Abstract
Synthetic radicals have intrinsic power for cascading and multifunctional reactions to construct diverse molecular scaffolds. In the previous review series, we covered 1,2-difunctionalizations, remote 1,3-, 1,4-, 1,5-, 1,6-, and 1,7-difunctionalizations, addition followed by cyclization reactions, and cycloaddition-initiated difunctionalizations. Presented in this paper are radical addition-initiated trifunctionalization reactions of alkenes, alkynes, and their derivatives. After the initial radical addition, there are different pathways, such as group or hydrogen atom transfer, cyclization, and radical coupling, to complete the second and third functionalizations.
Keywords:
radical; trifunctionalization; alkene; alkyne; addition; cyclization; atom transfer; group transfer 1. Introduction
Highly reactive synthetic radicals are feasible in making carbon–carbon and carbon–heteroatom bonds via a broad scope of transformations, including addition, cyclization, coupling, rearrangement, fragmentation, atom or group transfer, and single electron oxidation/reduction reactions [1,2]. Cascade radical transformations have intrinsic power in the construction of diverse molecular scaffolds and for incorporating multiple functional groups into the products. In addition to traditional methods for conducting radical reactions [3,4,5,6,7,8,9], recent developments in photoredox reactions [10,11,12,13], electrochemical reactions [14,15], and mechanoredox reactions [16] have paved new ways for synthetic radicals. From reaction efficiency, product diversity, and green chemistry considerations, cascade reactions and difunctionalization have a high pot-, atom-, and step-economy [17,18,19]. There are a number of reviews on radical difunctionalization reactions [20,21,22,23,24,25,26,27,28,29,30,31], including our recent articles on 1,2-difunctionalization of alkenes and alkynes (Scheme 1I) [32], remote 1,3-, 1,4-, 1,5-, 1,6-, and 1,7-difunctionalization of alkenes and alkynes (Scheme 1II) [33], addition/cyclization sequence of dienes, enynes, and related compounds (Scheme 1III) [34], and cyclization-initiated sequence for making cyclic compounds (Scheme 1IV) [35]. This paper covers radical trifunctionalization reactions reported in the last decade, while most papers were published in the last five years.
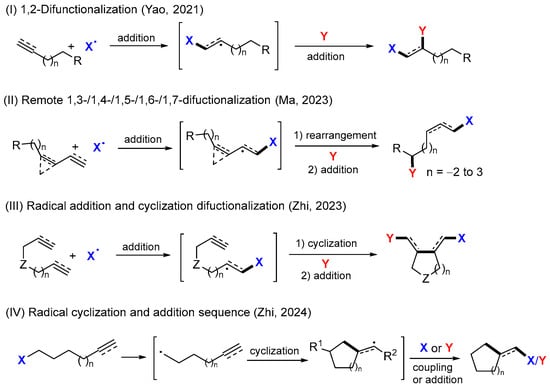
Scheme 1.
Radical difunctionalization reactions [32,33,34,35].
The radical trifunctionalization presented in this paper cannot be summarized by a single reaction scheme like that for the difunctional reactions shown in Scheme 1. Three representative radical trifunctionalization reactions are shown in Scheme 2. Alkenols or alkynols are employed as substrates in the Type-I reaction. The initial radical addition introduces X as the first group, followed by the atom or group transfer of Y as the second group. Oxidation of hydroxyl-carbon to carbonyl introduces the third group. The Type-II reaction employs alkynes as substrates. The addition of X radicals to alkynes, followed by 1,5-HAT (hydrogen atom transfer), cyclization, and coupling with Y, affords trifunctionalized products. The Type-III reaction employs alkenes or alkynes as substrates. The addition of X radicals, followed by oxygen trapping of the radical intermediates and enol functionalization with Y, gives the trifunctionalized products. The Type-III reaction could be extended to allenes and enynes. It is noteworthy that three new bonds generated during the trifunctionalization are shown in bold style in the products (Scheme 2).
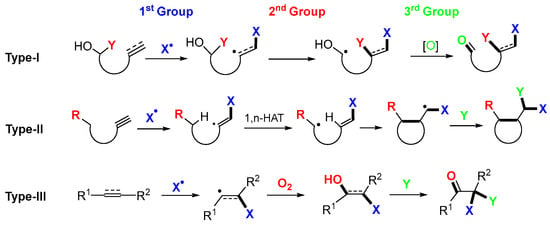
Scheme 2.
Three representative radical trifunctionalization reactions.
Some popular substrates for trifunctionalization, including alkenols/alkynols, alkenes/allenes, alkenylnitriles, and alkynoates, are shown in Figure 1.
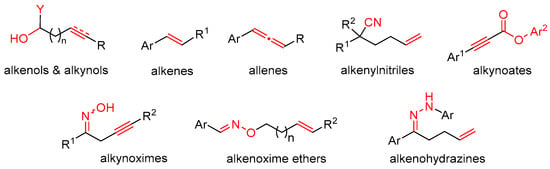
Figure 1.
Representative substrates for radical trifunctionalization reactions.
2. Reaction of Alkenols and Alkynols
Trifunctionalization of alkenols and alkynols are Type-I reactions shown in Scheme 3. They involve the addition of radical Y to form radicals I-A, followed by Y group migration to give radicals II-A, and the oxidation of OH to the carbonyl group (Scheme 3). In the reaction process, the key step is the Y group transfer. The Y group could be aryl, alkynyl, -CN, -CHO, phosphinoyl (-POR2), and sulfonyl (-SO2R) groups. It is noteworthy that functional group migration (FGM) could be a rearrangement process involving radical cyclization and cleavage. In the case of aryl migration, it is an ipso-cyclization/cleavage reaction process.
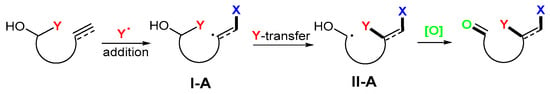
Scheme 3.
Trifunctionalization of Y-substituted alkenols and alkynols.
2.1. Reaction of Alkenols
The Zhu group is one of the pioneers who made contributions to the trifunctionalization of alkenols and alkynols [36,37]. They reported the first example of the trifunctionalization of alkenyl cyanohydrins in 2016 (Scheme 4) [38]. The key step of CN-transfer could be 1,3- to 1,6-shifts, but only 1,4- and 1,5-shifts give meaningful yields.
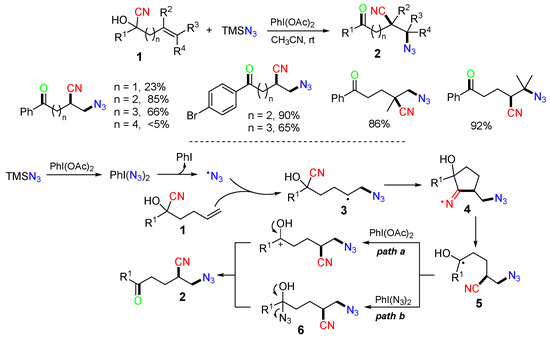
Scheme 4.
Reaction of alkenyl cyanohydrins involving CN shift.
The mechanism proposed in Scheme 4 suggested that the azido radical generated from the interaction of trimethylsilyl azide (TMSN3) with PhI(OAc)2 adds to the C=C bond of alkenyl cyanohydrins 1 to afford alkyl radicals 3, which then shifts the CN group through cyclic iminyl radicals 4 to form radicals 5. There could be two ways to convert radicals 5 to products 2: via oxidation with PhI(OAc)2 or through the formation of azidohydrins 6.
The Zhu group reported a method for distal heteroaryl shift in 2017 (Scheme 5) [39]. This method is good for 1,4-aryl shift since the migration via 5-membered ipso-radical intermediates is more favorable than other ring sizes. In the reaction process, a CF3 radical generated from the Langlois’ reagent (CF3SO2Na) and phenyliodine bis(trifluoroacetate) (PIFA) add to the C=C bond of hydroxy-substituted alkenes 7 to give alkyl radical intermediates 9, which then undergo 1,4-shift of the aryl group to form more stable hydroxyalkyl radicals 10, followed by oxidization to give products 8.
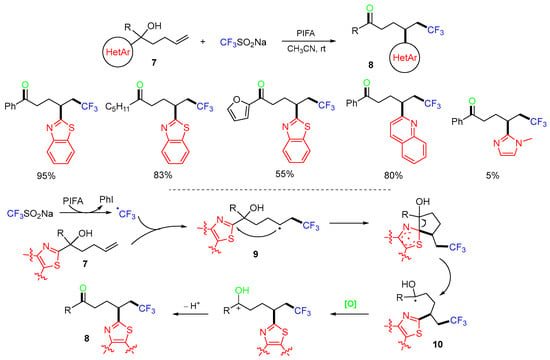
Scheme 5.
Reaction of heteroaryl alkenols involving heteroaryl shift.
The Zhu group extended the reaction scope for carbonyl migration (Scheme 6) [40]. The photoredox reaction of α-formyl-substituted alkenols 11 with fluoroalkyl bromides afforded formyl-migrated trifunctional products 12.
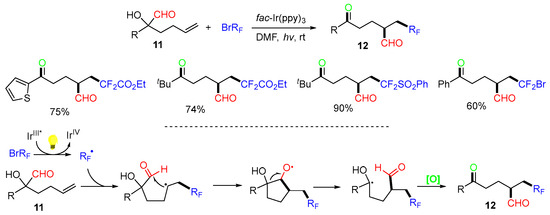
Scheme 6.
Reaction of α-carbonyl tertiary alcohols involving carbonyl shift.
A FeII-catalyzed reaction of alkenols 13 involving heteroaryl group shift to give 1,6- and 1,7-diketons 14 was reported by the Li group in 2020 (Scheme 7) [41]. The proposed mechanism suggested that the tBuO radical generated from di-tert-butyl peroxide (DTBP) in the presence of FeII abstracts hydrogen from aldehydes to afford acyl radicals 15. The addition of acyl radicals to the alkene moiety of alkenols 13 produces alkyl radicals 16, followed by intramolecular radical ipso-migration and FeIII-catalyzed oxidation to give products 14.
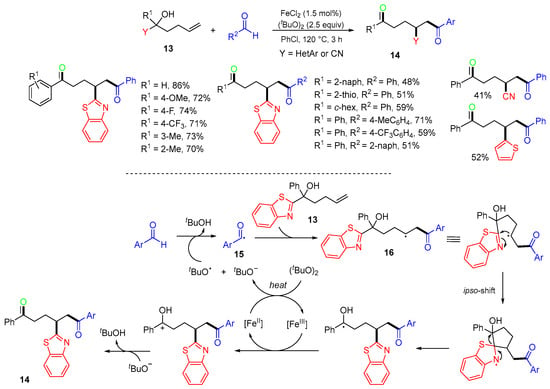
Scheme 7.
Fe-catalyzed reaction of alkenols involving heteroaryl migration.
An electrochemical reaction of alkenyl cyanohydrins 17 for making azidocyanated products 18 was reported by the Morrill group in 2022 (Scheme 8) [42]. The proposed mechanism suggested that the [MnIIX2N3]− generated from the interaction of MnIIX2 with NaN3 is oxidized at the graphite anode to give MnIIIX2N3, which delivers the azido radicals. The addition of azido radicals to alkene 17 to give secondary alkyl radicals 19, followed by cyclization, affords cyclic iminyl radicals 20. β-Scission of radicals 20 gives α-hydroxy alkyl radicals 21, which are oxidized at the anode to give final products 18. Hydrogen gas evolves through proton reduction at the cathode.
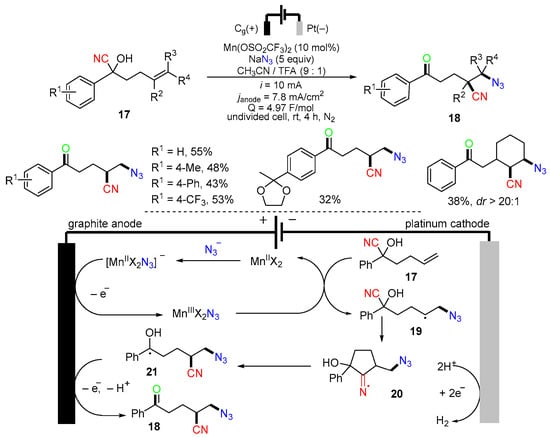
Scheme 8.
Electrochemical reaction alkenyl cyanohydrins involving 1,4-CN migration.
A three-component reaction of heteroaryl-substituted alkenols 22, aryldiazonium tetrafluoroborates, and 1,4-diazabicyclo [2.2.2]octane (DABCO)·(SO2)2 was reported by Wu’s group in 2021 [43]. Under catalyst-, base-, and additive-free conditions, the reaction gave products 23 in moderate to good yields (Scheme 9). The proposed mechanism suggested that sulfonyl radicals 24 generated from aryldiazonium tetrafluoroborate and DABCO·(SO2)2 undergo radical addition to alkenols 22 to afford carbon radicals 25, followed by cyclization to give the radicals 26. Ring-opening of radicals 26 forms more stabilized radicals 27, followed by oxidation to give products 23.
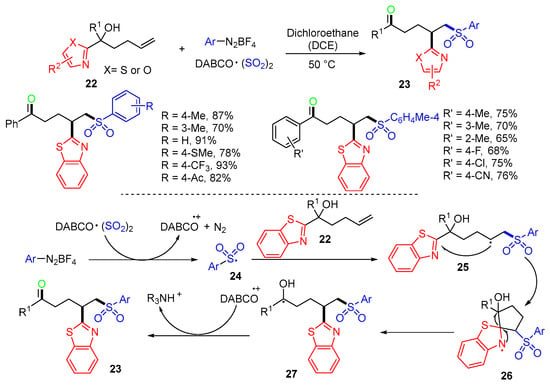
Scheme 9.
Reaction of alkenols involving heteroaryl shift.
Very recently, the Guo group reported a photoredox reaction of alkenols 28, DABCO·(SO2)2, and thianthrenium salts for the synthesis of alkylsulfonylated ketones 29 (Scheme 10) [44]. In the reaction process, phenethyl radicals generated from the thianthrenium are captured by SO2 from DABCO·(SO2)2 to generate phenethylsulfonyl radicals 30. The addition of radicals 30 to the terminal C=C bond carbon of tertiary alcohol 28 affords intermediates 31, which go through a five-membered cyclic transition state to give spiro N-radical 32. The ring opening of unstable spiro structures 32 gives more stable hydroxyalkyl radicals 33, which are oxidized to give final products 29 after deprotonation.
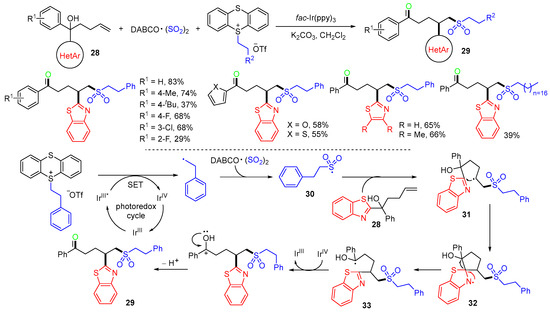
Scheme 10.
Photoredox reaction of alkenols involving heteroaryl shift.
A photoredox reaction for the synthesis of amino ketones was reported by the Xu group in 2022 [45]. The Cz-NI-catalyzed reaction of alkenols 34 with N-protected 1-aminopyridinium under LED irradiation afforded distal amino ketones 35 in moderate to excellent yields (Scheme 11). In the reaction process, the N-centered radical 36 generated from the N-protected 1-aminopyridinium adds to the C=C bond of hydroxyl-substituted alkenes 34 to give alkyl radical intermediates 37, which then undergo 1,4-shift of the heretoaryl group to give more stable hydroxyalkyl radicals 38, followed by oxidization and deprotonation to give the final products 35.
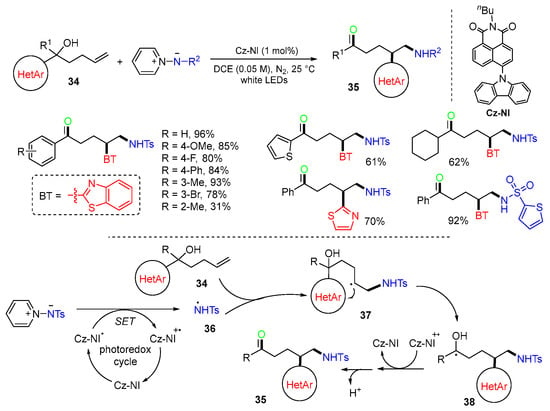
Scheme 11.
Photoredox reaction of alkenols involving heteroaryl shift.
The Feng group, in 2021, reported a straightforward photoredox reaction of alkenols 39 for the preparation of phosphonylated ketones 40, which undergo spontaneous Horner−Wadsworth−Emmons (HWE) olefination under a basic condition to give compounds 41 (Scheme 12) [46]. In the reaction process, single-electron transfer (SET) oxidation of bromophosphonates by photoexcited fac-Ir(ppy)3 generates radical 42, followed by addition to the terminal alkene of alkenols 39 to provide alkyl radical intermediates 43. The Y group 1,4-shift of radical 43 gives more stable hydroxyalkyl radicals 44, which are oxidized to give final products 40 after deprotonation.
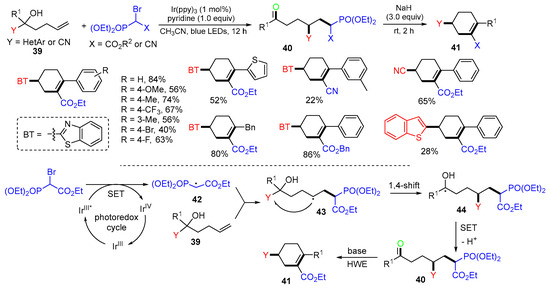
Scheme 12.
Synthesis of intramolecular HWE reaction.
The reaction of 1,4-enyn-3-ols 45 with alkylaldehydes for the synthesis of α,β-functionalized ketones 46 was reported by the Xiang group in 2021 (Scheme 13) [47]. Alkyl radicals, generated from the decarbonylation of alkylaldehydes using DTBP as an oxidant, add to the C=C bond of 1,4-enyn-3-ols 45, followed by an alkynyl shift through the three-membered ring intermediates 47 to give radicals 48. Oxidation of 48 with DTBP and deprotonation give products 46. A similar reaction for the synthesis of α-alkynyl ketones was reported by the Tu group [48].
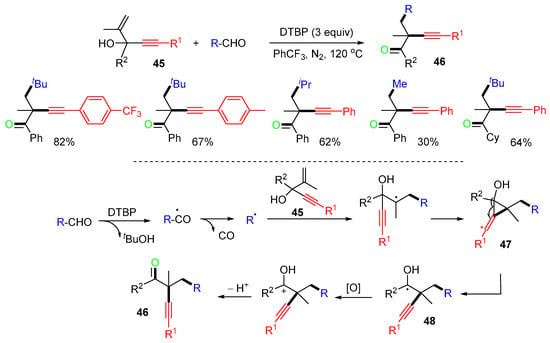
Scheme 13.
Reaction of 1,4-enyn-3-ols with alkylaldehydes.
The reaction of 1,4-enyn-3-ols 49 with nitriles for the synthesis of cyanolated ketones 50 was reported by Cheng and coworkers in 2021 (Scheme 14) [49]. The alkyl radicals generated from the reaction of nitriles using DTBP as an oxidant afforded products 50 in moderate yields.
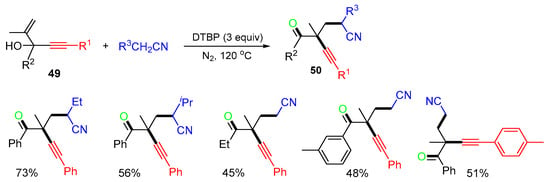
Scheme 14.
Reaction of 1,4-enyn-3-ols with alkyl nitriles.
The Chu group, in 2021, reported a reaction of diaryl allylic alcohols involving 1,2-aryl migration [50]. The reaction of 1,1-diarylprop-2-en-1-ols 51 and BrCF2CO2Et in the presence of sodium hydrosulfite as a radical initiator afforded products 52 in moderate to good yields (Scheme 15). In the reaction process, the CF2CO2Et radical generated from BrCF2CO2Et adds to the C=C bond of diaryl allylic alcohols 51 to form the alkyl radicals 53. Then, 1,2-aryl migration of 53 via the spiro[2,5]octadienyl radical intermediates 54 leads to the α-hydroxy carbon-centered radicals 55, which undergo a SET process to produce the final products 52 along with the regeneration of the CF2CO2Et radical. Some related methods for the radical trifunctionalization of diaryl allylic alcohols via 1,2-aryl migration by metal catalysis could be found in recent reviews [51,52].
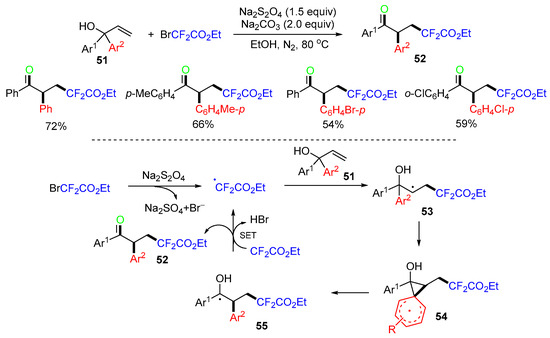
Scheme 15.
Reaction of allylic alcohols with ethyl bromodifluoroacetate.
The Han group introduced a trifunctionalization reaction involving a phosphinoyl group shift in 2022 [53]. The reaction of homoallylic alcohols 56, R2PCl, and fluoroalkyl iodides (RF-I) under the irradiation of visible light afforded products 57 in good to excellent yields (Scheme 16). The proposed mechanism suggested that the nC4F9 radical generated from nC4F9-I adds to the C=C bond of 58 to afford alkyl radicals 59. Radical cyclization onto the phosphine affords cyclic phosphine radicals 60, followed by β-fragmentation of the C–O bond to form C-radicals 61, which are trapped by nC4F9-I through an iodine-atom transfer process to give products 57.
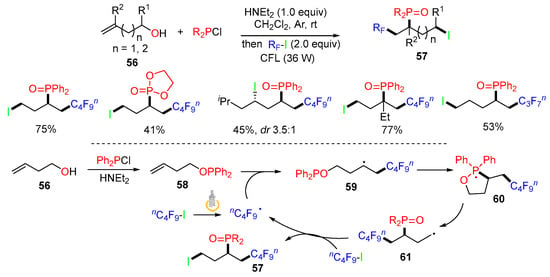
Scheme 16.
Reaction of alkenols involving phosphinoyl group shift.
In 2022, Molander and coworkers reported a photoredox reaction of cinnamyl alcohols 62 with ethyl bromodifluoroacetate under blue LED irradiation to give brominated α,α-difluoro-γ-lactones 63 in moderate to good yields in a diastereoselective manner (Scheme 17) [54]. In the reaction process, the CF2CO2Et radical generated from BrCF2CO2Et adds to the C=C bond of cinnamyl alcohols 62, followed by lactonization and the Br-atom transfer process to give products 63.
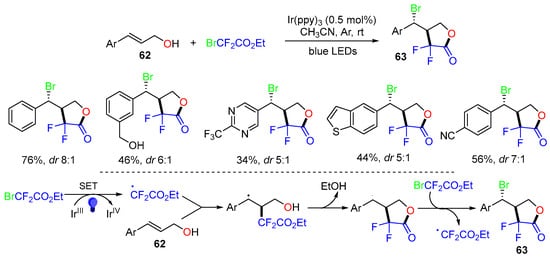
Scheme 17.
Trifunctionalization of cinnamyl alcohols with ethyl bromodifluoroacetates.
A photoredox reaction of heterocyclic-substituted azidyl homoallylic alcohols 64 with cycloketone oxime esters was reported by the Guo group in 2021 [55]. The reaction via 1,4-heteroaryl migration from the C-center to the N-center affords a variety of cyanoalkylacylated β-enamino ketone 65 in moderate-to-good yields under continuous-flow conditions (Scheme 18). The reaction process involves the addition of cyanoalkyl radical 66 to the C=C bond of homoallylic alcohols 64 with the loss of N2, followed by the C-center to the N-center heteroaryl migration through the key spiro radicals 67, single electron oxidation, and further deprotonation to give products 65.
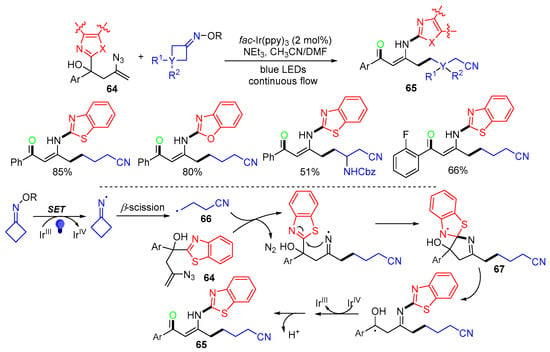
Scheme 18.
Reaction of azidyl homoallylic alcohols with cycloketone oxime esters.
In 2022, the Ye group introduced an electrochemical reaction of alkenols 68 with Na2S2O5 and MeOH for the synthesis of ketosulfonate esters 69 (Scheme 19) [56]. The sulfite radical anion, generated from the anode oxidation of Na2S2O5, is trapped by MeOH to afford the methoxysulfonyl radical, which then adds to the C=C bond of alkenols 68 to yield radicals 70. Functional group migration (FGM), anodic SET oxidation, and deprotonation afford products 69.
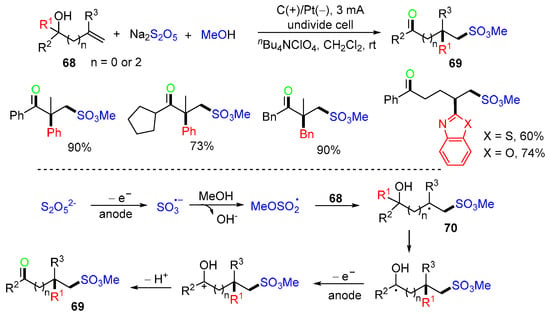
Scheme 19.
Electrochemical reaction of alkenols with Na2S2O5 and MeOH.
2.2. Reaction of Alkynols
Alkynol-based radical reactions are presented in this section. The addition of a radical to the double bond of alkenols generates alkyl radical intermediates, while the addition of a radical to the triple bond of alkynols generates more reactive vinyl radical intermediates, which could be more favorable for FGM as well as HAT in the trifunctionalization reactions.
In 2014, Liang’s group reported a Cu-catalyzed reaction of homopropargylic alcohols 71 with Togni’s II reagent for the synthesis of CF3-containing 3-buten-1-one derivatives 72 (Scheme 20) [57]. The proposed mechanism suggested that the CF3 radical, generated from the reaction of Togni’s II reagent and CuI species, adds to the alkyne of homopropargylic alcohols 71 to give radicals 73, followed by 5-ipso cyclization to form radicals 74, and then radicals 75 for 1,4-aryl migration. Finally, single-electron oxidation of radical 75 with CuII species furnishes product 72 and a regenerated CuI catalyst.
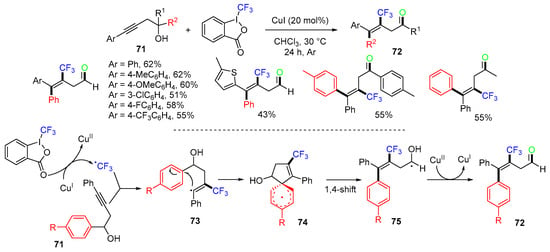
Scheme 20.
Cu-catalyzed reaction of alkynols involving 1,4-aryl migration.
In 2019, the Zhu group reported a CF3 radical addition-initiated reaction of alkynols 76 for the synthesis of CF3-containing 5-hexen-1-ones 77 under mild photoredox reaction conditions (Scheme 21) [58]. The proposed mechanism suggested that the addition of CF3 radicals to alkynols 76 generates alkenyl radicals 78, which undergo 1,5-HAT to produce alkyl radicals 79. Cyclization of alkyl radicals 79 affords cyclopentanol intermediates 80, which undergo C–C bond cleavage to give stereoselective E-olefins 77.
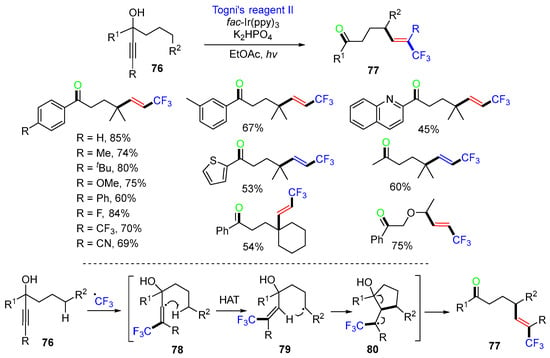
Scheme 21.
Regioselective reaction of alkynols involving 1,5-HAT.
The Zhu group, in 2017, reported a carbodifluoroalkylation reaction of homopropargylic alcohols 81 under photoredox conditions (Scheme 22) [59]. In this reaction, BrCF2CO2Et is used as a source for difluorination. The proposed mechanism suggested that the active state IrIII* generated from photocatalyst fac-Ir(ppy)3 reacts with BrCF2CO2Et through a SET to give radical 83, which adds to the alkyne moiety of homopropargylic alcohols 81 to give radicals 84, followed by 5-ipso cyclization and cleavage of radicals 85 to give hydroxyalkyl radicals 86. SET oxidation and then deprotonation of radicals 86 give products 82.
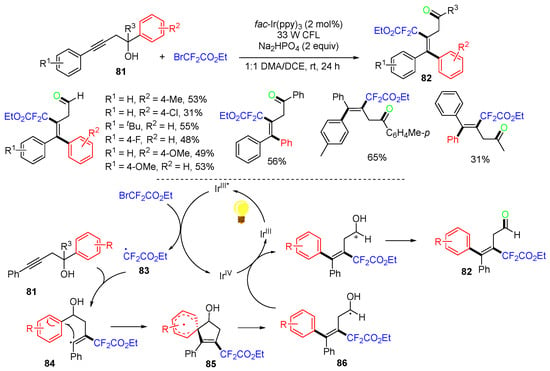
Scheme 22.
Reaction of homopropargylic alkenols involving 1,4-aryl migration.
A photoredox reaction of propargyl alcohols 87, K2S2O5, and cycloketone oxime esters for the synthesis of cyanoalkylated vinyl sulfone 88 was reported by the Wu group in 2021 (Scheme 23) [60]. The initial step of the synthesis is the irradiation of Eosin Y under blue light to form excited Eosin Y*, which undergoes SET with cycloketone oxime ester to form iminyl radical 89 and then cyanoalkyl radical 90 after the C–C bond cleavage. The reaction of radical 90 with K2S2O5 leads to the formation of cyanoalkylsulfonyl radical 91, which adds to propargyl alcohols 87 to form radicals 92. 1,5-HAT of vinyl radicals 92 results in alkyl radicals 93, which undergo cyclization to give radicals 94. The C–C bond cleavage of 94 gives vinyl-migrated radicals 95, which undergo SET with Eosin Y•+ to give carbocations 96 and then products 88 after deprotonation.
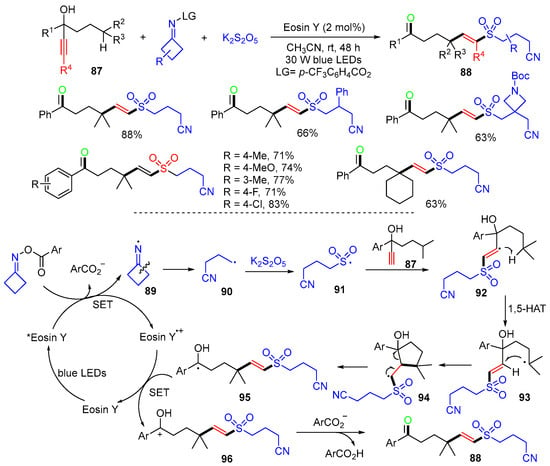
Scheme 23.
Reaction of alkenols involving 1,4-vinyl migration.
2.3. Reaction of Alkenol Derivatives
Other than the reactions of alkenols and alkynols presented above, alkenol derivatives could be used for the trifunctionalization reactions.
In 2016, the Liu group reported the CN migration reaction of TMS-protected alkenyl cyanohydrins 97. The reaction process involves the addition of R radicals (CF3, N3, P(O)Ph2, C4F9, and Ts radicals), remote CN group migration, and converting of OTMS to carbonyl group to afford cyano ketones 98 (Scheme 24) [61].
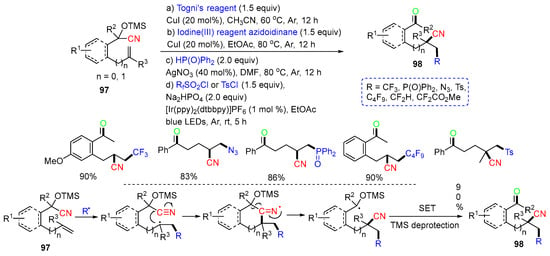
Scheme 24.
Reaction of TMS-protected alkenyl cyanohydrins involving CN migration.
A Cu-catalyzed aminoheteroarylation of alcohol-, amino-, and ether-containing alkenes 99 was reported by the Liu group in 2021 [62]. The second functional group, X, was derived from X’ (OH, OMe, or NHR). The reaction process involves the addition of amino radicals generated from the BzONR2R3 to the C=C bond of alkene 99, followed by heteroaryl migration, SET, and deprotonation to afford the final product 100 (Scheme 25).
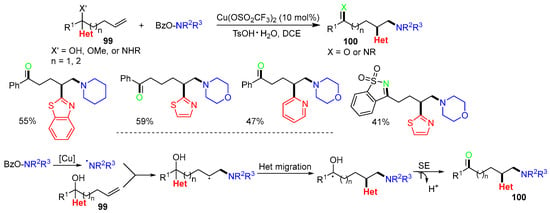
Scheme 25.
Reaction of functionalized alkenes involving heterocyclic group migration.
A photoredox-catalyzed reaction of α-aryl allyl alcohol TMS ethers 101 for the preparation of difluoromethyl-containing ketones 102 was reported by the Dolbier group in 2022 (Scheme 26) [63]. The proposed mechanism suggested that the CF2H radical derived from HCF2SO2Cl adds to the terminal carbon of alkenes 101 to form radical 103, followed by aryl ipso-migration to give radicals 104 and Ir-catalyzed oxidative SET to afford products 102 after deprotection. It is noteworthy that if there are two aryl groups that could undergo migration, the rr is used to indicate the selectivity of two aryl groups.
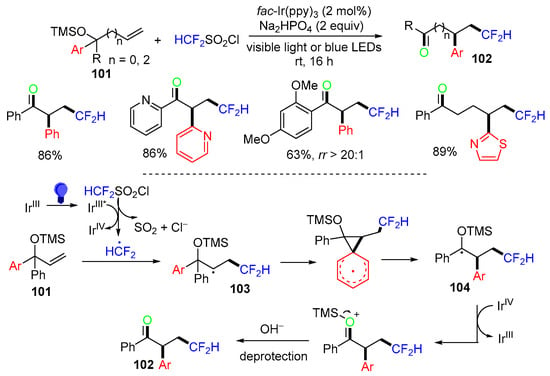
Scheme 26.
Reaction of TMS-protected alkenols involving Ar migration.
A photoredox reaction of α-aryl allyl alcohol TMS ethers 105 with gem-bromonitroalkanes for the synthesis of α-aryl-γ-nitro ketones 106 was reported by the Jiao group in 2023 (Scheme 27) [64]. The reaction process involves the addition of α-nitrocyclobutyl radicals, generated from the reaction of gem-bromonitrocylcobutane with photoexcited fac-*Ir(ppy)3 and Ag+ to the C=C bond of 105, to generate radicals 107, which undergo aryl ipso-migration to give α-trimethylsilyloxy radical 108, followed by SET oxidation to give products 106 after deprotection of TMS.
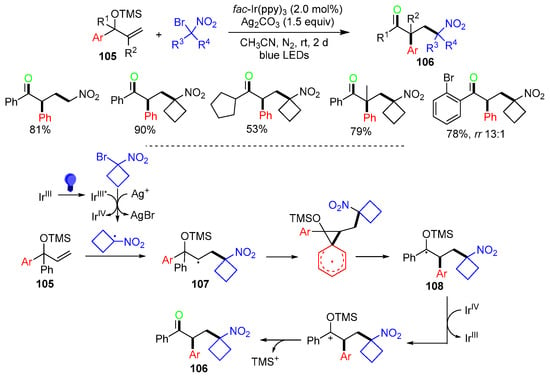
Scheme 27.
Reaction of α-aryl allyl alcohol TMS ethers.
A radical reaction of alkenyl anilines 109 and 1-azido-2-isocyanoarenes involving denitrogenative radical cyclization for making 2-substituted benzoimidazoles 110 was reported by the group in 2017 (Scheme 28) [65]. The reaction process involves the generation of azidal radicals 111, releasing nitrogen gas, followed by radical cyclization to form radicals 112. Radicals 112 add to 1-azido-2-isocyano-3,5-dimethylbenzene, followed by denitrogenative cyclization to form benzoimidazole radicals 113, and then products 110 after HAT.
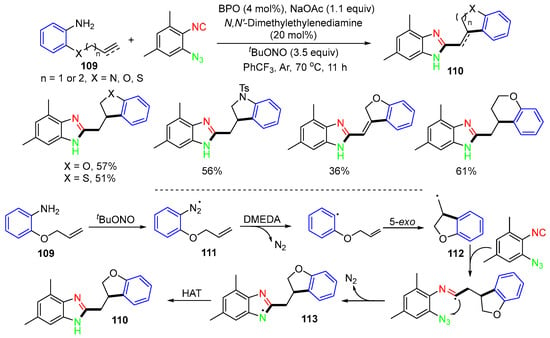
Scheme 28.
Reaction of 2-(allyloxy)-aniline for the synthesis of 2-substituted benzoimidazoles.
3. Reaction of Alkenes and Allenes
The trifunctionalization reaction of non-activated alkenes generally undergoes the Type-III pathway shown in Scheme 2. The initial radical addition gives the intermediate radicals I-B, which are oxidized to carbon cations and then react with O2, H2O, RCN, or other species to introduce the second and third functional groups (Scheme 29A). The reaction of functionalized alkenes could be designed for 1,2-group migration (1,2-GM), such as that shown in Scheme 29B. In the racial addition of allenes, a radical added to the terminal carbon of allenes generates vinyl radicals (like the reaction of alkynes), while a radical added to the middle carbon of allenes generates alkyl radicals (similar to the reaction of alkenes).
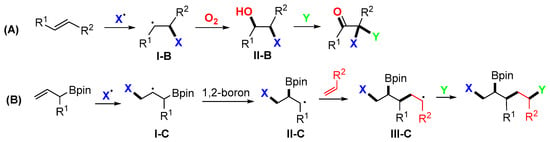
Scheme 29.
Representative pathways for trifunctionalization of alkenes.
3.1. Reaction of Alkenes
A visible-light-induced reaction of alkenes 114 with oxime esters for making functionalized ketones 115 was reported by Ma and coworkers in 2018 (Scheme 30) [66]. The reaction involves the generation of iminyl radicals, which are rearranged to alkyl radicals 116 via 1,5-HAT. The first functionalization of alkene 114 is the addition of alkyl radicals 116, followed by SET oxidation to cations 117, which react with H2O, followed by the hydrolysis of the emine group to afford products 115.
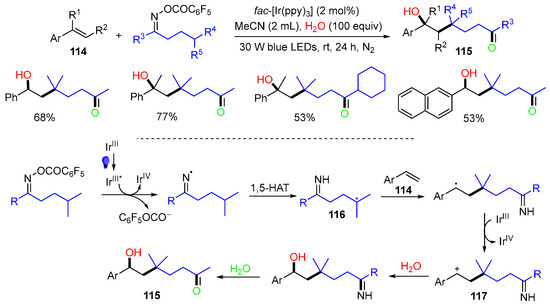
Scheme 30.
Reaction of aryl alkenes with oxime esters.
Another iminyl radical-initiated reaction of alkenes 118 was reported by the Golagani group in 2023 (Scheme 31) [67]. Under the catalysis of ferrocene, homolytic N–O bond cleavage of cycloketone oxime esters gives cyclic iminyl radicals, which convert to the CN-alkyl radical 120 after ring opening. The addition of the radical 120 to the terminal position of the aryl alkene 118 gives radicals 121, followed by SET oxidization and then nucleophilic attack by nitriles to give 122, which undergo Mumm rearrangement to give products 119.
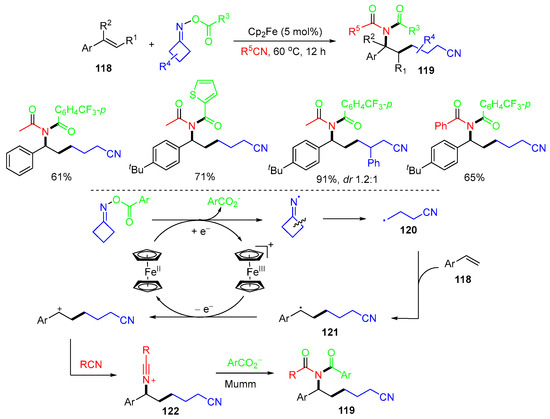
Scheme 31.
Reaction of alkenes with cycloketone oxime esters.
In 2018, the Xu group introduced a Cu-catalyzed reaction of alkenes 123 for the synthesis of functionalized β-keto thiosulfones 124. The arylsulfonyl radical derived from PhSO2SR adds to styrenes 123, followed by Cu-assisted oxidation with O2 gives 125. Formation of Cu-complex 126 followed by reductive elimination of L[CuI] affords products 124 (Scheme 32) [68].
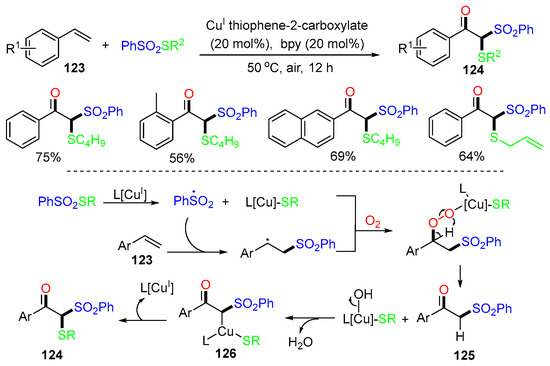
Scheme 32.
Reaction of styrenes for making S-alkyl benzenesulfonothioates.
A method for the reaction of ethylene 127 with bifunctional sulfonyl reagents and electron-deficient heterocyclic compounds for the synthesis of diheteroarylated products 128 was reported by the Zhu group in 2023 (Scheme 33) [69]. In the reaction process, difluoromethyl radicals 129 derived from a sulfonyl reagent are added to ethylene 127 to afford alkyl radical 130, which undergoes desulfonylative 1,2-heterocyclic group migration via ipso-rearrangement to give 131. The Minisci-type addition of 131 to quinoxalinone affords product 128 after rearomatization.
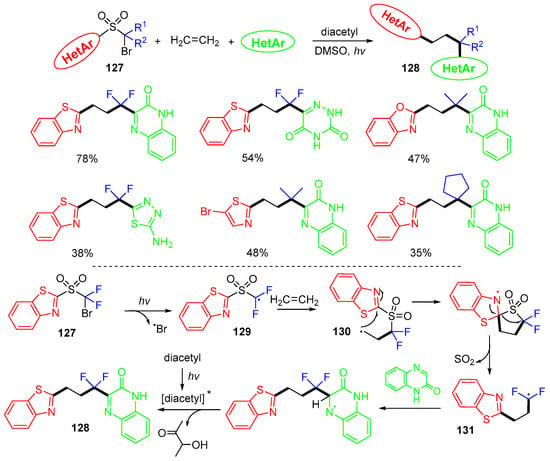
Scheme 33.
Reaction of ethylene involving desulfonylative 1,2-heterocyclic group migration.
3.2. Reaction of Functionalized Alkenes
Other than the Type-III radical trifunctionalization reaction of alkenes shown above, there are a couple of special cases involving the 1,2-group migration. In 2023, the Glorius group reported a reaction involving 1,2-boron shift of alkenes 132 and imine-based bifunctional reagents for making 1,2,5-trifunctionalized products 133 (Scheme 34) [70]. Under photoredox conditions, mine-based reagents release X and iminyl radicals. The X radicals add to allylboronic esters 132, followed by a 1,2-boron shift to form radicals 134. Then, the addition of radicals 134 to the second alkenes, followed by coupling with an iminyl radical, gives products 133.
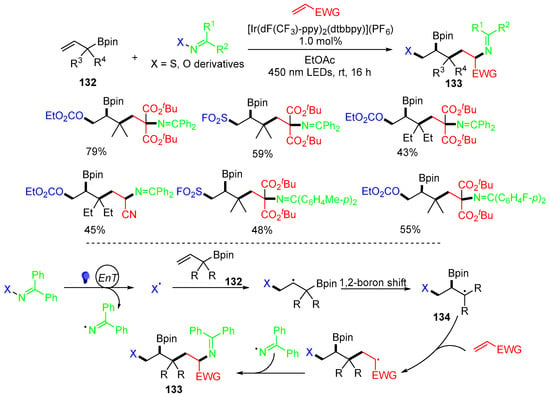
Scheme 34.
Reaction of alkenes involving 1,2-boron shift.
Duan and coworkers, in 2013, reported a reaction of hydrazones 135 with radical trapping agents TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) or DIAD (diisopropyl azodicarboxylate) to afford functionalized pyrazolines 136 with good stereoselectivity (Scheme 35) [71]. In the reaction involving TEMPO, initial hydrazone radicals are generated by the reaction of hydrazone 135 with TEMPO. The radicals undergo 1,5-HAT to form allyl radicals 137, which are trapped by TEMPO, followed by hydrogen atom abstraction with TEMPO to generate second hydrazone radicals 138. The 5-exo-trig cyclization of 138 generates pyrazoline-containing radicals, which are trapped by TEMPO to give products 136. It is noteworthy that TEMPO is used as a hydrazone radical initiator as well as a carbon radical scavenger.
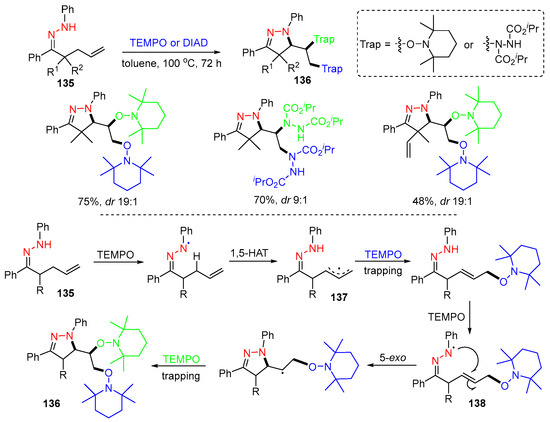
Scheme 35.
Reaction of alkenyl hydrazones with TEMPO or DIAD.
The Wang group, in 2021, reported a visible-light-promoted radical sulfonyl–Smiles rearrangement reaction of butenyl benzothiazole sulfone 139 with thiosulfonates or selenosulfonates for making 1,2,4-trifunctionalized butane products 140 (Scheme 36) [72]. The proposed mechanism suggested that the sulfonyl radical derived from thiosulfonates adds to the terminal double bond of butenyl benzothiazole sulfones 139 to form intermediate radicals 141. Ipso-radical cyclization of 141 followed by ring-opening and desulfonylation afford heteroaryl group migrated radicals 142. Trapping 142 by the phenylthio radical affords products 140. Other heterocyclic groups containing butenyl sulfones could be used for this reaction [73].
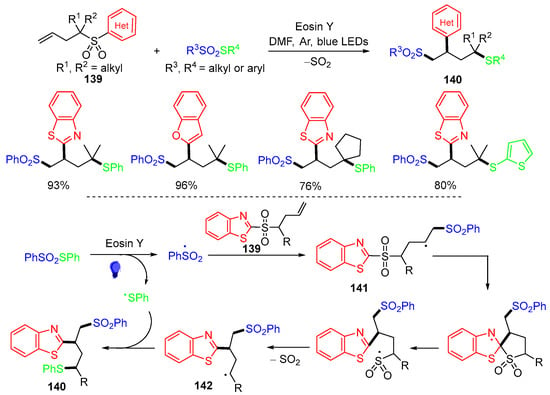
Scheme 36.
Reaction of butenyl benzothiazole sulfones and analogs.
A radical 1,2,3-tricarbofunctionalization of α-vinyl-β-ketoesters was reported by the Bao group in 2022. The reaction of α-vinyl-β-ketoesters 143 and alkynyl triflones in the presence of azodiisobutyronitrile (AIBN) as the radical initiator afforded trifunctional products 144 with excellent diastereoselectivity (Scheme 37) [74]. The reaction process involves the addition of a CF3 radical to the C=C bond of α-vinyl-β-ketoesters 143, followed by ring expansion to form radicals 145. The addition of 145 to alkynyl triflones, followed by the elimination of SO2 and CF3 radicals, gives products 144.
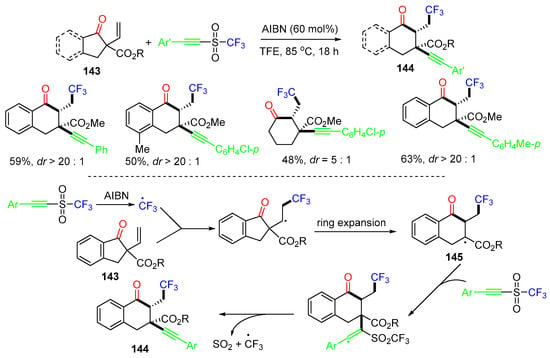
Scheme 37.
Reaction of α-vinyl-β-ketoesters.
The Shu group, in 2024, introduced a set of reactions of vinyl azides 146 and N-hydroxyphthal-imide (NHPI) with cyanoarenes esters for the synthesis of α-tertiary primary amines 147 or with aryl aldehydes for α,α’,α’-trisubstituted primary amines 148 (Scheme 38) [75]. In the reaction involving cyanoarenes, alkyl radicals generated from NHPI under photoredox catalysis are added to vinyl azides to afford the iminyl radicals 149 after denitrogenation. HAT of 149, followed by proton transfer from PyH+ and SET reduction afford radicals 150. Meanwhile, the reduction of cyanoarenes occurs through a proton-coupled electron transfer (PCET) process to give persistent radicals 151. The coupling of 150 with 151 and the elimination of HCN result in products 147.
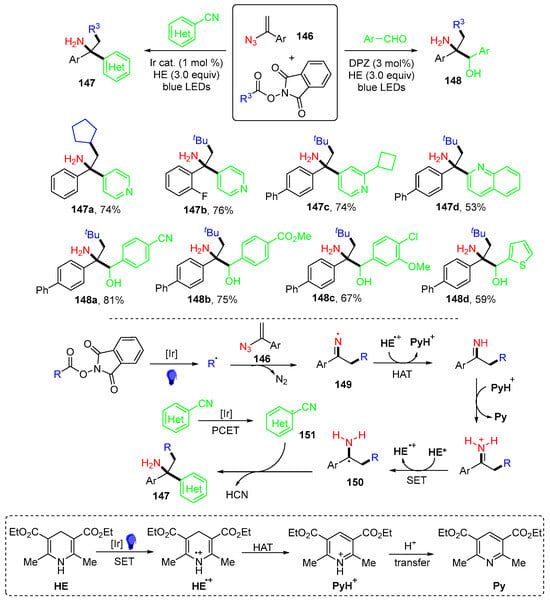
Scheme 38.
Reaction of vinyl azides and NHPI with cyanoarenes or aryl aldehydes.
3.3. Reaction of Allenes
The addition of a radical to allenes could happen at the terminal carbon of allenes to generate vinyl radicals (similar to the reaction of alkynes) or at the middle carbon of allenes to generate alkyl (allylic) radicals (similar to the reaction of alkenes). Two examples in this section involve the inter- and intramolecular radical addition of allenes.
A reaction for O,N,O-trifunctionalization of allenes 152 with nitrosoarenes and oxygen was reported by the Liu group in 2017. The reaction was carried out at −15 °C to afford 3-hydroxy-1-ketonyl-2-imine oxides 153 in good yields (Scheme 39) [76]. The proposed mechanism suggested that nitrosobenzene adds to the arylallene 152 to form 1,4-diradicals 154, followed by the capture of oxygen and 3-exo-trig cyclization to form diradicals 155. Intramolecular radical coupling of 155 affords dioxygen-containing oxacycle 156 and then product 153 after ring-opening rearrangement.
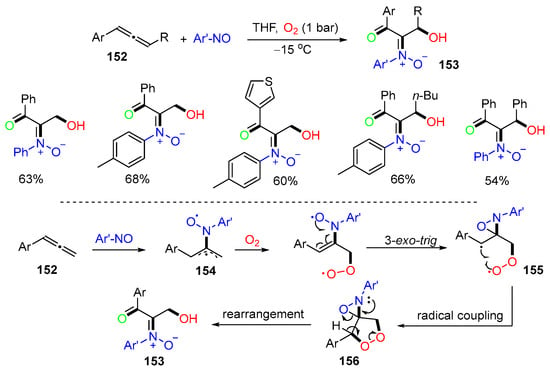
Scheme 39.
Reaction of allenes for O,N,O-trifunctionalization.
A photo-induced radical reaction of aryloxyamino-containing allenes 157 with TEMPO derivatives (R2N-O●) for the synthesis of TEMPO-substituted lactams 158 was reported by the Schomaker group in 2021 [77]. The reaction was carried out under 440 nm light irradiation in the presence of K2CO3 (Scheme 40). In the reaction process, the N-centered radicals 159 derived from aryloxyamino-containing allenes 157 add to the proximal C=C bond of allene 157 to generate vinyl radicals 160, which are trapped by TEMPO, followed by the elimination of TEMP to afford radicals 161, which are coupled with TEMPO again to form products 158.
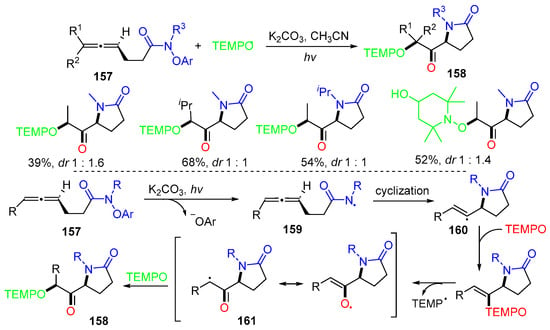
Scheme 40.
Reaction of aryloxyamino-containing allenes with TEMPO derivatives.
4. Reaction of Alkynes and Enynes
Depending on the substrate structures and reaction conditions, alkynes could undergo trifunctionalization reactions without group migration of intermediate radicals (Scheme 41A) or involving 1,5-HAT, as shown in Scheme 41B.
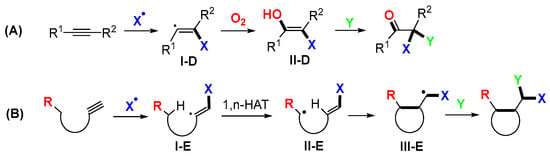
Scheme 41.
Two kinds of trifunctionalization reactions of alkynes.
4.1. Reaction of Alkynes
A photo-oxidative trifunctionalization of aryl alkynes was reported by the Itoh group in 2010 [78]. The reaction of aromatic alkynes 162 and HBr (aq.) in the presence of molecular oxygen afforded α,α-dibromoacetophenones 163 in good yields (Scheme 42). The proposed mechanism suggested that the Br radical, formed under visible light irradiation of HBr in the presence of O2, adds to phenylacetylenes 162 to generate vinyl radicals 164, which are oxidized by oxygen, followed by the H atom abstraction from HBr and reduction with HBr to provide bromoenols 165. Trapping of Br2 by 165 affords dibromoacetophenone 163.
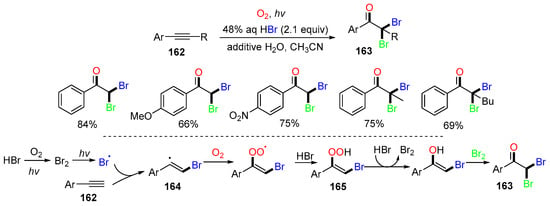
Scheme 42.
Reaction of aryl alkynes with HBr and oxygen.
A reaction of phenylpropiolic acids 166 with benzaldehydes using DTBP as an oxidant for the synthesis of 2-aroyl-3-phenylindenones 167 was reported by the Jafarpour group in 2020 (Scheme 43) [79]. The α-carbonyl radicals 168, generated from the reaction of aldehydes with DTBP, add to phenylpropiolic acids 166 to afford vinyl radicals 169, which undergo radical aromatic substitution, SET oxidation, and deprotonation to afford indenone-2-carboxylic acids 170. The addition of radicals 168 to 170 affords radicals 171, which undergo SET oxidation and decarboxylation to give products 167.
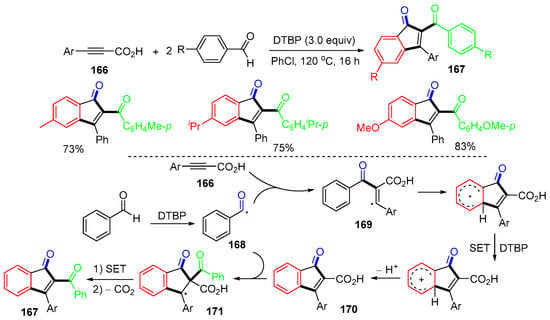
Scheme 43.
Reaction of phenylpropiolic acids with benzaldehydes.
In 2022, the Huang group reported an electrochemical reaction using TEMPO as the redox catalyst for the triamination of aryl alkynes 172 with anilines for the synthesis of functionalized indolines 173 (Scheme 44) [80]. The N-centered radicals generated from aryl alkynes 172 undergo 5-endo-dig cyclization to give radicals 174, followed by couplings with anilines and the oxidation on the anode to give products 173.
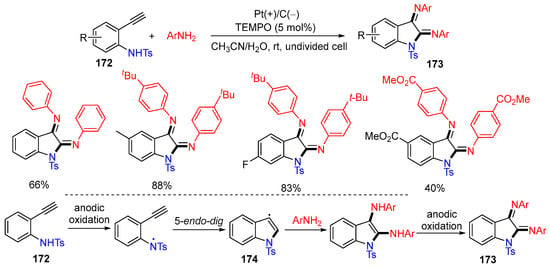
Scheme 44.
Electrochemical triamination of alkynes with anilines.
In 2024, the Zhu group reported a photoredox reaction of aliphatic alkynes 175 with sulfones for the synthesis of functionalized E-allyl alcohol 176 (Scheme 45) [81]. The reaction processes involve the formation of the initial alkyl radical for addition to alkynes 175 to form vinyl radicals 177, followed by the ipso-cyclization of 177 and desulfonylative ring-opening to give 1,2-heterocyclic group-migrated radicals 178. SET oxidation of 178 is followed by trapping with H2O to afford Z-allyl alcohols 176, which are isomerized to E-allyl alcohols 176.
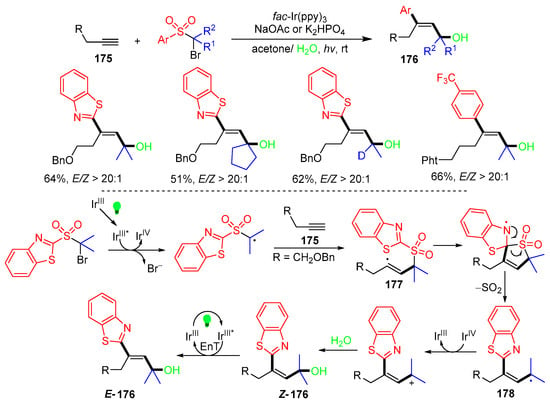
Scheme 45.
Reaction of aliphatic alkynes with sulfones.
The Studer group, in 2020, introduced a 1,1,2-trifunctionalization of terminal alkynes involving 1,5-HAT of intermediate vinyl radicals. The reaction of alkynyl triflones with alkynes 179 in the presence of dibenzoyl peroxide (BPO) or AIBN as the radical initiator furnished the substituted cyclopentanes 180 in moderate to good yields (Scheme 46) [82]. The reaction process involves the addition of the CF3 radical to alkynes 179, followed by 1,5-HAT to form radicals 181. Cyclization of 181 and trapping with alkynyl triflones afford radicals 182. Products 180 are produced after the fragmentation of the CF3SO2 group from 182.
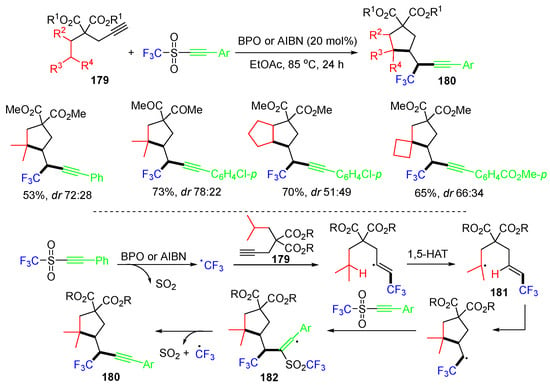
Scheme 46.
Reaction of alkynes with alkynyl triflones involving 1,5-HAT.
Another Cu-catalyzed 1,2,5-trifunctionalization reaction of alkynes 183 involving 1,5-HAT and using the SPh group as the transient directing group was reported by the Zhu group in 2022 to afford functionalized aldehydes 184 in good to excellent yields (Scheme 47) [83]. The proposed mechanism suggested that the electrophilic CCl3 radical, generated from SET oxidation of XCCl3 (X = Cl or Br), attacks at the C2 position of RS-attached alkynes that were generated from 183 to give RS-attached vinyl radicals 185, which undergo 1,5-HAT and Cu-assisted halogen atom transfer to give 186, followed by SN2′-type substitution and elimination of the PhS group and Cl anion to form products 184.
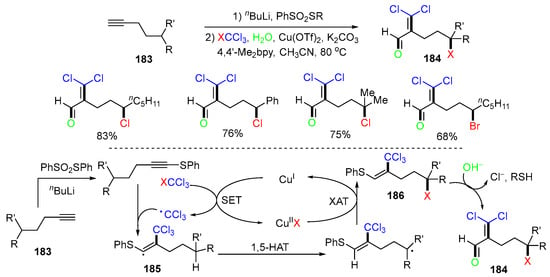
Scheme 47.
Reaction of alkynes using SPh as the transient directing group.
4.2. Reaction of Enynes
In 2020, the Li group reported an In-catalyzed [3+2] annulation of 1,3-enynes 187 to form functionalized isoxazoles 188 (Scheme 48) [84]. In the reaction process, the sulfonyl radicals derived from sodium sulfinates add to the C=C bond of enynes 187 to give sulfonyl-containing alkyl radicals 189, which have resonance structures of 190. Radical cross-coupling of 190 with NO radical, followed by isomerization and intramolecular nucleophilic annulation, affords products 188.
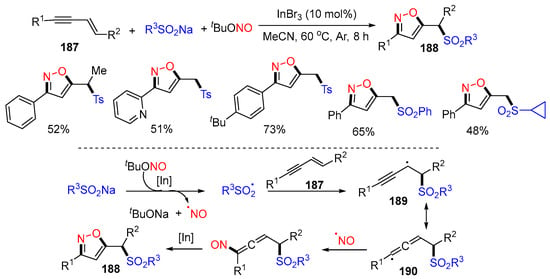
Scheme 48.
Trifunctionalization of 1,3-enynes with sodium sulfinates and TBN.
5. Reaction of Alkenylnitriles
Alkenes bearing a CN group at γ-position are good substrates for trifunctionalizations since the CN group could be relocated via 1,4-migration after the radical addition (Scheme 49). The CN group migration involves the formation of radicals I-F, the cleavage of cyclic iminyl radicals II-F, and the 1,4-shift to radicals III-F.
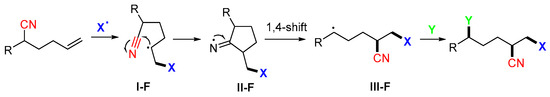
Scheme 49.
General pathway for the reaction of alkenylnitriles.
A trifunctionalization reaction of hexenenitriles 191 with TMSN3 was reported by the Zhu group in 2022 (Scheme 50) [85]. In this synthesis, the N3 radical generated from the reaction of TMSN3 and PIFA adds to hexenenitriles 191 to form alkyl radicals 193, which undergo 1,4-CN group migration followed by trapping the second N3 group to give products 192. The evaluation of the chain length for the CN group migration indicated that the 1,5-CN migration also readily proceeded to give the desired product. Meanwhile, the 1,3-CN migration only gave a trace amount of the products.
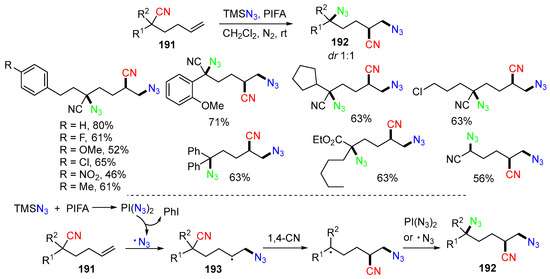
Scheme 50.
Reaction of hexenenitriles involving 1,4-CN migration.
The Zhu group, in 2023, reported another hexenenitrile-based reaction for the synthesis of trisubstituted alkenes (Scheme 51) [86]. The reaction of hexenenitriles 194 with aryl sulfonyl chlorides under blue LED irradiation and in the presence of fac-Ir(ppy)3 at room temperature gave products 195 in moderate to good yields. The proposed mechanism suggested that the excited IrIII* species interacts with sulfonyl chlorides to form sulfonyl radicals, which add to hexenenitriles 194, followed by 1,4-CN migration to give stabilized benzyl radicals 196. SET oxidation of radicals 196 with IrIV, followed by base-promoted deprotonation, gives products 195.
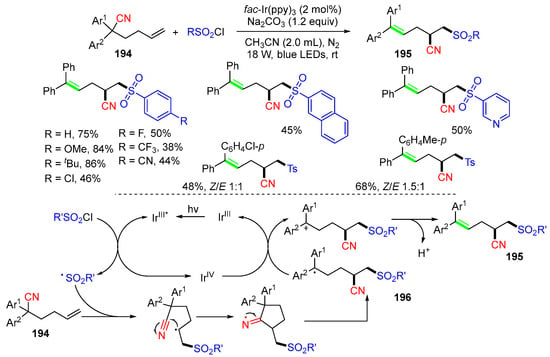
Scheme 51.
Reaction of hexenenitriles with aryl sulfonyl chlorides involving 1,4-CN migration.
In 2022, Chen and Zhu’s groups reported a photoreaction system of hexenenitriles 197 using Tongi’s reagent II as a radical source for making 1,2,5 trifuctionalized alkanes 198 (Scheme 52) [87]. Other than the formation of CF3 radicals, the reaction mechanism is similar to that shown in Scheme 51.
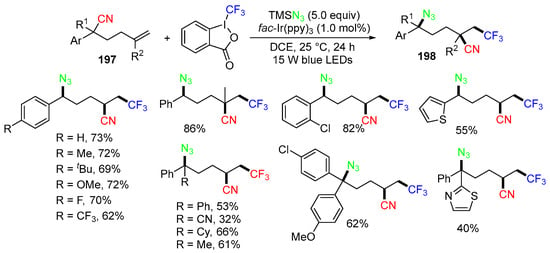
Scheme 52.
Reaction of hexenenitriles with Tongi’s reagent II involving 1,4-CN migration.
Very recently, Du and coworkers developed an N-heterocyclic carbene organocatalytic reaction of hexenenitriles 199 with Tongi’s reagent II and aldehydes for making functionalized ketones 200 (Scheme 53) [88]. The proposed mechanism suggested that the reaction of aldehydes and NHC 201 gives Breslow intermediates 202, which interact with Togni’s reagent II to afford NHC-bound ketyl radicals 203 and CF3 radicals. The addition of the CF3 radical to the C=C bond of hexenenitriles 199 gives alkyl radicals 204, followed by 1,4-CN migration to give radical 205, which then couples with the radicals 203 to afford products 200 with the loss of NHC 201 for the next catalytic cycle. The density functional theory (DFT) calculation results showed that the reaction pathway involving 1,4-CN migration is energetically more favorable compared to the pathway without CN migration.
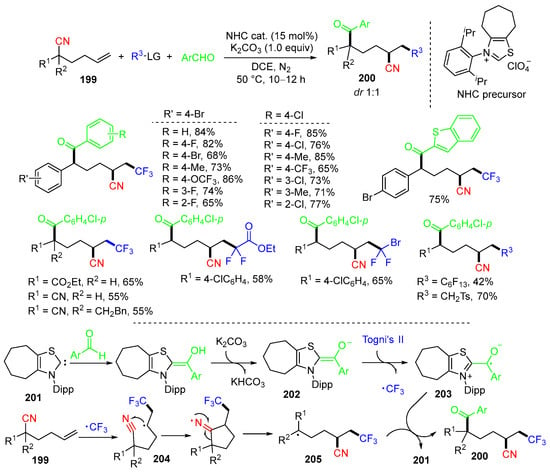
Scheme 53.
NHC-catalyzed reaction of hexenenitriles.
6. Reaction of Alkynoates
Aryl alkynoates bearing two aryl groups are good substrates for trifunctionalization reactions. The red-colored aryl group could be relocated via the processes of ipso-cyclization and cleavage (Scheme 54). The Ar group on the terminal carbon of alkyne directs the addition of an X radical to the carbon to form aryl-stabilized vinyl radicals I-G, followed by ipso-cyclization and cleavage to form radicals II-G. The decarboxylated radicals III-G are then functionalized by a Y group.
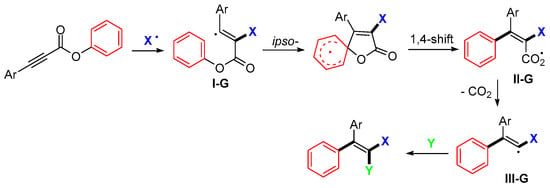
Scheme 54.
General pathway for the reaction of aryl alkynoates.
The Han group, in 2016, reported a reaction of alkynoates 206 for cascade iodination-triggered iodination, aryl migration, decarboxylation, and the second iodination for making functionalized alkenes 207 (Scheme 55) [89]. The proposed mechanism indicated that iodine radicals generated from N-iodosuccinimide (NIS) add to the triple bond of alkynoates 206 to afford vinyl radicals 208, which undergo 1,4-aryl migration to give carboxyl radicals 209. Decarboxylation of 209 followed by the second iodination gives 1,1-diiodoalkene products 207. It was found that N-bromosuccinimide (NBS), but not N-chlorosuccinimide (NCS), could be used to replace NIS for the reaction.
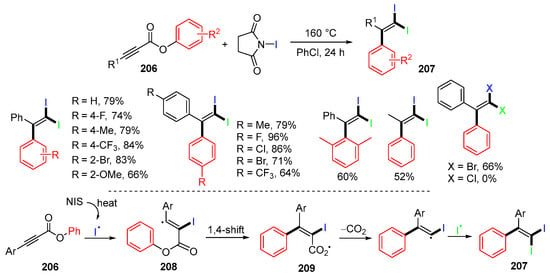
Scheme 55.
Reaction of alkynoates with NIS involving 1,4-aryl migration.
The Baidya group, in 2019, modified the reaction conditions and used diphenyl diselenides and other reactants to react with aryl alkynoates 210 to afford tetrasubstituted gem-diselenoalkene 211 in good to excellent yields (Scheme 56) [90]. The reaction process is similar to that shown in Scheme 55, which involves the addition of an aryl selenium radical followed by 1,4-aryl migration, decarboxylation, and coupling with a second aryl selenium radical to give products 211.
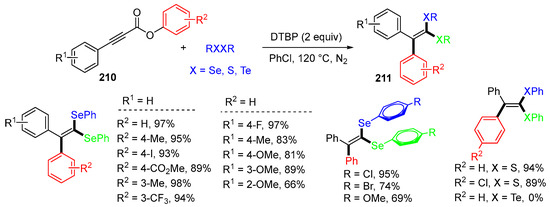
Scheme 56.
Reaction of alkynoates with dichalcogenides involving 1,4-aryl migration.
A visible-light-mediated reaction of alkynoates 212 with NBS for the synthesis of 3-bromocoumarins 213 was reported by the She group in 2017 (Scheme 57) [91]. The proposed mechanism suggested that the addition of Br radicals to alkynoates 212 affords vinyl radicals 214, which undergo 1,4-aryl migration to give carboxyl radicals 215. Instead of decarboxylation, as shown in Scheme 54, radicals 215 undergo cyclization, followed by aromatization, to afford products 213. It is noteworthy that under the photoreaction conditions, no decarboxylation of radical 215 occurred, which is different from that shown in Scheme 55.
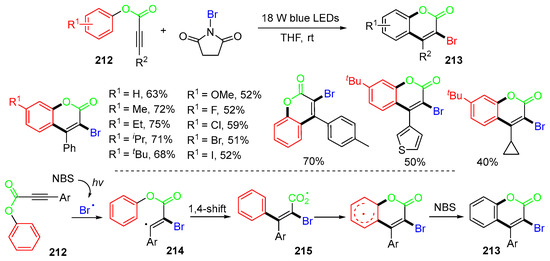
Scheme 57.
Photo reaction of alkynoates with NBS involving 1,4-aryl migration.
The Srimani group, in 2022, introduced two photoredox reaction conditions for the trifunctionalization of alkynoates 216 with diphenyl- and dialkyldiselenides (Scheme 58) [92]. The two reaction processes share the same intermediates 219, which are generated by the addition of selenium radicals to alkynoates followed by 1,4-aryl migration. For the reaction to make products 217, the Eosin Y photocatalyst transfers an electron to carboxyl radicals 219 to form carboxylate anions 220, which leads to the formation of products after protonation with HCl. For the reaction to make 1,1-diselenide alkenes 218, carboxyl radicals 219 undergo decarboxylation to form vinyl radical 221, followed by coupling with selenium radicals to give the products.
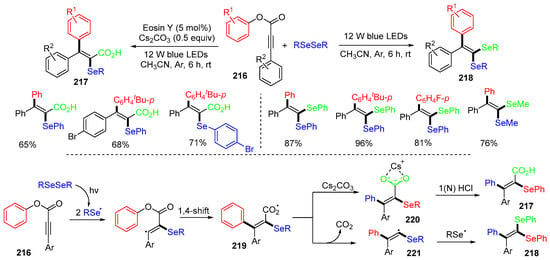
Scheme 58.
Reaction of aryl alkynoates with diphenyldiselenides.
7. Reaction of Oximes, Oxime Ethers, and Oxime Esters
Oximes and derivatives have unique =N–O–R functional groups, which readily undergo homolytic cleavage of N–O or O–R bonds to form iminyl or iminoxyl radicals. The iminyl or iminoxyl radicals I-H could serve as the initial radicals in the trifunctionalization reactions (Scheme 59A). For oximes tethered with unsaturated carbon bonds, the reaction could be initiated by the addition of radical X to the carbon bond to form radicals II-H and then undergo cyclization or group transfer reactions involving the oxime group (Scheme 59B).
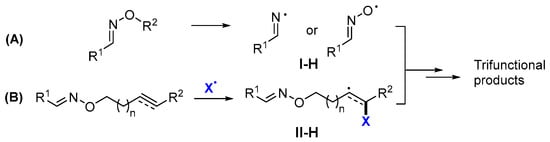
Scheme 59.
Two pathways for the radical reaction of oximes and derivatives.
Han and coworkers, in 2017, reported a Cu-catalyzed dioxygen activation reaction of alkynyl ketoximes 222 and 223 in the synthesis of isoxazoline/cyclic nitrone-featured α-ketols 224 and 225 (Scheme 60) [93]. The mechanism investigation revealed that the initial iminoxyl radicals 226 and the resonance structures 227 can undergo dichotomous O-/N-atom 5-exo-dig cyclization to the alkyne group, followed by oxygen activation, peroxy radical 4-endo-trig cyclization, and O–O bond cleavage to give oxy radicals 228 or 229, which leads to the formation of products 224 or 225 after hydrogen abstraction.
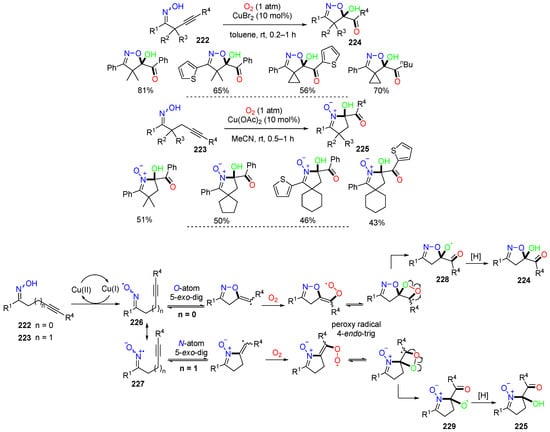
Scheme 60.
Reaction of γ,δ-alkynyl ketoximes.
The Han group, in 2021, introduced a reaction of alkenyl diphenyl ketoximes 230/231 for the synthesis of fluoroalkylated aminoketones 232/233 (Scheme 61) [94]. The reaction process was initiated with the addition of fluoroalkyl radicals to the C=C bond and involved a 1,4/5-amino shift. In the case of reaction with Togni’s reagent II, the CF3 radical attacks the C=C double bond of ketoxime ether 230 to form alkyl radicals 234, followed by a kinetically favorable 5/6-exo-trig cyclization and N–O bond cleavage to afford oxy radicals 235. Oxidation of 235, followed by HAT and hydrolysis, affords the final product 232. Other than Togni’s reagent II, fluoroalkyl iodides could be used for the synthesis of different kinds of fluoroalkyl-substituted products 233.
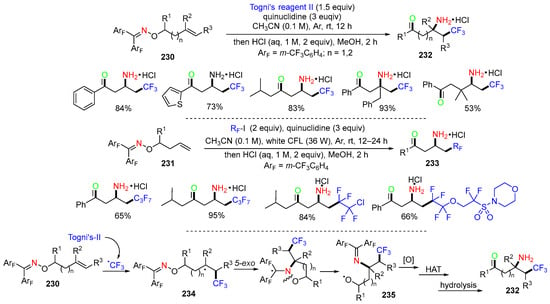
Scheme 61.
Reaction of alkenyl diphenyl ketoxime ethers.
A reaction of oxime-derived peresters 236 with azadienes for diastereoselective synthesis of highly condensed cyclic products 237 was reported by the Wei group in 2022 (Scheme 62) [95]. In the reaction process, the iminyl radicals 238 derived from oxime-derived peresters 236 initiated the cascade radical reactions of 1,5-HAT and addition to azadienes to form 239, followed by 1,7-HAT, 6-endo, and then 5-endo cyclizations to give radicals 240, which then led to the formation of products 237 after SET oxidation.
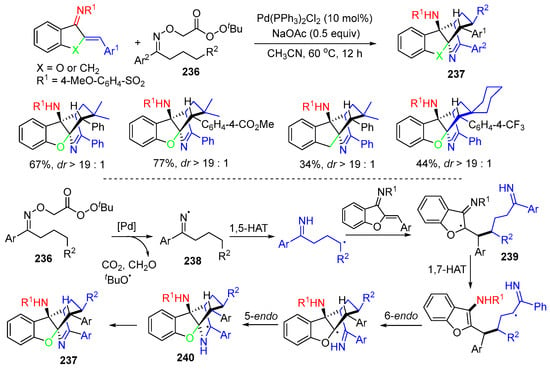
Scheme 62.
Reaction of oxime-derived peresters with azadienes.
A reaction of alkenyl oxime ethers 241 with sodium metabisulfite and aryldiazonium tetrafluoroborates for the synthesis of Boc-protected β-amino sulfones 242 was reported by the Wu group in 2023 (Scheme 63) [96]. In the alkene aminosulfonylation process, arylsulfonyl radicals, which are generated from the reaction of sodium metabisulfite and aryldiazonium tetrafluoroborates, are added to alkene 241 to form carbon radicals 243, followed by 1,4-iminyl group migration to give oxy radicals 244. Oxidative SET with aryldiazonium tetrafluoroborates and protonation afford 245, which undergo hydrolysis and Boc protection to give Boc-protected β-amino sulfone 242.
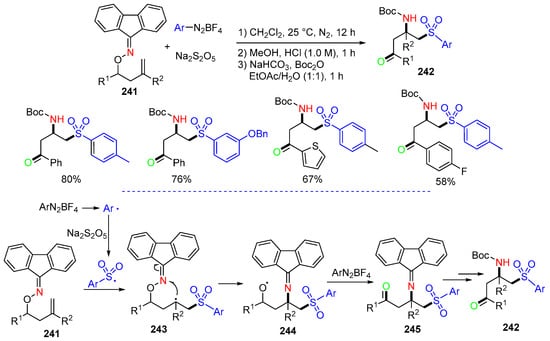
Scheme 63.
Trifunctionalization of alkenyl oxime ethers.
Other than the oximes and oxime ethers discussed above, oxime esters could be used for the trifunctionalization reaction. A reaction of oxime esters 246 and vinyl azides for the dearomatic synthesis of spiroaminals 247 was developed by the Yi group in 2021 (Scheme 64) [97]. In the reaction process, photoredox-induced iminyl radicals undergo 5-exo cyclization to form pyrroline-containing radicals 248, which are then added to vinyl azides to generate second iminyl radicals 249 after elimination of N2. 1,5-HAT of 249, followed by 5-endo cyclization, affords radicals 250 with spiroring scaffold. SET of 250 and proton transfer of 251 produce the spirobi[pyrrolines] products 247.
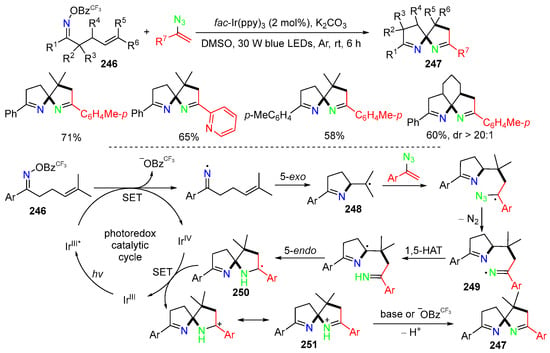
Scheme 64.
Reaction of oxime estersfor for the synthesis of spirobi[pyrrolines] products.
The opening of strained rings is a good approach for remote functionalization. In 2023, the Guo group reported a Fe-catalyzed reaction of cycloketone oxime esters 252 with trifluoromethylalkenes and TMSN3. The reaction involves a process of cycloketone oxime esters 252 ring opening, radical addition to trifluoromethylalkenes, and azidation with TMSN3 to afford products 253 (Scheme 65) [98].
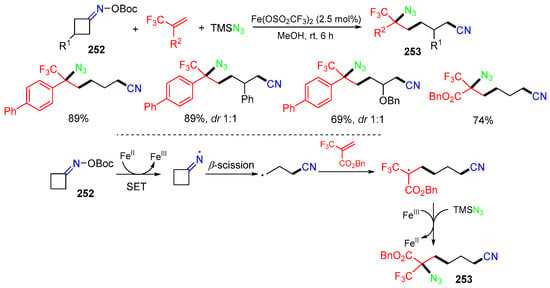
Scheme 65.
Reaction of cycloketone oxime esters.
8. Summary and Conclusions
Radical trifunctionalization is a relatively new research area. Many of the papers covered in this article were published in the last five years. The effort to promote highly efficient synthesis, product diversity, and sustainable chemistry makes the topic attractive. The development of photoredox catalysis and electrochemical reactions has empowered cascade radical reactions, including di- and trifunctionalization reactions. This article summarizes the different substrates and synthetic strategies developed for radical trifunctionalization reactions. We hope this overview of the field will inspire readers and encourage more chemists to engage in the development of trifunctionalization reactions to make novel scaffolds with potential biological activities and other utilities. We believe that the discovery of new substrate systems and the development of new radical reaction techniques, including photoredox, electrochemical reactions, and transition-metal catalysts, are critical for the future growth of radical trifunctionalization reactions.
Author Contributions
Q.Z., X.M. and S.Z. conducted literature searches and original manuscript writing, and W.Z. conducted the revision and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chatgilialoglu, C.; Studer, A. (Eds.) Encyclopedia of Radicals in Chemistry, Biology and Materials; Wiley: Chichester, UK, 2012. [Google Scholar]
- Zarf, S.Z. Radical Reactions in Organic Synthesis; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Clark, A.J. Copper catalyzed atom transfer radical cyclization reactions. Eur. J. Org. Chem. 2016, 2016, 2231–2243. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, X.; Li, J.; Xiao, J.; Dong, Y.; Liu, H. Recent advances on palladium radical involved reactions. ACS Catal. 2015, 5, 6111–6137. [Google Scholar] [CrossRef]
- Ge, Y.; Tian, Y.; Wu, J.; Yan, Q.; Zheng, L.; Ren, Y.; Zhao, J.; Li, Z. Iron-promoted free radical cascade difunctionalization of unsaturated benzamides with silanes. Chem. Commun. 2020, 56, 12656–12659. [Google Scholar] [CrossRef] [PubMed]
- Bag, D.; Mahajan, S.; Sawant, S.D. Transition-metal-catalyzed carbohalogenative 1,2-difunctionalization of C−C multiple bonds. Adv. Synth. Catal. 2020, 362, 3948–3970. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Tang, Y.; Yan, S.; Li, G. Copper-catalyzed annulation-trifluoromethyl functionalization of enynones. Org. Lett. 2023, 25, 2509–2514. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hao, W.; Li, G.; Jiang, B. Catalytic benzannulation reactions of enynones for accessing heterocycle-incorporating diarylmethanes. Synlett 2023, 34, 243–248. [Google Scholar] [CrossRef]
- Zuo, H.; Yuan, Y.; Chen, X.; Wang, J.-Y.; Yan, S.; Zhang, Y.; Liu, J. Copper-catalyzed radical-induced annulation-halo(bi)cyanomethylation of indole-tethered 1,6-enynes toward pyrrolo [1,2-a]indoles. Adv. Synth. Catal. 2024. advanced article. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed]
- Bag, D.; Koura, H.; Sawant, S.D. Photo-induced 1,2-carbohalofunctionalization of C–C multiple bonds via ATRA pathway. Org. Biomol. Chem. 2020, 18, 8278–8293. [Google Scholar] [CrossRef]
- Dong, D.-Q.; Tian, B.-L.; Yang, H.; Wei, Z.-H.; Yang, S.-H.; Zhou, M.-Y.; Ding, C.-Z.; Wang, Y.-L.; Gao, J.-H.; Wang, S.-J.; et al. Visible light induced palladium-catalyzed reactions involving halogenated hydrocarbon (RX). Mol. Catal. 2023, 541, 113073. [Google Scholar] [CrossRef]
- Zuo, H.; Chen, X.; Zhang, Y.; Liu, J.-W.; Yan, S.-H.; Li, G.; Wang, J.-Y. Photocatalytic thio/selenosulfonylation-bicyclization of indole-tethered 1,6-enynes leading to substituted benzo[c]pyrrolo [1,2,3-lm]carbazoles. Org. Lett. 2024, 26, 3828–3833. [Google Scholar] [CrossRef]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic organic electrochemical methods since 2000: On the verge of a renaissance. Chem. Rev. 2017, 117, 13230–13319. [Google Scholar] [CrossRef]
- Ji, X.-S.; Zuo, H.-D.; Shen, Y.-T.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Electrochemical selective annulative amino-ketalization and amino-oxygenation of 1,6-enynes. Chem. Commun. 2022, 58, 10420–10423. [Google Scholar] [CrossRef]
- Leitch, J.A.; Browne, D.L. Mechanoredox chemistry as an emerging strategy in synthesis. Chem. Eur. J. 2021, 27, 9721–9726. [Google Scholar] [CrossRef]
- Clarke, P.A.; Santos, S.; Martin, W.H.C. Combining pot, atom and step economy (PASE) in organic synthesis. Synthesis of tetrahydropyran-4-ones. Green Chem. 2007, 9, 438–440. [Google Scholar] [CrossRef]
- Zhang, W.; Yi, W.-B. Pot, atom, and step economy (PASE) synthesis. In Springer Briefs in Green Chemistry for Sustainability; Sharma, S.K., Rajasthan, J., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, W. Recent developments on one-pot stepwise synthesis (OPSS) of small molecules. iScience 2022, 25, 105005. [Google Scholar] [CrossRef]
- Besset, T.; Poisson, T.; Pannecoucke, X. Direct vicinal difunctionalization of alkynes: An efficient approach towards the synthesis of highly functionalized fluorinated alkenes. Eur. J. Org. Chem. 2015, 2015, 2765–2789. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, G.; Zhao, Y.F. Recent progress toward organophosphorus compounds based on phosphorus-centered radical difunctionalizations. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 589–596. [Google Scholar] [CrossRef]
- Koike, T.; Akita, M. A versatile strategy for difunctionalization of carbon–carbon multiple bonds by photoredox catalysis. Org. Chem. Front. 2016, 3, 1345–1349. [Google Scholar] [CrossRef]
- Lan, X.-W.; Wang, N.-X.; Xing, Y. Recent advances in radical difunctionalization of simple alkenes. Eur. J. Org. Chem. 2017, 2017, 5821–5851. [Google Scholar] [CrossRef]
- Bao, X.Z.; Li, J.; Jiang, W.; Huo, C.D. Radical-mediated difunctionalization of styrenes. Synthesis 2019, 51, 4507–4530. [Google Scholar] [CrossRef]
- Lin, J.; Song, R.J.; Hu, M.; Li, J.H. Recent advances in the intermolecular oxidative difunctionalization of alkenes. Chem. Rec. 2019, 19, 440–451. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Xiao, Y.-T.; Yang, Y.-Z.; Song, R.-J.; Li, J.-H. Recent advances in silver-mediated radical difunctionalization of alkenes. Chem. Cat. Chem. 2020, 12, 5312–5329. [Google Scholar] [CrossRef]
- Siu, J.C.; Fu, N.K.; Lin, S. Catalyzing electrosynthesis: A homogeneous electrocatalytic approach to reaction discovery. Acc. Chem. Res. 2020, 53, 547–560. [Google Scholar] [CrossRef]
- Li, Z.L.; Fang, G.C.; Gu, Q.S.; Liu, X.Y. Recent advances in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev. 2020, 49, 32–48. [Google Scholar] [CrossRef]
- Jiang, H.; Studer, A. Intermolecular radical carboamination of alkenes. Chem. Soc. Rev. 2020, 49, 1790–1811. [Google Scholar] [CrossRef]
- Coppola, G.A.; Pillitteri, S.; Van der Eycken, E.V.; You, S.-L.; Sharma, U.K. Multicomponent reactions and photo/electrochemistry join forces: Atom economy meets energy efficiency. Chem. Soc. Rev. 2022, 51, 2313–2382. [Google Scholar] [CrossRef]
- Xia, D.; Shi, Y.; Jiang, L.; Li, Y.; Kong, J. Recent advances in the radical cascade reaction for constructing nitrogen heterocycles using azides as radical acceptors. Org. Biomol. Chem. 2024, 22, 5511–5523, advance article. [Google Scholar] [CrossRef]
- Yao, H.; Hu, W.; Zhang, W. Difunctionalization of alkenes and alkynes via intermolecular radical and nucleophilic additions. Molecules 2021, 26, 105. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Q.; Zhang, W. Remote radical 1,3-, 1,4-, 1,5-, 1,6-and 1,7-difunctionalization reactions. Molecules 2023, 28, 3027. [Google Scholar] [CrossRef]
- Zhi, S.; Yao, H.; Zhang, W. Difunctionalization of dienes, enynes and related compounds via sequential radical addition and cyclization reactions. Molecules 2023, 28, 1145. [Google Scholar] [CrossRef]
- Zhi, S.; Ma, X.; Zhang, W. Radical cyclization-initiated difunctionalization reactions of alkenes and alkynes. Molecules 2024, 29, 2559. [Google Scholar] [CrossRef]
- Wu, X.; Wu, S.; Zhu, C. Radical-mediated difunctionalization of unactivated alkenes through distal migration of functional groups. Tetrahedron Lett. 2018, 59, 1328–1336. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, C. Radical-mediated remote functional group migration. Acc. Chem. Res. 2020, 53, 1620–1636. [Google Scholar] [CrossRef]
- Wu, Z.; Ren, R.; Zhu, C. Combination of a cyano migration strategy and alkene difunctionalization: The elusive selective azidocyanation of unactivated olefins. Angew. Chem. Int. Ed. 2016, 55, 10821–10824. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, D.; Liu, Y.; Huan, L.; Zhu, C. Chemo-and regioselective distal heteroaryl ipso-migration: A general protocol for heteroarylation of unactivated alkenes. J. Am. Chem. Soc. 2017, 139, 1388–1391. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.; Xu, Y.; Wu, Z.; Zhu, C. Distal functional group migration for visible-light induced carbo-difluoroalkylation/monofluoroalkylation of unactivated alkenes. Adv. Synth. Catal. 2018, 360, 744–750. [Google Scholar] [CrossRef]
- Tian, T.; Wang, X.; Lv, L.; Li, Z. Iron-catalyzed acylation-functionalization of unactivated alkenes with aldehydes. Chem. Commun. 2020, 56, 14637–14640. [Google Scholar] [CrossRef]
- Seastram, A.C.; Hareram, M.D.; Knight, T.; Morrill, L.C. Electrochemical alkene azidocyanation via 1,4-nitrile migration. Chem. Commun. 2022, 58, 8658–8661. [Google Scholar] [CrossRef]
- Yang, M.; Chang, X.; Ye, S.; Ding, Q.; Wu, J. Generation of heteroaryl-substituted sulfonyl compounds from sulfur dioxide via remote heteroaryl ipso-migration. J. Org. Chem. 2021, 86, 15177–15184. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, J.; Lv, H.; Qin, L.; Duan, X.; Wang, J.; Wu, M.; Chen, B.; Qiu, J.; Guo, K. Visible-light-induced selective alkylsulfonylation of unactivated alkenes via remote heteroaryl migrations. Green Synth. Catal. 2024, 5, 126–130. [Google Scholar] [CrossRef]
- Zhang, J.H.; Xiao, T.F.; Ji, Z.Q.; Chen, H.N.; Yan, P.J.; Luo, Y.C.; Xu, P.F.; Xu, G.Q. Organic photoredox catalytic amino-heteroarylation of unactivated olefins to access distal amino ketones. Chem. Commun. 2022, 58, 2882–2885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Zhang, C.; Feng, C. Multisubstituted cyclohexene construction through telescoped radical-addition induced remote functional group migration and Horner-Wadsworth-Emmons (HWE) olefination. Org. Lett. 2021, 23, 9611–9615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Chen, D.; Qin, Y.Q.; Deng, W.; Luo, Y.Y.; Xiang, J.N. Oxidative alkylation/alkynylation of terminal alkenes via alkylaldehyde decarbonylation and 1,2-alkynyl migration. Org. Biomol. Chem. 2021, 19, 3154–3158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, T.; Cai, P.; Jiang, B.; Tu, S. Study on tert-butyl radical-initiated 1,2-alkynyl migration. Chin. J. Org. Chem. 2021, 41, 2408–2416. [Google Scholar] [CrossRef]
- Jin, S.; Chen, F.; Qian, P.; Cheng, J. Cyanoalkylation/alkynylation of allylic alcohol through intramolecular radical 1,2-alkynyl migration. Org. Biomol. Chem. 2021, 19, 2416–2419. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Wang, X.; Chu, X. Difluoroalkylation/1,2-aryl migration of allylic alcohols under transition metal-free conditions. Tetrahedron Lett. 2021, 70, 153002. [Google Scholar] [CrossRef]
- Wu, X.; Ma, Z.; Feng, T.; Zhu, C. Radical-mediated rearrangements: Past, present, and future. Chem. Soc. Rev. 2021, 50, 11577–11613. [Google Scholar] [CrossRef] [PubMed]
- Kumar Nanda, S. Catalytic radical-polar crossover non-classical semipinacol rearrangements: The sustainable approach. Adv. Synth. Catal. 2023, 365, 834–853. [Google Scholar] [CrossRef]
- Xie, D.-T.; Chen, H.-L.; Wei, D.; Wei, B.-Y.; Li, Z.-H.; Zhang, J.-W.; Yu, W.; Han, B. Regioselective fluoroalkylphosphorylation of unactivated alkenes by radical-mediated alkoxyphosphine rearrangement. Angew. Chem. Int. Ed. 2022, 61, e202203398. [Google Scholar] [CrossRef]
- Dhungana, R.K.; Granados, A.; Ciccone, V.; Martin, R.T.; Majhi, J.; Sharique, M.; Gutierrez, O.; Molander, G.A. Trifunctionalization of cinnamyl alcohols provides access to brominated α,α-difluoro-γ-lactones via a photoinduced radical-polar-radical mechanism. ACS Catal. 2022, 12, 15750–15757. [Google Scholar] [CrossRef]
- Duan, X.; Sun, Q.; Yuan, X.; Qin, L.; Zhang, X.; Liu, J.; Wu, M.; Zhu, S.; Ma, C.; Qiu, J.; et al. Photoinduced remote heteroaryl migration accompanied by cyanoalkylacylation in continuous flow. Green Chem. 2021, 23, 8916–8921. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, M.; Qiu, Y.; Song, M.; Wang, H.; Yang, M.; Xie, W.; Wu, J.; Ye, S. Alkoxysulfonyl radical species: Acquisition and transformation towards sulfonate esters through electrochemistry. Chem. Sci. 2022, 13, 11785–11791. [Google Scholar] [CrossRef]
- Gao, P.; Shen, Y.W.; Fang, R.; Hao, X.H.; Qiu, Z.H.; Yang, F.; Yan, X.B.; Wang, Q.; Gong, X.J.; Liu, X.Y.; et al. Copper-catalyzed one-pot trifluoromethylation/aryl migration/carbonyl formation with homopropargylic alcohols. Angew. Chem. Int. Ed. 2014, 53, 7629–7633. [Google Scholar] [CrossRef]
- Wu, S.; Wu, X.; Wang, D.; Zhu, C. Regioselective vinylation of remote unactivated C(sp3)−H bonds: Access to complex fluoroalkylated alkenes. Angew. Chem. Int. Ed. 2019, 58, 1499–1503. [Google Scholar] [CrossRef]
- Zhou, N.; Xu, P.; Li, W.; Cheng, Y.; Zhu, C. Visible light promoted carbodifluoroalkylation of homopropargylic alcohols via concomitant 1,4-aryl migration. Acta Chim. Sin. 2017, 75, 60–65. [Google Scholar] [CrossRef][Green Version]
- Bao, P.; Yu, F.; He, F.-S.; Tang, Z.; Deng, W.-P.; Wu, J. Visible-light-induced remote C(sp3)−H sulfonylvinylation: Assembly of cyanoalkylated vinyl sulfones. Org. Chem. Front. 2021, 8, 4820–4825. [Google Scholar] [CrossRef]
- Wang, N.; Li, L.; Li, Z.; Yang, N.; Guo, Z.; Zhang, H.; Liu, X. Catalytic diverse radical-mediated 1,2-cyanofunctionalization of unactivated alkenes via synergistic remote cyano migration and protected strategies. Org. Lett. 2016, 18, 6026–6029. [Google Scholar] [CrossRef]
- Kwon, Y.; Zhang, W.; Wang, Q. Copper-catalyzed aminoheteroarylation of unactivated alkenes through distal heteroaryl migration. ACS Catal. 2021, 11, 8807–8817. [Google Scholar] [CrossRef]
- Lei, Z.; Wei, S.; Zhou, L.; Zhang, Z.; Lopez, S.E.; Dolbier, W.R. Photocatalytic difluoromethylarylation of unactivated alkenes via a (hetero)aryl neophyl-like radical migration. Org. Biomol. Chem. 2022, 20, 5712–5715. [Google Scholar] [CrossRef]
- Ma, S.; Guo, Y.; Liu, L.; Shi, L.; Lei, X.; Duan, X.; Jiao, P. Gem-bromonitroalkane involved radical 1,2-aryl migration of α,α-diaryl allyl alcohol TMS ether via visible-light photoredox catalysis. J. Org. Chem. 2023, 88, 474–4756. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mao, T.; Huang, J.; Zhu, Q. Denitrogenative imidoyl radical cyclization: Synthesis of 2-substituted benzoimidazoles from 1-azido-2-isocyanoarenes. Org. Lett. 2017, 19, 3223–3226. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-Y.; Guo, L.-N.; Gu, Y.-R.; Chen, L.; Duan, X.-H. Iminyl radical-mediated controlled hydroxyalkylation of remote C(sp3)−H bond via tandem 1,5-HAT and difunctionalization of aryl alkenes. Adv. Synth. Catal. 2018, 360, 4341–4347. [Google Scholar] [CrossRef]
- Golagani, D.; Ajmeera, S.; Erb, W.; Mongin, F.; Akondi, S.M. Ferrocene catalyzed redox-neutral difunctionalization of alkenes using cycloketone oxime esters: Access to distal imido-nitriles. Chem. Commun. 2023, 59, 9259–9262. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Thirupathi, N.; Tung, C.; Xu, Z. Copper-catalyzed oxidative trifunctionalization of olefins: An access to functionalized β-keto thiosulfones. J. Org. Chem. 2018, 83, 9449–9455. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, X.; Wu, X.; Liu, T.; Zhang, Z.; Wu, J.; Zhu, C. Metal-free radical difunctionalization of ethylene. Chem 2023, 9, 472–482. [Google Scholar] [CrossRef]
- Paulus, F.; Stein, C.; Heusel, C.; Stoffels, T.J.; Daniliuc, C.G.; Glorius, F. Three-component photochemical 1,2,5-trifunctionalizations of alkenes toward densely functionalized lynchpins. J. Am. Chem. Soc. 2023, 145, 23814–23823. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Yang, X.; Fang, R.; Peng, X.; Yu, W.; Han, B. Hydrazone radical promoted vicinal difunctionalization of alkenes and trifunctionalization of allyls: Synthesis of pyrazolines and tetrahydropyridazines. J. Org. Chem. 2013, 78, 10692–10704. [Google Scholar] [CrossRef]
- Liu, X.; Tian, S.; Jiang, Y.; Rao, W.; Wang, S. Visible-light-triggered sulfonylation/aryl migration/desulfonylation and C–S/Se bond formation reaction: 1,2,4-trifunctionalization of butenyl benzothiazole sulfone with thiosulfonate/selenosulfonates. Org. Lett. 2021, 23, 8246–8251. [Google Scholar] [CrossRef]
- Liu, X.; Fang, J.; Rao, W.; Shen, D.; Yang, Z.; Wang, S. Overcoming radical stability order via DABCO-triggered desulfurization: Visible-light-promoted 1,2,4-trifunctionalization of butenyl benzothiazole sulfone with thiosulfonate. J. Org. Chem. 2024, 89, 474–483. [Google Scholar] [CrossRef]
- Zhang, Q.; Chiou, M.F.; Ye, C.; Yuan, X.; Li, Y.; Bao, H. Radical 1,2,3-tricarbofunctionalization of α-vinyl-β-ketoesters enabled by a carbon shift from an all-carbon quaternary center. Chem. Sci. 2022, 13, 6836–6841. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Du, H.; Davies, P.W.; Shu, W. Synthesis of unprotected α-tertiary amines and 1,2-amino alcohols from vinyl azides by light induced denitrogenative alkylarylation/dialkylation. CCS Chem. 2024, 6, 1060–1070. [Google Scholar] [CrossRef]
- Liu, J.; Skaria, M.; Sharma, P.; Chiang, Y.W.; Liu, R.S. Ground-state dioxygen undergoes metal-free [3 + 2]-annulations with allenes and nitrosoarenes under ambient conditions. Chem. Sci. 2017, 8, 5482–5487. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.M.; Schomaker, J.M. Allene trifunctionalization via amidyl radical cyclization and TEMPO trapping. J. Org. Chem. 2021, 86, 8891–8899. [Google Scholar] [CrossRef]
- Nobuta, T.; Hirashima, S.; Tada, N.; Miura, T.; Itoh, A. Facile aerobic photo-oxidative syntheses of α,α-dibromoacetophenones from aromatic alkynes with 48% aq HBr. Tetrahedron Lett. 2010, 51, 4576–4578. [Google Scholar] [CrossRef]
- Jafarpour, F.; Azizzade, M.; Golpazir-Sorkheh, Y.; Navid, H.; Rajai-Daryasarei, S. Divergent synthesis of α-aroyloxy ketones and indenones: A controlled domino radical reaction for di- and trifunctionalization of alkynes. J. Org. Chem. 2020, 85, 8287–8294. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xu, H.; Liu, R.; Zheng, Y.; Huang, S. Electrochemical triamination of alkynes: Controllable synthesis of functionalized indolines and indoles. Green Chem. 2022, 24, 4476–4754. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Cao, Z.; Zhang, X.; Wang, X.; Li, J.; Zhu, C. E-selective radical difunctionalization of unactivated alkynes: Preparation of functionalized allyl alcohols from aliphatic alkynes. Adv. Sci. 2024, 11, e2309022. [Google Scholar] [CrossRef]
- Yang, Y.; Daniliuc, C.G.; Studer, A. 1,1,2-Trifunctionalization of terminal alkynes by radical addition-translocation-cyclization-trapping for the construction of highly substituted cyclopentanes. Angew. Chem. Int. Ed. 2021, 60, 2145–2148. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, F.; Shu, C.; Zhu, G. Copper-catalyzed 1,2,5-trifunctionalization of terminal alkynes using SR as a transient directing group for radical translocation. Chin. J. Chem. 2022, 40, 1667–1673. [Google Scholar] [CrossRef]
- Yue, X.; Hu, M.; He, X.; Wu, S.; Li, J.-H. A radical-mediated 1,3,4-trifunctionalization cascade of 1,3-enynes with sulfinates and tert-butyl nitrite: Facile access to sulfonyl isoxazoles. Chem. Commun. 2020, 56, 6253–6256. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, H.; Wu, X.; Zhu, C. Radical trifunctionalization of hexenenitrile via remote cyano migration. Chem. Commun. 2022, 58, 1005–1008. [Google Scholar] [CrossRef]
- Wang, Z.; Chang, C.; Chen, Y.; Wu, X.; Li, J.; Zhu, C. Remote desaturation of hexenenitriles by radical-mediated cyano migration. Tetrahedron 2023, 131, 133228. [Google Scholar] [CrossRef]
- Guo, K.; Gu, C.; Li, Y.; Xie, X.; Zhang, H.; Chen, K.; Zhu, Y. Photoredox catalyzed trifluoromethyl radical-triggered trifunctionalization of 5-hexenenitriles via cyano migration. Adv. Synth. Catal. 2022, 364, 1388–1393. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Li, J.; Wei, Z.; Feng, J.; Du, D. Organocatalytic radical relay trifunctionalization of unactivated alkenes by a combination of cyano migration and alkylacylation. Chem. Commun. 2023, 59, 5395–5398. [Google Scholar] [CrossRef]
- Ni, S.; Sha, W.; Zhang, L.; Xie, C.; Mei, H.; Han, J.; Pan, Y. N-iodosuccinimide-promoted cascade trifunctionalization of alkynoates: Access to 1,1-diiodoalkenes. Org. Lett. 2016, 18, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, H.; Ramakrishna, I.; Mandal, A.; Baidya, M. Atom transfer oxidative radical cascade of aryl alkynoates towards 1,1-dichalcogenide olefins. Chem. Asian J. 2019, 14, 4549–4552. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Li, J.; Liu, Z.; Sun, H.; Shi, H.; Wang, X.; Xie, X.; She, X. Visible-light-mediated radical cascade reaction: Synthesis of 3-bromocoumarins from alkynoates. Org. Biomol. Chem. 2017, 15, 8820–8826. [Google Scholar] [CrossRef]
- Roy, M.; Jamatia, R.; Samanta, A.; Mohar, K.; Srimani, D. Change in the product selectivity in the visible light-induced selenium radical-mediated 1,4-aryl migration process. Org. Lett. 2022, 24, 8180–8185. [Google Scholar] [CrossRef]
- Peng, X.; Wei, D.; Han, W.; Chen, F.; Yu, W.; Han, B. Dioxygen activation via Cu-catalyzed cascade radical reaction: An approach to isoxazoline/cyclic nitrone-featured α-ketols. ACS Catal. 2017, 7, 7830–7834. [Google Scholar] [CrossRef]
- Wei, D.; Liu, T.; He, Y.; Wei, B.; Pan, J.; Zhang, J.; Jiao, N.; Han, B. Radical 1,4/5-amino shift enables access to fluoroalkyl-containing primary β(γ)-aminoketones under metal-free conditions. Angew. Chem. Int. Ed. 2021, 60, 26308–26313. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Li, S.J.; Liu, Q.P.; Yu, N.; Li, Y.L.; Zhou, Y.Q.; He, K.C.; Lin, J.; Zheng, T.Y.; Lang, J.; et al. Iminyl radical-triggered relay annulation for the construction of bridged aza-tetracycles bearing four contiguous stereogenic centers. Chem. Sci. 2022, 13, 7283–7288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Yu, M.; Ye, S.; Wu, J. Construction of β-amino sulfones from sodium metabisulfite via a radical 1,4-amino migration. Org. Lett. 2023, 25, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Zhang, Z.; Yang, L.; Zhang, D.; Lu, J.; Wei, J.; Wei, S.; Fu, Q.; Du, X.; Yi, D. Nitrogen-radical-triggered trifunctionalizing ipso-spirocyclization of unactivated alkenes with vinyl azides: A modular access to spiroaminal frameworks. Adv. Synth. Catal. 2021, 363, 3762–3768. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Xu, L.; Gao, P.; Duan, X.; Guo, L.N. Fe-catalyzed alkylazidation of α-trifluoromethylalkenes: An access to quaternary stereocenters containing CF3 and N3 groups. Org. Lett. 2023, 25, 1336–1341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).