Abstract
Single-atom catalysts (SACs) have attracted extensive attention due to their unique catalytic properties and wide range of applications. Advanced characterization techniques, such as energy-dispersive X-ray spectroscopy, X-ray photoelectron spectroscopy, transmission electron microscopy, scanning electron microscopy, and X-ray absorption fine-structure spectroscopy, have been used to investigate the elemental compositions, structural morphologies, and chemical bonding states of SACs in detail, aiming at unraveling the catalytic mechanism. Meanwhile, theoretical calculations, such as quantum chemical calculations and kinetic simulations, were used to predict the catalytic reaction pathways, active sites, and reaction kinetic behaviors of SACs, providing theoretical guidance for the design and optimization of SACs. This review overviews advanced characterization techniques and theoretical calculations for SACs in Fenton-like chemistry. Moreover, this work highlights the importance of advanced characterization techniques and theoretical calculations in the study of SACs and provides perspectives on the potential applications of SACs in the field of environmental remediation and the challenges of practical engineering.
1. Introduction
In recent years, Fenton-like reactions that catalyze persulfate (peroxymonosulfate (PMS) and peroxydisulfate (PDS)) and other oxidants (peroxyacetic acid (PAA) and periodate (PI)) to produce reactive oxygen species (ROS) such as radicals or nonradicals to achieve efficient degradation of organic pollutants molecules have received widespread attention [1,2,3,4,5,6]. The focus of Fenton-like reactions is the design of superior catalysts to improve the activation efficiency and catalytic reaction kinetics. In homogeneous catalysis, the reactants, catalysts, and products are all in the same aqueous phase and have extensive contact areas, making it possible to obtain high catalytic efficiency with less catalyst addition [7,8,9,10]. However, the problem exists that homogeneous catalysts cannot be recovered after reaction, resulting in waste and, possibly, the pollution of water environments. Heterogeneous catalysts have the advantages of easy separation after reaction and recyclable use. However, the traditional heterogeneous catalysts with metal nanoparticles as the leading active site have poor catalytic performance due to the low utilization rate of metal atoms and the inability to expose the internal areas. Single-atom catalysts (SACs) are a class of materials that anchor a single metal atom to support [11,12,13,14,15]. The research on SACs has determined that dispersing metal particles at the atomic level maximizes the use of metal atoms and improves the selectivity of catalytic reactions [16,17,18,19,20]. Metals and coordination atoms are responsible for generating active sites in heterogeneous catalysis, similar to enzymes or homogeneous catalysts [21,22,23]. This utilization of metal atoms gives SACs an excellent performance and reduces the consumption of precious metals to produce low-cost catalysts [24,25]. Because of their unique advantages, SACs are widely used in energy and environmental applications, such as oxygen reduction reaction (ORR), carbon dioxide reduction reaction (CO2RR), oxygen evolution reaction (OER), and hydrogen evolution reaction (HER), and used as rechargeable battery electrodes [26,27,28,29,30,31,32]. However, the review of the application of SACs in Fenton-like chemistry is limited.
Determining the existence of isolated single atoms and their spatial distribution is the key to developing superior SACs [33,34,35,36,37]. Currently, the characterization methods of SACs are mainly divided into electron microscopy and spectroscopy, among which the spectroscopy mainly includes X-ray absorption near edge spectroscopy (XANES) and extended X-ray absorption fine structure spectroscopy (EXAFS), infrared spectroscopy and nuclear magnetic resonance (NMR) [38,39,40]. The most straightforward and convincing approach is to use electron microscopy techniques such as scanning tunneling microscope (STM) and atomic spherical correction electron microscopy (ACEM) to directly image individual atoms dispersed on the surface of a carrier with a high specific surface area. Moreover, XANES and EXAFS spectroscopy can also provide information on the dispersion and oxidation states of single atoms. In addition, by using suitable probe molecules (such as CO, NH3, and pyridine), infrared spectroscopy can also be used to assess the presence of individual metal atoms and, to a certain extent, quantify the percentage of individual metal atoms dispersed on the surface of the carrier [41,42]. In addition, the changes of the SACs in the catalytic reaction process, the proof of the actual active site, and the elucidation of the catalytic reaction mechanism have not been deeply understood [43,44]. The analysis of single atomic catalytic reaction mechanisms using theoretical calculation has been widely used [45,46]. Therefore, it is urgent to systematically summarize the advanced characterization methods and the theoretical calculation analysis of SACs reported so far.
In this work, the advanced characterization methods of SACs developed in recent years are summarized in detail, including electron microscopy and spectroscopy, and the characteristics, advantages, and disadvantages of each characterization method are analyzed. In addition, based on the work of SACs in Fenton-like chemistry, the current application direction of theoretical calculation is summarized systematically. The unique mechanism of SACs in catalysis is discussed, and theoretical guidance is provided for determining the real active center in the catalysis process and improving the catalytic performance of SACs through atomic-level regulation. Finally, the main problems facing the development of SACs are claimed, and the research challenges and prospects in single-atom catalysis are expounded.
2. Advanced Characterizations
In order to further study the electronic states and metal–carrier interactions of SACs, a variety of advanced characterization techniques are widely used. These techniques study the microscopic environment from different perspectives, which can deepen the understanding of the mechanism of SACs. Different characterization tools give respective information on the morphologies, structures, compositions, and pore properties. Until now, various emerging techniques, including aberration-corrected high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM), X-ray absorption fine structure (XAFS) measurement, infrared (IR) and NMR spectroscopy techniques, Raman, solid electron paramagnetic resonance (EPR), and Mössbauer spectroscopy, have been developed to investigate the detailed microstructure of SACs [14,15,47,48,49,50]. Given that a single technique usually cannot give comprehensive information, the complementary results are adopted using different tools to identify the SACs better and understand their comprehensive properties. The common SAC characterization methods applied in persulfates activation are categorized in Figure 1. Nowadays, advanced characterization tools used for identifying the morphologies, structures, and compositions of SACs include aberration-corrected HAADF-STEM, EXAFS, and XANES. Moreover, the electrochemical properties, such as reducibility and conductivity of SACs, can be investigated by electrochemical techniques, including linear sweep voltammetry (LSV), cyclic voltammetry (CV), and electrochemical impedance spectrum (EIS). Also, the concentrations of PMS/PDS during the reaction should be determined to measure the utilization of PMS/PDS by catalysts. In recent years, the theoretical calculation based on DFT has offered deep insight into the active sites and explored the reaction mechanism. It is worth noting that the combined various characterization methods enable us to understand the composite structures of SACs comprehensively. The objects and tools for the emerging characterization of SACs applied in persulfates activation are summarized, but not limited to, those listed below.
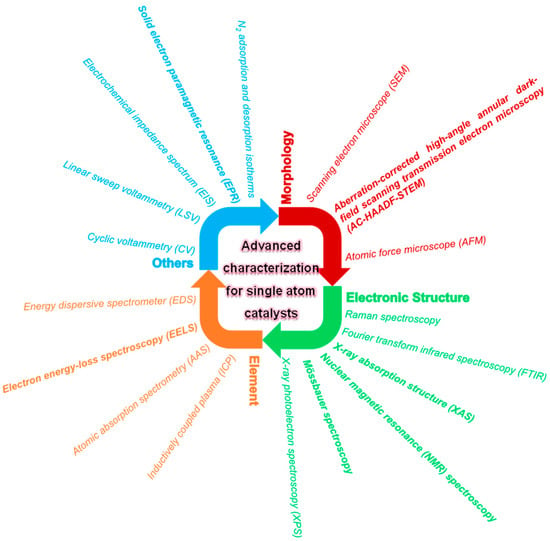
Figure 1.
Categorization of the common SACs characterization methods.
2.1. Electron Microscopy
The catalytic properties of SACs are bound up with their microstructures and compositions. Transmission electron microscopy (TEM) is the most commonly used microscope to elucidate the morphology, size, dispersity, and distribution of SACs. In some cases, even the crystal structure and porosity of the SACs could be identified by TEM [51,52,53]. To determine the size and morphology of SACs with a very tiny degree, various tools, such as high-resolution TEM (HR-TEM), HAADF-STEM, and selected area electron diffraction (SAED), are utilized complementarily to explain the crystallinity and also identify the phase based on the lattice fringe [54]. Based on the principle of Rutherford scattering, the image intensity collected from HAADF-STEM is proportional to the square of the atomic number for the selected element, which allows heavy atoms to show bright contrast compared with light atoms [55]. This tool can be readily applied to characterize the dispersity and distribution of metal elements in SACs. Li et al. clearly observed the dispersion of single Co/Fe atoms anchored on the porous N-doped graphene (FeCo-NC) derived from a Fe-Co Prussian blue analogue (PBA) by using aberration-corrected HAADF-STEM (Figure 2a–c) [56]. It can be observed that the light spots of single Co/Fe atoms were well dispersed across the entire graphene spheres. Similarly, a single Mn atom anchored on N-doped porous carbon (Mn-ISAs@CN) derived from Mn(acac)3@ZIF-8 was fabricated by Yang et al. [57]. The EDX mapping images (Figure 2d) revealed that the Mn, C, and N were distributed homogeneously throughout the structure. The HAADF-STEM images (Figure 2e,f) show that single Mn atoms are homogeneously dispersed in the N-doped porous carbon, which can be clearly observed by the isolated bright dots marked with red cycles (Figure 2f). In addition, Yang et al. also synthesized isolated diatomic Fe-Co anchored on N-doped porous carbon (FeCo@NC) based on Fe(acac)3/Co(acac)2@ZIF-8 [58]. HAADF-STEM and EDX mapping images also illustrated the homogeneous dispersion of Fe-Co single atoms (Figure 2g–i). The electron microscope image is intuitive and enables the observation of the morphology, particle size, and distribution of the catalyst, which facilitates our understanding of the microstructure of the catalyst. However, the field of view of the electron microscope is relatively limited, and usually only a localized area of the sample can be observed. Therefore, combined analyses with other characterizations tools may be required when a comprehensive understanding of the sample properties is necessary.
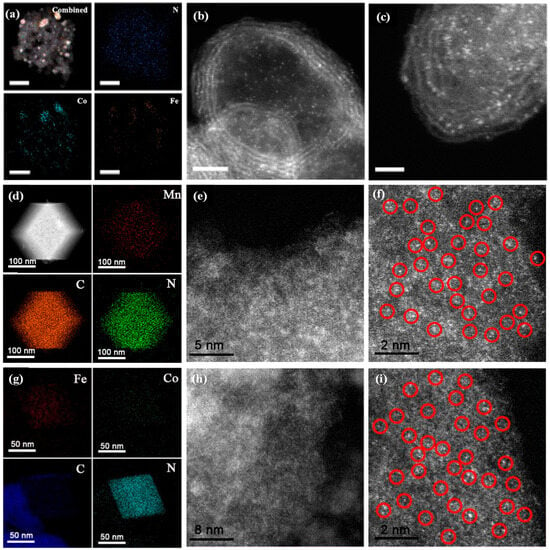
Figure 2.
(a) EDX mappings and (b,c) HAADF-STEM images of FeCo-NC-2. Scale bars: (a) 50 nm, (b) 2 nm, and (c) 1 nm. Reproduced with permission [56]. Copyright 2018, American Chemical Society. (d) Element mapping images and (e,f) HAADF-STEM images of the Mn-ISAs@CN, red circles represent individual dispersed metal atoms. Reproduced with permission [57]. Copyright 2020, Elsevier. (g) Element mapping images, and (h,i) HAADF-STEM images of the FeCo@NC-1, red circles represent individual dispersed metal atoms. Reproduced with permission [58]. Copyright 2020, Elsevier, The Netherlands.
2.2. Spectroscopy Techniques
X-ray absorption spectroscopy (XAS) is a widely used technology to determine the electronic structure of catalysts, especially accurately probing the size, coordination environment, and chemical form of single atoms in SACs structure [54,59,60]. The XAS characterization is usually performed at the synchrotron radiation sources, which provide intense and tunable X-ray beams. X-ray absorption fine-structure spectroscopy (XAFS) is associated with the excitation process of core electrons of the X-ray absorbing atom [61]. XAS can be divided into three regions: pre-edge region, near-edge region (i.e., XANES), and post-edge region (i.e., EXAFS) [62,63,64,65]. XANES and EXAFS are the most commonly used XAS characterization techniques. The XANES region, which results from the excitation of core electrons to the valence and conduction bands, can provide information about the electronic valence state and characteristics of concerned elements [4,66]. While the EXAFS region, originating from the scattering interactions of photoelectrons with the neighboring atoms, can provide vital information on the identity of nearest-neighboring elements, coordination values, and interatomic bond distances [61,62,66]. To date, XAS gradually plays a pivotal role among various materials characterization techniques with the development of synchrotron radiation sources. For instance, monitored with XAS measurements, the dominant coordination mode of the Zn-N4 site in hollow porous carbon (HPC) derived from ZIF-8 was studied by Yang et al. [67]. XANES spectra illustrated that the average oxidation state of Zn species was probably situated between Zn0 and Zn2+ (close to Zn2+). Moreover, the fingerprinting signal peaks of Zn-Zn interactions were not observed in the profile of HPC-800 in EXAFS spectra, revealing the atomic dispersion of Zn in HPC-800. EXAFS spectra further verified that the Zn-N4 was the dominant coordination mode of Zn atoms in HPC-800. Analogously, Yang et al. found that Mn-ISAs@CN is between those of the Mn foil and the Mn2O3, implying that the atomically dispersed Mn species carried positive charges (Figure 3a). The EXAFS spectrum of Mn-ISAs@CN exhibits a stronger peak at about 1.3 Å, originating from the shell of Mn-N scattering, indicating the presence of an isolated single Mn atom (Figure 3b). The corresponding fitting results reveal that the first shell coordination number of the single Mn atoms in the Mn-ISAs@CN is four (Figure 3c). In addition, Zeng et al. found that the valence state of Fe in FePc-CNT was close to 3+ in XANES spectra (Figure 3d) [68]. The k3-weighted Fourier-transform spectra of the Fe K edge EXAFS further confirmed that single Fe atoms with a FeN4 configuration were incorporated into FePc-CNT (Figure 3e,f). Furthermore, the Cu K-edge XANES analysis of Cu-SAC showed that the Cu state of Cu-SAC was located between CuO and Cu2O (Figure 3g) [69]. The EXAFS showed that the coordination structure of Cu-SAC was based on Cu-N4-C configuration (Figure 3h). The monatomic dispersion of Cu species is further proved by the wavelet transform spectrum (Figure 3i). The advantages of XAS derive from the extremely high resolution and accuracy, and as a non-destructive characterization, it allows the acquisition of the required information without destroying the original structure of the catalyst. XAS can also be used for in situ testing to analyze changes in elemental valence and structure during catalytic reactions. However, to obtain high-quality XAS data, fine sample preparation and processing are required, including controlling factors, such as sample purity, morphology, and size. Furthermore, XAS is sensitive to experimental conditions (e.g., temperature, pressure, atmosphere, etc.), and therefore, experiments need to be performed to avoid these conditions interfering with the experimental results.
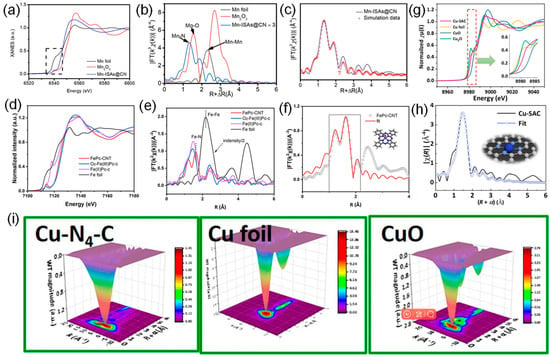
Figure 3.
(a) Normalized Mn K-edge XANES spectra of the Mn foil, Mn2O3 and Mn-ISAs@CN. (b) Fourier transform (FT) k3-weighted χ(k)-function of the EXAFS spectra of the Mn K-edge of the Mn-ISAs@CN. (c) Corresponding Mn K-edge EXAFS fitting curve of Mn-ISAs@CN. Reproduced with permission [57]. Copyright 2020, Elsevier. (d) Normalized Fe K-edge XANES spectrum and (e) k2-weighted FT-EXAFS spectrum of FePc-CNT, together with those of commercial O2-Fe(III)Pc (O2-Fe(III)Pc-c), commercial Fe(II)Pc (Fe(II)Pc-c), and Fe foil standards for comparison. (f) Experimental and fitted EXAFS spectrum in R-space for the FePc-CNT catalyst (R range, 1–2.2 Å). Reproduced with permission [68]. Copyright 2023, American Chemical Society. (g) XANES analysis of Cu-SAC. (h) EXAFS of Cu-SAC. (i) WT contour plots of Cu-SAC. Reproduced with permission [69]. Copyright 2024, National Academy of Science, USA.
The Raman spectrum is a useful tool to probe the structure of the SACs supported by carbon nanomaterials. Two distinguishable peaks in the Raman spectrum of the carbon materials are D band (1320–1355 cm−1) and G band (1570–1585 cm−1) [70]. The D band is related to the breathing mode of A1g symmetry involving phonons near the K-zone boundary, which becomes active in the presence of defects/disorders. The G band is related to the E2g stretching vibration mode of sp2 carbon, which is usually associated with sp2 sites in graphitic carbon. Thus, the intensity ratio of ID/IG in a Raman spectrum is a common criterion to evaluate the defect and disorder degree of carbon materials or the ordered crystal structures of carbon atoms (the graphitization degree) [71]. Furthermore, in situ Raman tests are often used to observe adsorbed-state PMS (PMS*) and high-valent metal species [72,73,74,75]. Wang et al. showed that the new band near 612 cm−1 was strong evidence of the formation of Cu(III) in the Cu(II)/PMS system (Figure 4a), since this was a characteristic Cu-O stretching vibrational band for Cu(III) [76]. Adsorption of PMS is an important step in its being activated. As shown in Figure 4b,c, PMS* can be efficiently observed by an in situ Raman test [72,73].
Mössbauer spectroscopy is a spectroscopic method for the study of energy-level transitions in atomic nuclei. It is particularly suitable for catalysts containing elements such as iron, cobalt, and nickel. The Mössbauer spectroscopy technique is known for its ultrahigh-energy resolution, which makes it possible to achieve a precise characterization of the purity of catalysts when characterizing single-atom catalysts. The Mössbauer technique is not limited to the characterization of catalyst purity and coordination environment but also provides insight into the electronic state and coordination structure changes of the catalytic center during the reaction [77,78]. The development of in situ Mössbauer spectroscopy has further enhanced its application in the characterization of single-atom catalysts. The technique can probe the real structure and property changes of catalytic sites in real-time and quantitatively under reaction conditions, thus revealing the actual process of catalytic reactions.
Fourier-transform infrared spectroscopy (FTIR) of CO adsorption is also a commonly used method to determine whether single atoms are formed. Taking advantage of the specific interactions between CO molecules and atoms on the catalyst surface, the vibrational frequencies of CO molecules change when they are adsorbed on the surface of the catalyst, and these changes are manifested as specific absorption peaks in the FTIR spectra. By detecting and analyzing the positions, intensities, and shapes of these absorption peaks, the adsorption configurations of CO molecules on the catalyst surface can be deduced, and then it can be judged whether single-atom active sites have been formed on the catalyst surface. Because the atomic structure on the surface of SACs is different from that of conventional catalysts, the way and configuration of CO molecules adsorbed on the surface of the catalysts will also be different, and this difference can be clearly reflected in FTIR spectroscopy. Therefore, FTIR spectroscopy has become an effective means to characterize SACs. However, this means also has certain limitations, such as the possible simultaneous presence of other adsorbed species (e.g., water, oxygen, etc.) on the catalyst surface, which may interfere with the FTIR spectra. The limited sensitivity may make the technique challenging to detect SACs with low metal loadings.
2.3. Electrochemical Techniques
Electrochemical techniques, including chronoamperometric experiments, cyclic voltammetry (CV), and electrochemical impedance spectrum (EIS) measurements, can investigate the electrochemical properties, such as the reducibility and conductivity of SACs, which can be linked to redox potential, surface defects, electron transfer rate, etc. [79]. Chronoamperometric experiments are a commonly used method to prove the interaction of PMS/PDS with catalysts. The electron transfer could be identified from the increased electrochemical current on the working electrode. Long et al. found that the current response was monitored at glassy carbon electrodes (GCEs) loaded with N-rGO-CoSA and N-rGO-800 after successive injections of IO4− and 4-CP (Figure 4a) [80]. After the injection of IO4−, the current on the electrodes covered by the two catalysts decreased significantly, which was caused by the electron transfer process after the formation of the complex between the catalyst and IO4−. The observed increase in current after the addition of 4-CP is attributed to the electron transfer process from the electron donor (4-CP) to the acceptor (complex). Therefore, the above results suggest that both N-rGO-CoSA and N-rGO-800 can act as electron shuttles to facilitate the electron transfer process from 4-CP to IO4−, leading to the nonradical oxidation of 4-CP. The increase in the current intensity of N-rGO-CoSA was more significant than that of N-rGO-800, indicating that the introduction of Co single atomic site greatly enhanced the catalytic performance. An EIS measurement can be applied to assess the electrical conductivity and electron transfer ability of catalysts [13,81,82]. It is well known that the smaller arc radius or semicircular radius of Nyquist plots indicates a lower charge-transfer resistance. The EIS of N-rGO-CoSA and N-rGO-800 shows that the electron transfer resistance (Ret) of the former is smaller than that of the latter. Therefore, the Co single atomic site endowed the catalyst carrier with higher conductivity and better electron transfer mediating ability, accelerating the kinetic process of 4-CP oxidation. Zhu et al. measured electrochemical impedance using a Nyquist diagram and equivalent fitting circuit [83] and found that the charge-transfer resistance (Rct) of CNFe2-0.6 was 566.80 Ω, much higher than that of CN (80.90 Ω) (Figure 4b). Since CN is a semiconductor, a higher CNFe2-0.6 Rct indicates poor conductivity, which is speculated to be due to the abundant nanopore disturbing the ordered electron flow. Furthermore, it is analyzed that the electron transfer mechanism dominates the degradation of pollutants. Electrochemical testing allows real-time measurements and monitoring of changes in catalyst performance during catalytic reactions, facilitating the understanding of catalyst deactivation mechanisms. Electrochemical techniques also typically have a high sensitivity for detecting minor current or voltage changes, making them uniquely suited for evaluating low-loading or high-activity SACs. However, the electrochemical testing process may be interfered with by a variety of factors, such as electrode materials, electrolyte composition, and test temperature. These interfering factors may affect the accuracy and reliability of the test results.
2.4. Other Characterization Techniques
Apart from the techniques discussed above, some other characterization approaches also have been widely employed to explore the structure and properties of SACs applied in SR-AOPs. N2 adsorption and desorption isotherm is the most typical physical adsorption process, which is an effective way to characterize the pore size distribution, pore volume, and surface area of SACs. The characters of pores (micropore, mesopore, and macropore) in SAC materials can be identified according to the type of isotherm (I–VI) and the possible type of hysteresis loop (H1-H5) in N2 adsorption and desorption isotherm. Additionally, the surface area and pore volume of SACs can be calculated via the corresponding calculation model, such as Langmuir and Brunauer–Emmett–Teller (BET), according to International Union of Pure and Applied Chemistry (IUPAC) classification. It is remarkable that, in almost all cases, the introduction of metal species will cause a decrease in the surface area of SACs to some extent, considering the mass occupation of metal species [54]. Likewise, the surface area of SACs presents an apparent decrease because the frameworks of the precursor tend to shrink, and many pores completely disappear after long-time harsh heat treatment [84]. Thermogravimetry (TG) is a powerful tool that can be used to characterize the thermostability and constituent of the materials via estimates of the correlation between quality and temperature of the catalyst at a specific temperature. Transient photocurrent tests of photoelectrochemical measurements are used to study the electron–hole-pair separation and carrier migration characteristics of photocatalysts and to evaluate the photocatalytic performance of the catalysts [81,85,86,87]. Qian et al. found that the photocurrent density of pure g-C3N4 and SA-Co-C3N4 is almost the same [88], but much smaller than that of SA-Co-CN/g-C3N4, indicating that the heterogeneous structure promotes the generation and transport of carriers, and the construction of SA-Co-CN/g-C3N4 heterogeneous structure can improve the separation and migration of carriers (Figure 4c). Electron paramagnetic resonance (EPR) spectroscopy is a direct and advanced technique for detecting oxygen vacancy and carbon material-defect levels [13,89]. It provides information about unpaired electrons on the surface of the material as a signal of oxygen vacancies and defects [90,91,92]. EPR spectroscopy is based on paramagnetic samples (with an unpaired electron) that, in a suitable magnetic field, can absorb electromagnetic radiation [86]. Liang et al. use solid-state EPR spectroscopy (Figure 4d), proving that nitrogen vacancy is formed during the construction of the Co-N2 coordination structure [93]. Co-N4 shows a slight paramagnetic signal with a g-value of about 2, which can be assigned to unpaired electrons, indicating the formation of a small number of nitrogen vacancies. The corresponding peak intensity of EPR in Co-N3 and Co-N2 increased significantly to a certain extent, respectively, indicating a large number of nitrogen vacancies in Co-N2 samples. In summary, there are various techniques for the characterizations of SACs, and each technique has its unique advantages and limitations. In practical applications, it is necessary to choose the appropriate characterizations technique or a combination of techniques according to the research purpose and sample characteristics in order to obtain comprehensive and accurate information.
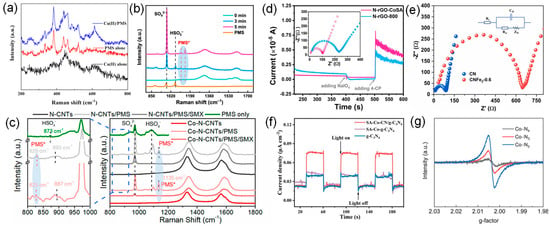
Figure 4.
(a) Raman spectra of Cu(II), PMS, and Cu(II)/PMS in the absence of pollutants. Reproduced with permission [76]. Copyright 2020, American Chemical Society. (b) In situ Raman patterns, PMS* represents the active composites formed by combining PMS with catalysts. Reproduced with permission [72]. Copyright 2023, American Chemical Society. (c) In situ Raman spectra of different oxidation systems, PMS* represents the active composites formed by combining PMS with catalysts. Reproduced with permission [73]. Copyright 2021, American Chemical Society. (d) Current response after the sequential addition of IO4− and 4-CP at the working electrode coated with the N-rGO-CoSA powder. The inset shows the EIS profiles of the N-rGO-CoSA and N-rGO-800 electrodes in the electrolyte solution. Reproduced with permission [80]. Copyright 2021, American Chemical Society. (e) Nyquist plots of CN and CNFe2-0.6-coated electrodes. Inset: equivalent fitting circuit. Reproduced with permission [83]. Copyright 2022, Elsevier. (f) Transient photocurrent responses of g-C3N4, SA-Co-g-C3N4, and SA-Co-CN/g-C3N4. Reproduced with permission [88]. Copyright 2022, John Wiley and Sons, USA. (g) Solid EPR spectra of Co-Nx. Reproduced with permission [93]. Copyright 2022, John Wiley and Sons, USA.
3. Theoretical Calculation
In addition to EXAFS and HAADF-STEM commonly used in SACs characterization, density functional theory (DFT) calculation is a powerful weapon to verify the presence state, reaction path, and specificity of catalysts [38,46,94]. Density functional theory is a quantum mechanical approach that studies the electronic structure of multi-electron systems. So, density functional theory has a wide range of applications in physics and chemistry, especially in studying molecular and condensed matter properties. The main focus in the electronic structure calculations is on the ground-state energy and wave function of the electrons, implying an atomic mean kinetic energy of 0, i.e., a system temperature of 0 K [95,96]. Such a setup simplifies the calculations and allows us to focus on the quantum-mechanical behavior of the electrons without having to take into account the effect of the temperature on the electron distribution [44,97]. A comparison of DFT calculations with experimental results at room temperature is a critical step in verifying the accuracy of theoretical predictions. Classical methods of electron structure theory, such as the Hartree–Fock method, are based on complex multi-electron wave functions [98,99]. The main goal of density functional theory is to replace the wave function with electron density as a fundamental quantity. The material science calculations of the DFT are often combined with related experiments as a supplement and extension to the experimental discipline. The mechanism behind the matter can be further explored by studying the structure of materials (e.g., bond length and vibration).
Applications of theoretical calculation in the field of SACs include studying the monatomic dispersion process, stability and electronic properties after loading, and catalysis processes [18,86,100,101]. In single-atom catalysis, the content that can be calculated mainly includes adsorption energy, state density, differential charge analysis, and catalytic reaction energy barrier. Among them, the adsorption energy (Eads) calculation reflects the capture ability of monatomic materials to reactants; the state density result reflects the electronic structure of materials; the difference charge analysis reflects the electron transfer between SACs materials and reactants; and the reaction energy barrier (ΔG) calculation can show that the energy change in the catalytic process demonstrates the activity and selectivity of catalysts [43,82,102,103]. Although SACs have received widespread attention, the changes in SACs in the catalytic reaction and the related mechanism of catalytic reaction have not been deeply understood. In addition, people still lack theoretical means to investigate the stability of single atomic sites in the reaction process, except for experimental observation. It is of great significance for designing and developing highly active SACs to analyze the stability of monatomic catalysts and the changes in small molecules and substrates in the catalytic reaction process utilizing DFT calculation.
Radicals (•OH and SO4•–) and nonradicals species (catalyst-mediated electron transfer mechanism, high-valent metal, and 1O2) are usually present in reactive oxygen species (ROS) in PMS-based SAC catalysts [49,104,105,106,107]. The adsorption and subsequent bond breaking of PMS molecules can be analyzed by theoretical calculations, and the reaction mechanism can be further explained. Zhang et al. performed DFT calculations to explore PMS adsorption on the possible catalytic sites [108]. Figure 5a shows that the adsorption energies on Fe–pyridinic N4–C, pyridinic N–C, Fe–pyrrolic N4–C, pyrrolic N–C, and Fe–graphitic N4–C were calculated to be −1.471, −0.729, −0.932, −0.455, and −0.174 eV, respectively. The O–O bond lengths (lO–O) in PMS were 1.562, 1.438, and 1.490 Å after adsorbing on the Fe–pyridinic N4–C, Fe–pyrrolic N4–C, and Fe–graphitic N4–C, respectively, which were elongated compared with original PMS molecules (1.410 Å). The results indicate that Fe–pyridinic N4–C interacts more strongly with HSO5− compared with other configuration models due to the more considerable adsorption energy and longer O–O bond length. Chu et al. demonstrated the critical role of the transfer of delocalized π-electrons in tetrapyridomacrocyclic (TPML) in facilitating O–O bond cleavage and stabilizing the surface-bound •OH [109]. The difference-charge and Bader-charge analyses were performed on two possible adsorption configurations: coordinating the Co atom with the O atom adjacent to the H atom (Type I adsorption) or the O atom adjacent to the S atom (Type II adsorption) of O–O bond (Figure 5b). A strong charge transfer from Co-TPML to PMS occurs via Type I adsorption configuration, elongating the O–O bond to 2.01 Å and enabling spontaneous dissociation of O–O bond to surface-bound •OH and free SO4•– species. In contrast to Type I adsorption, while strong adsorption of PMS on Co via Type II configuration also occurs, the charge transfer is minor, and the O–O bond of PMS is slightly elongated from 1.44 to 1.46 Å, indicating that the PMS–Co interaction via Type II adsorption configuration is insufficient for direct O–O bond dissociation. In addition, Song et al. designed unsaturated coordination of a single-atom Co-N3 catalyst in contrast to a conventional Co-N4 catalyst for the activation of PMS [110]. The DFT results showed that the unsaturated coordination model containing N vacancies (Co-N3) has more vital adsorption energy compared to the saturated coordination model (Co-N4, Co-N3C1, Co-N2C2, and Co-N1C3) (Figure 5c). The higher overlap between Co 3d and O 2p near the Fermi energy level, the narrower gap between the d-band center and the Fermi level, and the higher spin state indicated the strong interaction between the Co center and PMS and the enhanced adsorption capacity of the active center (Figure 5d,e).
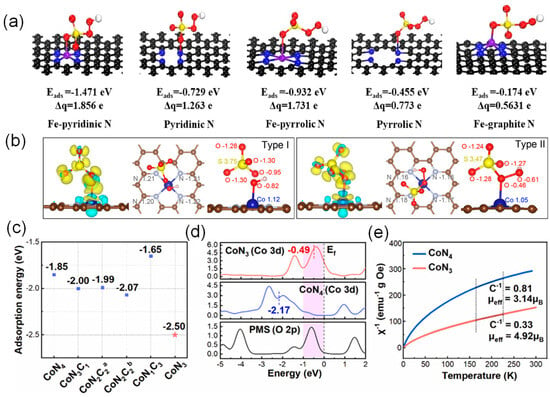
Figure 5.
(a) Adsorption energy on different FeNx–C and N–C sites and the corresponding O–O bond length in PMS and Bader charge after PMS adsorption. Reproduced with permission [108]. Copyright 2022, American Chemical Society. (b) Charge-density differences of PMS adsorbed onto Co-TPML via the coordination of O atom adjacent to the H atom (Type I) and O atom adjacent to the S atom (Type II) in the PMS bond. Yellow denotes the electron accumulation, and cyan represents the electron depletion. Reproduced with permission [109]. Copyright 2021, American Chemical Society. (c) Adsorption energy profiles of PMS toward different Co-N-C configuration models. (d) Projected density of states (PDOS) of Co 3d in CoN3, Co 3d in CoN4, and adsorbed O 2p in PMS. (e) Temperature-dependent inverse susceptibilities over CoN3 and CoN4 (C refers to Curie constant). Reproduced with permission [110]. Copyright 2023, Elsevier.
Recently, our group employed DFT to illustrate the effects of model catalysts with different electronic structures on the activation of PMS [72]. As displayed in Figure 6a, compared to CoPc/G, the Co 3d d-band center in CoPc/G-NH2 shifted positively from −1.997 eV to −0.077 eV, which led to a decreased occupancy and enhanced the adsorption strength of PMS. Additionally, the closer proximity of the d-band center to the Fermi level accelerated charge transfer at the reaction interface, thereby lowering both the free energy barrier and activation energy. Moreover, as shown in Figure 6b, the molecular orbitals analysis revealed that PMS activation was predominantly governed by the 3dz2 orbital in the Co(II) center and π* from the HSO5 group. The amino ligand modification notably enhanced the dz2 orbital compared to CoPc, which rendered CoPc-NH2 more prone to electron donation, consequently facilitating PMS activation. In addition, Zhou et al. integrate electron-deficient boron (B) or electron-rich phosphorus (P) heteroatoms into carbon substrates to tune the electronic structure of Cu-N4 sites for PMS activation [111]. As shown in Figure 6c, the partial density of states (PDOS) for the Cu centers, heteroatoms in the substrate, and oxygen of PMS adsorbed on the Cu sites were calculated. In apparent contrast to primary Cu-N4/C, the PDOS of Cu sites in Cu-N4/C-B displays a negative shift, indicating a decrease in the d-band center. Comparatively, the PDOS of Cu atoms in Cu-N4/C-P moves toward the opposite direction, with an increased d-band center. Additionally, the PDOS for PMS adsorbed on Cu centers shows that Cu-N4/C-B has a relatively weak interaction with PMS compared to Cu-N4/C. Therefore, heteroatom functionalization can effectively optimize the electronic structure of Cu-N4 sites, boosting the activation of PMS. Furthermore, DFT can also be used to calculate the Gibbs free energy of PMS activation from a thermodynamic point of view [112,113]. FeN4 structures were constructed to obtain single-atom catalysts for generating FeN4=O intermediate [113]. The corresponding energy profiles (surface-confined and unconfined systems) were performed to probe the insights of PMS activation and SMX oxidization mechanism on the FeN4 site (Figure 6d). The results showed that the total energy released at the FeN4 site during the reaction over the surface-confined system is much larger than that of the unconfined system, suggesting that the oxidation of SMX is thermodynamically favorable.
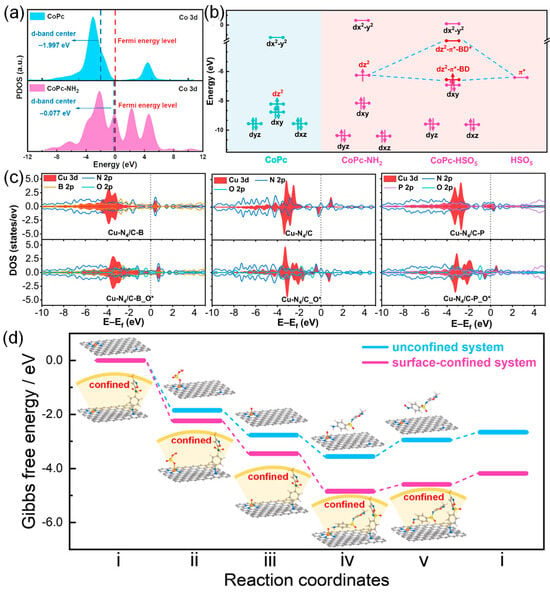
Figure 6.
(a) PDOS of Co 3d in CoPc and CoPc-NH2. (b) Energy levels of Co 3d orbital and orbital interactions between PMS and CoPc-NH2. (c) Reaction pathways of PMS activation at Co–N4 sites and corresponding Gibbs free energy (eV). Reproduced with permission [72]. Copyright 2023, American Chemical Society. (c) PDOS of Cu atom, heteroatoms in the substrate, and oxygen of PMS adsorbed on the Cu center (EF is marked in each graph with the black dashed line). Reproduced with permission [111]. Copyright 2022, National Academy of Science. (d) Reaction pathways of PMS activation at Fe–N4 sites and the corresponding Gibbs free energy (eV). Reproduced with permission [113]. Copyright 2023, American Chemical Society.
4. Conclusions and Perspectives
In this work, the advanced characterization methods and preparation strategies of SACs are reviewed, and the catalytic applications of SACs in Fenton-like chemistry and the application directions of theoretical calculation in single atomic catalysis are introduced. Although SACs have made significant progress in many fields, some research directions still need to solve some problems. There is still a long way to go before industrial application. At present, the following challenges need to be solved:
- (1)
- The changes in SACs in the catalytic reaction process and the related catalytic reaction mechanism still need to be further understood by employing theoretical calculations.
- (2)
- The stability of the single atomic site of SACs in the reaction process needs further improvement.
- (3)
- Under working conditions, direct knowledge of the dynamic changes in active sites during catalytic reactions is still limited. Real-time monitoring of real active sites by in situ measurements, such as in situ HAADF-STEM, XAS, and diffuse infrared Fourier-transform spectroscopy (DRIFTS), helps to clarify the catalytic mechanism.
- (4)
- It is still necessary to regulate the electronic structure of the single atomic active site through local electronic structure engineering to improve the intrinsic catalytic activity of the single atomic site and improve the catalytic reaction kinetics.
- (5)
- More efforts should be focused on the practical industrial application of SAC, and more attention should be paid to technical issues, reactor size, facility capacity, and reaction economics.
Author Contributions
Conceptualization, Z.X. and W.L.; methodology, Z.W. and B.H.; writing—original draft preparation, Z.X.; writing—review and editing, Z.X., Z.P., B.L. and W.L.; visualization, W.L.; funding acquisition, Z.P. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (grant No. 2021YFA1202500) and the China Postdoctoral Science Foundation (2022M710993).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gao, Y.; Zhu, Y.; Li, T.; Chen, Z.; Jiang, Q.; Zhao, Z.; Liang, X.; Hu, C. Unraveling the High-Activity Origin of Single-Atom Iron Catalysts for Organic Pollutant Oxidation via Peroxymonosulfate Activation. Environ. Sci. Technol. 2021, 55, 8318–8328. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Yin, M.; Chen, S.; Ouyang, C.; Huang, X.; Zhang, X. Single-Atom Fe-N4 Sites for Catalytic Ozonation to Selectively Induce a Nonradical Pathway toward Wastewater Purification. Environ. Sci. Technol. 2023, 57, 3623–3633. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xie, Y.; Xiao, J.; Zhao, Z.J.; Wang, Y.; Xu, Z.; Zhang, Y.; Yin, L.; Cao, H.; Gong, J. Single-Atom Mn-N4 Site-Catalyzed Peroxone Reaction for the Efficient Production of Hydroxyl Radicals in an Acidic Solution. J. Am. Chem. Soc. 2019, 141, 12005–12010. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Su, R.; Xu, F.; Xu, X.; Gao, B.; Li, Q. The Unique Fe3Mo3N Structure Bestowed Efficient Fenton-Like Performance of the Iron-Based Catalysts: The Double Enhancement of Radicals and Nonradicals. Adv. Mater. 2024, 36, 2311869. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cheng, X.; Zou, R.; Yong, X.; Pang, C.; Su, Y.; Zhang, Y. Simple modulation of Fe-based single atoms/clusters catalyst with acidic microenvironment for ultrafast Fenton-like reaction. Appl. Catal. B Environ. 2022, 304, 121009. [Google Scholar] [CrossRef]
- Huang, B.; Xiong, Z.; Zhou, P.; Zhang, H.; Pan, Z.; Yao, G.; Lai, B. Ultrafast degradation of contaminants in a trace cobalt(II) activated peroxymonosulfate process triggered through borate: Indispensable role of intermediate complex. J. Hazard. Mater. 2022, 424 Pt D, 127641. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Liang, S.; Zhu, L.; Hua, J.; Duan, W.; Yang, P.T.; Wang, S.L.; Wei, C.; Liu, C.; Feng, C. Fe2+/HClO Reaction Produces Fe(IV)O2+: An Enhanced Advanced Oxidation Process. Environ. Sci. Technol. 2020, 54, 6406–6414. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, P.; Zhang, D.; Xia, Y.; Li, Y.; Zeng, W.; Zhan, S.; Crittenden, J.C. Accelerating Fe(III)-Aqua Complex Reduction in an Efficient Solid-Liquid-Interfacial Fenton Reaction over the Mn-CNH Co-catalyst at Near-Neutral pH. Environ. Sci. Technol. 2021, 55, 13326–13334. [Google Scholar] [PubMed]
- Qi, Y.; Li, J.; Zhang, Y.; Cao, Q.; Si, Y.; Wu, Z.; Akram, M.; Xu, X. Novel lignin-based single atom catalysts as peroxymonosulfate activator for pollutants degradation: Role of single cobalt and electron transfer pathway. Appl. Catal. B Environ. 2021, 286, 119910. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wang, W.; Wu, D.; Wu, Q.; Hu, H. Highly selective production of singlet oxygen by manipulating the spin state of single-atom Co–N moieties and electron localization. Appl. Catal. B Environ. 2023, 324, 122248. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Z.; Hu, C.; Li, F. Understanding the Distance Effect of the Single-Atom Active Sites in Fenton-Like Reactions for Efficient Water Remediation. Adv. Sci. 2024, 11, 2307151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Liang, C.; Yin, L.; Yang, Y. Reconstructing the coordination environment of single atomic Fe-catalysts for boosting the Fenton-like degradation activities. Appl. Catal. B Environ. 2022, 315, 121536. [Google Scholar] [CrossRef]
- Liu, J.; Wei, Z.; Gong, Z.; Yan, M.; Hu, Y.; Zhao, S.; Ye, G.; Fei, H. Single-atom CoN4 sites with elongated bonding induced by phosphorus doping for efficient H2O2 electrosynthesis. Appl. Catal. B Environ. 2023, 324, 122267. [Google Scholar] [CrossRef]
- Fu, H.; Wei, J.; Chen, G.; Xu, M.; Liu, J.; Zhang, J.; Li, K.; Xu, Q.; Zou, Y.; Zhang, W.-X.; et al. Axial coordination tuning Fe single-atom catalysts for boosting H2O2 activation. Appl. Catal. B Environ. 2023, 321, 122012. [Google Scholar] [CrossRef]
- Li, X.; Cao, C.-S.; Hung, S.-F.; Lu, Y.-R.; Cai, W.; Rykov, A.I.; Miao, S.; Xi, S.; Yang, H.; Hu, Z.; et al. Identification of the Electronic and Structural Dynamics of Catalytic Centers in Single-Fe-Atom Material. Chem 2020, 6, 3440–3454. [Google Scholar] [CrossRef]
- Zhao, Z.; Tan, H.; Zhang, P.; Liang, X.; Li, T.; Gao, Y.; Hu, C. Turning the Inert Element Zinc into an Active Single-Atom Catalyst for Efficient Fenton-Like Chemistry. Angew. Chem. Int. Ed. Engl. 2023, 62, e202219178. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, C.; Jin, M.; He, J.; Zhang, H.; Ren, W.; Li, J.; Wang, D.; Li, Y. A Site Distance Effect Induced by Reactant Molecule Matchup in Single-Atom Catalysts for Fenton-Like Reactions. Angew. Chem. Int. Ed. Engl. 2022, 61, e202207268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Jiang, X.H.; Zhong, Z.A.; Tian, L.; Sun, Q.; Cui, Y.T.; Lu, X.; Zou, J.P.; Luo, S.L. Carbon Nitride Supported High-Loading Fe Single-Atom Catalyst for Activation of Peroxymonosulfate to Generate 1O2 with 100% Selectivity. Angew. Chem. Int. Ed. Engl. 2021, 60, 21751–21755. [Google Scholar] [CrossRef]
- Shao, S.; Cui, J.; Wang, K.; Yang, Z.; Li, L.; Zeng, S.; Cui, J.; Hu, C.; Zhao, Y. Efficient and Durable Single-Atom Fe Catalyst for Fenton-like Reaction via Mediated Electron-Transfer Mechanism. ACS EST Engg. 2022, 3, 36–44. [Google Scholar] [CrossRef]
- Tan, W.; Xie, S.; Le, D.; Diao, W.; Wang, M.; Low, K.B.; Austin, D.; Hong, S.; Gao, F.; Dong, L.; et al. Fine-tuned local coordination environment of Pt single atoms on ceria controls catalytic reactivity. Nat. Commun. 2022, 13, 7070. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Huang, D.; Gupta, S.; Weon, S.; Niu, J.; Stavitski, E.; Muhich, C.; Kim, J.H. Neighboring Pd single atoms surpass isolated single atoms for selective hydrodehalogenation catalysis. Nat. Commun. 2021, 12, 5179. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, M.; Huang, H.; Dong, C.; Dai, X.; Feng, G.; Lin, L.; Sun, D.; Yang, D.; Xie, L.; et al. Single Atom-Doped Nanosonosensitizers for Mutually Optimized Sono/Chemo-Nanodynamic Therapy of Triple Negative Breast Cancer. Adv. Sci. 2023, 10, e2206244. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xu, W. Synergistically enhanced single-atomic site catalysts for clean energy conversion. J. Mater. Chem. A 2022, 10, 5673–5698. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, W.J.; Zhang, J.; Lovell, E.C.; Amal, R.; Han, Z.; Lu, X. Anchoring Sites Engineering in Single-Atom Catalysts for Highly Efficient Electrochemical Energy Conversion Reactions. Adv. Mater. 2021, 33, e2102801. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Sun, H.; Lin, L.; Mu, Q.; Yang, B.; Su, Y.; Wu, H.; Lyu, F.; Zhong, J.; Deng, Z.; et al. A hierarchical Single-Atom Ni-N3-C catalyst for electrochemical CO2 reduction to CO with Near-Unity faradaic efficiency in a broad potential range. Chem. Eng. J. 2022, 446, 137296. [Google Scholar] [CrossRef]
- Lyu, H.; Li, P.; Tang, J.; Zou, W.; Wang, P.; Gao, B.; Dong, L. Single-atom Mn anchored on N-doped graphene oxide for efficient adsorption-photocatalytic degradation of sulfanilamide in water: Electronic interaction and mineralization pathway. Chem. Eng. J. 2023, 454, 140120. [Google Scholar] [CrossRef]
- Ao, X.; Zhang, W.; Zhao, B.; Ding, Y.; Nam, G.; Soule, L.; Abdelhafiz, A.; Wang, C.; Liu, M. Atomically dispersed Fe–N–C decorated with Pt-alloy core–shell nanoparticles for improved activity and durability towards oxygen reduction. Energy Environ. Sci. 2020, 13, 3032–3040. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Liu, W.; Wu, J.; Li, Q.; Feng, Q.; Chen, Z.; Xiong, X.; Wang, D.; Lei, Y. Regulating the coordination structure of metal single atoms for efficient electrocatalytic CO2 reduction. Energy Environ. Sci. 2020, 13, 4609–4624. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zheng, L.; Yang, J.; Li, Y.; Wan, X.; Liu, X.; Zhang, X.; Yu, R.; Shui, J. Carbon black-supported FM–N–C (FM = Fe, Co, and Ni) single-atom catalysts synthesized by the self-catalysis of oxygen-coordinated ferrous metal atoms. J. Mater. Chem. A 2020, 8, 13166–13172. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, J.; Zhao, K.; Bi, Y. Spatially confined iron single-atom and potassium ion in carbon nitride toward efficient CO2 reduction. Appl. Catal. B Environ. 2022, 316, 121643. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Yang, Y.; Huan, D.; Su, H.; Zhu, K.; Shi, N.; Qi, Z.; Zheng, X.; Pan, H.; et al. Highly stable and efficient Pt single-atom catalyst for reversible proton-conducting solid oxide cells. Appl. Catal. B Environ. 2022, 316, 121627. [Google Scholar] [CrossRef]
- Huang, B.; Wu, Z.; Zhou, H.; Li, J.; Zhou, C.; Xiong, Z.; Pan, Z.; Yao, G.; Lai, B. Recent advances in single-atom catalysts for advanced oxidation processes in water purification. J. Hazard. Mater. 2021, 412, 125253. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Chen, D.; Hu, T.; Duan, X.; Shakir, I.; Huang, Y.; Duan, X. Single atom electrocatalysts supported on graphene or graphene-like carbons. Chem. Soc. Rev. 2019, 48, 5207–5241. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chang, X.; Lin, X.; Zhao, Z.J.; Gong, J. Theoretical insights into single-atom catalysts. Chem. Soc. Rev. 2020, 49, 8156–8178. [Google Scholar] [CrossRef]
- Zhao, D.; Zhuang, Z.; Cao, X.; Zhang, C.; Peng, Q.; Chen, C.; Li, Y. Atomic site electrocatalysts for water splitting, oxygen reduction and selective oxidation. Chem. Soc. Rev. 2020, 49, 2215–2264. [Google Scholar] [CrossRef]
- Jiao, P.; Ye, D.; Zhu, C.; Wu, S.; Qin, C.; An, C.; Hu, N.; Deng, Q. Non-precious transition metal single-atom catalysts for the oxygen reduction reaction: Progress and prospects. Nanoscale 2022, 14, 14322–14340. [Google Scholar] [CrossRef]
- Wu, X.; Kim, J.-H. Outlook on Single Atom Catalysts for Persulfate-Based Advanced Oxidation. ACS EST Eng. 2022, 2, 1776–1796. [Google Scholar] [CrossRef]
- Weon, S.; Huang, D.; Rigby, K.; Chu, C.; Wu, X.; Kim, J.-H. Environmental Materials beyond and below the Nanoscale: Single-Atom Catalysts. ACS EST Eng. 2020, 1, 157–172. [Google Scholar] [CrossRef]
- Shang, Y.; Xu, X.; Gao, B.; Wang, S.; Duan, X. Single-atom catalysis in advanced oxidation processes for environmental remediation. Chem. Soc. Rev. 2021, 50, 5281–5322. [Google Scholar] [CrossRef]
- Hou, C.-C.; Wang, H.-F.; Li, C.; Xu, Q. From metal–organic frameworks to single/dual-atom and cluster metal catalysts for energy applications. Energy Environ. Sci. 2020, 13, 1658–1693. [Google Scholar] [CrossRef]
- Yan, Y.; Cheng, H.; Qu, Z.; Yu, R.; Liu, F.; Ma, Q.; Zhao, S.; Hu, H.; Cheng, Y.; Yang, C.; et al. Recent progress on the synthesis and oxygen reduction applications of Fe-based single-atom and double-atom catalysts. J. Mater. Chem. A 2021, 9, 19489–19507. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, Y.; Duan, X.; Liu, Y.; Wang, S. Density Functional Theory Calculations for Insight into the Heterocatalyst Reactivity and Mechanism in Persulfate-Based Advanced Oxidation Reactions. ACS Catal. 2021, 11, 11129–11159. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Zou, Y.; Lin, L.; Li, B.; Li, X.-Y. Transition metal single-atom embedded on N-doped carbon as a catalyst for peroxymonosulfate activation: A DFT study. Chem. Eng. J. 2022, 437, 135428. [Google Scholar] [CrossRef]
- Xia, P.; Wang, C.; He, Q.; Ye, Z.; Sirés, I. MOF-derived single-atom catalysts: The next frontier in advanced oxidation for water treatment. Chem. Eng. J. 2023, 452, 139446. [Google Scholar] [CrossRef]
- Yang, P.; Long, Y.; Huang, W.; Liu, D. Single-atom copper embedded in two-dimensional MXene toward peroxymonosulfate activation to generate singlet oxygen with nearly 100% selectivity for enhanced Fenton-like reactions. Appl. Catal. B Environ. 2023, 324, 122245. [Google Scholar] [CrossRef]
- Duan, P.; Pan, J.; Du, W.; Yue, Q.; Gao, B.; Xu, X. Activation of Peroxymonosulfate via Mediated Electron Transfer Mechanism on Single–Atom Fe Catalyst for Effective Organic Pollutants Removal. Appl. Catal. B Environ. 2021, 299, 120714. [Google Scholar] [CrossRef]
- Li, X.; Hu, J.; Deng, Y.; Li, T.; Liu, Z.-Q.; Wang, Z. High stable photo-Fenton-like catalyst of FeP/Fe single atom-graphene oxide for long-term antibiotic tetracycline removal. Appl. Catal. B Environ. 2023, 324, 122243. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, J.; Jiang, Y.; Yang, S.; Yang, Y.; Wang, Z. FeCo bimetallic metal organic framework nanosheets as peroxymonosulfate activator for selective oxidation of organic pollutants. Chem. Eng. J. 2022, 443, 136483. [Google Scholar] [CrossRef]
- Bi, G.; Ding, R.; Song, J.; Luo, M.; Zhang, H.; Liu, M.; Huang, D.; Mu, Y. Discriminating the Active Ru Species Towards the Selective Generation of Singlet Oxygen from Peroxymonosulfate: Nanoparticles Surpass Single-Atom Catalysts. Angew. Chem. Int. Ed. Engl. 2024, 63, e202401551. [Google Scholar] [CrossRef]
- Yang, L.; Xiong, Z.; Li, J.; Wu, Z.; Zhao, X.; Zhao, C.; Zhou, Y.; Qian, Y.; Lai, B. Iron active sites encapsulated in N-doped graphite for efficiently selective degradation of emerging contaminants via peroxymonosulfate (PMS) activation: Inherent roles of adsorption and electron-transfer dominated nonradical mechanisms. Chem. Eng. J. 2022, 444, 136623. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.; Bingham, P.A.; Akiyama, K.; Kubuki, S. Carbon matrix with atomic dispersion of binary cobalt/iron-N sites as efficient peroxymonosulfate activator for organic pollutant oxidation. Chem. Eng. J. 2023, 451, 138574. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Liu, W.; Jiao, M.; Mou, K.; Zhang, X.; Liu, L. Amination strategy to boost the CO2 electroreduction current density of M–N/C single-atom catalysts to the industrial application level. Energy Environ. Sci. 2021, 14, 2349–2356. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.-L. Metal-organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, L.; Doyle-Davis, K.; Fu, X.; Luo, J.L.; Sun, X. Recent Advances in MOF-Derived Single Atom Catalysts for Electrochemical Applications. Adv. Energy Mater. 2020, 10, 2001561. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Xi, S.; Miao, S.; Ding, J.; Cai, W.; Liu, S.; Yang, X.; Yang, H.; Gao, J.; et al. Single Cobalt Atoms Anchored on Porous N-Doped Graphene with Dual Reaction Sites for Efficient Fenton-like Catalysis. J. Am. Chem. Soc. 2018, 140, 12469–12475. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zeng, D.; Zhang, Q.; Cui, R.; Hassan, M.; Dong, L.; Li, J.; He, Y. Single Mn atom anchored on N-doped porous carbon as highly efficient Fenton-like catalyst for the degradation of organic contaminants. Appl. Catal. B Environ. 2020, 279, 119363. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, D.; Li, J.; Dong, L.; Ong, W.-J.; He, Y. A highly efficient Fenton-like catalyst based on isolated diatomic Fe-Co anchored on N-doped porous carbon. Chem. Eng. J. 2021, 404, 126376. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Y.; Yu, G.; Yin, L.; Xiao, J.; Wang, Y.; Lv, W.; Sun, Z.; Kim, J.H.; Cao, H. Manipulating Selectivity of Hydroxyl Radical Generation by Single-Atom Catalysts in Catalytic Ozonation: Surface or Solution. Environ. Sci. Technol. 2022, 56, 17753–17762. [Google Scholar] [CrossRef]
- Wu, X.; Rigby, K.; Huang, D.; Hedtke, T.; Wang, X.; Chung, M.W.; Weon, S.; Stavitski, E.; Kim, J.H. Single-Atom Cobalt Incorporated in a 2D Graphene Oxide Membrane for Catalytic Pollutant Degradation. Environ. Sci. Technol. 2022, 56, 1341–1351. [Google Scholar] [CrossRef]
- Li, J.; Dou, X.; Qin, H.; Sun, Y.; Yin, D.; Guan, X. Characterization methods of zerovalent iron for water treatment and remediation. Water Res. 2018, 148, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Hutson, N.D.; Attwood, B.C.; Scheckel, K.G. XAS and XPS Characterization of Mercury Binding on Brominated Activated Carbon. Environ. Sci. Technol. 2007, 41, 1747–1752. [Google Scholar] [CrossRef]
- Tse, J.S.; Shaw, D.M.; Klug, D.D.; Patchkovskii, S.; Vankó, G.; Monaco, G.; Krisch, M. X-Ray Raman Spectroscopic Study of Water in the Condensed Phases. Phy. Rev. Lett. 2008, 100, 095502. [Google Scholar] [CrossRef]
- Yin, Y.; Shi, L.; Li, W.; Li, X.; Wu, H.; Ao, Z.; Tian, W.; Liu, S.; Wang, S.; Sun, H. Boosting Fenton-Like Reactions via Single Atom Fe Catalysis. Environ. Sci. Technol. 2019, 53, 11391–11400. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Song, J.; Lang, J.; Zhu, Y.; Dai, J.; Wei, Y.; Long, M.; Shao, Z.; Zhou, B.; Alvarez, P.J.J.; et al. Single-Atom MnN5 Catalytic Sites Enable Efficient Peroxymonosulfate Activation by Forming Highly Reactive Mn(IV)–Oxo Species. Environ. Sci. Technol. 2023, 57, 4266–4275. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Zhang, J.; Huang, Y.; Liu, B. In Situ/Operando Techniques for Characterization of Single-Atom Catalysts. ACS Catal. 2019, 9, 2521–2531. [Google Scholar] [CrossRef]
- Jiang, H.L.; Yang, Q.; Yang, C.C.; Lin, C.H. Metal-Organic-Framework-Derived Hollow N-Doped Porous Carbon with Ultrahigh Concentrations of Single Zn Atoms for Efficient Carbon Dioxide Conversion. Angew. Chem. Int. Ed. Engl. 2018, 58, 3511–3515. [Google Scholar]
- Zeng, Y.; Zhao, J.; Wang, S.; Ren, X.; Tan, Y.; Lu, Y.-R.; Xi, S.; Wang, J.; Jaouen, F.; Li, X.; et al. Unraveling the Electronic Structure and Dynamics of the Atomically Dispersed Iron Sites in Electrochemical CO2 Reduction. J. Am. Chem. Soc. 2023, 145, 15600–15610. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Shang, Y.; Yin, K.; Li, Q.; Gao, B.; Li, Y.; Duan, X.; Xu, X. Fenton- like activity and pathway modulation via single-atom sites and pollutants comediates the electron transfer process. Proc. Natl. Acad. Sci. USA 2024, 121, e2313387121. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Qiang, R.; Du, Y.; Chen, D.; Ma, W.; Wang, Y.; Xu, P.; Ma, J.; Zhao, H.; Han, X. Electromagnetic functionalized Co/C composites by in situ pyrolysis of metal-organic frameworks (ZIF-67). J. Alloys Compd. 2016, 681, 384–393. [Google Scholar] [CrossRef]
- Huang, B.; Ren, X.; Zhao, J.; Wu, Z.; Wang, X.; Song, X.; Li, X.; Liu, B.; Xiong, Z.; Lai, B. Modulating Electronic Structure Engineering of Atomically Dispersed Cobalt Catalyst in Fenton-like Reaction for Efficient Degradation of Organic Pollutants. Environ. Sci. Technol. 2023, 57, 14071–14081. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Zhu, Y.; Lang, J.; Zhang, J.; Cheng, S.; Zhou, B.; Zhang, L.; Alvarez, P.J.J.; Long, M. Spin-State-Dependent Peroxymonosulfate Activation of Single-Atom M–N Moieties via a Radical-Free Pathway. ACS Catal. 2021, 11, 9569–9577. [Google Scholar] [CrossRef]
- Liang, S.; Hu, X.; Xu, H.; Lei, Z.; Wei, C.; Feng, C. Mechanistic insight into the reaction pathway of peroxomonosulfate-initiated decomplexation of EDTA-NiII under alkaline conditions: Formation of high-valent Ni intermediate. Appl. Catal. B Environ. 2021, 296, 120375. [Google Scholar] [CrossRef]
- Yang, S.; Xu, S.; Tong, J.; Ding, D.; Wang, G.; Chen, R.; Jin, P.; Wang, X.C. Overlooked role of nitrogen dopant in carbon catalysts for peroxymonosulfate activation: Intrinsic defects or extrinsic defects? Appl. Catal. B Environ. 2021, 295, 120291. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Jiang, N.; Wang, Z.; Jiang, J.; Zhang, T. Trace Cupric Species Triggered Decomposition of Peroxymonosulfate and Degradation of Organic Pollutants: Cu(III) Being the Primary and Selective Intermediate Oxidant. Environ. Sci. Technol. 2020, 54, 4686–4694. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, B.; Li, Y.; Fan, X.; Zhang, F.; Zhang, G.; Peng, W. Facile Synthesis of Atomic Fe-N-C Materials and Dual Roles Investigation of Fe-N4 Sites in Fenton-Like Reactions. Adv. Sci. 2021, 8, e2101824. [Google Scholar] [CrossRef]
- Bao, Y.; Lian, C.; Huang, K.; Yu, H.; Liu, W.; Zhang, J.; Xing, M. Generating High-valent Iron-oxo identical withFe(IV)=O Complexes in Neutral Microenvironments through Peroxymonosulfate Activation by Zn-Fe Layered Double Hydroxides. Angew. Chem. Int. Ed. Engl. 2022, 61, e202209542. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-J.; Hu, W.-L.; Zhang, Q.-H.; Zhao, T.-T.; Luo, H.; Zhang, X.; Gu, L.; Hu, J.-S.; Wan, L.-J. From biological enzyme to single atomic Fe–N–C electrocatalyst for efficient oxygen reduction. Chem. Commun. 2018, 54, 1307–1310. [Google Scholar] [CrossRef]
- Long, Y.; Dai, J.; Zhao, S.; Su, Y.; Wang, Z.; Zhang, Z. Atomically Dispersed Cobalt Sites on Graphene as Efficient Periodate Activators for Selective Organic Pollutant Degradation. Environ. Sci. Technol. 2021, 55, 5357–5370. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Cao, D.; Wang, Y.; Zhu, Y. Peroxymonosulfate enhanced visible light photocatalytic degradation bisphenol A by single-atom dispersed Ag mesoporous g-C3N4 hybrid. Appl. Catal. B Environ. 2017, 211, 79–88. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C.; Yang, Y.; Zhang, S.; Yan, X.; Xiao, C.; Zhou, Y.; Zhu, Z.; Qi, J.; Sun, X.; et al. Mn-Co dual sites relay activation of peroxymonosulfate for accelerated decontamination. Appl. Catal. B Environ. 2023, 330, 122656. [Google Scholar] [CrossRef]
- Zhu, C.; Nie, Y.; Cun, F.; Wang, Y.; Tian, Z.; Liu, F. Two-step pyrolysis to anchor ultrahigh-density single-atom FeN5 sites on carbon nitride for efficient Fenton-like catalysis near 0 °C. Appl. Catal. B Environ. 2022, 319, 121900. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.; Tong, T.; Zhang, L.; Lin, K.-Y.A.; Han, X.; Du, Y. Nitrogen, phosphorus, and sulfur tri-doped hollow carbon shells derived from ZIF-67@poly (cyclotriphosphazene-co-4,4′-sulfonyldiphenol) as a robust catalyst of peroxymonosulfate activation for degradation of bisphenol A. Carbon 2018, 137, 291–303. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, W.; Liu, W.; Li, Y.; Ye, J.; Liang, J.; Tong, M. Activation of sulfite by single-atom Fe deposited graphitic carbon nitride for diclofenac removal: The synergetic effect of transition metal and photocatalysis. Chem. Eng. J. 2021, 407, 127167. [Google Scholar] [CrossRef]
- Zhang, C.; Bai, L.; Chen, M.; Sun, X.; Zhu, M.; Wu, Q.; Gao, X.; Zhang, Q.; Zheng, X.; Yu, Z.Q.; et al. Modulating the Site Density of Mo Single Atoms to Catch Adventitious O Atoms for Efficient H2O2 Oxidation with Light. Adv. Mater. 2023, 35, e2208704. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.; Feng, Y.; Zeng, Y.; Xie, Z.; Zhang, Q.; Su, Y.; Chen, P.; Liu, Y.; Yao, K.; et al. Novel ternary photocatalyst of single atom-dispersed silver and carbon quantum dots co-loaded with ultrathin g-C3N4 for broad spectrum photocatalytic degradation of naproxen. Appl. Catal. B Environ. 2018, 221, 510–520. [Google Scholar] [CrossRef]
- Qian, M.; Wu, X.L.; Lu, M.; Huang, L.; Li, W.; Lin, H.; Chen, J.; Wang, S.; Duan, X. Modulation of Charge Trapping by Island-like Single-Atom Cobalt Catalyst for Enhanced Photo-Fenton-Like reaction. Adv. Funct. Mater. 2023, 33, 2208688. [Google Scholar] [CrossRef]
- Duan, P.; Li, M.; Xu, X.; Yue, Q.; Gao, Y.; Gao, B. Understanding of interfacial molecular interactions and inner-sphere reaction mechanism in heterogeneous Fenton-like catalysis on Mn-N4 site. Appl. Catal. B Environ. 2024, 344, 123619. [Google Scholar] [CrossRef]
- Ni, W.; Liu, Z.; Zhang, Y.; Ma, C.; Deng, H.; Zhang, S.; Wang, S. Electroreduction of Carbon Dioxide Driven by the Intrinsic Defects in the Carbon Plane of a Single Fe-N4 Site. Adv. Mater. 2021, 33, e2003238. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Wang, X.; Yang, S.; Tan, Y.; Yuan, F.; Zheng, S.; Dionysiou, D.D.; Sun, Z. Defect Engineering Modulated Iron Single Atoms with Assist of Layered Clay for Enhanced Advanced Oxidation Processes. Small 2022, 18, 2204793. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, P.; Wang, C.; Gan, L.; Chen, X.; Zhang, P.; Wang, Y.; Li, H.; Wang, L.; Zhou, X.; et al. Unraveling the dual defect sites in graphite carbon nitride for ultra-high photocatalytic H2O2 evolution. Energy Environ. Sci. 2022, 15, 830–842. [Google Scholar] [CrossRef]
- Liang, X.; Wang, D.; Zhao, Z.; Li, T.; Gao, Y.; Hu, C. Coordination Number Dependent Catalytic Activity of Single-Atom Cobalt Catalysts for Fenton-Like Reaction. Adv. Funct. Mater. 2022, 32, 2203001. [Google Scholar] [CrossRef]
- Wei, Y.; Miao, J.; Ge, J.; Lang, J.; Yu, C.; Zhang, L.; Alvarez, P.J.J.; Long, M. Ultrahigh Peroxymonosulfate Utilization Efficiency over CuO Nanosheets via Heterogeneous Cu(III) Formation and Preferential Electron Transfer during Degradation of Phenols. Environ. Sci. Technol. 2022, 56, 8984–8992. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yao, Y.; Wang, Y.; Liu, Y. Tuning the electronic structure of transition metals embedded in nitrogen-doped graphene for electrocatalytic nitrogen reduction: A first-principles study. Nanoscale 2020, 12, 9696–9707. [Google Scholar] [CrossRef]
- Jiao, D.; Liu, Y.; Cai, Q.; Zhao, J. Coordination tunes the activity and selectivity of the nitrogen reduction reaction on single-atom iron catalysts: A computational study. J. Mater. Chem. A 2021, 9, 1240–1251. [Google Scholar] [CrossRef]
- Greiner, M.T.; Jones, T.E.; Beeg, S.; Zwiener, L.; Scherzer, M.; Girgsdies, F.; Piccinin, S.; Armbrüster, M.; Knop-Gericke, A.; Schlögl, R. Free-atom-like d states in single-atom alloy catalysts. Nat. Chem. 2018, 10, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shang, S.; Fan, Y.; Shih, K.; Li, X.-Y.; Lin, L. Incorporation of atomically dispersed cobalt in the 2D metal–organic framework of a lamellar membrane for highly efficient peroxymonosulfate activation. Appl. Catal. B Environ. 2023, 325, 122344. [Google Scholar] [CrossRef]
- Sun, Y.; Li, R.; Song, C.; Zhang, H.; Cheng, Y.; Nie, A.; Li, H.; Dionysiou, D.D.; Qian, J.; Pan, B. Origin of the improved reactivity of MoS2 single crystal by confining lattice Fe atom in peroxymonosulfate-based Fenton-like reaction. Appl. Catal. B Environ. 2021, 298, 120537. [Google Scholar] [CrossRef]
- Mi, X.; Wang, P.; Xu, S.; Su, L.; Zhong, H.; Wang, H.; Li, Y.; Zhan, S. Almost 100% Peroxymonosulfate Conversion to Singlet Oxygen on Single-Atom CoN2+2 Sites. Angew. Chem. Int. Ed. Engl. 2021, 60, 4588–4593. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, H.; Liu, C.; Zheng, L.; Liu, C.; Wang, J.O.; Liu, S.; Han, Y.; Gu, L.; Qian, J.; et al. Single-Atom Fe Catalysts for Fenton-Like Reactions: Roles of Different N Species. Adv. Mater. 2022, 34, e2110653. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Ye, Z.; Zhao, L.; Xue, Q.; Lanzalaco, S.; He, Q.; Qi, X.; Sirés, I. Tailoring single-atom FeN4 moieties as a robust heterogeneous catalyst for high-performance electro-Fenton treatment of organic pollutants. Appl. Catal. B Environ. 2023, 322, 122116. [Google Scholar] [CrossRef]
- Jiang, N.; Xu, H.; Wang, L.; Jiang, J.; Zhang, T. Nonradical Oxidation of Pollutants with Single-Atom-Fe(III)-Activated Persulfate: Fe(V) Being the Possible Intermediate Oxidant. Environ. Sci. Technol. 2020, 54, 14057–14065. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pei, Y.; Cao, M.; Yang, H.; Li, Y. Highly dispersed copper single-atom catalysts activated peroxymonosulfate for oxytetracycline removal from water: Mechanism and degradation pathway. Chem. Eng. J. 2022, 450, 138194. [Google Scholar] [CrossRef]
- Shang, Y.; Liu, X.; Li, Y.; Gao, Y.; Gao, B.; Xu, X.; Yue, Q. Boosting fenton-like reaction by reconstructed single Fe atom catalyst for oxidizing organics: Synergistic effect of conjugated π-π sp2 structured carbon and isolated Fe-N4 sites. Chem. Eng. J. 2022, 446, 137120. [Google Scholar] [CrossRef]
- Han, B.; Luo, Y.; Lin, Y.; Weng, B.; Xia, D.; Zhou, Y.; Guan, C.; Wang, Z.; Wei, X.; Jiang, J. Microenvironment engineering of single-atom catalysts for persulfate-based advanced oxidation processes. Chem. Eng. J. 2022, 447, 137551. [Google Scholar] [CrossRef]
- Chen, F.; Wu, X.-L.; Yang, L.; Chen, C.; Lin, H.; Chen, J. Efficient degradation and mineralization of antibiotics via heterogeneous activation of peroxymonosulfate by using graphene supported single-atom Cu catalyst. Chem. Eng. J. 2020, 394, 124904. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.; Akiyama, K.; Bingham, P.A.; Kubuki, S. Elucidating the Mechanistic Origin of a Spin State-Dependent FeNx-C Catalyst toward Organic Contaminant Oxidation via Peroxymonosulfate Activation. Environ. Sci. Technol. 2022, 56, 1321–1330. [Google Scholar] [CrossRef]
- Chu, C.; Yang, J.; Zhou, X.; Huang, D.; Qi, H.; Weon, S.; Li, J.; Elimelech, M.; Wang, A.; Kim, J.H. Cobalt Single Atoms on Tetrapyridomacrocyclic Support for Efficient Peroxymonosulfate Activation. Environ. Sci. Technol. 2021, 55, 1242–1250. [Google Scholar] [CrossRef]
- Song, J.; Hou, N.; Liu, X.; Antonietti, M.; Wang, Y.; Mu, Y. Unsaturated single-atom CoN3 sites for improved fenton-like reaction towards high-valent metal species. Appl. Catal. B 2023, 325, 122368. [Google Scholar] [CrossRef]
- Zhou, X.; Ke, M.K.; Huang, G.X.; Chen, C.; Chen, W.; Liang, K.; Qu, Y.; Yang, J.; Wang, Y.; Li, F.; et al. Identification of Fenton-like active Cu sites by heteroatom modulation of electronic density. Proc. Natl. Acad. Sci. USA 2022, 119, e2119492119. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Chen, H.; Li, W.; Ao, Z.; Wu, Y.N.; Guan, X. Single-Atom Fe Catalyst Outperforms Its Homogeneous Counterpart for Activating Peroxymonosulfate to Achieve Effective Degradation of Organic Contaminants. Environ. Sci. Technol. 2021, 55, 7034–7043. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wu, Z.; Wang, X.; Song, X.; Zhou, H.; Zhang, H.; Zhou, P.; Liu, W.; Xiong, Z.; Lai, B. Coupled Surface-Confinement Effect and Pore Engineering in a Single-Fe-Atom Catalyst for Ultrafast Fenton-like Reaction with High-Valent Iron-Oxo Complex Oxidation. Environ. Sci. Technol. 2023, 57, 15667–15679. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).