Comprehensive Comparison of Three Different Medicinal Parts of Eupatorium lindleyanum DC. Using the RRLC-Q-TOF-MS-Based Metabolic Profile and In Vitro Anti-Inflammatory Activity
Abstract
1. Introduction
2. Results
2.1. Identification of the Chemical Constituents of Different Medicinal Parts
2.1.1. Alkaloid Identification
2.1.2. Flavonoid Identification
2.1.3. Sesquiterpene Lactones Identification
2.1.4. Phenolic Acid Identification
2.1.5. Diterpene Identification
2.2. Analysis of Differential Composition of Different Medicinal Parts
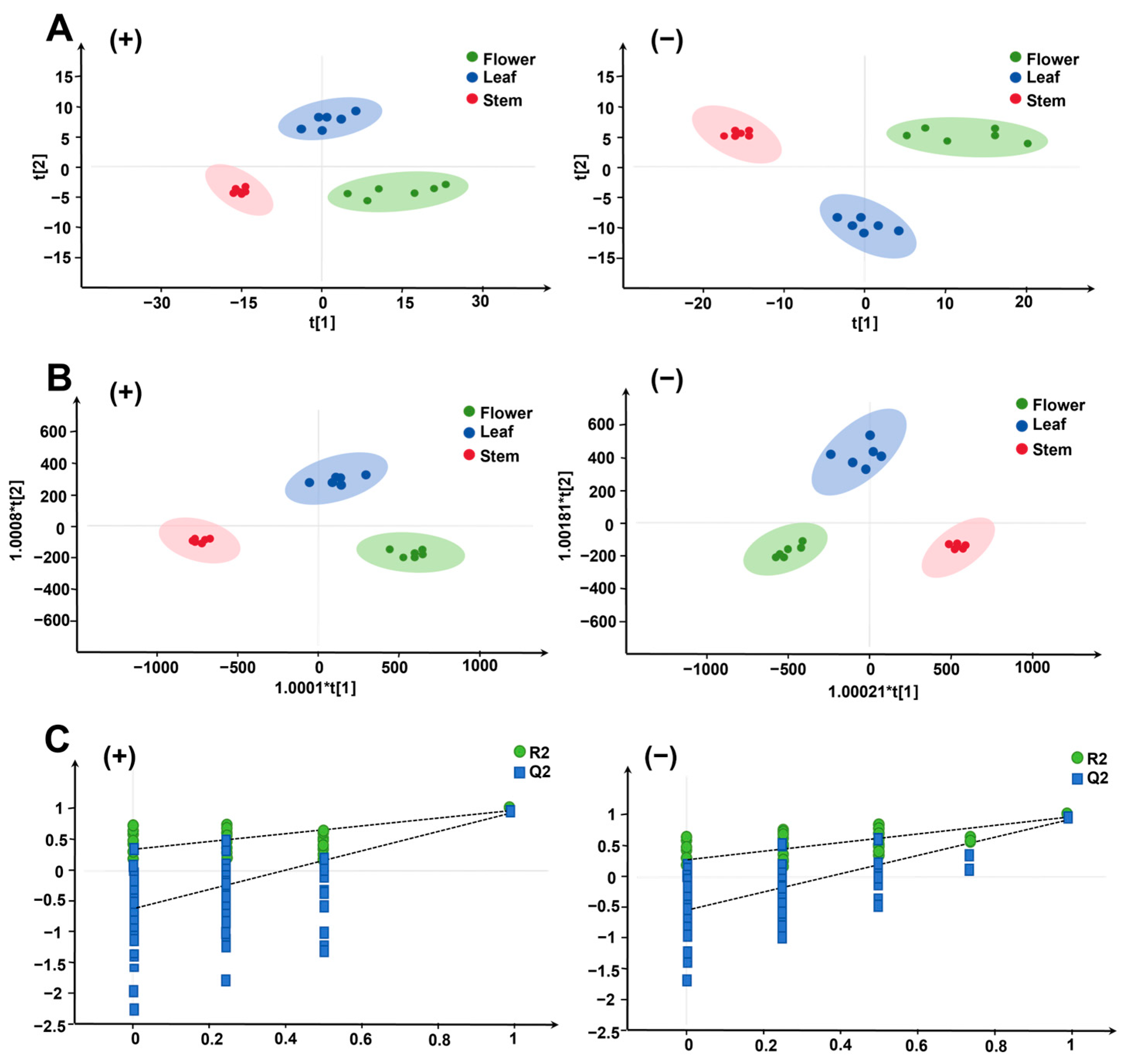
2.2.1. PCA and OPLS-DA Analysis
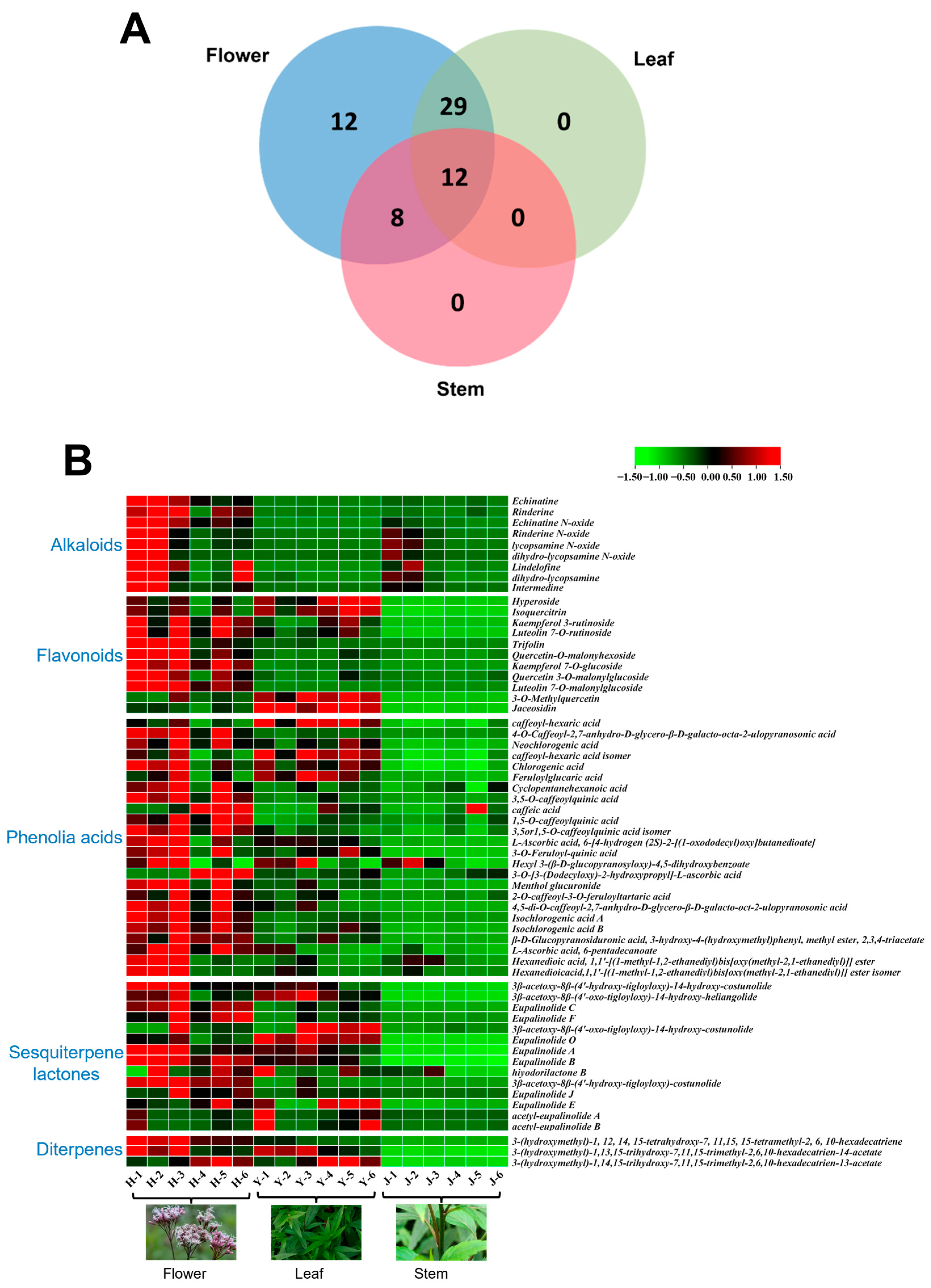
2.2.2. Wayne Analysis of Differential Components
2.2.3. Heat Map Visualization of Component Spectra
2.3. Quantitative Analysis of Four Representative Components by HPLC
2.3.1. Methodology Validation
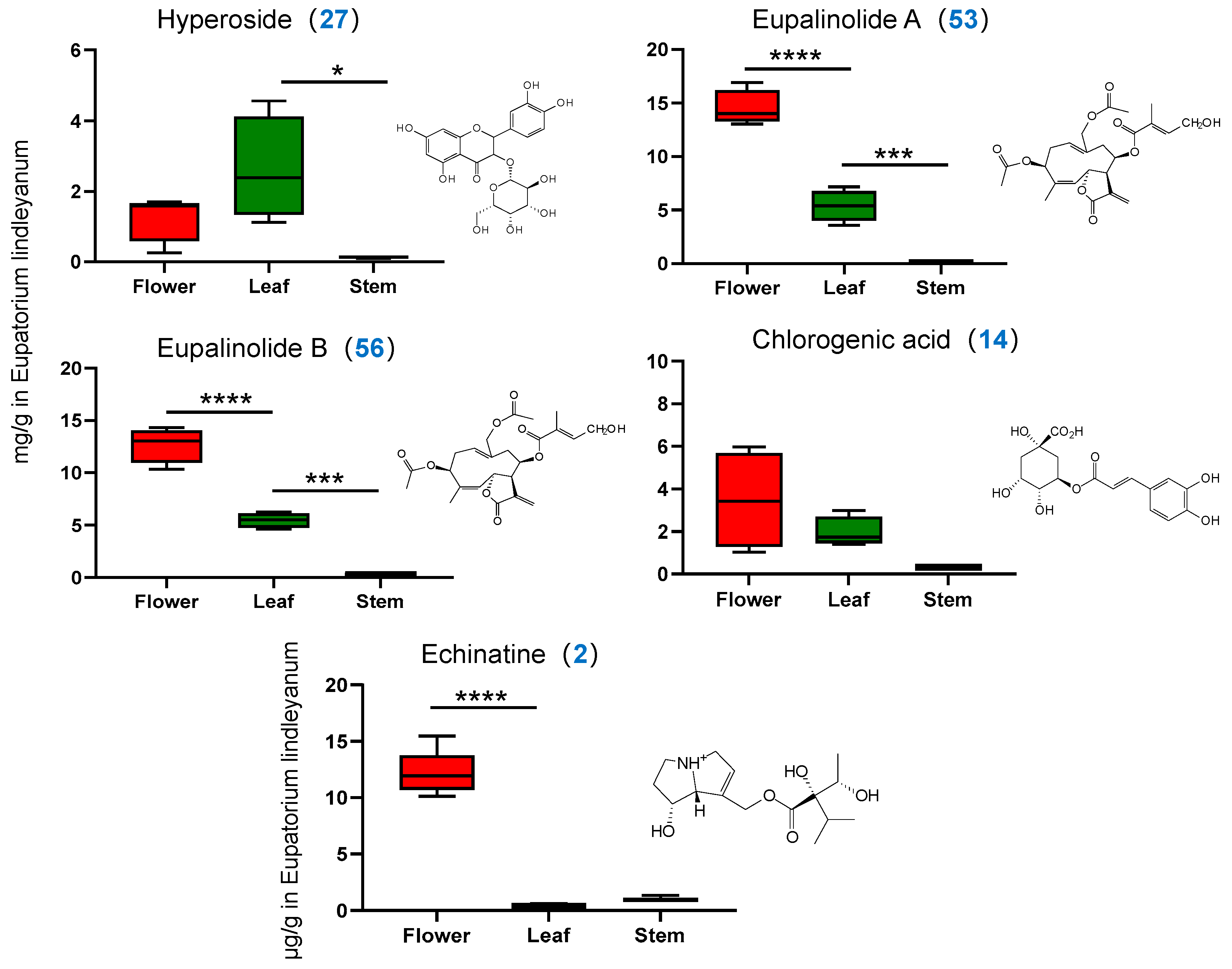
2.3.2. Determination of Content of Four Indicator Components
2.4. Quantitative Analysis of One Alkaloidal Constituent by UPLC-MS/MS
2.4.1. Methodology Validation
2.4.2. Determination of Alkaloid Content
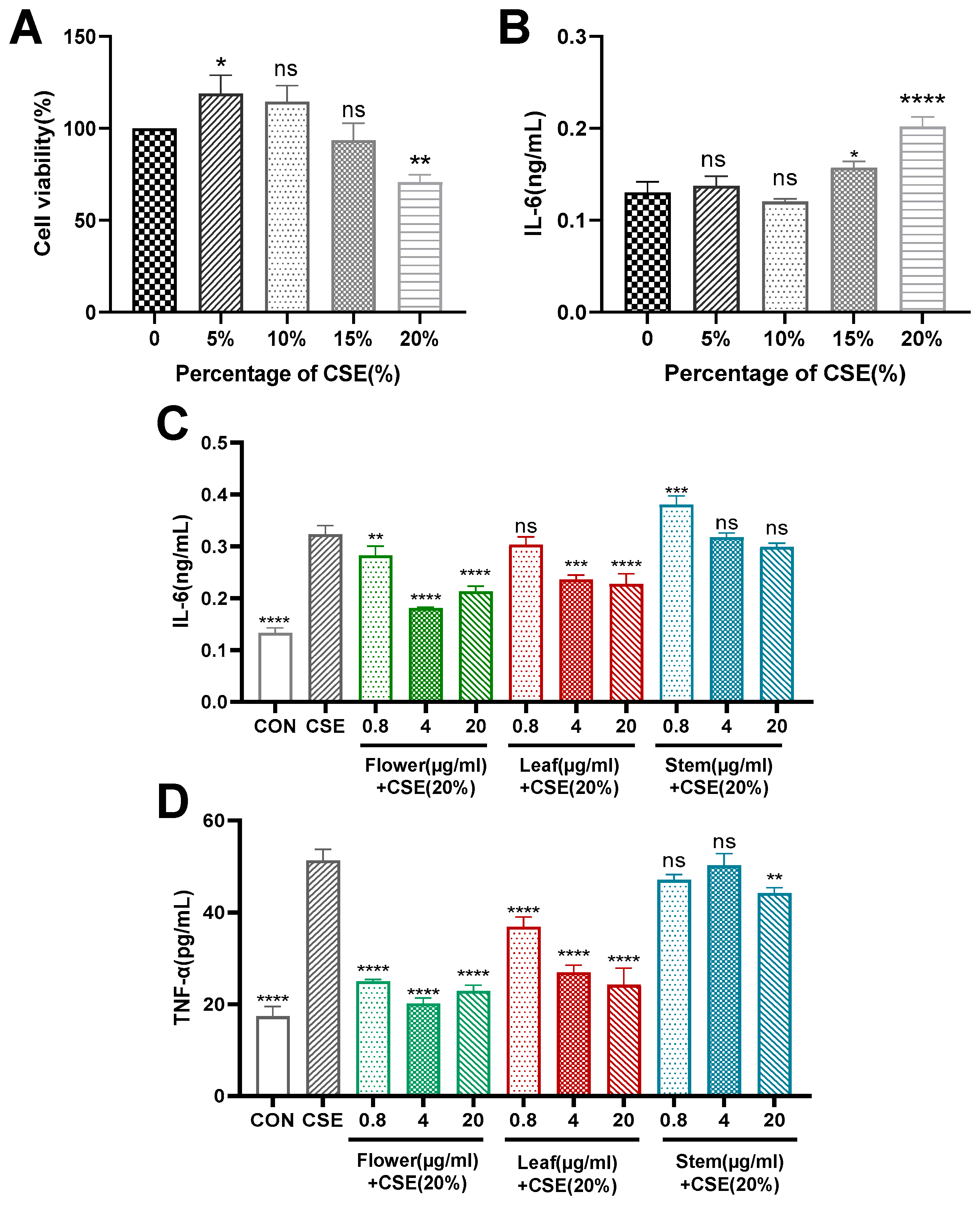
2.5. Study on the Anti-Inflammatory Activity of Different Medicinal Parts
2.5.1. Modelling Chronic Bronchitis In Vitro
2.5.2. Flower, Stem and Leaf Extracts Reduce CSE-Induced IL-6 and TNF-α Expression in 16HBE Cells
3. Discussion
4. Experimental Section
4.1. Plant Material, Standards and Reagents
4.2. Sample Preparation
4.3. Preparation of Standard Solutions
4.4. HPLC Chromatographic Conditions
4.5. RRLC-Q-TOF-MS Spectrometric Conditions
4.6. UPLC-TQ-MS/MS Spectrometric Conditions
4.7. Study on the Anti-Inflammatory Activity of Different Medicinal Parts
4.7.1. Cell Cultures
4.7.2. Preparation of Cigarette Smoke Extract (CSE)
4.7.3. In Vitro Chronic Bronchitis Modelling
4.7.4. ELISA for Inflammatory Factor Expression
4.8. Data Processing and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Qian, S.H.; Yang, N.Y.; Duan, J.A.; Yuan, L.H. Preliminary evaluation of the quality of different medicinal parts of Eupatorium lindleyanum. Res. Pract. Chin. Med. 2004, 5, 40–42. [Google Scholar] [CrossRef]
- Chu, C.; Yao, S.; Chen, J.; Wei, X.; Xia, L.; Chen, D.; Zhang, J. Eupatorium lindleyanum DC. sesquiterpenes fraction attenuates lipopolysaccharide-induced acute lung injury in mice. J. Ethnopharmacol. 2016, 185, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Y.; Tian, S.S. Research Progress on Chemical Composition and Pharmacological Effects of Eupatorium lindleyanum DC. in Chinese Herbal Medicine. Chin. J. Ethnomed. Ethnopharm. 2019, 28, 37–42. [Google Scholar] [CrossRef]
- Zhang, Y.P. Clinical observation of Eupatorium lindleyanum syrup for paediatric chronic bronchitis. J. Imaging Res. Med. Applications. 2017, 1, 141–142. [Google Scholar] [CrossRef]
- Peng, Y.R.; Dou, J.; Huang, F.; Qian, D.W. Experimental study on the anti-influenza virus of Compound Eupatorium lindleyanum DC. Capsule. Chin. Tradit. Pat. Med. 2008, 30, 650–654. [Google Scholar] [CrossRef]
- Hu, L.S. Study on the optimal harvesting period of Eupatorium lindleyanum DC. China Pharm. 2006, 02, 63–64. [Google Scholar] [CrossRef]
- Wang, X.F.; Ji, Y.; Jiang, H.L. Influence of soil properties on the mineral element content and pharmacological activity of Eupatorium lindleyanum DC. J. Anhui Agric. Sci. 2007, 11, 68–70. [Google Scholar] [CrossRef]
- Wang, F.; Zhong, H.H.; Fang, S.Q.; Zheng, Y.F.; Li, C.Y.; Peng, G.P.; Shen, X.C. Potential Anti-inflammatory Sesquiterpene Lactones from Eupatorium lindleyanum. Planta Med. 2018, 84, 123–128. [Google Scholar] [CrossRef]
- Zhong, H.H.; Fang, S.Q.; Chen, Y.J.; Li, C.Y.; Zheng, Y.F.; Peng, G.P. Chemical constituents and their anti-inflammatory activities of Eupatorium lindleyanum. Chin. Tradit. Pat. Med. 2017, 39, 329–333. [Google Scholar] [CrossRef]
- Huang, L.; Xu, D.; Chen, Y.; Fu, R.; Yue, S.; Yin, J.; Tang, Y. Qualitative and quantitative analysis of chemical components in Eupatorium lindleyanum DC. by ultra-performance liquid chromatography-mass spectrometry integrated with anti-inflammatory activity research. J. Sep. Sci. 2021, 17, 3174–3187. [Google Scholar] [CrossRef]
- He, H.X.; Kong, L.X.; Li, X.Y.; Zhou, Y.D. Effects of kaempferol and total flavonoids of Eupatorium lindleyanum on lipid-lowering and hemorheology in experimental hyperlipidemic rats compared with that. J. Army Med. Univ. 2014, 36, 1187–1189. [Google Scholar] [CrossRef]
- Wang, S.; Wu, H.X.; Lou, T.C.; Yu, S.F.; Xie, Y.P. Study on the in vivo and in vitro anti-breast cancer activity and mechanism of total flavonoids from Eupatorium lindleyanum DC. Chin. J. Mod. Appl. Pharm. 2020, 37, 2073–2080. [Google Scholar] [CrossRef]
- These, A.; Bodi, D.; Ronczka, S.; Lahrssen-Wiederholt, M.; Preiss-Weigert, A. Structural screening by multiple reaction monitoring as a new approach for tandem mass spectrometry: Presented for the determination of pyrrolizidine alkaloids in plants. Anal. Bioanal. Chem. 2013, 405, 9375–9383. [Google Scholar] [CrossRef]
- Zan, K.; Hu, X.; Li, Y.; Wang, Y.; Jin, H.; Zuo, T.; Ma, S. Simultaneous determination of eight pyrrolizidine alkaloids in various parts of Eupatorium lindleyanum by ultra-high-performance liquid chromatography tandem mass spectrometry and risk assessments based on a real-life exposure scenario. J. Sep. Sci. 2021, 44, 3237–3247. [Google Scholar] [CrossRef]
- Li, J.; Jiang, K.; Wang, L.J.; Yin, G.; Wang, J.; Wang, Y.; Jin, Y.B.; Li, Q.; Wang, T.J. HPLC-MS/MS determination of flavonoids in Gleditsiae Spina for its quality assessment. J. Sep. Sci. 2018, 41, 1752–1763. [Google Scholar] [CrossRef]
- Yang, N.Y.; Duan, J.A.; Shang, E.X.; Tian, L.J. Analysis of sesquiterpene lactones in Eupatorium lindleyanum by HPLC-PDA-ESI-MS/MS. Phytochem. Anal. 2010, 21, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.H.; Liu, X.; Wang, Y.H.; Song, X.X.; Yang, Y.; Li, Q. Analysis of the in vivo metabolites of Eupalinolide F in rats based on UPLC-Q-TOF-MS. Nat. Prod. Res. Dev. 2019, 31, 2137–2143. [Google Scholar] [CrossRef]
- Hernandez, J.; Müller, A.; Jaiswal, R.; Davalos, J.Z.; Kuhnert, N. Energy resolved mass spectrometry of chlorogenic acids and its application to isomer quantification by direct infusion tandem mass spectrometry. Phytochem. Anal. 2018, 29, 406–412. [Google Scholar] [CrossRef]
- Takenaka, M.; Yan, X.; Ono, H.; Yoshida, M.; Nagata, T.; Nakanishi, T. Caffeic acid derivatives in the roots of yacon (Smallanthus sonchifolius). J. Agric Food Chem. 2003, 51, 793–796. [Google Scholar] [CrossRef]
- Wu, S.Q.; Xu, N.Y.; Zhang, J.; Yao, S.; Chu, C.J. Three new acyclic diterpenoids from Eupatorium lindleyanum DC. J Asian Nat Prod Res. 2012, 14, 652–656. [Google Scholar] [CrossRef]
- Manevski, M.; Devadoss, D.; Long, C.; Singh, S.P.; Nasser, M.W.; Borchert, G.M.; Nair, M.N.; Rahman, I.; Sopori, M.; Chand, H.S. Increased Expression of LASI lncRNA Regulates the Cigarette Smoke and COPD Associated Airway Inflammation and Mucous Cell Hyperplasia. Front Immunol. 2022, 13, 803362. [Google Scholar] [CrossRef]
- Shang, L.Z.; Zhang, Z.J.; Xie, W.Y.; Zhang, L.Z.; Chang, X.H.; Hu, W.H. Effects of Aero-coumadin on tumour necrosis factor-alpha, macrophage inflammatory protein-2, myeloperoxidase, inflammatory cells and lung histopathological changes in rats with chronic obstructive pulmonary disease. Chin. J. Gerontol. 2015, 35, 5705–5708. [Google Scholar] [CrossRef]
- Yu, D.; Wang, F.; Ye, S.M.; Yang, S.; Yu, N.; Zhou, X.Y.; Zhang, N. Quercitrin protects human bronchial epithelial cells from oxidative damage. Open Med. 2022, 17, 375–383. [Google Scholar] [CrossRef]
- Jayachandran, M.; Wu, Z.; Ganesan, K.; Khalid, S.; Chung, S.; Xu, B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chem.-Biol. Interact. 2019, 303, 62–69. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Q.; Wang, T.; Liu, J.; Chen, D. Comparison of the Antioxidant Effects of Quercitrin and Isoquercitrin: Understanding the Role of the 6″-OH Group. Molecules. 2016, 21, 1246. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic acid: A review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef]
- Dusemund, B.; Nowak, N.; Sommerfeld, C.; Lindtner, O.; Schäfer, B.; Lampen, A. Risk assessment of pyrrolizidine alkaloids in food of plant and animal origin. Food Chem Toxicol. 2018, 115, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Wang, Y.H.; Wang, M.; Smillie, T.J.; Khan, I.A. Simultaneous determination of sesquiterpenes and pyrrolizidine alkaloids from the rhizomes of Petasites hybridus (L.) G.M. et Sch. and dietary supplements using UPLC-UV and HPLC-TOF-MS methods. J. Pharm. Biomed Anal. 2012, 70, 53–63. [Google Scholar] [CrossRef]
- Song, Z.; Lian, W.; He, Y.; Zhang, C.; Lin, G. Targeting erythrocyte-mediated hypoxia to alleviate lung injury induced by pyrrolizidine alkaloids. Arch Toxicol. 2023, 97, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Hulina-Tomašković, A.; Heijink, I.H.; Jonker, M.R.; Somborac-Bačura, A.; Grdić Rajković, M.; Rumora, L. Pro-inflammatory effects of extracellular Hsp70 and cigarette smoke in primary airway epithelial cells from COPD patients. Biochimie 2019, 156, 47–58. [Google Scholar] [CrossRef]
- Mais, E.; Alolga, R.N.; Wang, S.L.; Linus, L.O.; Yin, X.; Qi, L.W. A comparative UPLC-Q/TOF-MS-based metabolomics approach for distinguishing Zingiber officinale Roscoe of two geographical origins. Food Chem. 2018, 240, 239–244. [Google Scholar] [CrossRef] [PubMed]
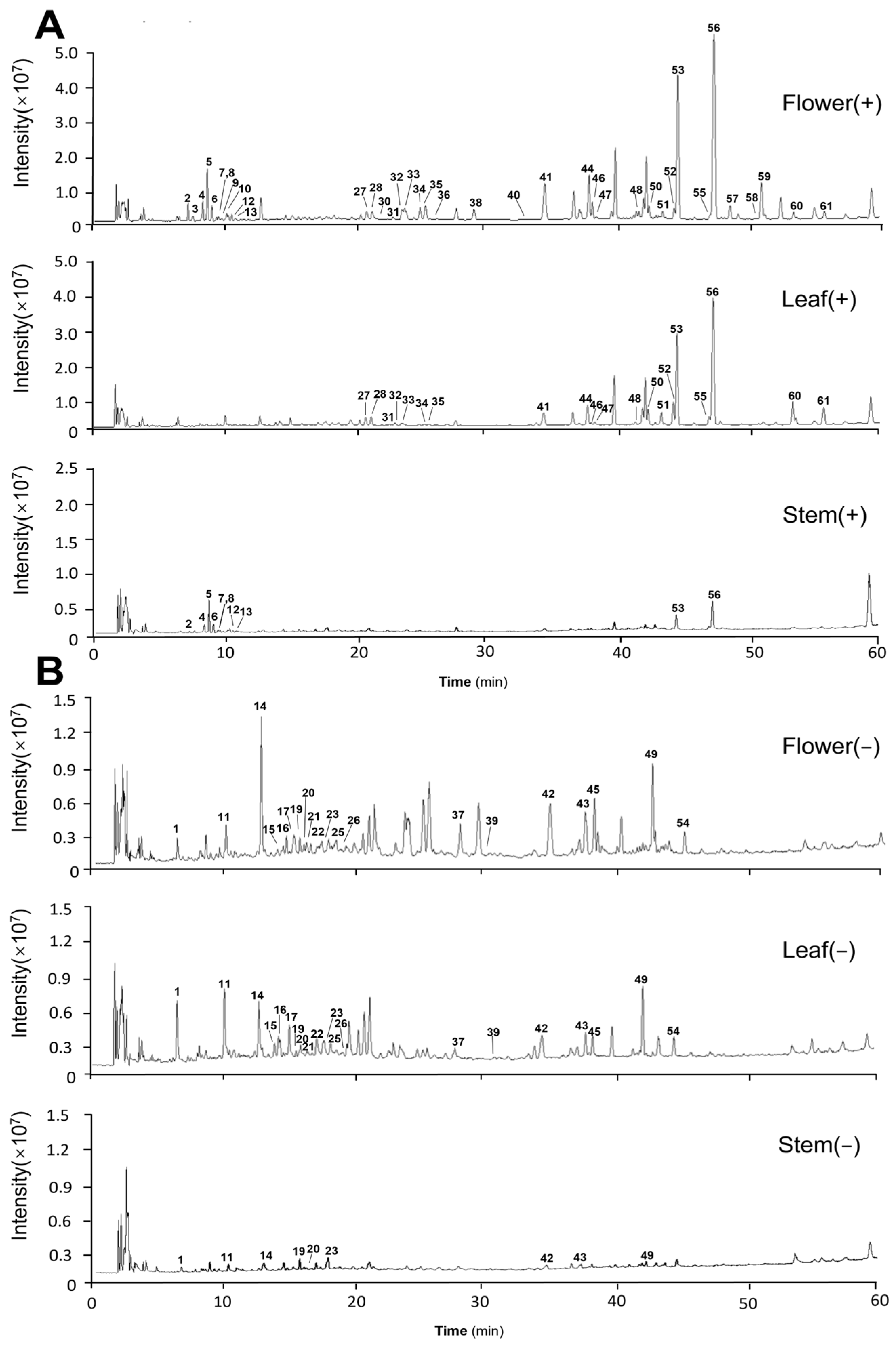

| NO. | RT (min) | Protonated Molecular Ion | Measured (m/z) | Characteristic Fragment Ions | Error (ppm) | Molecular Formula | Identification | Stem | Leaf | Flower |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.52 | [M-H]− | 371.0624 | 209.0318, 191.0195, 179.0352, 135.0452, 85.0307 | 1.1 | C15H16O11 | Caffeoyl-hexaric acid | + | + | + |
| 2 | 7.31 | [M+H]+ | 300.1804 | 156.1017, 138.0917, 120.0814, 94.0617 | −0.5 | C15H25NO5 | Echinatine * | + | − | + |
| 3 | 7.67 | [M+H]+ | 300.1804 | 156.1018, 138.0918, 120.0813, 94.0672 | −0.5 | C15H25NO5 | Rinderine | − | − | + |
| 4 | 8.43 | [M+H]+ | 316.1754 | 172.0964, 155.0943, 154.0864, 138.0918, 136.0762 | −0.2 | C15H25NO6 | Echinatine N-oxide | + | − | + |
| 5 | 8.77 | [M+H]+ | 316.1755 | 172.0956, 155.0932, 154.0854, 138.0910, 136.0755 | 0.1 | C15H25NO6 | Rinderine N-oxide | + | − | + |
| 6 | 9.13 | [M+H]+ | 316.1755 | 172.0973, 155.0948, 138.0924, 136.0770 | 0.1 | C15H25NO6 | Lycopsamine N-oxide | + | − | + |
| 7 | 9.48 | [M+H]+ | 318.1909 | 174.1126,156.1020,113.0848,82.0686 | −0.7 | C15H27NO6 | Dihydro-lycopsamine N-oxide | + | − | + |
| 8 | 9.69 | [M-H]− | 397.0776 | 235.0450, 179.0342, 161.0244, 135.0452, 133.0295 | −0.1 | C17H18O11 | 4-o-Caffeoyl-2,7-anhydro-d-glycero-β-d-galacto-octa-2-ulopyranosonic acid | + | − | + |
| 9 | 9.87 | [M+H]+ | 286.2013 | 142.1232, 124.1131, 96.0832 | −1.0 | C15H27NO4 | Lindelofine | − | − | + |
| 10 | 10.04 | [M-H]− | 353.0882 | 191.0572, 179.0358, 135.0453 | 1.1 | C16H18O9 | Neochlorogenic acid | − | − | + |
| 11 | 10.18 | [M-H]− | 371.0625 | 209.0299, 191.0204, 85.0307 | 1.4 | C15H16O11 | Caffeoyl-hexaric acid isomer | + | + | + |
| 12 | 10.38 | [M+H]+ | 302.1959 | 158.1173, 141.1149, 140.1074, 124.1130, 110.0978 | −1.0 | C15H27NO5 | Dihydro-lycopsamine | + | − | + |
| 13 | 10.65 | [M+H]+ | 300.1804 | 156.1018, 139.0996, 138.0916, 122.0970, 120.0815 | −0.5 | C15H25NO5 | Intermedine | + | − | + |
| 14 | 12.82 | [M-H]− | 353.0882 | 191.0547, 179.0336, 135.0441 | 1.1 | C16H18O9 | Chlorogenic acid * | + | + | + |
| 15 | 14.09 | [M-H]− | 385.0776 | 209.0301, 178.0294, 134.0360, 85.0305 | −0.1 | C16H18O11 | Feruloylglucaric acid | − | + | + |
| 16 | 14.73 | [M-H]−/ [M+Cl]−/ [M+FA-H]− | 343.1403/ 379.1169/ 389.1457 | 180.0784, 165.0551, 135.0442, 81.0352 | 1.3/ 1.0/ 1.0 | C16H24O8 | Cyclopentanehexanoic acid | − | + | + |
| 17 | 15.28 | [M-H]− | 533.0924 | 209.0300, 191.0197, 179.0347, 85.0306 | −2.4 | C24H26O14 | 3,5-O-caffeoylquinic acid | − | + | + |
| 18 | 15.45 | [M-H]− | 179.0362 | 135.0462 | −6.8 | C9H8O4 | Caffeic acid | − | − | + |
| 19 | 15.73 | [M-H]− | 533.0925 | 209.0301, 191.0189, 179.0342, 85.0306 | −2.2 | C24H22O14 | 1,5-O-caffeoylquinic acid | + | + | + |
| 20 | 16.25 | [M-H]− | 533.0924 | 209.0306, 191.0201, 179.0332, 85.0312 | −2.4 | C24H22O14 | 3,5or1,5-O-caffeoylquinic acid isomer | + | + | + |
| 21 | 16.54 | [M-H]− | 473.2021 | 265.1350, 247.1302, 203.1437, 119.0342, 93.0722 | −1.6 | C22H34O11 | l-Ascorbic acid,6-[4-hydrogen (2S)-2-[(1-oxododecyl)oxy]butanedioate] | − | + | + |
| 22 | 17.38 | [M-H]− | 367.1039 | 191.0560, 173.0445, 134.0373, 93.0354 | 1.2 | C17H20O9 | 3-O-Feruloyl-quinic acid | − | + | + |
| 23 | 17.89 | [M-H]−/ [M+Cl]−/ [M+FA-H]− | 415.1605/ 451.1369/ 461.1657 | 191.0548, 149.0436, 131.0350, 89.0252 | −1.1/ −1.7/ −1.6 | C19H28O10 | Hexyl-3-(β-d-glucopyranosyloxy)-4,5-dihydroxybenzoate | + | + | + |
| 24 | 18.05 | [M+FA-H]− | 463.2539 | 255.1961, 159.0329, 113.0246, 89.0265 | −2.1 | C21H38O8 | 3-O-[3-(Dodecyloxy)-2-hydroxypropyl]-L-ascorbic acid | − | − | + |
| 25 | 18.46 | [M+FA-H]− | 377.1819 | 331.1754, 179.0551, 161.0443, 119.0345, 89.0251, 71.0154, 59.0162 | 0.5 | C16H28O7 | Menthol glucuronide | − | + | + |
| 26 | 19.21 | [M+FA-H]−/ [M+AcO-H]− | 533.092/ 547.1078 | 209.0300, 191.0200, 179.0347, 85.0302 | −3.2/ −2.8 | C23H20O12 | 2-O-caffeoyl-3-O-feruloyltartaric acid | − | + | + |
| 27 | 20.93 | [M+H]+ | 465.1017 | 303.0490, 257.0436, 229.0493, 201.0544, 165.0181, 153.0182, 137.0236 | −2.3 | C21H20O12 | Hyperoside * | − | + | + |
| 28 | 21.35 | [M+H]+ | 465.1019 | 303.0496, 257.0436, 229.0494, 201.0535, 165.0178, 153.0187, 85.0318 | −1.8 | C21H20O12 | Isoquercitrin | − | + | + |
| 29 | 21.49 | [M-H]− | 559.1078 | 397.0790, 235.0469, 179.0355, 161.0245, 135.0445 | −2.7 | C26H24O14 | 4,5-di-O-caffeoyl-2,7-anhydro-d-glycero-β-d-galacto-oct-2-ulopyranosonic acid | − | + | + |
| 30 | 21.68 | [M+H]+ | 595.1642 | 287.0552 | −2.6 | C27H30O15 | Kaempferol 3-rutinoside | − | − | + |
| 31 | 22.94 | [M+H]+ | 595.1641 | 287.0548 | −2.8 | C27H30O15 | Luteolin 7-O-rutinoside | − | + | + |
| 32 | 23.63 | [M+H]+ | 449.1073 | 287.0548, 258.0512, 213.0547, 165.0180, 153.0187, 121.0296, 85.0317 | −1.2 | C21H20O11 | Trifolin | − | + | + |
| 33 | 23.95 | [M+H]+ | 551.1015 | 303.0496, 257.0440, 165.0185, 85.0319 | −3.0 | C24H22O15 | Quercetin-O-malonyhexoside | − | + | + |
| 34 | 25.01 | [M+H]+ | 449.1070 | 287.0545, 241.0494, 213.0544, 153.0185, 121.0293, 85.0316 | −1.9 | C21H20O11 | Kaempferol 7-O-glucoside | − | + | + |
| 35 | 25.45 | [M-H]− | 515.1190 | 191.0554, 179.0346, 135.0447 | −1.0 | C25H24O12 | Isochlorogenic acid A | − | + | + |
| 36 | 25.92 | [M+H]+ | 551.1011 | 303.0488, 257.0413, 165.0179, 85.0321 | −3.7 | C24H22O15 | Quercetin 3-O-malonylglucoside | − | − | + |
| 37 | 27.77 | [M-H]− | 515.1188 | 191.0564, 179.0353, 135.0453 | −1.4 | C25H24O12 | Isochlorogenic acid B | − | + | + |
| 38 | 29.13 | [M+H]+ | 535.1057 | 287.0545, 258.0520, 213.0544, 153.0188, 85.0319 | −4.7 | C24H22O14 | Luteolin 7-O-malonylglucoside | − | − | + |
| 39 | 30.07 | [M-H]− | 455.1192 | 209.0272, 191.0223, 147.0279, 85.0305 | −0.7 | C20H24O12 | β-d-Glucopyranosiduronicacid,3-hydroxy-4-(hydroxymethyl)phenyl, methylester,2,3,4-triacetate | − | + | + |
| 40 | 32.61 | [M+Cl]−/ [M+FA-H]− | 435.2150/ 445.2438 | 179.0562, 143.0296, 89.0245 | −1.2/ −1.1 | C21H36O7 | l-Ascorbic acid, 6-pentadecanoate | − | − | + |
| 41 | 34.50 | [M+NH4]+/ [M+Na]+ | 438.2112/ 443.1664 | 383.1466, 267.0990, 237.0881, 181.1025 | −2.4/ −2.8 | C22H28O8 | 3β-acetoxy-8β-(4′-hydroxy-tigloyloxy)-14-hydroxy-costunolide | − | + | + |
| 42 | 34.67 | [M-H]−/ [M+Cl]−/ [M+FA-H]− | 447.2225/ 483.1991/ 493.2286 | 315.1919, 161.0455, 85.0308 | −2.4/ −2.4/ −0.9 | C21H36O10 | Hexanedioicacid,1,1′-[(1-methyl-1,2-ethanediyl)bis[oxy(methyl-2,1-ethanediyl)]] ester | + | + | + |
| 43 | 37.27 | [M-H]−/ [M+Cl]−/ [M+FA-H]− | 447.2228/ 483.1991/ 493.2283 | 315.1806, 191.0563, 161.0455 | −1.7/ −2.4/ −1.5 | C21H36O10 | Hexanedioicacid,1,1′-[(1-methyl-1,2-ethanediyl)bis[oxy(methyl-2,1-ethanediyl)]] ester isomer | + | + | + |
| 44 | 37.83 | [M+H]+/ [M+NH4]+/ [M+Na]+ | 419.1689/ 436.1954/ 441.1497 | 267.2083, 243.0872, 197.0955, 181.0654 | −2.7/ −2.7/ −5.2 | C22H26O8 | 3β-acetoxy-8β-(4′-oxo-tigloyloxy)-14-hydroxy-heliangolide | − | + | + |
| 45 | 37.88 | [M-H]−/ [M+Cl]−/ [M+FA-H]− | 355.2496/ 391.2257/ 401.2544 | 231.1793, 161.1863, 123.0797 | 1.7/ 0.1/ −0.2 | C20H36O5 | 3-(hydroxymethyl)-1,12,14,15-tetrahydroxy-7,11,15,15-tetramethyl-2,6,10-hexadecatriene | − | + | + |
| 46 | 38.14 | [M+H]+ | 421.1844 | 227.1052, 209.0959, 199.1094, 181.1010, 165.0703 | −3.1 | C22H26O8 | Eupalinolide C | − | + | + |
| 47 | 38.42 | [M+H]+ | 317.0657 | 302.0425, 168.0046, 140.0107, 137.0243 | −1.2 | C16H12O7 | 3-O-Methylquercetin | − | + | + |
| 48 | 41.68 | [M+H]+ | 421.1846 | 209.0919, 199.0769, 181.1015, 165.0704 | −2.6 | C22H28O8 | Eupalinolide F | − | + | + |
| 49 | 42.26 | [M-H]−/ [M+Cl]−/ [M+FA-H]− | 397.2594/ 433.2358/ 443.2646 | 59.0161 | −0.4/ −1.0/ −1.0 | C22H38O6 | 3-(hydroxymethyl)-1,13,15-trihydroxy-7,11,15-trimethyl-2,6,10-hexadecatrien-14-acetate | + | + | + |
| 50 | 42.46 | [M+H]+/ [M+NH4]+/ [M+Na]+ | 419.1693/ 436.1954/ 441.1511 | 267.2095, 243.0978, 225.0911, 197.0960, 165.0672, 154.0776 | −1.8/ −2.7/ −2.0 | C22H26O8 | 3β-acetoxy-8β-(4′-oxo-tigloyloxy)-14-hydroxy-costunolide | − | + | + |
| 51 | 43.48 | [M+H]+ | 331.0806 | 316.0570, 301.0367, 273.0399, 245.0441 | −1.9 | C17H14O7 | Jaceosidin | − | + | + |
| 52 | 44.38 | [M+H]+/ [M+NH4]+/ [M+Na]+ | 419.1697/436.1955/ 441.1510 | 243.1078, 225.0942, 197.0979, 154.0779 | −0.8/ −2.5/ −2.2 | C22H26O8 | Eupalinolide O | − | + | + |
| 53 | 44.66 | [M+H]+/ [M+NH4]+/ [M+Na]+ | 463.1944/480.2212/485.1769 | 227.1070, 209.0962, 199.0751, 181.1006, 165.0695 | −4.0/ −3.3/ −2.7 | C24H30O9 | Eupalinolide A * | + | + | + |
| 54 | 44.67 | [M-H]− [M+Cl]−/ [M+FA-H]− | 397.2595/ 433.2361/ 443.2650 | 59.0158 | −0.2/ −0.3/ −0.1 | C22H38O6 | 3-(hydroxymethyl)-1,14,15-trihydroxy-7,11,15-trimethyl-2,6,10-hexadecatrien-13-acetate | − | + | + |
| 55 | 47.10 | [M+H]+ | 405.1913 | 183.1125, 153.0691 | 1.3 | C22H28O7 | hiyodorilactone B | − | + | + |
| 56 | 47.42 | [M+H]+ | 463.1949 | 227.1051, 209.0965, 181.1010, 165.0695 | −2.9 | C24H30O9 | Eupalinolide B * | + | + | + |
| 57 | 48.64 | [M+H]+/ [M+NH4]+/ [M+Na]+ | 405.1901/ 422.2162/ 427.1713 | 195.0812, 183.1176, 168.0937, 153.0698 | −1.7/ −2.7/ −3.3 | C22H28O7 | 3β-acetoxy-8β-(4′-hydroxy-tigloyloxy)- costunolide | − | − | + |
| 58 | 50.24 | [M+H]+ | 405.1894 | 183.1219, 179.0852, 165.0693, 153.0705 | −3.4 | C22H28O7 | Eupalinolide J | − | − | + |
| 59 | 51.26 | [M+NH4]+/ [M+Na]+ | 478.2069/ 483.1624 | 225.1020, 197.0946, 181.1020 | −0.5/ −0.3 | C24H28O9 | Eupalinolide E | − | − | + |
| 60 | 53.49 | [M+H]+/ [M+NH4]+/ [M+Na]+ | 505.2057/ 522.2325/ 527.1879 | 209.0963, 199.0748, 181.1019 | −2.2/ −1.7/ −1.6 | C26H32O10 | acetyl-eupalinolide A | − | + | + |
| 61 | 55.84 | [M+H]+/ [M+NH4]+/ [M+Na]+ | 505.2052/ 522.2320/ 527.1872 | 209.0956, 199.1117, 181.1009 | −3.2/ −2.6/ −3.0 | C26H32O10 | acetyl-eupalinolide B | − | + | + |
| Detection Methods | Analyte | Linearity | LOD (µg·mL−1) | LOQ (µg·mL−1) | ||
|---|---|---|---|---|---|---|
| Calibration Curve | r2 | Range (µg·mL−1) | ||||
| HPLC-PDA | Hyperoside (27) | Y = 9870.2X − 11136 | 0.9999 | 3.47~222 | 1.16 | 3.47 |
| HPLC-PDA | Eupalinolide A (53) | Y = 11271X + 37666 | 0.9999 | 8.30~531 | 2.77 | 8.30 |
| HPLC-PDA | Eupalinolide B (56) | Y = 17940X − 15365 | 0.9999 | 7.79~510 | 2.67 | 7.97 |
| HPLC-PDA | Chlorogenic acid (14) | Y = 5346.4X − 25388 | 0.9999 | 7.56~484 | 2.52 | 7.56 |
| UPLC−MS/MS | Echinatine (2) | Y = 88139X + 255621 | 0.9973 | 0.000664~0.170 | 0.000050 | 0.000150 |
| Samples | Batch No. | Regions |
|---|---|---|
| S1 | 20220805 | Jiangsu |
| S2 | 20220818 | Jiangsu |
| S3 | 20220826 | Jiangsu |
| S4 | 20220902 | Jiangsu |
| S5 | 20220921 | Jiangsu |
| S6 | 20221001 | Jiangsu |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Zheng, C.; Xue, S.; Gao, Y.; Chen, G.; Shan, C.; Ding, N.; Peng, G.; Li, C.; Zheng, Y. Comprehensive Comparison of Three Different Medicinal Parts of Eupatorium lindleyanum DC. Using the RRLC-Q-TOF-MS-Based Metabolic Profile and In Vitro Anti-Inflammatory Activity. Molecules 2024, 29, 3551. https://doi.org/10.3390/molecules29153551
Lu J, Zheng C, Xue S, Gao Y, Chen G, Shan C, Ding N, Peng G, Li C, Zheng Y. Comprehensive Comparison of Three Different Medicinal Parts of Eupatorium lindleyanum DC. Using the RRLC-Q-TOF-MS-Based Metabolic Profile and In Vitro Anti-Inflammatory Activity. Molecules. 2024; 29(15):3551. https://doi.org/10.3390/molecules29153551
Chicago/Turabian StyleLu, Jiaojiao, Chengbo Zheng, Simin Xue, Ye Gao, Guijin Chen, Chenxiao Shan, Ning Ding, Guoping Peng, Cunyu Li, and Yunfeng Zheng. 2024. "Comprehensive Comparison of Three Different Medicinal Parts of Eupatorium lindleyanum DC. Using the RRLC-Q-TOF-MS-Based Metabolic Profile and In Vitro Anti-Inflammatory Activity" Molecules 29, no. 15: 3551. https://doi.org/10.3390/molecules29153551
APA StyleLu, J., Zheng, C., Xue, S., Gao, Y., Chen, G., Shan, C., Ding, N., Peng, G., Li, C., & Zheng, Y. (2024). Comprehensive Comparison of Three Different Medicinal Parts of Eupatorium lindleyanum DC. Using the RRLC-Q-TOF-MS-Based Metabolic Profile and In Vitro Anti-Inflammatory Activity. Molecules, 29(15), 3551. https://doi.org/10.3390/molecules29153551






