Comparative Analysis of Hydrosol Volatile Components of Citrus × Aurantium ‘Daidai’ and Citrus × Aurantium L. Dried Buds with Different Extraction Processes Using Headspace-Solid-Phase Microextraction with Gas Chromatography–Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of Volatile Components of CAVA IH and CAVA UH
2.2. Analysis of Volatile Components of CADB IH and CADB UH
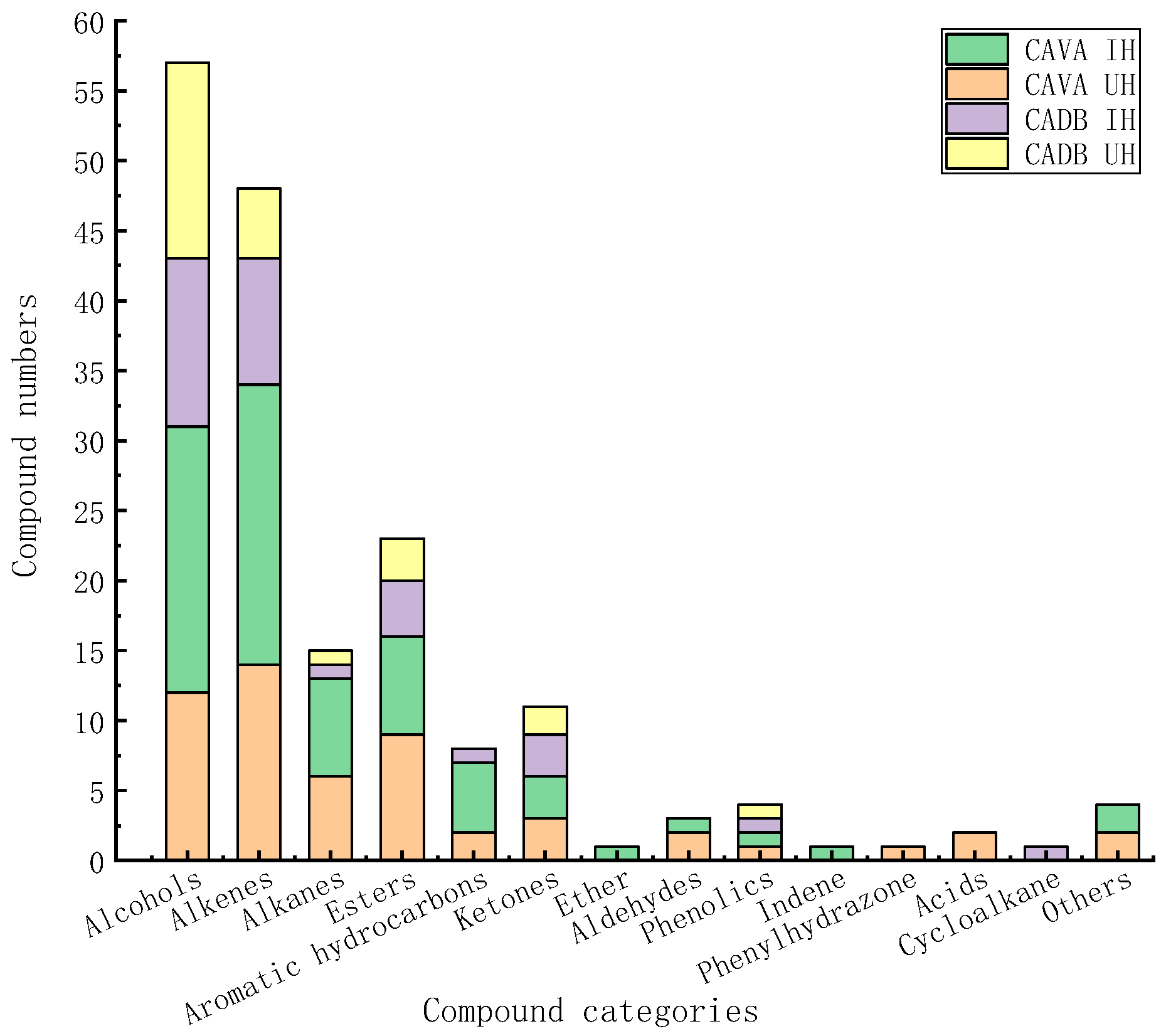
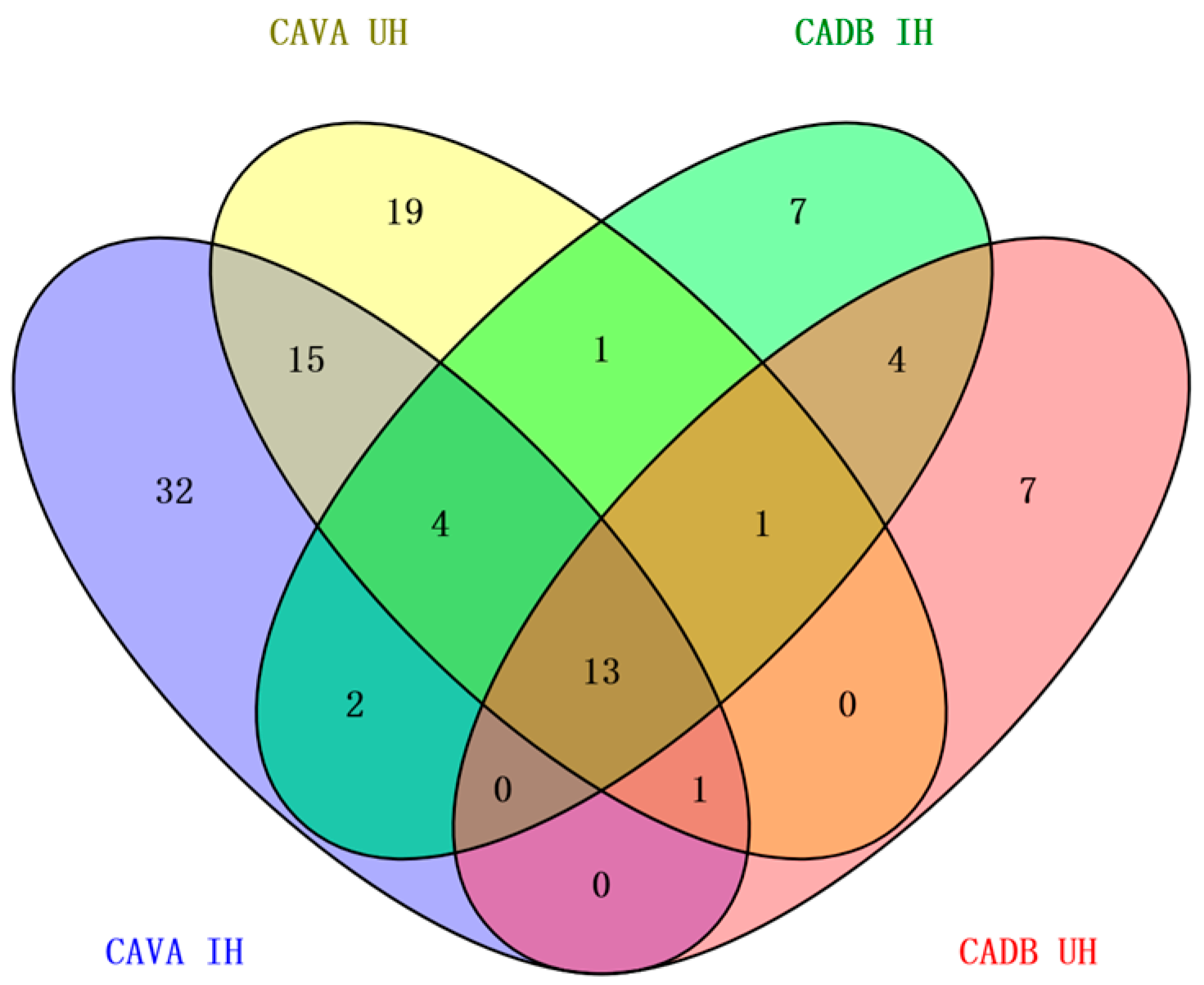
2.3. Comprehensive Analysis of Volatile Components of CAVA Hydrosols and CADB Hydrosols
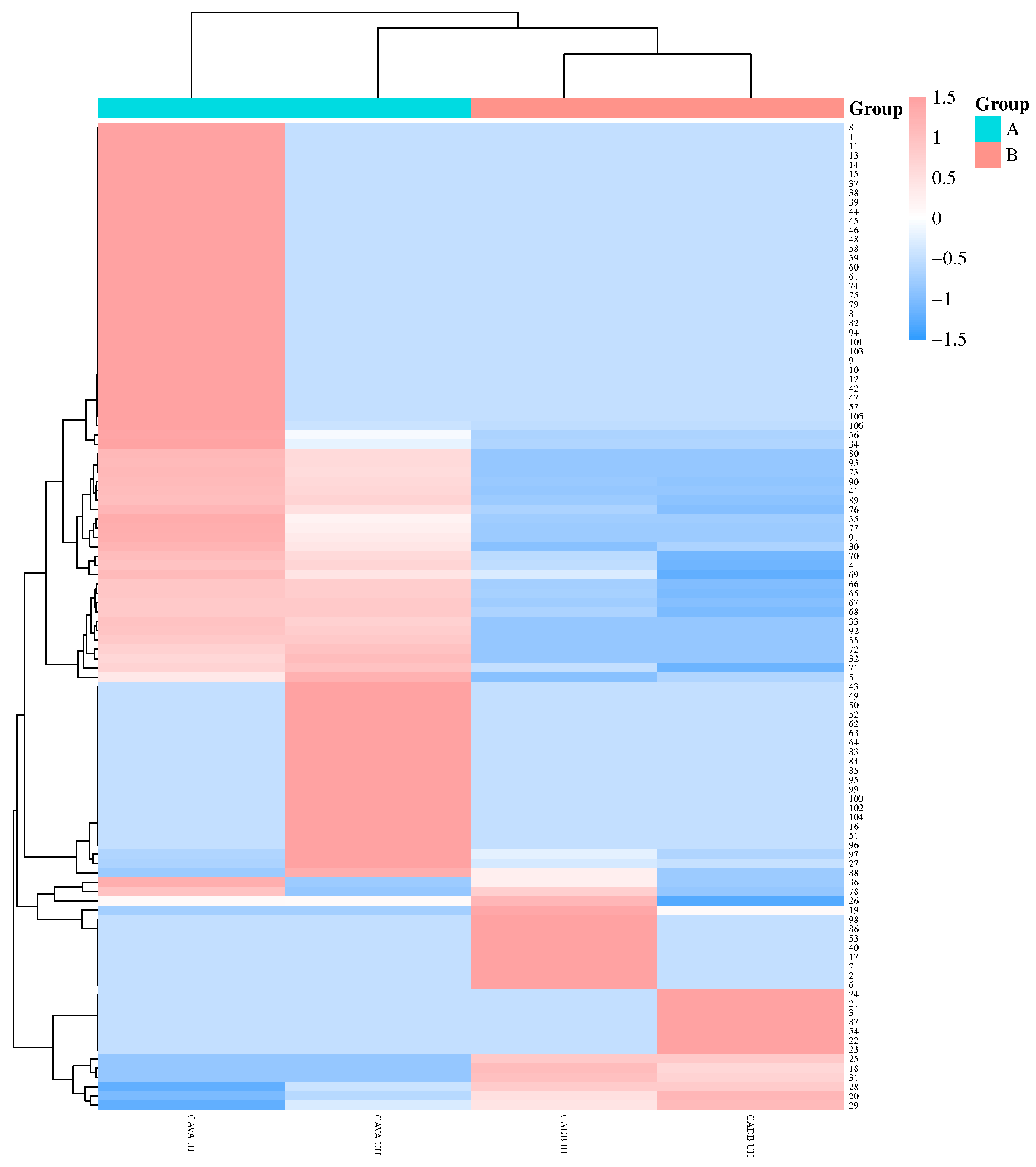
2.4. Cluster Analysis of Volatile Components of CAVA Hydrosols and CADB Hydrosols
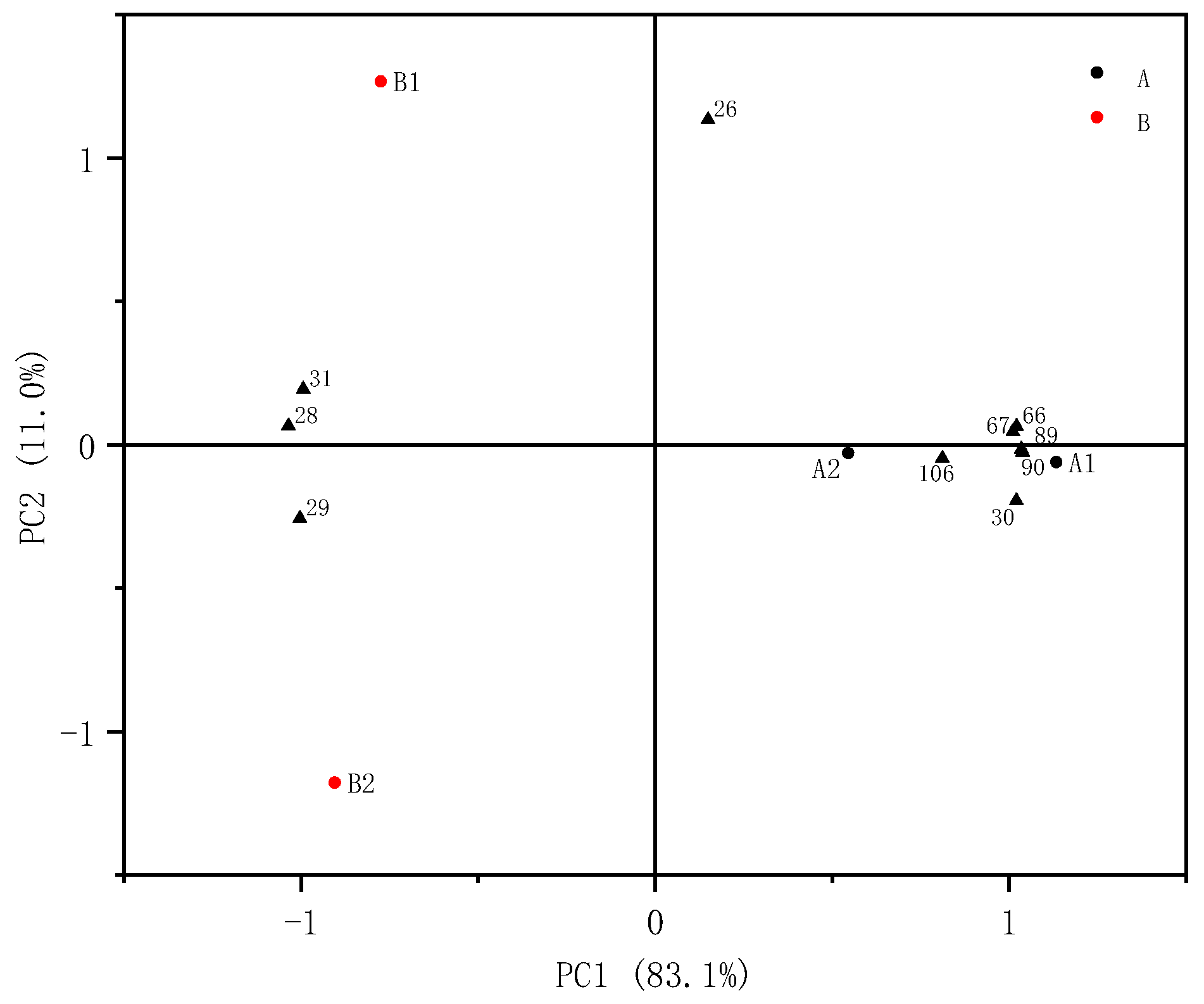
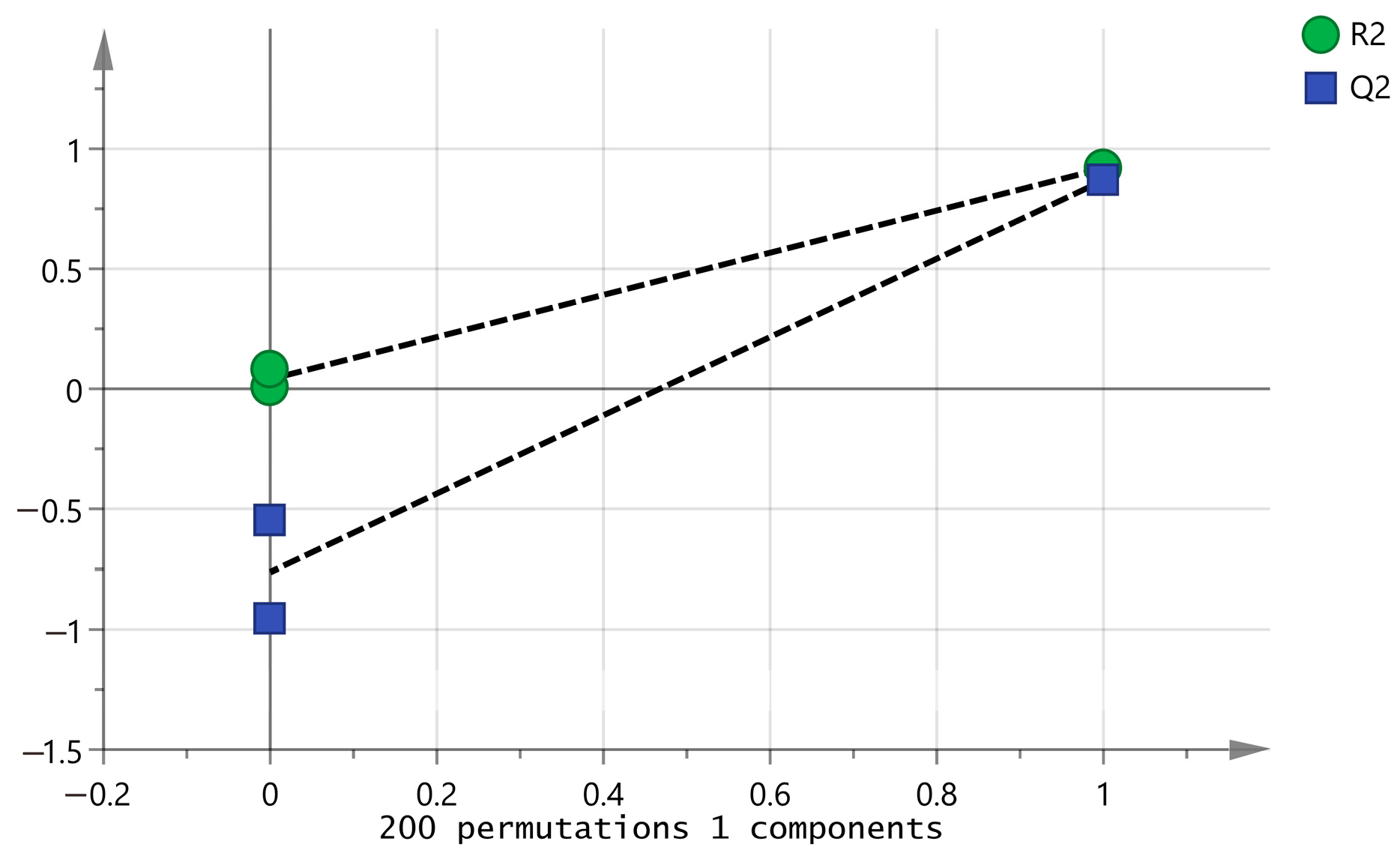
2.5. PCA Analysis of Volatile Components of CAVA Hydrosols and CADB Hydrosols
2.6. OVA Analysis of Volatile Components of CAVA Hydrosols and CADB Hydrosols
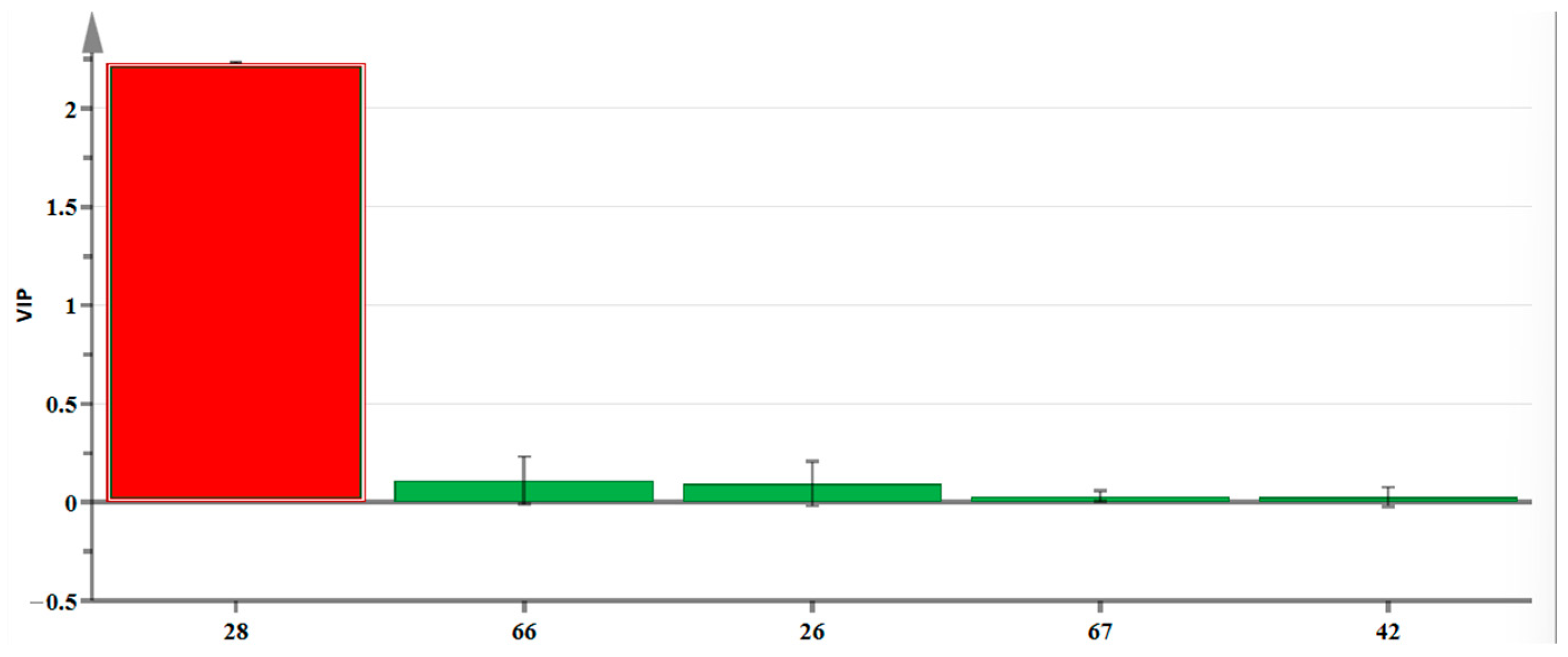
2.7. OPLS-DA Analysis of Volatile Components of CAVA Hydrosols and CADB Hydrosols
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals and Reagents
3.3. Preparation of Samples
3.4. Determination of Volatile Components
3.4.1. Headspace Solid-Phase Microextraction
3.4.2. GC-MS Analysis
3.5. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; p. 257. [Google Scholar]
- Sarrou, E.; Chatzopoulou, P.; Dimassi-Theriou, K.; Therios, I. Volatile constituents and antioxidant activity of peel, flowers and leaf oils of Citrus aurantium L. growing in Greece. Molecules 2013, 18, 10639–10647. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Yang, Y.J.; Lee, Y.H.; Jung, H.Y.; Kim, T.D.; Noh, J.Y.; Lee, S.J.; Yoon, S.R. Aurantii Fructus Immaturus enhances natural killer cytolytic activity and anticancer efficacy in vitro and in vivo. Front. Med. 2022, 9, 973681. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.H.; Jiang, M.Y.; Deng, B.; Zhang, Z.; Fu, Q.; Fu, C.M. Aurantii Fructus: A systematic review of ethnopharmacology, phytochemistry and pharmacology. Phytochem. Rev. 2021, 20, 909–944. [Google Scholar] [CrossRef]
- Li, H.H.; Liu, X.; Huang, S. Chinese Aurantii Fructus industry development status and Jiangjin Aurantii Fructus development prospect analysis. China Fruit News 2022, 39, 17–20. [Google Scholar]
- National Administration of Traditional Chinese Medicine; Editorial Committee of Chinese Materia Medica. Chinese Materia Medica; Shanghai Science and Technology Press: Shanghai, China, 1999; pp. 884–885. [Google Scholar]
- Chinese Academy of Sciences Editorial Committee of Flora of China. Flora of China; Science Press: Beijing, China, 1997; p. 194. [Google Scholar]
- Shen, C.Y.; Wang, T.X.; Zhang, X.M.; Jiang, J.G. Various Antioxidant Effects Were Attributed to Different Components in the Dried Blossoms of Citrus aurantium L. var. amara Engl. J. Agric. Food Chem. 2017, 65, 6087–6092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Huang, T.; Liao, X.N.; Zhou, Y.H.; Chen, S.X.; Chen, J.; Xiong, W.M. Extraction of camphor tree essential oil by steam distillation and supercritical CO2 extraction. Molecules 2022, 27, 5385. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Hao, Y.F.; Hao, Z.X.; Jiang, J.G.; Liu, Q.; Shen, Q.; Liu, L.; Yi, Y.K.; Shen, C.Y. Inhibitory effect of chloroform extracts from Citrus aurantium L. var. amara Engl. on fat accumulation. Phytomedicine 2021, 90, 153634. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Wan, L.; Wang, T.X.; Jiang, J.G. Citrus aurantium L. var. amara Engl. inhibited lipid accumulation in 3T3-L1 cells and Caenorhabditis elegans and prevented obesity in high-fat diet-fed mice. Pharmacol. Res. 2019, 147, 104347. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.F.; Yan, M.; Wang, Z.; Jiang, M.P.; Yan, B.; Shen, C.Y. Optimization of the extract from flower of Citrus aurantium L. var. amara Engl. and its inhibition of lipid accumulation. J. Food Biochem. 2022, 46, e14332. [Google Scholar] [CrossRef]
- Boussaada, O.; Chemli, R. Chemical composition of essential oils from flowers, leaves and peel of Citrus aurantium L. var. amara from Tunisia. J. Essent. Oil Bear. Plants 2006, 9, 133–139. [Google Scholar] [CrossRef]
- Wang, X.S.; Kapoor, V.; Smythe, G.A. Extraction and chromatography-mass spectrometric analysis of the active principles from selected Chinese herbs and other medicinal plants. Am. J. Chin. Med. 2003, 31, 927–944. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, S.; Karimi, S.; Abbasi, H.; Assari, M. Sequential ultrasound-microwave technique as an efficient method for extraction of essential oil from Lavandula coronopifolia Poir. Food Meas. Charact. 2022, 16, 377–390. [Google Scholar] [CrossRef]
- Mohagheghniapour, A.; Saharkhiz, M.J.; Golmakani, M.T.; Niakousari, M. Variations in chemical compositions of essential oil from sour orange (Citrus aurantium L.) blossoms by different isolation methods. Sustain. Chem. Pharm. 2018, 10, 118–124. [Google Scholar] [CrossRef]
- Bressanello, D.; Liberto, E.; Cordero, C.; Rubiolo, P.; Pellegrino, G.; Ruosi, M.R.; Bicchi, C. Coffee aroma: Chemometric comparison of the chemical information provided by three different samplings combined with GC–MS to describe the sensory properties in cup. Food Chem. 2017, 214, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Cécile, L.; Céline, C.; Frédéric, C. Fate and control of pathogenic and spoilage micro -organisms in orange blossom (Citrus aurantium) and rose flower (Rosa centifolia) hydrosols. J. Appl. Microbiol. 2016, 121, 1568–1579. [Google Scholar] [CrossRef]
- Değirmenci, H.; Erkurt, H. Relationship between volatile components, antimicrobial and antioxidant properties of the essential oil, hydrosol and extracts of Citrus aurantium L. flowers. Infect. Public Health 2020, 13, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Pan, Z.P.; Xiao, Y.; Liang, Z.E.N.; Fu, F.H. Study on the composition and the antioxidant activity of essential oil from flowers of Citrus aurantium L. var. amara Engl. Food Mach. 2020, 36, 165–170. [Google Scholar] [CrossRef]
- Družić, J.; Jerković, I.; Marijanović, Z.; Roje, M. Chemical biodiversity of the leaf and flower essential oils of Citrus aurantium L. from Dubrovnik area (Croatia) in comparison with Citrus sinensis L. Osbeck cv. Washington navel, Citrus sinensis L. Osbeck cv. Tarocco and Citrus sinensis L. Osbeck cv. Doppio Sanguigno. EssEntial Oil Res. 2016, 28, 283–291. [Google Scholar] [CrossRef]
- Li, S.S.; Yang, M.; Wu, W.J. Comparison of botanical traits and volatile constituents of grapefruit, lemon and Citrus aurantium ‘Daidai’. South. Chin. Fruit Trees 2022, 51, 35–39. [Google Scholar] [CrossRef]
- Lu, X.M.; Zhao, C.Y.; Shi, H.; Liao, Y.C.; Xu, F.; Du, H.J.; Xiao, H.; Zheng, J.K. Nutrients and bioactives in citrus fruits: Different citrus varieties, fruit parts, and growth stages. Crit. Rev. Food Sci. Nutr. 2023, 63, 2018–2041. [Google Scholar] [CrossRef]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An overview of bioactive flavonoids from citrus fruits. Appl. Sci. 2021, 12, 29. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.C.; Leeuwen, C.V.; Dubourdieu, D. Examples of Perceptive Interactions Involved in Specific “Red-” and “Black-berry” Aromas in Red Wines. J. Agric. Food Chem. 2009, 57, 3702–3708. [Google Scholar] [CrossRef]
- Lv, P.; Zhong, L.; Jiang, N.; Jian, S.H. Effect of different assisted extraction method on volatile compounds of the essential oil from Citrus aurantium L. var. amara Engl. Cereals Oils 2018, 31, 52–54. [Google Scholar]
- Xiao, Q.M.; Zhang, X.Y.; Wang, J.; Lv, Y.C. Comparative analysis on aromatic components of sun-dried green tea from different townships in Menghai county. Sci. Technol. Food Ind. 2023, 44, 332–339. [Google Scholar] [CrossRef]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf. B Biointerfaces 2018, 171, 566–578. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Hăncianu, M.; Costache, I.I.; Miron, A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour Fragr. J. 2014, 29, 193–219. [Google Scholar] [CrossRef]
- Song, S.Q.; Tong, Y.Z.; Feng, T.; Zhu, J.C.; Wang, Y.F.; Sun, M.; Yao, L.Y.; Xu, Z.M. Multidimensional analysis of odorous compounds in finger citron fruit (Citrus medica L. var. sarcodactylis Swingle) and identification of key aroma compounds. Food Sci. 2017, 38, 94–100. [Google Scholar]
- Chen, Y.; Zhang, L.L.; Wang, W.Y.; Wang, G.G. Recent updates on bioactive properties of α-terpineol. J. Essent. Oil Res. 2023, 35, 274–288. [Google Scholar] [CrossRef]
- Ma, M.J.; Su, X.X.; Tan, X.Y.; Niu, Y.; Li, R.Y.; Bian, Q. Analysis of volatile flavor compounds in spicy sausages. Meat Res. 2022, 36, 28–34. [Google Scholar]
- Do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.D.C.; Góis Ruiz, A.L.T.; Ann Foglio, M.; De Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, J.L.; Hu, J.L.; Shi, Y.X.; Huang, P.; Ding, D.K. Screening of key aroma components in essential oils from citrus peels of different cultivars. Sci. Technol. Food Ind. 2023, 44, 259–269. [Google Scholar] [CrossRef]
- Celuppi, L.C.M.; Capelezzo, A.P.; Cima, L.B.; Zeferino, R.C.F.; Carniel, T.A.; Zanetti, M.; De Mello, J.M.M.; Fiori, M.A.; Riella, H.G. Microbiological, thermal and mechanical performance of cellulose acetate films with geranyl acetate. Int. J. Biol. Macromol. 2023, 228, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Ping, H.R.; Wang, Y.P.; Chen, J.N.; Wang, J.H. Analysis of taste and aroma differences between Miao and Shui ethnic red sour soup in Guizhou. Sci. Technol. Food Ind. 2023, 45, 273–283. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef]
- Xun, Z.L.; Ma, X.H.; Huang, L.P.; Wang, M.; Zhao, Q.F. Analysis of berry aroma compounds from 47 table grape germplasm resources. J. Shanxi Agric. Univ. (Nat. Sci. Ed.) 2023, 43, 83–91. [Google Scholar] [CrossRef]
- Silva, B.O.; Orlando, J.B.; Pires, C.L.; Hiruma-Lima, C.A.; De Mascarenhas Gaivao, I.; Perazzo, F.F.; Maistro, E.L. Genotoxicity induced by nerol, an essential oil present in citric plants using human peripheral blood mononuclear cells (PBMC) and HepG2/C3A cells as a model. J. Toxicol. Environ. Health 2021, 84, 518–528. [Google Scholar] [CrossRef]
- Su, L.D.; Luo, X.Y.; Duan, L.L.; Xiong, S.L. Optimization of processing technology and analysis of volatile flavor substances of garlic-flavor hot pot dipping sauce. China Condiment 2023, 48, 145–151. [Google Scholar]
- Zhou, X.; Zhou, J.S.; Liu, T.Y.; Jiang, L.W.; Liu, Y.; Yin, S.X.; Rong, Z.X.; Chen, H. Analysis of the effects in the Flavor Anchovy during circulating boiling brine based on HS-SPME-GC-MS. Sci. Technol. Food Ind. 2023, 44, 320–328. [Google Scholar] [CrossRef]
- Almarzooqi, S.; Venkataraman, B.; Raj, V.; Alkuwaiti, S.A.A.; Das, K.M.; Collin, P.D.; Adrian, T.E.; Subramanya, S.B. β-Myrcene mitigates colon inflammation by inhibiting MAP kinase and NF-κB signaling pathways. Molecules 2022, 27, 8744. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Chen, X.Y.; Wang, C.Q.; Jiao, B.N. Difference analysis of volatile components in Orah mandarin (Citrus reticulata Blanco) from different areas. Food Ferment. Ind. 2023, 1–11. [Google Scholar] [CrossRef]
- Opdyke, D.L.J. Neryl acetate. Food Cosmet. Toxicol. 1976, 14, 625. [Google Scholar] [CrossRef]
- Gabriele, A.; Giovanna, A.; Vasileios, B.; De Lourdes Bastos, M.; Georges, B.; Andrew, C.; Cocconcelli, P.S.; Gerhard, F.; Boris, K.; Maryline, K. Safety and efficacy of α, β-unsaturated straight-chain and branched-chain aliphatic primary alcohols, aldehydes, acids and esters belonging to chemical group 3 when used as flavourings for all animal species. Efsa J. 2016, 14, e04512. [Google Scholar] [CrossRef]
- Yin, J.; Jiang, J.; Liu, W.J.; Liao, F.; Hu, A.F.; Huang, F.F.; Liu, J.L.; Yang, J. Combined GC-O and GC-MS identification of key aroma constituents in the essential oil of yangmei leaves. South China Fruits 2017, 46, 64–71. [Google Scholar] [CrossRef]
- García-Casas, I.; Montes, A.; De Los Santos, D.M.; Valor, D.; Pereyra, C.; De la Ossa, E.M. Generation of high-porosity cerium oxide nanoparticles and their functionalization with caryophyllene oxide using supercritical carbon dioxide. J. Supercrit. Fluids 2023, 196, 105901. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Zeng, W.X.; Zhang, J.Z.; Zeng, J.L.; Zhang, X.M.; Zeng, L.K.; Ling, C.J. Analysis on quality components and aroma characteristics of Hakka roasted green tea. Mod. Food Sci. Technol. 2023, 39, 242–252. [Google Scholar] [CrossRef]
- Deng, X.Y.; Xue, J.S.; Li, H.Y.; Ma, Z.Q.; Fu, Q.; Qu, R.; Ma, S.P. Geraniol produces antidepressant-like effects in a chronic unpredictable mild stress mice model. Physiol. Behav. 2015, 152, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Ho, C.T.; Schwab, W.; Wan, X.C. Effect of the roasting degree on flavor quality of large-leaf yellow tea. Food Chem. 2021, 347, 129016. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Liu, W.; He, S.; Hu, X.Q.; Zhang, J.H.; Shan, Y. Analysis of volatile components in three varieties of kumquat by headspace solid phase microextraction-gas chromatography-mass spectrometry. Food Sci. 2021, 42, 176–184. [Google Scholar] [CrossRef]
- Wu, Q.H.; Jiang, W.; Yang, J.Y.; Si, X.X.; Yi, A.; Wang, M.J.; Zhao, Y.; Shan, S.Y.; Zhang, F.M. Effect of solvent extraction on the key aroma components of Tamarindus indica L. pulp. J. Food Compos. Anal. 2023, 123, 105613. [Google Scholar] [CrossRef]
- Zeng, L.; Fu, Y.Q.; Huang, J.S.; Wang, J.R.; Jin, S.; Yin, J.F.; Xu, Y.Q. Comparative analysis of volatile compounds in Tieguanyin with different types based on HS–SPME–GC–MS. Foods 2022, 11, 1530. [Google Scholar] [CrossRef]
| No. | Retention Time (min) | Compound | Molecular Formula | Compound Type | Absolute Content (mg/kg) | Relative Content (%) |
|---|---|---|---|---|---|---|
| 1 | 6.434 | 3-Heptanone,4-methyl | C8H16O | Ketone | 0.0005 | 0.01 |
| 2 | 6.785 | 2-Heptanone,3-methyl- | C8H16O | Ketone | 0.0029 | 0.07 |
| 3 | 7.103 | 3-Phenyl-2-butanol | C10H14O | Alcohol | 0.0009 | 0.02 |
| 4 | 7.353 | Benzene,1-ethyl-2-methyl | C9H12 | Aromatic hydrocarbon | 0.0009 | 0.02 |
| 5 | 7.505 | Benzene,1,2,3-trimethyl- | C9H12 | Aromatic hydrocarbon | 0.0143 | 0.33 |
| 6 | 7.615 | Nonane,5-methylene- | C10H20 | Alkene | 0.0039 | 0.09 |
| 7 | 7.725 | Allyl butyrate | C7H12O2 | Ester | 0.0039 | 0.09 |
| 8 | 7.806 | Diallyl carbonate | C7H10O3 | Ester | 0.0003 | 0.01 |
| 9 | 7.865 | 2-Norpinene-2-ethanol,6,6-dimethyl- | C11H18O | Alcohol | 0.0022 | 0.05 |
| 10 | 8.256 | β-Myrcene | C10H16 | Alkene | 0.0769 | 1.80 |
| 11 | 8.656 | α-Phellandrene | C10H16 | Alkene | 0.0117 | 0.28 |
| 12 | 8.749 | trans, trans-2,8-Decadiene | C10H18 | Alkene | 0.0059 | 0.14 |
| 13 | 9.064 | α-Terpinene | C10H16 | Alkene | 0.0130 | 0.31 |
| 14 | 9.232 | p-Cymene | C10H14 | Aromatic hydrocarbon | 0.0024 | 0.06 |
| 15 | 9.354 | o-Cymene | C10H14 | Aromatic hydrocarbon | 0.0085 | 0.20 |
| 16 | 9.455 | D-Limonene | C10H16 | Alkene | 0.0937 | 2.20 |
| 17 | 9.827 | trans-β-Ocimene | C10H16 | Alkene | 0.0221 | 0.52 |
| 18 | 10.174 | cis-β-Ocimene | C10H16 | Alkene | 0.0316 | 0.74 |
| 19 | 10.491 | γ-Terpinene | C10H16 | Alkene | 0.0085 | 0.20 |
| 20 | 10.972 | α-Methyl-α-[4-methyl-3-pentenyl] oriranemethanol | C10H18O2 | Alcohol | 0.0125 | 0.29 |
| 21 | 11.513 | 2-Carene | C10H16 | Alkene | 0.0275 | 0.64 |
| 22 | 12.166 | Linalool | C10H18O | Alcohol | 1.5674 | 36.79 |
| 23 | 13.006 | 1,3,8-p-Menthatriene | C10H14 | Alkene | 0.0235 | 0.55 |
| 24 | 13.430 | Neo-alloocimene, stab. | C10H16 | Alkene | 0.0091 | 0.21 |
| 25 | 14.624 | Terpinen-4-ol | C10H18O | Alcohol | 0.0073 | 0.17 |
| 26 | 15.149 | α-Terpineol | C10H18O | Alcohol | 0.4013 | 9.42 |
| 27 | 16.126 | Octahydro-5-(2-octyldecyl)-4,7-methano-1H-indene | C28H52 | Indene | 0.0004 | 0.01 |
| 28 | 16.451 | Nerol | C10H18O | Alcohol | 0.0841 | 1.97 |
| 29 | 17.061 | D-Carvone | C10H14O | Ketone | 0.0071 | 0.17 |
| 30 | 17.366 | trans-Geraniol | C10H18O | Alcohol | 0.3119 | 7.32 |
| 31 | 17.903 | Butanenitrile | C4H7N | Other | 0.0020 | 0.05 |
| 32 | 18.085 | 4,6-Dimethyldodecane | C14H30 | Alkane | 0.0019 | 0.04 |
| 33 | 18.272 | Isoborneol | C10H18O | Alcohol | 0.0018 | 0.04 |
| 34 | 18.561 | Ethylene glycol diallyl ether | C8H14O2 | Ether | 0.0006 | 0.01 |
| 35 | 18.668 | Butanal,4-[(tetrahydro-2H-pyran-2-yl)oxy]- | C9H16O3 | Aldehyde | 0.0013 | 0.03 |
| 36 | 19.035 | 2,3,5,8-Tetramethyldecane | C14H30 | Alkane | 0.0004 | 0.01 |
| 37 | 19.478 | Cadala-1(10),3,8-triene | C15H22 | Alkene | 0.0007 | 0.02 |
| 38 | 19.619 | 2,6,11-Trimethyldodecane | C15H32 | Alkane | 0.0008 | 0.02 |
| 39 | 20.465 | α-Terpinyl acetate | C12H20O2 | Ester | 0.0047 | 0.11 |
| 40 | 21.093 | Neryl acetate | C12H20O2 | Ester | 0.0647 | 1.52 |
| 41 | 21.903 | Geranyl acetate | C12H20O2 | Ester | 0.1066 | 2.50 |
| 42 | 22.528 | Tetradecane | C14H30 | Alkane | 0.0006 | 0.01 |
| 43 | 24.016 | Artemesia alcohol | C10H18O | Alcohol | 0.0022 | 0.05 |
| 44 | 24.478 | 7-Tetracyclo[6.2.1.0(3.8)0(3.9)]undecanol, 4,4,11,11-tetramethyl- | C15H24O | Alcohol | 0.0100 | 0.24 |
| 45 | 24.677 | (Z)-β-farnesene | C15H24 | Alkene | 0.0035 | 0.08 |
| 46 | 24.802 | 4,5-dimethyl-Biphenylene,1,2,3,6,7,8,8a,8b-octahydro-4,5-dimethyl- | C14H20 | Aromatic hydrocarbon | 0.0045 | 0.11 |
| 47 | 25.221 | Dehydro aromadendrene | C15H22 | Alkene | 0.0039 | 0.09 |
| 48 | 25.714 | 1-Heptatriacotanol | C37H76O | Alcohol | 0.0029 | 0.07 |
| 49 | 25.849 | α-Muurolene | C15H24 | Alkene | 0.0027 | 0.06 |
| 50 | 26.210 | γ-Muurolene | C15H24 | Alkene | 0.0045 | 0.11 |
| 51 | 26.304 | Phenol,2,4-bis(1,1-dimethylethyl)- | C14H22O | Phenolic | 0.0049 | 0.12 |
| 52 | 26.437 | Calamenene | C15H22 | Alkene | 0.0064 | 0.15 |
| 53 | 26.671 | 8,9-dehydro-Neoisolongifolene | C15H22 | Alkene | 0.0033 | 0.08 |
| 54 | 26.917 | 2-Chlorobenzoic acid, dodec-9-ynyl ester | C19H25ClO2 | Ester | 0.0066 | 0.15 |
| 55 | 27.417 | trans-Nerolidol | C15H26O | Alcohol | 0.0319 | 0.75 |
| 56 | 27.682 | Spathulenol | C15H24O | Alcohol | 0.1937 | 4.55 |
| 57 | 27.773 | Caryophyllene oxide | C15H24O | Other | 0.0426 | 1.00 |
| 58 | 27.960 | (+)-Viridiflorol | C15H26O | Alcohol | 0.0084 | 0.20 |
| 59 | 28.069 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | C16H30O4 | Ester | 0.0081 | 0.19 |
| 60 | 28.195 | Ledol | C15H26O | Alcohol | 0.0097 | 0.23 |
| 61 | 29.183 | α-Cadinol | C15H26O | Alcohol | 0.0249 | 0.58 |
| 62 | 29.481 | Tricyclo[5.2.2.0(1,6)]undecan-3-ol,2-methylene-6,8,8-trimethyl | C15H24O | Alcohol | 0.0136 | 0.32 |
| 63 | 29.772 | 7R,8R-8-Hydroxy-4-isopropylidene-7-methylbicyclo[5.3.1]undec-1-ene | C15H24O | Alkene | 0.0090 | 0.21 |
| 64 | 30.067 | 10-Methylnonadecane | C20H42 | Alkane | 0.0021 | 0.05 |
| 65 | 30.172 | 5-Butylnonane | C13H28 | Alkane | 0.0011 | 0.03 |
| 66 | 30.336 | 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol | C15H24O | Alcohol | 0.0024 | 0.06 |
| 67 | 31.239 | Azulene,1,4-dimethyl-7-(1-methylethyl)- | C15H18 | Alkane | 0.0130 | 0.31 |
| No. | Retention Time (min) | Compound | Molecular Formula | Compound Type | Absolute Content (mg/kg) | Relative Content (%) |
|---|---|---|---|---|---|---|
| 1 | 6.761 | 2-Heptanone,3-methyl- | C8H16O | Ketone | 0.0027 | 0.06 |
| 2 | 7.091 | Bicyclo[3.1.0]hexane-6,6-dicarbonitrile | C8H8N2 | Other | 0.0011 | 0.02 |
| 3 | 7.493 | Benzene,1,2,3-trimethyl- | C9H12 | Aromatic hydrocarbon | 0.0123 | 0.26 |
| 4 | 7.603 | 2-Methyl-1-hexene | C7H14 | Alkene | 0.0030 | 0.06 |
| 5 | 7.710 | Allyl butyrate | C7H12O2 | Ester | 0.0024 | 0.05 |
| 6 | 8.243 | β-Myrcene | C10H16 | Alkene | 0.0810 | 1.73 |
| 7 | 8.644 | α-Phellandrene | C10H16 | Alkene | 0.0093 | 0.20 |
| 8 | 8.737 | cis-7-Dodecen-1-ol | C12H24O | Alcohol | 0.0046 | 0.10 |
| 9 | 9.053 | α-Terpinene | C10H16 | Alkene | 0.0111 | 0.24 |
| 10 | 9.220 | m-Cymene | C10H14 | Aromatic hydrocarbon | 0.0026 | 0.06 |
| 11 | 9.447 | D-Limonene | C10H16 | Alkene | 0.1025 | 2.19 |
| 12 | 9.819 | trans-β-Ocimene | C10H16 | Alkene | 0.0243 | 0.52 |
| 13 | 10.167 | cis-β-Ocimene | C10H16 | Alkene | 0.0331 | 0.71 |
| 14 | 10.483 | γ-Terpinene | C10H16 | Alkene | 0.0106 | 0.23 |
| 15 | 10.964 | α-Methyl-α-[4-methyl-3-pentenyl] oriranemethanol | C10H18O2 | Alcohol | 0.0178 | 0.38 |
| 16 | 11.510 | 2-Carene | C10H16 | Alkene | 0.0340 | 0.73 |
| 17 | 12.162 | Linalool | C10H18O | Alcohol | 2.0741 | 44.38 |
| 18 | 13.008 | 1,3,8-p-Menthatriene | C10H14 | Alkene | 0.0187 | 0.40 |
| 19 | 13.426 | Neo-alloocimene, stab. | C10H16 | Alkene | 0.0070 | 0.15 |
| 20 | 14.619 | Terpinen-4-ol | C10H18O | Alcohol | 0.0101 | 0.22 |
| 21 | 15.145 | α-Terpineol | C10H18O | Alcohol | 0.5052 | 10.81 |
| 22 | 16.136 | Levoverbenone | C10H14O | Ketone | 0.0021 | 0.04 |
| 23 | 16.450 | Nerol | C10H18O | Alcohol | 0.0914 | 1.96 |
| 24 | 16.969 | D-Carvone | C10H14O | Ketone | 0.0132 | 0.28 |
| 25 | 17.353 | trans-Geraniol | C10H18O | Alcohol | 0.3413 | 7.30 |
| 26 | 17.986 | (E)-citral | C10H16O | Aldehyde | 0.0013 | 0.03 |
| 27 | 18.055 | 4-Methylundecane | C12H26 | Alkane | 0.0020 | 0.04 |
| 28 | 18.282 | Citronellal | C10H18O | Aldehyde | 0.0022 | 0.05 |
| 29 | 18.666 | Isohexylpentyl sulfite | C11H24O3S | Ester | 0.0010 | 0.02 |
| 30 | 19.050 | Oxalic acid, allyl nonyl ester | C14H24O4 | Ester | 0.0008 | 0.02 |
| 31 | 20.463 | α-Terpinyl acetate | C12H20O2 | Ester | 0.0047 | 0.10 |
| 32 | 21.099 | Neryl acetate | C12H20O2 | Ester | 0.0597 | 1.28 |
| 33 | 21.914 | Geranyl acetate | C12H20O2 | Ester | 0.0909 | 1.94 |
| 34 | 22.540 | Tetradecane | C14H30 | Alkane | 0.0006 | 0.01 |
| 35 | 24.029 | Isovaleric anhydride | C10H18O3 | Acid | 0.0013 | 0.03 |
| 36 | 24.478 | 7-Tetracyclo[6.2.1.0(3.8)0(3.9)]undecanol, 4,4,11,11-tetramethyl- | C15H24O | Alcohol | 0.0096 | 0.21 |
| 37 | 24.686 | trans-Caryophyllene | C15H24 | Alkene | 0.0011 | 0.02 |
| 38 | 24.798 | 4,5-dehydro-Isolongifolene | C15H22 | Alkene | 0.0020 | 0.04 |
| 39 | 25.541 | Dotriacontane | C32H66 | Alkane | 0.0021 | 0.04 |
| 40 | 25.731 | n-Eicosane | C20H42 | Alkane | 0.0023 | 0.05 |
| 41 | 26.308 | Phenol,2,4-bis(1,1-dimethylethyl)- | C14H22O | Phenolic | 0.0079 | 0.17 |
| 42 | 26.676 | 8,9-dehydro-Neoisolongifolene | C15H22 | Alkene | 0.0017 | 0.04 |
| 43 | 26.920 | 4,6-Cholestadiene-3-one, 2,4-dinitrophenylhydrazone | C33H46N4O4 | Phenylhydrazone | 0.0031 | 0.07 |
| 44 | 27.097 | Caryophyllene oxide | C15H24O | Other | 0.0020 | 0.04 |
| 45 | 27.458 | trans-Nerolidol | C15H26O | Alcohol | 0.0076 | 0.16 |
| 46 | 27.669 | Spathulenol | C15H24O | Alcohol | 0.1626 | 3.48 |
| 47 | 28.070 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | C16H30O4 | Ester | 0.0067 | 0.14 |
| 48 | 28.881 | (−)-Spathulenol | C15H24O | Alcohol | 0.0283 | 0.61 |
| 49 | 29.188 | α-Cadinol | C15H26O | Alcohol | 0.0119 | 0.26 |
| 50 | 29.546 | 3,5-Dehydro-6-methoxy-trimethylacetate-cholest-22-en-21-ol | C33H54O3 | Acid | 0.0075 | 0.16 |
| 51 | 29.869 | Oxalic acid, 6-ethyloct-3-yl propyl ester | C15H28O4 | Ester | 0.0050 | 0.11 |
| 52 | 30.748 | Tetracosane | C24H50 | Alkane | 0.0019 | 0.04 |
| 53 | 31.263 | Guaiazulene | C15H18 | Alkane | 0.0043 | 0.09 |
| 54 | 31.579 | Oxalic acid, allyl hexadecyl ester | C21H38O4 | Ester | 0.0016 | 0.03 |
| No. | Retention Time (min) | Compound | Molecular Formula | Compound Type | Absolute Content (mg/kg) | Relative Content (%) |
|---|---|---|---|---|---|---|
| 1 | 11.223 | 2-Heptanone,3-methyl- | C8H16O | Ketone | 0.0015 | 0.02 |
| 2 | 12.883 | β-Myrcene | C10H16 | Alkene | 0.0570 | 0.93 |
| 3 | 13.259 | α-Phellandrene | C10H16 | Alkene | 0.0065 | 0.11 |
| 4 | 13.689 | α-Terpinene | C10H16 | Alkene | 0.0047 | 0.08 |
| 5 | 13.922 | Neopentyl dihydrocinnamate | C14H20O2 | Ester | 0.0012 | 0.02 |
| 6 | 13.987 | Benzene,1-ethyl-3,5-dimethyl- | C10H14 | Aromatic hydrocarbon | 0.0017 | 0.03 |
| 7 | 14.079 | D-Limonene | C10H16 | Alkene | 0.0464 | 0.76 |
| 8 | 14.531 | trans-β-Ocimene | C10H16 | Alkene | 0.0125 | 0.20 |
| 9 | 14.863 | cis-β-Ocimene | C10H16 | Alkene | 0.0185 | 0.30 |
| 10 | 15.136 | γ-Terpinene | C10H16 | Alkene | 0.0042 | 0.07 |
| 11 | 15.605 | α-Methyl-α-[4-methyl-3-pentenyl] oriranemethanol | C10H18O2 | Alcohol | 0.0360 | 0.59 |
| 12 | 16.806 | Linalool | C10H18O | Alcohol | 3.4712 | 56.54 |
| 13 | 17.272 | (−)-Carveol | C10H16O | Alcohol | 0.0024 | 0.04 |
| 14 | 17.485 | Cosmene | C10H14 | Alkene | 0.0088 | 0.14 |
| 15 | 17.720 | (−)-trans-Pinocarveol | C10H16O | Alcohol | 0.0054 | 0.09 |
| 16 | 17.859 | 1-Methylene-2-methyl-3-isopropenylcyclopentane | C10H16 | Cycloalkane | 0.0043 | 0.07 |
| 17 | 18.430 | Pinocarvone | C10H14O | Ketone | 0.0023 | 0.04 |
| 18 | 18.971 | (−)-Terpinen-4-ol | C10H16 | Alcohol | 0.0193 | 0.31 |
| 19 | 19.483 | α-Terpineol | C10H18O | Alcohol | 0.7361 | 11.99 |
| 20 | 20.379 | cis-Carveol | C10H16O | Alcohol | 0.0127 | 0.21 |
| 21 | 20.534 | Nerol | C10H18O | Alcohol | 0.2149 | 3.50 |
| 22 | 20.944 | (−)-Carvone | C10H14O | Ketone | 0.0036 | 0.06 |
| 23 | 21.192 | trans-Geraniol | C10H18O | Alcohol | 0.4865 | 7.92 |
| 24 | 21.789 | Isoborneol | C10H18O | Alcohol | 0.0012 | 0.02 |
| 25 | 22.000 | Sulfurous acid,isohexyl pentyl ester | C11H24O3S | Ester | 0.0009 | 0.01 |
| 26 | 23.381 | Neryl acetate | C12H20O2 | Ester | 0.0123 | 0.20 |
| 27 | 23.771 | Geranyl acetate | C12H20O2 | Ester | 0.0134 | 0.22 |
| 28 | 24.718 | Dehydro aromadendrene | C15H22 | Alkene | 0.0047 | 0.08 |
| 29 | 25.627 | Phenol,2,4-bis(1,1-dimethylethyl)- | C14H22O | Phenolic | 0.0083 | 0.13 |
| 30 | 25.884 | 7,9-Dimethylhexadecane | C18H38 | Alkane | 0.0013 | 0.02 |
| 31 | 26.492 | Spathulenol | C15H24O | Alcohol | 0.1041 | 1.70 |
| 32 | 27.285 | (−)-Spathulenol | C15H24O | Alcohol | 0.0054 | 0.09 |
| No. | Retention Time (min) | Compound | Molecular Formula | Compound Type | Absolute Content (mg/kg) | Relative Content (%) |
|---|---|---|---|---|---|---|
| 1 | 11.282 | 3,4-Dimethyl-2-hexanone | C8H16O | Ketone | 0.0097 | 0.18 |
| 2 | 12.885 | β-Myrcene | C10H16 | Alkene | 0.0435 | 0.79 |
| 3 | 14.081 | D-Limonene | C10H16 | Alkene | 0.0310 | 0.56 |
| 4 | 14.537 | trans-β-Ocimene | C10H16 | Alkene | 0.0072 | 0.13 |
| 5 | 14.874 | cis-β-Ocimene | C10H16 | Alkene | 0.0115 | 0.21 |
| 6 | 15.608 | cis-Linalool oxide (furanoid) | C10H18O2 | Alcohol | 0.0399 | 0.72 |
| 7 | 16.081 | 3,4-Dimethylbenzyl alcohol | C9H12O | Alcohol | 0.0028 | 0.05 |
| 8 | 16.159 | trans-Linalool oxide (furans) | C10H18O2 | Alcohol | 0.0165 | 0.30 |
| 9 | 16.788 | Linalool | C10H18O | Alcohol | 3.1096 | 56.44 |
| 10 | 17.490 | Cosmene | C10H14 | Alkene | 0.0029 | 0.05 |
| 11 | 17.716 | (−)-trans-Pinocarveol | C10H16O | Alcohol | 0.0038 | 0.07 |
| 12 | 18.979 | (−)-Terpinen-4-ol | C10H16 | Alcohol | 0.0169 | 0.31 |
| 13 | 19.493 | α-Terpineol | C10H18O | Alcohol | 0.7186 | 13.04 |
| 14 | 20.370 | cis-Carveol | C10H16O | Alcohol | 0.0043 | 0.08 |
| 15 | 20.536 | Nerol | C10H18O | Alcohol | 0.1795 | 3.26 |
| 16 | 20.931 | D-Carvone | C10H14O | Ketone | 0.0024 | 0.04 |
| 17 | 21.190 | trans-Geraniol | C10H18O | Alcohol | 0.3591 | 6.52 |
| 18 | 21.643 | 5-Ethyl-5-methyldecane | C13H28 | Alkane | 0.0016 | 0.03 |
| 19 | 21.795 | Glutaric acid, tridec-2-yn-1-yl 2-decyl ester | C28H50O4 | Ester | 0.0017 | 0.03 |
| 20 | 23.379 | Neryl acetate | C12H20O2 | Ester | 0.0069 | 0.13 |
| 21 | 23.774 | Geranyl acetate | C12H20O2 | Ester | 0.0074 | 0.13 |
| 22 | 24.718 | 4a,5-Dimethyl-3-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalen-1-ol | C15H24O | Alcohol | 0.0021 | 0.04 |
| 23 | 24.764 | (+)-Cycloheterophyllin-5-ol | C15H24O | Alcohol | 0.0025 | 0.05 |
| 24 | 25.627 | 2,4-Bis(1,1-dimethylethyl) phenol | C14H22O | Phenolic | 0.0065 | 0.12 |
| 25 | 26.491 | Spathulenol | C15H24O | Alcohol | 0.1147 | 2.08 |
| 26 | 27.281 | (−)-Spathulenol | C15H24O | Alcohol | 0.0034 | 0.06 |
| Compound Categories | CAVA IH | CAVA UH | CADB IH | CADB UH | |
|---|---|---|---|---|---|
| Specific compounds | Alcohols | 8 | 1 | 1 | 4 |
| Alkenes | 8 | 3 | - | - | |
| Esters | 2 | 3 | 1 | 1 | |
| Alkanes | 5 | 4 | 1 | 1 | |
| Aromatic hydrocarbons | 4 | 1 | 1 | - | |
| Ketones | 1 | 1 | 2 | 1 | |
| Aldehydes | 1 | 2 | - | - | |
| Ether | 1 | - | - | - | |
| Indene | 1 | - | - | - | |
| Acids | - | 2 | - | - | |
| Phenylhydrazone | - | 1 | - | - | |
| Cycloalkane | - | - | 1 | - | |
| Others | 1 | 1 | - | - | |
| Total | - | 32 | 19 | 7 | 7 |
| Shared compounds | Alkenes | 4 | |||
| Alcohols | 6 | ||||
| Esters | 2 | ||||
| Phenolic | 1 | ||||
| Total | - | 13 | |||
| No. | Compounds | Odor Descriptors | Odor Threshold (mg/kg) | OAV | |||
|---|---|---|---|---|---|---|---|
| CAVA IH | CAVA UH | CADB IH | CADB UH | ||||
| Ketone | |||||||
| 1 | 3-Heptanone,4-methyl # (1) | - | - | - | - | - | |
| 2 | (−)-Carvone # (2) | Grass, menthol | 0.007 | - | - | 0.514 | - |
| 3 | 3,4-Dimethyl-2-hexanone # (3) | - | - | - | - | - | |
| 4 | 2-Heptanone,3-methyl- (4) | - | - | - | - | - | |
| 5 | D-Carvone (5) | Floral, lingering orchid | 0.16 | 0.044 | 0.083 | - | 0.015 |
| 6 | Pinocarvone # (6) | Lingering orchid | - | - | - | - | - |
| 7 | Levoverbenone # (7) | Strong minty, camphoraceous | - | - | - | - | - |
| Alcohol | |||||||
| 1 | 3-Phenyl-2-butanol # (8) | - | - | - | - | - | |
| 2 | 2-Norpinene-2-ethanol,6,6-dimethyl- # (9) | - | - | - | - | - | |
| 3 | Artemesia alcohol # (10) | - | - | - | - | - | |
| 4 | 1-Heptatriacotanol # (11) | Grease fragrance | - | - | - | - | - |
| 5 | (+)-Viridiflorol # (12) | Peppery, spicy, camphoraceous | - | - | - | - | - |
| 6 | Ledol # (13) | Tea, fruit sweetness | - | - | - | - | - |
| 7 | Tricyclo[5.2.2.0(1,6)]undecan-3-ol,2-methylene-6,8,8-trimethyl # (14) | - | - | - | - | - | |
| 8 | 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol # (15) | - | - | - | - | - | |
| 9 | cis-7-Dodecen-1-ol # (16) | - | - | - | - | - | |
| 10 | (-)-Carveol # (17) | Mint, green, herbal, coriander, spicy | 0.25 | - | - | 0.010 | - |
| 11 | (-)-trans-Pinocarveol (18) | Green, wood | - | - | - | - | - |
| 12 | cis-Carveol (19) | Citrus, spearmint | 0.25 | - | - | 0.051 | 0.017 |
| 13 | cis-Linalool oxide (furanoid) * (20) | Earthy, floral, sweet wood | 0.32 | 0.039 | 0.056 | 0.113 | 0.125 |
| 14 | 3,4-Dimethylbenzyl alcohol # (21) | - | - | - | - | - | |
| 15 | trans-Linalool oxide (furans) # (22) | sweet scent | 0.32 | - | - | - | 0.052 |
| 16 | 4a,5-Dimethyl-3-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalen-1-ol # (23) | - | - | - | - | - | |
| 17 | (+)-Cycloisolongifol-5-ol # (24) | - | - | - | - | - | |
| 18 | (−)-Terpinen-4-ol (25) | Nutmeg, musk | 1.2 | - | - | 0.016 | 0.014 |
| 19 | trans-Geraniol (26) | Passion fruit, lemon, rose | 0.0075 | 41.587 | 45.507 | 64.867 | 47.880 |
| 20 | (−)-Spathulenol (27) | Pungent, loamy, woody | - | - | - | - | - |
| 21 | Linalool * (28) | Floral (lily of the valley, rose, lilac), citrus, woody | 0.00022 | 7124.55 | 9427.73 | 15778.182 | 14134.545 |
| 22 | α-Terpineol * (29) | Lilac floral, citrus, woody | 1.2 | 0.334 | 0.421 | 0.613 | 0.599 |
| 23 | Spathulenol * (30) | Earthy, herbal, fruity | - | - | - | - | - |
| 24 | Nerol * (31) | Flowery, grassy | 0.68 | 0.124 | 0.134 | 0.316 | 0.264 |
| 25 | Terpinen-4-ol (32) | Wooden, fresh | 1.2 | 0.006 | 0.008 | - | - |
| 26 | 7-Tetracyclo[6.2.1.0(3.8)0(3.9)]undecanol, 4,4,11,11-tetramethyl- (33) | - | - | - | - | - | |
| 27 | trans-Nerolidol (34) | Fir, pine incense | 0.25 | 0.128 | 0.030 | - | - |
| 28 | α-Cadinol (35) | Herbs, herbal medicine, mullein | - | - | - | - | - |
| 29 | Isoborneol (36) | Camphoraceous | 0.0085 | 0.212 | - | 0.141 | - |
| Aromatic hydrocarbon | |||||||
| 1 | Benzene,1-ethyl-2-methyl # (37) | - | - | - | - | - | |
| 2 | p-Cymene # (38) | Subtle citrus, carrot | 0.005 | 0.48 | - | - | - |
| 3 | 4,5-dimethyl-Biphenylene,1,2,3,6,7,8,8a,8b-octahydro-4,5-dimethyl- # (39) | - | - | - | - | - | |
| 4 | Benzene,1-ethyl-3,5-dimethyl- # (40) | - | - | - | - | - | |
| 5 | Benzene,1,2,3-trimethyl- (41) | Aromatic | - | - | - | - | - |
| 6 | o-Cymene # (42) | 0.004 | 2.125 | - | - | - | |
| 7 | m-Cymene # (43) | 0.8 | - | 0.003 | - | - | |
| Alkane | |||||||
| 1 | 4,6-Dimethyldodecane # (44) | - | - | - | - | - | |
| 2 | 2,3,5,8-Tetramethyldecane # (45) | - | - | - | - | - | |
| 3 | 2,6,11-Trimethyldodecane # (46) | Tasteless | - | - | - | - | - |
| 4 | 10-Methylnonadecane # (47) | - | - | - | - | - | |
| 5 | 5-Butylnonane # (48) | - | - | - | - | - | |
| 6 | 4-Methylundecane # (49) | - | - | - | - | - | |
| 7 | Dotriacontane # (50) | Tasteless | - | - | - | - | - |
| 8 | n-Eicosane # (51) | Glutinous rice | - | - | - | - | - |
| 9 | Tetracosane # (52) | Tasteless | - | - | - | - | - |
| 10 | 7,9-Dimethylhexadecane # (53) | - | - | - | - | - | |
| 11 | 5-Ethyl-5-methyldecane # (54) | - | - | - | - | - | |
| 12 | Tetradecane (55) | Waxiness | 0.005 | 0.120 | 0.120 | - | - |
| 13 | Guaiazulene (56) | - | - | - | - | - | |
| Alkene | |||||||
| 1 | Nonane,5-methylene- # (57) | - | - | - | - | - | |
| 2 | (Z)-β-farnesene # (58) | Citrus, floral | 0.087 | 0.040 | - | - | - |
| 3 | α-Muurolene # (59) | Fresh flower | 0.0075 | 0.360 | - | - | - |
| 4 | γ-Muurolene # (60) | Fresh flower | - | - | - | - | - |
| 5 | 7R,8R-8-Hydroxy-4-isopropylidene-7-methylbicyclo [5.3.1] undec-1-ene # (61) | - | - | - | - | - | |
| 6 | 2-Methyl-1-hexene # (62) | - | - | - | - | - | |
| 7 | trans-Caryophyllene # (63) | Sweet, woody, peppery | 0.064 | - | 0.017 | - | - |
| 8 | 4,5-dehydro-Isolongifolene # (64) | - | - | - | - | - | |
| 9 | cis-β-Ocimene * (65) | Grassy, floral with orange blossom oil | 0.034 | 0.929 | 0.974 | 0.544 | 0.338 |
| 10 | β-Myrcene * (66) | Peppery, spicy, floral | 0.0012 | 64.083 | 67.500 | 47.500 | 36.250 |
| 11 | D-Limonene * (67) | Citrus, lemon | 0.034 | 2.756 | 3.015 | 1.365 | 0.912 |
| 12 | trans-β-Ocimene * (68) | Green, citrus, mint | 0.034 | 0.650 | 0.715 | 0.368 | 0.212 |
| 13 | α-Phellandrene (69) | Black pepper | 0.036 | 0.325 | 0.258 | 0.181 | - |
| 14 | α-Terpinene (70) | Woody, tea | 0.08 | 0.163 | 0.139 | 0.059 | - |
| 15 | γ-Terpinene (71) | Citrus | 1 | 0.009 | 0.011 | 0.004 | - |
| 16 | 2-Carene (72) | Green grassy | 0.037 | 0.743 | 0.919 | - | - |
| 17 | Neo-alloocimene, stab. (73) | Grassy, floral with orange blossom oil | 0.034 | 0.268 | 0.206 | - | - |
| 18 | trans, trans-2,8-Decadiene # (74) | - | - | - | - | - | |
| 19 | Calamenene # (75) | Vanilla, Camphoraceous, medicinal | - | - | - | - | - |
| 20 | Cosmene * (76) | - | - | - | - | - | |
| 21 | 8,9-dehydro-Neoisolongifolene (77) | - | - | - | - | - | |
| 22 | Dehydro aromadendrene (78) | - | - | - | - | - | |
| 23 | Cadala-1(10),3,8-triene # (79) | - | - | - | - | - | |
| 24 | 1,3,8-p-Menthatriene # (80) | - | - | - | - | - | |
| Ester | |||||||
| 1 | 2-Chlorobenzoic acid, dodec-9-ynyl ester # (81) | - | - | - | - | - | |
| 2 | Diallyl carbonate # (82) | - | - | - | - | - | |
| 3 | Oxalic acid, allyl nonyl ester # (83) | - | - | - | - | - | |
| 4 | Oxalic acid, 6-ethyloct-3-yl propyl ester # (84) | - | - | - | - | - | |
| 5 | Oxalic acid, allyl hexadecyl ester # (85) | - | - | - | - | - | |
| 6 | Neopentyl dihydrocinnamate # (86) | - | - | - | - | - | |
| 7 | Glutaric acid, tridec-2-yn-1-yl 2-decyl ester # (87) | - | - | - | - | - | |
| 8 | Sulfurous acid,isohexyl pentyl ester (88) | - | - | - | - | - | |
| 9 | Neryl acetate * (89) | Rose fragrance, honey sweetness | 2 | 0.032 | 0.030 | 0.006 | 0.003 |
| 10 | Geranyl acetate * (90) | Rose, lavender | 0.15 | 0.711 | 0.606 | 0.089 | 0.049 |
| 11 | Allyl butyrate (91) | - | - | - | - | - | |
| 12 | α-Terpinyl acetate (92) | Pine fragrance, woody fragrance | 2.5 | 0.002 | 0.002 | - | - |
| 13 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate (93) | - | - | - | - | - | |
| Aldehyde | |||||||
| 1 | Butanal,4-[(tetrahydro-2H-pyran-2-yl) oxy]- # (94) | - | - | - | - | - | |
| 2 | (E)-citral # (95) | - | - | - | - | - | |
| 3 | Citronellal # (96) | Fruity | 0.0035 | - | 0.629 | - | - |
| Phenolic | |||||||
| 1 | 2,4-Bis(1,1-dimethylethyl) phenol * (97) | Phenolic, resinous | 0.5 | 0.010 | 0.016 | 0.017 | 0.013 |
| Cycloalkane | |||||||
| 1 | 1-Methylene-2-methyl-3-isopropenylcyclopentane # (98) | - | - | - | - | - | |
| Acid | |||||||
| 1 | Isovaleric anhydride # (99) | - | - | - | - | - | |
| 2 | 3,5-Dehydro-6-methoxy-trimethylacetate-cholest-22-en-21-ol # (100) | - | - | - | - | - | |
| Ether | |||||||
| 1 | Ethylene glycol diallyl ether # (101) | - | - | - | - | - | |
| Phenylhydrazone | |||||||
| 1 | 4,6-Cholestadiene-3-one, 2,4-dinitrophenylhydrazone # (102) | - | - | - | - | - | |
| Indene | |||||||
| 1 | Octahydro-5-(2-octyldecyl)-4,7-methano-1H-indene # (103) | - | - | - | - | - | |
| Other | |||||||
| 1 | Bicyclo[3.1.0]hexane-6,6-dicarbonitrile # (104) | - | - | - | - | - | |
| 2 | Butanenitrile # (105) | - | - | - | - | - | |
| 3 | Caryophyllene oxide (106) | Pungent, woody, clove, some sweetness | 0.41 | 0.112 | 0.005 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Xue, H.; Ma, B.; Guo, X.; Xia, Y.; Yang, Y.; Xu, K.; Li, T.; Luo, X. Comparative Analysis of Hydrosol Volatile Components of Citrus × Aurantium ‘Daidai’ and Citrus × Aurantium L. Dried Buds with Different Extraction Processes Using Headspace-Solid-Phase Microextraction with Gas Chromatography–Mass Spectrometry. Molecules 2024, 29, 3498. https://doi.org/10.3390/molecules29153498
Xie X, Xue H, Ma B, Guo X, Xia Y, Yang Y, Xu K, Li T, Luo X. Comparative Analysis of Hydrosol Volatile Components of Citrus × Aurantium ‘Daidai’ and Citrus × Aurantium L. Dried Buds with Different Extraction Processes Using Headspace-Solid-Phase Microextraction with Gas Chromatography–Mass Spectrometry. Molecules. 2024; 29(15):3498. https://doi.org/10.3390/molecules29153498
Chicago/Turabian StyleXie, Xinyue, Huiling Xue, Baoshan Ma, Xiaoqian Guo, Yanli Xia, Yuxia Yang, Ke Xu, Ting Li, and Xia Luo. 2024. "Comparative Analysis of Hydrosol Volatile Components of Citrus × Aurantium ‘Daidai’ and Citrus × Aurantium L. Dried Buds with Different Extraction Processes Using Headspace-Solid-Phase Microextraction with Gas Chromatography–Mass Spectrometry" Molecules 29, no. 15: 3498. https://doi.org/10.3390/molecules29153498
APA StyleXie, X., Xue, H., Ma, B., Guo, X., Xia, Y., Yang, Y., Xu, K., Li, T., & Luo, X. (2024). Comparative Analysis of Hydrosol Volatile Components of Citrus × Aurantium ‘Daidai’ and Citrus × Aurantium L. Dried Buds with Different Extraction Processes Using Headspace-Solid-Phase Microextraction with Gas Chromatography–Mass Spectrometry. Molecules, 29(15), 3498. https://doi.org/10.3390/molecules29153498








