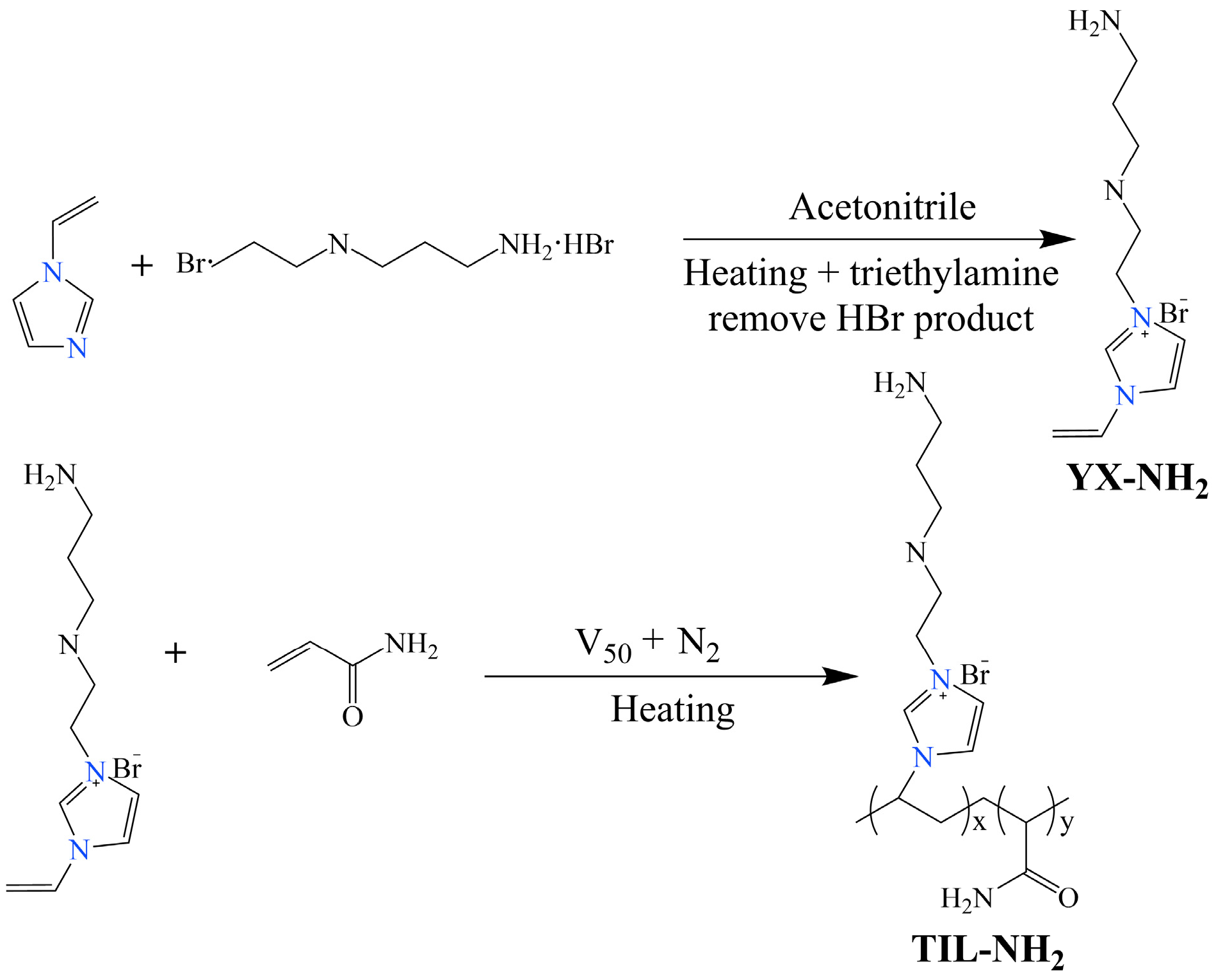
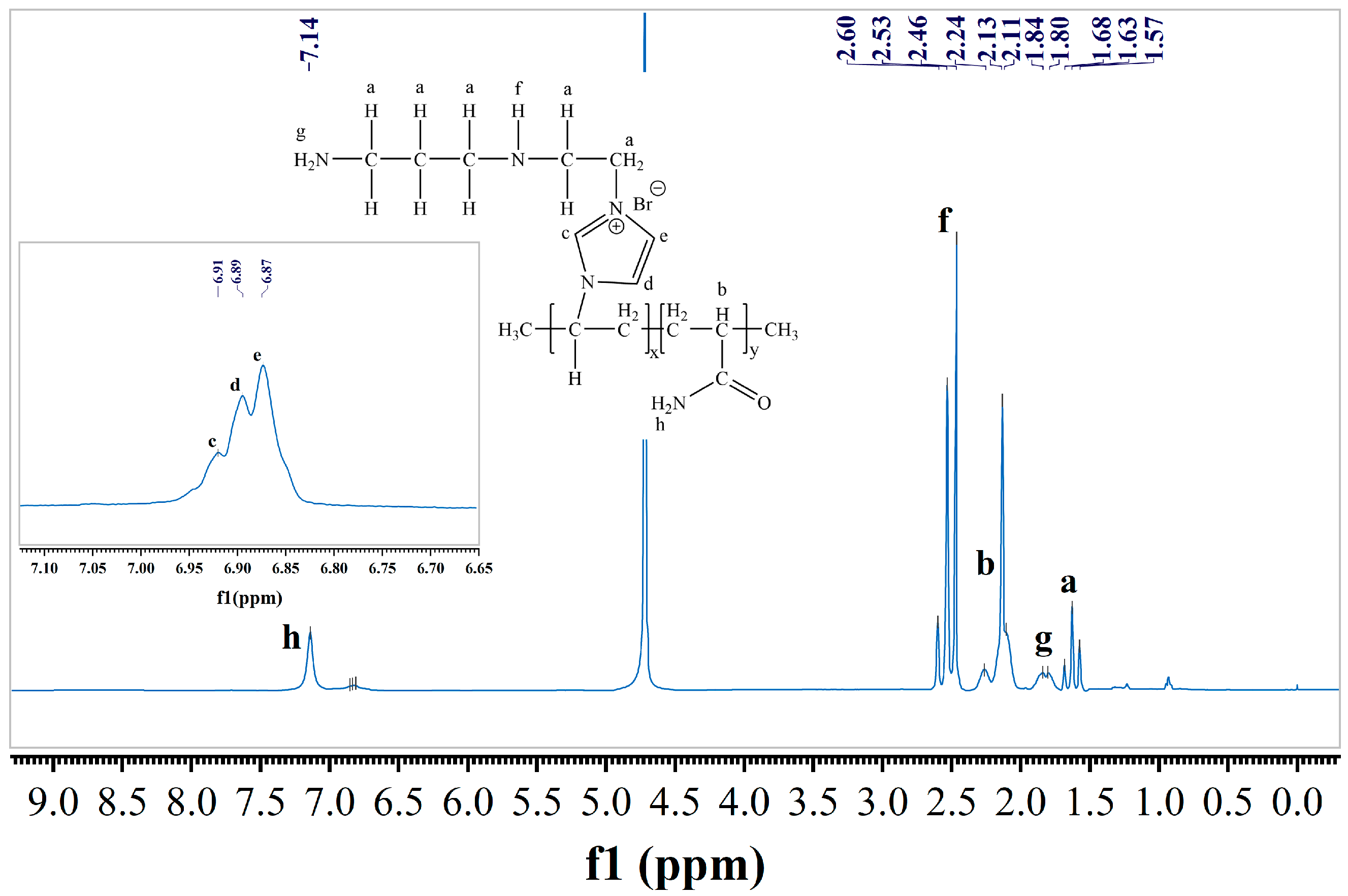
3.1. Synthesis of the Inhibitor TIL-NH2
First, 12.3 g of 1-vinylimidazole was added to 70 mL of acetonitrile solvent and then the mixture was poured into a three-necked flask equipped with a reflux condenser. The system was heated to 80 °C and 25.6 g of N-(2-bromoethyl)-1,3-propanediamine dihydrobromide was added. The reaction mixture was stirred and refluxed at 80 °C for 24 h. After the reaction, triethylamine was added to remove HBr, forming triethylamine hydrochloride. The mixture was filtered, and the precipitate was washed several times with anhydrous ethanol. The precipitate was then dried in a vacuum oven at 45 °C for 24 h, yielding a light orange solid monomer (YX-NH2) with a yield of 84.2%.
Appropriate amounts of the YX-NH
2 monomer and acrylamide were dissolved in deionized water and placed in a three-necked flask equipped with a reflux condenser. The pH was adjusted to 5, and the mixture was heated to the desired temperature under reflux. A small amount of V-50 initiator was added under nitrogen protection, and the polymerization reaction was carried out with stirring at 200 r/min for several hours at a constant temperature. After the reaction, the mixture was transferred to a rotary evaporator and distilled under reduced pressure for 2.5 h. The resulting orange-red viscous solid product was the polyionic polymer TIL-NH
2. The reaction mechanism is shown in
Figure 1:
To assess the reproducibility of the synthesis process, we conducted three repeated experiments and recorded the yield and purity of each experiment. By calculating the yield of each experiment, we obtained the average yield and calculated its standard deviation to evaluate the differences in yield among different batches.
This section uses high-performance liquid chromatography (HPLC) to analyze the purity of each batch of TIL-NH2 samples. First, each batch of TIL-NH2 samples was dissolved in water and filtered through a 0.45 µm filter membrane to ensure that there were no particulates in the sample solution. Next, separation was performed using a C18 reverse-phase column. The mobile phase was a suitable system for TIL-NH2 (water–methanol system), and gradient elution was applied. The flow rate was set to 1.0 mL/min. An appropriate UV detection wavelength of 210 nm was selected to maximize the absorption signal of the target compound, and each injection volume was 10–20 µL. The samples were injected into the HPLC system, chromatograms were recorded, and the purity of the samples was calculated by comparing the peak area in the chromatogram of the sample to the peak area of the standard.
Assuming the retention time of the target product is t
R, the peak area
Asample at this time point was measured and compared to the peak area
Astandard of the standard. The purity was calculated using the following formula:
Each experiment was conducted following the same synthesis procedure to ensure consistency in experimental conditions. Finally, the TIL-NH2 from different batches was tested for its performance in inhibiting shale hydration swelling and dispersion at the same concentration. The inhibition effect of each batch was recorded and compared.
The data for yield, purity, and inhibition effect of different batches are shown in
Table 1.
Based on the data in
Table 1, it can be seen that the yield of TIL-NH
2 across different batches is stable, with an average value of 84.17% and a standard deviation of 0.35%, indicating good reproducibility of the synthesis process. HPLC analysis results show that the purity of different batches is above 98%, with a standard deviation of 0.30%, indicating minimal differences in purity among batches. The inhibition effect of different batches of TIL-NH
2 on shale hydration swelling and dispersion is consistent, ranging from 84.4% to 86.2%. These results demonstrate that the synthesis process of TIL-NH
2 is stable and reliable and that differences in yield and purity have little impact on its performance.
3.3. Inhibition Mechanism Analysis of TIL-NH2
3.3.1. Particle Size Analysis
The experimental results of testing the particle size distribution in this section are shown in
Figure 5 and
Figure 6.
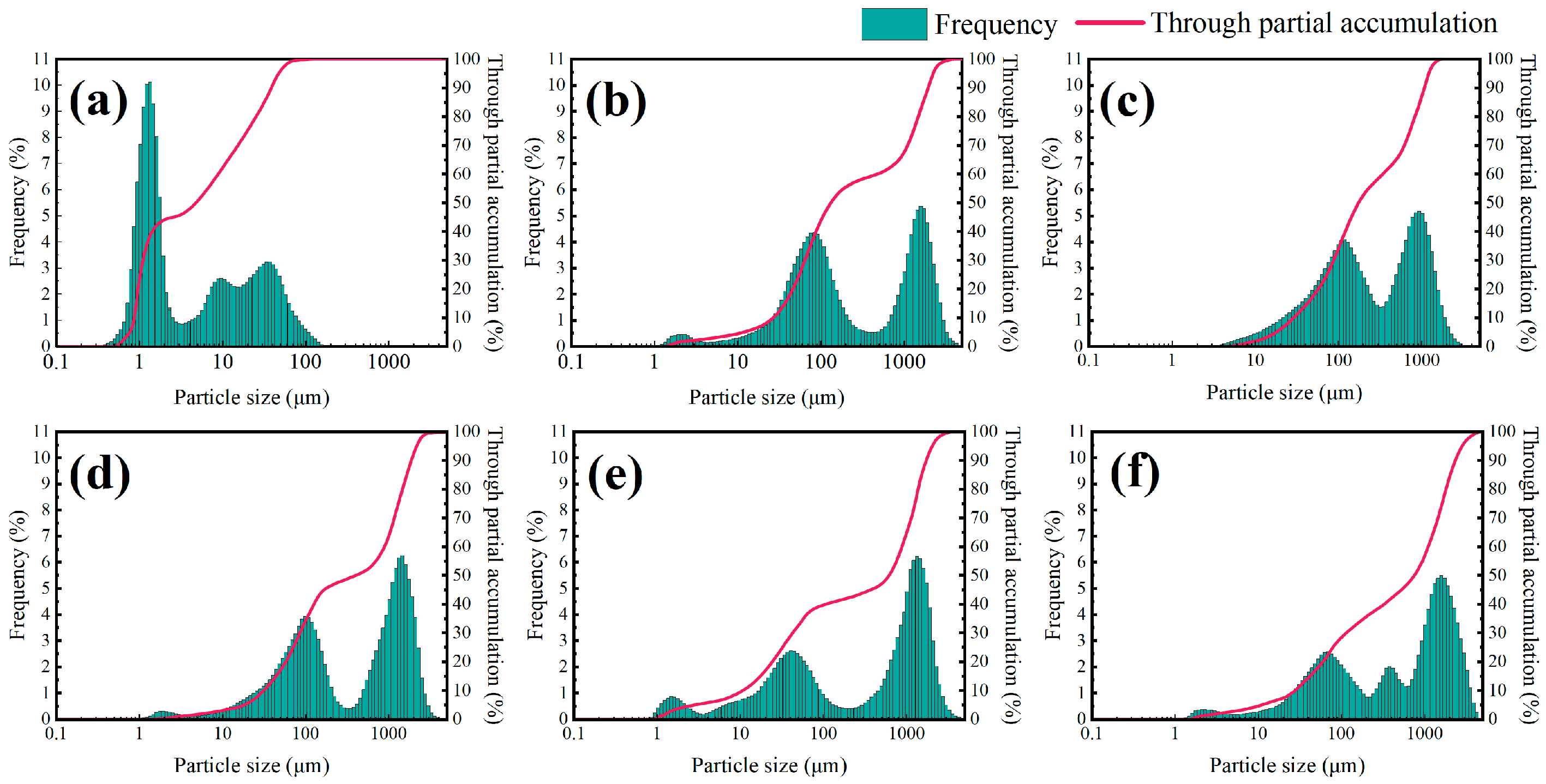
Figure 5 shows the particle size distribution curves of a 4% shale powder matrix and the addition of TIL-NH
2 at different concentrations. Compared with the shale powder matrix, the addition of TIL-NH
2 significantly decreased the particle content in the 0.1 μm and 10 μm ranges, shifted the particle content in the 10 μm~100 μm range to the right, and increased the particle content in the over 100 μm range, with a new peak appearing. This indicates that TIL-NH
2 altered the dispersion state of particles in the shale powder matrix, promoting particle aggregation and inhibiting particle hydration and dispersion.
As the concentration of TIL-NH2 increased, the content of particles in the 10 μm~100 μm range continued to decrease, while the content of particles over 100 μm continued to increase, reflecting an increase in both the average and median particle size of the shale powder matrix. The polyionic polymer TIL-NH2 can aggregate surrounding shale powder particles by bridging them through the polymer molecular chains, showing higher aggregation efficiency compared with small-molecule ionic liquids. When the concentration of TIL-NH2 exceeded 0.9%, the average and median particle sizes of the shale powder base slurry exceeded 300 μm, effectively suppressing the hydration and dispersion of shale powder.
Additionally, the figure shows that the particle size accumulation curve of the shale powder matrix is significantly higher than that after adding TIL-NH2, indicating that the particle distribution in the shale powder matrix is more uniform. In contrast, the particle distribution after adding TIL-NH2 is more uneven, with larger particle aggregates, suggesting that TIL-NH2 significantly inhibits the hydration and dispersion of shale powder.
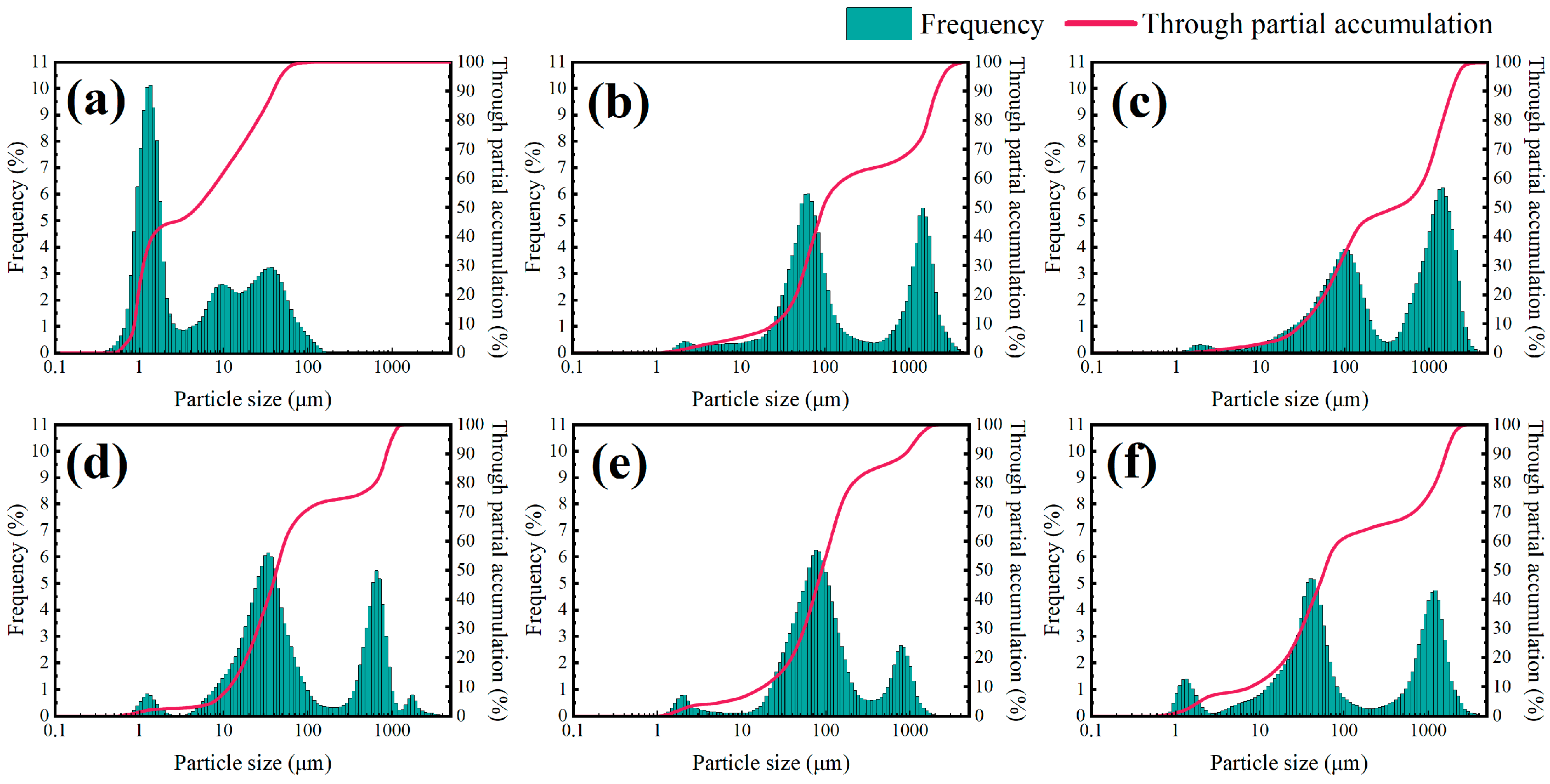
Figure 6 shows the particle size distribution curves and particle size accumulation curves of a 4% shale powder matrix with different concentrations of inhibitors. The figure demonstrates that all types of inhibitors can improve the hydration and dispersion phenomena of particles in the shale powder matrix. Among the inhibitors, 5% KCl and 2% NW-1, as small-molecule cationic inhibitors, increased the median particle size of the shale powder matrix from 5.2871 μm to 57.9824 μm and 40.7652 μm, respectively, with similar inhibition effects.
DEM and polyether amine, as amine polymers, more effectively inhibited the hydration and dispersion of particles in the shale powder matrix, with median particle sizes reaching 73.3025 μm and 63.5874 μm, respectively. The imidazole cation and amine group are two inhibitory functional groups in the linear polymer TIL-NH2, which can effectively inhibit the hydration and dispersion of particles in the shale powder matrix. Consequently, the median and average particle sizes for 0.9% TIL-NH2 were higher than those for the other inhibitors, with both values exceeding 320 μm.
Additionally, the particle size distribution curve and the particle size accumulation curve of the shale powder matrix were lower than those with the added inhibitor. This indicates that the particles in the shale powder matrix were more homogeneously distributed, while the particles became more unevenly distributed with the addition of the inhibitor, forming larger particle aggregates. This demonstrates that the inhibitor significantly inhibited the hydration and dispersion of shale powder.
3.3.2. Contact Angle Analysis
The contact angle of the TIL-NH
2 aqueous solution with a shale powder cake measured in this section is shown in
Figure 7.
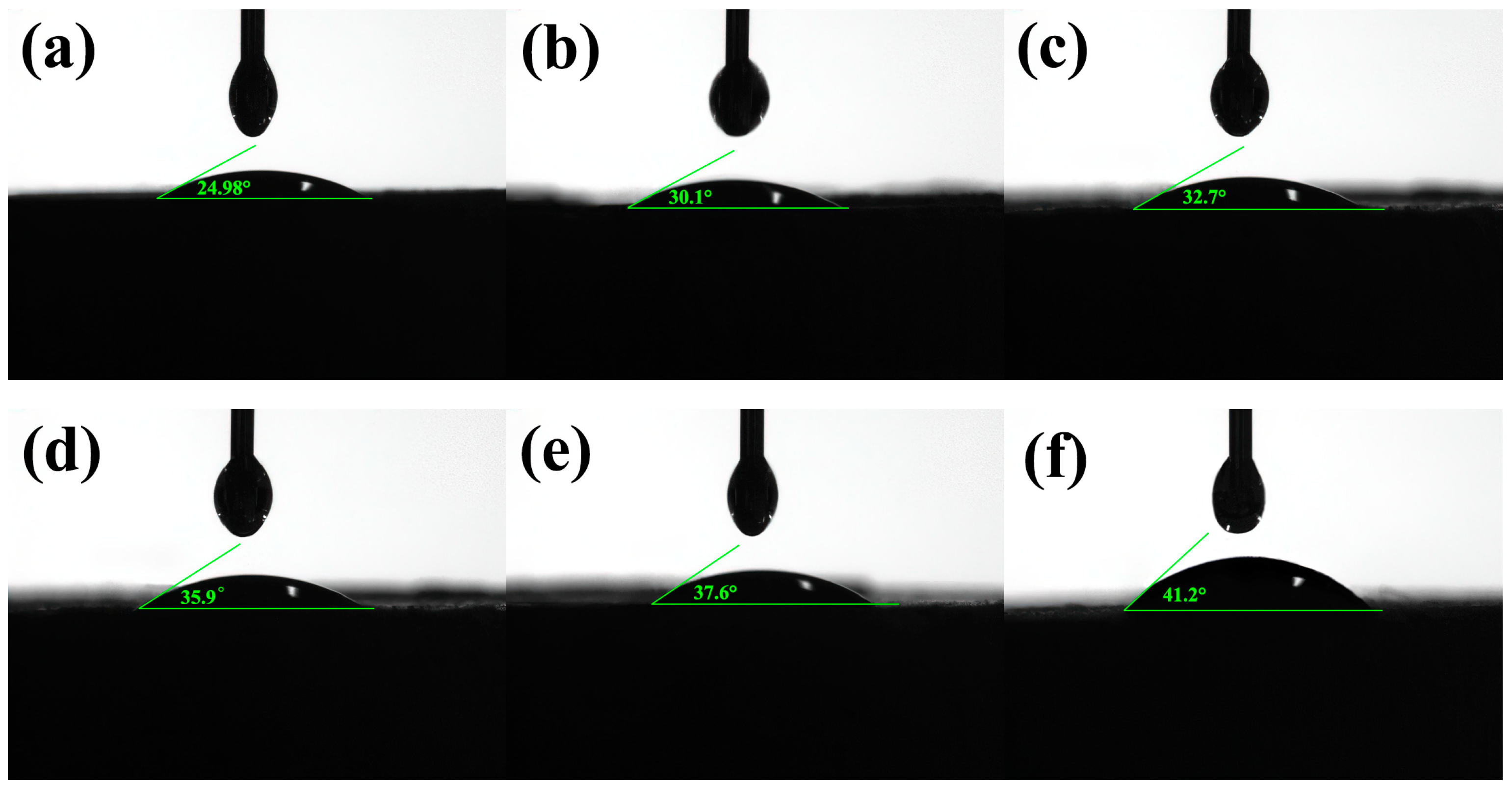
Figure 7 shows that the contact angles of the TIL-NH
2 inhibitor solutions are all less than 90°, indicating that water is wettable to shale. Since the main clay mineral of shale powder is illite (64.5%), the inhibitor does not affect the wetting state of water on shale. With pure water, the contact angle between the water and the shale powder cake was 24.98°. After adding TIL-NH
2, the contact angles increased, indicating that the inhibitor attenuates the wetting and intrusion effect of water on illite.
When the concentrations of TIL-NH2 were 1.2% and 1.5%, the contact angles increased to 37.6° and 41.2°, respectively, which were 50.5% and 64.9% higher than pure water. This indicates that TIL-NH2 increases the hydrophobicity of the shale surface, forming a hydrophobic film that prevents water molecules from adsorbing and invading the illite. The hydrophobic film reduces the hydration force of illite, decreasing the wettability of water on shale.
As an inhibitor, TIL-NH2 reduces the wetting and intrusion rate of water on shale, prolonging the formation time of a mudcake and helping reduce filtration loss. The increase in contact angle reflects an increase in solid–liquid interfacial tension and surface energy, making it harder for water molecules to move to the shale surface, thereby improving the inhibition capability.
To evaluate the stability of the hydrophobic film on the shale surface treated with TIL-NH
2 after long-term exposure to water and other drilling fluids, the following experiment was conducted. First, shale samples were prepared and cleaned with deionized water and ethanol to ensure the surface was free of dirt and impurities. The samples were then immersed in a 1 wt% TIL-NH
2 solution for 24 h to form a uniform hydrophobic film. The treated samples were subsequently immersed in deionized water and various drilling fluids (water-based drilling fluid, oil-based drilling fluid, and synthetic-based drilling fluid), with temperature (25 °C) and humidity controlled in an incubator to simulate actual drilling conditions. At predetermined soaking times (1 day, 7 days, 14 days, and 30 days), the samples were removed and dried, and their contact angles were measured using a contact angle meter. Measurements were taken at multiple locations on each sample to reduce error. The experimental results are shown in
Table 2.
(1) Short-Term Stability (1–7 Days):
In the short term, the contact angles of all samples showed a slight decrease, but overall changes were minimal. The contact angles in the deionized water and various drilling fluids decreased from the initial 41.2° to 40.8°–40.5° after 1 day and to 39.5°–39.2° after 7 days. This result indicates that TIL-NH2 maintains good hydrophobicity in the short term, with stable inhibition effects.
(2) Long-Term Stability (14–30 Days):
After longer soaking times, the contact angles decreased significantly. After 14 days, the contact angles in the deionized water decreased to 37.0°, in the water-based drilling fluid to 36.8°, in the oil-based drilling fluid to 37.2°, and in the synthetic-based drilling fluid to 36.9°. After 30 days, the contact angles further decreased to 33.5°–33.2°, indicating that the stability of the hydrophobic film gradually weakened over time.
(3) Effect of Different Drilling Fluids:
The impact of different drilling fluids on the stability of the hydrophobic film showed some differences. Overall, the contact angle changes were the smallest in the oil-based drilling fluid, indicating that the oil-based drilling fluid had the weakest erosive effect on the hydrophobic film. The water-based and synthetic-based drilling fluids had similar impacts, both leading to significant decreases in contact angles, while the effect of the deionized water was intermediate.
The reasons for the decrease in hydrophobicity include the gradual erosion of the TIL-NH2 hydrophobic film by water molecules over long-term soaking, which damages its integrity and hydrophobicity, and the chemical components in water-based and synthetic-based drilling fluids interacting with TIL-NH2, weakening the stability of the hydrophobic film. In contrast, the oil-based drilling fluid remains relatively stable as it does not contain water molecules, reducing competitive interactions with the hydrophobic film. In the short term (1–7 days), the hydrophobic film on the shale surface treated with TIL-NH2 effectively inhibits water penetration and shale hydration swelling, ensuring short-term inhibition effects. However, in the long term (14–30 days), as hydrophobicity decreases, the inhibition effect of TIL-NH2 may weaken, indicating that additional measures are needed to ensure the effectiveness of TIL-NH2 during extended drilling operations.
3.3.3. Zeta Potential Analysis
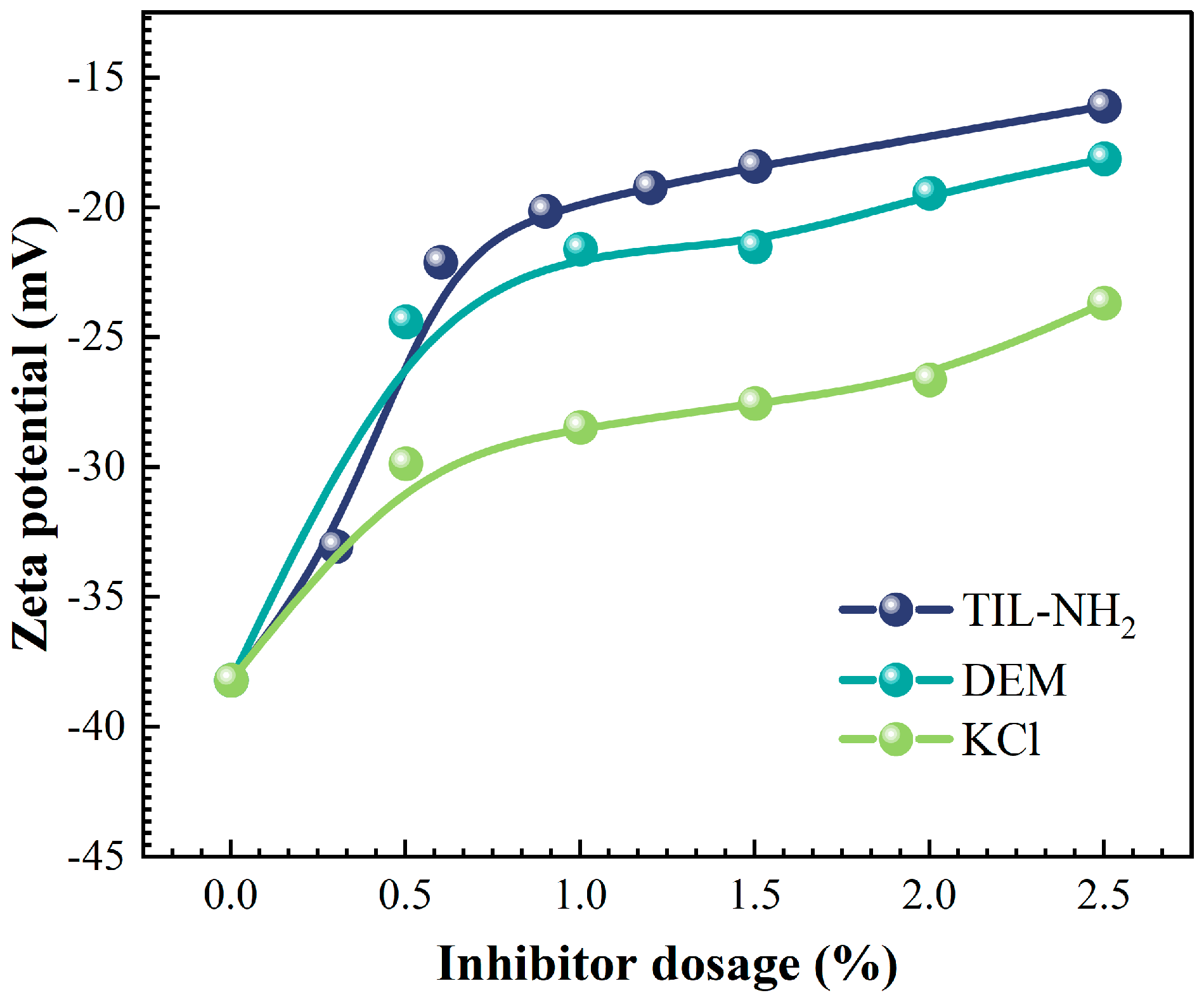
Figure 8 shows the effect of TIL-NH
2, polyamine DEM, and KCl on the zeta potential of the clay mineral illite at different concentrations.
Figure 8 shows that the zeta potential of illite was −38.2 mV. As the inhibitor concentration increased, the absolute value of the zeta potential significantly decreased, indicating that the inhibitor reduced the surface charge of illite. TIL-NH
2 significantly reduced the zeta potential of illite even at a lower concentration. At a 0.6% addition, the zeta potential was −22.1 mV, which was higher than the zeta potential values of polyamines DEM and KCl at a 1% addition. The absolute value of the zeta potential changed less at concentrations above 0.9%, remaining between −16 mV and −19 mV. This suggests that the ionic liquid significantly affects the diffusive bilayer state of illite agglomerates, reducing the tendency of water molecules to penetrate the illite layer.
The cationic structure on the TIL-NH2 polymer chain gives it polyelectrolyte properties with multiple charges, which are linearly adsorbed onto the negatively charged illite surface through charge interactions, electrostatic forces, and hydrogen bonding. The imidazole five-membered ring structure of TIL-NH2 eliminates the need for amine protonation and improves inhibition efficiency. The cationic groups in TIL-NH2 neutralize the negative charge on the surface of illite, reducing the electrostatic repulsive force between clay particles. The long-chain organic groups cover the hydrophilic groups on the surface of illite, reducing the structured water layer and lowering hydration swelling, thereby improving the mechanical properties of the shale.
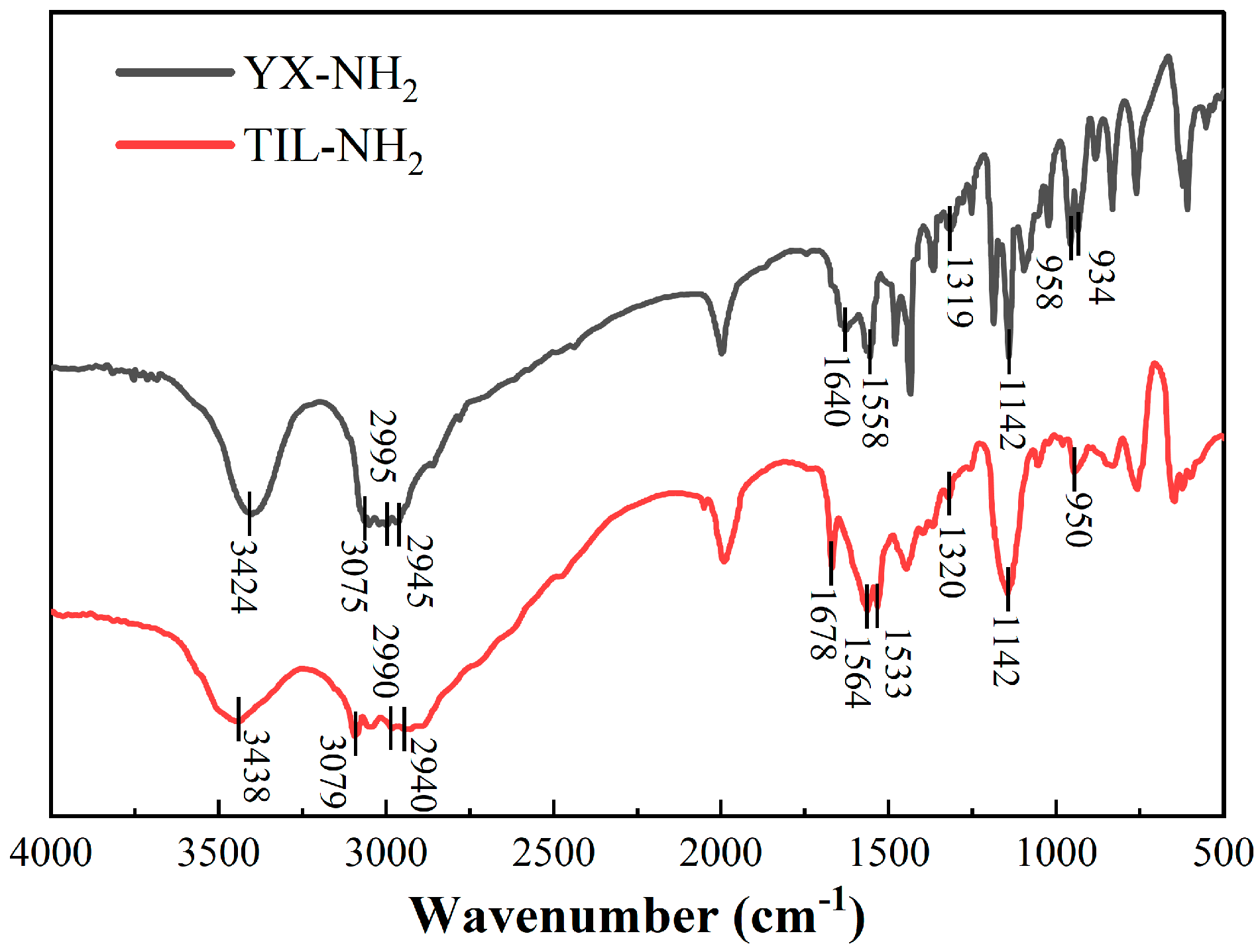
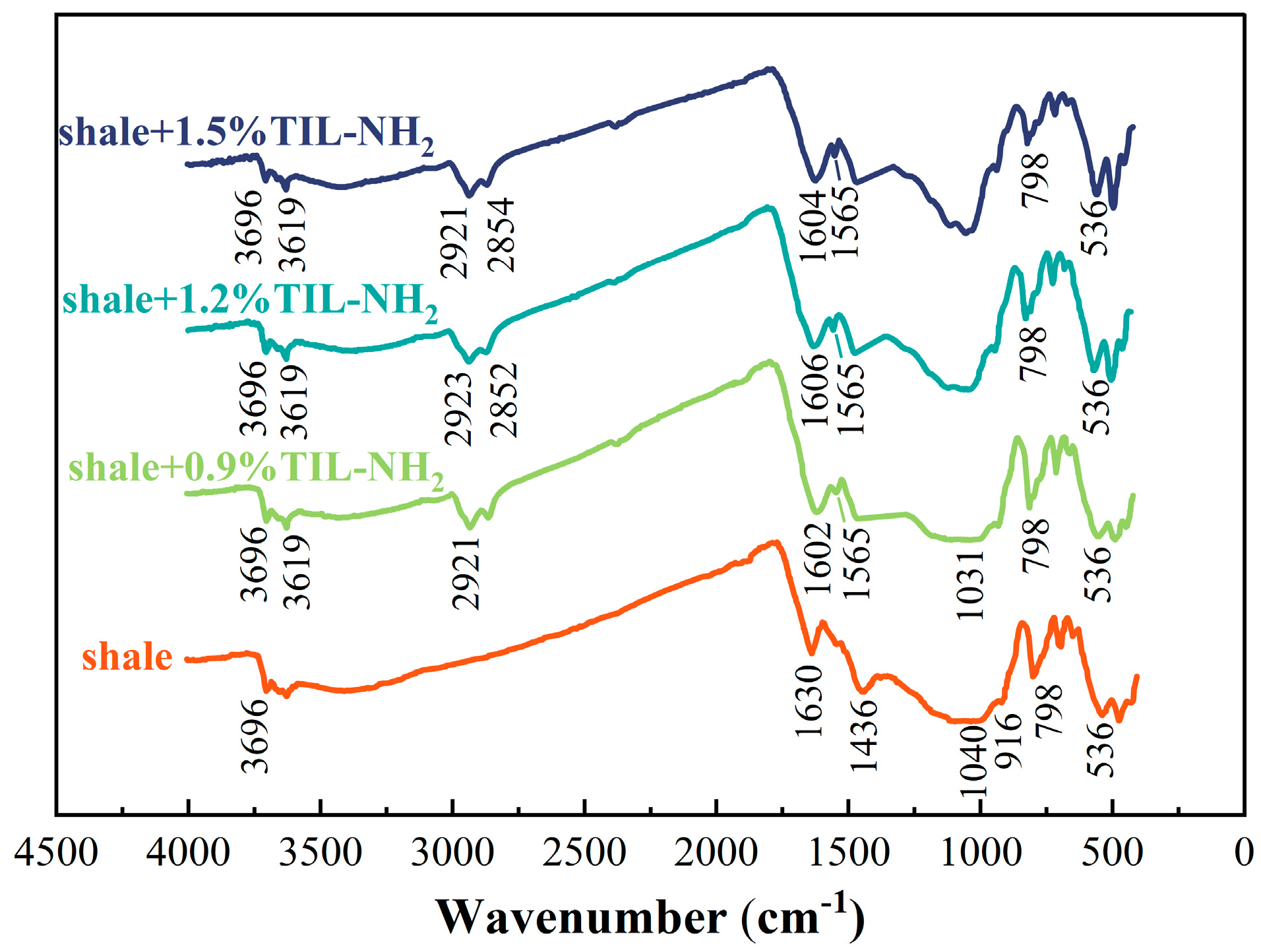
3.3.4. Infrared Spectral Analysis
Figure 9 shows the infrared spectra of shale powder before and after treatment with different concentrations of TIL-NH
2.
Figure 9 shows that the addition of TIL-NH
2 did not change the main structure of the shale. The FT-IR spectra of the shale showed the stretching vibrational peak of O-H in the illite structure at 3695 cm
−1, the characteristic O-H bending vibrational peak of water at 1634 cm
−1, the bending vibrational peak of CH
3 at 1435 cm
−1, the stretching vibrational peak of Si-O at 1042 cm
−1, and the bending vibrational peak of Al-OH at 918 cm
−1. At TIL-NH
2 concentrations of 0.6–1.5%, a new O-H stretching vibration absorption peak appeared at 3616 cm
−1, and a shoulder signal of symmetric stretching vibration of the C-H bond in -CH
2- was found at 2852 cm
−1. Additionally, overlapping peaks of the N=C bond stretching vibration on the cationic imidazole ring, the N-H bond bending vibration in primary amines, and the N-H bending vibration in amides in the structure of TIL-NH
2 were detected near 1565 cm
−1.
These results indicate that the molecular chain of TIL-NH
2 polymer adsorbs onto illite in shale. By comparing with the infrared spectra of TIL-NH
2 molecules in
Figure 2, it is observed that the intensity of the overlapping peaks around 1560 cm
−1 is significantly weakened when TIL-NH
2 fully interacts with shale powders, and a new peak appears around 1604 cm
−1. This is because the N-H of the primary amide in TIL-NH
2 forms a stabilized hydrogen-bonding structure with O on the surface of illite, causing a shift in the N-H vibrational frequency to higher frequencies. The original peak mainly consists of the N=C stretching vibration of the imidazole ring. The disappearance of the peak at 1630 cm
−1 compared with the shale spectra suggests that TIL-NH
2 reduces water adsorption on the surface of shale particles and between layers.
These results demonstrate the advantage of TIL-NH2 side group multifunctionality. The imidazole cation adsorbs on illite through electrostatic interaction, while the primary amine forms intermolecular interaction with the illite surface through hydrogen bonding. Together, they allow TIL-NH2 to adsorb tightly onto illite, preventing water molecules from entering and leading to the dispersion and hydration of illite. This reduces the repulsive force and hydration expansion force between shale powder particles, increasing their aggregation and stability, and suppressing shale dispersion. This explains the inherent reason for the aggregation of shale powder particles treated with TIL-NH2 in the particle size distribution experiments.
3.3.5. XRD Analysis
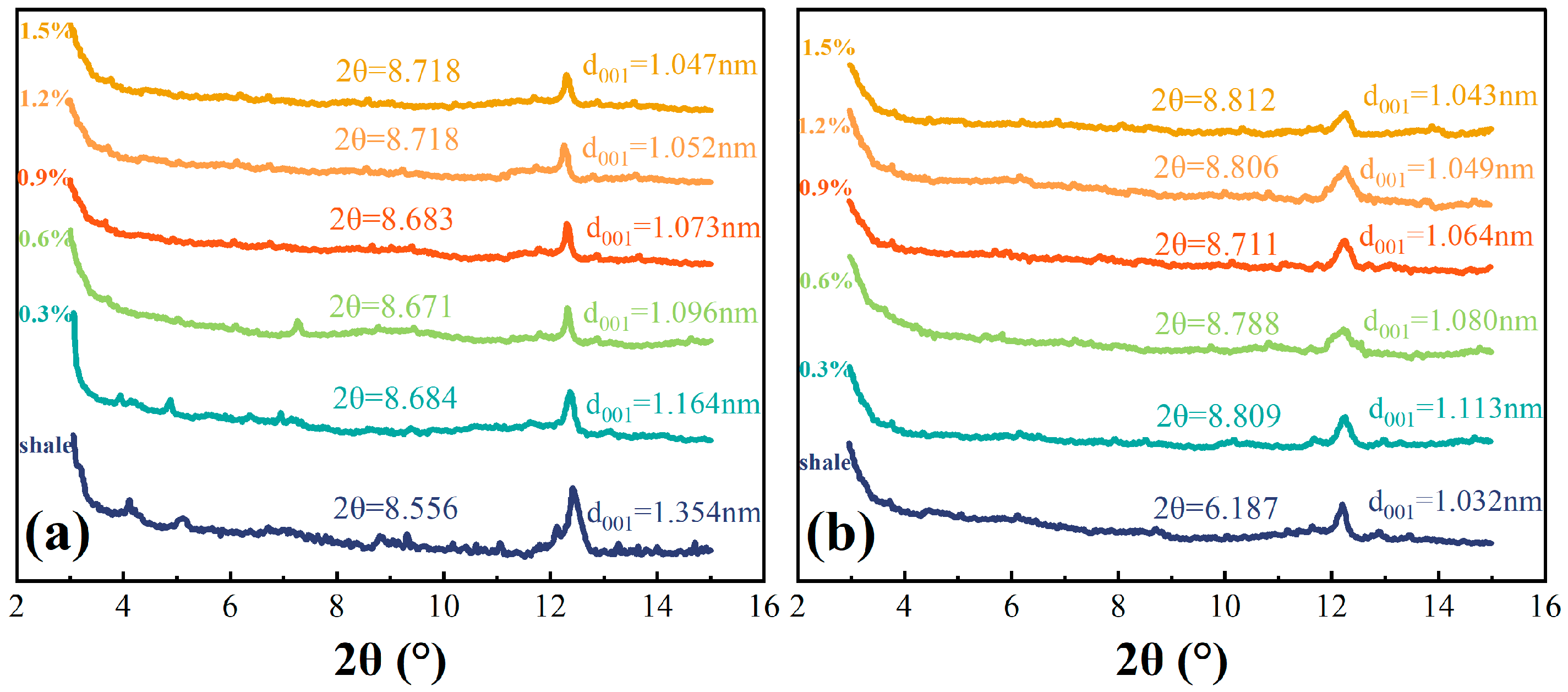
The X-ray diffraction spectral studies of the dry and wet shale powder samples determined in this section are shown in
Figure 10.
Figure 10 shows that TIL-NH
2 can effectively inhibit the hydration of illite, resulting in interlayer spacings of 1.032 nm and 1.354 nm for shale in dry and wet conditions, respectively. TIL-NH
2 contains an imidazole cationic ring and a primary amine group, which adsorb strongly on the crystalline surfaces of illite to form dense and stable chemical bonds. This adsorption maintains charge equilibrium, prevents water from entering the interlayer, and reduces the interaction between water and the illite surface, leading to a reduction in interlayer spacing and the expulsion of interlayer water.
When the concentration of TIL-NH2 exceeds 0.9%, the interlayer spacing can be reduced to 1.073 nm. Unlike small molecule cations, the imidazole cationic ring is present in each repeating unit of TIL-NH2. When some cationic rings undergo electrostatic attraction between layers, other cationic rings are also induced to be attracted, achieving charge balance because of interactions and restrictions among chain segments. Additionally, the rigid and planar imidazole rings of TIL-NH2 can arrange in a tight monolayer structure among the layers to form a hydrophobic membrane.
At a higher concentration (1.5%), the interlayer spacings of shale in dry and wet states were 1.043 nm and 1.047 nm, respectively, and did not change significantly with increasing TIL-NH2 concentration. This indicates that the hydration inhibition effect of TIL-NH2 on illite reached saturation, reflecting the monolayer arrangement of TIL-NH2 in the interlayer voids.
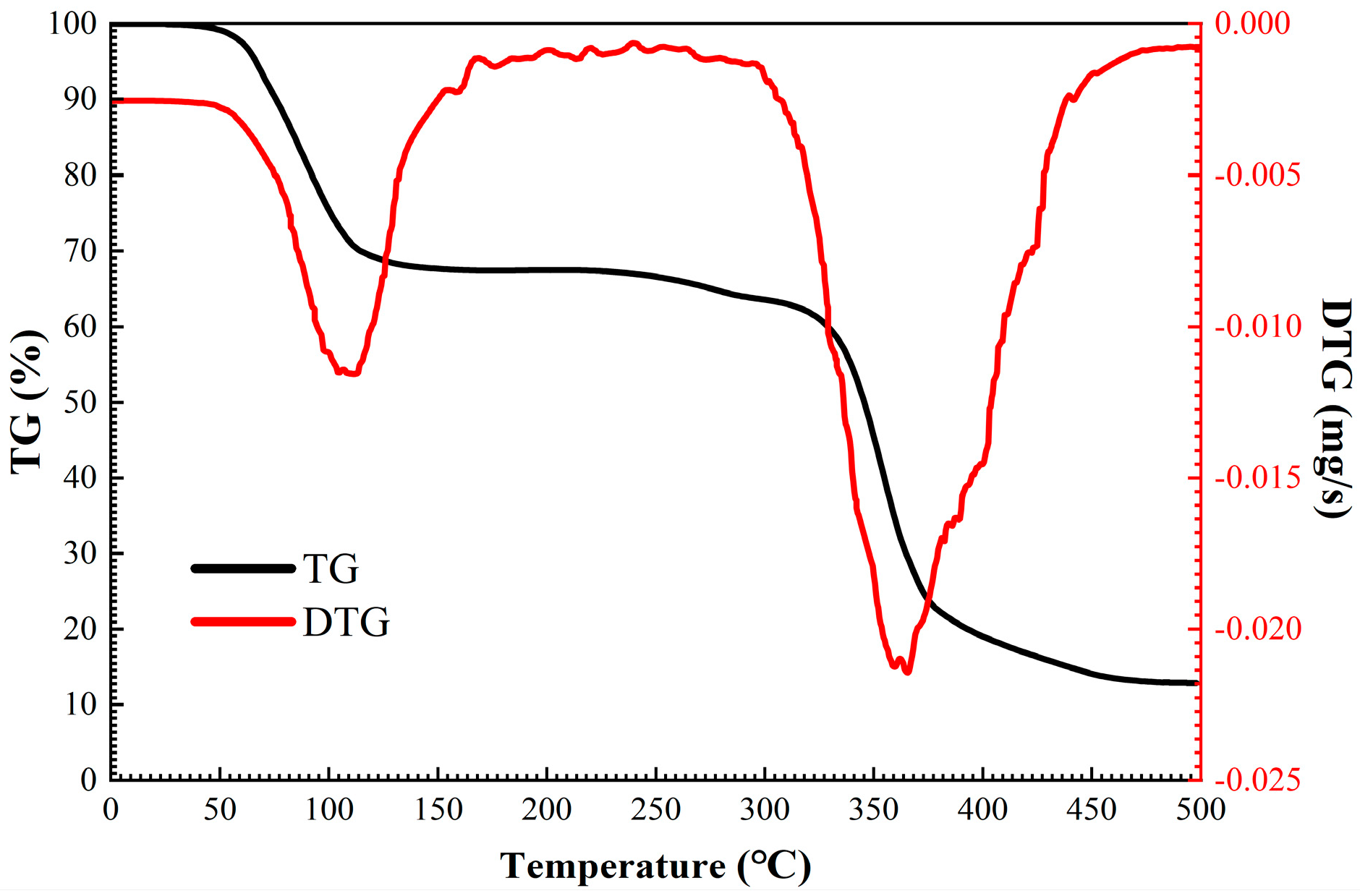
3.3.6. Thermogravimetric Analysis
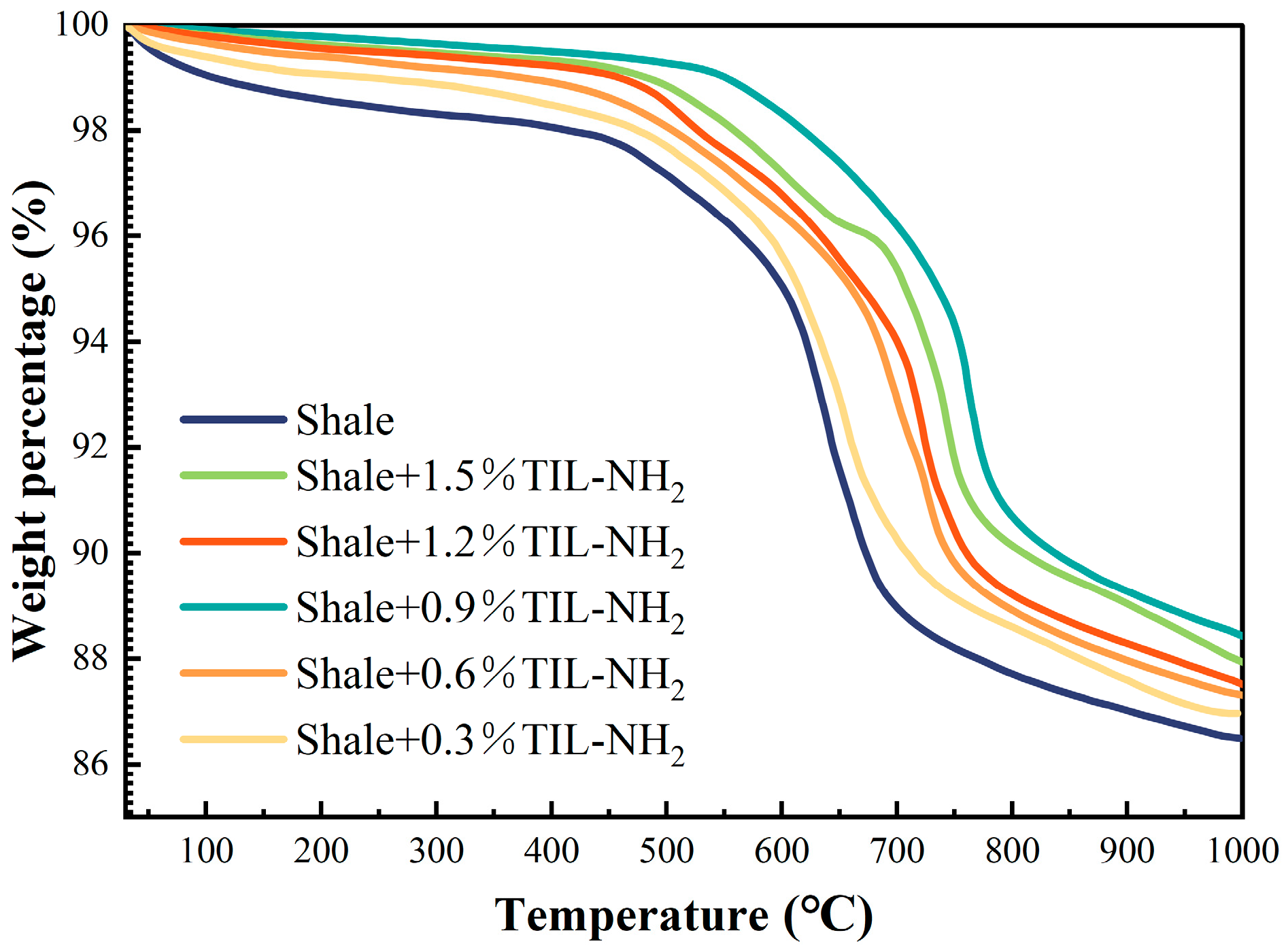
In this section, thermogravimetric tests were carried out on pure shale flour and shale flour treated with 0.3–1.5% TIL-NH
2, and the results are shown in
Figure 11.
Figure 11 and
Table 3 illustrate that TIL-NH
2 significantly impacts the heat-loss curve and the content of various adsorbed waters in shale powders. According to the research by Xie Gang et al., different types of adsorbed water have specific weight loss temperature ranges as follows: free water loses weight between 70 °C and 80 °C, weakly bound water loses weight between 135 °C and 145 °C, and strongly bound water loses weight between 205 °C and 215 °C.
TIL-NH2, a multifunctional group inhibitor, exhibits a strong inhibition effect. At 80 °C, the free water content in the shale powder was calculated to be 0.7900%, the weakly bound water content was 0.4185%, and the strongly bound water content was 0.2646%. The low-concentration, high-efficiency property of TIL-NH2 is evident in lowering the weakly bound water content; when the TIL-NH2 concentration was 0.9%, the weakly bound water content dropped to 0.0951%, a reduction of over 78%. This aligns with previous analyses showing that TIL-NH2 adsorbs on the illite surface through cationic electrostatic interaction and primary amine hydrogen bonding, thereby compressing the diffusion layer and reducing the weakly bound water content.
Additionally, TIL-NH2 is temperature-resistant, and it reduces the strongly bound water content after treatment in the temperature range of 145 °C to 215 °C. The results were consistent across different TIL-NH2 concentrations, with each showing a reduction of more than 40% in strongly bound water. When the concentration of TIL-NH2 reached 0.9%, the shale powder had the most significant reduction in strongly bound water content, down to 0.0911%, which is more than 65% less than the blank shale powder. This indicates that TIL-NH2 replaces the water molecules that were originally strongly bound to the illite crystal surface, forming strong coordination with the illite lattice surface through electrostatic interactions and hydrogen bonding, thereby inhibiting the hydration degree on the illite surface. This conclusion is consistent with the reduced pattern of illite layer spacing shown by XRD tests.
3.3.7. Micro-Morphological Analysis
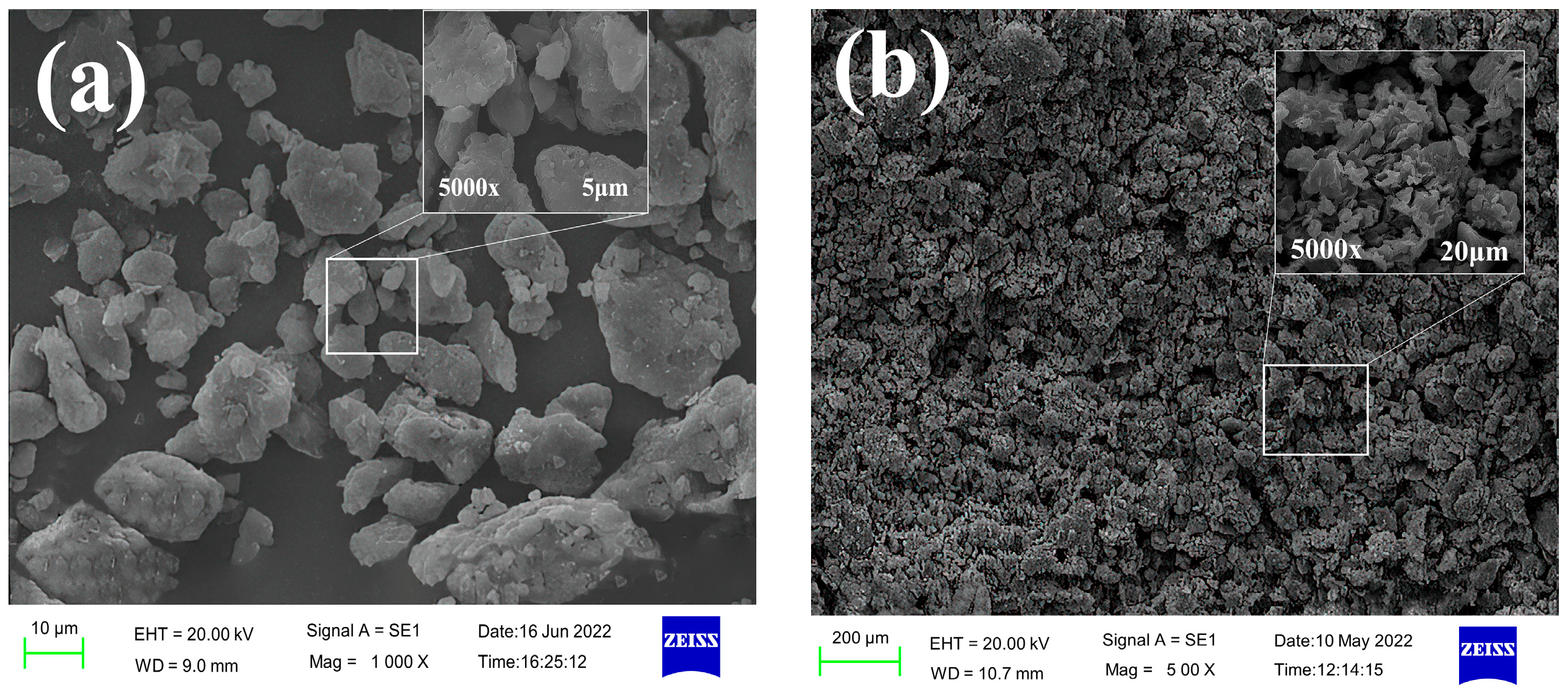
In this section, environmental scanning electron microscopy (SEM) was used to analyze and study the microscopic interaction between the inhibitor and the shale powder. The resulting SEM images are shown in
Figure 12.
Figure 12 shows that the wet shale powder in the clear water group was dispersed and had an uneven surface, while the wet shale powder treated with TIL-NH
2 exhibited a special micro-morphology. After treatments with 0.3%, 0.9%, and 1.5% TIL-NH
2, the size of the wet shale powder increased significantly, with magnification ranging from ×500 to ×5000. The inhibition effect approached saturation at a TIL-NH
2 dosage of 0.9%, where the wet shale powder appeared as tightly stacked flower-like structures under ×500 magnification. These structures were not truly separated and remained connected despite voids.
The size of these clusters of wet shale powder exceeded 100 μm, consistent with the size distribution of the TIL-NH2 concentration. Under ×5000 magnification, the interaction among the wet shale powders was more clearly observed. Larger wet shale powders were covered with a thin film on the surface, indicating that the inhibitor molecules effectively encapsulated the wet shale powder particles. The TIL-NH2 molecules played an excellent encapsulation role. Additionally, larger wet shale powders attracted smaller ones to accumulate around them, connecting through linear stretching to form strong bonds. This reflects the aggregation and settling of shale powder particles observed in the particle size distribution experiments.
These results indicate that TIL-NH2 strongly adsorbs onto illite through bifunctional groups, greatly inhibiting the hydration and dispersion of wet shale powders and maintaining their tight structure. This shows that microscopic interactions are closely related to macroscopic changes.
3.4. Inhibition Mechanism of TIL-NH2
The design concept of TIL-NH2 is to impart versatility to the polymer from a molecular structure perspective. To achieve this, the monomer of TIL-NH2 needs diverse properties. The molecular structure is dendritic, with amine groups and imidazole cationic five-membered ring structures introduced on the side groups of the molecule. The amine group acts as an inhibitory functional group, while the imidazolium cationic five-membered ring structure possesses properties of both an inhibitory and a temperature-resistant functional group.
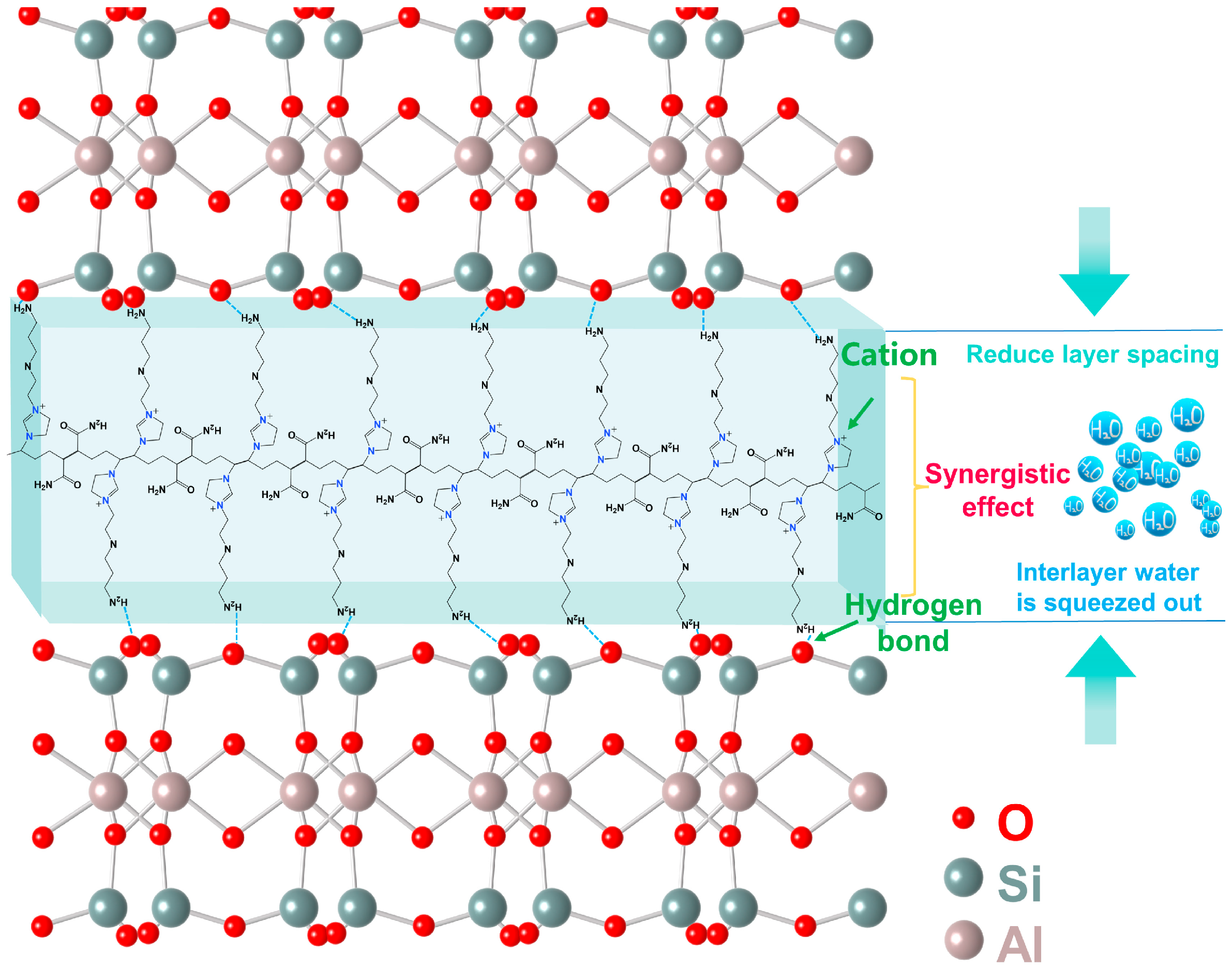
From the experimental analysis, it is evident that TIL-NH2 improves the wettability of water on shale powder and slows the rate of water penetration into illite. TIL-NH2 has a positive charge that counteracts the negative charge of illite, reducing the ionic concentration difference between the layers of illite. This brings the crystalline layers closer together, expelling the interlayer water, reducing the interlayer distance, and inhibiting the hydration of illite.
In the zeta potential test, TIL-NH2 adsorbed on the surface of illite and compressed the thickness of the diffusion layer around it. Infrared spectroscopy showed an increase in the frequency of N-H bond bending vibration peaks after the reaction of TIL-NH2 with shale powder, indicating that the amine group in TIL-NH2 formed a hydrogen bonding structure (N-H⋯O) with illite.
Thus, TIL-NH2 plays a dual inhibitory role: the imidazole cation adsorbs quickly and efficiently with illite, while the amine group at the end of TIL-NH2 forms a hydrogen bond with the oxygen atoms on the surface of illite. These effects are complementary and enhance the inhibition effect of TIL-NH2 on illite.
The reduction in various adsorbed water contents of shale powder by TIL-NH2 can be quantitatively measured by the heat loss weight test. The weakly bound water content is reduced from 0.4185% to 0.0951%, and the strongly bound water content is reduced from 0.2646% to 0.0911%. Scanning electron microscope images show that shale powder particles treated with TIL-NH2 exhibit an irregular bulk shape, with no obvious separation or cracks at lower magnifications, indicating low hydration and strong inter-particle bonding of illite.
Figure 13 provides a schematic diagram of the inhibition mechanism, explaining the mechanism of the inhibitory effect on illite.
In summary, the presence of primary amine groups and cationic imidazole rings is the fundamental reason for the inherent inhibition properties of the polymer inhibitor TIL-NH2. The adsorption mechanism between TIL-NH2 and illite involves both electrostatic interactions and hydrogen bonding, which work synergistically to enhance the inhibitory ability of TIL-NH2.