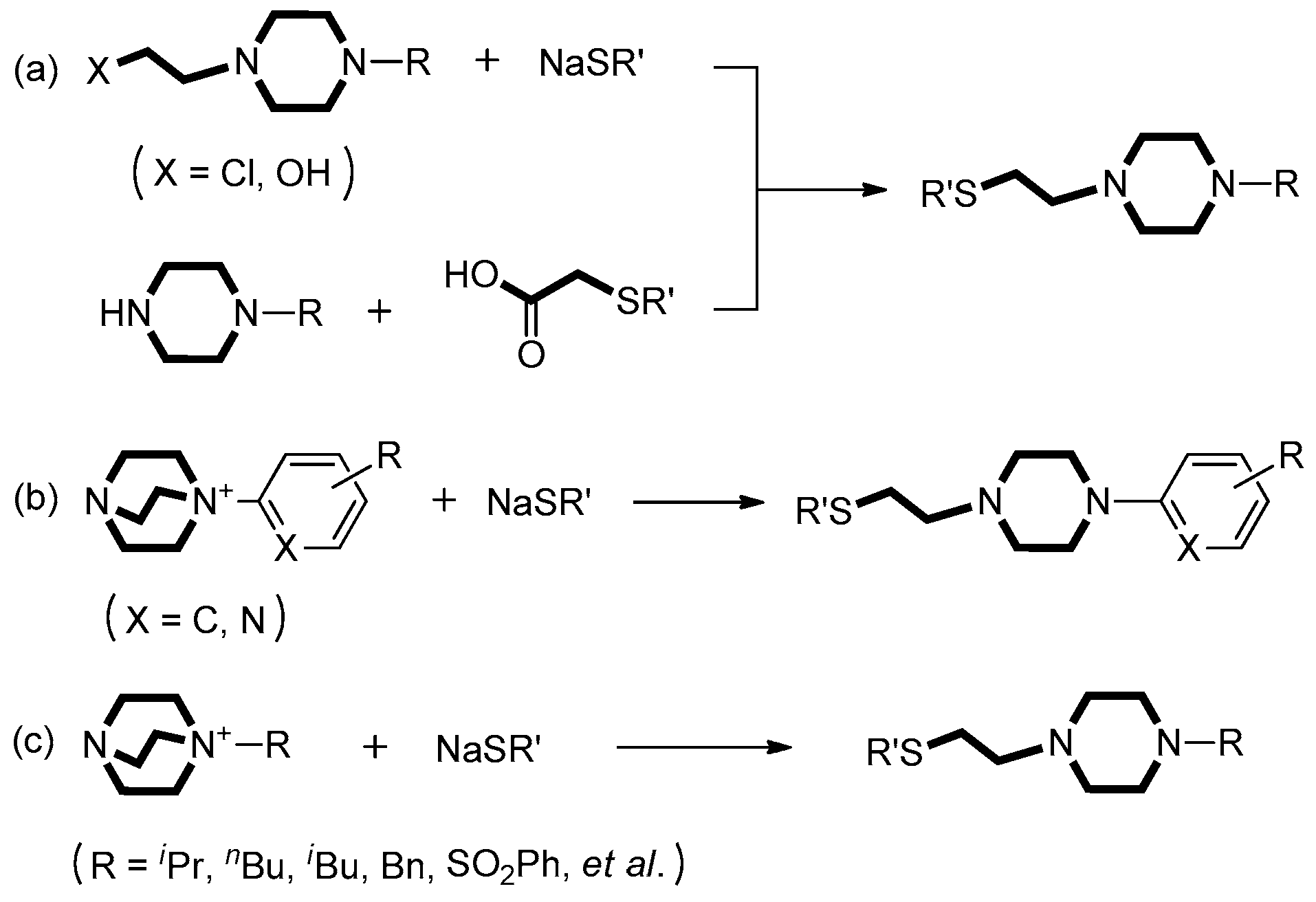
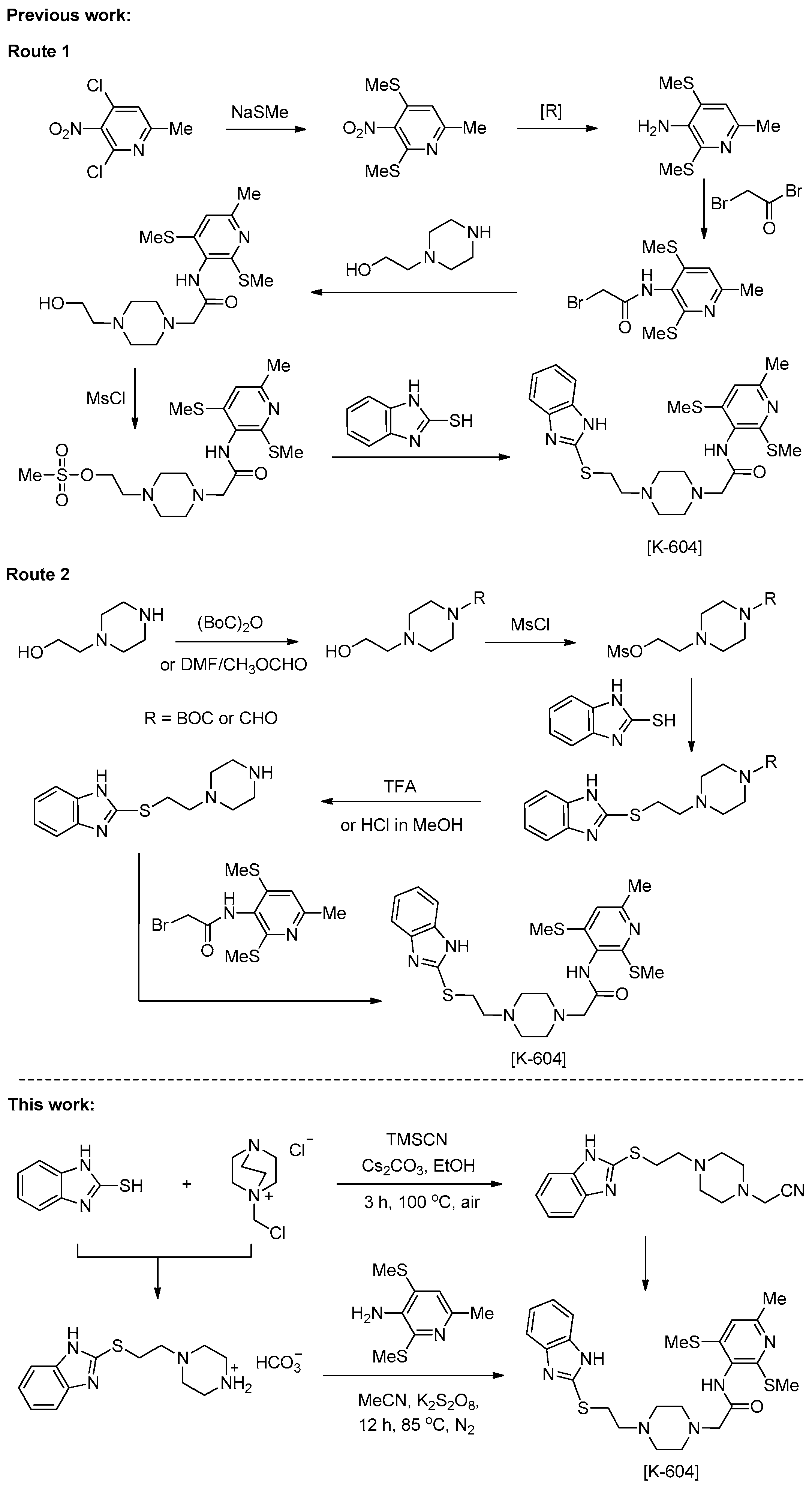
1. Introduction

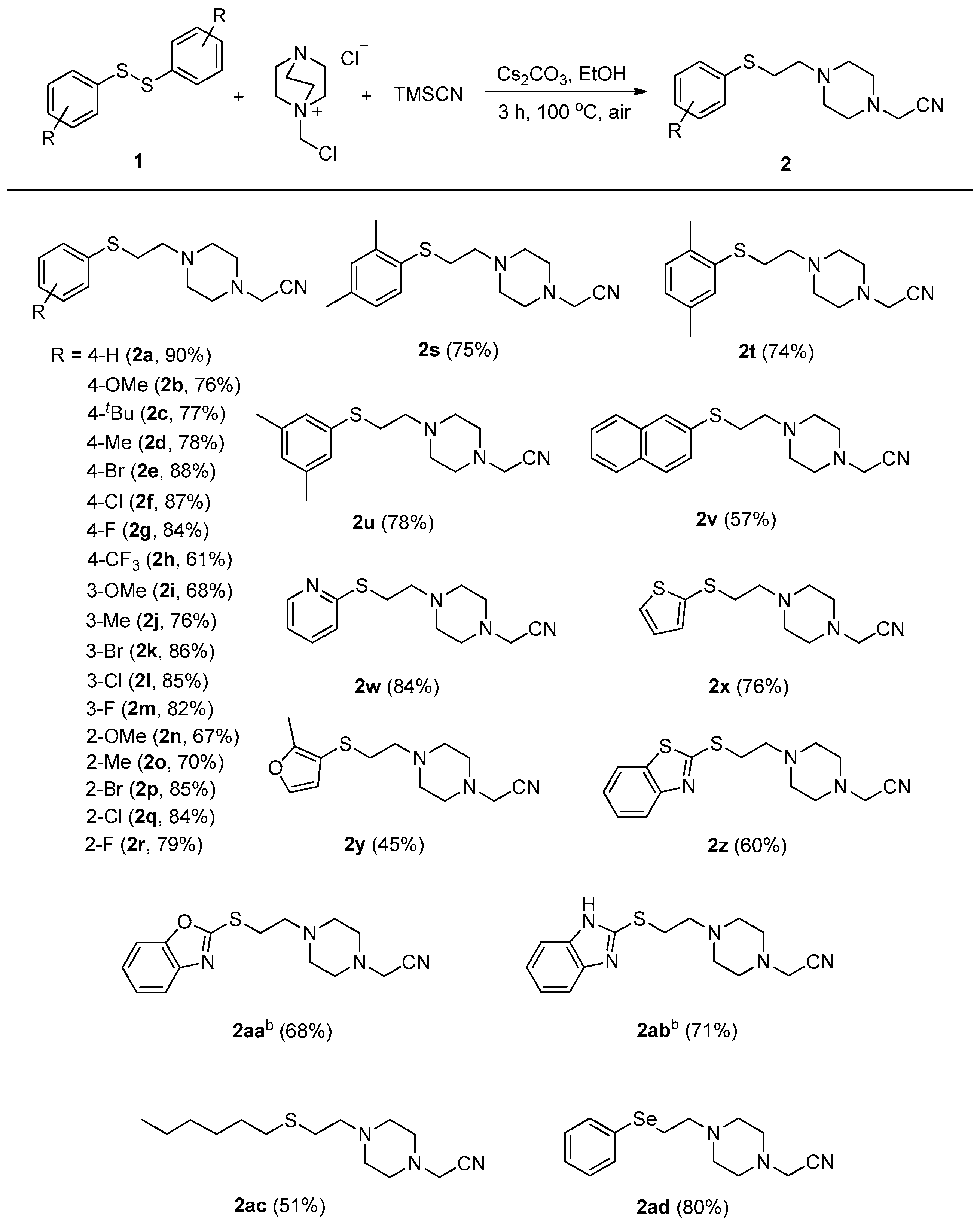
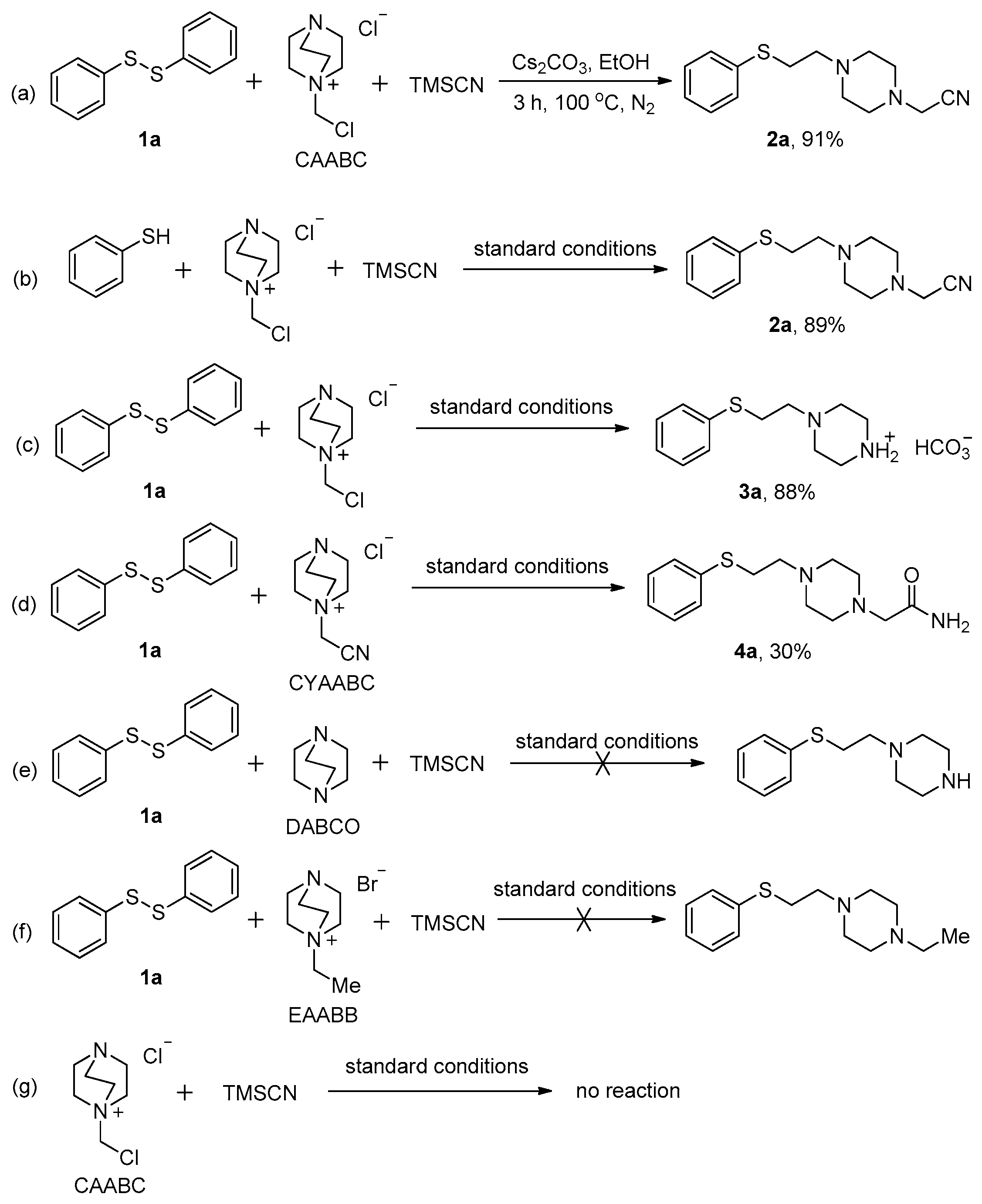
2. Results and Discussion
3. Experimental Section
3.1. General Preparations
3.2. Synthesis Procedures
3.2.1. General Procedure for the Synthesis of Compounds 2
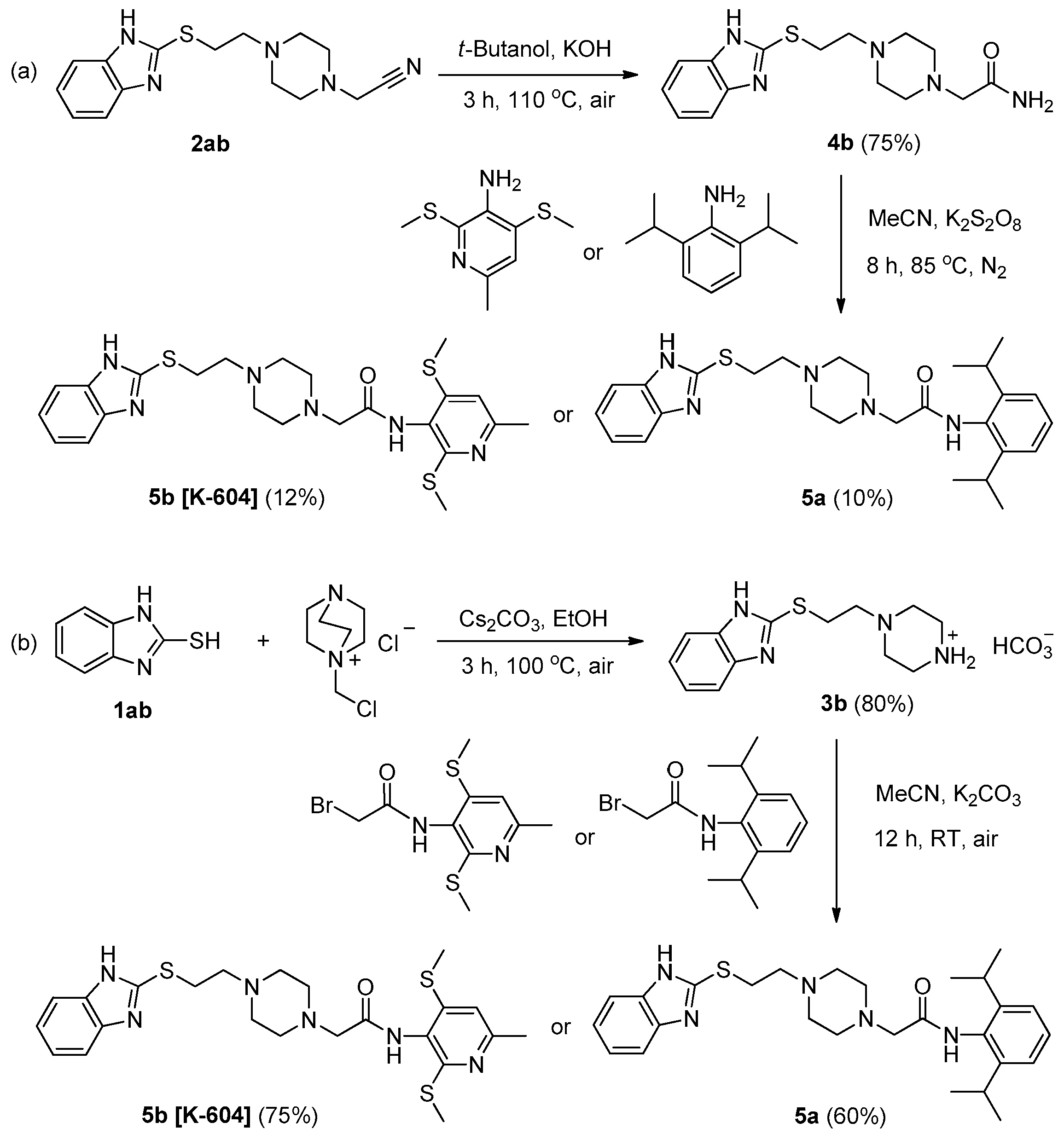
3.2.2. Experimental Procedures for the Synthesis of ACAT-1 Inhibitors 5a and 5b
- (a)
- Method 1:
- (b)
- Method 2:
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomass, S.I.; Pfahler, J.; Mautone, N.; Rovere, A.; Esposito, C.; Passeri, D.; Pellicciari, R.; Novellino, E.; Pannek, M.; Steegborn, C.; et al. Synthesis and Evaluation of Cyclic Secondary Amine Substituted Phenyl and Benzyl Ni-trofuranyl Amides as Novel Antituberculosis Agents. ACS Med. Chem. Lett. 2020, 11, 862–868. [Google Scholar]
- Geng, F.; Cheng, X.; Wu, X.; Yoo, J.Y.; Cheng, C.; Guo, J.Y.; Mo, X.; Ru, P.; Hurwitz, B.; Kim, S.-H.; et al. Inhibition of SOAT1 Suppresses Glioblastoma Growth via Blocking SREBP-1-Mediated Lipogenesis. Clin. Cancer Res. 2016, 22, 5337–5348. [Google Scholar] [CrossRef] [PubMed]
- Ohmoto, T.; Nishitsuji, K.; Yoshitani, N.; Mizuguchi, M.; Yanagisawa, Y.; Saito, H.; Sakashita, N. K604, a Specific Acyl-CoA: Cholesterol Acyltrans-ferase 1 Inhibitor, Suppresses Proliferation of U251-MG Glio-blastoma Cells. Mol. Med. Rep. 2015, 12, 6037–6042. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Bai, Y.; Xiong, Y.; Zhang, J.; Chen, S.; Zheng, X.; Meng, X.; Li, L.; Wang, J.; Xu, C.; et al. Potentiating the Antitumour Response of CD8+T Cells by Modulating Cholesterol Metabolism. Nature 2016, 531, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Ballet, S.; Mauborgne, A.; Bourgoin, S.; Caussade, F.; Cloarec, A.; Hamon, M.; Cesselin, F.; Collin, E. Effects of the Analgesic Drug up 26-91 on the in vivo Release of Substance P and Cal-citonin Gene-Related Peptide from the Rat Spinal Cord. Fundam. Clin. Pharmacol. 1997, 11, 151–199. [Google Scholar]
- Corsano, S.; Strappaghetti, G.; Codagnone, A.; Scapicchi, R.; Marucci, G. Synthesis and Pharmacological Activity of Some New Pyridazinones. Eur. J. Med. Chem. 1992, 21, 545–549. [Google Scholar] [CrossRef]
- Qian, H.; Zhao, X.; Yan, R.; Yao, X.; Gao, S.; Sun, X.; Du, X.; Yang, H.; Wong, C.C.L.; Yan, N. Structural Basis for Catalysis and Substrate Specificity of Human ACAT1. Nature 2020, 581, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, K.; Kawamine, K.; Ozaki, C.; Ohgiya, T.; Edano, T.; Yoshinaka, Y.; Tsunenari, Y. Discovery of Clinical Candidate 2-(4-(2-((1H-Benzo[d]imidazol-2-yl)thio)ethyl)piperazin-1-yl)-N-(6-methyl-2,4-bis(methylthio)pyridin-3-yl)acetamide Hydrochloride [K-604], an Aqueous-Soluble Acyl-CoA: Cho-lesterol O-Acyltransferase-1 Inhibitor. J. Med. Chem. 2018, 61, 10635–10650. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-Y.; Chang, C.C.Y.; Bryleva, E.; Rogers, M.A.; Murphy, S.R. Neuronal Cholesterol Esterification by ACAT1 in Alzheimer’s Disease. IUBMB Life 2010, 62, 261–267. [Google Scholar] [CrossRef]
- Shibuya, Y.; Chang, C.C.Y.; Huang, L.-H.; Bryleva, E.Y.; Chang, T.-Y. Inhibiting ACAT1/SOAT1 in Microglia Stimulates Au-tophagy-Mediated Lysosomal Proteolysis and Increases A1-42 Clearance. J. Neurosci. 2014, 34, 14484–14501. [Google Scholar] [CrossRef]
- Yamanaka, K.; Urano, Y.; Takabe, W.; Saito, Y.; Noguchi, N. Induction of Apoptosis and Necroptosis by 24(S)-Hydroxycholesterol is Dependent on Activity of Acyl-CoA: Cholesterol Acyltrans-ferase 1. Cell Death Dis. 2014, 5, e990. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, Y.; Chang, C.C.Y.; Chang, T.-Y. ACAT1/SOAT1 as a Therapeutic Target for Alzheimer’s Disease. Future Med. Chem. 2015, 7, 2451–2467. [Google Scholar] [CrossRef]
- Shibuya, K.; Watanabe, T.; Urano, Y.; Takabe, W.; Noguchi, N.; Kitagishi, H. Synthesis of 24(S)-Hydroxycholesterol Esters Responsible for the Induction of Neuronal Cell Death. Bioorg. Med. Chem. 2016, 24, 2559–2566. [Google Scholar] [CrossRef]
- Takabe, W.; Urano, Y.; Vo, D.-K.H.; Shibuya, K.; Tanno, M.; Kitagishi, H.; Fujimoto, T. Esterification of 24(S)-Hydroxycholesterol Induces Formation of Atypical Lipid Droplet-Likestructures, Leading to Neuronal Cell Death. J. Lipid Res. 2016, 57, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gu, D.; Lee, S.S.-Y.; Song, B.; Bandyopadhyay, S.; Chen, S.; Konieczny, S.F.; Ratliff, T.L.; Liu, X.; Xie, J.; et al. Abrogating Cholesterol Esterification Suppresses Growth and Metastasis of Pancreatic Cancer. Oncogene 2016, 35, 6378–6388. [Google Scholar] [CrossRef] [PubMed]
- Saraon, P.; Trudel, D.; Kron, K.; Dmitromano-lakis, A.; Trachtenberg, J.; Bapat, B.; Kwast, T.V.D.; Jarvi, K.A.; Diamandis, E.P. Evaluation and Prognostic Significance of ACAT1as a Marker of Prostate Cancer Progression. Prostate 2014, 74, 372–380. [Google Scholar] [CrossRef]
- Mulas, M.F.; Abete, C.; Pulisci, D.; Pani, A.; Massidda, B.; Dess, D.; Mandas, A. Cholesterol Esters as Growth Regulators of Lymphocytic Leukaemia Cells. Cell Prolif. 2011, 44, 360–371. [Google Scholar] [CrossRef]
- Stevenson, E.R.; Wilkinson, M.L.; Abramova, E.; Guo, C.J.; Gow, A.J. Intratracheal Administration of Acyl Coenzyme a Acyltransferase-1 Inhibitor K-604 Reduces Pulmonary Inflammation following Bleomycin-Induced Lung Injury. J. Pharmacol. Exp. Ther. 2020, 382, 356–365. [Google Scholar] [CrossRef]
- Siracusa, M.A.; Salerno, L.; Modica, M.N.; Pittala, V.; Romeo, G.; Amato, M.E.; Nowak, M.; Bojarski, A.J.; Mereghetti, I.; Cagnotto, A.; et al. Synthesis of New Arylpiperazinylalkylthiobenzimidazole, Benzothiazole, or Benzoxazole Derivatives as Potent and Selective 5-HT1A Serotonin Receptor Ligands. J. Med. Chem. 2008, 51, 4529–4538. [Google Scholar] [CrossRef]
- Shibuya, K.; Miura, T.; Ohgiya, T.; Omichi, K.; Tsunenari, Y. Syntheses and Pharmacokinetic Evaluations of Four Metabolites of 2-(4-(2-((1H-benzo[d]imidazol-2-yl)thio)ethyl)piperazin-1-yl)-N-(6-methyl-2,4-bis-(methylthio)pyridin-3-yl)acetamide Hydro-chloride [K-604], an AcylCoA: Cholesterol O-acyltransferase-1 Inhibitor. Bioorg. Med. Chem. 2020, 28, 115457. [Google Scholar]
- Maffuid, K.A.; Koyioni, M.; Torrice, C.D.; Murphy, W.A.; Mewada, H.K.; Koutentis, P.A.; Crona, D.J.; Asquith, C.R.M. Design and Evaluation of 1,2,3-Dithiazoles and Fused 1,2,4-Dithiazines as Anti-Cancer Agents. Bioorg. Med. Chem. Lett. 2021, 43, 128078. [Google Scholar] [CrossRef] [PubMed]
- Linciano, P.; Sorbi, C.; Rossino, G.; Rossi, D.; Marsala, A.; Denora, N.; Bedeschi, M.; Marino, N.; Miserocchi, G.; Dondio, G.; et al. Novel S1R Agonists Counteracting NMDA Excitotoxicity and Oxidative Stress: A Step forward in the Discovery of Neuroprotective Agents. Eur. J. Med. Chem. 2023, 249, 115163. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yuan, Q.; Chen, M.; Guo, M.; Huang, H. Multicomponent Reactions with Cyclic Tertiary Amines Enabled by Facile C-N Bond Cleavage. Angew. Chem. Int. Ed. 2017, 56, 5101–5105. [Google Scholar] [CrossRef] [PubMed]
- Bugaenko, D.I.; Yurovskaya, M.A.; Karchava, A.V. Qua-ternary N-(2-Pyridyl)-DABCO Salts: One-Pot in Situ For-mation from Pyridine-N-oxides and Reactions with Nucleo-philes: A Mild and Selective Route to Substituted N-(2-Pyridyl)-N′-ethylpiperazines. J. Org. Chem. 2017, 82, 2136–2149. [Google Scholar] [CrossRef] [PubMed]
- Min, G.; Seo, J.; Ko, H.M. Three-Component Reactions of Arynes, Amines, and Nucleophiles via a One-Pot Process. J. Org. Chem. 2018, 83, 8417–8425. [Google Scholar] [CrossRef] [PubMed]
- Maras, N.; Polanc, S.; Kocevar, M. Ring-Opening Reactions of 1,4-Diazabicyclo[2.2.2]octane (DABCO) Derived Quater-nary Ammonium Salts with Phenols and Related Nucleophiles. Org. Biomol. Chem. 2012, 10, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Bugaenko, D.I.; Yurovskaya, M.A.; Karchava, A.V. N-Arylation of DABCO with Diaryliodonium Salts: General Synthesis of N-Aryl-DABCO Salts as Precursors for 1,4-Disubstituted Piperazines. Org. Lett. 2018, 20, 6389–6393. [Google Scholar] [CrossRef] [PubMed]
- Asar, F.J.; Soleymani, F.; Hooshmand, S.E.; Halimehjani, A.Z. Direct Synthesis of Piperazines Containing Dithiocarbamate Derivatives via DABCO Bond Cleavage. Tetrahedron Lett. 2020, 61, 152610. [Google Scholar] [CrossRef]
- Hajizadeh, F.; Mojtahedi, M.M.; Abaee, M.S. One-Pot Four-Component Synthesis of Novelisothiourea-Ethylene-Tethered-Piperazinederivatives. RSC Adv. 2023, 13, 32772–32777. [Google Scholar] [CrossRef]
- Shibuya, K.; Kawamine, K.; Sato, Y.; Miura, T.; Ozaki, C.; Edano, T.; Hirata, M. Novel Cyclic Diamine Compounds and Medicine Containing the Same. U.S. Patent 6,969,711, 29 November 2011. [Google Scholar]
- Shibuya, K.; Ohgiya, T.; Sato, Y.; Miura, T. Processes for Production of Cyclic Diamine Compounds or Salts Thereof. U.S. Patent 6,998,486, 14 February 2006. [Google Scholar]
- Shibuya, K.; Tosaka, A. Method for Producing Cyclic Diamine Derivative. U.S. Patent 7,576,203, 18 September 2009. [Google Scholar]


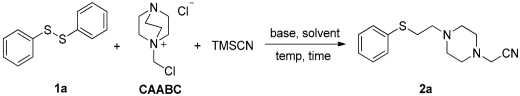 | |||||
|---|---|---|---|---|---|
| En | Base | Solvent | Temp/°C | Time/h | Yield b |
| 1 | Cs2CO3 | EtOH | 100 | 3 | 90% |
| 2 | K2CO3 | EtOH | 100 | 3 | 73% |
| 3 | Na2CO3 | EtOH | 100 | 3 | 57% |
| 4 | KOH | EtOH | 100 | 3 | 35% |
| 5 | tBuOK | EtOH | 100 | 3 | 30% |
| 6 | Cs2CO3 | EtOH/H2O (7:3) | 100 | 3 | 55% |
| 7 | Cs2CO3 | H2O | 100 | 3 | trace |
| 8 | Cs2CO3 | MeOH | 100 | 3 | 75% |
| 9 | Cs2CO3 | DMF | 100 | 3 | none |
| 10 | Cs2CO3 | DMSO | 100 | 3 | none |
| 11 | Cs2CO3 | EtOH | 120 | 3 | 88% |
| 12 | Cs2CO3 | EtOH | 80 | 3 | 82% |
| 13 | Cs2CO3 | EtOH | 100 | 6 | 87% |
| 14 | Cs2CO3 | EtOH | 100 | 1 | 72% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Zhu, T.; Li, Y.; Huang, D. Chain Extension of Piperazine in Ethanol: Synthesis of 2-(4-(2-(Phenylthio)ethyl)piperazinyl)acetonitriles and ACAT-1 Inhibitors. Molecules 2024, 29, 3723. https://doi.org/10.3390/molecules29163723
Huang Y, Zhu T, Li Y, Huang D. Chain Extension of Piperazine in Ethanol: Synthesis of 2-(4-(2-(Phenylthio)ethyl)piperazinyl)acetonitriles and ACAT-1 Inhibitors. Molecules. 2024; 29(16):3723. https://doi.org/10.3390/molecules29163723
Chicago/Turabian StyleHuang, Ying, Tingyu Zhu, Yinghua Li, and Deguang Huang. 2024. "Chain Extension of Piperazine in Ethanol: Synthesis of 2-(4-(2-(Phenylthio)ethyl)piperazinyl)acetonitriles and ACAT-1 Inhibitors" Molecules 29, no. 16: 3723. https://doi.org/10.3390/molecules29163723
APA StyleHuang, Y., Zhu, T., Li, Y., & Huang, D. (2024). Chain Extension of Piperazine in Ethanol: Synthesis of 2-(4-(2-(Phenylthio)ethyl)piperazinyl)acetonitriles and ACAT-1 Inhibitors. Molecules, 29(16), 3723. https://doi.org/10.3390/molecules29163723





