Tungsten Molecular Species in Deuterium Plasmas in Contact with Sputtered W Surfaces
Abstract
1. Introduction
2. Experimental Setups and Methods
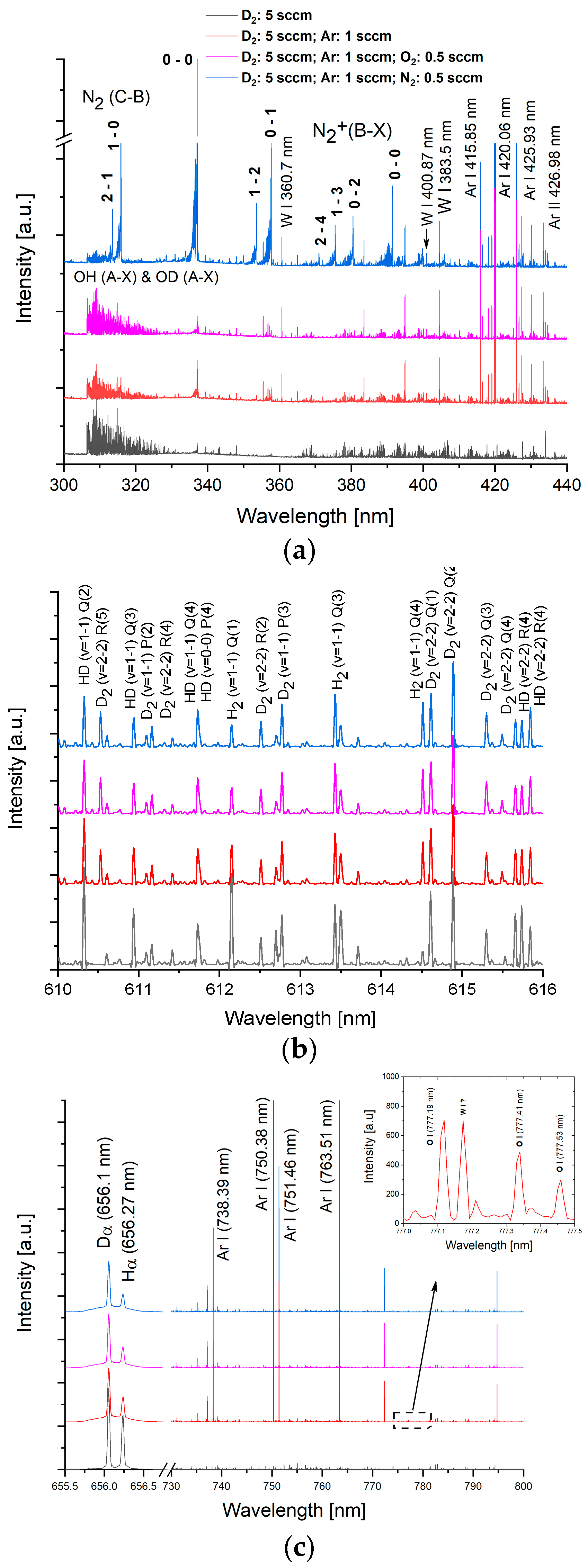
2.1. Generation of W Plasma and Characterization Techniques
2.2. Mass Spectra Processing Methodology
3. Results
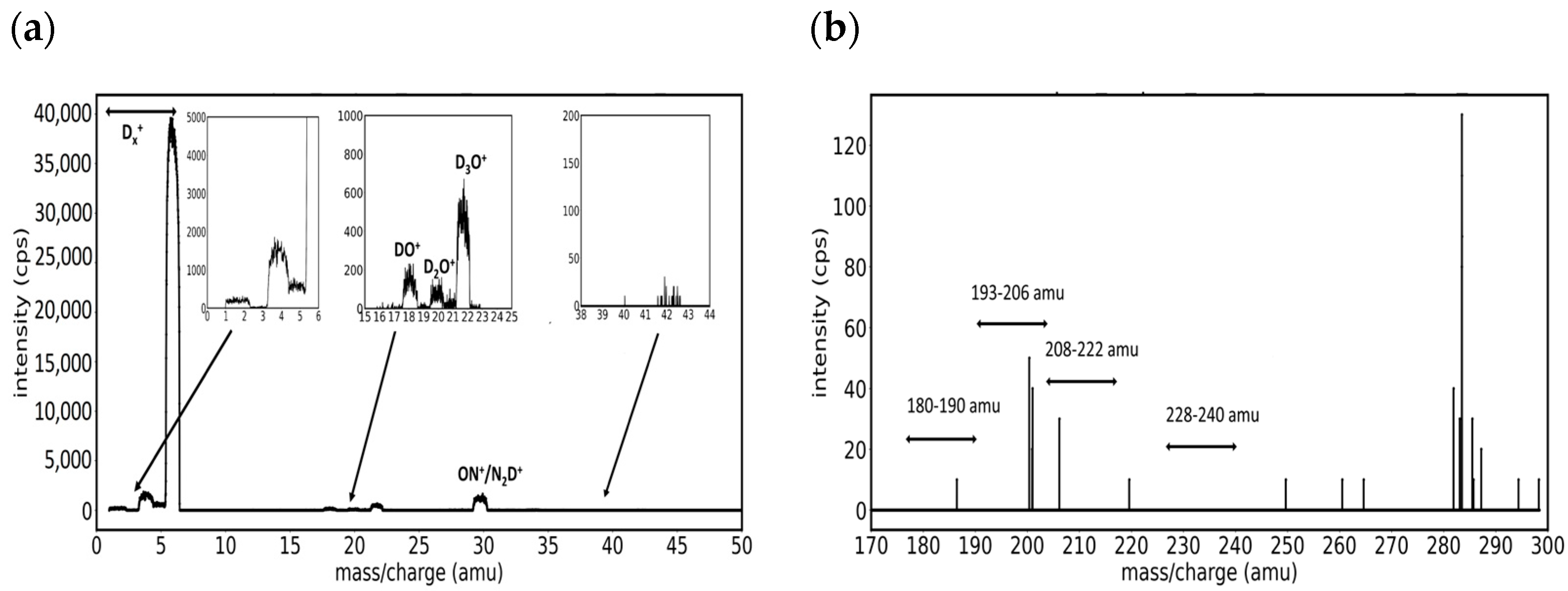
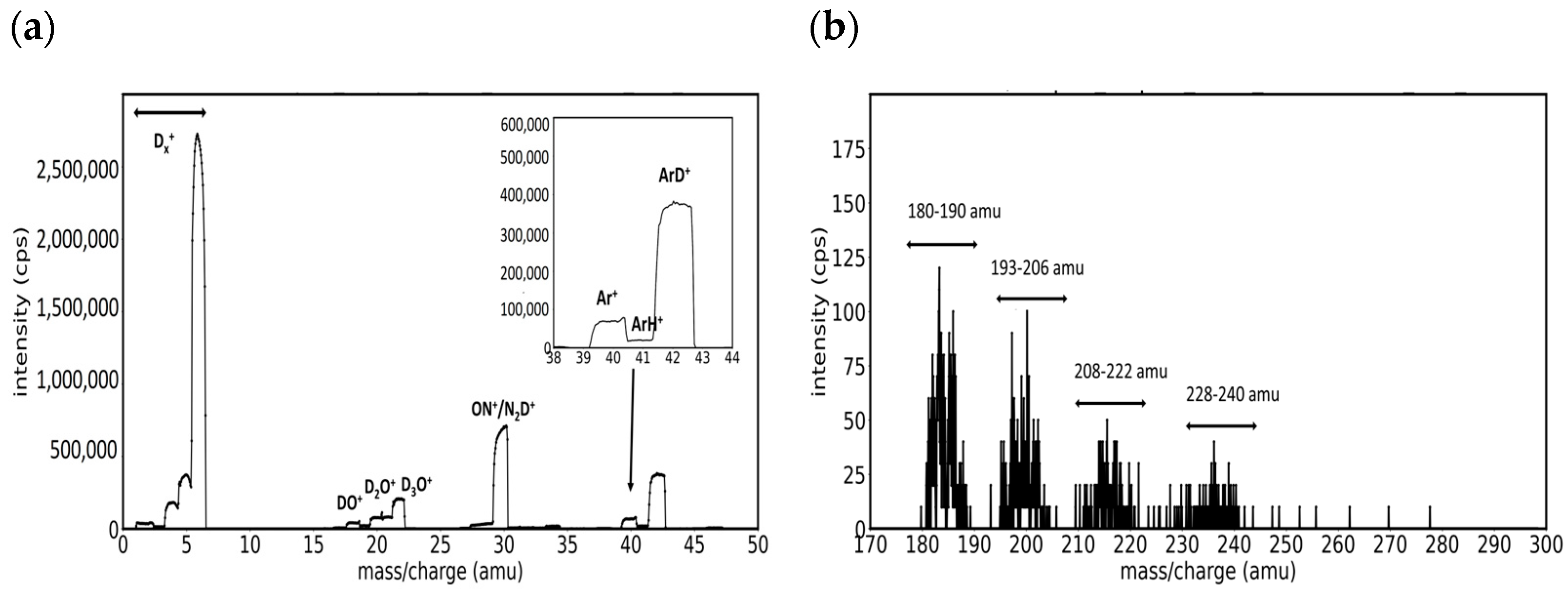
3.1. Mass Spectra of W/D2 and W/Ar/D2 Plasmas in the Presence of Impurities
3.2. Mass Spectra of W/D2 Plasmas Intentionally Injected with Ar, N2, and O2 Gases
- Group A. Mass peaks in the range of 180–190 amu
- Group B. Mass peaks in the range of 193–206 amu
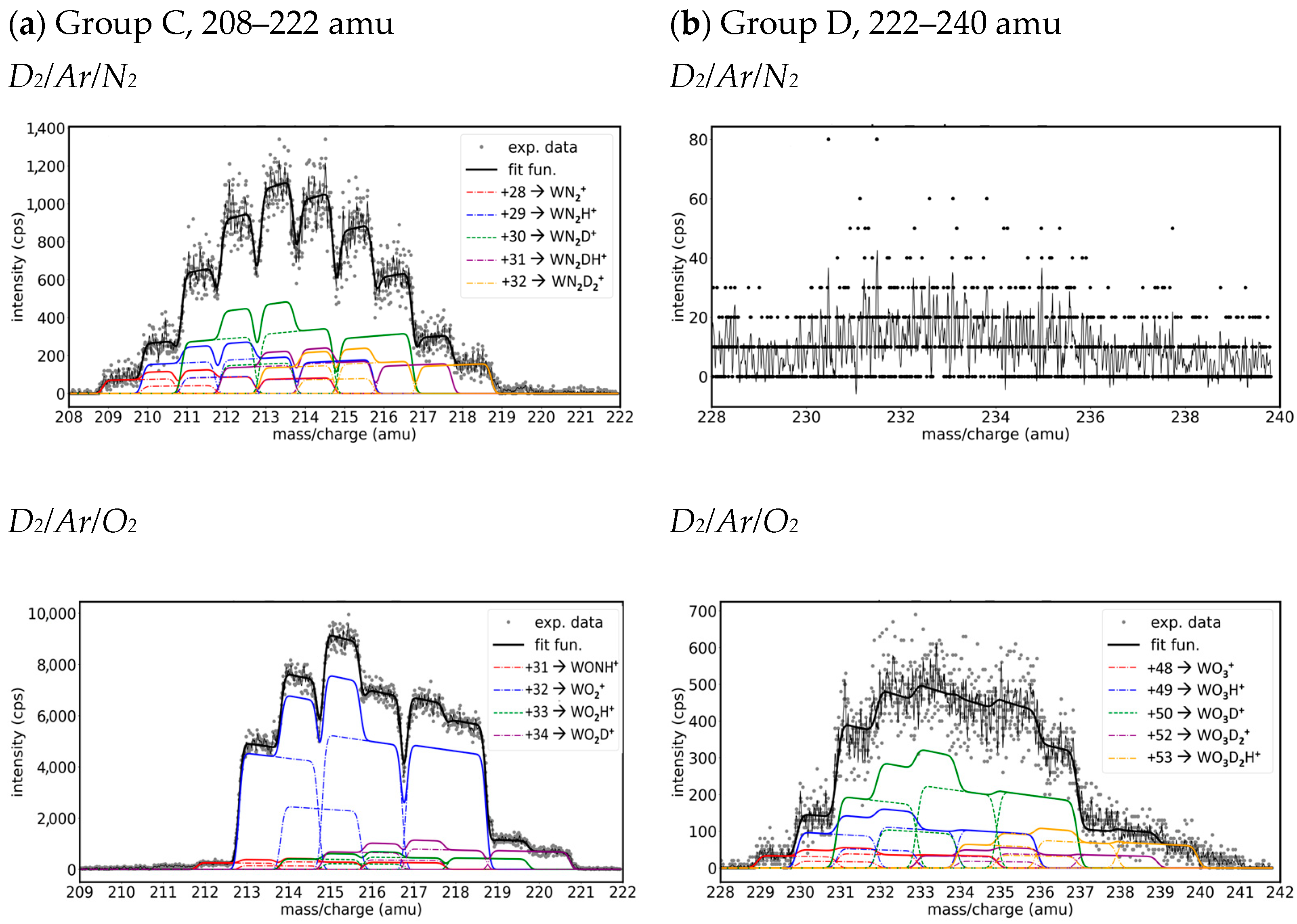
- Group C. Mass peaks in the range of 208–222 amu
- Group D. Mass peaks in the range of 228–240 amu
4. Discussion of the Mechanisms of Formation of Tungsten Molecular Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lassner, E.; Schubert, W.-D. Tungsten: Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds; Kluwer Academic: New York, NY, USA, 1999; p. 447. ISBN 978-0-306-45053-2. [Google Scholar]
- Khripunov, B.I.; Koidan, V.S.; Ryazanov, A.I.; Gureev, V.M.; Kornienko, S.N.; Latushkin, S.T.; Rupyshev, A.S.; Semenov, E.V.; Kulikauskas, V.S.; Zatekin, V.V. Study of Tungsten as a Plasma-Facing Material for a Fusion Reactor. Phys. Procedia 2015, 71, 63–67. [Google Scholar] [CrossRef]
- Skinner, H.; Haasz, C.A.A.; Alimov, V.K.; Bekris, N.; Causey, R.A.; Clarck, R.E.H.; Coad, J.P.; Davis, J.W.; Doerner, R.P.; Mayer, M.; et al. Recent advances on hydrogen retention in ITER’S plasma facing materials: Beryllium, carbon and tungsten. Fusion Sci. Technol. 2008, 54, 891–945. [Google Scholar] [CrossRef]
- Zhang, T.; Xie, Z.; Liu, C.; Xiong, Y. The tungsten-based plasma-facing materials. In Fusion Energy; Aamir, S., Ed.; IntechOpen: London, UK, 2019; pp. 1–19. [Google Scholar]
- Linke, J.; Du, J.; Loewenhoff, T.; Pintsuk, G.; Spilker, B.; Steudel, I.; Wirtz, M. Challenges for plasma-facing components in nuclear fusion. Matter. Radiat. Extremes 2019, 4, 5. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, Z.; Zhang, S.; Qin, Z.; Yu, X.; Chen, Z.; Yu, J. Dust explosion in fusion reactors: Explosion characteristics and reaction mechanism of tungsten micro-powder. Combust. Flame 2023, 248, 112551. [Google Scholar] [CrossRef]
- Ratynskaia, S.; Bortolon, A.; Krasheninnikov, S.I. Dust and powder in fusion plasmas: Recent developments in theory, modeling, and experiments. Rev. Mod. Plasma Phys. 2022, 6, 20. [Google Scholar] [CrossRef]
- Rosanvallon, S.; Grisolia, C.; Delaporte, P.; Worms, J.; Onofri, F.; Hong, S.H.; Counsell, G.; Winter, J. Dust in ITER: Diagnostics and removal techniques. J. Nucl. Mater. 2009, 386–388, 882–883. [Google Scholar] [CrossRef]
- Martin, J.D.; Coppins, M.; Counsell, G.F. Motion and lifetime of dust grains in a tokamak plasma. J. Nucl. Mater. 2005, 337–339, 114–118. [Google Scholar] [CrossRef]
- Hitzler, F. Radiative Cooling of the Divertor Plasma with Argon and Nitrogen Seeding in the ASDEX Upgrade Tokamak. Ph.D. Thesis, Technische Universität München, Max-Planck-Institut für Plasmaphysik, Munich, Germany, 2020. [Google Scholar]
- Stoica, S.D.; Vizireanu, S.; Acsente, T.; Dinescu, G. Hybrid Nanomaterial Architectures: Combining Layers of Carbon Nanowalls, Nanotubes, and Particles. Plasma Chem. Plasma Process. 2018, 38, 695–706. [Google Scholar] [CrossRef]
- Bekarevich, R.; Motrescu, I.; Rahachou, A.; Nagatsu, M. Mass spectrometric study of ammonia/methane surface-wave plasma applied to low-temperature growth of carbon nanomaterials. J. Phys. D Appl. Phys. 2015, 48, 045201. [Google Scholar] [CrossRef]
- Thiry, D.; Konstantinidis, S.; Cornil, J.; Snyders, R. Plasma diagnostics for the low-pressure plasma polymerization process: A critical review. Thin Solid Films 2016, 606, 19–44. [Google Scholar] [CrossRef]
- Benedikt, J.; Hecimovic, A.; Ellerweg, D.; von Keudell, A. Quadrupole mass spectrometry of reactive plasmas. J. Phys. D Appl. Phys. 2012, 45, 403001. [Google Scholar] [CrossRef]
- Satulu, V.; Ionita, M.D.; Vizireanu, S.; Mitu, B.; Dinescu, G. Plasma Processing with Fluorine Chemistry for Modification of Surfaces Wettability. Molecules 2016, 21, 1711. [Google Scholar] [CrossRef] [PubMed]
- Pokorný, P.; Musil, J.; Lancok, J.; Fitl, P.; Novotný, M.; Bulír, J.; Vlcek, J. Mass spectrometry investigation of magnetron sputtering discharges. Vacuum 2017, 143, 438–444. [Google Scholar] [CrossRef]
- Matthews, G.F.; Schustereder, W.; Cant, N.; Erents, S.K.; Vince, J.; Qayyum, A.; Mair, C.; Scheier, P.; Märk, T.D. Ion optics evaluation of the plasma ion mass spectrometer (PIMS) designed for the JET tokamak. Int. J. Mass Spectrom. 2003, 223–224, 45–53. [Google Scholar] [CrossRef]
- Matthews, G.F.; Elder, D.; McCracken, G.M.; Monk, R.D.; Pitts, R.A.; Samm, U.; Schweer, B.; Stangeby, P.C. Plasma ion mass spectrometry in the TEXTOR boundary. J. Nucl. Mater. 1992, 196–198, 253–257. [Google Scholar] [CrossRef]
- Aruev, N.N.; Boltenkov, B.S.; Novokhatsky, A.N. Mass-spectrometric measurements of helium isotopes in structural materials of the Globus-M tokamak. Int. J. Mass Spectrom. 2013, 351, 76–80. [Google Scholar] [CrossRef]
- Alipour, R.; Ghoranneviss, M.; Salar Elahi, A. First investigation on plasma impurities of the IR-T1 tokamak. AIP Adv. 2017, 7, 115303. [Google Scholar] [CrossRef]
- Ushakov, A.; Verlaan, A.; Stephan, U.; Steinke, O.; de Bock, M.; Maniscalco, M.P.; Verhoeff, P. ITER visible spectroscopy reference system first mirror plasma cleaning in radio-frequency gas discharge—Circuit design and plasma effects. Fusion Eng. Des. 2020, 154, 111546. [Google Scholar] [CrossRef]
- Thorman, A.; Litherland-Smith, E.; Menmuir, S.; Hawkes, N.; O’Mullane, M.; Delabie, E.; Lomanowski, B.; Fontdecaba, J.M.; Scully, S.; JET Contributors. Visible spectroscopy of highly charged tungsten ions with the JET charge exchange diagnostic. Phys. Scr. 2021, 96, 125631. [Google Scholar] [CrossRef]
- Craciun, C.; Stoica, S.D.; Mitu, B.M.; Acsente, T.; Dinescu, G. Mass Spectra Fitting as Diagnostic Tool for Magnetron Plasmas Generated in Ar and Ar/H2 Gases with Tungsten Targets. Molecules 2023, 28, 5664. [Google Scholar] [CrossRef]
- Stoica, S.D.; Craciun, C.; Acsente, T.; Mitu, B.; Dinescu, G. Evidence for molecular tungsten ionic species presence in impurity-seeded hydrogen plasma in contact with W surfaces. Plasma Process. Polym. 2024, 21, e2300227. [Google Scholar] [CrossRef]
- Shermukhamedov, S.; Probst, M. Modelling the impact of argon atoms on a tungsten surface. Eur. Phys. J. D 2022, 76, 169. [Google Scholar] [CrossRef]
- Brezinsek, S.; Pospieszczyk, A.; Sergienko, G.; Dux, R.; Cavedon, M.; Faitsch, M.; Krieger, K. Chemically assisted physical sputtering of Tungsten: Identification via the 6Π→6Σ+ transition of WD in TEXTOR and ASDEX Upgrade plasmas. Nucl. Mater. Energy 2019, 18, 50–55. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, F.; Brezinsek, S.; Yu, L.; Meng, L.Y.; Zhao, P.A.; Ye, D.W.; Hu, Z.H.; Zhang, Y.; Ding, R.; et al. Spectroscopic investigation of the tungsten deuteride sputtering in the EAST divertor. Nucl. Mater. Energy 2022, 33, 101265. [Google Scholar] [CrossRef]
- Gao, L.; Jacob, W.; Von Toussaint, U.; Manhard, A.; Balden, M.; Schmid, K.; Schwarz-Selinger, T. Deuterium supersaturation in low-energy plasma-loaded tungsten surfaces. Nucl. Fusion 2016, 57, 016026. [Google Scholar] [CrossRef]
- Gao, L.; Wilde, M.; Manhard, A.; Von Toussaint, U.; Jacob, W. Hydrogen atom-ion synergy in surface lattice modification at sub-threshold energy. Acta Mater. 2020, 201, 55–62. [Google Scholar] [CrossRef]
- Gao, L.; Yi, X.; Wilde, M.; Schwarz-Selinger, T.; Linsmeier, C. Early-stage structure and evolution mechanism of hydrogen supersaturated surface layers on tungsten under low-energy plasma exposure. Acta Mater. 2023, 256, 119137. [Google Scholar] [CrossRef]
- Brezinsek, S.; Stamp, M.F.; Nishijima, D.; Borodin, D.; Devaux, S.; Krieger, K.; Kirschner, A. Study of physical and chemical assisted physical sputtering of beryllium in the JET ITER-like wall. Nucl. Fusion 2014, 54, 103001. [Google Scholar] [CrossRef]
- Lyashenko, A.; Safi, E.; Polvi, J.; Djurabekova, F.; Nordlund, K. Computational study of tungsten sputtering by nitrogen. J. Nucl. Mater. 2020, 542, 152465. [Google Scholar] [CrossRef]
- Schmid, K.; Manhard, A.; Linsmeier, C.; Wiltner, A.; Schwarz-Selinger, T.; Jacob, W.; Mändl, S. Interaction of nitrogen plasmas with tungsten. Nucl. Fusion 2010, 50, 025006. [Google Scholar] [CrossRef]
- Wendel, J. Thermodynamics and Kinetics of Tungsten Oxidation and Tungsten Oxide Sublimation in the Temperature Interval 200–1100 °C. Master’s Thesis, Lund University, Lund, Sweden, 2014. [Google Scholar]
- Carpen, L.G.; Acsente, T.; Sătulu, V.; Matei, E.; Vizireanu, S.; Biță, B.I.; Dinescu, G. Hybrid nanostructures obtained by transport and condensation of tungsten oxide vapours onto CNW templates. Nanomaterials 2021, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, A.; Gijbels, R. Effect of small amounts of hydrogen added to argon glow discharges: Hybrid Monte Carlo–fluid model. Phys. Rev. E 2002, 65, 056402. [Google Scholar] [CrossRef]
- Karnopp, J.; Magaldi, B.; Sagás, J.; Pessoa, R. The effect of excited species on the collisional energy of argon inductively coupled plasmas: A global model study. Plasma 2022, 5, 30–43. [Google Scholar] [CrossRef]
- Merkt, F.; Höveler, K.; Deiglmayr, J. Reactions of H2, HD, and D2 with H2+, HD+, and D2+: Product-Channel Branching Ratios and Simple Models. J. Phys. Chem. Lett. 2022, 13, 864–871. [Google Scholar] [CrossRef]
- Plašil, R.; Roučka, Š.; Kovalenko, A.; Tran, T.D.; Rednyk, S.; Dohnal, P.; Glosík, J. Reaction of N+ ion with H2, HD, and D2 at low temperatures: Experimental study of the pathway to deuterated nitrogen-containing molecules in the interstellar medium. Astrophys. J. 2022, 941, 144. [Google Scholar] [CrossRef]
- Babu, S.S.; Rudolph, M.; Ryan, P.J.; Fischer, J.; Lundin, D.; Bradley, J.W.; Gudmundsson, J.T. High power impulse magnetron sputtering of tungsten: A comparison of experimental and modelling results. Plasma Sources Sci. Technol. 2023, 32, 034003. [Google Scholar] [CrossRef]
- Kostomarov, D.; Bagdasarov, K.; Antonov, E. Formation of complex tungsten oxides at high temperatures in vacuum. Dokl. Chem. 2012, 446, 204. [Google Scholar] [CrossRef]
- Schissel, P.O.; Trulson, O.C. Mass-Spectrometric Study of the Oxidation of Tungsten. J. Chem. Phys. 1965, 43, 737–743. [Google Scholar] [CrossRef]
- Batty, J.C.; Stickney, R.E. Quasiequilibrium treatment of gas-solid reactions. III. Rate of volatilization of tungsten by high-temperature oxidation. Oxid. Met. 1971, 3, 331–355. [Google Scholar]
- Kluge, S.; Wiggers, H.; Schulz, C. Mass spectrometric analysis of clusters and nanoparticles during the gas-phase synthesis of tungsten oxide. Proc. Combust. Inst. 2017, 36, 1037–1044. [Google Scholar] [CrossRef]
- Vizireanu, S.; Stoica, S.D.; Luculescu, C.; Nistor, L.C.; Mitu, B.; Dinescu, G. Plasma techniques for nanostructured carbon materials synthesis. A case study: Carbon nanowall growth by low pressure expanding RF plasma. Plasma Sources Sci. Technol. 2010, 19, 034016. [Google Scholar] [CrossRef]
- Arnas, C.; Campos, A.; Diez, M.; Peillon, S.; Martin, C.; Hassouni, K.; Tsitrone, E. Micron-sized dust and nanoparticles produced in the WEST tokamak. Nucl. Mater. Energy 2023, 36, 101471. [Google Scholar] [CrossRef]



| Regions 180–189 amu and 193–206 amu | Region 208–222 amu | Region 228–240 amu | ||||||
|---|---|---|---|---|---|---|---|---|
| Mass Shift (amu) | Centre of the Peak (amu) | Pos. Ions | Mass Shift (amu) | Centre of the Peak (amu) | Pos. Ions | Mass Shift (amu) | Centre of the Peak (amu) | Pos. Ions |
| 0 | 182,183,184,186 | W+ | 28 | 210,211,212,214 | WN2+ | 48 | 230,231,232,234 | WO3+ |
| 1 | 183,184,185,187 | WH+ | 29 | 211,212,213,215 | WN2H+ | 49 | 231,232,233,235 | WO3H+ |
| 2 | 184,185,186,188 | WD+ | 30 | 212,213,214,216 | WN2H2+ WN2D+ WNO+ | 50 | 232,233,234,236 | WO3H2+ WO3D+ |
| 14 | 196,197,188,200 | WN+ | 31 | 213,214,215,217 | WN2H3+ WN2DH+ WNOH+ | 52 | 234,235,236,238 | WO3H4+ WO3D2+ |
| 15 | 197,198,189,201 | WNH+ | 32 | 214,215,216,218 | WN2H4+ WN2D2+ WNOH2+ WNOD+ WO2+ | 53 | 235,236,237,239 | WO3H5+ WO3D2H+ |
| 16 | 198,199,200,202 | WNH2+ WND+ WO+ | 33 | 215,216,217,219 | WN2H5+ WN2D2H+ WNOH3+ WNODH+ WO2H+ | |||
| 17 | 199,200,201,203 | WNH3+ WNDH+ WOH+ | 34 | 216,217,218,220 | WN2H6+ WN2D3+ WNOH4+ WNOD2+ WO2H2+ WO2D+ | |||
| 18 | 200,201,202,204 | WNH4+ WND2+ WOH2+ WOD+ | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinescu, G.; Craciun, C.; Stoica, S.D.; Constantin, C.; Mitu, B.M.; Acsente, T. Tungsten Molecular Species in Deuterium Plasmas in Contact with Sputtered W Surfaces. Molecules 2024, 29, 3539. https://doi.org/10.3390/molecules29153539
Dinescu G, Craciun C, Stoica SD, Constantin C, Mitu BM, Acsente T. Tungsten Molecular Species in Deuterium Plasmas in Contact with Sputtered W Surfaces. Molecules. 2024; 29(15):3539. https://doi.org/10.3390/molecules29153539
Chicago/Turabian StyleDinescu, Gheorghe, Cristina Craciun, Silviu Daniel Stoica, Catalin Constantin, Bogdana Maria Mitu, and Tomy Acsente. 2024. "Tungsten Molecular Species in Deuterium Plasmas in Contact with Sputtered W Surfaces" Molecules 29, no. 15: 3539. https://doi.org/10.3390/molecules29153539
APA StyleDinescu, G., Craciun, C., Stoica, S. D., Constantin, C., Mitu, B. M., & Acsente, T. (2024). Tungsten Molecular Species in Deuterium Plasmas in Contact with Sputtered W Surfaces. Molecules, 29(15), 3539. https://doi.org/10.3390/molecules29153539








