Synthesis, Computational Study, and In Vitro α-Glucosidase Inhibitory Action of Thiourea Derivatives Based on 3-Aminopyridin-2(1H)-Ones
Abstract
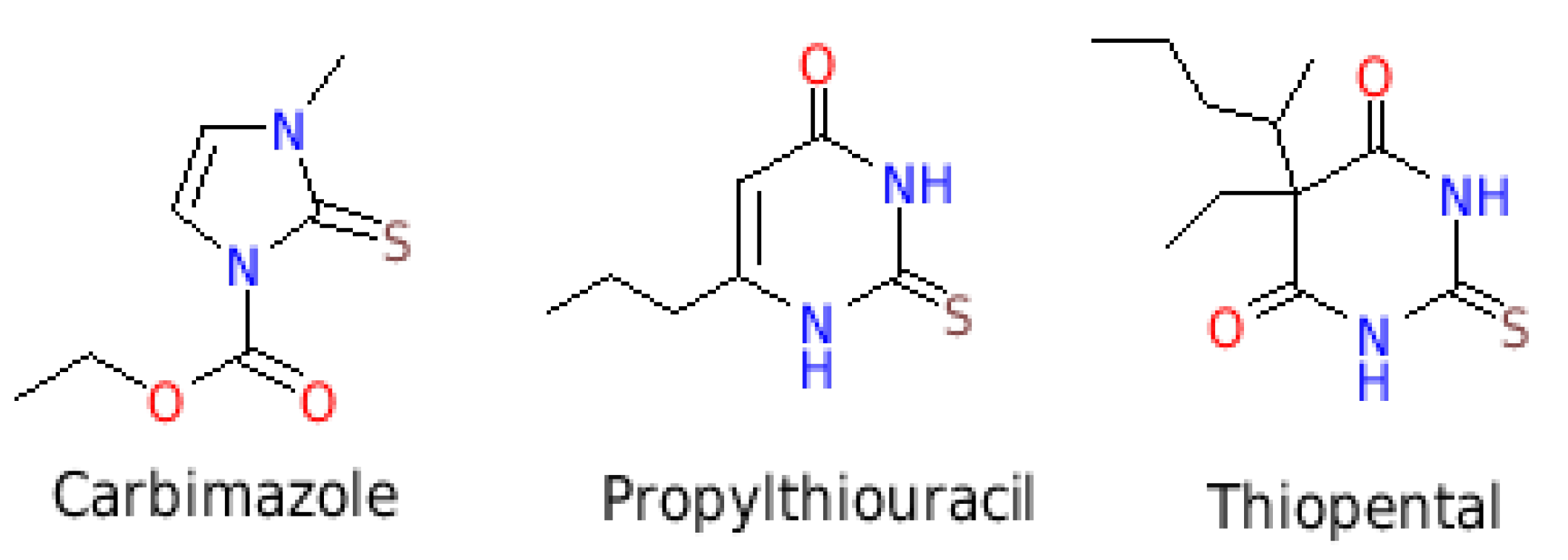
1. Introduction
2. Results and Discussion
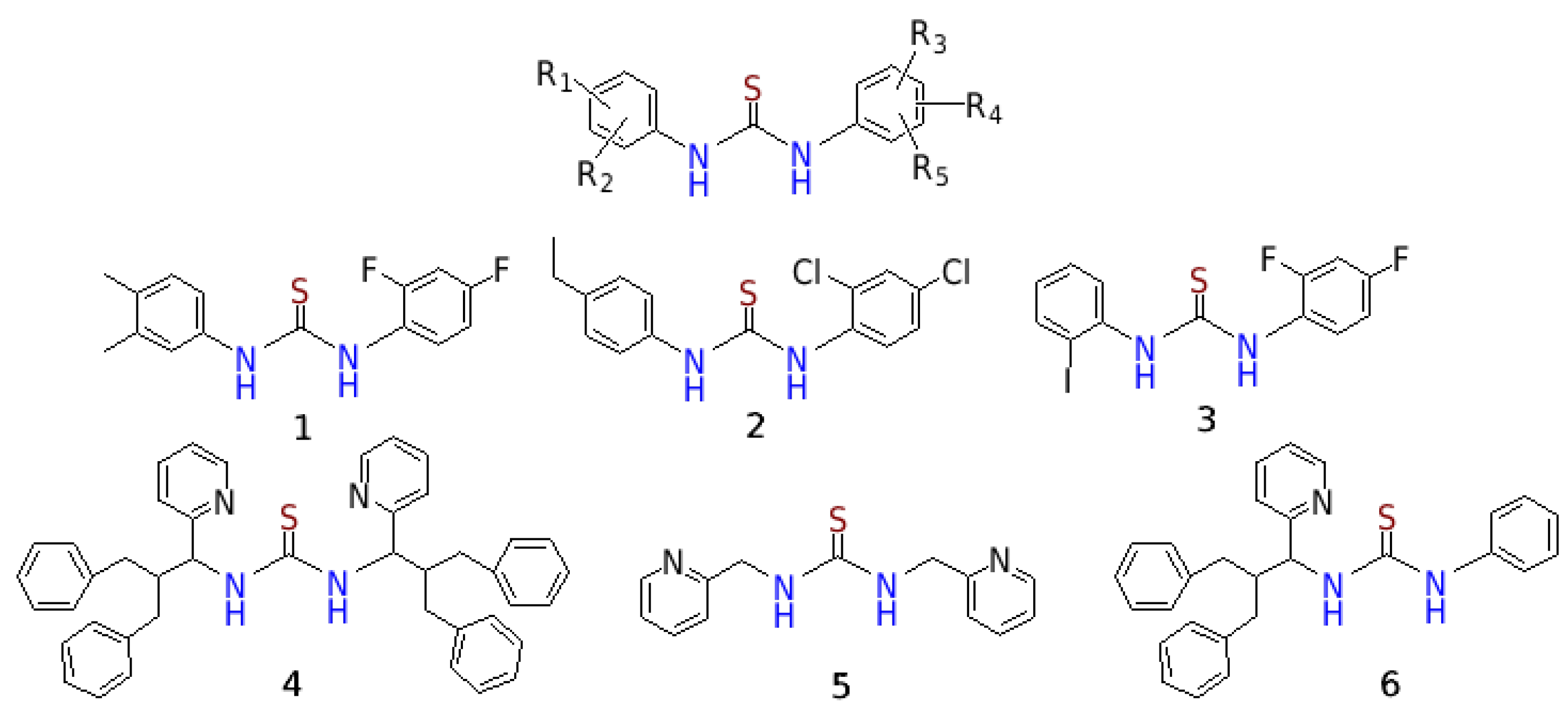
2.1. Chemistry
2.2. In Vitro α-Glucosidase Inhibition Assay
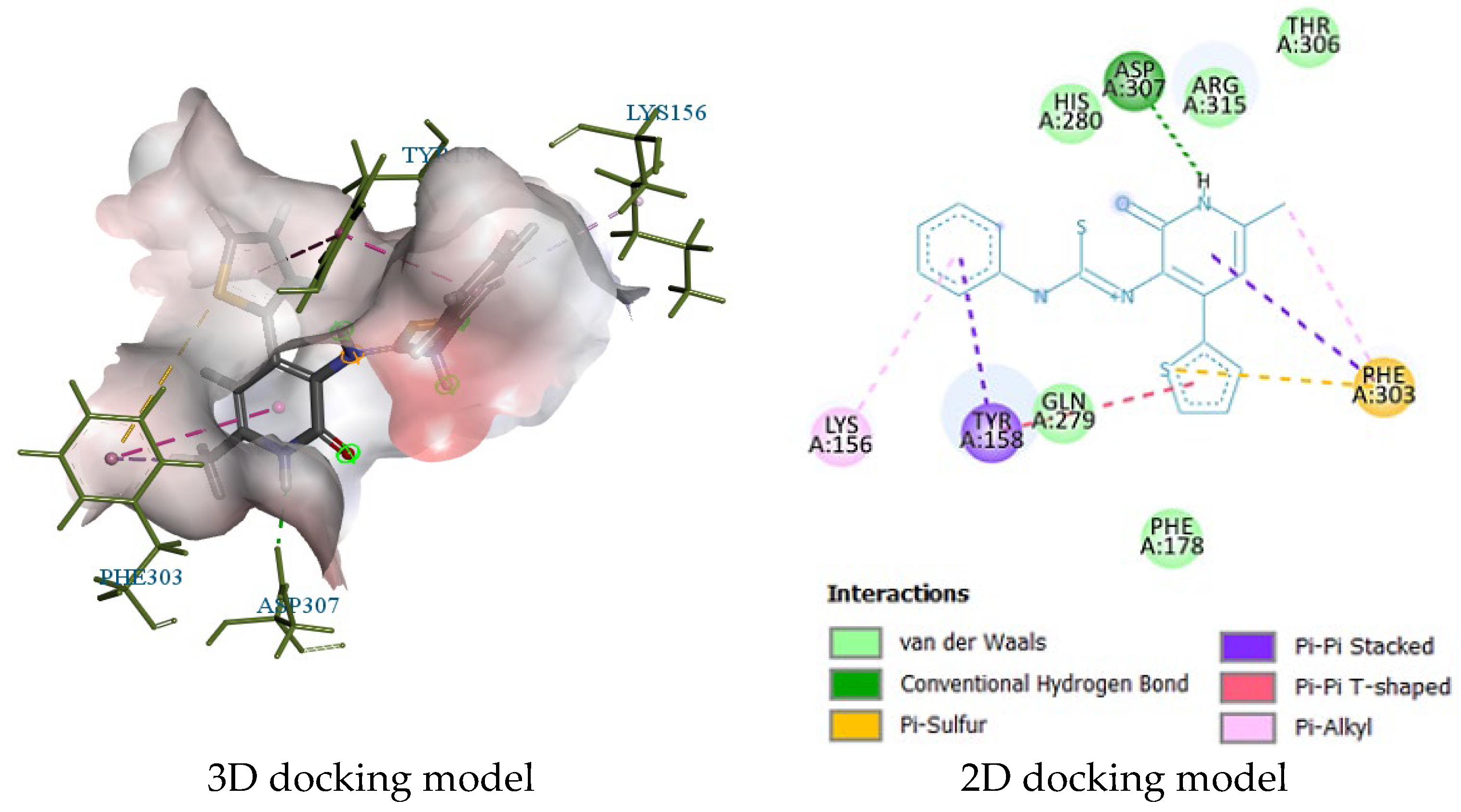
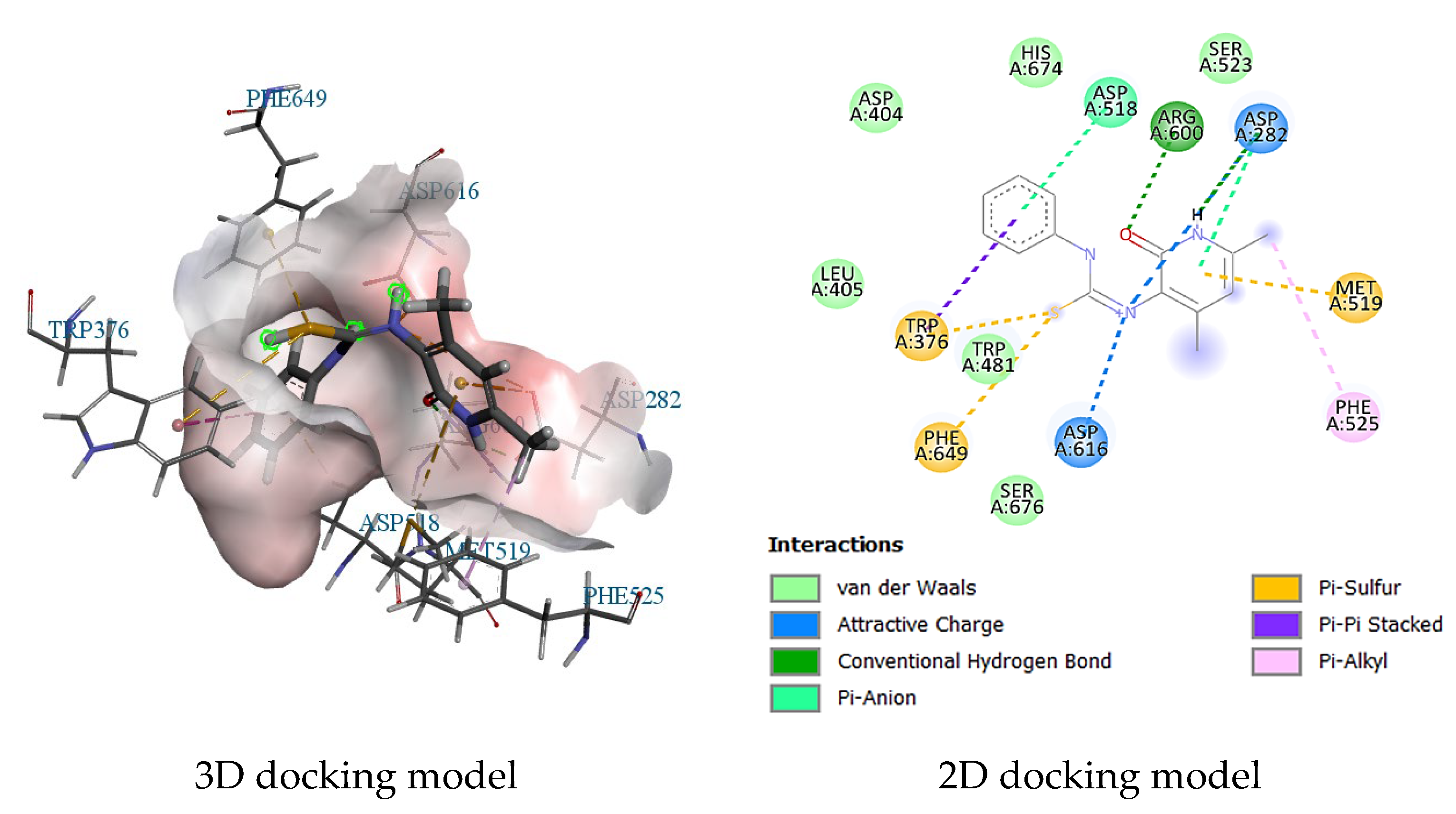
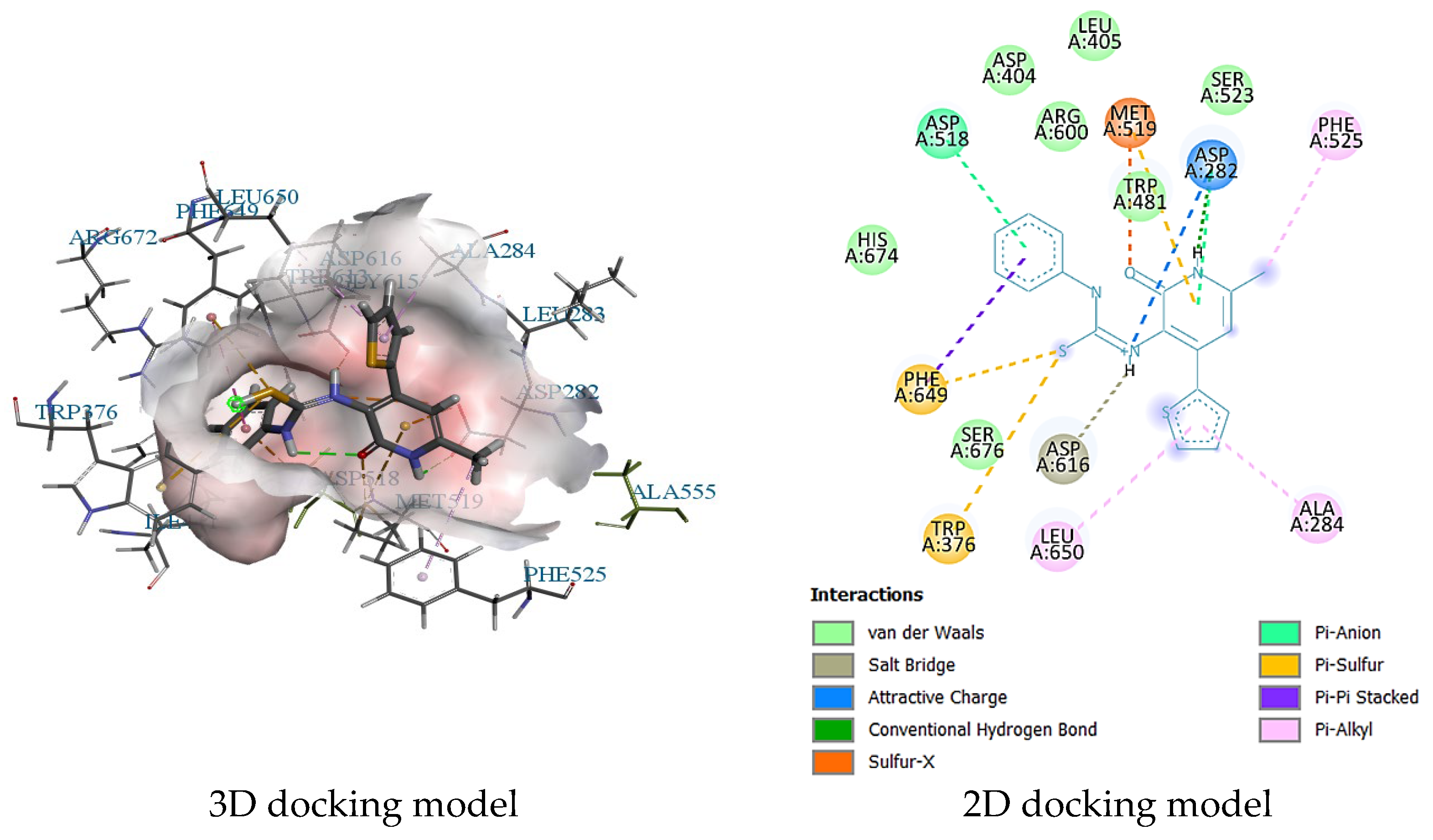
2.3. Molecular Docking
3. Experimental Procedures
3.1. Materials and Methods
3.2. Synthesis of Thiourea Derivatives: The General Methodology
3.3. Biological Tests
In Vitro Assay of α-Glucosidase Inhibitory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119–109125. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.statista.com/topics/1723/diabetes/ (accessed on 20 May 2024).
- Akhter, S.; Ullah, S.; Yousuf, S.; Wahab, A.; Siddiqui, H.; Choudhary, M.I. Synthesis, crystal structure and Hirshfeld Surface analysis of benzamide derivatives of thiourea as potent inhibitors of α-glucosidase in-vitro. Bioorg. Chem. 2021, 107, 104531–104539. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, S.T.; Gunal, S.E.; Ekizoglu, M.; Kelekci, N.G.; Erdem, S.S.; Bulak, E.; Frey, W.; Dogan, I. Thioureas and their cyclized derivatives: Synthesis, conformational analysis and antimicrobial evaluation. J. Mol. Struct. 2019, 1179, 40–56. [Google Scholar] [CrossRef]
- Bai, W.; Ji, J.; Huang, Q.; Wei, W. Synthesis and evaluation of new thiourea derivatives as antitumor and antiangiogenic agents. Tetrahedron Lett. 2020, 61, 152366–152372. [Google Scholar] [CrossRef]
- Korkmaz, N.; Obaidi, O.A.; Senturk, M.; Astley, D.; Ekinci, D.; Supuran, C.T. Synthesis and biological activity of novel thiourea derivatives as carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2015, 30, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Agili, F.A. Biological Applications of Thiourea Derivatives: Detailed Review. Chemistry 2024, 6, 435–468. [Google Scholar] [CrossRef]
- Riccardo, R.; Giada, M.; Andrea, C.; Antimo, G.; Emidio, C. Recent advances in urea- and thiourea-containing compounds: Focus on innovative approaches in medicinal chemistry and organic synthesis. RSC Med. Chem. 2021, 12, 1046–1064. [Google Scholar] [CrossRef]
- Krzywik, J.; Maj, E.; Nasulewics-Goldeman, A.; Mozga, W.; Wietrzyk, J.; Huczynski, A. Synthesis and antiproliferative screening of novel doubly modified colchicines containing urea, thiourea and guanidine moieties. Bioorg. Med. Chem. Lett. 2021, 47, 128197–128202. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://go.drugbank.com/drugs/DB00389 (accessed on 20 May 2024).
- Available online: https://go.drugbank.com/drugs/DB00550 (accessed on 20 May 2024).
- Available online: https://go.drugbank.com/drugs/DB00599 (accessed on 20 May 2024).
- Faidallah, H.M.; Al-Mohammadi, M.M.; Alamry, K.A.; Khan, K.A. Synthesis and biological evaluation of fluoropyrazolesulfonylurea and thiourea derivatives as possible antidiabetic agents. J. Enzym. Inhib. Med. Chem. 2016, 31 (Suppl. S1), 157–163. [Google Scholar] [CrossRef]
- Khan, I.; Rehman, W.; Rahim, F.; Hussain, R.; Khan, S.; Rasheed, L.; Alanazi, A.S.; Hefnawy, M.; Alanazi, M.M.; Shah, S.A.A.; et al. Synthesis, in vitro biological analysis and molecular docking studies of new thiadiazole-based thiourea derivatives as dual inhibitors of a-amylase and a-glucosidase. Arab. J. Chem. 2023, 16, 105078–105087. [Google Scholar] [CrossRef]
- Tok, F.; Cakir, C.; Çam, D.; Kirpat, M.M.; Sicak, Y. Synthesis, Characterization and biological evaluation of novel thiourea derivatives. Clin. Exp. Health Sci. 2022, 12, 533–540. [Google Scholar] [CrossRef]
- Dayma, V.; Chopra, J.; Sharma, P.; Dwivedi, A.; Tripathi, I.P.; Bhargava, A.; Murugesan, V.; Goswami, A.K.; Baroliya, P.K. Synthesis, antidiabetic, antioxidant and anti-inflammatory activities of novel hydroxytriazenes based on sulpha drugs. Heliyon 2020, 6, 4787–4796. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Patujo, J.; Mushtaq, I.; Ishtiaq, A.; Tahir, M.N.; Bibi, S.; Khan, M.S.; Ullah, N.; Mustafa, G.; Mirza, B.; et al. Anti-diabetic potential, crystal structure, molecular docking, DFT, and optical-electrochemical studies of new dimethyl and diethyl carbamoyl-N, N′-disubstituted based thioureas. J. Mol. Struct. 2022, 1253, 132207–132218. [Google Scholar] [CrossRef]
- Ovais, S.; Pushpalatha, H.; Reddy, G.B.; Rathore, P.; Bashir, R.; Yaseen, S.; Dheyaa, A.; Yaseen, R.; Tanwar, O.; Akthar, M.; et al. Synthesis and biological evaluation of some new pyrazoline substituted benzenesulfonylurea/thiourea derivatives as antihyperglycaemic agents and aldose reductase inhibitors. Eur. J. Med. Chem. 2014, 80, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Hassan, M.; Khan, K.M.; Sajid, M.; Umar, M.; Hassan, S.; Ullah, A.; El-Serehy, H.A.; Charifi, W.; Yasmin, H. Thiourea derivatives inhibit key diabetes-associated enzymes and advanced glycation end-product formation as a treatment for diabetes mellitus. Int. Union. Biochem. Mol. Biol. 2023, 75, 161–180. [Google Scholar] [CrossRef]
- Naz, S.; Zahoor, M.; Umar, M.N.; AlQahtany, F.S.; Elnahas, Y.M.; Ullah, R. In vivo glucose-6-phosphatase inhibitory, toxicity and antidiabetic potentials of 2-picolylamine thioureas in Swiss albino mice. Saudi J. Biol. Sci. 2020, 27, 3267–3273. [Google Scholar] [CrossRef] [PubMed]
- Kulakov, I.V.; Matsukevich, M.V.; Shulgau, Z.T.; Sergazy, S.; Seilkhanov, T.M.; Puzari, A.; Fisyuk, A.S. Synthesis and antiradical activity of 4-aryl(hetaryl)-substituted 3-aminopyridin-2(1H)-ones. Chem. Heterocycl. Compd. 2015, 51, 991–996. [Google Scholar] [CrossRef]
- Kusakabe, K.I.; Tada, Y.; Iso, Y.; Sakagami, M.; Morioka, Y.; Chomei, N.; Shinonome, S.; Kawamoto, K.; Takenaka, H.; Yasui, K.; et al. Design, synthesis, and binding mode prediction of 2-pyridone-based selective CB2 receptor agonists. Bioorganic Med. Chem. 2013, 21, 2045–2055. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Fan, X.; Chakaravarty, D.; Xiang, B.; Scannevin, R.H.; Huang, Z.; Ma, J.; Burke, S.L.; Karnachi, P.; Rhodes, K.J.; et al. 1-Hydroxy-2-pyridinone-based MMP inhibitors: Synthesis and biological evaluation for the treatment of ischemic stroke. Bioorganic Med. Chem. Lett. 2008, 18, 409–413. [Google Scholar] [CrossRef]
- Ward, A.; Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Amrinone: A Preliminary Review of its Pharmacological Properties and Therapeutic Use. Drugs 1983, 26, 468–502. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Wai, J.S.; Thomas, C.M.; Levin, R.B.; O’Brien, J.A.; Goldman, M.E. Synthesis and Evaluation of 2-Pyridinone Derivatives as HIV-1 Specific Reverse Transcriptase Inhibitors. 1. Phthalimidoalkyl and -alkylamino Analogs. J. Med. Chem. 1992, 35, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Saari, W.S.; Wai, J.S.; Fisher, T.E.; Thomas, C.M.; Hoffman, J.M.; Rooney, C.S.; Smith, A.M.; Jones, J.H.; Bamberger, D.L.; Goldman, M.E.; et al. Synthesis and Evaluation of 2-Pyridinone Derivatives as HIV-1-Specific Reverse Transcriptase Inhibitors. 2. Analogues of 3-Aminopyridin-2(1H)-one. J. Med. Chem. 1992, 35, 3792–3802. [Google Scholar] [CrossRef] [PubMed]
- Verissimo, E.; Berry, N.; Gibbons, P.; Cristiano, M.L.S.; Rosenthal, P.J.; Gut, J.; Ward, S.A.; O’Neill, P.M. Design and synthesis of novel 2-pyridone peptidomimetic falcipain 2/3 inhibitors. Bioorganic Med. Chem. Lett. 2008, 18, 4210–4214. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Hudson, T.H.; Kyle, D.E.; Lin, A.J. Synthesis and in vitro studies of novel pyrimidinyl peptidomimetics as potential antimalarial therapeutic agents. J. Med. Chem. 2002, 45, 3491–3496. [Google Scholar] [CrossRef] [PubMed]
- Kulakov, I.V.; Palamarchuk, I.V.; Shulgau, Z.T.; Seilkhanov, T.M.; Gatilov, Y.V.; Fisyuk, A.S. Synthesis, structure and biological activity 3-(arylmethyl)aminopyridine-2(1H)-ones and 1H-pyrido[2,3-b][1,4]oxazin-2(3H)-ones. J. Mol. Struct. 2018, 1166, 262–269. [Google Scholar] [CrossRef]
- Sergazy, S.; Shulgau, Z.; Zhulikeyeva, A.; Ramankulov, Y.; Palamarchuk, I.V.; Kulakov, I.V. Cytoprotective activity of newly synthesized 3-(arylmethylamino)-6-methyl-4-phenylpyridin-2(1H)-ones derivatives. Molecules 2022, 27, 5362–5379. [Google Scholar] [CrossRef]
- Palamarchuk, I.V.; Shulgau, Z.T.; Kharitonova, M.A.; Kulakov, I.V. Synthesis and neurotropic activity of new 3-(arylmethyl)aminopyridine-2(1H)-one. Chem. Pap. 2021, 75, 4729–4739. [Google Scholar] [CrossRef]
- Palamarchuk, I.V.; Shulgau, Z.T.; Dautov, A.Y.; Sergazy, S.D.; Kulakov, I.V. Design, synthesis, spectroscopic characterization, computational analysis, and in vitro α-amylase and α-glucosidase evaluation of 3-aminopyridin-2(1H)-one based novel monothiooxamides and 1,3,4-thiadiazoles. Org. Biomol. Chem. 2022, 20, 8962–8976. [Google Scholar] [CrossRef] [PubMed]
- Shulgau, Z.; Palamarchuk, I.V.; Sergazy, S.; Urazbayeva, A.; Ramankulov, Y.; Kulakov, I.V. Synthesis, Computational Study, and In Vitro α-Glucosidase Inhibitory Action of 1,3,4-Thiadiazole Derivatives of 3-Aminopyridin-2(1H)-ones. Pharmaceuticals 2024, 17, 377–391. [Google Scholar] [CrossRef]
- Kislyi, V.P.; Shestopalov, A.M.; Kagramanov, N.D.; Semenov, V.V. Synthesis of 3-nitropyrid-2(1H)-ones from C-nitroacetamide and 1,3-dicarbonyl compounds. Russ. Chem. Bull. 1997, 46, 539–542. [Google Scholar] [CrossRef]
- Kulakov, I.V.; Nikitina, O.S.; Fisyuk, A.S.; Goncharov, D.S.; Shul’Gau, Z.T.; Gulyaev, A.E. Synthesis and intramolecular cyclization of N-acyl- and N-allyl-N’-(2-oxo-1,2-dihydro-pyridin-3-yl)thiourea. Chem. Heterocycl. Compd. 2014, 50, 670–676. [Google Scholar] [CrossRef]
- Kulakov, I.V.; Nurkenov, O.A.; Turdybekov, D.M.; Ibragimov, B.T.; Talipov, S.A.; Zhambekov, Z.M.; Ainabaev, A.A.; Turdybekov, K.M. Synthesis of thiourea derivatives of the alkaloid anabasine and crystal structure of N-(anabasino1-thiocarbonyl)furan-2-carboxamide. Chem. Nat. Compd. 2009, 45, 209–212. [Google Scholar] [CrossRef]
- Kulakov, I.V.; Nurkenov, O.A.; Turdybekov, D.M.; Ainabaev, A.A.; Turdybekov, K.M.; Gazaliev, A.M. Synthesis and crystal structure of cytisino-N-(2-hydroxyethyl)-thiocarbamide. Chem. Nat. Compd. 2009, 45, 66–68. [Google Scholar] [CrossRef]
- Nasima, A.; Uzma, P.; Pervaiz, A.C.; Aamer, S.; Waseem, S.S.; Fouzia, P.; Aneela, J.; Hammad, I.; Muhammad, I.M.; Atteeque, A.; et al. Investigation of Newly Synthesized Bis-Acyl-Thiourea Derivatives of 4-Nitrobenzene-1,2-Diamine for Their DNA Binding, Urease Inhibition, and Anti-Brain-Tumor Activities. Molecules 2023, 28, 2707. [Google Scholar] [CrossRef] [PubMed]
- Shai, L.J.; Masoko, P.; Mokgotho, M.P.; Magano, S.R.; Mogale, A.M.; Boaduo, N.; Eloff, J.N. Yeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa. S. Afr. J. Bot. 2010, 76, 465–470. [Google Scholar] [CrossRef]
- Yamamoto, K.; Miyake, H.; Kusunoki, M.; Osaki, S. Crystal structures of isomaltase from Saccharomyces cerevisiae and in complex with its competitive inhibitor maltose. FEBS J. 2010, 277, 4205–4214. [Google Scholar] [CrossRef] [PubMed]
- Roig-Zamboni, V.; Cobucci-Ponzano, B.; Lacono, R.; Ferrara, M.C.; Germany, S.; Bourne, Y.; Parenti, G.; Moracci, M.; Sulzenbacher, G. Structure of human lysosomal acid α-glucosidase—A guide for the treatment of Pompe disease. Nat. Commun. 2017, 8, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.rcsb.org/ (accessed on 20 May 2024).
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comp. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef]
- Nipun, T.S.; Khatib, A.; Ibrahim, Z.; Ahmed, Q.U.; Redzwan, I.E.; Saiman, M.Z.; Supandi, F.; Primaharinastiti, R.; El-Seedi, H.R. Characterization of α-Glucosidase Inhibitors from Psychotria malayana Jack Leaves Extract Using LC-MS-Based Multivariate Data Analysis and In-Silico Molecular Docking. Molecules 2020, 25, 5885. [Google Scholar] [CrossRef] [PubMed]
- Junejo, J.A.; Zaman, K.; Rudrapal, M.; Celik, I.; Attah, E.I. Antidiabetic bioactive compounds from Tetrastigma angustifolia (Roxb.) Deb and Oxalis debilis Kunth.: Validation of ethnomedicinal claim by in vitro and in silico studies. S. Afr. J. Bot. 2021, 143, 164–175. [Google Scholar] [CrossRef]
- Discovery Studio. Dassault Systemes BIOVIA, Discovery Studio Modelling Environment, Release 4.5; Dassault Systemes: San Diego, CA, USA, 2015. [Google Scholar]
- Wei, S.; Abas, F.; Wai, K.; Yusoff, K. In Vitro and in Silico Evaluations of Diarylpentanoid Series as α-Glucosidase Inhibitor. Bioorg. Med. Chem. Lett. 2018, 28, 302–309. [Google Scholar]


| Compound | Structure | Inhibitory Activity of Test Compounds at a Concentration of 15 mM Against the α-Glucosidase Enzyme (%) | IC50 (mM) |
|---|---|---|---|
| 8a | 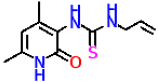 | 23.3 ± 1.7 | 16.64 ± 1.57 |
| 8b | 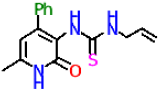 | 26.9 ± 2.5 | 19.79 ± 2.55 |
| 8c | 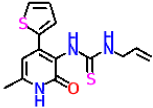 | No activity | No activity |
| 9a | 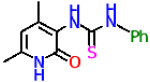 | 56.6 ± 2.2 | 9.77 ± 0.83 |
| 9b | 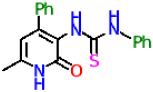 | 35.2 ± 3.4 | 21.79 ± 2.65 |
| 9c | 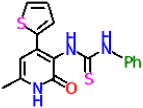 | 41.2 ± 2.5 | 12.94 ± 1.08 |
| 10a | 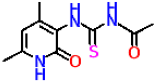 | No activity | No activity |
| 10b | 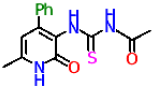 | No activity | No activity |
| 10c | 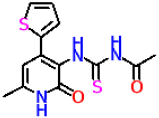 | No activity | No activity |
| 11a | 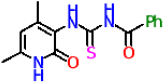 | No activity | No activity |
| 11b | 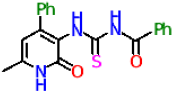 | No activity | No activity |
| 11c | 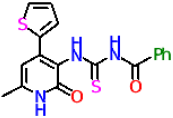 | No activity | No activity |
| Acarbose | 46.1 ± 4.6 | 11.96 ± 1.34 |
| Receptor | 3A4A | 5NN8 | |
|---|---|---|---|
| Ligand | |||
| Acarbose | −8.4 | −8.0 | |
| 8a | −6.8 | −5.8 | |
| 8b | −7.8 | −6.5 | |
| 8c | −6.6 | −6.0 | |
| 9a | −8.6 | −8.2 | |
| 9b | −7.6 | −7.2 | |
| 9c | −8.2 | −7.8 | |
| 10a | −6.9 | −6.2 | |
| 10b | −7.6 | −7.8 | |
| 10c | −6.9 | −6.8 | |
| 11a | −8.5 | −7.6 | |
| 11b | −8.3 | −7.8 | |
| 11c | −8.6 | −7.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shulgau, Z.; Palamarchuk, I.; Sergazy, S.; Urazbayeva, A.; Gulyayev, A.; Ramankulov, Y.; Kulakov, I. Synthesis, Computational Study, and In Vitro α-Glucosidase Inhibitory Action of Thiourea Derivatives Based on 3-Aminopyridin-2(1H)-Ones. Molecules 2024, 29, 3627. https://doi.org/10.3390/molecules29153627
Shulgau Z, Palamarchuk I, Sergazy S, Urazbayeva A, Gulyayev A, Ramankulov Y, Kulakov I. Synthesis, Computational Study, and In Vitro α-Glucosidase Inhibitory Action of Thiourea Derivatives Based on 3-Aminopyridin-2(1H)-Ones. Molecules. 2024; 29(15):3627. https://doi.org/10.3390/molecules29153627
Chicago/Turabian StyleShulgau, Zarina, Irina Palamarchuk, Shynggys Sergazy, Assel Urazbayeva, Alexander Gulyayev, Yerlan Ramankulov, and Ivan Kulakov. 2024. "Synthesis, Computational Study, and In Vitro α-Glucosidase Inhibitory Action of Thiourea Derivatives Based on 3-Aminopyridin-2(1H)-Ones" Molecules 29, no. 15: 3627. https://doi.org/10.3390/molecules29153627
APA StyleShulgau, Z., Palamarchuk, I., Sergazy, S., Urazbayeva, A., Gulyayev, A., Ramankulov, Y., & Kulakov, I. (2024). Synthesis, Computational Study, and In Vitro α-Glucosidase Inhibitory Action of Thiourea Derivatives Based on 3-Aminopyridin-2(1H)-Ones. Molecules, 29(15), 3627. https://doi.org/10.3390/molecules29153627







