Comprehensive Review of Synthesis, Optical Properties and Applications of Heteroarylphosphonates and Their Derivatives
Abstract
1. Introduction
2. Optical Properties, Synthesis and Applications of Heteroarylphosphonates and Their Derivatives
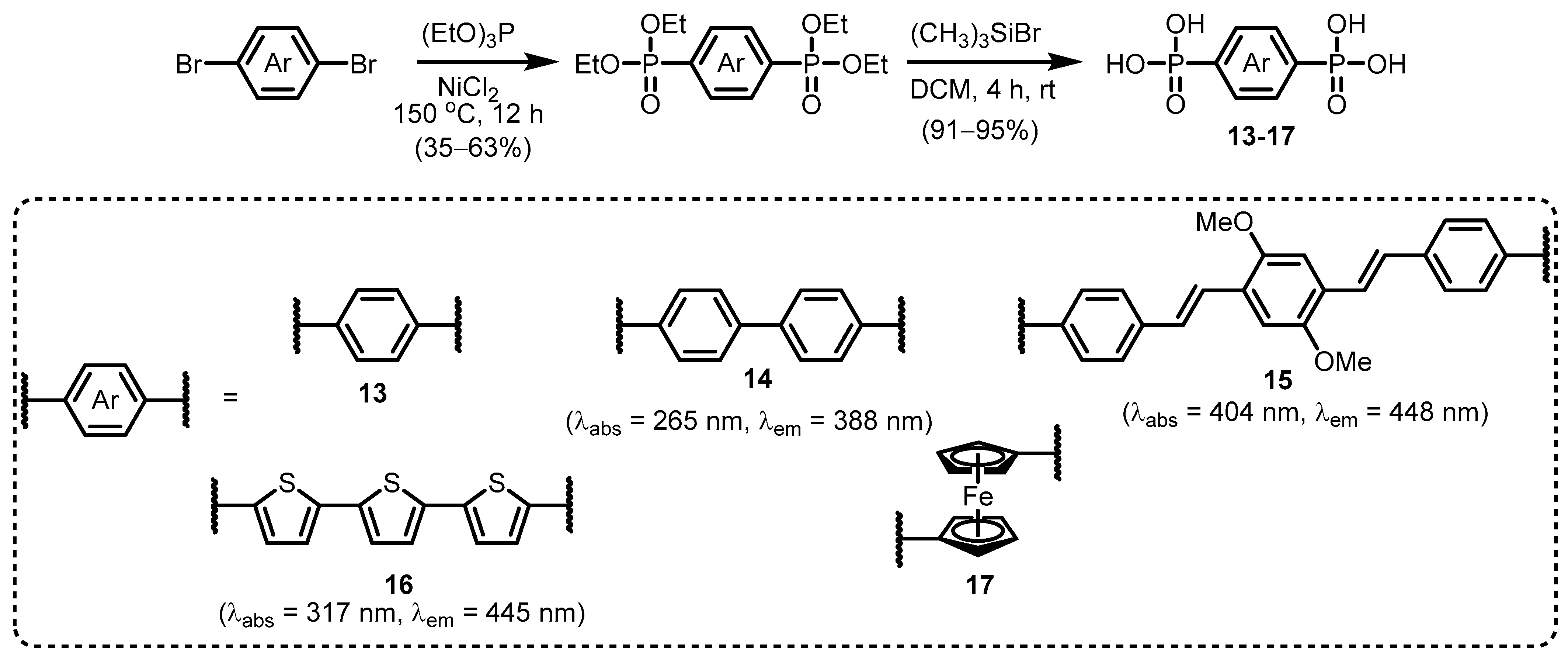
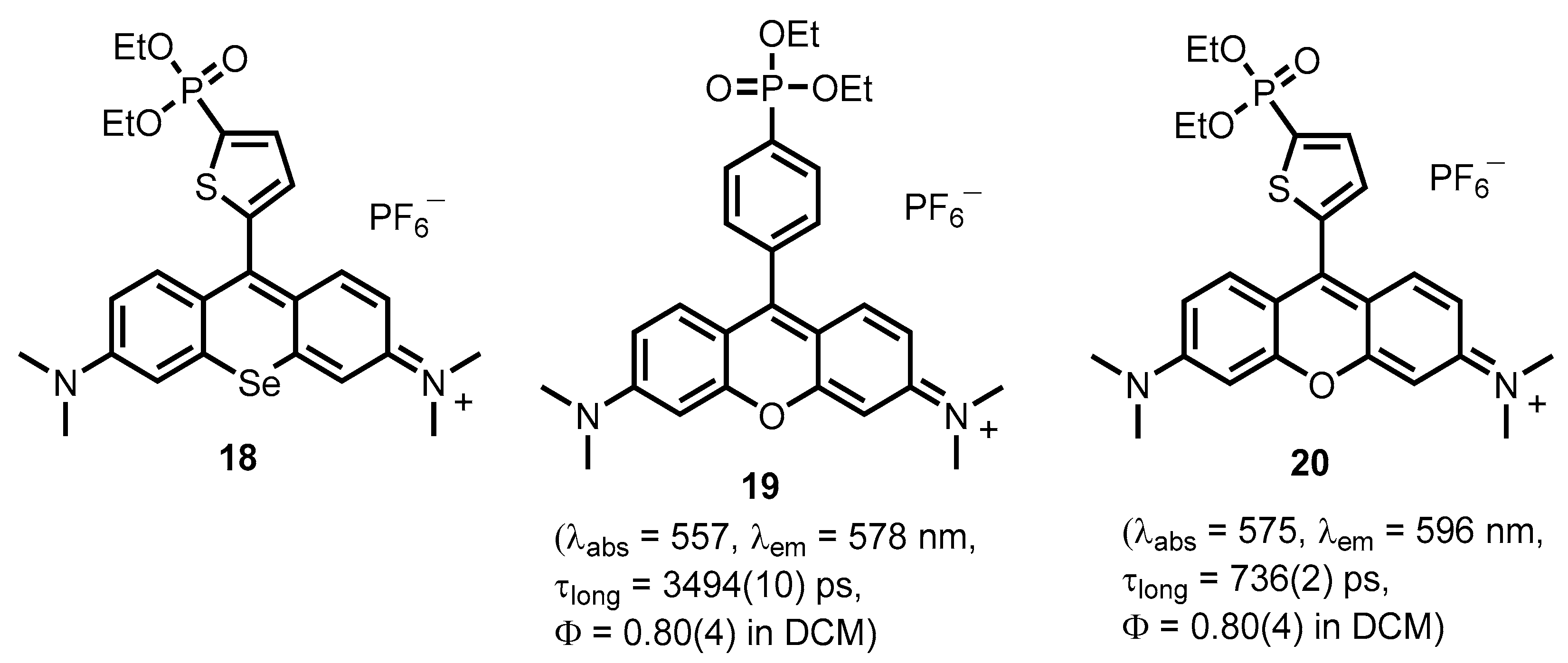
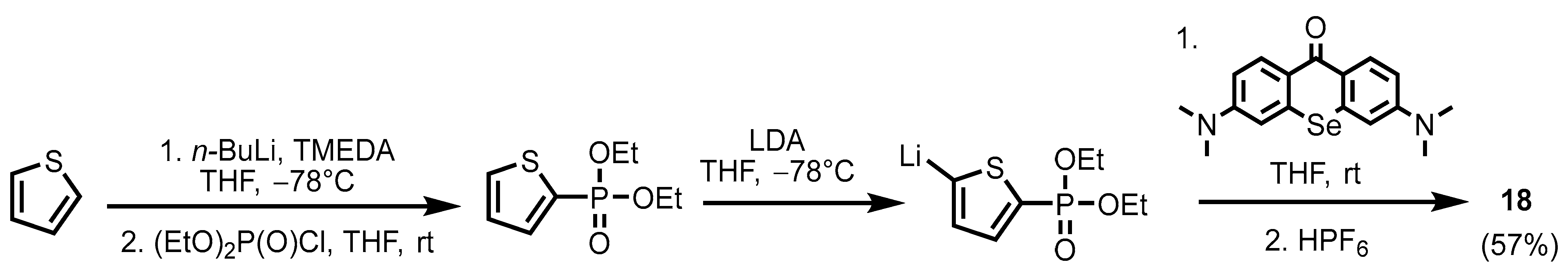
2.1. Heteroarylphosphonates with Heteroaryl Sulfur Atom
2.2. Heteroarylphosphonates with Heteroaryl Nitrogen
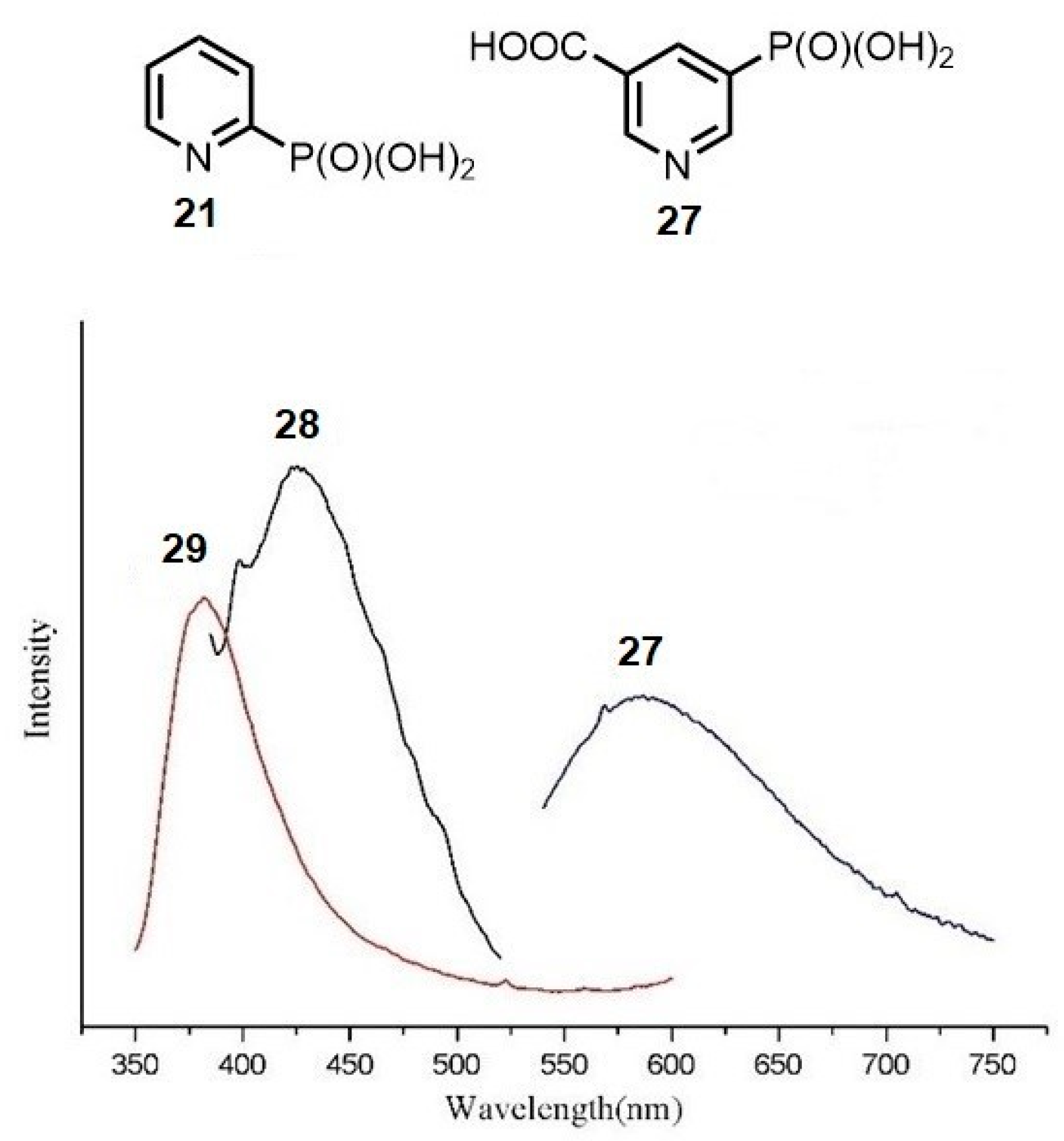
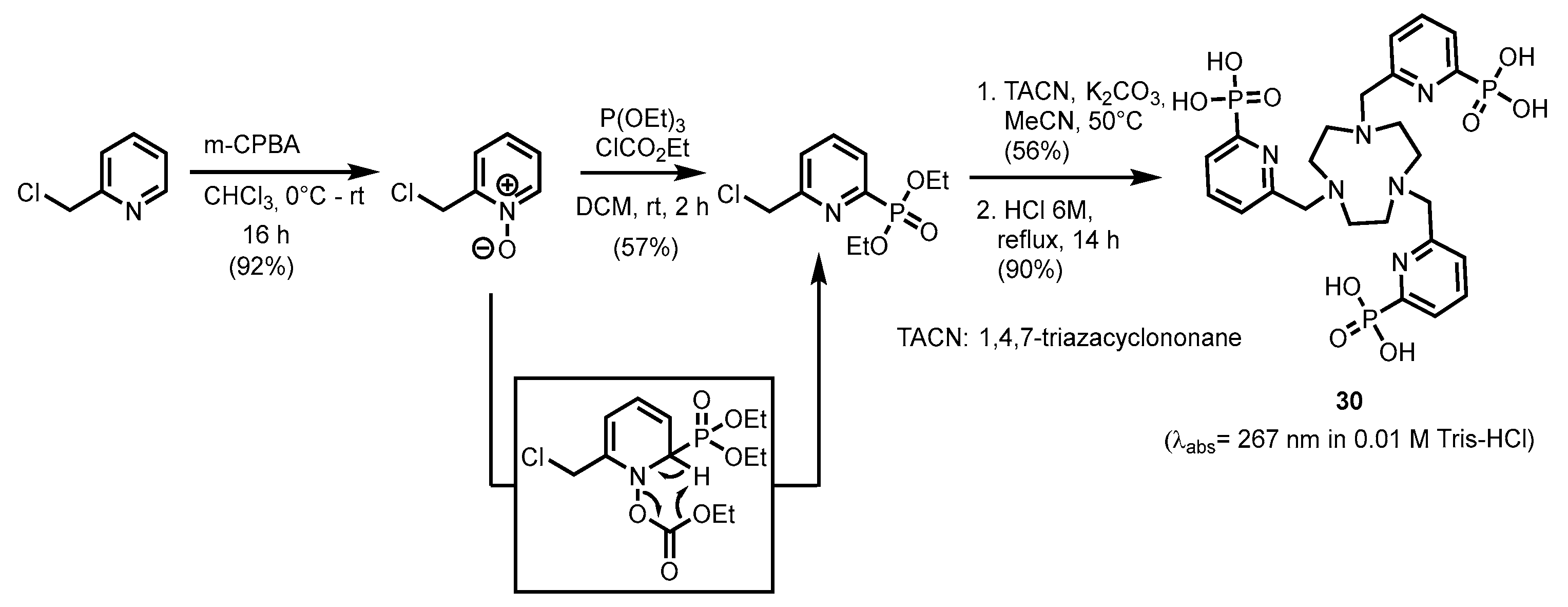
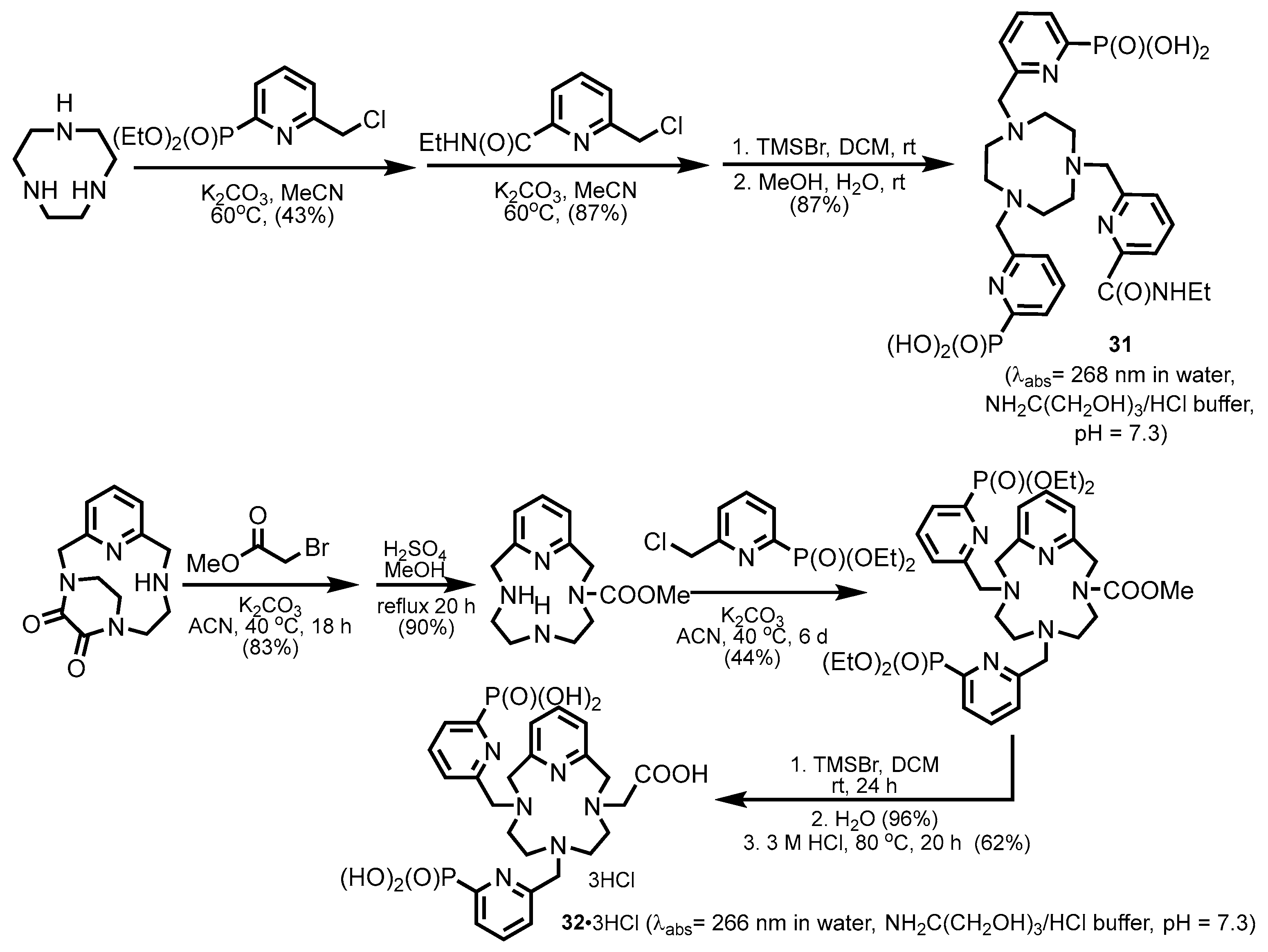
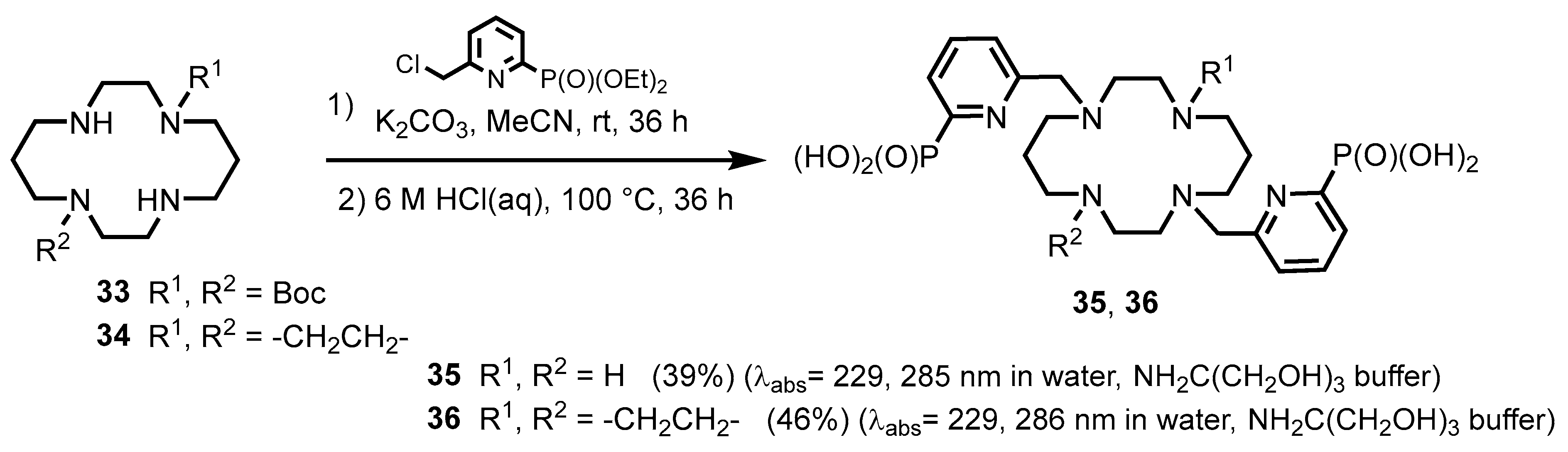
2.2.1. Pyridylphosphonic Acids and Derivatives
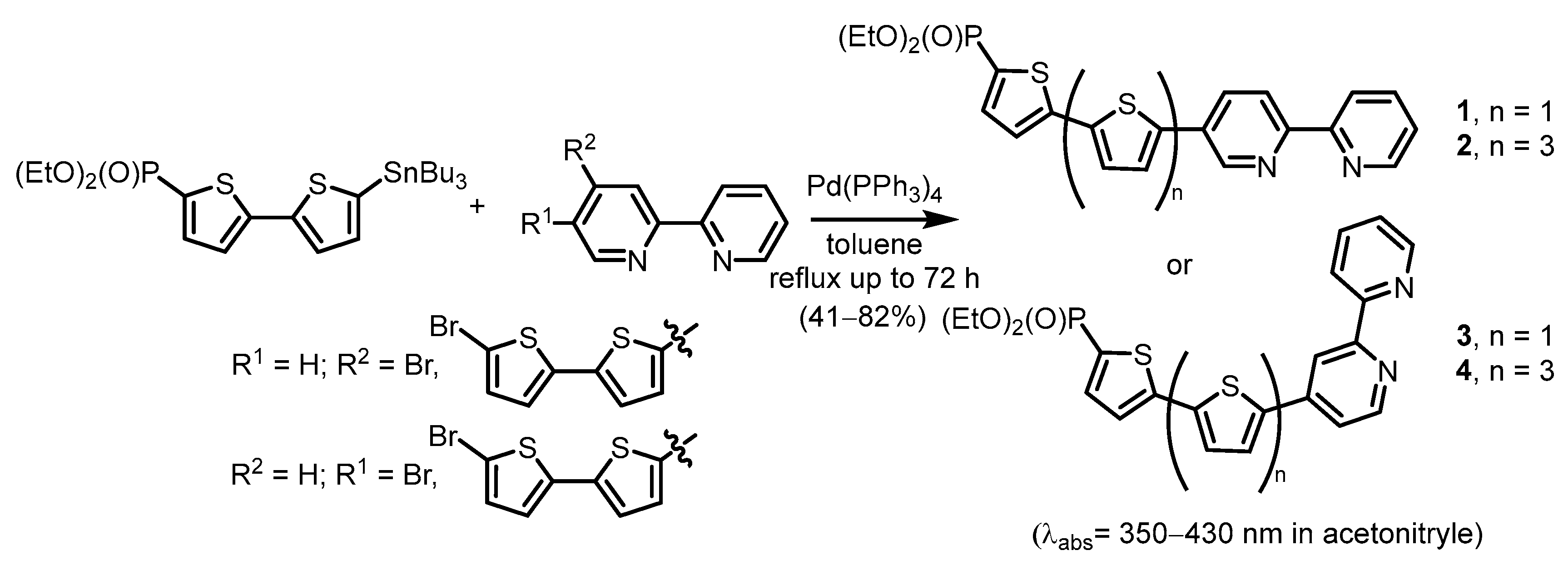
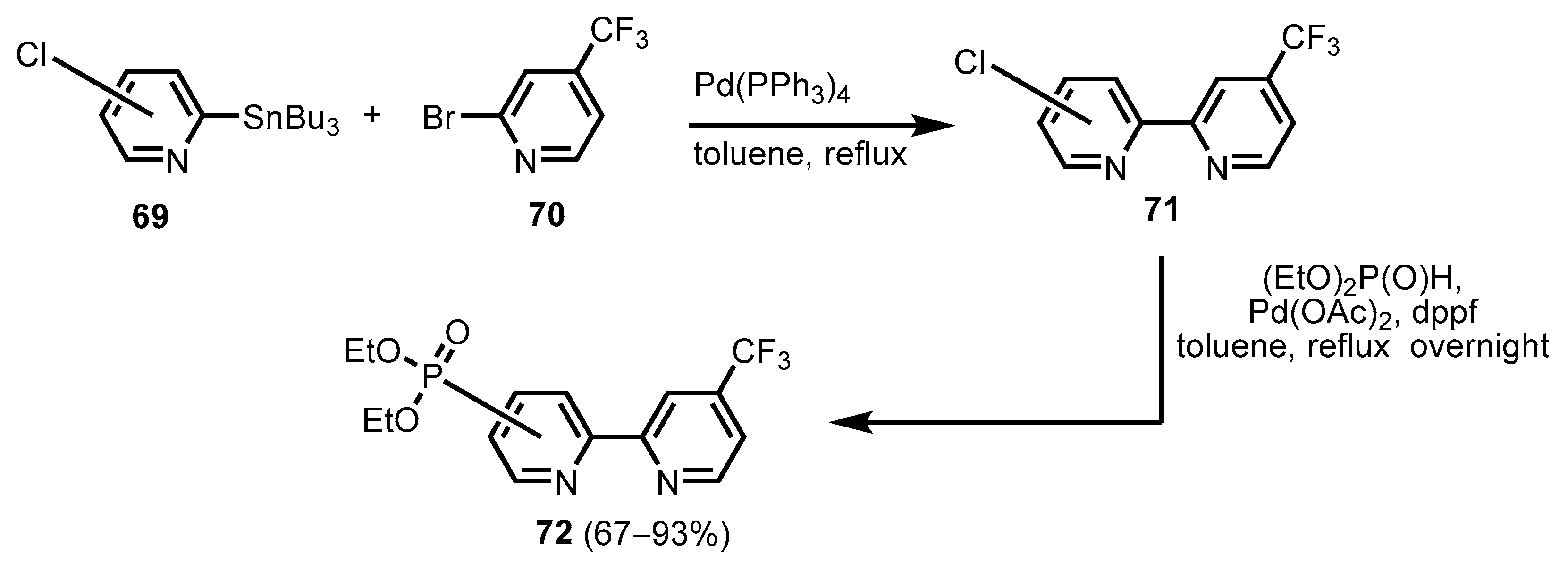
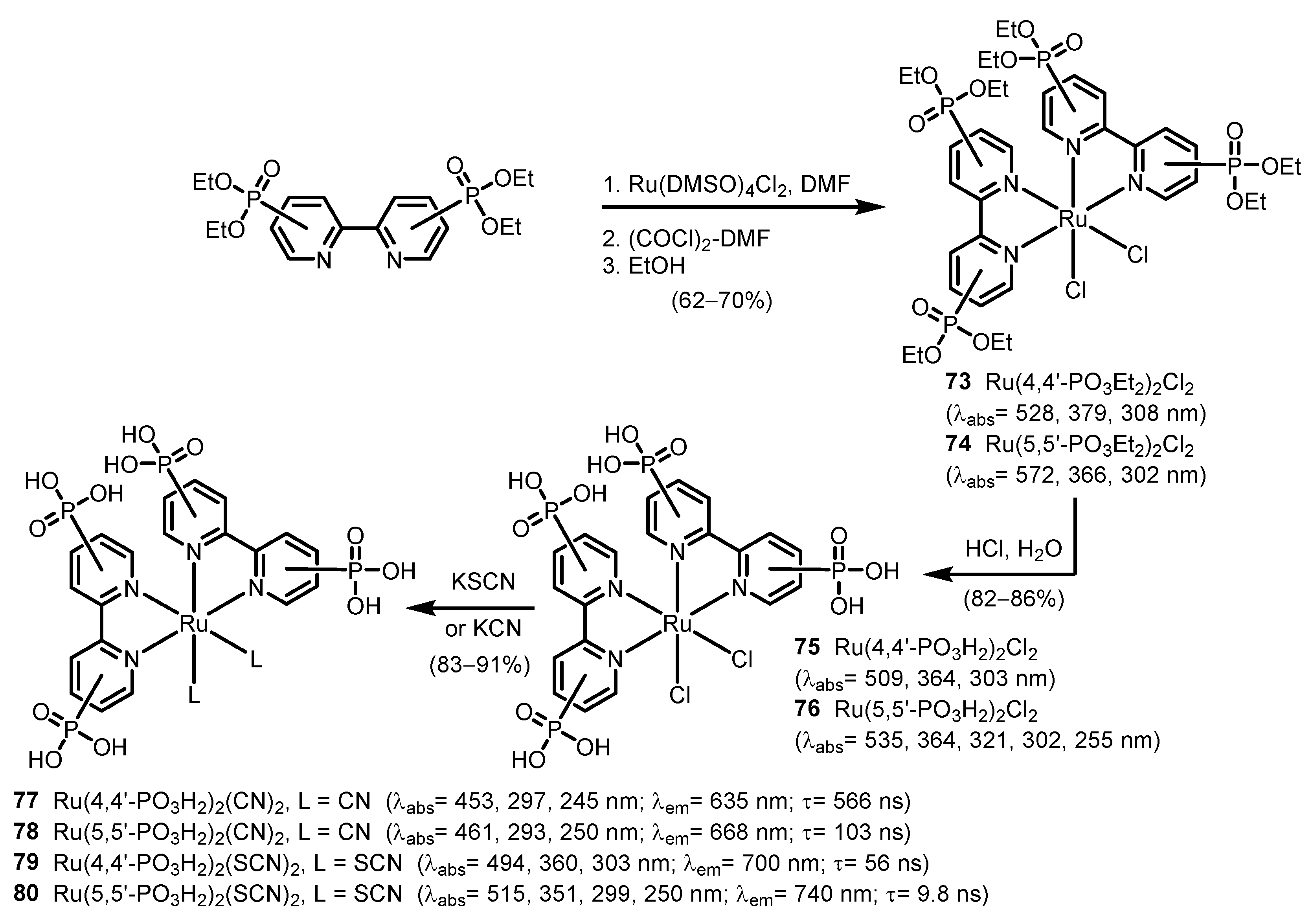
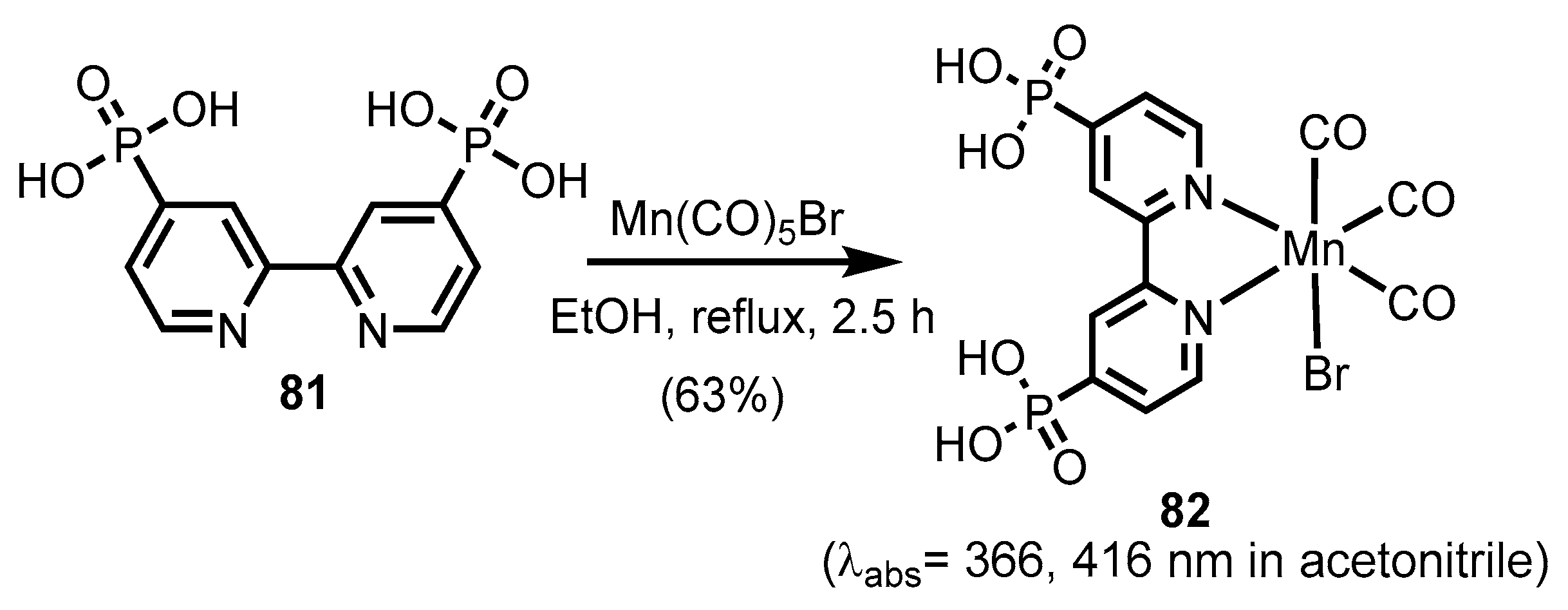
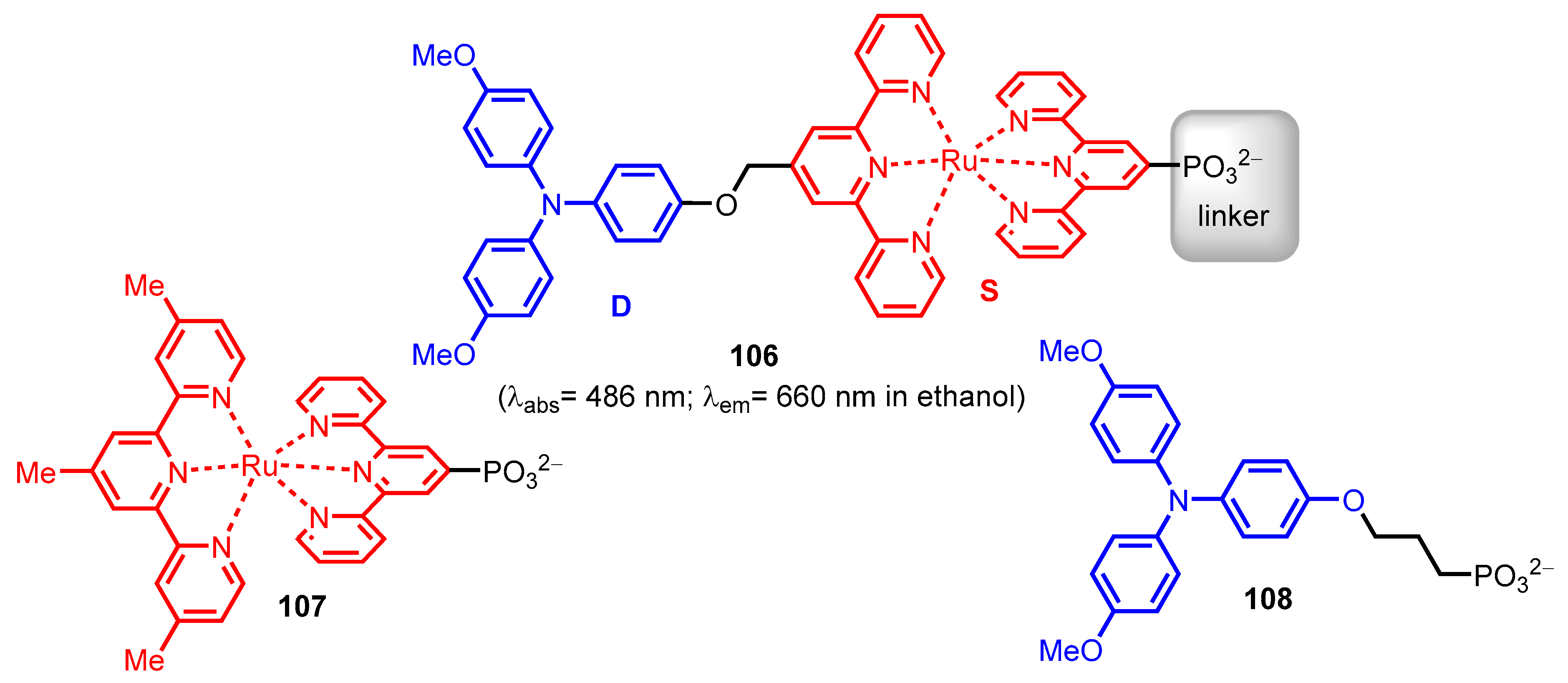
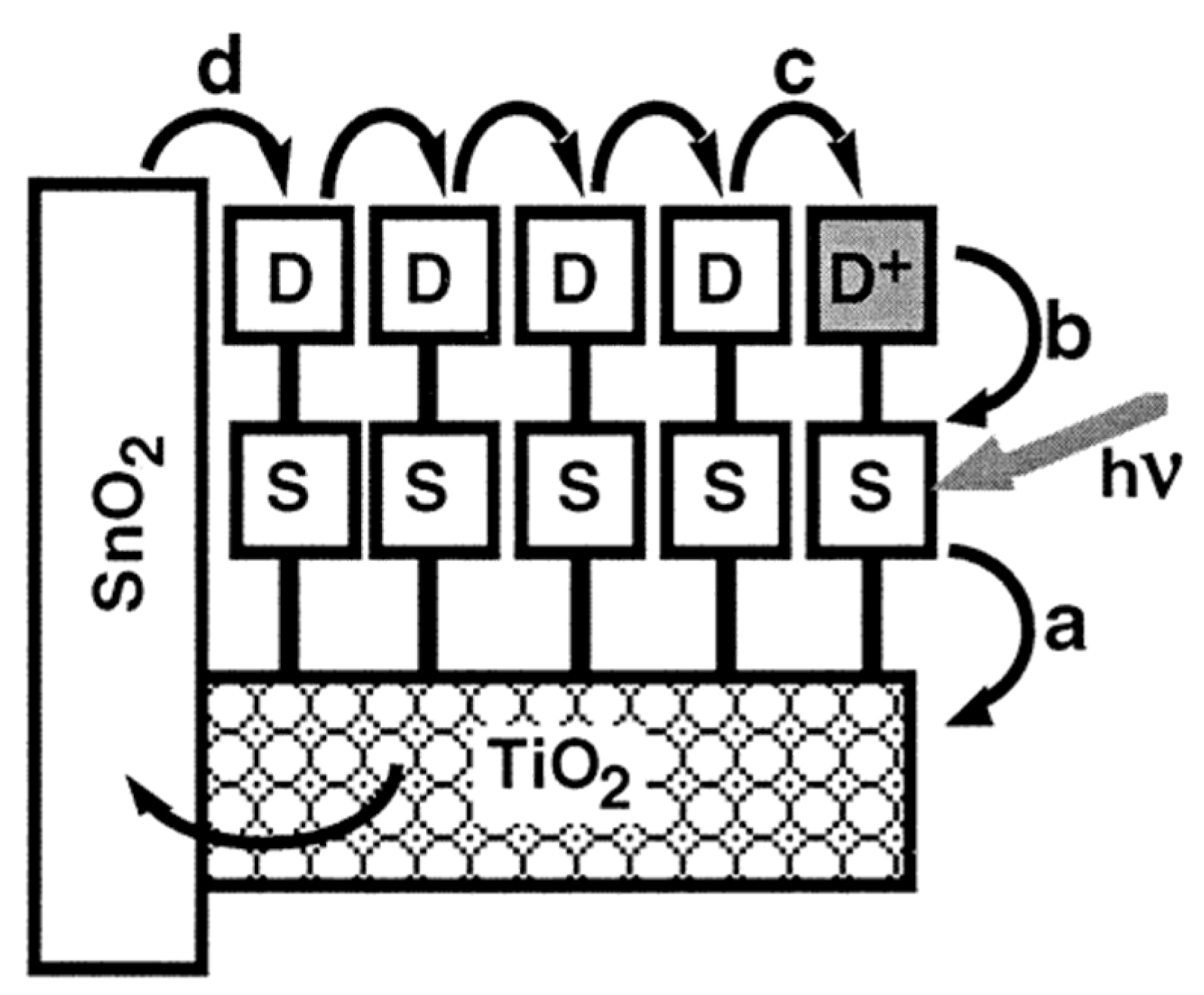
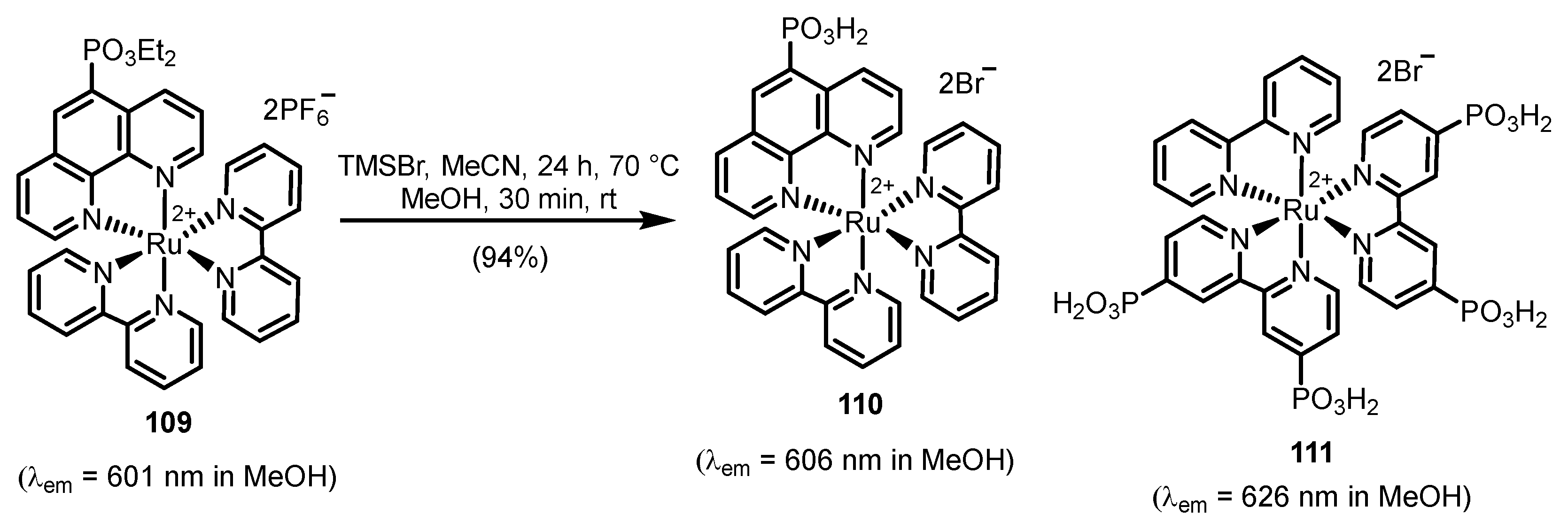
2.2.2. Bipyridinephosphonic Acids and Derivatives
2.2.3. Terpyridinephosphonic Acids and Derivatives
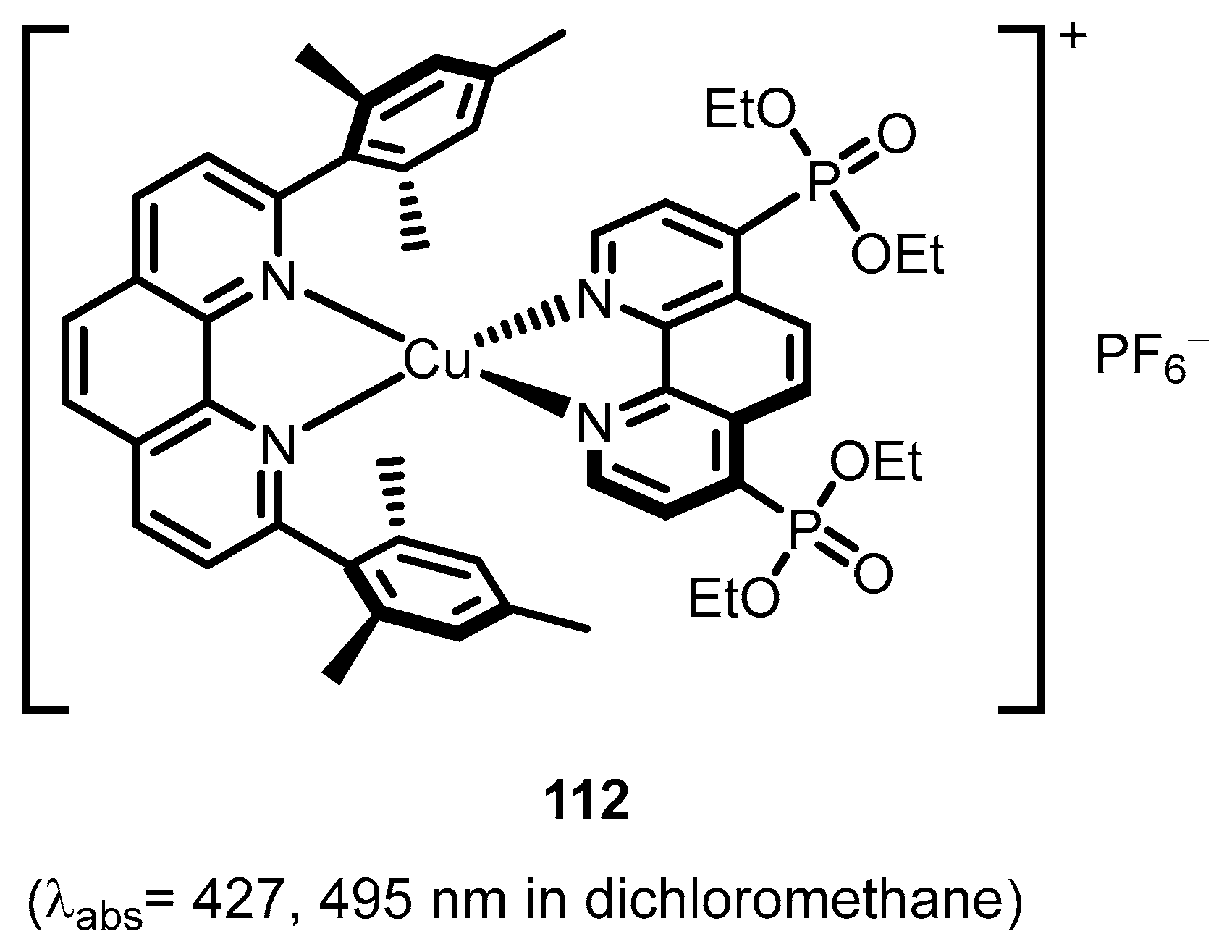
2.2.4. Phenantrolinephosphonic Acids and Derivatives
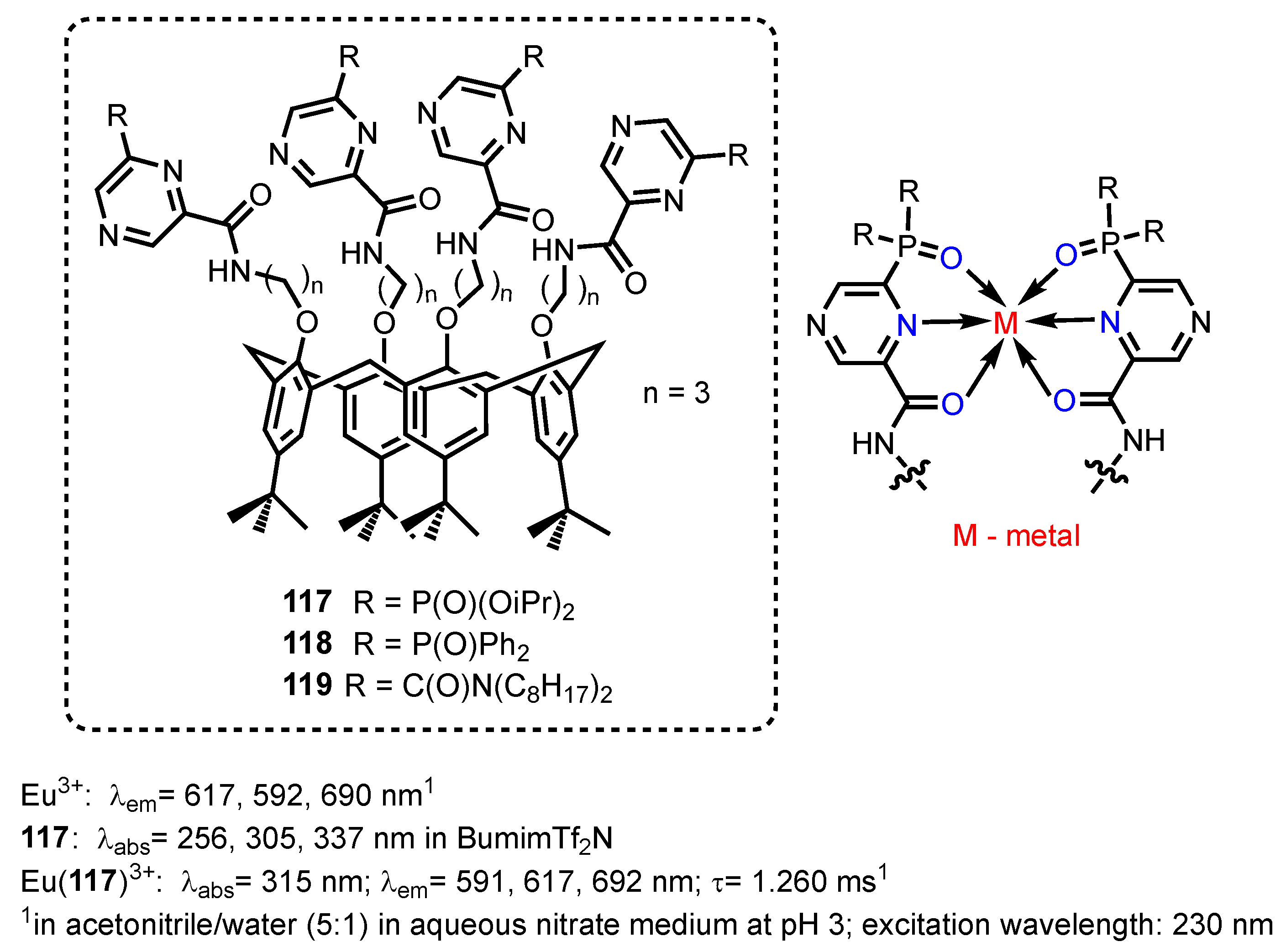
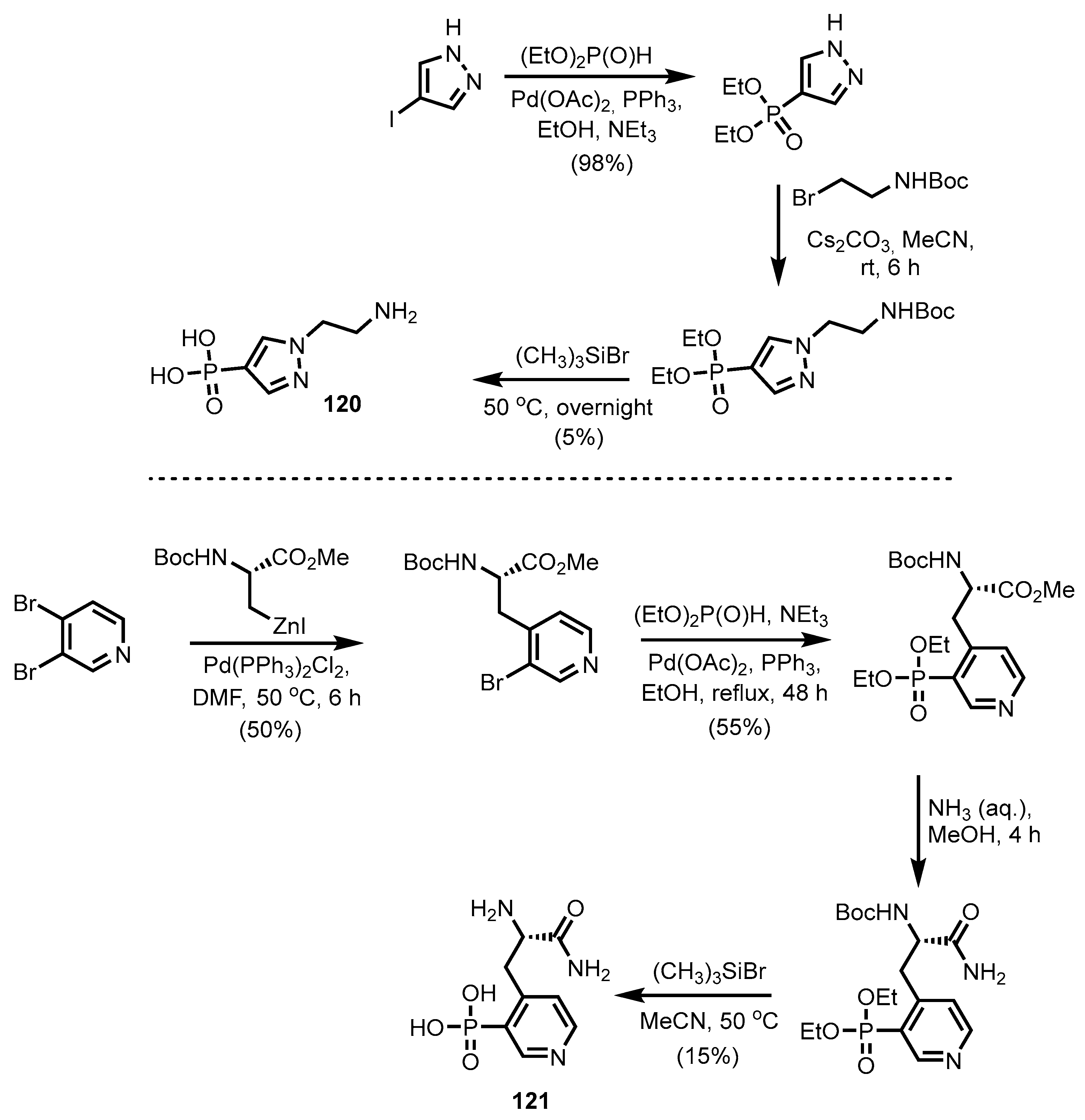
2.2.5. Pyrazine-, Pyrazole- and Phenoxazinonephosphonic Acids and Derivatives
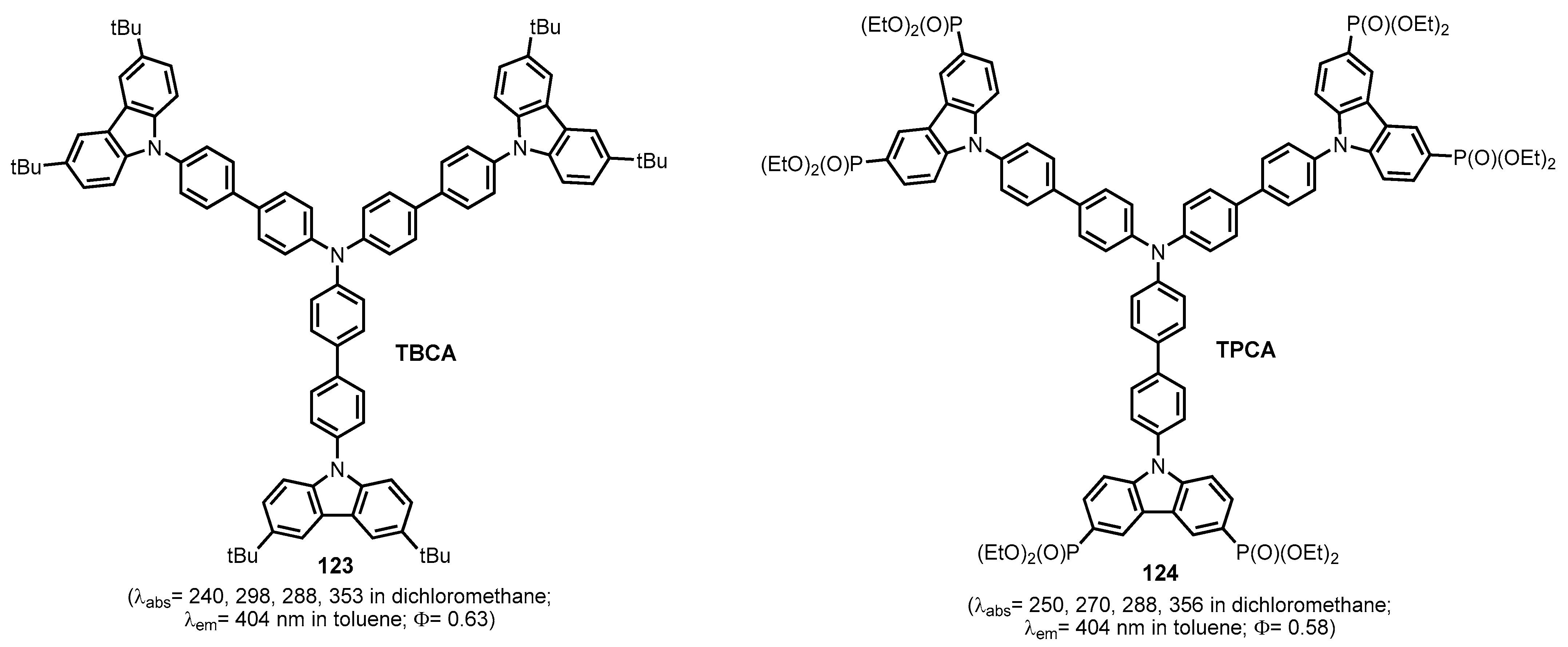
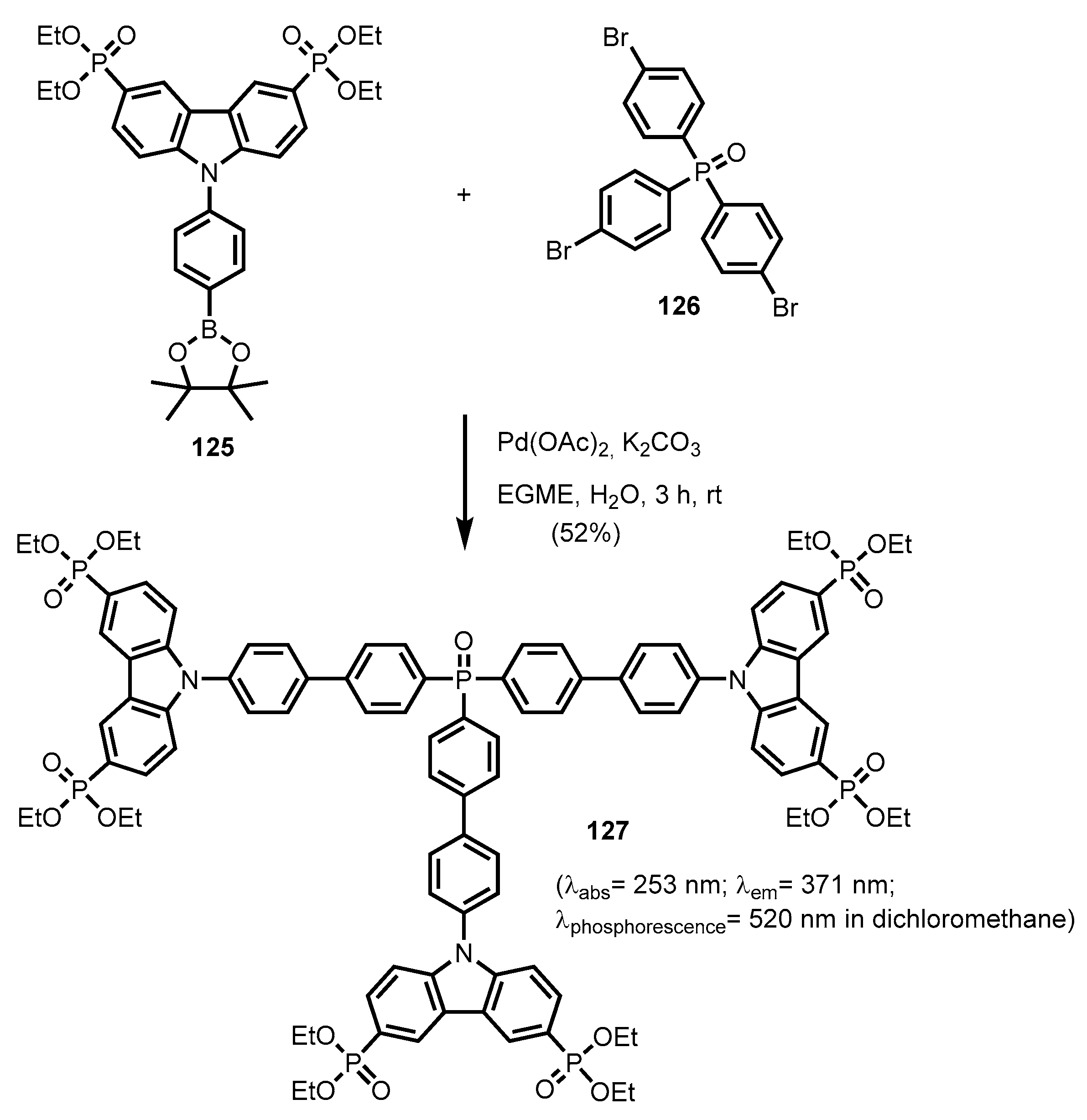
2.2.6. Carbazolephosphonic Acids and Derivatives
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bałczewski, P.; Owsianik, K. Quinquevalent phosphorus acids. In Organophosphorus Chemistry: Volume 52; Higham, L.J., Allen, D.W., Eds.; Royal Society of Chemistry: London, UK, 2024; pp. 109–231. [Google Scholar]
- Koprowski, M.; Owsianik, K.; Knopik, Ł.; Vivek, V.; Romaniuk, A.; Różycka-Sokołowska, E.; Bałczewski, P. Comprehensive Review on Synthesis, Properties, and Applications of Phosphorus (PIII, PIV, PV) Substituted Acenes with More Than Two Fused Benzene Rings. Molecules 2022, 27, 6611. [Google Scholar] [CrossRef] [PubMed]
- Khalkhali, F.S.; Kowsari, E.; Ramakrishna, S.; Eqbalpour, M.; Gheibi, M.; Esmaili, H. A review on the photosensitizers used for enhancing the photoelectrochemical performance of hydrogen production with emphasis on a novel toxicity assessment framework. Int. J. Hydrogen Energy 2024, 51, 990–1022. [Google Scholar] [CrossRef]
- Charpentier, C.; Salaam, J.; Nonat, A.; Carniato, F.; Jeannin, O.; Brandariz, I.; Esteban-Gomez, D.; Platas-Iglesias, C.; Charbonnière, L.J.; Botta, M. pH-Dependent Hydration Change in a Gd-Based MRI Contrast Agent with a Phosphonated Ligand. Chem. Eur. J. 2020, 26, 5407–5418. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, W.; Hui, Q.; Yang, Z. Chapter 4—Biosensors for bacteria detection. In Advanced Sensor Technology; Barhoum, A., Altintas, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 81–123. [Google Scholar]
- Hasegawa, M.; Ohmagari, H.; Tanaka, H.; Machida, K. Luminescence of lanthanide complexes: From fundamental to prospective approaches related to water- and molecular-stimuli. J. Photochem. Photobiol. C Photochem. Rev. 2022, 50, 100484. [Google Scholar] [CrossRef]
- Virender; Chauhan, A.; Kumar, A.; Singh, G.; Solovev, A.A.; Xiong, J.; Liu, X.; Mohan, B. Photonic properties and applications of multi-functional organo-lanthanide complexes: Recent advances. J. Rare Earths 2024, 42, 16–27. [Google Scholar] [CrossRef]
- Arya, M.; Heera, S.; Meenu, P.; Deepa, K.G. Organic-inorganic hybrid materials and architectures in optoelectronic devices: Recent advancements. ChemPhysMater 2024, 3, 252–272. [Google Scholar] [CrossRef]
- Mauri, L.; Colombo, A.; Dragonetti, C.; Roberto, D.; Fagnani, F. Recent Investigations on Thiocyanate-Free Ruthenium(II) 2,2′-Bipyridyl Complexes for Dye-Sensitized Solar Cells. Molecules 2021, 26, 7638. [Google Scholar] [CrossRef] [PubMed]
- Laschuk, N.O.; Ebralidze, I.I.; Spasyuk, D.; Zenkina, O.V. Multi-Readout Logic Gate for the Selective Detection of Metal Ions at the Parts Per Billion Level. Eur. J. Inorg. Chem. 2016, 2016, 3530–3535. [Google Scholar] [CrossRef]
- Duan, T.; Chen, Q.; Hu, D.; Lv, J.; Yu, D.; Li, G.; Lu, S. Oligothiophene-based photovoltaic materials for organic solar cells: Rise, plateau, and revival. Trends Chem. 2022, 4, 773–791. [Google Scholar] [CrossRef]
- Iftikhar, R.; Khan, F.Z.; Naeem, N. Recent synthetic strategies of small heterocyclic organic molecules with optoelectronic applications: A review. Mol. Divers. 2024, 28, 271–307. [Google Scholar] [CrossRef]
- Bair, J.S.; Harrison, R.G. Synthesis and Optical Properties of Bifunctional Thiophene Molecules Coordinated to Ruthenium. J. Org. Chem. 2007, 72, 6653–6661. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.L.; Zhao, Q.; Zhang, Y.; Wang, J.; Lawson, C.M.; Gray, G.M. Synthesis, linear and nonlinear optical properties of phosphonato-substituted bithiophenes derived from 2,2′-biphenol. Dalton Trans. 2013, 42, 14281–14297. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.L.; French, D.N.; Zhao, Q.; Wang, J.; Zhang, Y.; Hamilton, T.P.; Lawson, C.M.; Gray, G.M. Synthesis, Linear, and Nonlinear Optical Properties of Phosphonato-Substituted Bithiophenes Derived from 2,2-Dimethyl-1,3-propanediol. Eur. J. Inorg. Chem. 2017, 2017, 4077–4093. [Google Scholar] [CrossRef]
- Horne, J.C.; Blanchard, G.J. The Role of Substrate Identity in Determining Monolayer Motional Relaxation Dynamics. J. Am. Chem. Soc. 1998, 120, 6336–6344. [Google Scholar] [CrossRef]
- Byrd, H.; Whipps, S.; Pike, J.K.; Ma, J.; Nagler, S.E.; Talham, D.R. Role of the template layer in organizing self-assembled films: Zirconium phosphonate monolayers and multilayers at a Langmuir-Blodgett template. J. Am. Chem. Soc. 1994, 116, 295–301. [Google Scholar] [CrossRef]
- Lo, C.-Y.; Chen, C.-H.; Tsai, T.W.T.; Zhang, L.; Lim, T.-S.; Fann, W.; Chan, J.C.C.; Luh, T.-Y. Efficient Energy and Electron Transfer between Donor and Acceptor Chromophores in Aluminophosphate Hybrid Materials. J. Chin. Chem. Soc. 2010, 57, 539–546. [Google Scholar] [CrossRef]
- Mulhern, K.R.; Orchard, A.; Watson, D.F.; Detty, M.R. Influence of Surface-Attachment Functionality on the Aggregation, Persistence, and Electron-Transfer Reactivity of Chalcogenorhodamine Dyes on TiO2. Langmuir 2012, 28, 7071–7082. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, R.P.; Mark, M.F.; Mark, D.J.; Kryman, M.W.; Hill, J.E.; Brennessel, W.W.; Detty, M.R.; Eisenberg, R.; McCamant, D.W. A comparative study of the photophysics of phenyl, thienyl, and chalcogen substituted rhodamine dyes. Photochem. Photobiol. Sci. 2016, 15, 1417–1432. [Google Scholar] [CrossRef]
- Sheokand, M.; Rout, Y.; Misra, R. Recent development of pyridine based charge transporting materials for organic light-emitting diodes and perovskite solar cells. J. Mater. Chem. C 2022, 10, 6992–7017. [Google Scholar] [CrossRef]
- Saeed, A.; Faisal, M.; Larik Ali, F.; El-Seedi, R.H.; Channar Ali, P.; Shahzad, D. Recent Progress in Pyridine Containing Heterocycles as High Performance Host Materials for Blue PHOLEDs. Mini-Rev. Org. Chem. 2018, 15, 261–273. [Google Scholar] [CrossRef]
- dos Santos, P.L.; Chen, D.; Rajamalli, P.; Matulaitis, T.; Cordes, D.B.; Slawin, A.M.Z.; Jacquemin, D.; Zysman-Colman, E.; Samuel, I.D.W. Use of Pyrimidine and Pyrazine Bridges as a Design Strategy To Improve the Performance of Thermally Activated Delayed Fluorescence Organic Light Emitting Diodes. ACS Appl. Mater. Interfaces 2019, 11, 45171–45179. [Google Scholar] [CrossRef]
- Chaudhran, P.A.; Sharma, A. Progress in the Development of Imidazopyridine-Based Fluorescent Probes for Diverse Applications. Crit. Rev. Anal. Chem. 2022, 1–18. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Cheng, X.; Xie, Y.; Zhao, J.; Weng, J. Recent advances in versatile pyridazine-cored materials: Principles, applications, and challenges. J. Mater. Chem. C 2023, 11, 5563–5584. [Google Scholar] [CrossRef]
- Ng, X.Y.; Fong, K.W.; Kiew, L.V.; Chung, P.Y.; Liew, Y.K.; Delsuc, N.; Zulkefeli, M.; Low, M.L. Ruthenium(II) polypyridyl complexes as emerging photosensitisers for antibacterial photodynamic therapy. J. Inorg. Biochem. 2024, 250, 112425. [Google Scholar] [CrossRef] [PubMed]
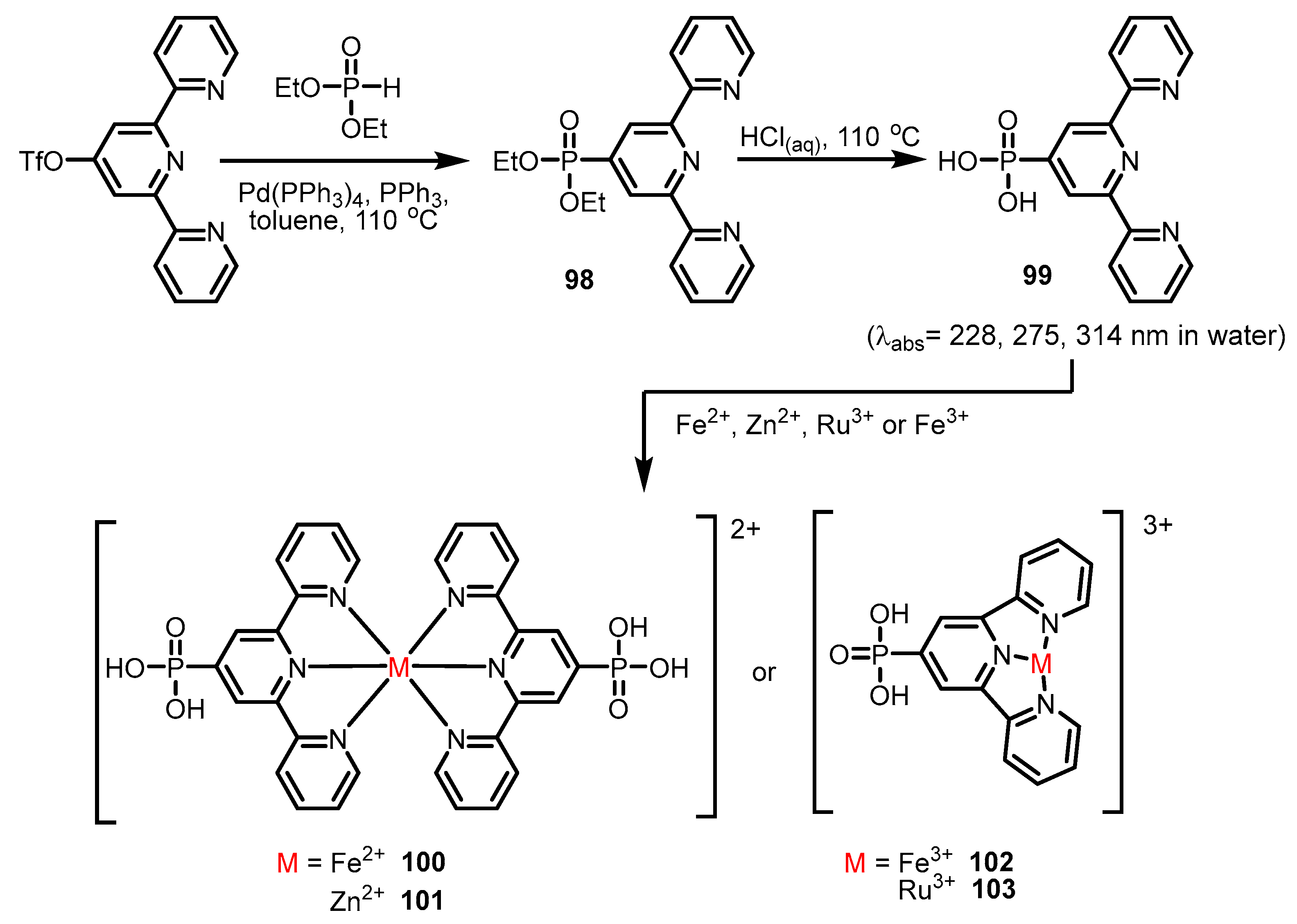
- Fry, J.A.; Samanamu, C.R.; Montchamp, J.-L.; Richards, A.F. A Mild Synthetic Route to Zinc, Cadmium, and Silver Polymers with (2-Pyridyl)phosphonic Acid: Synthesis and Analysis. Eur. J. Inorg. Chem. 2008, 2008, 463–470. [Google Scholar] [CrossRef]
- Chen, R.; Chen, Y.; Zeng, X.; Li, J.; Luo, X. Transition metal phosphonates with pyridyl-based phosphonic acids: Synthesis, characterization and luminescence properties. J. Coord. Chem. 2017, 70, 949–959. [Google Scholar] [CrossRef]
- Salaam, J.; Tabti, L.; Bahamyirou, S.; Lecointre, A.; Hernandez Alba, O.; Jeannin, O.; Camerel, F.; Cianférani, S.; Bentouhami, E.; Nonat, A.M.; et al. Formation of Mono- and Polynuclear Luminescent Lanthanide Complexes based on the Coordination of Preorganized Phosphonated Pyridines. Inorg. Chem. 2018, 57, 6095–6106. [Google Scholar] [CrossRef] [PubMed]
- Hamon, N.; Godec, L.; Jourdain, E.; Lucio-Martínez, F.; Platas-Iglesias, C.; Beyler, M.; Charbonnière, L.J.; Tripier, R. Synthesis and Photophysical Properties of Lanthanide Pyridinylphosphonic Tacn and Pyclen Derivatives: From Mononuclear Complexes to Supramolecular Heteronuclear Assemblies. Inorg. Chem. 2023, 62, 18940–18954. [Google Scholar] [CrossRef] [PubMed]
- Knighton, R.C.; Soro, L.K.; Troadec, T.; Mazan, V.; Nonat, A.M.; Elhabiri, M.; Saffon-Merceron, N.; Djenad, S.; Tripier, R.; Charbonnière, L.J. Formation of Heteropolynuclear Lanthanide Complexes Using Macrocyclic Phosphonated Cyclam-Based Ligands. Inorg. Chem. 2020, 59, 10311–10327. [Google Scholar] [CrossRef]
- Zeng, D.; Ren, M.; Bao, S.-S.; Li, L.; Zheng, L.-M. A luminescent heptanuclear DyIr6 complex showing field-induced slow magnetization relaxation. Chem. Commun. 2014, 50, 8356–8359. [Google Scholar] [CrossRef]
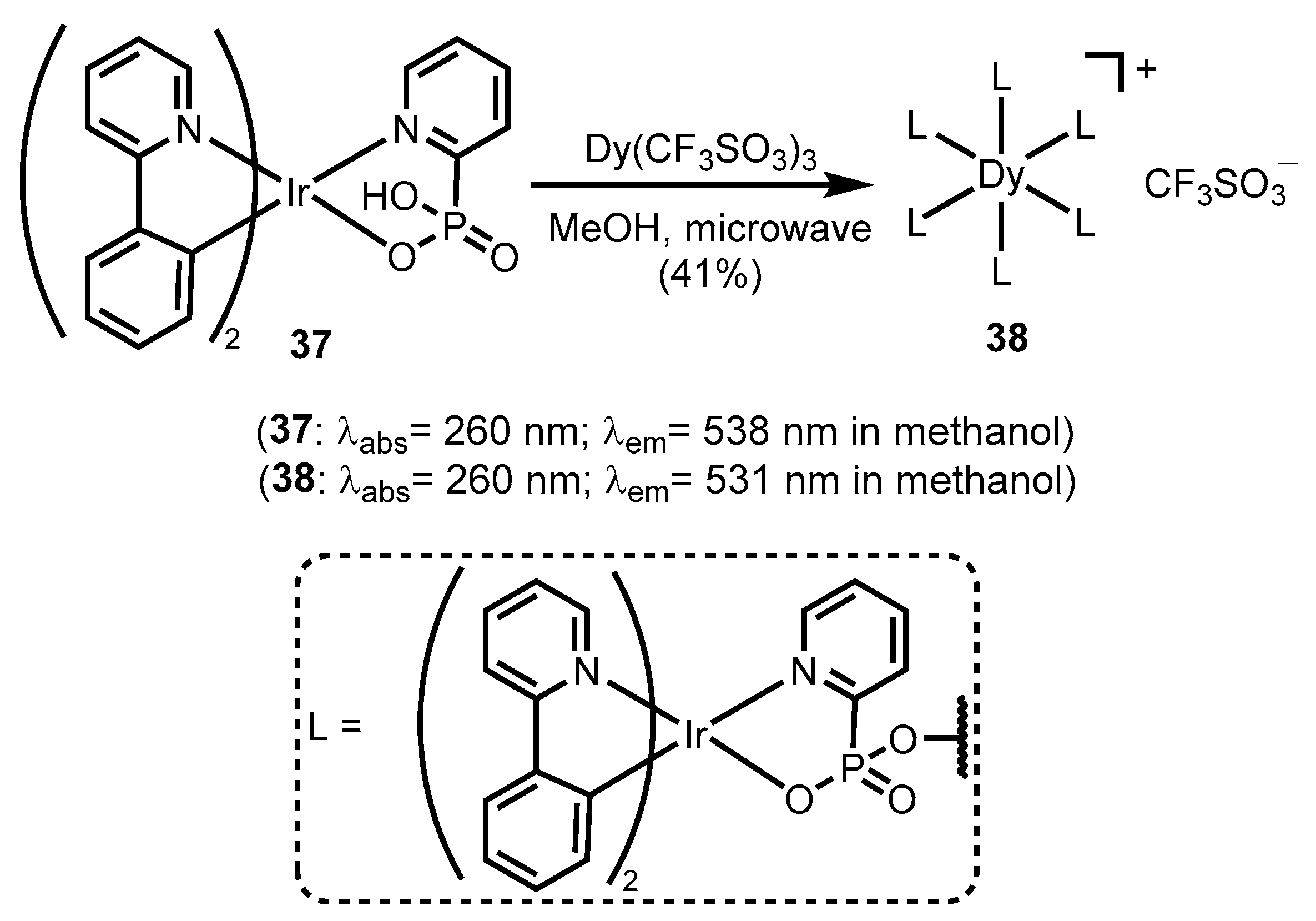
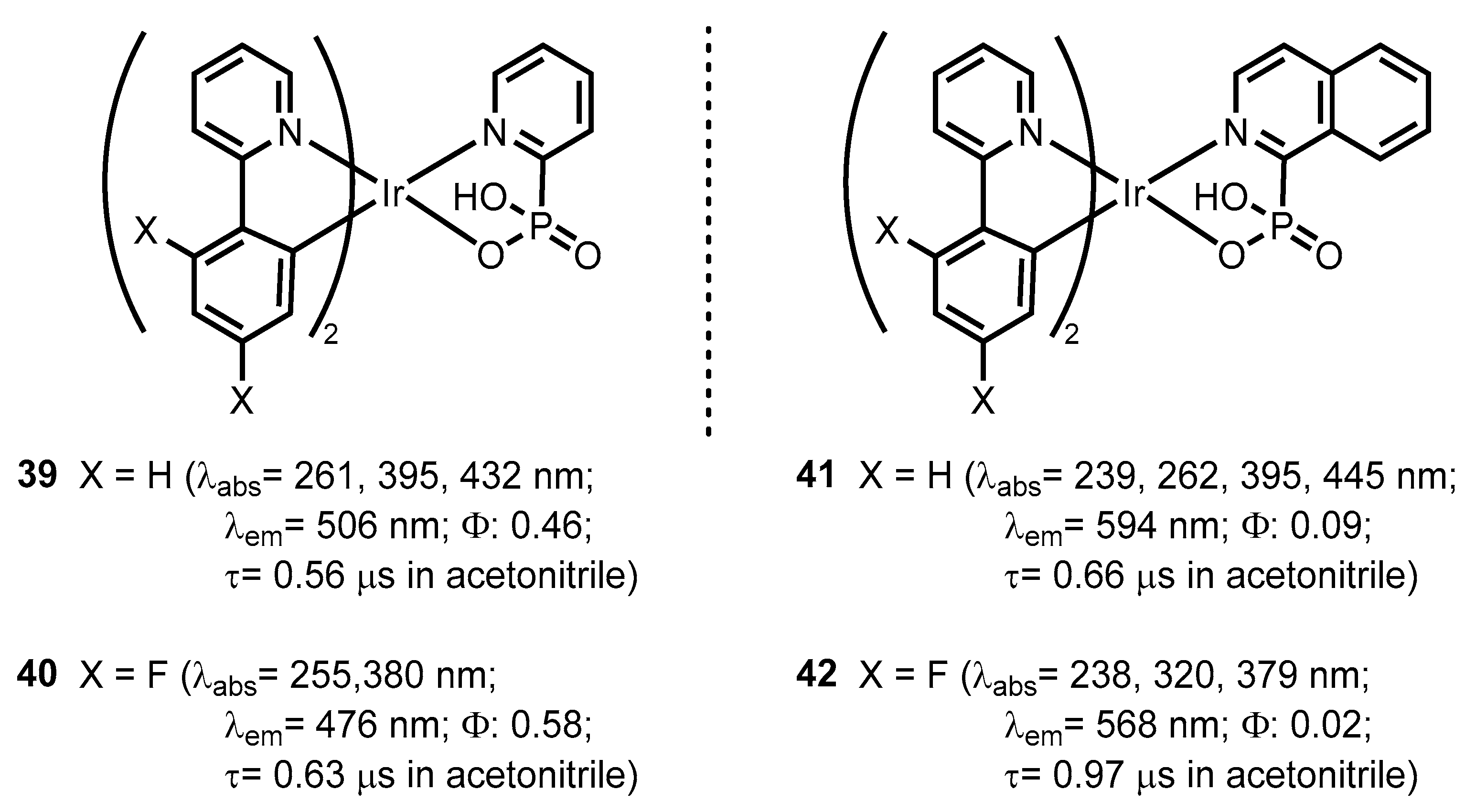
- Zeng, D.; Yuan, X.-A.; Liu, J.-C.; Li, L.; Wang, L.-P.; Qin, M.-F.; Bao, S.-S.; Ma, J.; Zheng, L.-M. Cyclometalated Iridium(III) Complexes Incorporating Aromatic Phosphonate Ligands: Syntheses, Structures, and Tunable Optical Properties. ACS Omega 2019, 4, 16543–16550. [Google Scholar] [CrossRef] [PubMed]
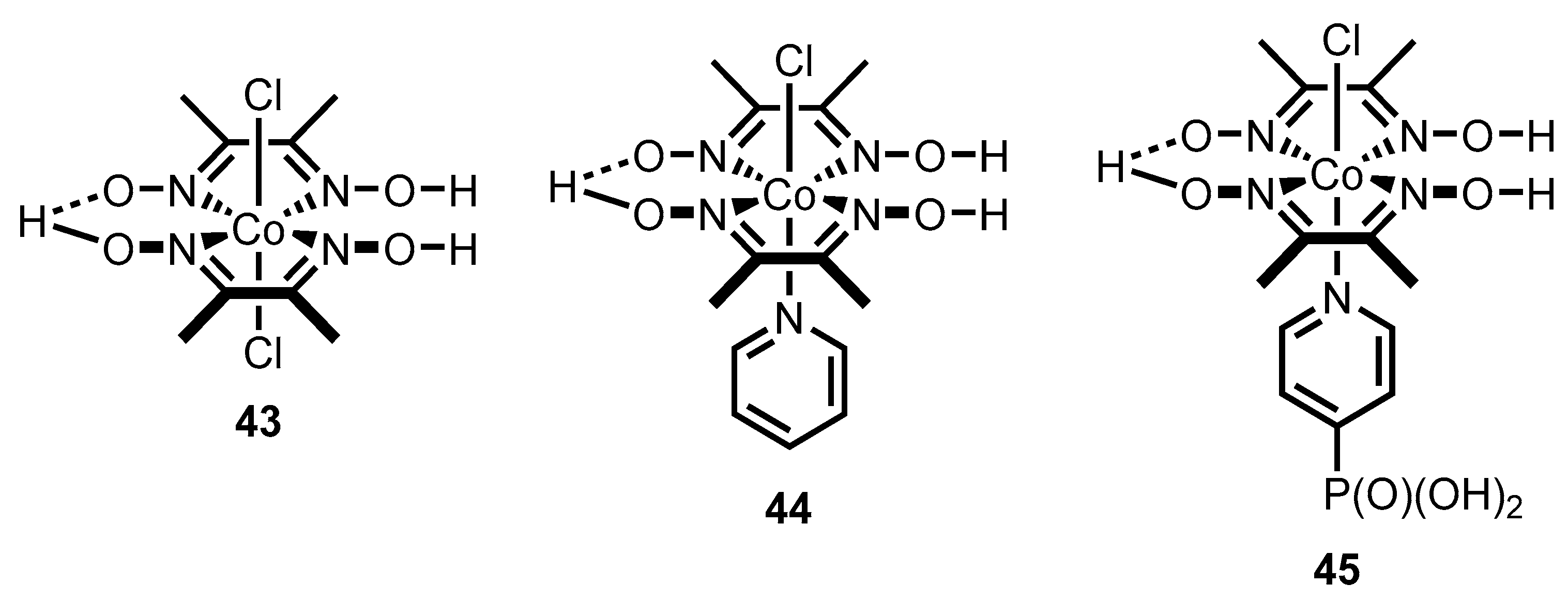
- Lawson, T.; Gentleman, A.S.; Pinnell, J.; Eisenschmidt, A.; Antón-García, D.; Frosz, M.H.; Reisner, E.; Euser, T.G. In situ Detection of Cobaloxime Intermediates During Photocatalysis Using Hollow-Core Photonic Crystal Fiber Microreactors. Angew. Chem. Int. Ed. 2023, 62, e202214788. [Google Scholar] [CrossRef] [PubMed]
- Lakadamyali, F.; Reisner, E. Photocatalytic H2 evolution from neutral water with a molecular cobalt catalyst on a dye-sensitised TiO2 nanoparticle. Chem. Commun. 2011, 47, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, A.-S.; Comby, S.; Baud, M.; De Piano, C.; Duhot, C.; Bünzli, J.-C.G. Luminescent Lanthanide Helicates Self-Assembled from Ditopic Ligands Bearing Phosphonic Acid or Phosphoester Units. Inorg. Chem. 2009, 48, 10687–10696. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verma, P.K.; Mahanty, B.; Bhattacharyya, A.; Matveev, P.I.; Borisova, N.E.; Kalmykov, S.N.; Mohapatra, P.K. Pyridine Diphosphonate Ligand for Stabilization of Tetravalent Uranium and Neptunium in Aqueous Medium under Aerobic Conditions. Inorg. Chem. 2024, 63, 3348–3358. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Ansari, S.A.; Matveev, P.I.; Zakirova, G.G.; Borisova, N.E.; Petrov, V.G.; Sumyanova, T.; Verma, P.K.; Kalmykov, S.N.; Mohapatra, P.K. Unfolding the complexation and extraction of Am3+ and Eu3+ using N-heterocyclic aromatic diphosphonic acids: A combined experimental and DFT study. Dalton Trans. 2019, 48, 16279–16288. [Google Scholar] [CrossRef] [PubMed]
- Charbonnière, L.J.; Weibel, N.; Retailleau, P.; Ziessel, R. Relationship Between the Ligand Structure and the Luminescent Properties of Water-Soluble Lanthanide Complexes Containing Bis(bipyridine) Anionic Arms. Chem. Eur. J. 2007, 13, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, C.; Salaam, J.; Lecointre, A.; Jeannin, O.; Nonat, A.; Charbonnière, L.J. Phosphonated Podand Type Ligand for the Complexation of Lanthanide CationsPhosphonated Podand Type Ligand for the Complexation of Lanthanide Cations. Eur. J. Inorg. Chem. 2019, 2019, 2168–2174. [Google Scholar] [CrossRef]
- Purnama, I.; Salmahaminati; Abe, M.; Hada, M.; Kubo, Y.; Mulyana, J.Y. Factors influencing the photoelectrochemical device performance sensitized by ruthenium polypyridyl dyes. Dalton Trans. 2019, 48, 688–695. [Google Scholar] [CrossRef]
- Ruile, S.; Kohle, O.; Péchy, P.; Grätzel, M. Novel sensitisers for photovoltaic cells. Structural variations of Ru(II) complexes containing 2,6-bis(1-methylbenzimidazol-2-yl)pyridine. Inorg. Chim. Acta 1997, 261, 129–140. [Google Scholar] [CrossRef]
- Wang, L.; Shaffer, D.W.; Manbeck, G.F.; Polyansky, D.E.; Concepcion, J.J. High-Redox-Potential Chromophores for Visible-Light-Driven Water Oxidation at Low pH. ACS Catal. 2020, 10, 580–585. [Google Scholar] [CrossRef]
- Zabri, H.; Gillaizeau, I.; Bignozzi, C.A.; Caramori, S.; Charlot, M.-F.; Cano-Boquera, J.; Odobel, F. Synthesis and Comprehensive Characterizations of New cis-RuL2X2 (X = Cl, CN, and NCS) Sensitizers for Nanocrystalline TiO2 Solar Cell Using Bis-Phosphonated Bipyridine Ligands (L). Inorg. Chem. 2003, 42, 6655–6666. [Google Scholar] [CrossRef] [PubMed]
- Zabri, H.; Odobel, F.; Altobello, S.; Caramori, S.; Bignozzi, C.A. Efficient osmium sensitizers containing 2,2′-bipyridine-4,4′-bisphosphonic acid ligand. J. Photochem. Photobiol. A Chem. 2004, 166, 99–106. [Google Scholar] [CrossRef]
- Rosser, T.E.; Windle, C.D.; Reisner, E. Electrocatalytic and Solar-Driven CO2 Reduction to CO with a Molecular Manganese Catalyst Immobilized on Mesoporous TiO2. Angew. Chem. Int. Ed. 2016, 55, 7388–7392. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, Q.; Ho, P.-Y.; Yiu, S.-C.; Suramitr, S.; Hannongbua, S.; Ho, C.-L. Development of Aldehyde Functionalized Iridium(III) Complexes Photosensitizers with Strong Visible-Light Absorption for Photocatalytic Hydrogen Generation from Water. Inorganics 2023, 11, 110. [Google Scholar] [CrossRef]
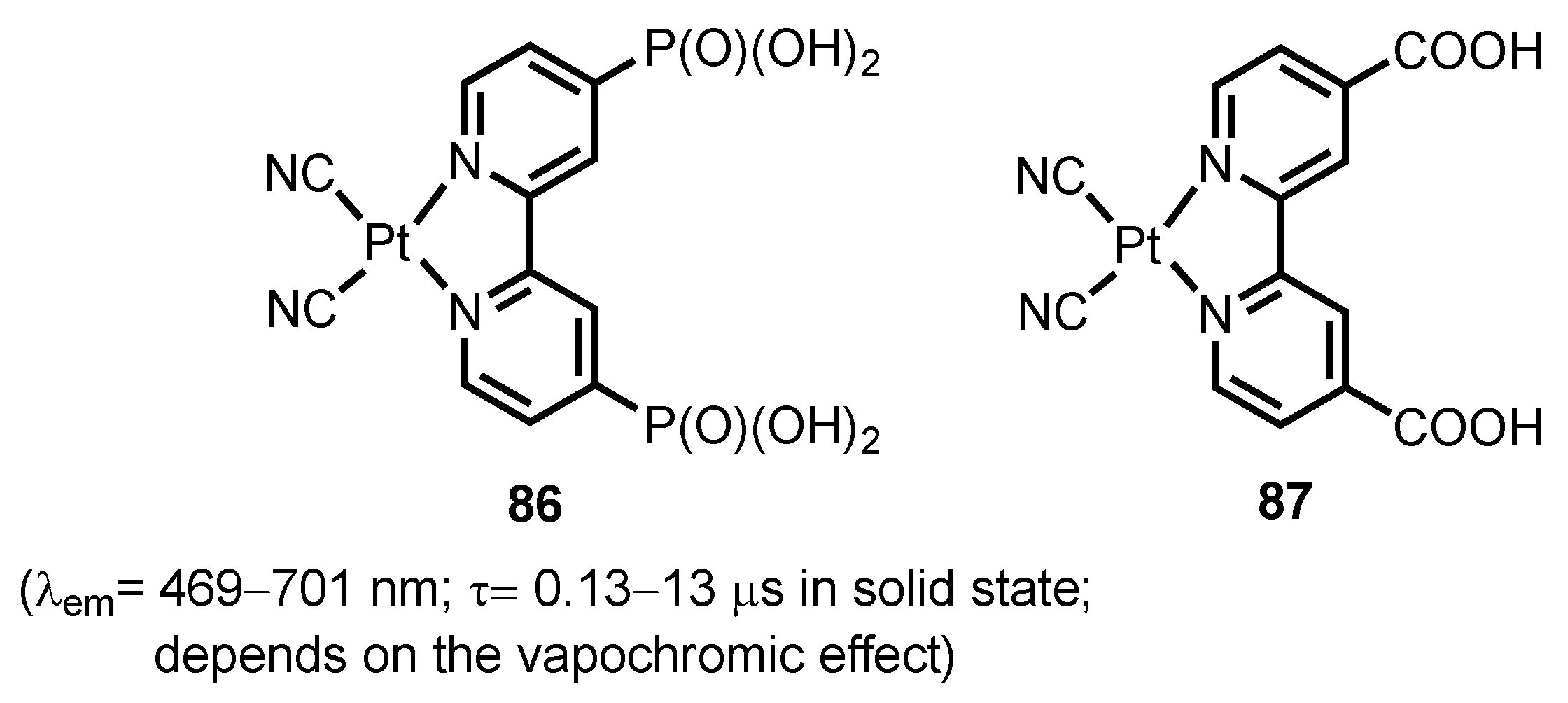
- Kobayashi, A.; Yamamoto, N.; Shigeta, Y.; Yoshida, M.; Kato, M. Two-way vapochromism of a luminescent platinum(ii) complex with phosphonic-acid-functionalized bipyridine ligand. Dalton Trans. 2018, 47, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
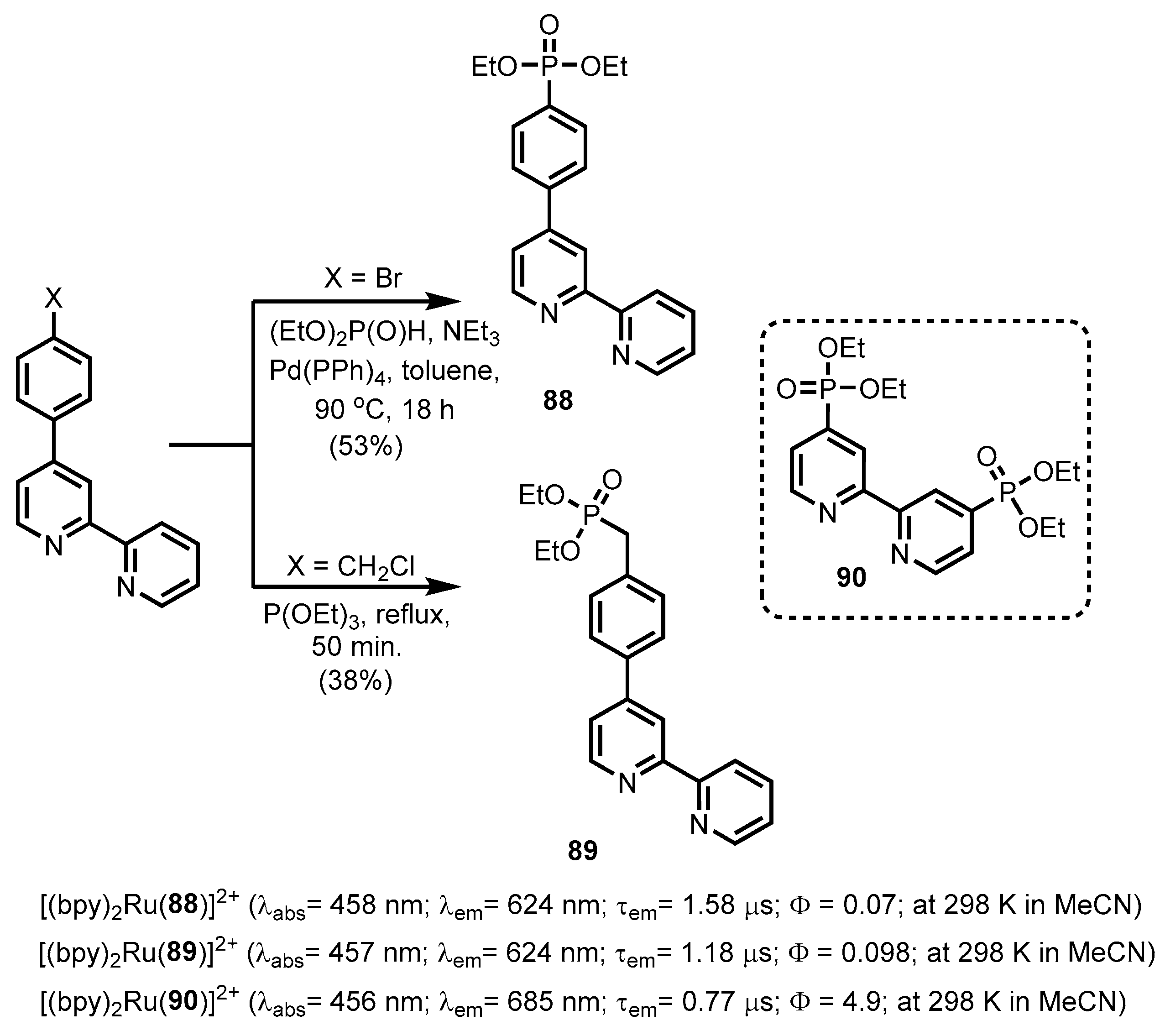
- Montalti, M.; Wadhwa, S.; Kim, W.Y.; Kipp, R.A.; Schmehl, R.H. Luminescent Ruthenium(II) Bipyridyl−Phosphonic Acid Complexes: pH Dependent Photophysical Behavior and Quenching with Divalent Metal Ions. Inorg. Chem. 2000, 39, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Comby, S.; Imbert, D.; Chauvin, A.-S.; Bünzli, J.-C.G.; Charbonnière, L.J.; Ziessel, R.F. Influence of Anionic Functions on the Coordination and Photophysical Properties of Lanthanide(III) Complexes with Tridentate Bipyridines. Inorg. Chem. 2004, 43, 7369–7379. [Google Scholar] [CrossRef] [PubMed]
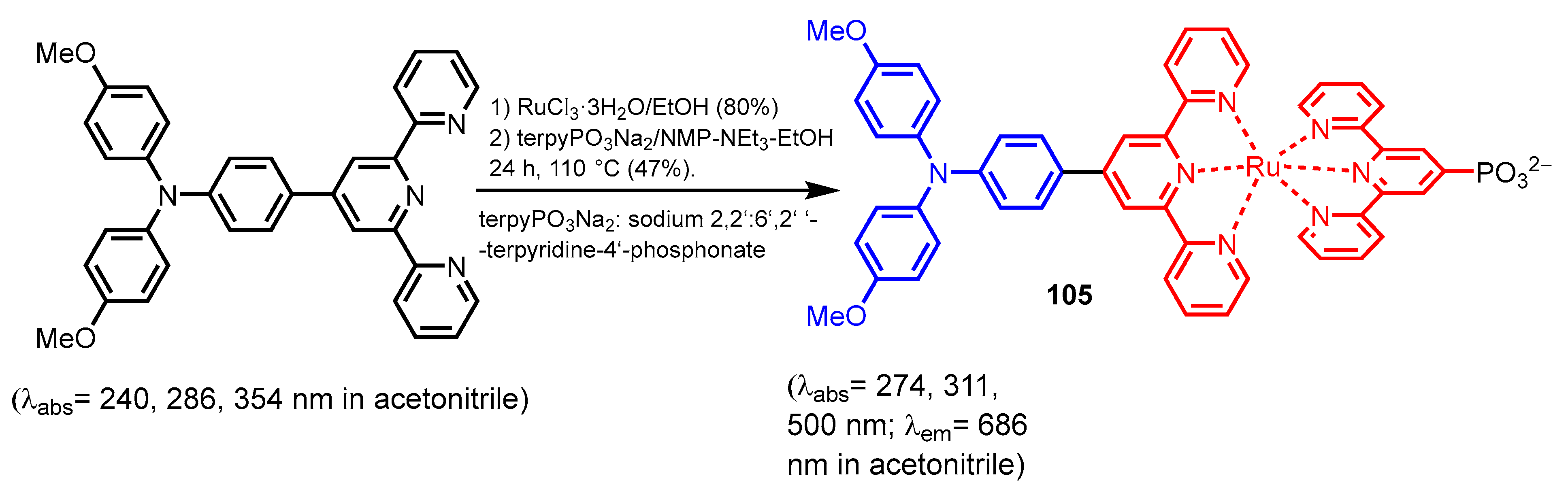
- Herath, H.N.K.; MacRae, A.L.; Ugrinov, A.; Morello, G.R.; Parent, A.R. Electrochemical Properties of Ru Polypyridyl Phosphonates. Eur. J. Inorg. Chem. 2023, 26, e202200747. [Google Scholar] [CrossRef]
- Ferrer, Í.; Fontrodona, X.; Roig, A.; Rodríguez, M.; Romero, I. A Recoverable Ruthenium Aqua Complex Supported on Silica Particles: An Efficient Epoxidation Catalyst. Chem. Eur. J. 2017, 23, 4096–4107. [Google Scholar] [CrossRef]
- Bonhôte, P.; Moser, J.-E.; Humphry-Baker, R.; Vlachopoulos, N.; Zakeeruddin, S.M.; Walder, L.; Grätzel, M. Long-Lived Photoinduced Charge Separation and Redox-Type Photochromism on Mesoporous Oxide Films Sensitized by Molecular Dyads. J. Am. Chem. Soc. 1999, 121, 1324–1336. [Google Scholar] [CrossRef]
- Amthor, S.; Keil, P.; Nauroozi, D.; Perleth, D.; Rau, S. A Phosphonate Substituent in a 1,10-Phenanthroline Ligand Boosts Light-Driven Catalytic Water Oxidation Performance Sensitized by Ruthenium Chromophores. Eur. J. Inorg. Chem. 2021, 2021, 4790–4798. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Z.-L.; Phelan, B.T.; Lynch, V.M.; Chen, L.X.; Mulfort, K.L. Changing Directions: Influence of Ligand Electronics on the Directionality and Kinetics of Photoinduced Charge Transfer in Cu(I)Diimine Complexes. Inorg. Chem. 2023, 62, 14368–14376. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-S.; Tang, X.-Y.; Yin, W.-Y.; Wu, B.; Xue, F.-F.; Yuan, R.-X.; Roy, S. Zinc and cadmium 2-pyrazinephosphonates: Syntheses, structures and luminescent properties. Dalton Trans. 2012, 41, 2340–2345. [Google Scholar] [CrossRef]
- Ansari, S.A.; Mohapatra, P.K.; Sengupta, A.; Nikishkin, N.I.; Huskens, J.; Verboom, W. An Insight into the Complexation of Pyrazine-Functionalized Calix [4]arenes with Am3+ and Eu3+—Solvent Extraction and Luminescence Studies in Room-Temperature Ionic Liquids. Eur. J. Inorg. Chem. 2014, 2014, 5689–5697. [Google Scholar] [CrossRef]
- Ansari, S.A.; Mohapatra, P.K.; Verboom, W.; Zhang, Z.; Dau, P.D.; Gibson, J.K.; Rao, L. Binding of pyrazine-functionalized calix [4]arene ligands with lanthanides in an ionic liquid: Thermodynamics and coordination modes. Dalton Trans. 2015, 44, 6416–6422. [Google Scholar] [CrossRef]
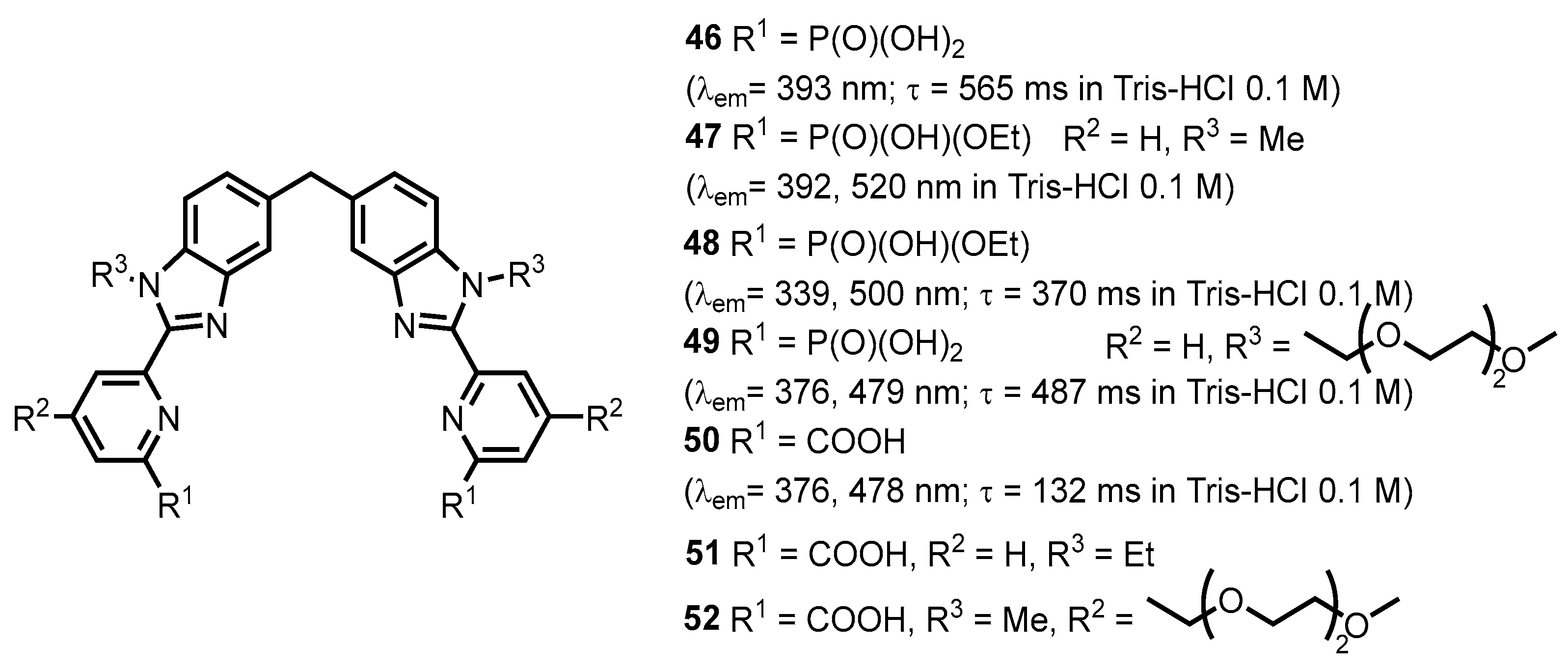
- Makwana, M.V.; dos Santos Souza, C.; Pickup, B.T.; Thompson, M.J.; Lomada, S.K.; Feng, Y.; Wieland, T.; Jackson, R.F.W.; Muimo, R. Chemical Tools for Studying Phosphohistidine: Generation of Selective τ-Phosphohistidine and π-Phosphohistidine Antibodies. ChemBioChem 2023, 24, e202300182. [Google Scholar] [CrossRef]
- Bruyneel, F.; D’Auria, L.; Payen, O.; Courtoy, P.J.; Marchand-Brynaert, J. Live-Cell Imaging with Water-Soluble Aminophenoxazinone Dyes Synthesised through Laccase Biocatalysis. ChemBioChem 2010, 11, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ding, J.; Wang, L.; Jing, X.; Wang, F. A solution-processable phosphonate functionalized deep-blue fluorescent emitter for efficient single-layer small molecule organic light-emitting diodes. Chem. Commun. 2012, 48, 8970–8972. [Google Scholar] [CrossRef]
- Li, X.; Bai, K.; Wang, S.; Ding, J.; Wang, L. An arylphosphine oxide and phosphonate combination as a solution processable electron injection layer for power-efficient PLEDs. J. Mater. Chem. C 2019, 7, 2633–2639. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, L.; Ding, J.; Wang, L.; Jing, X.; Wang, F. An alcohol-soluble and ion-free electron transporting material functionalized with phosphonate groups for solution-processed multilayer PLEDs. Chem. Commun. 2016, 52, 12052–12055. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Wang, C.; Wang, S.; Ding, J. Bulky Passivation by an Electroactive Phosphonate Dendrimer for High-Performance Perovskite Quantum Dot Light-Emitting Diodes. J. Phys. Chem. C 2024, 128, 877–884. [Google Scholar] [CrossRef]



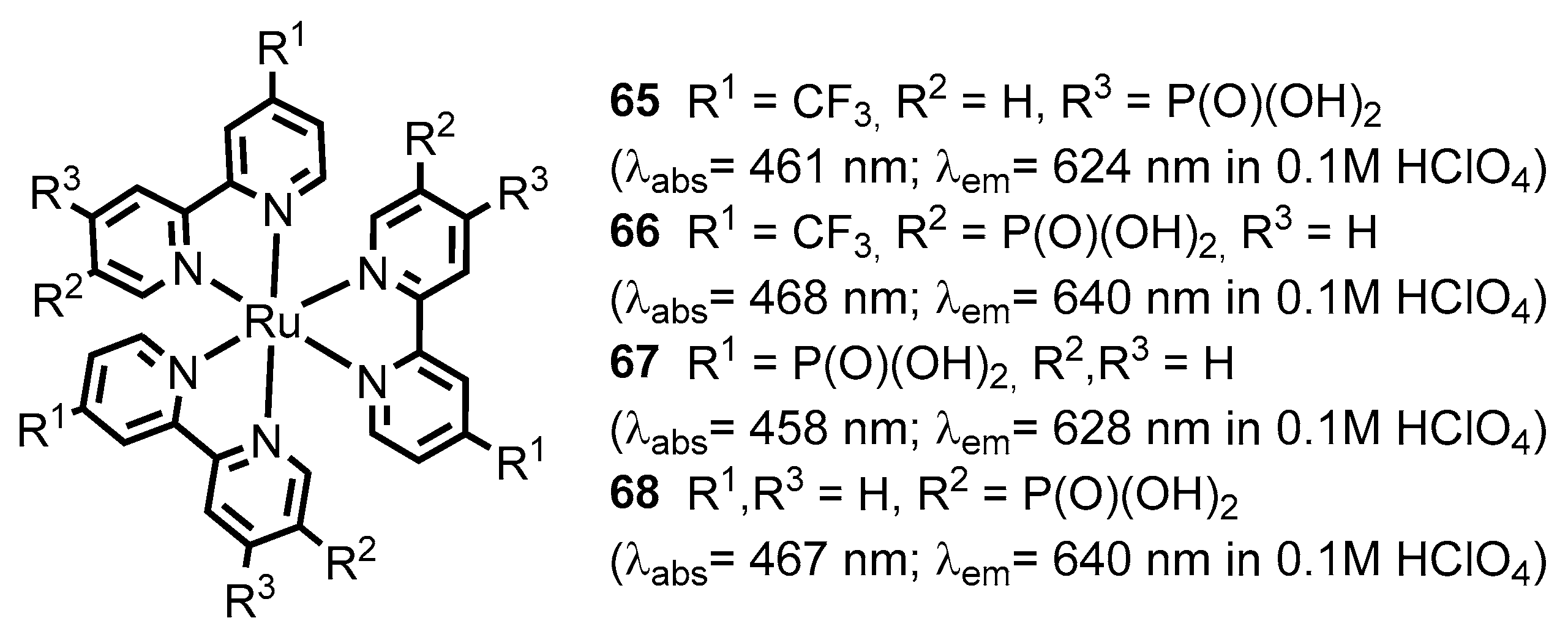









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owsianik, K.; Romaniuk, A.; Turek, M.; Bałczewski, P. Comprehensive Review of Synthesis, Optical Properties and Applications of Heteroarylphosphonates and Their Derivatives. Molecules 2024, 29, 3691. https://doi.org/10.3390/molecules29153691
Owsianik K, Romaniuk A, Turek M, Bałczewski P. Comprehensive Review of Synthesis, Optical Properties and Applications of Heteroarylphosphonates and Their Derivatives. Molecules. 2024; 29(15):3691. https://doi.org/10.3390/molecules29153691
Chicago/Turabian StyleOwsianik, Krzysztof, Adrian Romaniuk, Marika Turek, and Piotr Bałczewski. 2024. "Comprehensive Review of Synthesis, Optical Properties and Applications of Heteroarylphosphonates and Their Derivatives" Molecules 29, no. 15: 3691. https://doi.org/10.3390/molecules29153691
APA StyleOwsianik, K., Romaniuk, A., Turek, M., & Bałczewski, P. (2024). Comprehensive Review of Synthesis, Optical Properties and Applications of Heteroarylphosphonates and Their Derivatives. Molecules, 29(15), 3691. https://doi.org/10.3390/molecules29153691







