Effects of Ellagic Acid on Vaginal Innate Immune Mediators and HPV16 Infection In Vitro
Abstract
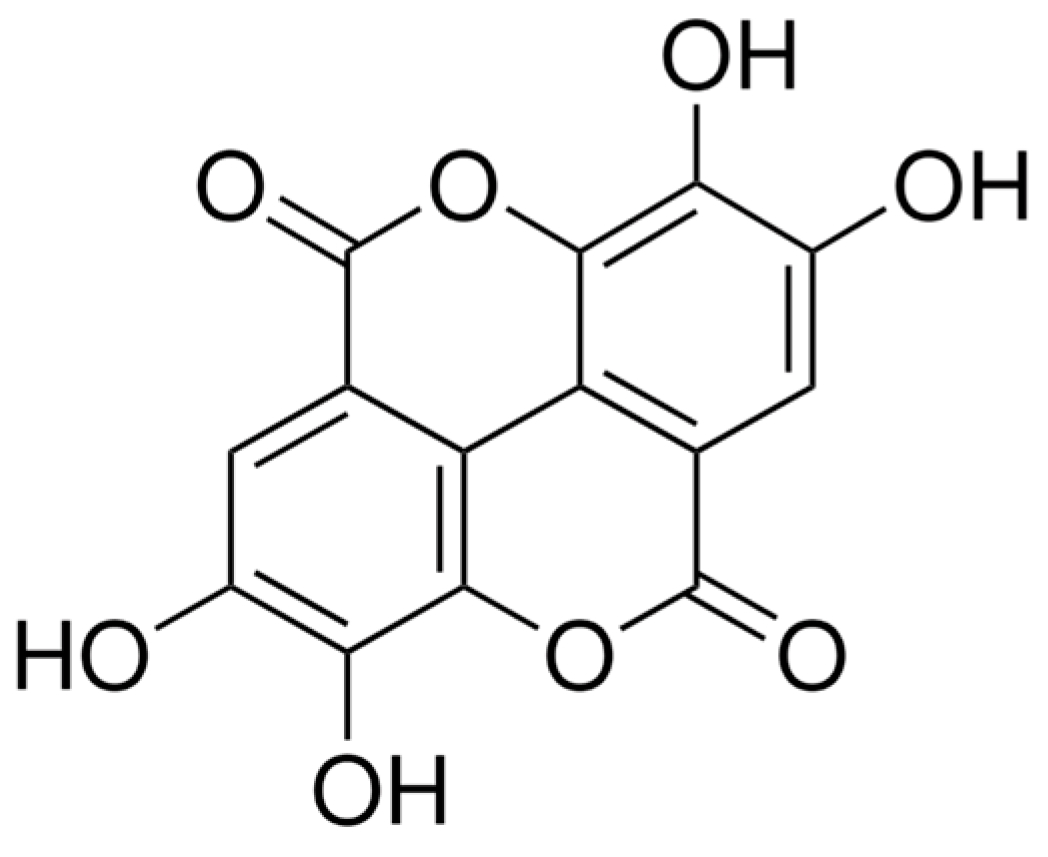
1. Introduction
2. Results
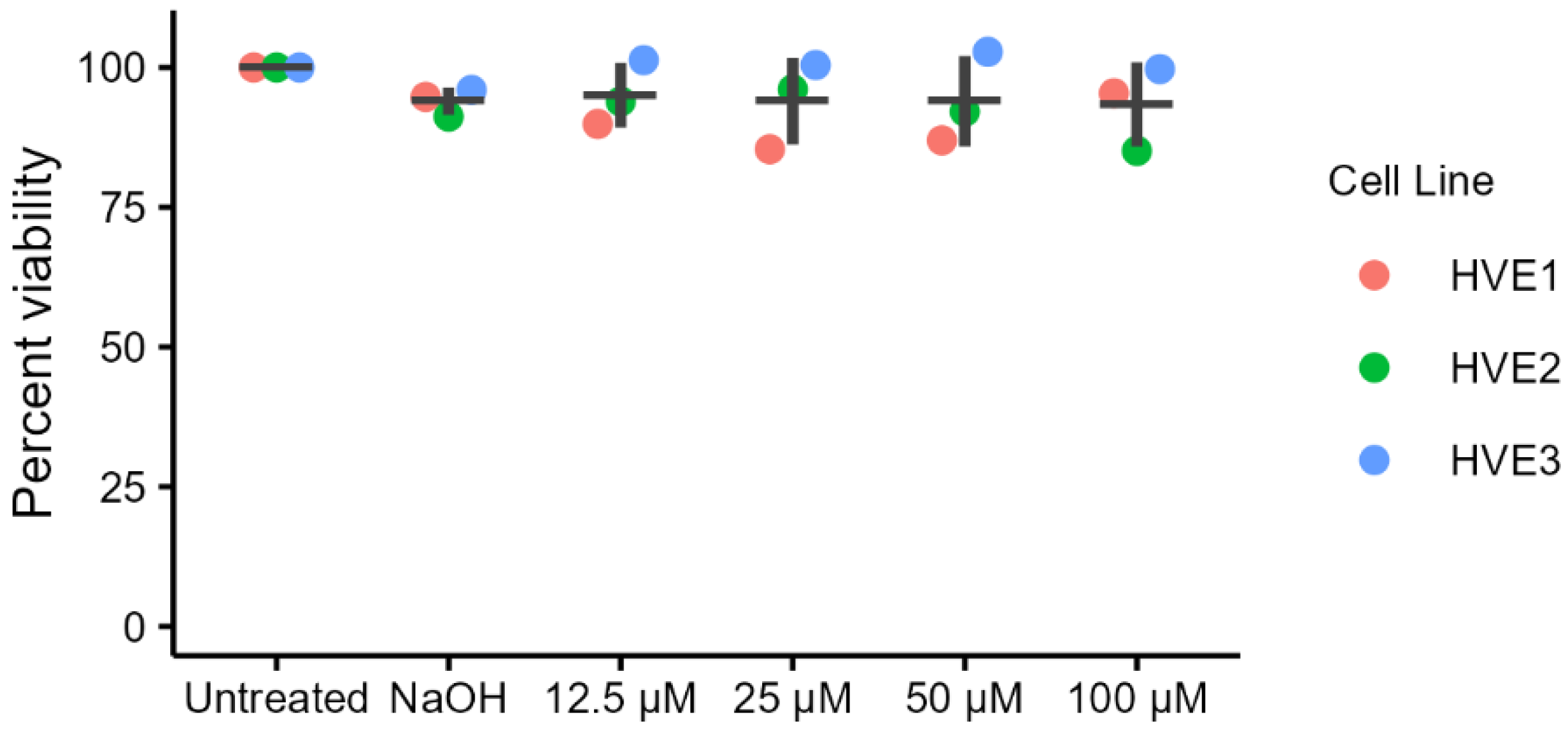
2.1. Cytotoxicity of Ellagic Acid in Primary Human Vaginal Epithelial Cells (HVEs)
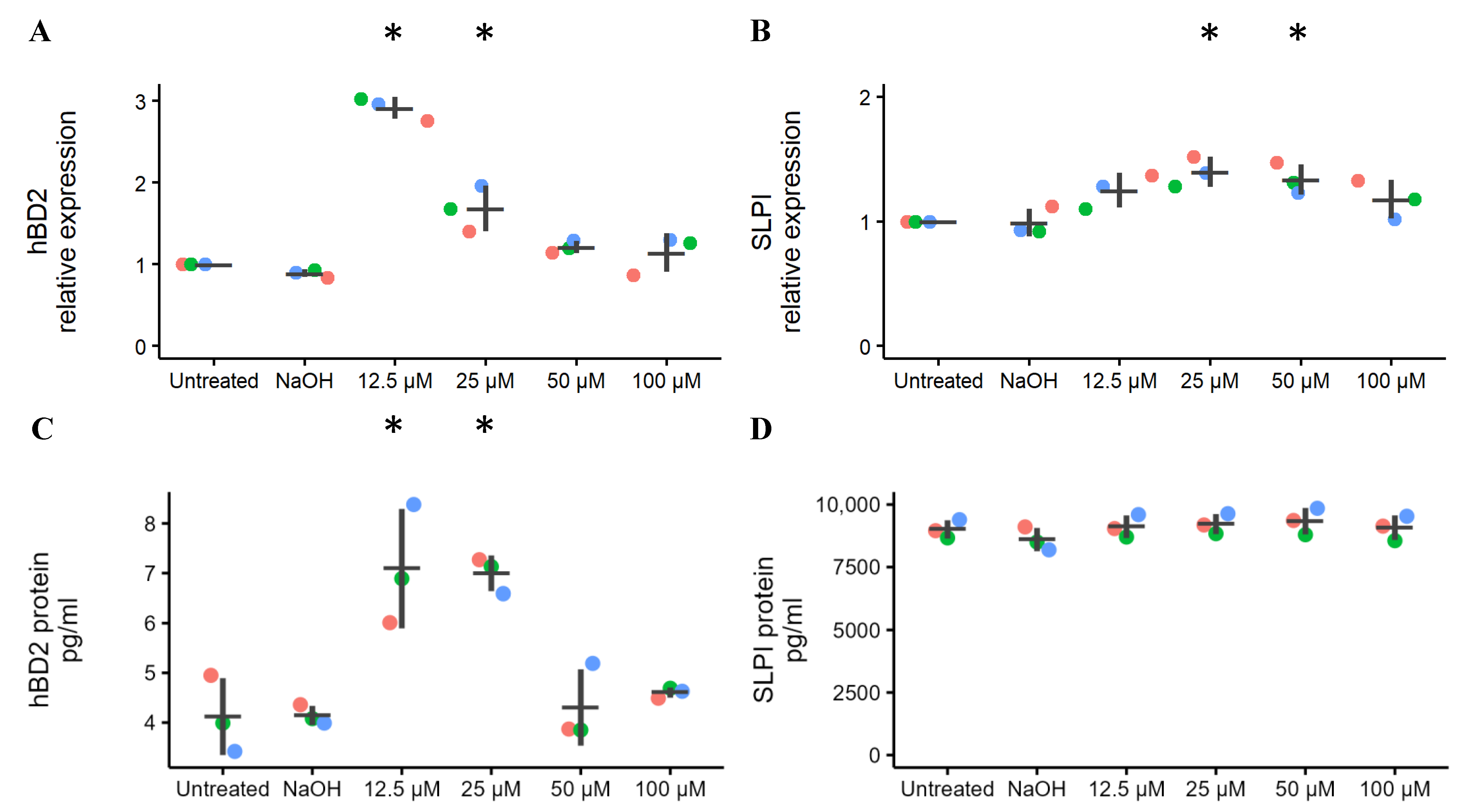
2.2. Effects of Ellagic Acid on the Expression of Vaginal Innate Immunity
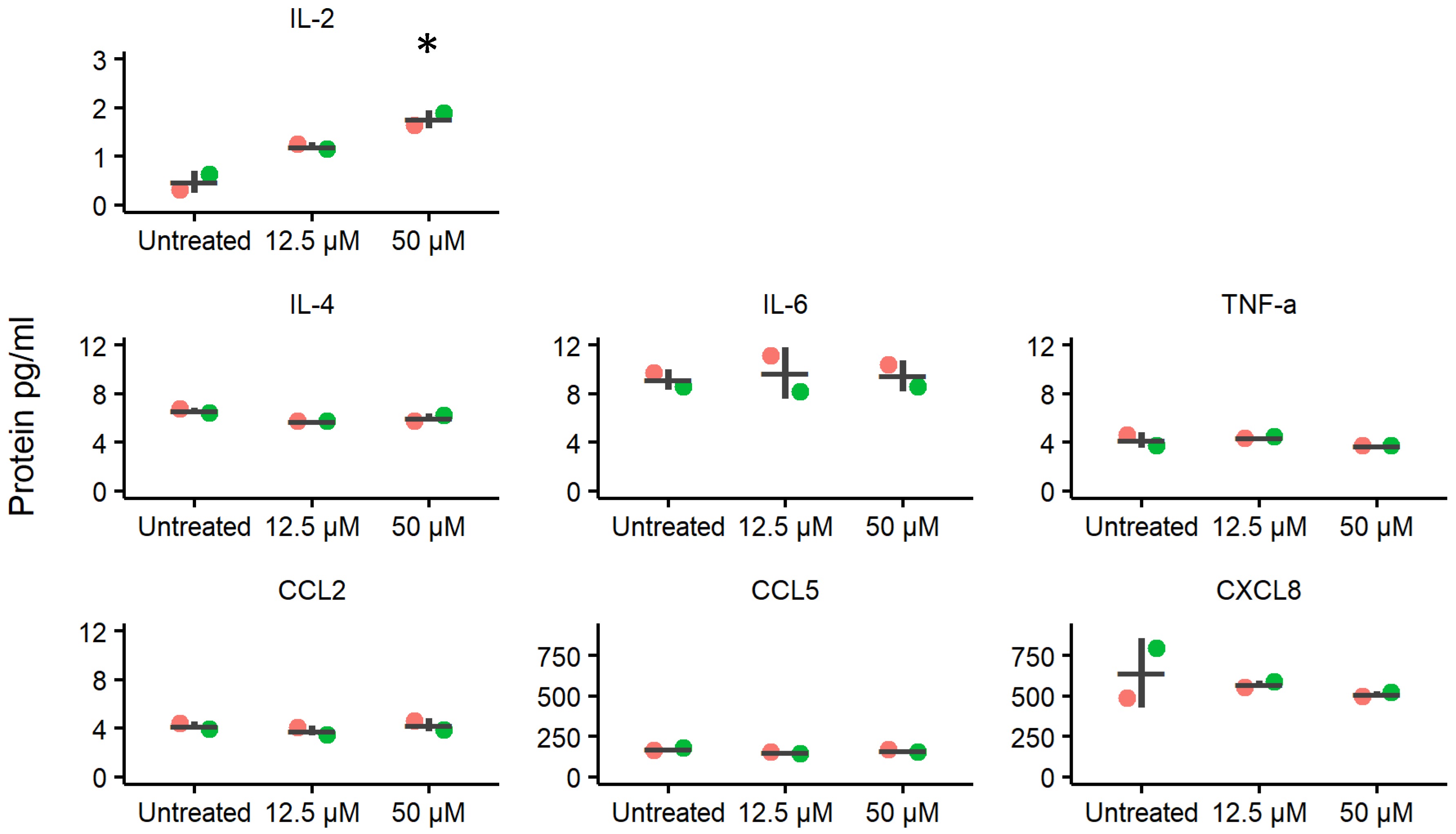
2.3. Effects of Ellagic Acid on Cytokine and Chemokine Secretion Levels
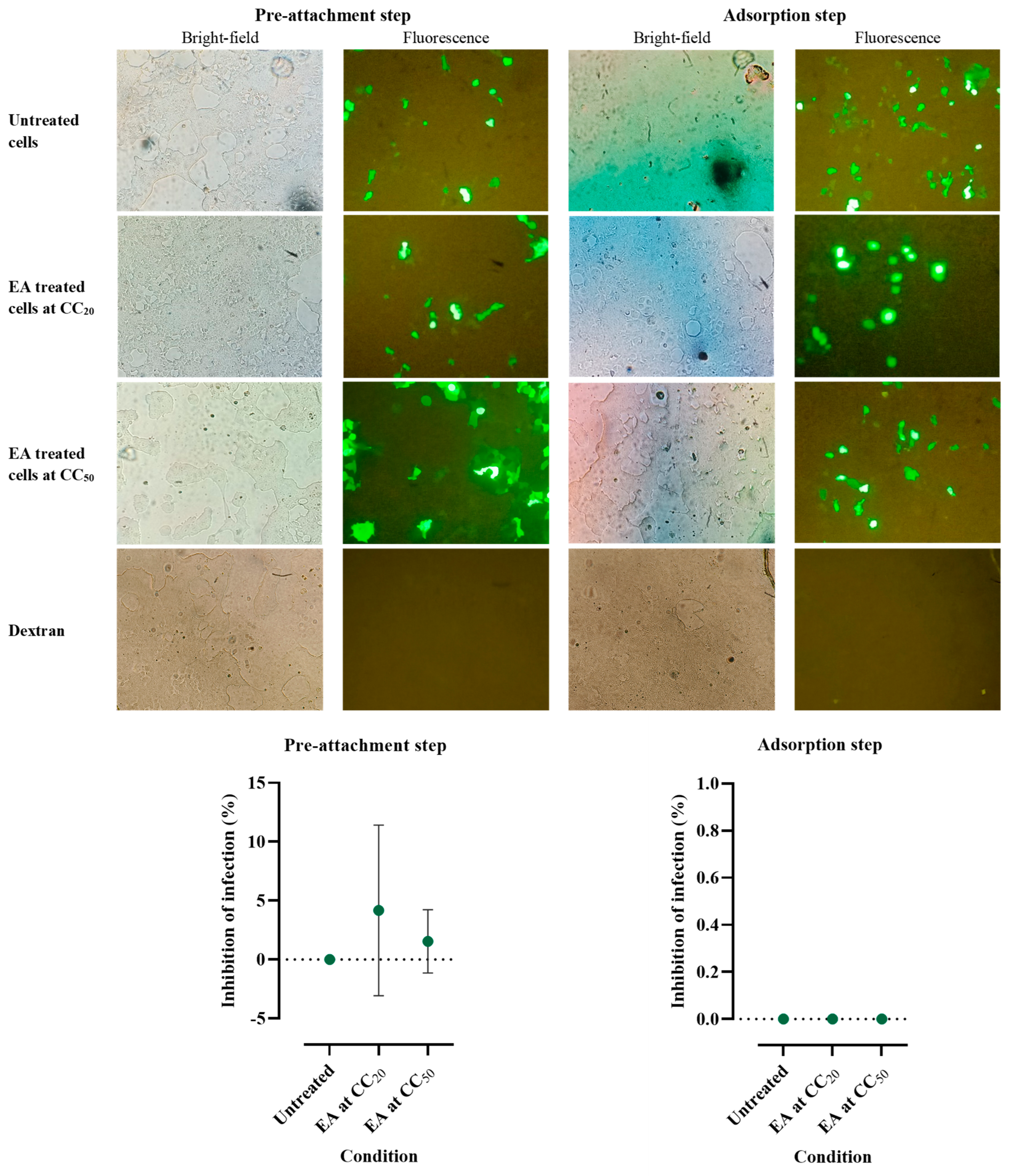
2.4. Effects of Ellagic Acid on HPV16 Pseudovirus Infection
3. Discussion
4. Materials and Methods
4.1. Ellagic Acid Preparation
4.2. Primary Human Vaginal Epithelial Cell Preparation
4.3. Isolation of HVEs
4.4. Cytotoxicity of Ellagic Acid in HVEs
4.5. Treatment of HVEs with Ellagic Acid
4.6. cDNA Preparation and Quantitative Real-Time PCR
4.7. Detection of Immune Mediator Concentrations in Culture Medium
4.8. hBD2 and SLPI Immunoassays
4.9. Multiple Human Cytokines and Chemokines by Luminex Assay
4.10. Cytotoxicity of Ellagic Acid in HEK-293FT Cells
4.11. Production of HPV16 Pseudovirus
4.12. HPV Attachment Assays
4.12.1. Ellagic Acid Added to HPV before HPV Attachment to Cells
4.12.2. Ellagic Acid Added to Cells after HPV Attachment to Cells
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- da Silva, R.L.; da Silva Batista, Z.; Bastos, G.R.; Cunha, A.P.A.; Figueiredo, F.V.; de Castro, L.O.; Dos Anjos Pereira, L.; da Silva, M.; Vidal, F.C.B.; Barros, M.C.; et al. Role of HPV 16 variants among cervical carcinoma samples from Northeastern Brazil. BMC Women’s Health 2020, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25 (Suppl. S1), 2–23. [Google Scholar] [CrossRef] [PubMed]
- Hang, D.; Yin, Y.; Han, J.; Jiang, J.; Ma, H.; Xie, S.; Feng, X.; Zhang, K.; Hu, Z.; Shen, H.; et al. Analysis of human papillomavirus 16 variants and risk for cervical cancer in Chinese population. Virology 2016, 488, 156–161. [Google Scholar] [CrossRef]
- Larsen, S.B.; Cowley, C.J.; Fuchs, E. Epithelial cells: Liaisons of immunity. Curr. Opin. Immunol. 2020, 62, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, B.; Patel, M.; Fahey, J.; Wira, C. Endocrine control of mucosal immunity in the female reproductive tract: Impact of environmental disruptors. Mol. Cell. Endocrinol. 2012, 354, 85–93. [Google Scholar] [CrossRef]
- Majchrzak-Gorecka, M.; Majewski, P.; Grygier, B.; Murzyn, K.; Cichy, J. Secretory leukocyte protease inhibitor (SLPI), a multifunctional protein in the host defense response. Cytokine Growth Factor Rev. 2016, 28, 79–93. [Google Scholar] [CrossRef]
- Meade, K.G.; O’Farrelly, C. beta-Defensins: Farming the Microbiome for Homeostasis and Health. Front. Immunol. 2018, 9, 3072. [Google Scholar] [CrossRef]
- Zhai, Y.J.; Feng, Y.; Ma, X.; Ma, F. Defensins: Defenders of human reproductive health. Hum. Reprod. Update 2023, 29, 126–154. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, J.P.; Sun, L.; Lu, W.; Gartner, S.; Garzino-Demo, A. Human Beta-Defensin 2 and 3 Inhibit HIV-1 Replication in Macrophages. Front. Cell. Infect. Microbiol. 2021, 11, 535352. [Google Scholar] [CrossRef]
- Nugteren, S.; Samsom, J.N. Secretory Leukocyte Protease Inhibitor (SLPI) in mucosal tissues: Protects against inflammation, but promotes cancer. Cytokine Growth Factor Rev. 2021, 59, 22–35. [Google Scholar] [CrossRef]
- Woodham, A.W.; Da Silva, D.M.; Skeate, J.G.; Raff, A.B.; Ambroso, M.R.; Brand, H.E.; Isas, J.M.; Langen, R.; Kast, W.M. The S100A10 subunit of the annexin A2 heterotetramer facilitates L2-mediated human papillomavirus infection. PLoS ONE 2012, 7, e43519. [Google Scholar] [CrossRef] [PubMed]
- Quabius, E.S.; Gorogh, T.; Fischer, G.S.; Hoffmann, A.S.; Gebhard, M.; Evert, M.; Beule, A.; Maune, S.; Knecht, R.; Ovari, A.; et al. The antileukoprotease secretory leukocyte protease inhibitor (SLPI) and its role in the prevention of HPV-infections in head and neck squamous cell carcinoma. Cancer Lett. 2015, 357, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Gholiof, M.; Adamson-De Luca, E.; Wessels, J.M. The female reproductive tract microbiotas, inflammation, and gynecological conditions. Front. Reprod. Health 2022, 4, 963752. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.L.; Giner, R.M.; Marin, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Sarkar, A.; Ghosh, P.; Ganguly, M.; Karmakar, B.C.; Saha, D.R.; Halder, A.; Chowdhury, A.; Mukhopadhyay, A.K. Antimicrobial activity of ellagic acid against Helicobacter pylori isolates from India and during infections in mice. J. Antimicrob. Chemother. 2018, 73, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Jodynis-Liebert, J. Potential of the ellagic acid-derived gut microbiota metabolite—Urolithin A in gastrointestinal protection. World J. Gastroenterol. 2020, 26, 3170–3181. [Google Scholar] [CrossRef] [PubMed]
- Nittayananta, W.; Promsong, A.; Levy, C.; Hladik, F.; Chaitaveep, N.; Ungphaiboon, S.; Tewtrakul, S.; Satthakarn, S. Microbicide Containing Ellagic Acid Can Inhibit HIV-1 Infection. Molecules 2022, 27, 7941. [Google Scholar] [CrossRef] [PubMed]
- Promsong, A.; Chuenchitra, T.; Saipin, K.; Tewtrakul, S.; Panichayupakaranant, P.; Satthakarn, S.; Nittayananta, W. Ellagic acid inhibits HIV-1 infection in vitro: Potential role as a novel microbicide. Oral Dis. 2018, 24, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Promsong, A.; Chung, W.O.; Satthakarn, S.; Nittayananta, W. Ellagic acid modulates the expression of oral innate immune mediators: Potential role in mucosal protection. J. Oral Pathol. Med. 2015, 44, 214–221. [Google Scholar] [CrossRef]
- Moscato, G.M.F.; Galanti, F.; Criscuolo, A.A.; Ciotti, M.; Riccio, S.; Sorge, R.; Andreoni, M.; Sesti, F. A retrospective study on two cohorts of immunocompetent women treated with nonavalent HPV vaccine vs. Ellagic acid complex: Outcome of the evolution of persistent cervical HPV infection. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5509–5519. [Google Scholar] [CrossRef]
- Cai, T.; Rizzo, M.; Liguori, G.; Palumbo, M.; Palmieri, A.; Gallelli, L. The Role of Ellagic Acid and Annona muricata in the Management of Human Papillomavirus (HPV)-Related Genital Lesions: A Systematic Review. Uro 2023, 3, 54–60. [Google Scholar] [CrossRef]
- Fesahat, F.; Firouzabadi, A.M.; Zare-Zardini, H.; Imani, M. Roles of Different beta-Defensins in the Human Reproductive System: A Review Study. Am. J. Mens. Health 2023, 17, 15579883231182673. [Google Scholar] [CrossRef]
- Patel, M.V.; Fahey, J.V.; Rossoll, R.M.; Wira, C.R. Innate immunity in the vagina (part I): Estradiol inhibits HBD2 and elafin secretion by human vaginal epithelial cells. Am. J. Reprod. Immunol. 2013, 69, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Hickey, D.K.; Patel, M.V.; Fahey, J.V.; Wira, C.R. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: Stratification and integration of immune protection against the transmission of sexually transmitted infections. J. Reprod. Immunol. 2011, 88, 185–194. [Google Scholar] [CrossRef]
- Lafferty, M.K.; Sun, L.; Christensen-Quick, A.; Lu, W.; Garzino-Demo, A. Human Beta Defensin 2 Selectively Inhibits HIV-1 in Highly Permissive CCR6(+)CD4(+) T Cells. Viruses 2017, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Pol, J.G.; Caudana, P.; Paillet, J.; Piaggio, E.; Kroemer, G. Effects of interleukin-2 in immunostimulation and immunosup-pression. J. Exp. Med. 2020, 217, e20191247. [Google Scholar] [CrossRef]
- Santoni, G.; Boccanera, M.; Adriani, D.; Lucciarini, R.; Amantini, C.; Morrone, S.; Cassone, A.; De Bernardis, F. Immune cell-mediated protection against vaginal candidiasis: Evidence for a major role of vaginal CD4(+) T cells and possible participation of other local lymphocyte effectors. Infect. Immun. 2002, 70, 4791–4797. [Google Scholar] [CrossRef]
- Fakhry, C.; Marks, M.A.; Gilman, R.H.; Cabrerra, L.; Yori, P.; Kosek, M.; Gravitt, P.E. Comparison of the immune mi-croenvironment of the oral cavity and cervix in healthy women. Cytokine 2013, 64, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, P.; Kok, T.W.; Burrell, C.J. AZT blocks down-regulation of IL-2 and IFN-gamma gene expression in HIV acutely infected cells. Arch. Virol. 1997, 142, 1035–1043. [Google Scholar] [CrossRef]
- Hu, R.; Oyaizu, N.; Kalyanaraman, V.S.; Pahwa, S. HIV-1 gp160 as a modifier of Th1 and Th2 cytokine response: gp160 suppresses interferon-gamma and interleukin-2 production concomitantly with enhanced interleukin-4 production in vitro. Clin. Immunol. Immunopathol. 1994, 73, 245–251. [Google Scholar] [CrossRef]
- Collette, Y.; Chang, H.L.; Cerdan, C.; Chambost, H.; Algarte, M.; Mawas, C.; Imbert, J.; Burny, A.; Olive, D. Specific Th1 cytokine down-regulation associated with primary clinically derived human immunodeficiency virus type 1 Nef gene-induced expression. J. Immunol. 1996, 156, 360–370. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, Y.; Zhang, S.; Luo, H.; Zhang, H. HIV-1 Infection-Induced Suppression of the Let-7i/IL-2 Axis Contributes to CD4(+) T Cell Death. Sci. Rep. 2016, 6, 25341. [Google Scholar] [CrossRef]
- Koussounadis, A.; Langdon, S.P.; Um, I.H.; Harrison, D.J.; Smith, V.A. Relationship between differentially expressed mRNA and mRNA-protein correlations in a xenograft model system. Sci. Rep. 2015, 5, 10775. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Aishwarya, V.; Solaipriya, S.; Sivaramakrishnan, V. Role of ellagic acid for the prevention and treatment of liver diseases. Phytother. Res. 2021, 35, 2925–2944. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.M.; Wang, Z.H.; Liu, C.H.; Chen, C.S. Ellagic acid inhibits IL-1beta-induced cell adhesion molecule expression in human umbilical vein endothelial cells. Br. J. Nutr. 2007, 97, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef]
- Piazza, S.; Fumagalli, M.; Martinelli, G.; Pozzoli, C.; Maranta, N.; Angarano, M.; Sangiovanni, E.; Dell’Agli, M. Hydro-lyzable Tannins in the Management of Th1, Th2 and Th17 Inflammatory-Related Diseases. Molecules 2022, 27, 7593. [Google Scholar] [CrossRef]
- Boozari, M.; Butler, A.E.; Sahebkar, A. Impact of curcumin on toll-like receptors. J. Cell. Physiol. 2019, 234, 12471–12482. [Google Scholar] [CrossRef]
- Perez-Cano, F.J.; Massot-Cladera, M.; Rodriguez-Lagunas, M.J.; Castell, M. Flavonoids Affect Host-Microbiota Crosstalk through TLR Modulation. Antioxidants 2014, 3, 649–670. [Google Scholar] [CrossRef]
- D’Abramo, C.M.; Archambault, J. Small molecule inhibitors of human papillomavirus protein—Protein interactions. Open Virol. J. 2011, 5, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Rheinwald, J.G.; Green, H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing col-onies from single cells. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.; Liu, X.; Meyers, C.; Schlegel, R.; McBride, A.A. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J. Clin. Investig. 2010, 120, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ory, V.; Chapman, S.; Yuan, H.; Albanese, C.; Kallakury, B.; Timofeeva, O.A.; Nealon, C.; Dakic, A.; Simic, V.; et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am. J. Pathol. 2012, 180, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Gornalusse, G.G.; Valdez, R.; Fenkart, G.; Vojtech, L.; Fleming, L.M.; Pandey, U.; Hughes, S.M.; Levy, C.N.; Dela Cruz, E.J.; Calienes, F.L.; et al. Mechanisms of Endogenous HIV-1 Reactivation by Endocervical Epithelial Cells. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gornalusse, G.G.; Kim, Y.; Pandey, U.; Hladik, F.; Vojtech, L. Potent Restriction of Sexual Zika Virus Infection by the Lipid Fraction of Extracellular Vesicles in Semen. Front. Microbiol. 2020, 11, 574054. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C., Baell, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Ekalaksananan, T.; Sookmai, W.; Fangkham, S.; Pientong, C.; Aromdee, C.; Seubsasana, S.; Kongyingyoes, B. Activity of andro-grapholide and its derivatives on HPV16 pseudovirus infection and viral oncogene expression in cervical carcinoma cells. Nutr. Cancer 2015, 67, 687–696. [Google Scholar] [CrossRef]
- RCore Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; 1.1.463 edn; RStudio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Auguie, B. gridExtra: Miscellaneous Functions for “Grid” Graphics. Available online: https://rdrr.io/cran/gridExtra/ (accessed on 25 July 2024).
- Kassambara, A. ggpubr: g’gplot2’ Based Publication Ready Plots. Available online: https://rdrr.io/github/kassambara/ggpubr/ (accessed on 25 July 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2 ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H. Tidyverse: Easily Install and Load the ‘Tidyverse’. Available online: https://rdrr.io/cran/tidyverse/ (accessed on 25 July 2024).
- Wickham, H.; Seidel, D. Scales: Scale Functions for Visualization. Available online: https://scales.r-lib.org (accessed on 25 July 2024).
- Wilke, C.O. cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. Available online: https://wilkelab.org/cowplot/ (accessed on 25 July 2024).
| SLPI (NM_003064) Exon Location: 3–4 | Forward primer: 5′-CAAGCGTGACTTGAAGTGTTG-3′ Reverse primer: 5′-GAAAGGACCTGGACCACAC-3′ Probe: 5′-/56-FAM/AGGAGATAC/ZEN/AAGACCCTCATGGCTGA/3IABkFQ/-3′ |
| hBD2 (DEFB4A) (NM_004942) Exon Location: 1–2 | Forward primer: 5′-TCCTGGTGAAGCTCCCA-3′ Reverse primer: 5′-CGCCTATACCACCAAAAACAC-3′ Probe: 5′-/56-FAM/AGGAGATAC/ZEN/AAGACCCTCATGGCTGA/3IABkFQ/-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Promsong, A.; Chuerduangphui, J.; Levy, C.N.; Hladik, F.; Satthakarn, S.; Nittayananta, W. Effects of Ellagic Acid on Vaginal Innate Immune Mediators and HPV16 Infection In Vitro. Molecules 2024, 29, 3630. https://doi.org/10.3390/molecules29153630
Promsong A, Chuerduangphui J, Levy CN, Hladik F, Satthakarn S, Nittayananta W. Effects of Ellagic Acid on Vaginal Innate Immune Mediators and HPV16 Infection In Vitro. Molecules. 2024; 29(15):3630. https://doi.org/10.3390/molecules29153630
Chicago/Turabian StylePromsong, Aornrutai, Jureeporn Chuerduangphui, Claire N. Levy, Florian Hladik, Surada Satthakarn, and Wipawee Nittayananta. 2024. "Effects of Ellagic Acid on Vaginal Innate Immune Mediators and HPV16 Infection In Vitro" Molecules 29, no. 15: 3630. https://doi.org/10.3390/molecules29153630
APA StylePromsong, A., Chuerduangphui, J., Levy, C. N., Hladik, F., Satthakarn, S., & Nittayananta, W. (2024). Effects of Ellagic Acid on Vaginal Innate Immune Mediators and HPV16 Infection In Vitro. Molecules, 29(15), 3630. https://doi.org/10.3390/molecules29153630






