The Influence of Zinc Oxide and Zinc Stearate on the Antimicrobial Activity of Coatings Containing Raspberry and Chokeberry Extracts
Abstract
1. Introduction
2. Results and Discussion
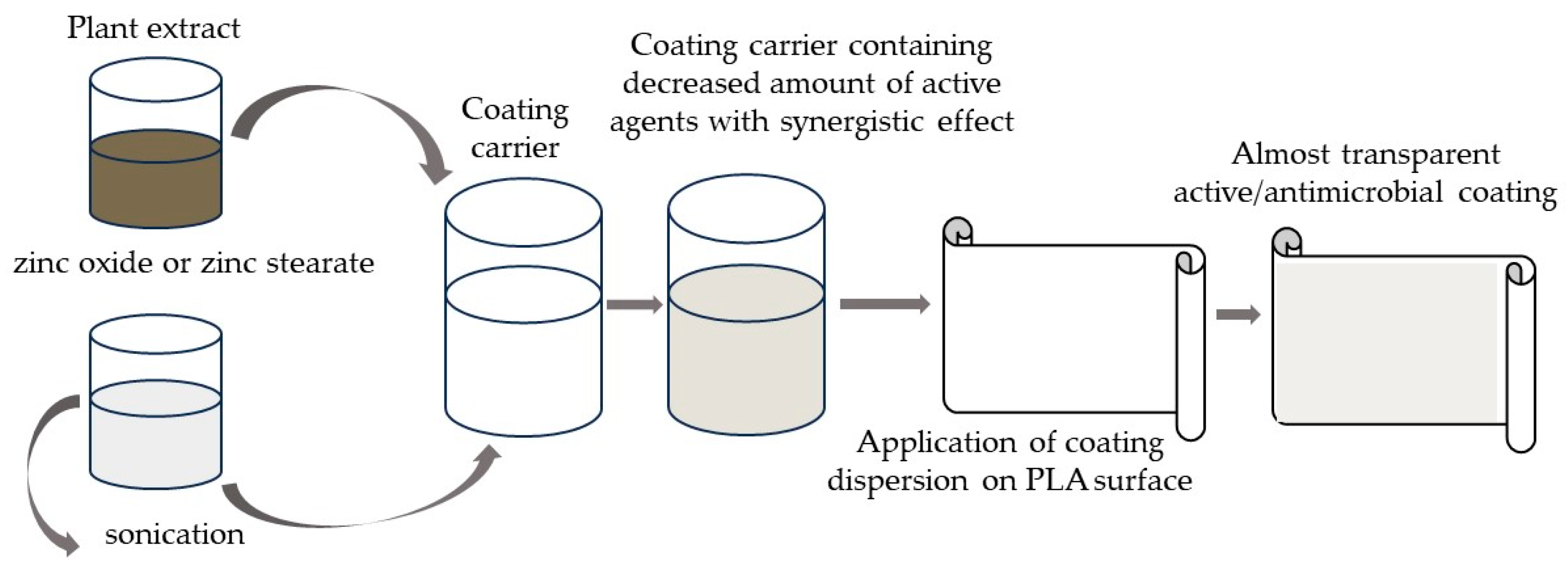
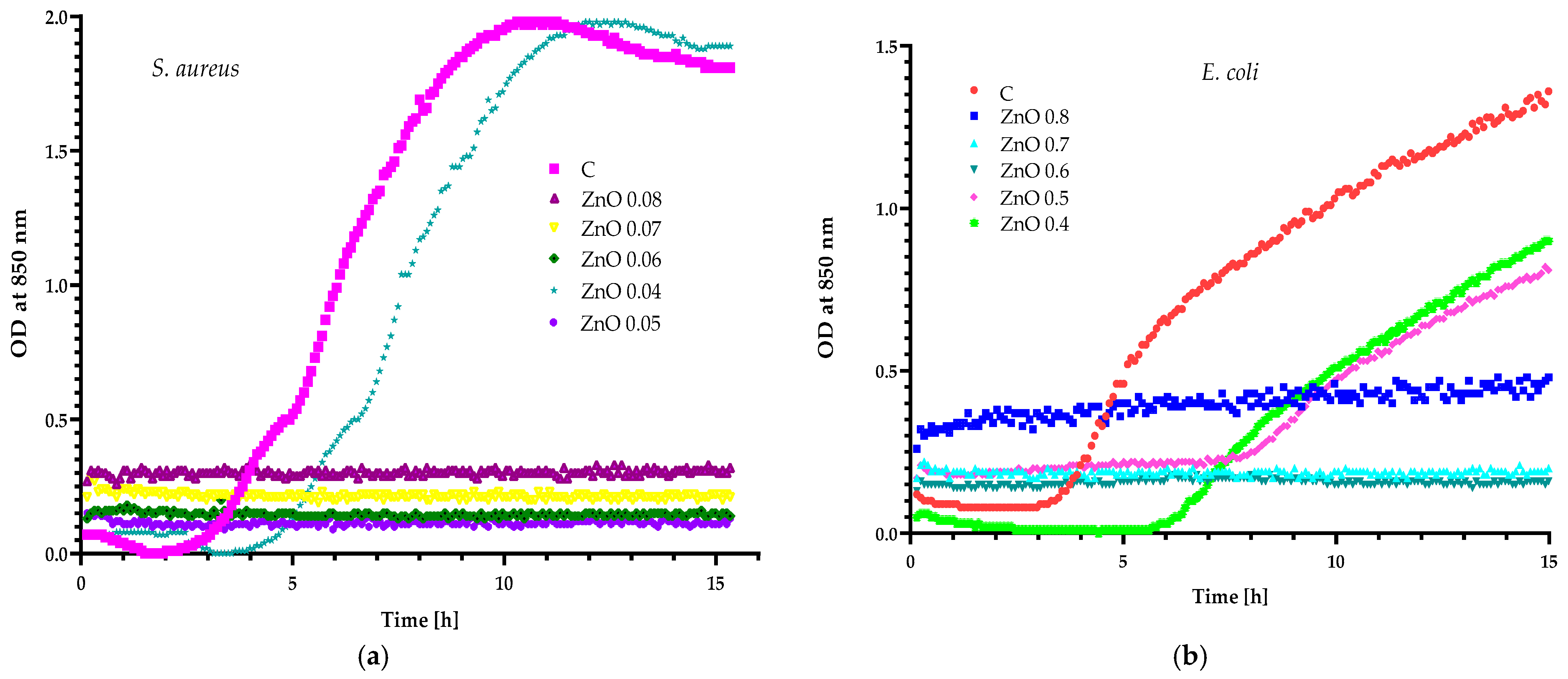
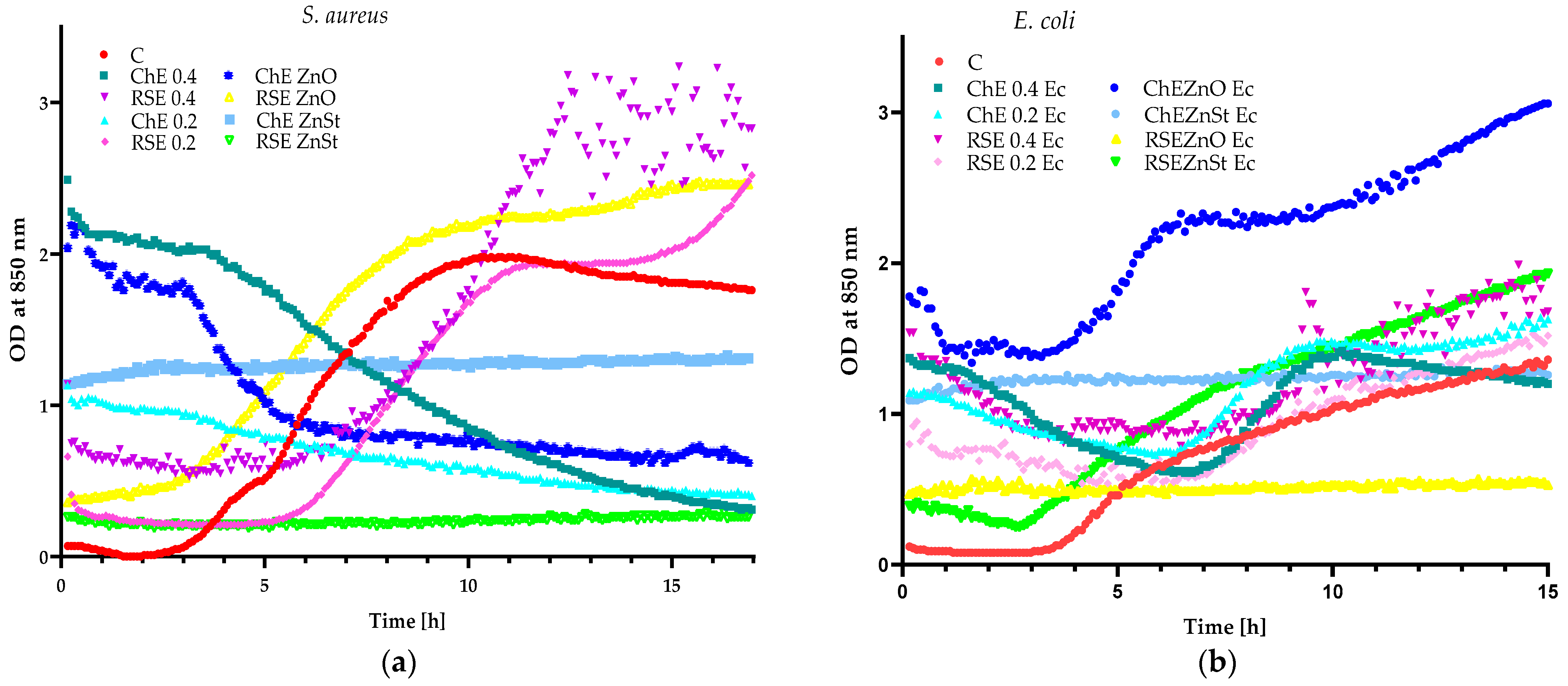
2.1. Preliminary Research Work
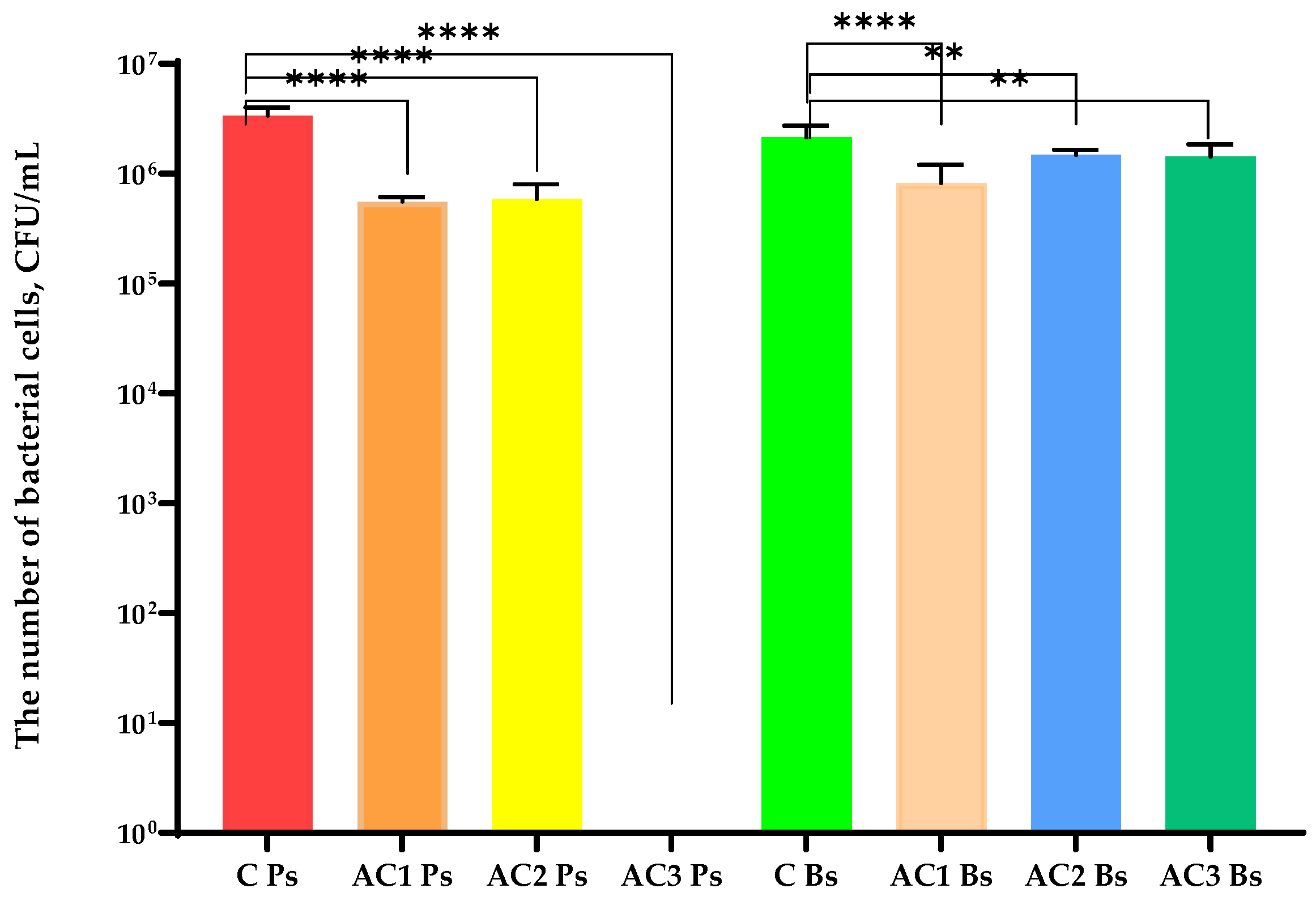
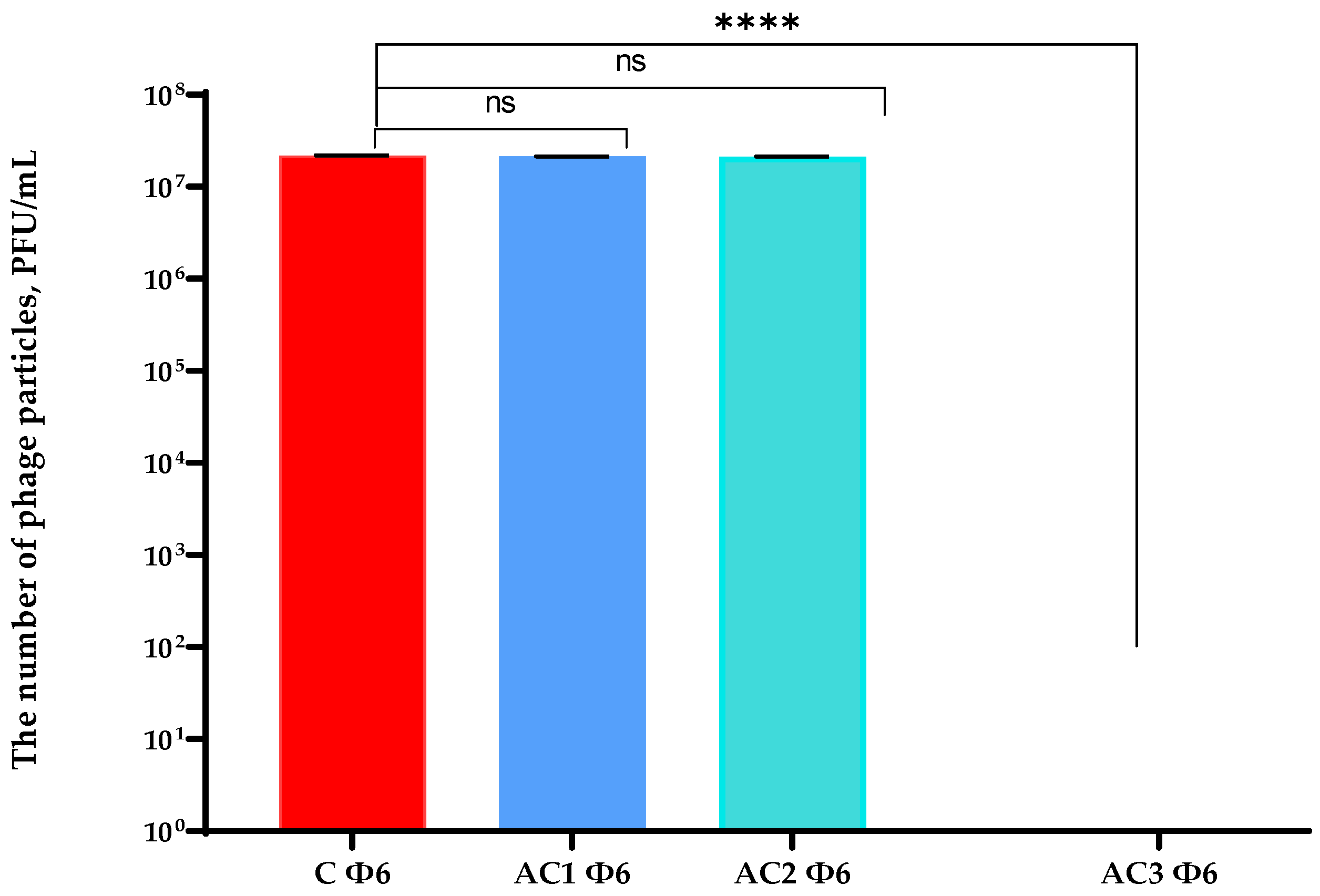
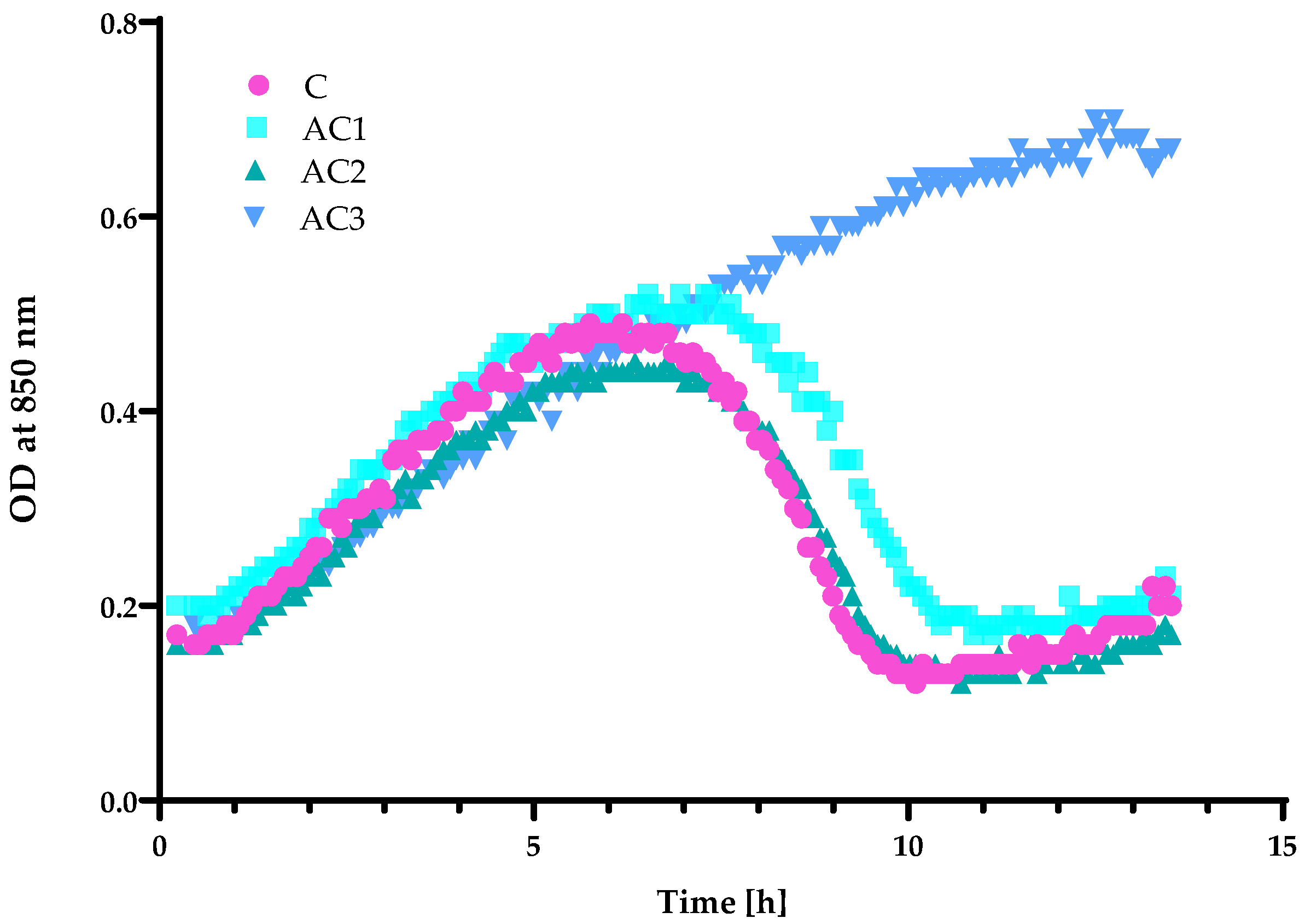
2.2. Antibacterial and Antiviral Properties Analysis of the Coatings
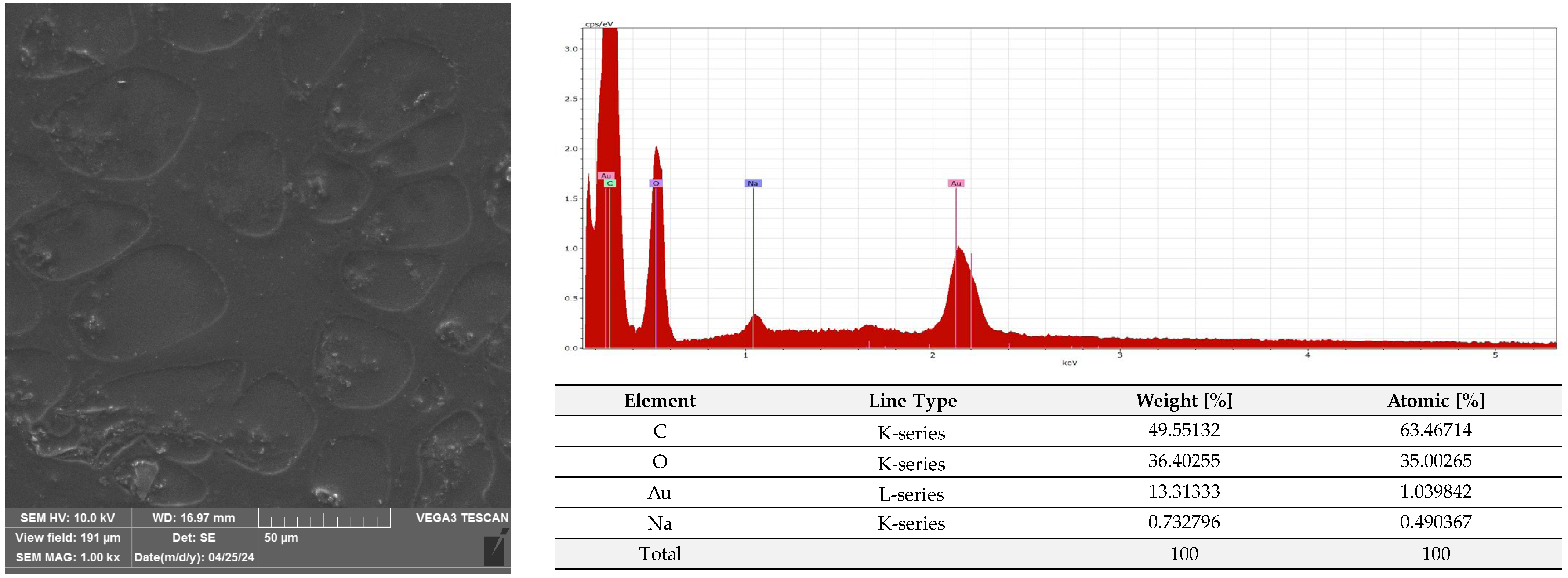
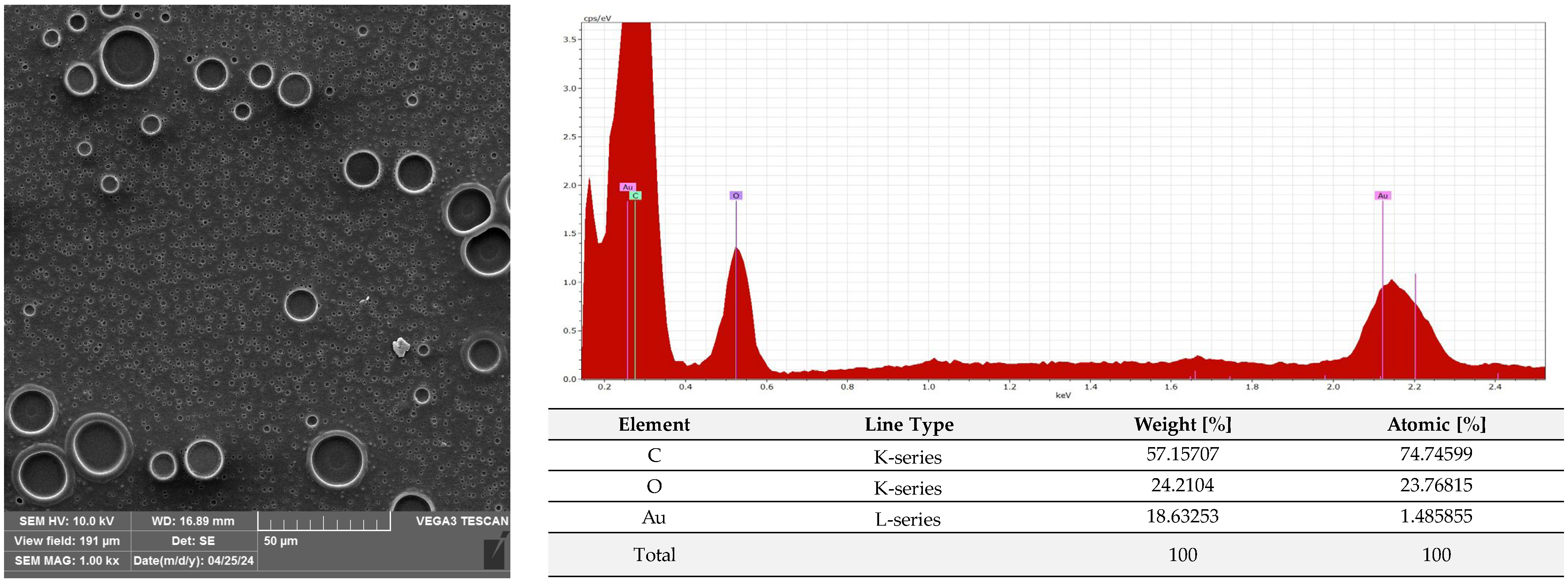
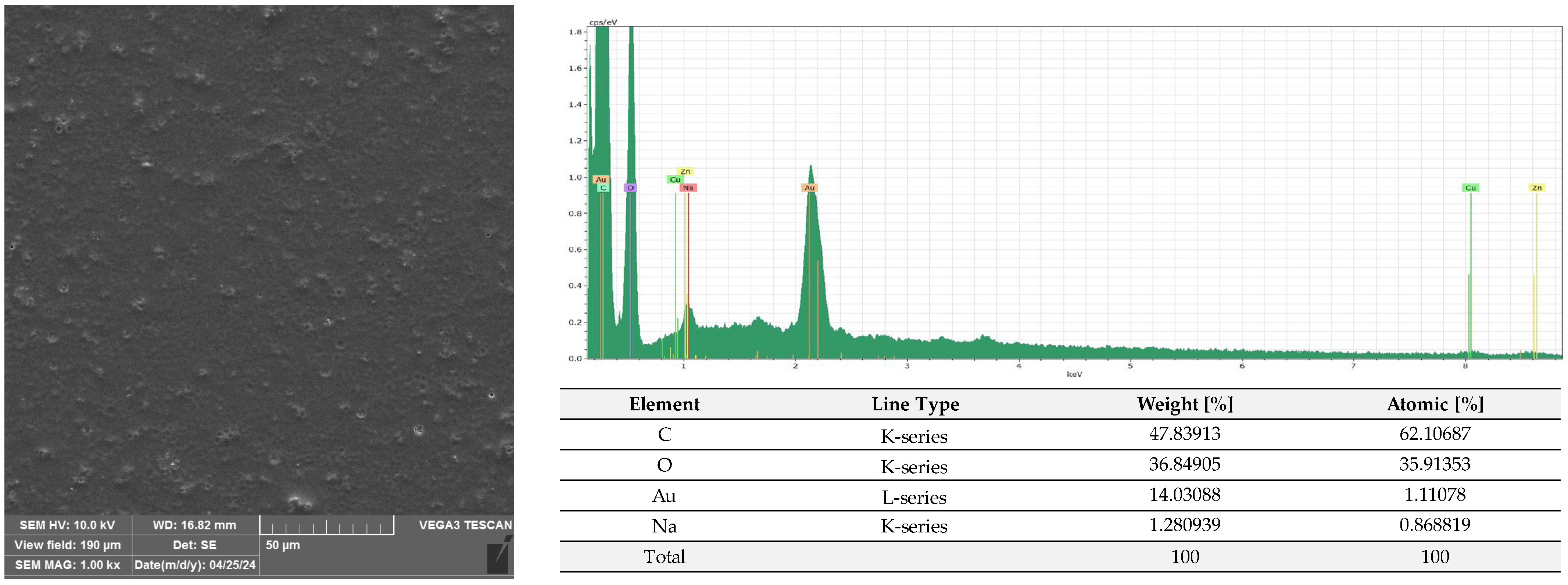
2.3. Microscopic Analysis of Films Covered with the Active Coatings
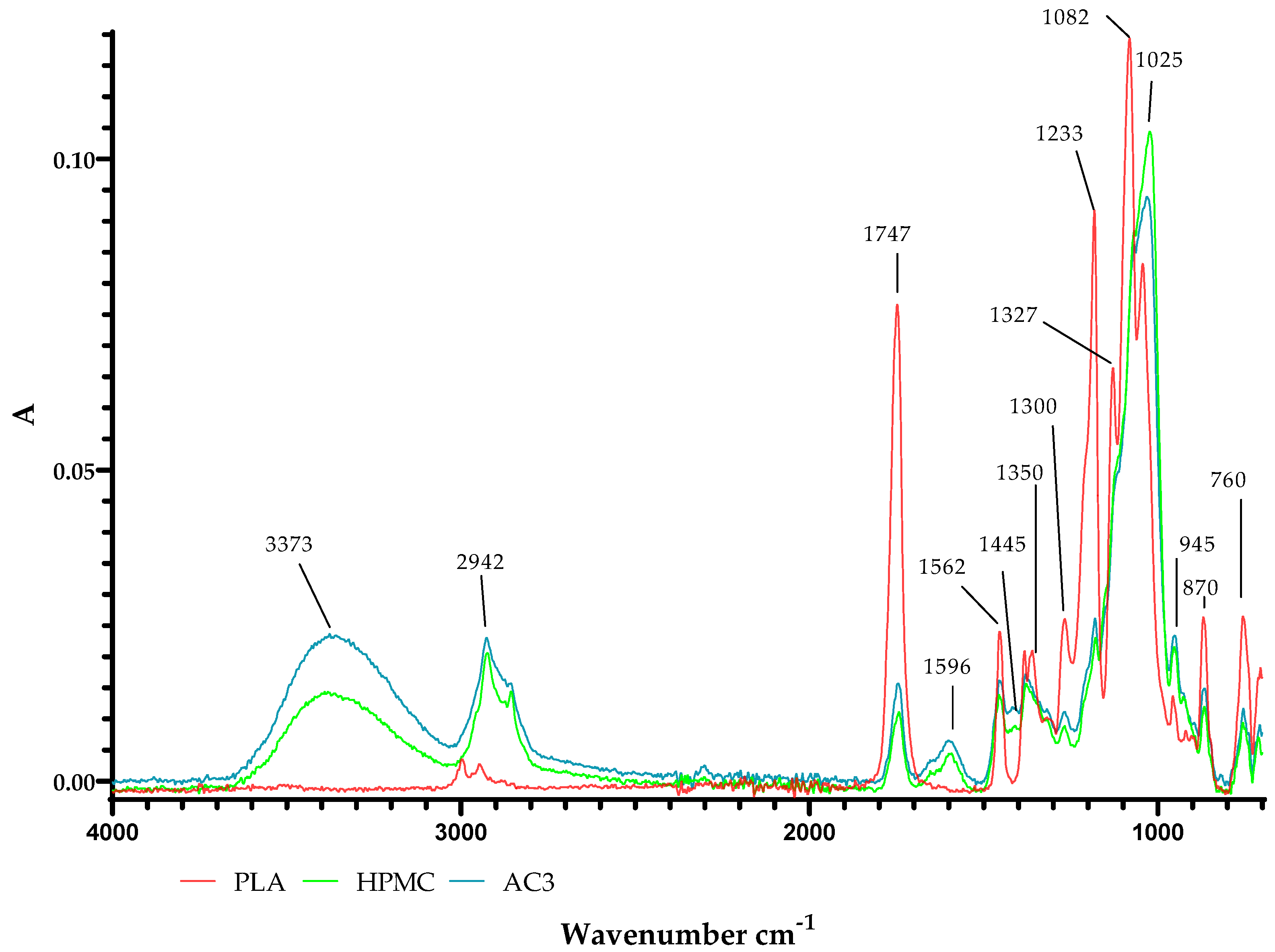
2.4. Results of ATR-FTIR Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Extracts Preparation
3.2.2. Preliminary Analysis of Extracts and Zinc Oxide
3.2.3. Analysis of Synergistic Effect between Extracts and Zinc Oxide and Stearate
3.2.4. Coating System Preparation
3.2.5. Antibacterial and Antiviral Analysis of the Coatings
3.2.6. SEM and EDS Analysis
3.2.7. ATR-FTIR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef]
- Huang, F.; Cai, X.; Hou, X.; Zhang, Y.; Liu, J.; Yang, L.; Liu, Y.; Liu, J. A dynamic covalent polymeric antimicrobial for conquering drug-resistant bacterial infection. Exploration 2022, 2, 20210145. [Google Scholar] [CrossRef] [PubMed]
- Abebe, G.M. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Hind. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef] [PubMed]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyżowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Shin, Y. Antibacterial and in vitro antidementia effects of aronia (Aronia melanocarpa) leaf extracts. Food Sci. Biotechnol. 2020, 29, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhu, J.; Tong, Y.; Kong, Y.; Tan, C.; Wang, M.; Meng, X. Antibacterial characteristics and mechanisms of action of Aronia melanocarpa anthocyanins against Escherichia coli. LWT 2021, 150, 112018. [Google Scholar] [CrossRef]
- Peng, M.; Jiang, C.; Jing, H.; Du, X.; Fan, X.; Zhang, Y.; Wang, H. Comparison of different extraction methods on yield, purity, antioxidant, and antibacterial activities of proanthocyanidins from chokeberry (Aronia melanocarpa). Food Meas. 2022, 16, 2049–2059. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyżowska, A.; Śniadowska, M.; Otlewska, A.; Żyżelewicz, D. Antibacterial mechanisms of Aronia melanocarpa (Michx.), Chaenomeles superba Lindl. and Cornus mas L. leaf extracts. Food Chem. 2021, 350, 129218. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, H.; Kim, J.; Bae, D.; Song, K.Y.; Chon, J.W.; Lee, J.M.; Kim, S.H.; Lim, H.W.; Seo, K.H. Antibacterial Activity of Crude Aronia melanocarpa (Black Chokeberry) Extracts against Bacillus cereus, Staphylococcus aureus, Cronobacter sakazakii, and Salmonella Enteritidis in Various Dairy Foods: Preliminary Study. J. Milk Sci. Biotechnol. 2018, 36, 155–163. [Google Scholar] [CrossRef]
- Ordon, M.; Nawrotek, P.; Stachurska, X.; Mizielińska, M. Polyethylene films coated with antibacterial and antiviral layers based on CO2 extracts of raspberry seeds, of pomegranate seeds and of rosemary. Coatings 2021, 11, 1179. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Ali Mahdi, A.; Al-Ansi, W.; Mohammed, J.K.; Wei, M.; Yao, W. Evaluation of bioactive compounds and antibacterial activity of Pulicaria jaubertii extract obtained by supercritical and conventional methods. J. Food Meas. Charact. 2021, 15, 449–456. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.K. Review of functional and pharmacological activities of berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef]
- Denev, P.; Číž, M.; Kratchanova, M.; Blazheva, D. Black chokeberry (Aronia melanocarpa) polyphenols reveal different antioxidant, antimicrobial and neutrophil-modulating activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef]
- Lajarrige, A.; Gontard, N.; Gaucel, S.; Peyron, S. Evaluation of the Food Contact Suitability of Aged Bio-Nanocomposite Materials Dedicated to Food Packaging Applications. Appl. Sci. 2020, 10, 877. [Google Scholar] [CrossRef]
- Jordá-Reolid, M.; Ibáñez-García, A.; Catani, L.; Martínez-García, A. Development of Blends to Improve Flexibility of Biodegradable Polymers. Polymers 2022, 14, 5223. [Google Scholar] [CrossRef] [PubMed]
- De Luca, S.; Milanese, D.; Gallichi-Nottiani, D.; Cavazza, A.; Sciancalepore, C. Poly(lactic acid) and Its Blends for Packaging Application: A Review. Clean Technol. 2023, 5, 1304. [Google Scholar] [CrossRef]
- Wang, Q.; Bobadilla, S.; Espert, M.; Sanz, T.; Salvador, A. Shortening replacement by hydroxypropyl methylcellulose-based oleogels obtained by different indirect approaches. Texture and sensory properties of baked puff pastry. Food Hydrocoll. 2024, 153, 109936. [Google Scholar] [CrossRef]
- Jayalakshmi, K.; Ismayil; Hegde, S.; Monteiro, J. Investigating the properties of hydroxy propyl methyl cellulose based magnesium ion-conducting solid polymer electrolytes for primary battery applications. J. Energ. Storage 2024, 89, 111575. [Google Scholar] [CrossRef]
- Yao, J.; Zhi, H.; Shi, Q.; Zhang, Y.; Feng, J.; Liu, J.; Huang, H.; Xie, X. Tannic Acid Interfacial Modification of Prochloraz Ethyl Cellulose Nanoparticles for Enhancing the Antimicrobial Effect and Biosafety of Fungicides. ACS Appl. Mater Interfaces 2023, 15, 41324–41336. [Google Scholar] [CrossRef]
- Acquavia, M.A.; Benítez, J.J.; Bianco, G.; Crescenzi, M.A.; Hierrezuelo, J.; Grifé-Ruiz, M.; Romero, D.; Guzmán-Puyol, S.; Heredia-Guerrero, J.A. Incorporation of bioactive compounds from avocado by-products to ethyl cellulose-reinforced paper for food packaging applications. Food Chem. 2023, 429, 136906. [Google Scholar] [CrossRef]
- Yang, X.; Rao, J.; Shen, C.; Lian, H.; Wang, D.; Wu, D.; Chen, K. Natamycin-Loaded Ethyl Cellulose/PVP Films Developed by Microfluidic Spinning for Active Packaging. Foods 2024, 13, 132. [Google Scholar] [CrossRef]
- Ordon, M.; Burdajewicz, W.; Sternal, J.; Okręglicki, M.; Mizielińska, M. The Antibacterial Effect of the Films Coated with the Layers Based on Uncaria tomentosa and Formitopsis betulina Extracts and ZnO Nanoparticles and Their Influence on the Secondary Shelf-Life of Sliced Cooked Ham. Appl. Sci. 2023, 13, 8853. [Google Scholar] [CrossRef]
- Ordon, M.; Nawrotek, P.; Stachurska, X.; Schmidt, A.; Mizielińska, M. Mixtures of Scutellaria baicalensis and Glycyrrhiza L. Extracts as Antibacterial and Antiviral Agents in Active Coatings. Coatings 2021, 11, 1438. [Google Scholar] [CrossRef]
- Mizielińska, M.; Nawrotek, P.; Stachurska, X.; Ordon, M.; Bartkowiak, A. Packaging Covered with Antiviral and Antibacterial Coatings Based on ZnO Nanoparticles Supplemented with Geraniol and Carvacrol. Int. J. Mol. Sci. 2021, 22, 1717. [Google Scholar] [CrossRef]
- Mohammadipour-Nodoushan, R.; Shekarriz, S.; Shariatinia, Z.; Heydari, A.; Montazer, M. Improved cotton fabrics properties using zinc oxide-based nanomaterials: A review. Int. J. Biol. Macromol. 2023, 242, 124916. [Google Scholar] [CrossRef]
- Gu, B.; Cai, J.; Peng, G.; Zhou, H.; Zhang, W.; Zhang, D.; Gong, D. Metal organic framework-loaded biohybrid magnetic microrobots for enhanced antibacterial treatment. Colloids Surf. A 2024, 685, 133295. [Google Scholar] [CrossRef]
- Mizielińska, M.; Bartkowiak, A. The Influence of the Q-SUN and UV-B Irradiation on the Antiviral Properties of the PP Films Covered with the Coatings Based on ZnO Nanoparticles and TiO2. Coatings 2024, 14, 125. [Google Scholar] [CrossRef]
- Mania, S.; Cieślik, M.; Konzorski, M.; Święcikowski, P.; Nelson, A.; Banach, A.; Tylingo, R. The Synergistic Microbiological Effects of Industrial Produced Packaging Polyethylene Films Incorporated with Zinc Nanoparticles. Polymers 2020, 12, 1198. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.F.F.; Coimbra, J.S.R.; Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess. Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef]
- Chaudhry, Q.; Scotter, M.; Blackburn, J.; Ross, B.; Boxall, A.; Castle, L.; Aitken, R.; Watkins, R. Applications and implications of nanotechnologies for the food sector. Food Addit. Contam. Part A 2008, 25, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Mizielińska, M.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. The Influence of Accelerated UV-A and Q-SUN Irradiation on the Antimicrobial Properties of Coatings Containing ZnO Nanoparticles. Molecules 2017, 22, 1556. [Google Scholar] [CrossRef] [PubMed]
- Tania, I.S.; Ali, M. Coating of ZnO Nanoparticle on Cotton Fabric to Create a Functional Textile with Enhanced Mechanical Properties. Polymers 2021, 13, 2701. [Google Scholar] [CrossRef] [PubMed]
- Jamnongkan, T.; Sirichaicharoenkol, K.; Kongsomboon, V.; Srinuan, J.; Srisawat, N.; Pangon, A.; Mongkholrattanasit, R.; Tammasakchai, A.; Huang, C.-F. Innovative Electrospun Nanofiber Mats Based on Polylactic Acid Composited with Silver Nanoparticles for Medical Applications. Polymers 2024, 16, 409. [Google Scholar] [CrossRef] [PubMed]
- Baghi, F.; Gharsallaoui, A.; Dumas, E.; Agusti, G.; Ghnimi, S. Characterization of antimicrobial multilayer film based on ethylcellulose-pectin incorporated with nanoemulsions of trans-cinnamaldehyde essential oil. Food Chem. X 2024, 22, 101261. [Google Scholar] [CrossRef] [PubMed]
- Kaloper, S.; Plohl, O.; Možina, S.S.; Vesel, A.; Šimat, V.; Lidija Fras Zemljič, L.F. Exploring chitosan-plant extract bilayer coatings: Advancements in active food packaging via polypropylene modification. Int. J. Biol. Macromol. 2024, 270, 132308. [Google Scholar] [CrossRef] [PubMed]
- Ordon, M.; Burdajewicz, W.; Pitucha, J.; Tarnowiecka-Kuca, A.; Mizielińska, M. Influence of Active Packaging Covered with Coatings Containing Mixtures of Glycyrrhiza L. and Scutellaria baicalensis Extracts on the Microbial Purity and Texture of Sliced Chicken Sausages. Coatings 2023, 13, 795. [Google Scholar] [CrossRef]
- Li, Q.-S.; He, H.-W.; Fan, Z.-Z.; Zhao, R.-H.; Chen, F.-X.; Zhou, R.; Ning, X. Preparation and Performance of Ultra-Fine Polypropylene Antibacterial Fibers via Melt Electrospinning. Polymers 2020, 12, 606. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, L.; Liu, T.; Liu, M.; Yang, Y.; Liu, G.; Zhao, H.; Chen, P.; Fu, S.; Zhang, J.Y.; et al. Poly-(lactic acid) composite films comprising carvacrol and cellulose nanocrystal–zinc oxide with synergistic antibacterial effects. Int. J. Biol. Macromol. 2024, 266, 130937. [Google Scholar] [CrossRef]
- Carvalho, P.; Sampaio, P.; Azevedo, S.; Vaz, C.; Espinós, J.P.; Teixeira, V.; Carneiro, J.O. Influence of thickness and coatings morphology in the antimicrobial performance of zinc oxide coatings. Appl. Surf. Sci. 2024, 307, 548–557. [Google Scholar] [CrossRef]
- Sarhadi, H.; Shahdadi, F.; Salehi Sardoei, A.; Hatami, M.; Ghorbanpour, M. Investigation of physio-mechanical, antioxidant and antimicrobial properties of starch–zinc oxide nanoparticles active films reinforced with Ferula gummosa Boiss essential oil. Sci. Rep. 2024, 14, 5789. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.N.; de Matos Fonseca, J.; Feldhaus, H.K.; Soares, L.S.; Valencia, G.A.; de Campos, C.E.M.; Di Luccio, M.; Monteiro, A.R. Physical and morphological properties of hydroxypropyl methylcellulose films with curcumin polymorphs. Food Hydrocoll. 2019, 97, 105217. [Google Scholar] [CrossRef]
- Halász, K.; Csóka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric pH indicator film application. Food Packag. Shelf Life 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Ćujić, N.; Trifković, K.; Bugarski, B.; Ibrić, S.; Pljevljakušić, D.; Šavikin, K. Chokeberry (Aronia melanocarpa L.) extract loaded in alginate and alginate/inulin system. Ind. Crops Prod. 2016, 86, 120–131. [Google Scholar] [CrossRef]
- Janković, B.; Marinović-Cincović, M.; Janković, M. TG-DTA-FTIR analysis and isoconversional reaction profiles for thermal and thermo-oxidative degradation processes in black chokeberry (Aronia melanocarpa). Chem. Pap. 2016, 70, 1094–1105. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Kataru, B.A.S.; Sravani, N.; Vigneshwari, T.; Panneerselvam, A.; Rajeswari, D.V. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano 2018, 3, 48–55. [Google Scholar] [CrossRef]
- Sachadyn-Król, M.; Budziak-Wieczorek, I.; Jackowska, I. The Visibility of Changes in the Antioxidant Compound Profiles of Strawberry and Raspberry Fruits Subjected to Different Storage Conditions Using ATR-FTIR and Chemometrics. Antioxidants 2023, 12, 1719. [Google Scholar] [CrossRef]
- Kubra, I.R.; Kumar, D.; Rao, L.J.M. Effect of microwave-assisted extraction on the release of polyphenols from ginger (Zingiber officinale). Int. J. Food Sci. Tech. 2013, 48, 1828–1833. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave Assisted Extraction—An Innovative and Promising Extraction Tool for Medicinal Plant Research. Pharmacogn. Rev. 2007, 1, 1. Available online: http://www.phcogrev.com (accessed on 1 January 2020).
- E 2180-01; ASTM Standard Test Method for Determining the Activity of Incorporated Antimicrobial Agent(s) in Polymeric or Hydrophobic Materials. ASTM International: West Conshohocken, PA, USA, 2002. Available online: https://standards.globalspec.com/std/4472809/astm-e2180-18 (accessed on 1 January 2020).
- Bhetwal, A.; Maharjan, A.; Shakya, S.; Satyal, D.; Ghimire, S.; Khanal, P.R.; Parajuli, N.P. Isolation of Potential Phages against Multidrug-Resistant Bacterial Isolates: Promising Agents in the Rivers of Kathmandu, Nepal. BioMed. Res. Int. 2017, 2017, 3723254. [Google Scholar] [CrossRef]
- Bonilla, N.; Rojas, M.J.; Cruz, G.N.F.; Hung, S.H.; Rohwer, F.; Barr, J.J. Phage on tap–a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. PeerJ 2016, 4, 2261. [Google Scholar] [CrossRef] [PubMed]
- ISO_22196-2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2011. Available online: https://www.iso.org/standard/54431.html (accessed on 1 January 2020).
- Skaradzińska, A.; Ochocka, M.; Śliwka, P.; Kuźmińska-Bajora, M.; Skaradziński, G.; Friese, A.; Roschanski, N.; Murugaiyan, J.; Roesler, U. Bacteriophage amplification–A comparison of selected methods. J. Virol. Methods 2020, 282, 113856. [Google Scholar] [CrossRef] [PubMed]







| Material | Synergistic Effect | Active Agent | Ref. | |
|---|---|---|---|---|
| 1 | 2 | |||
| Polyethylene film (PE) | + | zinc oxide | geraniol/carvacrol | [24] |
| Modified PLA composites | + | nanocrystal–zinc oxide | carvacrol | [28] |
| LDPE/LLDPE | + | zinc oxide nanoparticles | zinc stearate nanoparticles | [36] |
| PET (Polyethylene terephthalate) | + | zinc oxide nanoparticles | Ag nanoparticles | [37] |
| Starch nanocomposite | + | zinc oxide nanoparticles | Ferula gummosa essential oil | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizielińska, M.; Bartkowiak, A. The Influence of Zinc Oxide and Zinc Stearate on the Antimicrobial Activity of Coatings Containing Raspberry and Chokeberry Extracts. Molecules 2024, 29, 3493. https://doi.org/10.3390/molecules29153493
Mizielińska M, Bartkowiak A. The Influence of Zinc Oxide and Zinc Stearate on the Antimicrobial Activity of Coatings Containing Raspberry and Chokeberry Extracts. Molecules. 2024; 29(15):3493. https://doi.org/10.3390/molecules29153493
Chicago/Turabian StyleMizielińska, Małgorzata, and Artur Bartkowiak. 2024. "The Influence of Zinc Oxide and Zinc Stearate on the Antimicrobial Activity of Coatings Containing Raspberry and Chokeberry Extracts" Molecules 29, no. 15: 3493. https://doi.org/10.3390/molecules29153493
APA StyleMizielińska, M., & Bartkowiak, A. (2024). The Influence of Zinc Oxide and Zinc Stearate on the Antimicrobial Activity of Coatings Containing Raspberry and Chokeberry Extracts. Molecules, 29(15), 3493. https://doi.org/10.3390/molecules29153493







