Research Progress on the Anti-Cancer Effects of Astragalus membranaceus Saponins and Their Mechanisms of Action
Abstract
1. Introduction
2. Triterpenoid Saponins
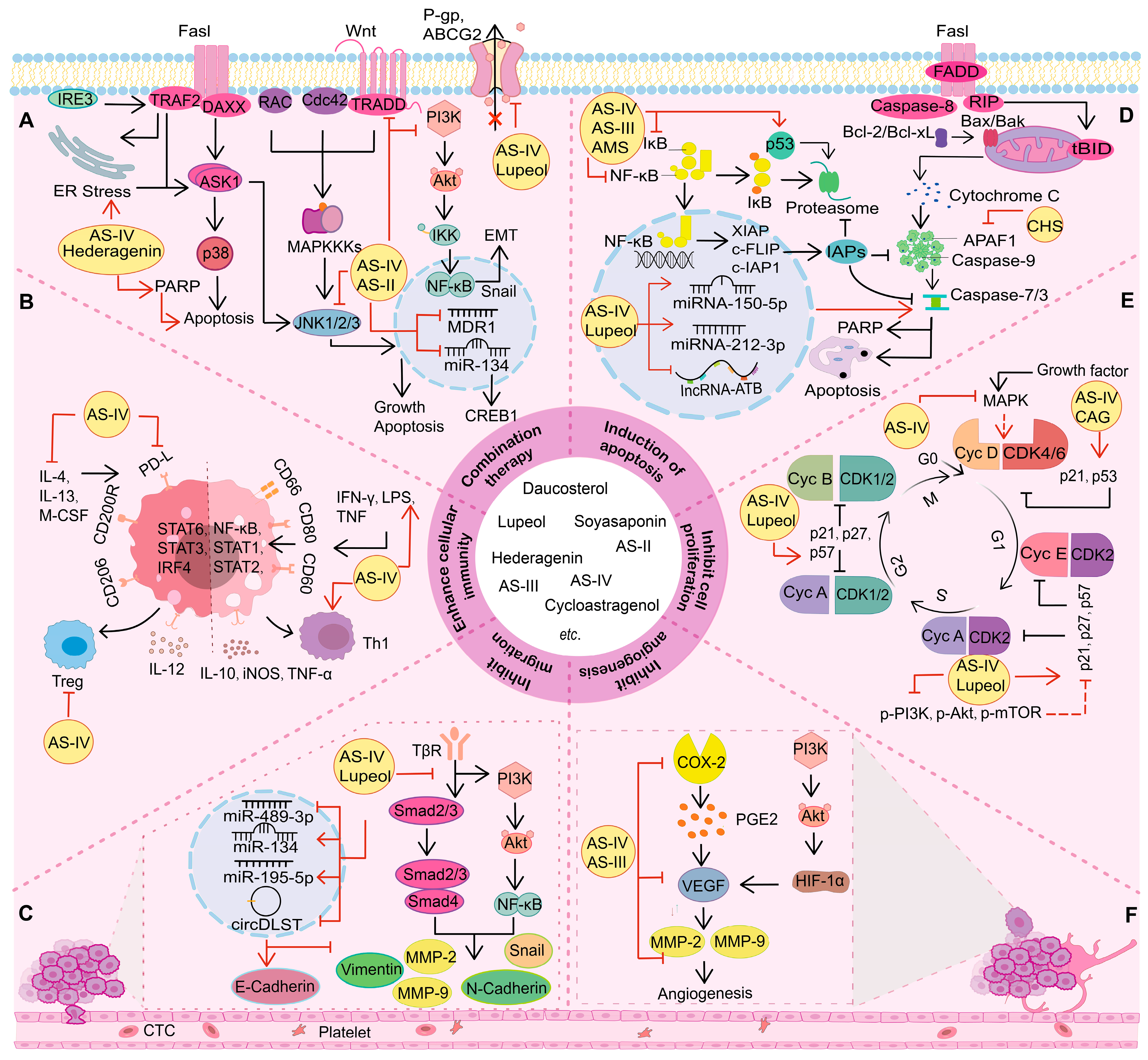
3. Anti-Cancer Effect of the Extracts of A. membranaceus Saponins
3.1. Inhibition of Cell Proliferation
3.2. Regulation of Cell Death
3.2.1. Induction of Apoptosis
3.2.2. Regulation of Autophagy
3.3. Inhibition of Tumor Cell Metastasis
3.4. Inhibition of Angiogenesis
3.5. Enhancement of Cellular Immunity
3.5.1. Enhancement of Cellular Nonspecific Immunity
3.5.2. Enhancement of Specific Immunity
3.6. Combination Therapy
3.6.1. Improvement of Multidrug Resistance
3.6.2. Increase of Sensitivity to Anti-Cancer Agents
3.6.3. Amelioration of Side Effects and Toxicity
4. Discussion and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lane, D.S.; Smith, R.A. Cancer Screening: Patient and Population Strategies. Med. Clin. N. Am. 2023, 107, 989–999. [Google Scholar] [CrossRef]
- Sankaranarayanan, R. Screening for cancer in low- and middle-income countries. Ann. Glob. Health 2014, 80, 412. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Basu, P. Cancer screening and early diagnosis in low and middle income countries: Current situation and future perspectives. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2018, 61, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Tsuji, Y. Updated Adjuvant Chemotherapy for Gastric Cancer. J. Clin. Med. 2023, 12, 6727. [Google Scholar] [CrossRef]
- Leon-Ferre, R.A.; Goetz, M.P. Advances in systemic therapies for triple negative breast cancer. BMJ 2023, 381, e071674. [Google Scholar] [CrossRef]
- Chen, C.; Liu, X.; Chang, C.Y.; Wang, H.Y.; Wang, R.F. The Interplay between T Cells and Cancer: The Basis of Immunotherapy. Genes 2023, 14, 1008. [Google Scholar] [CrossRef] [PubMed]
- Vaghari-Tabari, M.; Jafari-Gharabaghlou, D.; Mohammadi, M.; Hashemzadeh, M.S. Zinc Oxide Nanoparticles and Cancer Chemotherapy: Helpful Tools for Enhancing Chemo-sensitivity and Reducing Side Effects? Biol. Trace Elem. Res. 2023, 202, 1878–1900. [Google Scholar] [CrossRef]
- Sahin, T.K.; Bilir, B.; Kucuk, O. Modulation of inflammation by phytochemicals to enhance efficacy and reduce toxicity of cancer chemotherapy. Crit. Rev. Food Sci. Nutr. 2023, 63, 2494–2508. [Google Scholar] [CrossRef]
- Davodabadi, F.; Sajjadi, S.F.; Sarhadi, M.; Mirghasemi, S.; Hezaveh, M.N.; Khosravi, S.; Andani, M.K.; Cordani, M.; Basiri, M.; Ghavami, S. Cancer chemotherapy resistance: Mechanisms and recent breakthrough in targeted drug delivery. Eur. J. Pharmacol. 2023, 958, 176013. [Google Scholar] [CrossRef]
- Guo, X.; Gao, C.Y.; Yang, D.H.; Li, S.L. Exosomal circular RNAs: A chief culprit in cancer chemotherapy resistance. Drug Resist. Updates 2023, 67, 100937. [Google Scholar] [CrossRef]
- Nassar, A.; Abdelhamid, A.; Ramsay, G.; Bekheit, M. Chronomodulated Administration of Chemotherapy in Advanced Colorectal Cancer: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e36522. [Google Scholar] [CrossRef] [PubMed]
- Paraskeva, A.; Triantafyllidis, A.; Kazantzi, M.; Theodosopoulos, T. Indications, Safety, Efficacy and Survival Benefit of Intraperitoneal Chemotherapy in Patients with Advanced Gastric Cancer. Cancer Diagn. Progn. 2022, 3, 9–16. [Google Scholar] [CrossRef]
- Peng, Y.; Deng, X.; Yang, S.; Nie, W.; Tang, Y. Progress in Mechanism of Astragalus membranaceus and Its Chemical Constituents on Multiple Sclerosis. Chin. J. Integr. Med. 2022, 29, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Song, X.M.; Dan, L.W.; Tang, J.M.; Jiang, Y.; Deng, C.; Zhang, D.D.; Li, Y.Z.; Wang, W. Astragali Radix comprehensive review of its botany, phytochemistry, pharmacology and clinical application. Arch. Pharm. Res. 2024, 47, 165–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Yang, X.; Wei, J.R.; Chen, N.M.H.; Xu, J.P.; Bi, Y.Q.; Yang, M.; Gong, X.; Li, Z.Y.; Ren, K.; et al. Ethnopharmacology, Phytochemistry, Pharmacology, Toxicology and Clinical Applications of Radix Astragali. Chin. J. Integr. Med. 2021, 27, 229–240. [Google Scholar] [CrossRef]
- Dai, Y.J.; Guo, M.; Jiang, L.; Gao, J.R. Network pharmacology-based identification of miRNA expression of Astragalus membranaceus in the treatment of diabetic nephropathy. Medicine 2022, 101, e28747. [Google Scholar] [CrossRef] [PubMed]
- Ghabeshi, S.; Mousavizadeh, L.; Ghasemi, S. Enhancing the Antiviral Potential and Anti-inflammatory Properties of Astragalus membranaceus: A Comprehensive Review. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2023, 22, 211–219. [Google Scholar] [CrossRef]
- Li, Z.; Qi, J.; Guo, T.; Li, J. Research progress of Astragalus membranaceus in treating peritoneal metastatic cancer. J. Ethnopharmacol. 2023, 305, 116086. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, H.; Chen, J.; Chen, X.; Wen, Y.; Xu, L. Extract from Astragalus membranaceus inhibit breast cancer cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC Complement. Altern. Med. 2018, 18, 83. [Google Scholar] [CrossRef]
- Tin, M.M.; Cho, C.H.; Chan, K.; James, A.E.; Ko, J.K. Astragalus saponins induce growth inhibition and apoptosis in human colon cancer cells and tumor xenograft. Carcinogenesis 2007, 28, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Meng, D.D.; Zhang, Q.Y.; Wang, J. Advances in research on the anti-tumor mechanism of Astragalus polysaccharides. Front. Oncol. 2024, 14, 1334915. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, M.; Chai, Z.; Zhang, J.; Cao, K.; Deng, L.; Liu, Y.; Jiao, C.; Zou, G.-M.; Wu, J.; et al. Anticancer effects and mechanisms of astragaloside-IV (Review). Oncol. Rep. 2023, 49, 5. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.Y.; Li, J.J.; Yang, D.L.; Li, M.F.; Wei, J. Biosynthesis and Pharmacological Activities of Flavonoids, Triterpene Saponins and Polysaccharides Derived from Astragalus membranaceus. Molecules 2023, 28, 5018. [Google Scholar] [CrossRef] [PubMed]
- Su, H.f.; Shaker, S.; Kuang, Y.; Zhang, M.; Ye, M.; Qiao, X. Phytochemistry and cardiovascular protective effects of Huang-Qi (Astragali Radix). Med. Res. Rev. 2021, 41, 1999–2038. [Google Scholar] [CrossRef] [PubMed]
- Ionkova, I.; Shkondrov, A.; Krasteva, I.; Ionkov, T. Recent progress in phytochemistry, pharmacology and biotechnology of Astragalus saponins. Phytochem. Rev. 2014, 13, 343–374. [Google Scholar] [CrossRef]
- Kim, J.S.; Yean, M.H.; Lee, E.J.; Jung, H.S.; Lee, J.Y.; Kim, Y.J.; Kang, S.S. Two new cycloartane saponins from the roots of Astragalus membranaceus. Chem. Pharm. Bull. 2008, 56, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Savran, T.; Gülcemal, D.; Masullo, M.; Karayıldırım, T.; Polat, E. Cycloartane Glycosides from Astragalus erinaceus. Rec. Nat. Prod. 2012, 6, 230–236. [Google Scholar]
- Iskenderov, D.A.; Keneshov, B.M.; Isaev, M.I. Triterpene glycosides from Astragalus and their genins. LXXVI. Glycosides from A. sieversianus. Chem. Nat. Compd. 2008, 44, 319–323. [Google Scholar] [CrossRef]
- Polat, E.; Bedir, E.; Perrone, A.; Piacente, S.; Alankus-Caliskan, O. Triterpenoid saponins from Astragalus wiedemannianus Fischer. Phytochemistry 2010, 71, 658–662. [Google Scholar] [CrossRef]
- Gülcemal, D.; Masullo, M.; Bedir, E.; Festa, M.; Karayıldırım, T.; Alankus-Caliskan, O.; Piacente, S. Triterpene Glycosides fromAstragalus angustifolius. Planta Medica 2012, 78, 720–729. [Google Scholar] [CrossRef]
- Krasteva, I.; Nikolov, S. Flavonoids in Astragalus corniculatus. Química Nova 2008, 31, 59–60. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, M.; Xu, L.L.; Yi, Y.; Wang, L.L.; Wang, H.T.; Wang, Z.; Xing, J.T.; Li, P.; Zhang, X.H.; et al. Identification of oxidosqualene cyclases associated with saponin biosynthesis from Astragalus membranaceus reveals a conserved motif important for catalytic function. J. Adv. Res. 2023, 43, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Liu, Y.; Wang, Q.; Zhang, B. Astragaloside IV inhibits human colorectal cancer cell growth. Front. Biosci.-Landmark 2019, 24, 597–606. [Google Scholar]
- Liu, F.; Ran, F.; He, H.Q.; Chen, L.y. Astragaloside IV Exerts Anti-tumor Effect on Murine Colorectal Cancer by Re-educating Tumor-Associated Macrophage. Arch. Immunol. Ther. Exp. 2020, 68, 33. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, K.K.; Mok, N.; Wong, C.; Cho, C.; Ko, J. Astragalus saponins modulate mTOR and ERK signaling to promote apoptosis through the extrinsic pathway in HT-29 colon cancer cells. Int. J. Mol. Med. 2010, 26, 341–349. [Google Scholar] [PubMed]
- Hwang, S.T.; Kim, C.; Lee, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.M.; Sethi, G.; Ahn, K.S. Cycloastragenol can negate constitutive STAT3 activation and promote paclitaxel-induced apoptosis in human gastric cancer cells. Phytomedicine 2019, 59, 152907. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Sekar, V.; Majumder, B.; Mehrotra, D.G.; Banerjee, S.; Bhowmick, A.K.; Alam, N.; Mandal, G.K.; Biswas, J.; Majumder, P.K.; et al. CDKN2A-p53 mediated antitumor effect of Lupeol in head and neck cancer. Cell. Oncol. 2016, 40, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Wang, L.F.; Wang, Y.S.; Dong, S.Y.; Yang, S.J.; Guan, Y.F.; Wu, X. Astragaloside IV inhibits cell proliferation in vulvar squamous cell carcinoma through the TGF-β/Smad signaling pathway. Dermatol. Ther. 2018, 32, e12802. [Google Scholar] [CrossRef]
- Li, B.; Wang, F.; Liu, N.; Shen, W.; Huang, T. Astragaloside IV inhibits progression of glioma via blocking MAPK/ERK signaling pathway. Biochem. Biophys. Res. Commun. 2017, 491, 98–103. [Google Scholar] [CrossRef]
- Wang, S.M.; Tang, L.L.; Chen, F.Y. Astragaloside III from Astragalus membranaceus antagonizes breast cancer growth. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 183–186. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhao, Y.; Peng, Z.; LI, S.; Wang, A.; Zheng, X. Effects of Astragaloside on Biological Characteristics of Non-Small Cell Lung Cancer Cells via the Phosphatidylinositol 3-Kinase/Protein Kinase B/Mechanistic Target of Rapamycin Pathway. Indian. J. Pharm. Sci. 2021, 83, 865–870. [Google Scholar] [CrossRef]
- Guo, Z.W.; Xia, Y.J.; Hao, G.; Gao, Z.Z.; Li, R.; Yang, Y. In vitro analysis on inhibitory effect of sodium arsenite combined with astragaloside IV on HepG2 liver cancer cells. Alex. Eng. J. 2021, 60, 5749–5764. [Google Scholar] [CrossRef]
- Wang, S.X.; Mou, J.G.; Cui, L.S.; Wang, X.G.; Zhang, Z.Q. Astragaloside IV inhibits cell proliferation of colorectal cancer cell lines through down-regulation of B7-H3. Biomed. Pharmacother. 2018, 102, 1037–1044. [Google Scholar] [CrossRef]
- Zhao, C.K.; She, T.T.; Wang, L.X.; Su, Y.H.; Qu, L.K.; Gao, Y.J.; Xu, S.; Cai, S.Q.; Shou, C.C. Daucosterol inhibits cancer cell proliferation by inducing autophagy through reactive oxygen species-dependent manner. Life Sci. 2015, 137, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Che, S.T.; Wu, S.; Yu, P. Lupeol induces autophagy and apoptosis with reduced cancer stem-like properties in retinoblastoma via phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin inhibition. J. Pharm. Pharmacol. 2022, 74, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Afaq, F.; Adhami, V.M.; Mukhtar, H. Lupeol modulates NF-κB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene 2004, 23, 5203–5214. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.L.; Hou, S.; Shi, Z.H.; Xu, W.J.; Wang, Q.; He, Y.H.; Gong, Y.F.; Fang, Z.R.; Yang, Y. Astragaloside IV inhibits hepatocellular carcinoma by continually suppressing the development of fibrosis and regulating pSmad3C/3L and Nrf2/HO-1 pathways. J. Ethnopharmacol. 2021, 279, 114350. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Jiang, X.Y.; Wei, C.S.; Xing, Y.F.; Tong, G.D. Astragaloside IV suppresses development of hepatocellular carcinoma by regulating miR-150-5p/β-catenin axis. Environ. Toxicol. Pharmacol. 2020, 78, 103397. [Google Scholar] [CrossRef]
- Jia, L.W.; Lv, D.Y.; Zhang, S.; Wang, Z.Y.; Zhou, B. Astragaloside IV Inhibits the Progression of Non-Small Cell Lung Cancer Through the Akt/GSK-3β/β-Catenin Pathway. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2019, 27, 503–508. [Google Scholar] [CrossRef]
- Cheng, L.; Shi, L.; Wu, J.; Zhou, X.J.; Li, X.X.; Sun, X.; Zhu, L.; Xia, T.S.; Ding, Q. A hederagenin saponin isolated from Clematis ganpiniana induces apoptosis in breast cancer cells via the mitochondrial pathway. Oncol. Lett. 2017, 15, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Hsu, H.H.; Chu, Y.Y.; Cheng, S.F.; Shen, C.Y.; Lin, Y.J.; Chen, R.J.; Viswanadha, V.P.; Lin, Y.M.; Huang, C.Y. Lupeol alters ER stress-signaling pathway by downregulating ABCG2 expression to induce Oxaliplatin-resistant LoVo colorectal cancer cell apoptosis. Environ. Toxicol. 2018, 33, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.X.Z.; Zhou, J.Y.; Li, Y.; Zou, X.; Wu, J.; Gu, J.F.; Yuan, J.R.; Zhao, B.J.; Feng, L.; Jia, X.B.; et al. Hederagenin from the leaves of ivy (Hedera helix L.) induces apoptosis in human LoVo colon cells through the mitochondrial pathway. Complement. Altern. Med. 2014, 14, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Fei, Z.; Wei, N. Chemosensitive effects of Astragaloside IV in osteosarcoma cells via induction of apoptosis and regulation of caspase-dependent Fas/FasL signaling. Pharmacol. Rep. 2017, 69, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Wang, H.C.; Hsu, F.T.; Lu, C.H.; Lai, C.K.; Chung, J.G.; Kuo, Y.C. Astragaloside IV Induces Apoptosis, G1-Phase Arrest and Inhibits Anti-apoptotic Signaling in Hepatocellular Carcinoma. In Vivo 2020, 34, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, X.L.; Li, H.X.; Lu, Q.; Sun, G.P.; Chen, H.J. Astragalus Mongholicus Regulate the Toll-Like-Receptor 4 Meditated Signal Transduction of Dendritic Cells to Restrain Stomach Cancer Cells. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, K.; Cho, C.; Ko, J. A novel anticancer effect of Astragalus saponins: Transcriptional activation of NSAID-activated gene. Int. J. Cancer 2009, 125, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Rajavel, T.; Banu Priya, G.; Suryanarayanan, V.; Singh, S.K.; Pandima Devi, K. Daucosterol disturbs redox homeostasis and elicits oxidative-stress mediated apoptosis in A549 cells via targeting thioredoxin reductase by a p53 dependent mechanism. Eur. J. Pharmacol. 2019, 855, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Jia, R.F.; Wang, G.; Yang, J.H. Astragaloside attenuates the progression of prostate cancer cells through endoplasmic reticulum stress pathways. Oncol. Lett. 2018, 16, 3901–3906. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.T.; Wang, Y.; Peng, F. Astragaloside IV sensitizes non-small cell lung cancer cells to cisplatin by suppressing endoplasmic reticulum stress and autophagy. J. Thorac. Dis. 2020, 12, 3715–3724. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, X.Y.; Cuiping, C.; Pu, R.; Yin, W.H. Study on the Anticancer Effect of an Astragaloside- and Chlorogenic Acid-Containing Herbal Medicine (RLT-03) in Breast Cancer. Evid. Based Complement. Altern. Med. 2020, 2020, 1515081. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Ye, Y.; Chen, H.Y. Astragaloside IV inhibits cell migration and viability of hepatocellular carcinoma cells via suppressing long noncoding RNA-ATB. Biomed. Pharmacother. 2018, 99, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Min, T.R.; Park, H.J.; Ha, K.T.; Chi, G.Y.; Choi, Y.H.; Park, S.H. Suppression of EGFR/STAT3 activity by lupeol contributes to the induction of the apoptosis of human non-small cell lung cancer cells. Int. J. Oncol. 2019, 55, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.T.; Li, D.H.; Liu, Y.; Ren, H.; Yang, X.; Luo, W.T. Astragaloside IV Inhibits the Proliferation of Human Uterine Leiomyomas by Targeting IDO1. Cancers 2022, 14, 4424. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.H.; He, C.L.; Xu, F.T.; Xu, X.Y.; Liu, L.L.; Xu, M.J.; Guo, Z.; Wang, Y.L.; Liao, J.H.; Li, Y.H. Lupeol inhibits osteosarcoma progression by up-regulation of HMGA2 via regulating miR-212-3p. J. Orthop. Surg. Res. 2020, 15, 374. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Baek, S.; Shin, D.; Lee, J.; Roh, J.L. Hederagenin Induces Apoptosis in Cisplatin-Resistant Head and Neck Cancer Cells by Inhibiting the Nrf2-ARE Antioxidant Pathway. Oxidative Med. Cell. Longev. 2017, 2017, 5498908. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Shen, X.H.; Zhang, Y.; Wang, S.Y.; Zhou, L.J. Astragaloside IV suppresses transforming growth factor-β1-induced epithelial–mesenchymal transition through inhibition of Wnt/β-catenin pathway in glioma U251 cells. Biosci. Biotechnol. Biochem. 2020, 84, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.D.; Ma, D.N.; Ren, Z.G.; Zhu, X.D.; Wang, C.H.; Wang, Y.C.; Ye, B.G.; Cao, M.Q.; Gao, D.M.; Tang, Z.Y. Astragaloside IV inhibits metastasis in hepatoma cells through the suppression of epithelial-mesenchymal transition via the Akt/GSK-3β/β-catenin pathway. Oncol. Rep. 2017, 37, 1725–1735. [Google Scholar] [CrossRef]
- Hsu, M.J.; Peng, S.F.; Chueh, F.S.; Tsai, C.H.; Tsai, F.J.; Huang, C.Y.; Tang, C.H.; Yang, J.S.; Hsu, Y.M.; Huang, W.W.; et al. Lupeol suppresses migration and invasion via p38/MAPK and PI3K/Akt signaling pathways in human osteosarcoma U-2 OS cells. Biosci. Biotechnol. Biochem. 2019, 83, 1729–1739. [Google Scholar] [CrossRef]
- Cheng, X.D.; Gu, J.F.; Zhang, M.H.; Yuan, J.R.; Zhao, B.J.; Jiang, J.; Jia, X.B. Astragaloside IV inhibits migration and invasion in human lung cancer A549 cells via regulating PKC-α-ERK1/2-NF-κB pathway. Int. Immunopharmacol. 2014, 23, 304–313. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; Qin, X.K.; Huang, H.M.; Nie, C. Astragaloside IV inhibits the invasion and metastasis of SiHa cervical cancer cells via the TGF-β1-mediated PI3K and MAPK pathways. Oncol. Rep. 2019, 41, 2975–2986. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Chen, X.; Gao, J.X.; Yang, H.; Duan, Y.; Feng, Y.X.; He, X.; Gong, X.Q.; Wang, H.J.; Wu, X.L.; et al. Astragaloside III Enhances Anti-Tumor Response of NK Cells by Elevating NKG2D and IFN-γ. Front. Pharmacol. 2019, 10, 898–899. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Gao, Y.B.; Tian, N.X.; Wang, T.; Shi, Y.M.; Xu, J.Y.; Wu, B.J. Astragaloside IV inhibits glucose-induced epithelial-mesenchymal transition of podocytes through autophagy enhancement via the SIRT-NF-κB p65 axis. Sci. Rep. 2019, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- Li, F.G.; Cao, K.; Wang, M.Y.; Liu, Y.; Zhang, Y. Astragaloside IV exhibits anti-tumor function in gastric cancer via targeting circRNA dihydrolipoamide S-succinyltransferase (circDLST)/miR-489-3p/eukaryotic translation initiation factor 4A1(EIF4A1) pathway. Bioengineered 2022, 13, 10112–10123. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, H.; Wang, D.S. Protective role of astragaloside IV in gastric cancer through regulation of microRNA-195-5p-mediated PD-L1. Immunopharmacol. Immunotoxicol. 2021, 43, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Su, L.; Chen, D.G.; Zheng, W.Y.; Liu, Y. Astragaloside IV Induced miR-134 Expression Reduces EMT and Increases Chemotherapeutic Sensitivity by Suppressing CREB1 Signaling in Colorectal Cancer Cell Line SW-480. Cell. Physiol. Biochem. 2017, 43, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.F.; Zheng, W.H.; Jin, L. Astragaloside IV inhibits cell proliferation and metastasis of breast cancer via promoting the long noncoding RNA TRHDE-AS1. J. Nat. Med. 2020, 75, 156–166. [Google Scholar] [CrossRef]
- Chang, W.W.; Yu, C.Y.; Lin, T.W.; Wang, P.H.; Tsai, Y.C. Soyasaponin I decreases the expression of α2,3-linked sialic acid on the cell surface and suppresses the metastatic potential of B16F10 melanoma cells. Biochem. Biophys. Res. Commun. 2006, 341, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Lin, T.W.; Chang, W.W.; Wu, C.Y.; Lo, W.H.; Wang, P.H.; Tsai, Y.C. Soyasaponin I-modified invasive behavior of cancer by changing cell surface sialic acids. Gynecol. Oncol. 2005, 96, 415–422. [Google Scholar] [CrossRef]
- Jiang, K.; Lu, Q.; Li, Q.; Ji, Y.J.; Chen, W.L.; Xue, X.H. Astragaloside IV inhibits breast cancer cell invasion by suppressing Vav3 mediated Rac1/MAPK signaling. Int. Immunopharmacol. 2017, 42, 195–202. [Google Scholar] [CrossRef]
- Nguedia, M.Y.; Tueche, A.B.; Yaya, A.J.G.; Yadji, V.; Ndinteh, D.T.; Njamen, D.; Zingue, S. Daucosterol from Crateva adansoniiDC (Capparaceae) reduces 7,12-dimethylbenz(a)anthracene-induced mammary tumors in Wistar rats. Environ. Toxicol. 2020, 35, 1125–1136. [Google Scholar] [CrossRef]
- Wang, J.; Cui, L.; Feng, L.; Zhang, Z.H.; Song, J.; Liu, D.; Jia, X.B. Isoalantolactone inhibits the migration and invasion of human breast cancer MDA-MB-231 cells via suppression of the p38 MAPK/NF-κB signaling pathway. Oncol. Rep. 2016, 36, 1269–1276. [Google Scholar] [CrossRef]
- Yu, Y.Q.; Hao, J.Q.; Wang, L.; Zheng, X.J.; Xie, C.X.; Liu, H.L.; Wu, J.; Qiao, S.; Shi, J.H. Astragaloside IV antagonizes the malignant progression of breast cancer induced by macrophage M2 polarization through the TGF-β-regulated Akt/Foxo1 pathway. Pathol. Res. Pract. 2023, 249, 154766. [Google Scholar] [CrossRef]
- Zhang, L.L.; Li, Q.; Zhong, D.S.; Zhang, W.J.; Sun, X.J.; Zhu, Y. MCM5 Aggravates the HDAC1-Mediated Malignant Progression of Lung Cancer. Front. Cell Dev. Biol. 2021, 9, 669132. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Yuan, R.H.; Cao, Q.W.; Wang, M.; Ren, D.Z.; Huang, X.Y.; Wu, M.; Zhang, L.P.; Zhao, X.R.; Huo, X.P.; et al. Astragaloside III activates TACE/ADAM17-ependent anti-inflammatory and growth factor signaling in endothelial cells in a p38-dependent fashion. Phytother. Res. 2020, 34, 1096–1107. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Zhang, X.X.; Feng, Q.; Chen, J.D.; Shen, L.; Yu, P.; Yang, L.; Chen, D.H.; Zhang, H.W.; Sun, W.X.; et al. Astragaloside IV exerts angiogenesis and cardioprotection after myocardial infarction via regulating PTEN/PI3K/Akt signaling pathway. Life Sci. 2019, 227, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, C.; Su, Y.; Yang, C.; Xia, Q.; Xu, D.j. Astragaloside II sensitizes human hepatocellular carcinoma cells to 5-fluorouracil via suppression of autophagy. J. Pharm. Pharmacol. 2017, 69, 743–752. [Google Scholar] [CrossRef]
- You, X.J.; Wu, Y.R.; Li, Q.X.; Sheng, W.; Zhou, Q.; Fu, W. Astragalus-Scorpion Drug Pair Inhibits the Development of Prostate Cancer by Regulating GDPD4-2/PI3K/AKT/mTOR Pathway and Autophagy. Front. Pharmacol. 2022, 13, 895696. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.L.; He, Z.H.; Cai, Y.T. Quantitative proteomics analysis of differentially expressed proteins induced by astragaloside IV in cervical cancer cell invasion. Cell. Mol. Biol. Lett. 2020, 25, 25. [Google Scholar] [CrossRef]
- Ying, Y.; Sun, C.B.; Zhang, S.Q.; Chen, B.J.; Yu, J.Z.; Liu, F.Y.; Wen, J.; Hou, J.; Han, S.S.; Yan, J.Y.; et al. Induction of autophagy via the TLR4/NF-κB signaling pathway by astragaloside IV contributes to the amelioration of inflammation in RAW264.7 cells. Biomed. Pharmacother. 2021, 137, 111271. [Google Scholar] [CrossRef]
- Qu, X.Y.; Gao, H.; Tao, L.N.; Zhang, Y.M.; Zhai, J.H.; Sun, J.M.; Song, Y.Q.; Zhang, S.X. Astragaloside IV protects against cisplatin-induced liver and kidney injury via autophagy-mediated inhibition of NLRP3 in rats. J. Toxicol. Sci. 2019, 44, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Z.; Wen, J.; Ying, Y.; Yin, W.; Zhang, S.Q.; Pang, W.L.; Wang, C.; Bian, Y.; Yuan, J.L.; Yan, J.Y.; et al. Astragaloside trigger autophagy: Implication a potential therapeutic strategy for pulmonary fibrosis. Biomed. Pharmacother. 2022, 154, 113603. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Huang, X.P.; Liao, T.T.; Li, G.S.; Yu, X.J.; You, Y.D.; Huang, Y.X. Daucosterol induces autophagic-dependent apoptosis in prostate cancer via JNK activation. Biosci. Trends 2019, 13, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zheng, Y.H.; Que, Z.J.; Zhang, L.J.; Lin, S.C.; Le, V.; Liu, J.W.; Tian, J.H. Astragaloside IV inhibits progression of lung cancer by mediating immune function of Tregs and CTLs by interfering with IDO. J. Cancer Res. Clin. Oncol. 2014, 140, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Cui, W.Q.; Wei, Y.; Cui, J.; Qiu, J.; Hu, L.L.; Gong, W.Y.; Dong, J.C.; Liu, B.J. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J. Exp. Clin. Cancer Res. 2018, 37, 472. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, S.Y.; Song, L.Y.; Liu, M.; Sun, Z.Q.; Liu, J.B. Astragaloside IV antagonizes M2 phenotype macrophage polarization-evoked ovarian cancer cell malignant progression by suppressing the HMGB1-TLR4 axis. Mol. Immunol. 2021, 130, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, Y.C.; Hu, G.Y.; Song, X.L.; Wang, X.S.; Li, X.Q.; Xu, X.Y. Astragaloside IV suppresses the migration and EMT progression of cervical cancer cells by inhibiting macrophage M2 polarization through TGFβ/Smad2/3 signaling. Funct. Integr. Genom. 2023, 23, 133. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.T.; Liu, J.Q.; Lu, X.T.; Chen, F.X.; Zhou, Z.H.; Wang, T.; Zhu, S.P.; Fei, S.J. The enhanced effect of lupeol on the destruction of gastric cancer cells by NK cells. Int. Immunopharmacol. 2013, 16, 332–340. [Google Scholar] [CrossRef]
- Li, Y.; Meng, T.; Hao, N.; Tao, H.Q.; Zou, S.P.; Li, M.M.; Ming, P.F.; Ding, H.Y.; Dong, J.H.; Feng, S.B.; et al. Immune regulation mechanism of Astragaloside IV on RAW264.7 cells through activating the NF-κB/MAPK signaling pathway. Int. Immunopharmacol. 2017, 49, 38–49. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Cai, T.T.; Zeng, X.H.; Cai, D.K.; Chen, Y.X.; Huang, X.J.; Gan, H.N.; Zhuo, J.C.; Zhao, Z.M.; Pan, H.F.; et al. Astragaloside IV reverses MNNG-induced precancerous lesions of gastric carcinoma in rats: Regulation on glycolysis through miRNA-34a/LDHA pathway. Phytother. Res. 2018, 32, 1364–1372. [Google Scholar] [CrossRef]
- Wang, Z.F.; Ma, D.G.; Zhu, Z.; Mu, Y.P.; Yang, Y.Y.; Feng, L.; Yang, H.; Liang, J.Q.; Liu, Y.Y.; Liu, L.; et al. Astragaloside IV inhibits pathological functions of gastric cancer-associated fibroblasts. World J. Gastroenterol. 2017, 23, 8512–8525. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, Y.; Wu, T.T.; Li, Y.S.; Wang, Q. Astragaloside IV Attenuates Programmed Death-Ligand 1-Mediated Immunosuppression during Liver Cancer Development via the miR-135b-5p/CNDP1 Axis. Cancers 2023, 15, 5048. [Google Scholar] [CrossRef]
- Wang, H.; Wei, L.Y.; Mao, D.X.; Che, X.Y.; Ye, X.T.; Liu, Y.P.; Chen, Y. Combination of oxymatrine (Om) and astragaloside IV (As) enhances the infiltration and function of TILs in triple-negative breast cancer (TNBC). Int. Immunopharmacol. 2023, 125, 111026. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, X.D.; Liu, Q.M.; Ho, I.h.; Wei, X.L.; Yin, T.; Zhan, Y.J.; Zhang, W.J.; Zhang, W.B.; Chen, B.N.; et al. Hederagenin potentiated cisplatin- and paclitaxel-mediated cytotoxicity by impairing autophagy in lung cancer cells. Cell Death Dis. 2020, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Q.; Chen, H.; Guo, Q.; Zhang, L.; Zhang, Z.; Li, Y. Astragaloside IV enhanced carboplatin sensitivity in prostate cancer by suppressing AKT/NF-κB signaling pathway. Biochem. Cell Biol. 2021, 99, 214–222. [Google Scholar] [CrossRef]
- He, C.S.; Liu, Y.C.; Xu, Z.P.; Dai, P.C.; Chen, X.W.; Jin, D.H. Astragaloside IV Enhances Cisplatin Chemosensitivity in Non-Small Cell Lung Cancer Cells Through Inhibition of B7-H3. Cell. Physiol. Biochem. 2016, 40, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Li, Y.; Li, S.L.; Luo, H.F. Astragaloside IV Enhances Cisplatin Chemosensitivity in Human Colorectal Cancer via Regulating NOTCH3. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2016, 24, 447–453. [Google Scholar] [CrossRef]
- Dai, P.C.; Liu, D.L.; Zhang, L.; Ye, J.; Wang, Q.; Zhang, H.W.; Lin, X.H.; Lai, G.X. Astragaloside IV sensitizes non-small cell lung cancer cells to gefitinib potentially via regulation of SIRT6. Tumor Biol. 2017, 39, 69755. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Dai, Y.; Liu, W.P.; Wang, N.; Cai, Y.L.; Wang, S.Q.; Zhang, F.X.; Liu, P.X.; Chen, Q.J.; Wang, Z.Y. Astragaloside IV enhances taxol chemosensitivity of breast cancer via caveolin-1-targeting oxidant damage. J. Cell. Physiol. 2018, 234, 4277–4290. [Google Scholar] [CrossRef]
- Qu, X.Y.; Gao, H.; Zhai, J.H.; Sun, J.M.; Tao, L.N.; Zhang, Y.M.; Song, Y.Q.; Hu, T.T. Astragaloside IV enhances cisplatin chemosensitivity in hepatocellular carcinoma by suppressing MRP2. Eur. J. Pharm. Sci. 2020, 148, 105325. [Google Scholar] [CrossRef]
- Wang, P.P.; Xu, D.J.; Huang, C.; Wang, W.P.; Xu, W.K. Astragaloside IV reduces the expression level of P-glycoprotein in multidrug-resistant human hepatic cancer cell lines. Mol. Med. Rep. 2014, 9, 2131–2137. [Google Scholar] [CrossRef]
- Wang, P.P.; Luan, J.J.; Xu, W.K.; Wang, L.; Xu, D.J.; Yang, C.Y.; Zhu, Y.H.; Wang, Y.Q. Astragaloside IV downregulates the expression of MDR1 in Bel-7402/FU human hepatic cancer cells by inhibiting the JNK/c-Jun/AP-1 signaling pathway. Mol. Med. Rep. 2017, 16, 2761–2766. [Google Scholar] [CrossRef]
- Lin, J.M.; Fang, L.J.; Li, H.; Li, Z.X.; Lyu, L.M.; Wang, H.Y.; Xiao, J. Astragaloside IV alleviates doxorubicin induced cardiomyopathy by inhibiting NADPH oxidase derived oxidative stress. Eur. J. Pharmacol. 2019, 859, 172490. [Google Scholar] [CrossRef]
- Lou, Y.M.; Guo, Z.Z.; Zhu, Y.F.; Zhang, G.Y.; Wang, Y.; Qi, X.X.; Lu, L.L.; Liu, Z.Q.; Wu, J.J. Astragali radix and its main bioactive compounds activate the Nrf2-mediated signaling pathway to induce P-glycoprotein and breast cancer resistance protein. J. Ethnopharmacol. 2019, 228, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.F.; Guan, P.; Qin, L.Y.; Wang, J.X.; Wang, N.; Ji, E.S. Astragaloside IV inhibits adriamycin-induced cardiac ferroptosis by enhancing Nrf2 signaling. Mol. Cell. Biochem. 2021, 476, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.C.; Zhang, P.; Yang, L.; Song, P.; Zhao, J.; Wang, H.Z.; Zhao, Y.J.; Cao, L. Astragaloside IV Alleviates Doxorubicin-Induced Cardiotoxicity by Inhibiting Cardiomyocyte Pyroptosis through the SIRT1/NLRP3 Pathway. Am. J. Chin. Med. 2024, 52, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.H.; Liu, X.H.; Jia, J.; Zhong, X.; Han, R.Y.; Tan, R.Z.; Wang, L. Hederagenin ameliorates cisplatin-induced acute kidney injury via inhibiting long non-coding RNA A330074k22Rik/Axin2/β-catenin signalling pathway. Int. Immunopharmacol. 2022, 112, 109247. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.R. A tumor suppressor DLC1: The functions and signal pathways. J. Cell. Physiol. 2019, 235, 4999–5007. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.H.; Wei, W.Y. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022, 32, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Banik, N.L.; Patel, S.J.; Ray, S.K. Curcumin activated both receptor-mediated and mitochondria-mediated proteolytic pathways for apoptosis in human glioblastoma T98G cells. Neurosci. Lett. 2006, 407, 53–58. [Google Scholar] [CrossRef]
- Bagci, E.; Vodovotz, Y.; Billiar, T.; Ermentrout, G.; Bahar, I. Bistability in Apoptosis: Roles of Bax, Bcl-2, and Mitochondrial Permeability Transition Pores. Biophys. J. 2006, 90, 1546–1559. [Google Scholar] [CrossRef]
- Li, L.; Li, G.; Chen, M.B.; Cai, R.Z. Astragaloside IV enhances the sensibility of lung adenocarcinoma cells to bevacizumab by inhibiting autophagy. Drug Dev. Res. 2021, 83, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2019, 24, 4771–4778. [Google Scholar] [CrossRef]
- Cubillos Ruiz, J.R.; Bettigole, S.E.; Glimcher, L.H. Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer. Cell 2017, 168, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Xia, R.X.; Cheng, C.B.; Yang, M.Z.; Zeng, Q.S.; Xia, H.L.; Li, J.J. Mechanism of apoptosis in human leukemia NB4 cells induced by total astragalosides. Zhonghua Zhong Liu Za Zhi [Chin. J. Oncol.] 2011, 33, 345–348. [Google Scholar]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; He, S.K.; Ma, B.Y. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12–27. [Google Scholar] [CrossRef]
- Mortezaee, K.; Majidpoor, J.; Kharazinejad, E. Epithelial-mesenchymal transition in cancer stemness and heterogeneity: Updated. Med. Oncol. 2022, 39, 193. [Google Scholar] [CrossRef]
- Kai, F.; Drain, A.P.; Weaver, V.M. The Extracellular Matrix Modulates the Metastatic Journey. Dev. Cell 2019, 49, 332–346. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Zhang, H.Y. Astragaloside IV inhibits cell invasion and metastasis in vulvar squamous cell carcinoma through the TGF-β1/FAK/AKT signaling pathway. Ginekol. Pol. 2022, 93, 179–184. [Google Scholar] [CrossRef]
- Kong, P.F.; Tang, X.M.; Liu, F.; Tang, X.G. Astragaloside IV regulates circ-0001615 and miR-873-5p/LASP1 axis to suppress colorectal cancer cell progression. Chem. Biol. Drug Des. 2024, 103, e14423. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Goradel, N.H.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in cancer: A review. J. Cell. Physiol. 2018, 234, 5683–5699. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Jeong, J.H.; Choi, Y.H.; Park, S.H. Hexane Fraction of Adenophora triphylla var. japonica Root Extract Inhibits Angiogenesis and Endothelial Cell-Induced Erlotinib Resistance in Lung Cancer Cells. Molecules 2024, 29, 597. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.Y.; Yu, Y.D.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2020, 124, 359–367. [Google Scholar] [CrossRef]
- Liang, J.J.; Yang, C.Y.; Li, P.C.; Zhang, M.L.; Xie, X.Q.; Xie, X.T.; Chen, Y.L.; Wang, Q.; Zhou, L.; Luo, X. Astragaloside IV inhibits AOM/DSS-induced colitis-associated tumorigenesis via activation of PPARγ signaling in mice. Phytomedicine 2023, 121, 155116. [Google Scholar] [CrossRef]
- Li, Y.J.; Lei, Y.H.; Yao, N.; Wang, C.R.; Hu, N.; Ye, W.C.; Zhang, D.M.; Chen, Z.S. Autophagy and multidrug resistance in cancer. Chin. J. Cancer 2017, 36, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Lu, J.J. Combination strategies for first-line treatment of patients with unresectable hepatocellular carcinoma: Prospect of natural products. Chin. J. Nat. Med. 2024, 22, 1–3. [Google Scholar] [CrossRef]
- Ping, G.; Haowei, S.; Xiaofei, J.; Shuhan, G.; Xiaohong, Z.; Weijuan, G. Mechanism of astragaloside IV regulating NLRP3 through LOC102555978 to attenuate cerebral ischemia reperfusion induced microglia pyroptosis. Int. Immunopharmacol. 2024, 131, 111862. [Google Scholar]
- Zhang, L.J.; Liu, Y.; Lei, X.; Liu, X.W.; Sun, H.; Liu, S.J. Astragaloside II enhanced sensitivity of ovarian cancer cells to cisplatin via triggering apoptosis and autophagy. Cell Biol. Int. 2023, 47, 1600–1613. [Google Scholar] [CrossRef]
- Guo, J.; Le, Y.; Yuan, A.; Liu, J.; Chen, H.; Qiu, J.; Wang, C.; Dou, X.; Yuan, X.; Lu, D. Astragaloside IV ameliorates cisplatin-induced liver injury by modulating ferroptosis-dependent pathways. J. Ethnopharmacol. 2024, 328, 118080. [Google Scholar] [CrossRef]
- Gong, Y.F.; Hou, S.; Xu, J.C.; Chen, Y.; Zhu, L.L.; Xu, Y.Y.; Chen, Y.Q.; Li, M.M.; Li, L.L.; Yang, J.J.; et al. Amelioratory effects of astragaloside IV on hepatocarcinogenesis via Nrf2-mediated pSmad3C/3L transformation. Phytomedicine 2023, 117, 154903. [Google Scholar] [CrossRef]
- Qin, W.T.; Li, S.L.; Cheng, Z.J.; Xue, W.L.; Tian, M.Y.; Mou, F.F.; Guo, H.D.; Shao, S.J.; Liu, B.N. Astragaloside IV attenuates sunitinib-associated cardiotoxicity by inhibiting COUP-TFII. Heliyon 2024, 10, e24779. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.Y.; Tan, X.Y.; Hu, Q.C.; Tang, J.; Wang, Y.Y.; He, C.Y.; He, Z.J.; Li, B.; Fu, X.X.; Du, Q.Y. A systematic review of astragaloside IV effects on animal models of diabetes mellitus and its complications. Heliyon 2024, 10, e26863. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Z.; Niu, Y.Y.; Tian, L.K.; Zhang, T.C.; Zhou, S.J.; Wang, L.N.; Sun, J.; Wumiti, T.; Chen, Z.W.; Zhou, Q.F.; et al. Astragaloside IV alleviates macrophage senescence and d-galactose-induced bone loss in mice through STING/NF-κB pathway. Int. Immunopharmacol. 2024, 129, 111588. [Google Scholar] [CrossRef] [PubMed]
- You, X.J.; Qiu, J.F.; Li, Q.X.; Zhang, Q.; Sheng, W.; Cao, Y.G.; Fu, W. Astragaloside IV-PESV inhibits prostate cancer tumor growth by restoring gut microbiota and microbial metabolic homeostasis via the AGE-RAGE pathway. BMC Cancer 2024, 24, 272. [Google Scholar] [CrossRef]
- Jan, S.; Iram, S.; Bashir, O.; Shah, S.N.; Kamal, M.A.; Rahman, S.; Kim, J.; Jan, A.T. Unleashed Treasures of Solanaceae: Mechanistic Insights into Phytochemicals with Therapeutic Potential for Combatting Human Diseases. Plants 2024, 13, 724. [Google Scholar] [CrossRef]
- Chinnadurai, A.; Breadner, D.; Baloush, Z.; Lohmann, A.E.; Black, M.; D’Souza, D.; Welch, S. Adjuvant carboplatin and paclitaxel with “sandwich” method radiotherapy for stage III or IV endometrial cancer: Long-term followup at a single-institution. J. Gynecol. Oncol. 2023, 35, 1455–4664. [Google Scholar] [CrossRef]
- Ding, H.; Huang, X.P.; Liu, X.D.; Li, Y.L.; Tang, S.; Xiong, H.L.; Huang, M.T.; Li, Y.; Liu, C.X.; Zhang, W.; et al. Effects of borneol combined with astragaloside IV and Panax notoginseng saponins regulation of microglia polarization to promote neurogenesis after cerebral ischaemia. J. Pharm. Pharmacol. 2023, 75, 940–950. [Google Scholar] [CrossRef]
- Elekofehinti, O.O.; Iwaloye, O.; Olawale, F.; Ariyo, E.O. Saponins in Cancer Treatment: Current Progress and Future Prospects. Pathophysiology 2021, 28, 250–272. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, Y.; Huang, J.; Wang, B.; Gong, Y.; Fang, Y.; Liu, Y.; Wang, S.; Guo, Y.; Wang, H.; et al. Anti-tumor effects and mechanisms of Astragalus membranaceus (AM) and its specific immunopotentiation: Status and prospect. J. Ethnopharmacol. 2020, 258, 112797. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Sheng, F.Y.; Yang, S.Y.; Liu, Y.; Zou, L.; Zhang, L.L. A comprehensive review on the medicinal usage of Podocarpus species: Phytochemistry and pharmacology. J. Ethnopharmacol. 2023, 310, 116401. [Google Scholar] [CrossRef]
- Zhang, L.L.; Sheng, F.Y.; Yang, Y.; Hu, Y.F.; Li, W.; Huang, G.Y.; Wu, M.Y.; Gong, Y.; Zhang, P.; Zou, L. Integrative transcriptomics and proteomics analyses to reveal the therapeutic effect and mechanism of Buxue Yimu Pills in medical-induced incomplete abortion rats. J. Ethnopharmacol. 2023, 305, 116113. [Google Scholar] [CrossRef] [PubMed]
- Song, S.Y.; Li, Y.N.; Liu, X.; Yu, J.Y.; Li, Z.; Liang, K.X.; Wang, S.P.; Zhang, J.Y. Study on the Biotransformation and Activities of Astragalosides from Astragali Radix In Vitro and In Vivo. J. Agric. Food Chem. 2023, 71, 17924–17946. [Google Scholar] [CrossRef] [PubMed]
- Song, S.S.; Wang, R.Y.; Li, Z.H.; Yang, Y.; Wang, T.T.; Qing, L.S.; Luo, P. Role of simulated in vitro gastrointestinal digestion on biotransformation and bioactivity of astragalosides from Radix Astragali. J. Pharm. Biomed. Anal. 2023, 231, 115414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, X.; Qin, X.Y.; Zhang, Y.; Guo, N.; Zhu, H.L.; Fu, Y.J. A solid preparation of phytochemicals: Improvement of the solubility and bioavailability of astragaloside IV based on β-cyclodextrin microencapsulation. Chem. Pap. 2023, 77, 6491–6503. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, L.X.; Wang, J.; Xu, X.L.; Ni, S.T.; Liu, M.; Hu, K.L. Nose to brain delivery of Astragaloside IV by β-Asarone modified chitosan nanoparticles for multiple sclerosis therapy. Int. J. Pharm. 2023, 644, 123351. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Luo, Y.J.; Chen, Y.C.; Ma, Y.Q.; Yue, P.F.; Yang, M. Novel breviscapine nanocrystals modified by panax notoginseng saponins for enhancing bioavailability and synergistic anti-platelet aggregation effect. Colloids Surf. B Biointerfaces 2019, 175, 333–342. [Google Scholar] [CrossRef]
- Wang, C.X.; Yue, G.J.; Qin, N.K.; Zhang, W.Q.; Yu, M.Q.; Wang, L.T.; Xu, G.; Ma, Q. Study on the Preparation Technology of Doxorubicin Hydrochloride-astragaloside IV Liposome. J. Liaoning Univ. Tcm 2021, 23, 32–37. [Google Scholar]
- Enchev, P.; Zarev, Y.; Ionkova, I. Biotechnological approaches for sustainable production of astragaloside I, II and IV from endemic species of Astracantha aitosensis (Ivan.) and Astragalus membranaceus (fisch.) by in vitro cultures. Pharmacia 2023, 70, 1449–1453. [Google Scholar] [CrossRef]
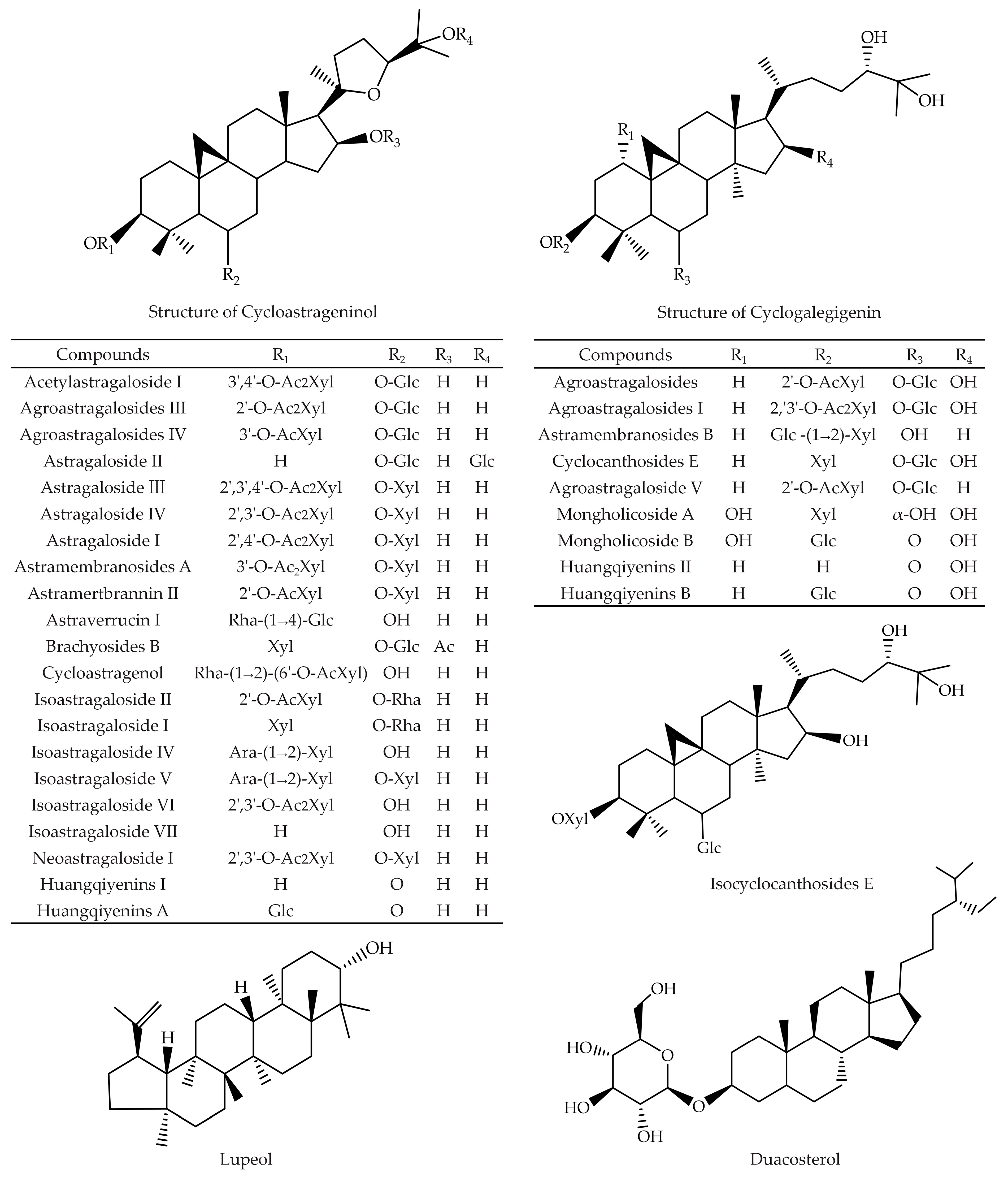
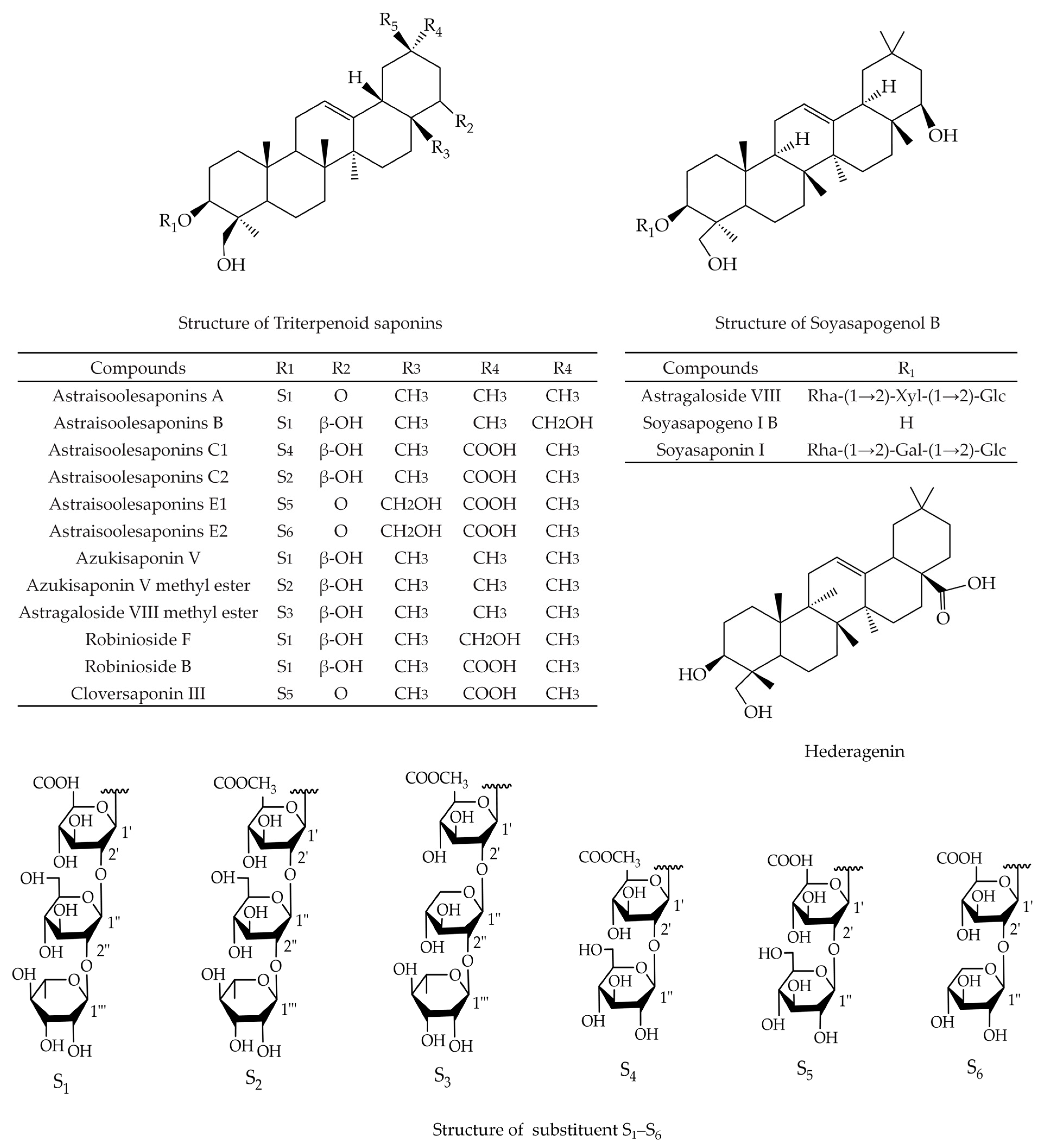
| Pharmacological Activity | Components | Models | Administration Design | Mechanisms | Refs. |
|---|---|---|---|---|---|
| Inhibition of cell proliferation | AS-IV | HT29, SW480, BALB/c mice | 25, 50 μM; 24 h; 20 mg/kg; 30 days; i.g. | p21, Cyt C, and Omi↑ | [34] |
| AS-IV | CT26 | 10, 50, and 100 nM; 48 h | B7-H3, Cyclin D1, and CDK4↓ | [35] | |
| AST | HT29 | 94 μM; 24, 48, and 72 h; | mTOR/NF-κB pathway↓; PTEN↑ | [36] | |
| CAG | SNU1, SNU16 | 10, 30, and 50 μM; 8 h SNU16: IC50 5 μM | p-STAT3, STAT3, and JAK1/2↓; caspase-3 and PARP cleavage↑ | [37] | |
| Lupeol | HEp2, UPCI | 50 μM; 72 h; HEp2: IC50 53.51 μM UPCI: IC50 52.44 μM | p53 and p16↑; Cyclin D1↓ | [38] | |
| AS-IV | SW962 | 255 μM; 24 h | Cyclin D1↓; Bax, cleaved caspase-3, p21, and p53↑ | [39] | |
| AS-IV | U251 | 50, 75, and 100 μM; 24 h | Ki67, PCNA, MMP-2, MMP-9, and MAPK/ERK pathway↓ | [40] | |
| AS-III | MDA-MB-231, nude mice | 13, 65, and 130 nM; 24, 48 h; 10, 20 mg/kg; 30 days; i.p. | mTOR/NF-κB pathway↓ | [41] | |
| AS-IV | A549 | 125 μM; 24 h; A549: IC50 32.2 μM | p-PI3K, p-AKt, and p-mTOR↓ | [42] | |
| AS-IV, NaAsO2 | HepG2 | 4 μM NaAsO2, 1 μM AS-IV; 48 or 72 h | PI3K/AKt/mTOR pathway↓ | [43] | |
| AS-IV | SW620, HT116 | 0, 6, 12, 25, 62, and 126 nM; 24, 48, and 72 h | miR-29c↑; B7-H3 and NF- κB↓ | [44] | |
| DS | MCF7, AGS, MGC803, Murine H22 hepatoma allograft model | 5, 25, 50, and 100 μM; 48 h; MCG803: IC50 19.96 μM, BGC823: IC50 3.13 μM, AGS: IC50 24.19 μM, MCF-7: IC50 16.95 μM 0.5, 2.5 mg/kg; 7 days; i.g. | ROS production and LC3-II↑ | [45] | |
| Lupeol | Y79, WERI-Rb-1 | 10, 20, and 40 μM; 24 h; Y79: IC50 47.1 μM, WERI-Rb-1: IC50 26.7 μM | Bax/Bcl-2 and LC3 II↑; Ki67, Bcl-2, and PI3K/AKt/mTOR pathway↓ | [46] | |
| Lupeol | CD-1 mice | 12, 24 mM; 30 min | iNOS↑; COX-2, and PI3K/Akt pathway↓ | [47] | |
| AS-IV | HSC-T6, HepG2, C57BL/6J | 5, 10, and 20 μM; 24 h; 20, 40, and 80 mg/kg; 20 weeks; i.g. | Nrf2/HO-1 and p-Smad3C/p21 pathway↑; p-Smad3L/c-Myc pathway↓ | [48] | |
| AS-IV | SMMC7721, Huh7 | 6, 12, 25, 50, 100, 125, 150, 225, and 255 μM; 24 h; 20 μg/mL; 24 h | miR-1505p↑; β-catenin and CTNNB1↓ | [49] | |
| Induction of apoptosis | AS-IV | HCC827, A549, NCI-H1299 | 15 and 30 nM; 48 h | cleavage of caspase-3↑; Akt/GSK-3β/β-catenin pathway↓ | [50] |
| AS-IV | HT29, SW480, BALB/c nu/nu mice | 12, 25, and 50 μM; 24 h; 20 mg/kg; 30 days; i.g. | Cyt C, Omi, and p21↑ | [34] | |
| AS-III | MCF7, MDA-MB-231, nude mice | 13, 65, and 130 nM; 24, 48 h; 10, 20 mg/kg; every two days, 30 days; i.p. | mTOR/NF-κB pathway↓ | [41] | |
| HDG | MCF7, MDA-MB-231 | 0.2, 0.8, 4.2, and 21.2 μM; 24 h; | caspase-3 and caspase-9↑; Apaf-1 and Cyt C↓ | [51] | |
| Lupeol | Oxaliplatin- R LoVo, nude mice | 0, 20, 50, 100, and 150 μM; 24 h; LoVo: IC50 15 μM 150 mM; every two days, 12 days; i.p. | ER stress↑; ABCG2↓ | [52] | |
| HDG | LoVo cells | 1.0, 2.0 μM; 24, 48 h; LoVo: IC50 1.39 μM at 24 h and 1.17 μM at 48 h | Bax, caspase-3, caspase-9, Bcl-2, procaspase-9, procaspase-3, and PARP↑ | [53] | |
| AS-IV | MG63, 143B, BALB/c nu/nu mice | 20, 40, 60, 80, and 100 μM; 48 h; 20 mg/kg; 28 days; i.g. | cleaved caspase-8, cleaved caspase-3, cleaved PARP, Fas, and FasL expression↑ | [54] | |
| AS-IV | SK-Hep1, Hep3B | 0, 200, and 400 μM; 48 h | cleavage of caspase-3, caspase-8, and caspase-9↑; XIAP, MCL1, and CFLIP↓ | [55] | |
| AS-IV | HT29 | 75 μM; 1 h | PTEN↑; reduce the binding activity of NF-κB to DNA, activate the activity of ERK 1/2 | [36] | |
| AMs | MKN45, C57/BL female mouse | 76–102 nM; 24 h; 250 mg/kg; 6 days; i.p. | TLR4 and CD11c↑; IκB-αby↓ | [56] | |
| AST | MGC803, MKN45, HCT116, MCF7, HepG2, HT29, Caco2, DLD1 | 94, 125 μM; 24 h, 48 h; HCT116: IC50 94 μM | NAG-1, Egr-1, and PARP cleavage↑; PI3K/Akt pathway↓ | [57] | |
| AS-IV | SW962 | 255 μM; 24 h | Bax, and cleaved caspase-3↑; Bcl-2, Bcl-xl, and TGF-β1/FAK/Akt pathway↓ | [39] | |
| DS | A549 | 25, 50, 100, and 200 μM; 72 h; A549: IC50 20.9 μM | PARP and caspase-3↑; EGFR and STAT3↓ | [58] | |
| AS | DU145 | 20, 50, and 100 nM; 72 h | IRE1, p-PERK, AFT4, AFT6, BiP, CHOP, and caspase-12 mRNA↑ | [59] | |
| AS-IV | A549Cis, H1299Cis | 3, 5, 10, and 20 nM; 24 h | PERK, LC3 II/I ratio, GRP78, and Beclin1↓ | [60] | |
| Lupeol | HEp2, UPCI | 50 μM; 24h; Hep-2: IC50 53.51 μM, UPCI: IC50 52.44 μM | p53 and p16↑; Cyclin D1↓ | [38] | |
| AS and CGA | 4T1, EMT6, BT549, MDA-MB-231, SPF-BALB/c mice | 2, 6 mM; 72 h; 4T1: IC50 6.7 mM, EMT6: IC50 5.6 mM, BT-549: IC50 7.3 mM, MDA-MB-231: IC50 1.8 mM 20 mg/g; 21 days; p.o. | RTK, VEGF, EGF, IL-10, TGF-β, and CD34↓ | [61] | |
| AS-IV | SMMC7721, Huh7 | 200 μM; 48 h | IL11/STAT3 signaling↑; lncRNA-ATB↓ | [62] | |
| Lupeol | H1299, A549, H460, WI38 | 125 and 250 μM; 72 h | PARP↑; EGFR and STAT3↓ | [63] | |
| HDG | MCF7, MDA-MB-231 | 0.2, 0.8, 4.2, and 21.2 μM; 24 h | caspase-3 and caspase-9↑; Apaf-1 and Cyt C↓ | [51] | |
| AS-IV | ULM1, ULM2, SD rats | 1, 10, 50, 100, 200, 300, and 400 µM; 24 h; ULM1: IC50 205.9 µM ULM2: IC50 215.0 µM 0.5, 4 mg/kg; 10 weeks; i.p. | IDO1↑; PTEN/PI3K/Akt pathway↓ | [64] | |
| DS | MCF7, BGC823, MGC803, Murine H22 hepatoma allograft model | 0.01, 0.1, 1, 3, 10, 30, and 100 mΜ; 48 h; MGC803: IC50 19.96 μM BGC823: IC50 3.13 μM, MCF-7: IC50 16.95 μM 0.5, 2.5 mg/kg; 7 days; p.o. | ROS production, and LC3-II↑ | [45] | |
| Lupeol | MNNG/HOS, MG63 | 30 mM; 24 h | miR-212-3p↑; HMGA2↓ | [65] | |
| HDG | SNU1041, SNU1066, SNU1076, BALB/c nu/nu mice | 50 and 100 μM; 72 h; 200 mg/kg; 35 days; i.p. | cleaved PARP, cleaved caspase-3, and Bax↑; Nrf2-ARE pathway↓ | [66] | |
| Inhibition of cell migration and invasion | AS-IV | U251 | 25, 50, and 100 μM; 48 h | vimentin, N-cadherin, β-catenin, Cyclin D1, and Wnt/β-catenin pathway↓ | [67] |
| AS-IV | Huh7, MHCC97-H | 1, 6, 12, 62, and 125 μM; 24, 48, and 72 h | E-cadherin↑; vimentin, α-SMA, N-cadherin, and Akt/GSK-3β/β-catenin pathway↓ | [68] | |
| AS-IV | HCC827, A549, NCI-H1299 | 4, 8, 15, and 30 nM; 48 h | cleavage of caspase-3 and Bcl-2↑; Bax and Akt/GSK-3β/β-catenin pathway↓ | [50] | |
| Lupeol | U2OS | 0, 5, 10, 15, 20, and 25 μM; 12 or 24 h | uPA, MMP-2, MMP-9, N-cadherin, β-catenin, p38, and PI3K/Akt/GSK-3β pathway↓ | [69] | |
| AS-IV | A549 | 5, 10, and 20 μM; 24 h | MMP-2, MMP-9, integrin β1, and PKC-α-ERK1/2-NF-κB pathway↓ | [70] | |
| AS-IV | SiHa, BALB/c nude mice | 62, 255, and 1000 μM; 24 h; SiHa: IC50 800.39 μM 120 mg/kg; 21 days; i.g. | TGF-β1, N-cadherin, Vimentin, and E-cadherin↑; MAPK and PI3K↓ | [71] | |
| AS-IV | A549 | 5, 10, and 20 μM; 24 h | MMP-2, MMP-9, integrin β1, and PKC-α-ERK1/2-NF-κB pathway↓ | [70] | |
| AS-IV | SW962 | 125, 255 μM; 24 h | E-cadherin, TGF-β1, FAK, and Akt↓ | [72] | |
| AS-IV | Immortalized mouse podocyte cell line, C57BL/6 J mice | 25, 50, and 100 μM; 48 h; 40 mg/kg; 12 weeks; i.g. | Nephrin, E-cadherin, and SIRT1↑; NF-κB, TGF-β, N-cadherin, α-SMA, Beclin 1, and LC3 II↓ | [73] | |
| AS-IV | HGC27, MKN45, nude mice | 10, 20, and 40 μM; 24, 48 h; 40 mg/kg; 21 days; i.p. | EIF4A1↑ Regulate circDLST/miR489-3p/EIF4A1 axis. circDLST↓ | [74] | |
| AS-IV | SGC7901, MGC803 | 6, 12, 31, and 62 μM; 24 h | miR-195-5p and miR-424-5p↑; PD-L1↓ | [75] | |
| AS-IV | SW480 | 6, 12, 31, and 62 μM; 24 or 48 h | miR-134↑; CREB1↓ | [76] | |
| AS-IV | MCF7, MDA-MB-231, MDA-MB-468, BALB/c nude mice | 25, 50, and 100 μM; 48 h; 20 mg/kg; every three days, 14 days; i.p. | TRHDE-AS1↑; MMP-2 and MMP-9↓ | [77] | |
| AS-IV | SMMC7721, Huh7 | 200 μM; 48 h | lncRNA-ATB↓ | [62] | |
| SsaI | B16F10, NIH/3T3, C57BL/6J male mice | 25, 50, and 75 μM; 24 h | α2, 3-linked sialic acids↓ | [78] | |
| SsaI | MCF7, MDA-MB-231 | 5, 25, 50, and 100 μM; 48, 72 h; | α2,3-sialylations and ST3Gal IV↓ | [79] | |
| AS-IV | MDA-MB-231, BALB/c nude mice | 12, 25, 38, and 50 μM; 24 h; 20 mg/kg; every three days, 6 weeks; i.p. | p-ERK1/2, p-JNK, and Vav3↓, Rac1 signaling↓ | [80] | |
| Regulation of autophagy | DS | A549, NCI–H460, NCI–H23, L132, female Wistar | 25, 50, 100, and 200 μM; 24 h; 2.5, 10 mg/kg; 28 days; i.g. | MDA, CA 15-3, TC, TG, and HDL-C↓ | [81] |
| Isoalantolactone | MDA-MB-231 | 1, 2, and 4 µM; 24 h | MMP-2, MMP-9, p38, MAPK, NF-κB, and p65↓ | [82] | |
| AS-IV | THP1, BALB/c nude mice | 5, 10, 25, 50, and 100 μM; 24 h; 40 mg/kg; 20 days; i.p. | Akt/Foxo1 and TGF-β↓ | [83] | |
| Enhance cellular immunity | AS-IV | A549, H1975, BALB/c nude mice | 20 mg/kg; 20 days; p.o. | MCM5/HDAC1, HDAC1, and MCM5↓ | [84] |
| AS-III | bEnd.3 | 10, 100, and 1000 nM; 24 h | EGFR, ERK1/2, p38, and AKT↑ Inhibit PMA-induced EPCR shedding through MAPKs and PKC pathway. | [85] | |
| AS-IV | HUVECs, SD rats | 10, 20, 40, 80, and 160 μM; 18 h; 20, 50 mg/kg; 2 weeks; i.g. | VEGF and PTEN/PI3K/Akt pathway↑ | [86] | |
| AS-II | Bel7402, Bel7402/FU | 0, 40, 80, 160, and 320 μM; 48 h | LC3-II, Beclin-1, and MAPK/mTOR pathway↓ | [87] | |
| Astragalus–Scorpion | LNCap, BALB/c nude mice | A (1.17, 2.54, 3.9 g/kg); S (0.39, 0.585, 0.78 g/kg); 4 weeks; i.g. | GDPD4-2↑; PI3K, Akt, and mTOR↓ | [88] | |
| AS-IV | SiHa, HeLA, BALB/c nude mice | 5, 25, and 50 μM; 12 h; 12.5, 25, and 50 mg/kg; 35 days; i.g. | DCP1A and TMSB4X↑; LC3I/II↓ | [89] | |
| AS-IV | A549, H1299, A549cis, H1299cis | 3, 5, 10, and 20 nM; 24 h | PERK, LC3 II/I ratio, GRP78, and Beclin1↓ | [60] | |
| AS-IV | RAW264.7 | 31, 62, and 125 μM; 24 h | NLRP3↑; IL-1β, IL-18, ROS, and TLR4/NF-κB pathway↓ | [90] | |
| AS-IV | SW962 | 125, 255, 510, 765, and 1020 μM; 24 h | Induce G0/G1 phase arrest. TGF-βRII and Smad4↑ | [39] | |
| AS-IV | SD rats | 40, 80 mg/kg; 7 days; p.o. | caspase-1, IL-1β, and IL-18↑; NLRP3↓ | [91] | |
| AS-IV | Kunming mice, the human lung fibroblasts cell line | 6, 12, 25, 50, 100, 200, and 400 μM; 24 h; 50, 100, and 200 mg/kg; 8–35 days; i.g. | LC3B-II/LC3B-I ratio↑; Col-I, Col-II, ATG7, Ras, Raf, MEK, ERKs, CD3 T cells, and CD68 T cells↓ | [92] | |
| DS | LNCap, PC3 | 5, 10, 20, 40, and 80 μM; 48 h | cleaved caspase-3, cleaved caspase-9, Bax, LC3II/LC3I ratio, Beclin 1, and JNK↑ | [93] | |
| AS-IV | A549 | 1, 25, 62, and 125 nM; 24 h | CTLs↑; Tregs, IDO, and Akt/mTOR pathway↓ | [94] | |
| Combination therapy | AS-IV | A549, H1299, THP1, C57BL/6 J | 80 nM; 48 h; 40 mg/kg; 21 days; i.g. | AMPK, IL-13, and IL-4↓; suppress M2 polarization of macrophages. | [95] |
| AS-IV | THP1, SKOV3 | 1, 6, and 12 μM; 24 h | TGF-β, MMP-9, IL-10, HMGB1, and TLR4↓ | [96] | |
| AS-IV | THP1, HeLa, SiHa | 20, 40, 60, and 80 μM; 24 h; HeLa: IC50 56.70 μM THP1: IC50 85.34 μM | CD163, IL-10, TGFβ, CD206, p-Smad2, and p-Smad3↓ | [97] | |
| AS-III | CT26, BALB/c mice | 4, 12, 20, and 40 nM; 48 h; 50 mg/kg; 2-day intervals five times; i.v. | NKG2D, Fas, IFN-γ, and T-Bet↑ | [72] | |
| Lupeol | N87, BGC823, HGC27 | 1, 2, 4, 7, 14, 29, 58, 116, 232, and 464 μM; 72 h | PFP, CD107a, IFN-γ, Wnt/β-catenin, PI3K/Akt, NK cells, NO, and TLR4/NF-κB pathway↑ | [98] | |
| AS-IV | RAW264.7 | 31 to 125 μM; 24 h | p38, ERK, and JNK↓ | [99] | |
| AS-IV | SD rats | 50, 100 mg/kg; 10 weeks; p.o. | p53 and TP53↑; MCT1, MCT4, HIF-1α, and CD147↓ | [100] | |
| AS-IV | BGC823 | 10, 20, and 40 μM; 72 h | SOX2, NANOG, and miR-214↑; GCAFs and miR301a↓ | [101] | |
| AS-IV | 3LL-luc, C57BL/6 | 40 mg/kg; 4, 8, and 12 days; p.o. | CTLs↑; Tregs, IDO, and Akt/mTOR pathway↓ | [94] | |
| AS-IV | CT26, BALB/c female mice | 10, 50, and 100 nM; 48 h; 15 mg/kg; once every three days, three times; i.p. | B7-H3, CyclinD1, and CDK4↓ | [35] | |
| AS-IV | SiHa, HeLa | 5, 25, and 50 μM; 12 h; HeLa: IC50 0.49 ± 0.03 mM, SiHa: IC50 0.27 ± 0.03 mM; 12.5, 25, and 50 mg/kg; 35 days; i.g. | DCP1A and TMSB4X↑; LC3I/II↓ | [89] | |
| AS-IV | THLE2, Huh7, SMMC7721, BALB/c nude mice | 12, 25, 50, and 100 μM; 4 h; 50, 100, and 150 mg/kg; 40 days; i.g. | CNDP1↑; PD-L1 and miR-135b-5p↓ | [102] | |
| AS-IV/β-CD IC | CTLL2, 4T1, NIH3T3, BALB/c nude mice | 0.6, 1.2, 2.4, 4.8, and 9.6 μM; 24 h; 1.2, 1.6 mg/kg; 6 days; i.p. | SOD↑; MDA↓ | [103] | |
| AS-IV | SW620, HCT116 | 6, 12, 25, 62, and 125 nM; 48 h | miR-29c↑; B7-H3, and NF- κB↓ | [44] | |
| AS-IV | A549cis, H1299cis | 2, 5, 10, and 20 nM; 24 h | PERK, LC3 II/I, GRP78, and Beclin1↓ | [60] | |
| HDG | NCI-H1299, NCI-H1975, BALB/c nude mice | 50 μM; 4 or 24 h; NCI-H1299: IC50 101.4 Nm, NCI-H1975: IC50 22.61 nM 25 mg/kg; 11 days; i.h. | LC3-II/LC3-I ratio↑; p62↓ | [104] | |
| AS-II | Bel7402, Bel7402/FU | 40, 80, 160, and 320 μM; 48 h; | LC3-II, Beclin-1, and MAPK/mTOR pathway↓ | [87] | |
| AS-IV | MG63, 143B, BALB/c nu/nu mice | 20, 40, 60, 80, and 100 μM; 48 h; 20 mg/kg; 28 days; i.g. | cleaved caspase-8, cleaved caspase-3, cleaved PARP, Fas, and FasL expression↑ | [54] | |
| AS-IV | LNCap, PC3 | 10 μM; 72 h; 40 mg/kg; 24 days; i.p. | E-cadherin↑; p-IĸB, p-AKT, p-p65, N-cadherin, and Vimentin↓ | [105] | |
| AS-IV | A549, H1975, BALB/c nude mice | 20 mg/kg; once every two days, 20 days; i.g. | MCM5/HDAC1, HDAC1, and MCM5↓ | [84] | |
| AS-IV | SW480 | 0, 6, 12, 31, and 62 μM; 24 or 48 h | miR-134↑; CREB1↓ | [76] | |
| AS-IV | A549, HCC827, NCI-H1299 | 1, 3, 6, 12, 25, and 50 nM; 48 h | B7-H3↓; increase cisplatin cytotoxicity | [106] | |
| AS-IV | HCT116, SW480, NCM460 | 3, 6, 9, 12, and 18 nM; 48 h | NOTCH3↓ | [107] | |
| AS-IV | NCI-H1299, HCC827, A549 | 3, 7, 15, and 30 nM; 48 h | Increase gefitinib sensitivity. SIRT6↑ | [108] | |
| AS-IV | MCF7, MCF10A, MDA-MB-231 | 10, 20, 30, 40, 50, 60, 70, 80, and 90 μM; 48 h | eNOS/NO/ONOO− pathway↑; phosphorylation of CAV-1 and ERK/JNK↓ | [109] | |
| AS-IV | HepG2, H22, BALB/c nude mice | 0.4, 4, and 40 μM; 24 h; 50 mg/kg; 14 days; p.o. | MRP2↓ | [110] | |
| AS-IV | Bel7402, Bel7402/FU | 0.1, 0.2, 0.4, and 0.8 mM; 48 h | P-gp and mdr1↓ | [111] | |
| AS-IV | Bel7402/FU | 0.1 mM; 24 h | JNK, c-Jun, and APD-1 DNA binding activity↓ | [112] | |
| AS-IV | 4T1, EMT6, BT549, MDA-MB-231, SPF- BALB/c nude mice | 2.5 and 3 mM; 1.6 and 2.5 mM; 2 and 2.5 mM; 72 h; 4T1: IC50 6.7 mM, EMT6: IC50 5.6 mM, BT-549: IC50 7.3 mM, MDA-MB-231: IC50 1.8 mM 20 mg/g; 21 days; p.o. | RTK, VEGF, EGF, IL-10, TGF-β, and CD34↓ | [61] | |
| AS-IV | NRCMs, C57BL/6 | 40 mg/kg; 4 weeks; i.g. | MDA, 8-OhdG, NOX2, and NOX4↓ | [113] | |
| DS | A549, NCI–H460, NCI–H23, L132; female Wistar rats | 25, 50, 100, and 200 μM; 24, 48, and 72 h; 2.5, 10 mg/kg; 28 days; i.p. | MDA, CA 15-3, TC, TG, and HDL-C↓ | [81] | |
| AS-IV | BT549, MDA-MB-231, SPF- BALB/c nude mice | 40 mg/kg; 4 weeks; i.g. | Nrf2, ARE-luciferin, BCRP, ATP, and GPx4↑ | [114] | |
| HDG | Human LoVo cancer cell | 1.0, 2.0, and 4.0 μM; 48 h LoVo: IC50 1.17 μM | Bax↑; Bcl-2 and Bcl-xL↓ | [53] | |
| Lupeol | OXA-R LoVo cell | 50 μM; 24 h | ER stress↑; ABCG2↓ | [52] | |
| CAG | SNU1, SNU16 | 50 μM; 24 h; SNU16: IC50 5 μM | caspase-3 and PARP cleavage↑; p-STAT3, STAT3, JAK1, and JAK2↓ | [37] | |
| AS-IV | SD rats | 10 mg/kg; 5 weeks; i.g. | type I/III collagens, TGF-β, NOX2, and NOX4↓; promote nuclear Nrf2 level. | [115] | |
| AS-IV | C57BL/6J | 20 mg/kg; 5 days/week, 6 weeks; i.g. | SIRT1↑; NLRP3, caspase-1/GSDMD, and caspase-3/GSDME↓ | [116] | |
| AS-IV | SD rats | 40, 80 mg/kg; 1 week; i.g. | NLRP3 and pro-inflammatory cytokines↓ | [91] | |
| HDG | pTEC cells, C57BL/6J | 21, 42, and 63 μM; 20, 40 mg/kg; 3 days; i.p. | Axin2/β-catenin and lncRNA-A330074k22Rik↓ | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, F.; Yang, S.; Li, M.; Wang, J.; Liu, L.; Zhang, L. Research Progress on the Anti-Cancer Effects of Astragalus membranaceus Saponins and Their Mechanisms of Action. Molecules 2024, 29, 3388. https://doi.org/10.3390/molecules29143388
Sheng F, Yang S, Li M, Wang J, Liu L, Zhang L. Research Progress on the Anti-Cancer Effects of Astragalus membranaceus Saponins and Their Mechanisms of Action. Molecules. 2024; 29(14):3388. https://doi.org/10.3390/molecules29143388
Chicago/Turabian StyleSheng, Feiya, Siyu Yang, Mi Li, Jiaojiao Wang, Lianghong Liu, and Lele Zhang. 2024. "Research Progress on the Anti-Cancer Effects of Astragalus membranaceus Saponins and Their Mechanisms of Action" Molecules 29, no. 14: 3388. https://doi.org/10.3390/molecules29143388
APA StyleSheng, F., Yang, S., Li, M., Wang, J., Liu, L., & Zhang, L. (2024). Research Progress on the Anti-Cancer Effects of Astragalus membranaceus Saponins and Their Mechanisms of Action. Molecules, 29(14), 3388. https://doi.org/10.3390/molecules29143388







