Preparation and Properties of Composite Double-Network Gel for Inhibiting Coal Spontaneous Combustion
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of the Impact of Component Content on Gel Properties
- (1)
- The effects of CS, AM, and MBA content on gelation time.
- (2)
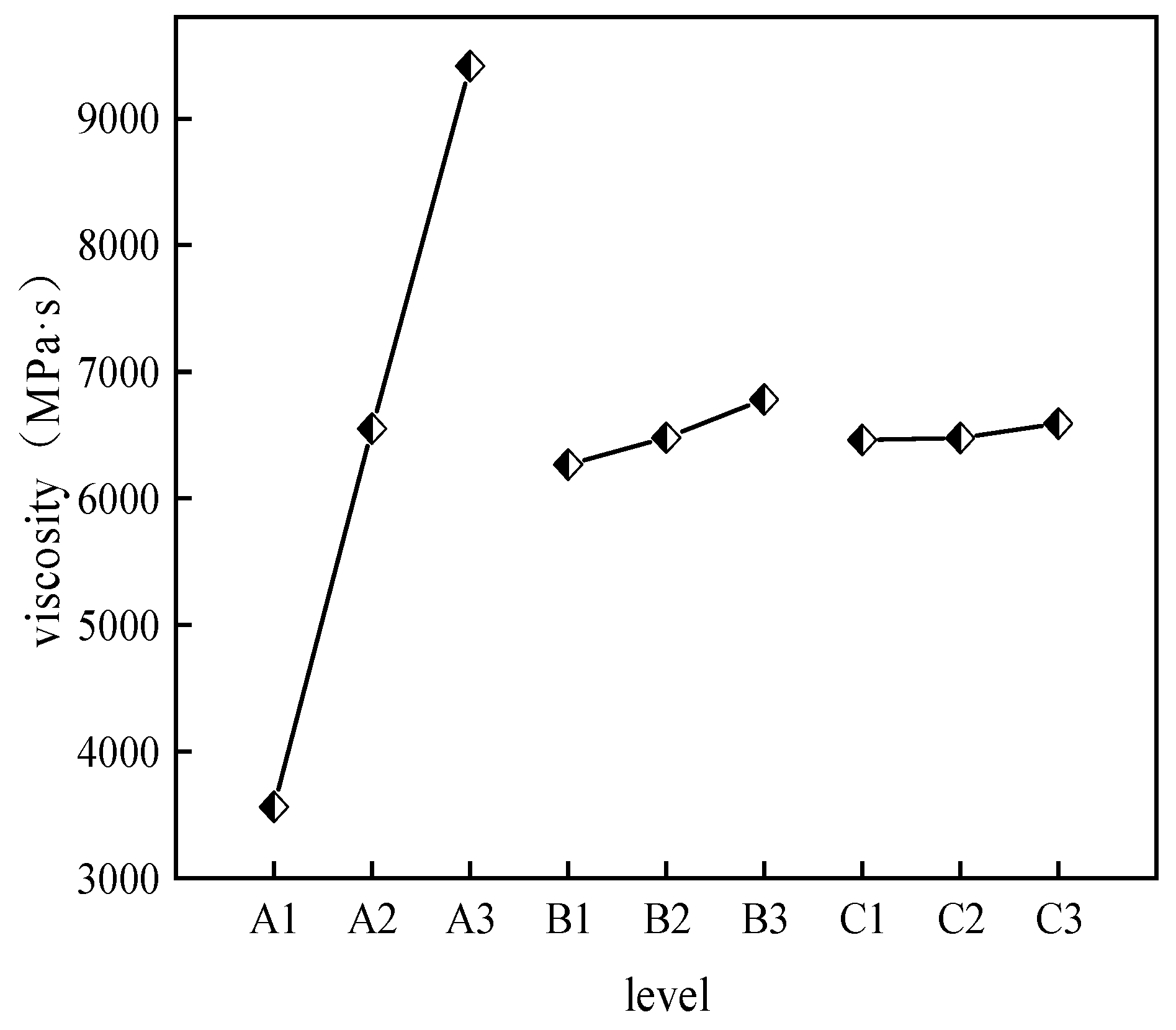
- The effects of CS, AM, and MBA content on viscosity.
- (3)
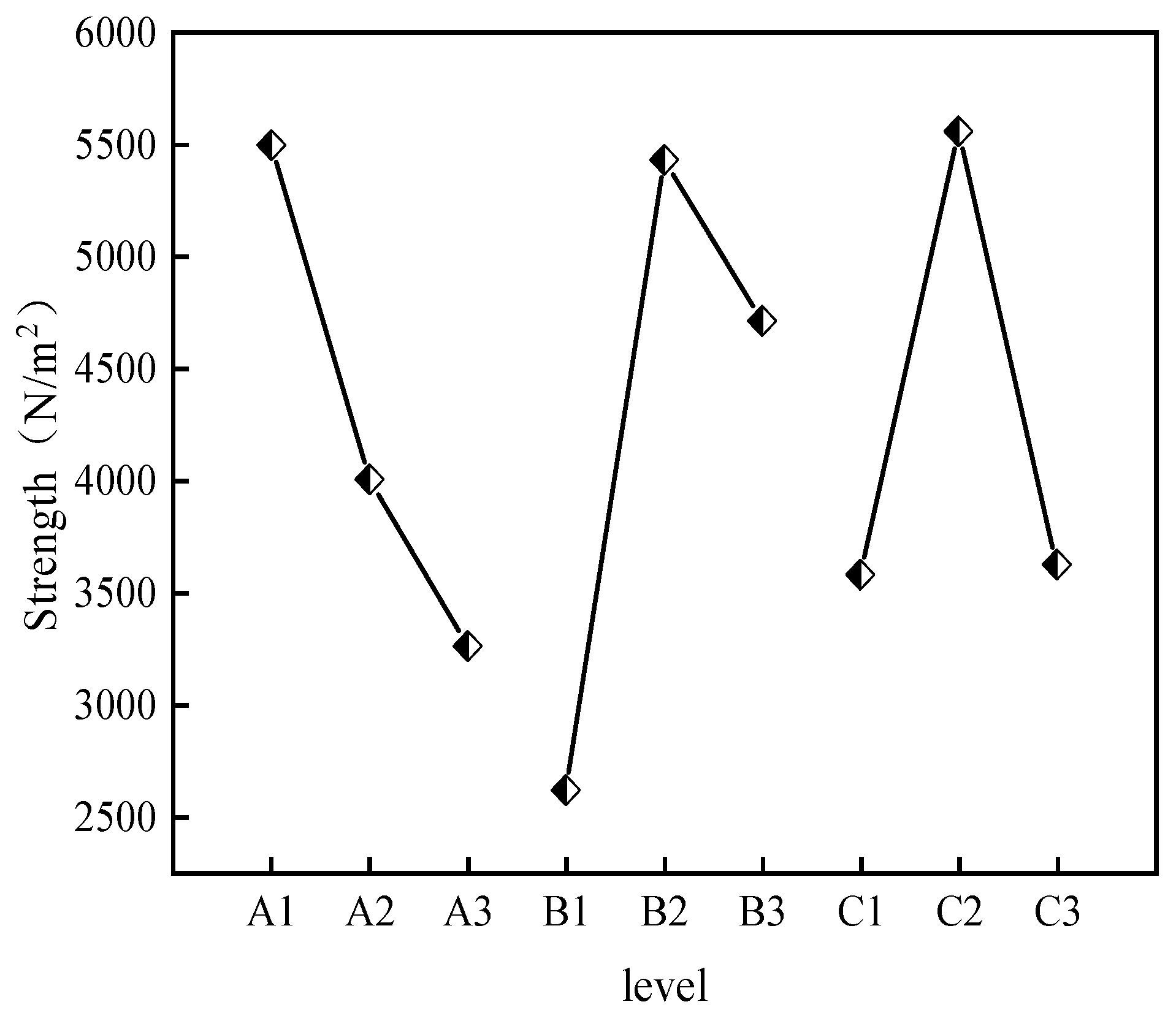
- Impact of CS, AM, and MBA Content on Strength
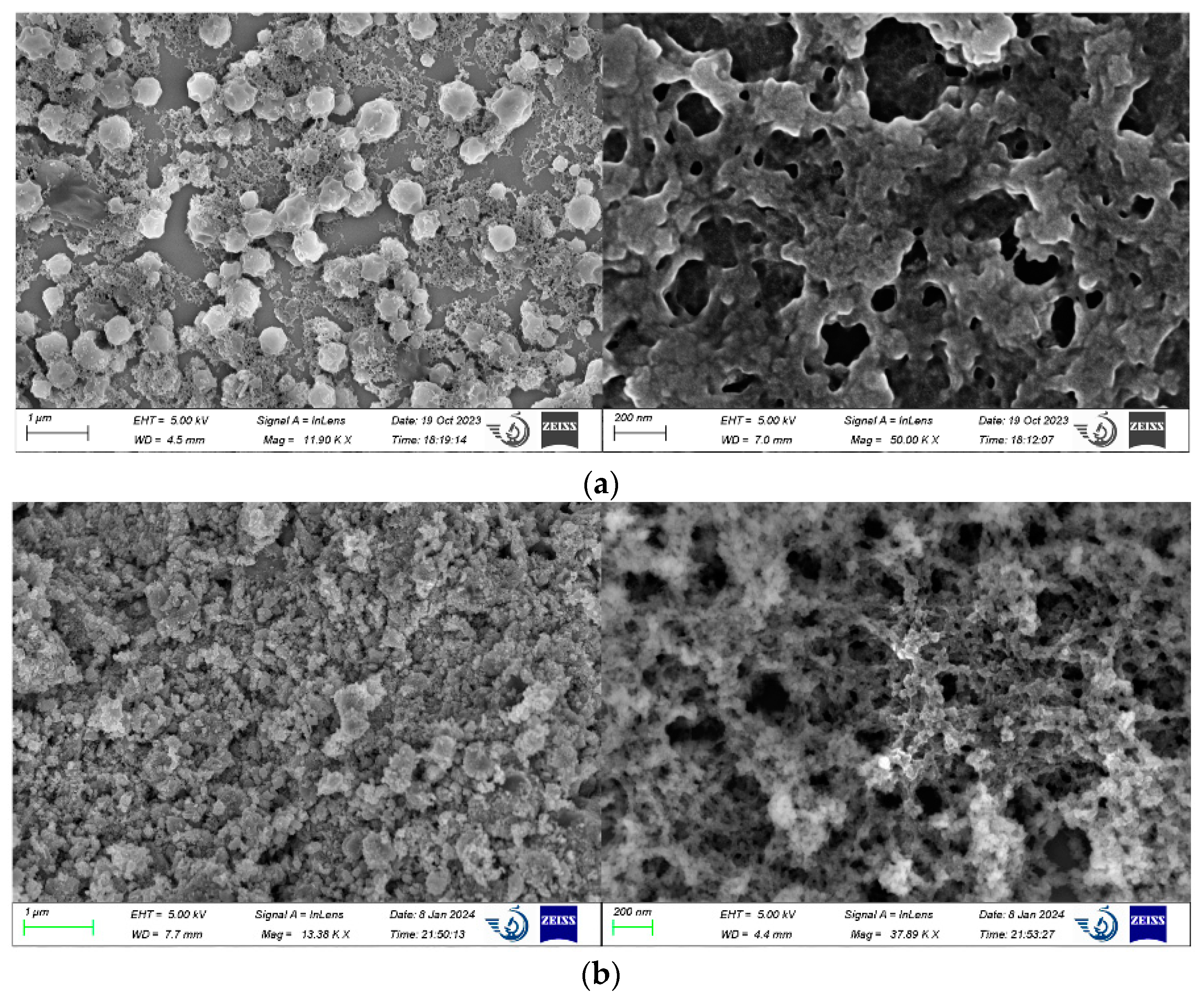
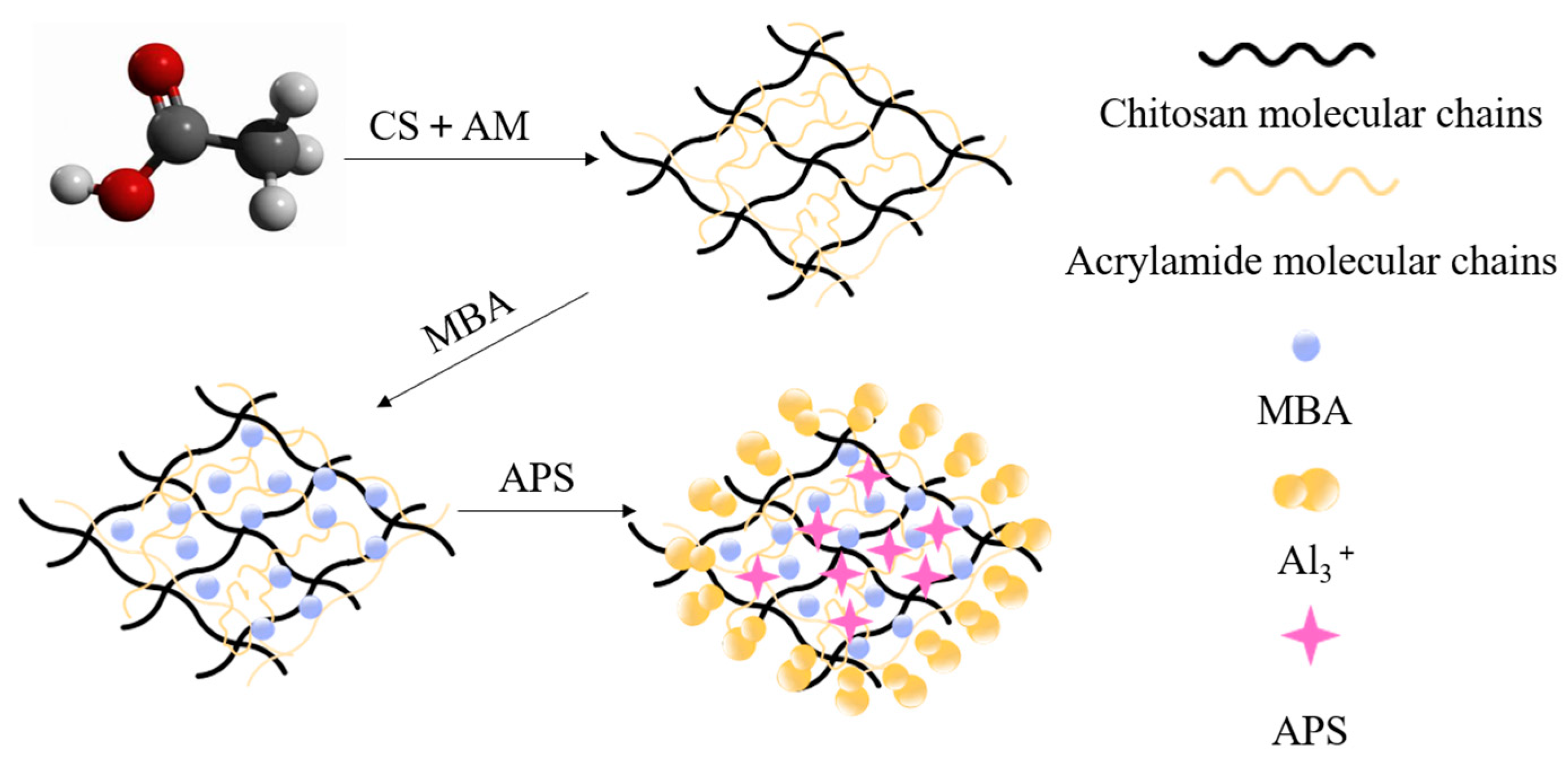
2.2. Microstructural Characterization of Double-Network Gel
2.3. Water Retention Analysis
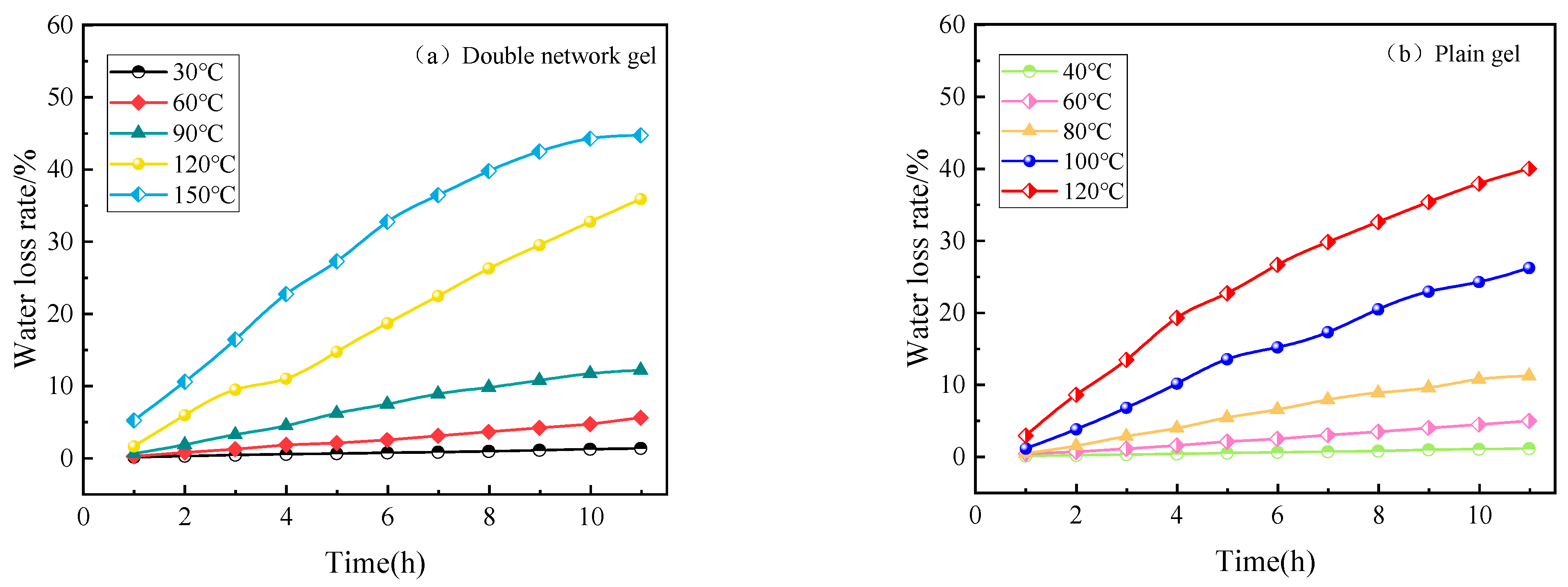
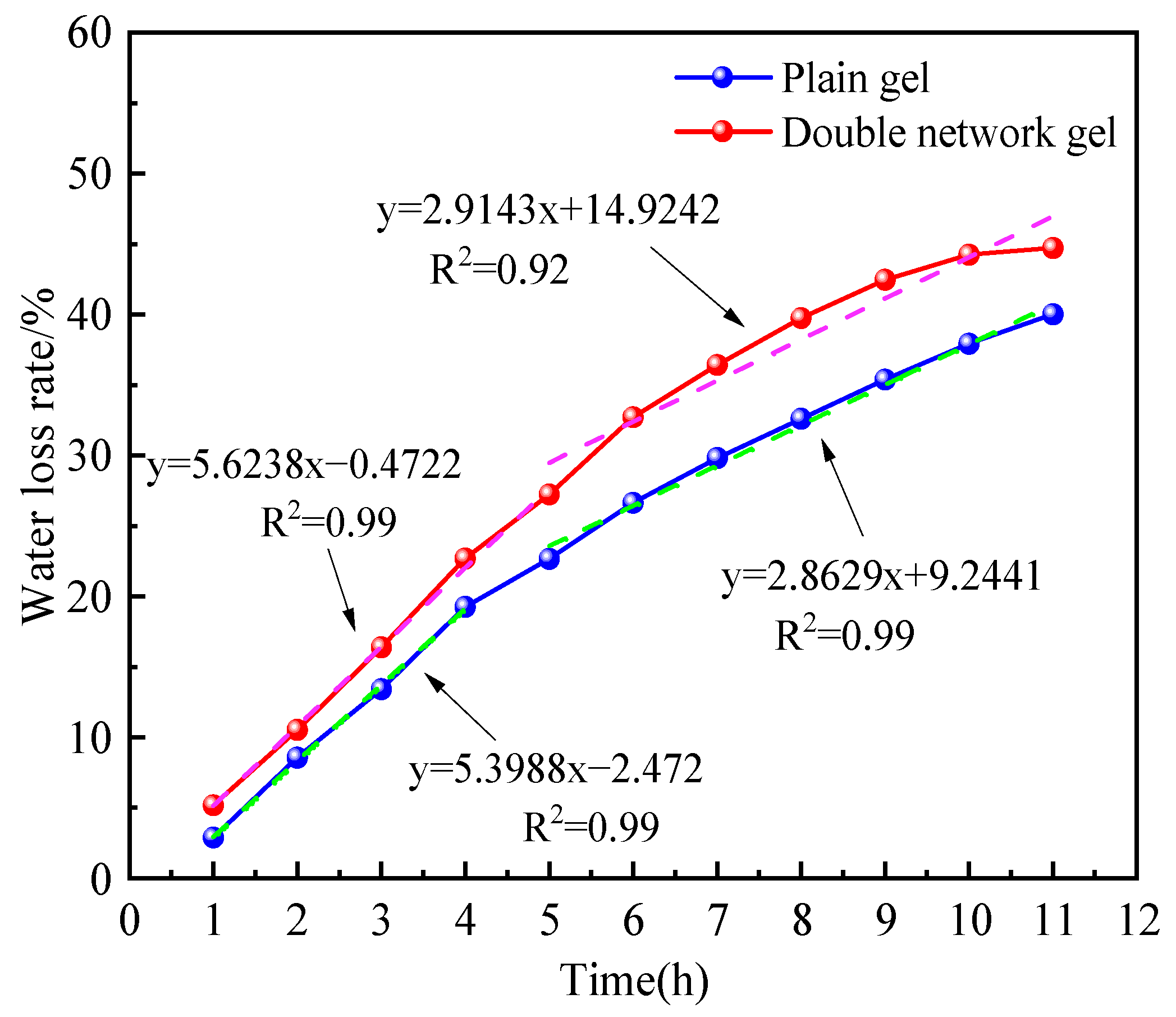
2.3.1. Water Retention Analysis at Constant Temperature
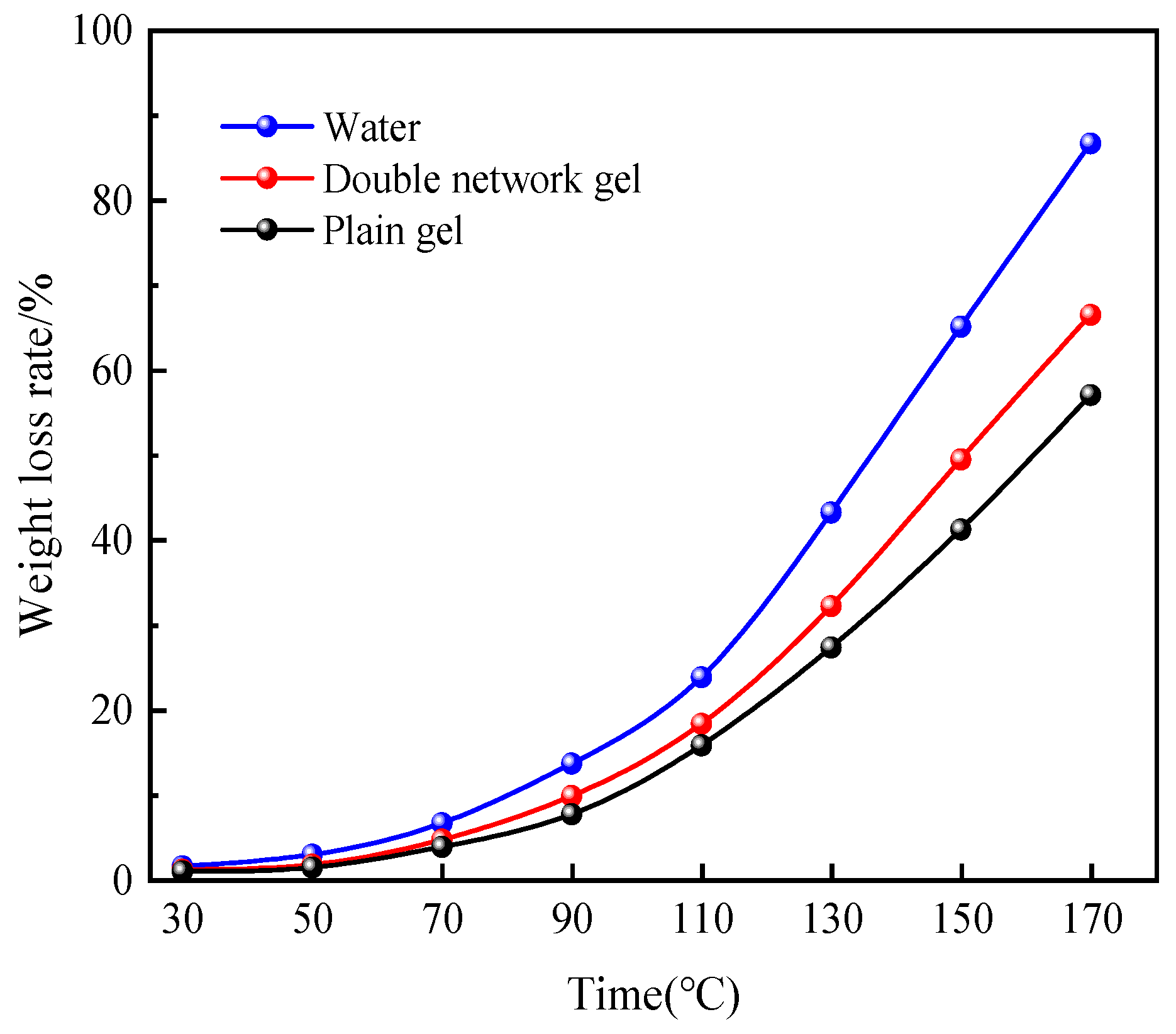
2.3.2. Water Retention Analysis under Heating
2.4. Mechanical Property Analysis
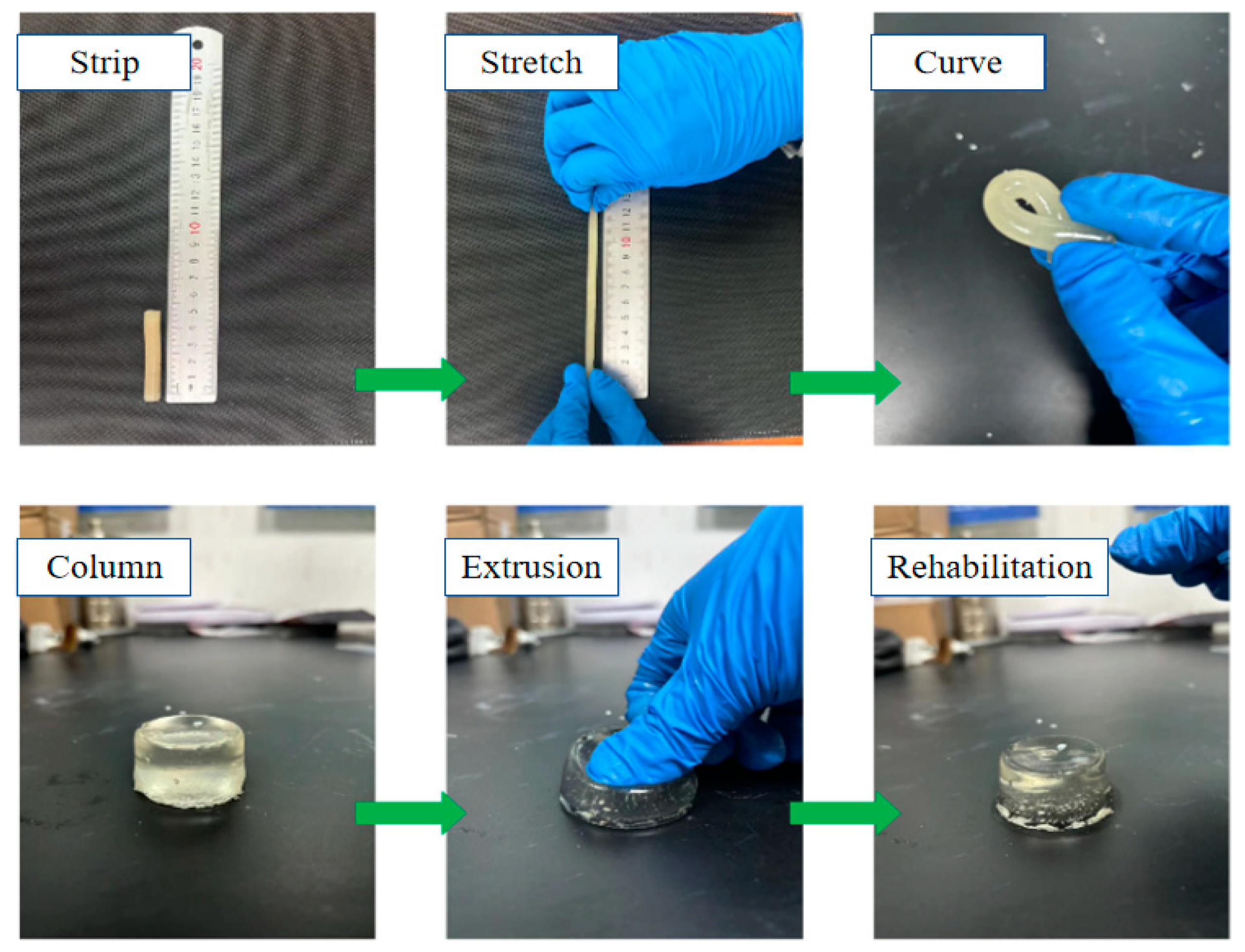
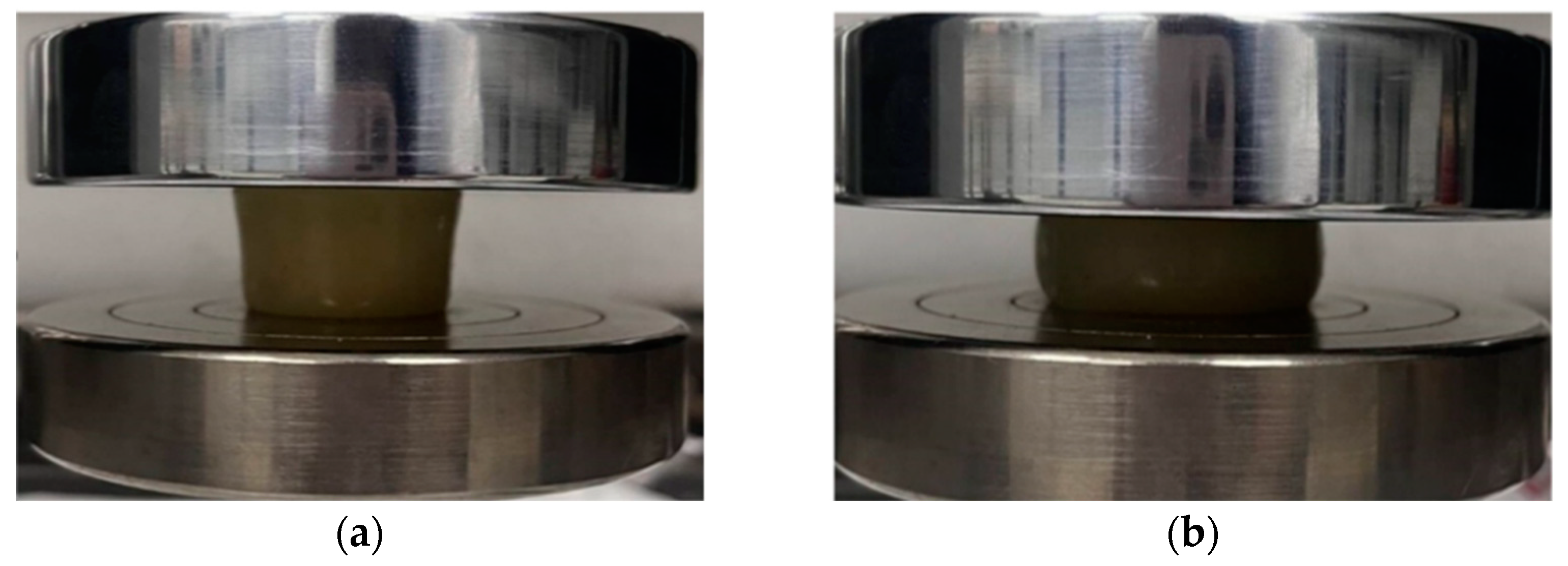
2.4.1. Analysis of Resistance to Damage
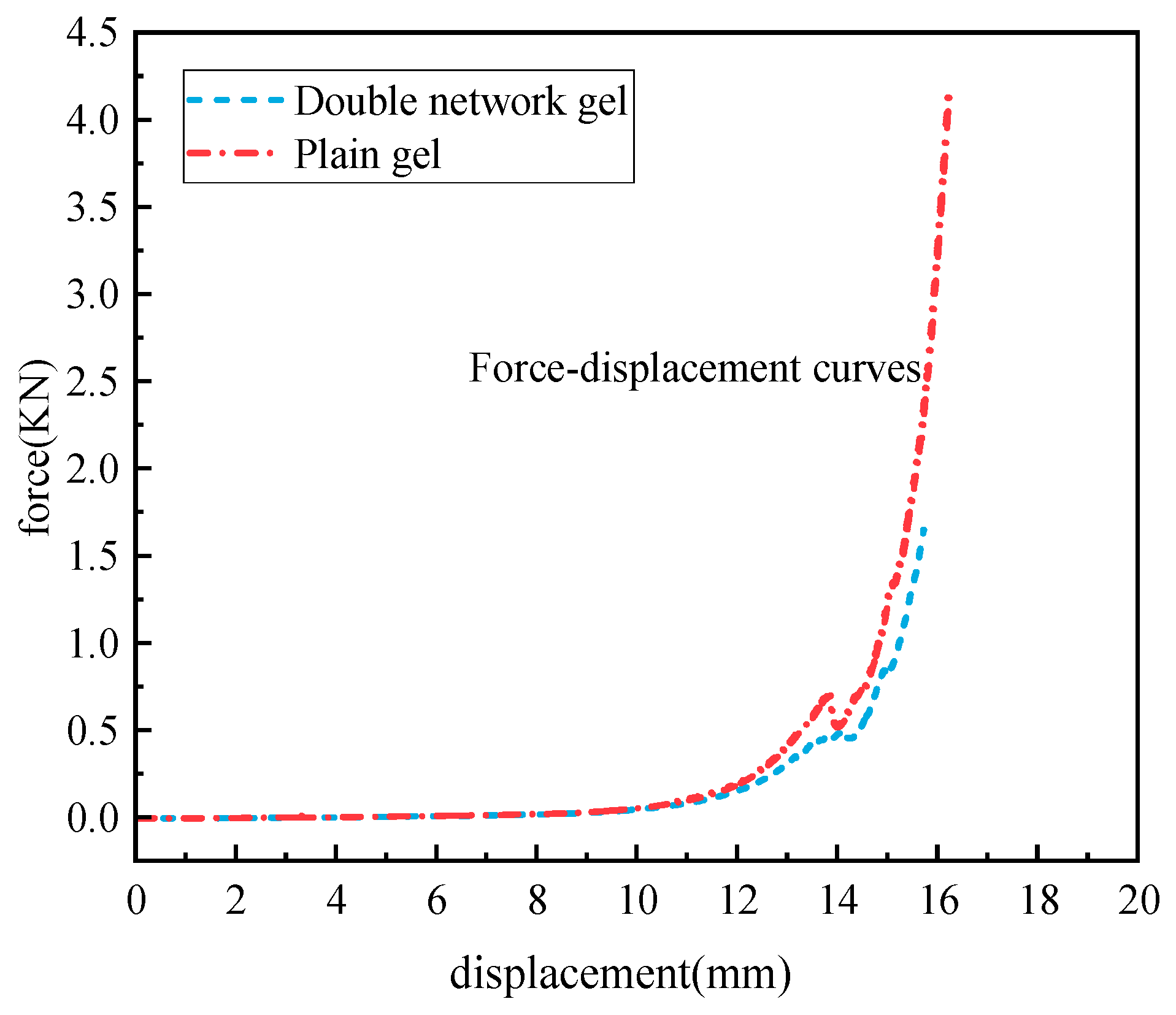
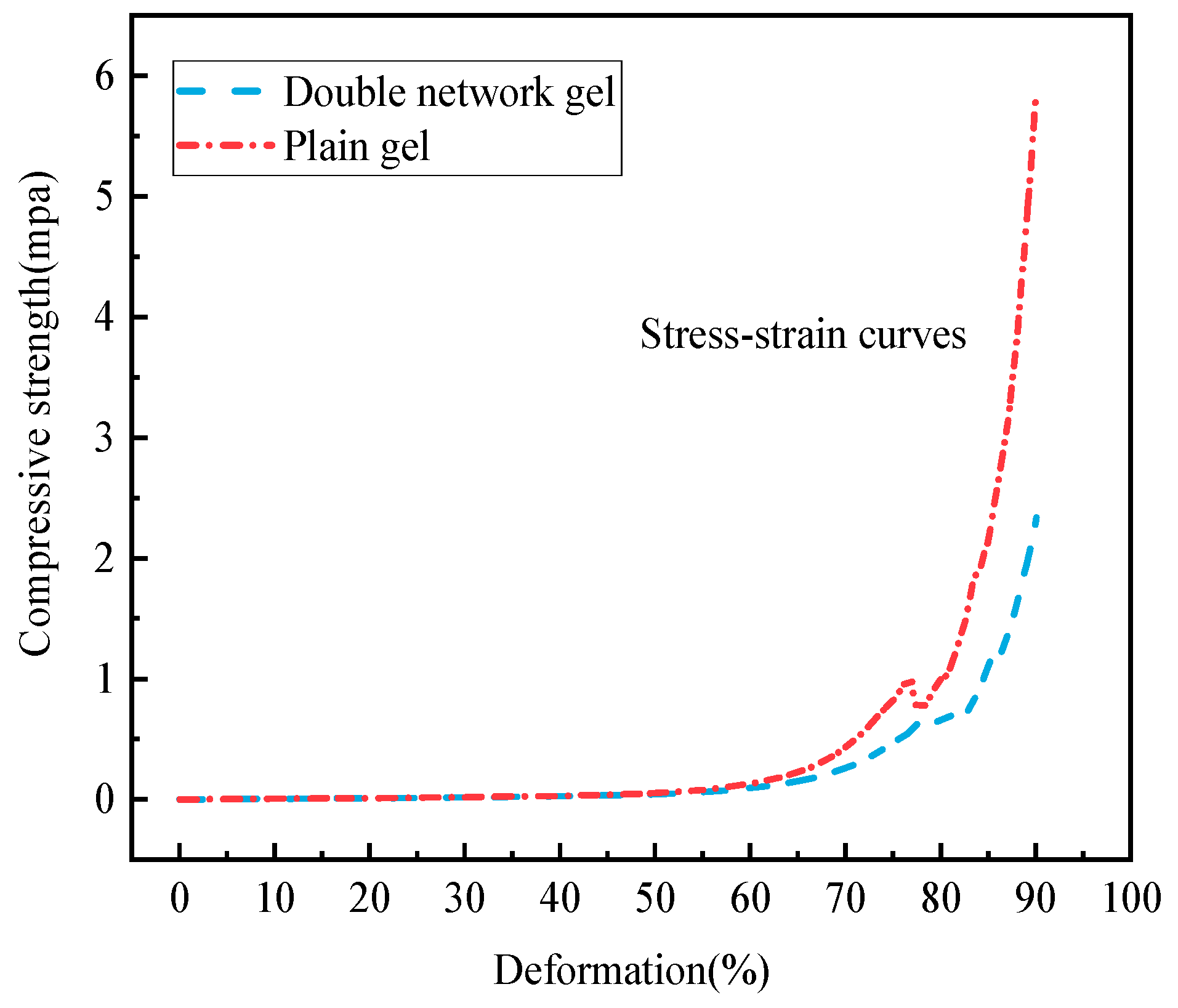
2.4.2. Compression Performance Analysis
2.5. Analysis of the Characteristics of Inhibiting Coal Spontaneous Combustion
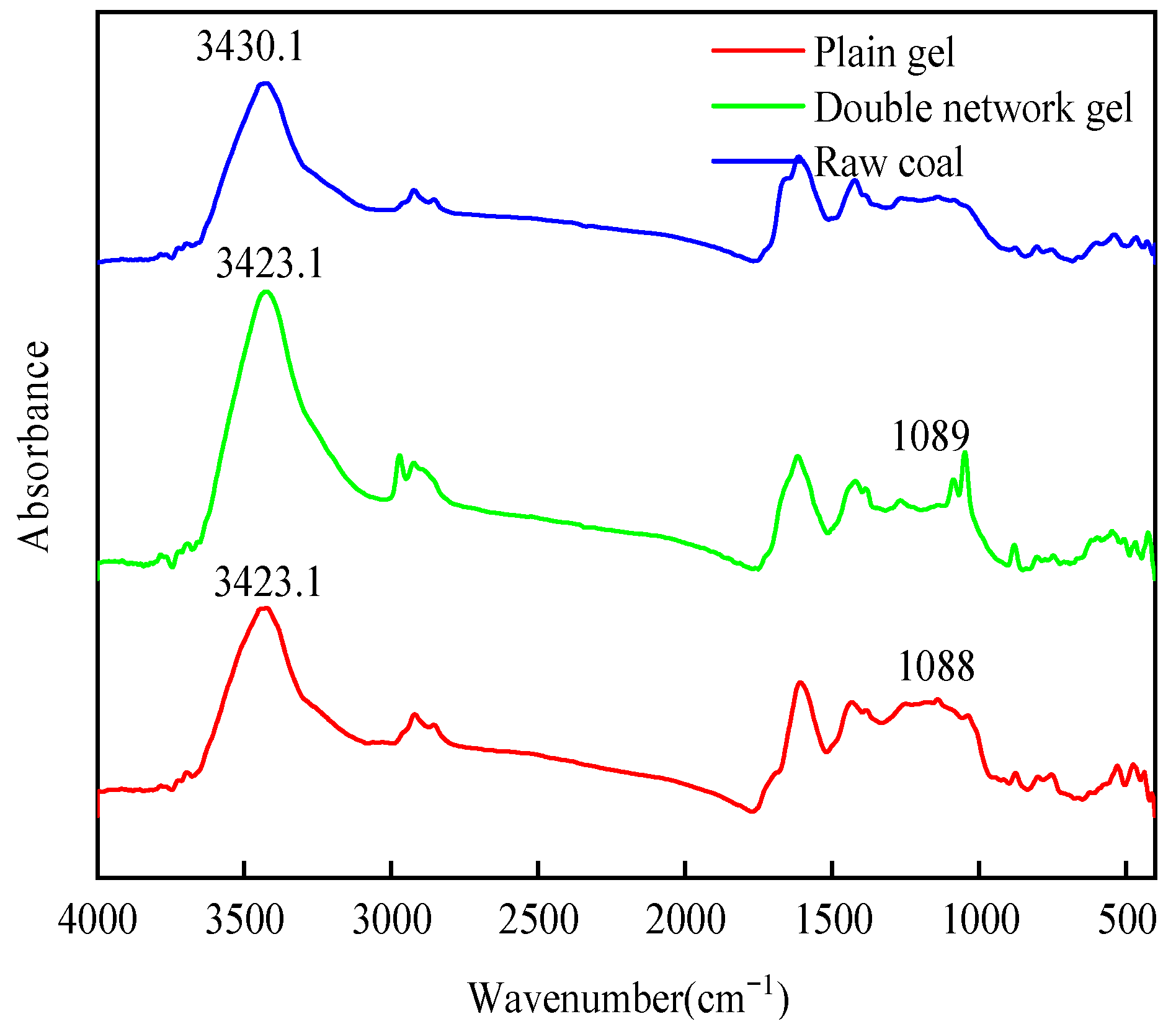
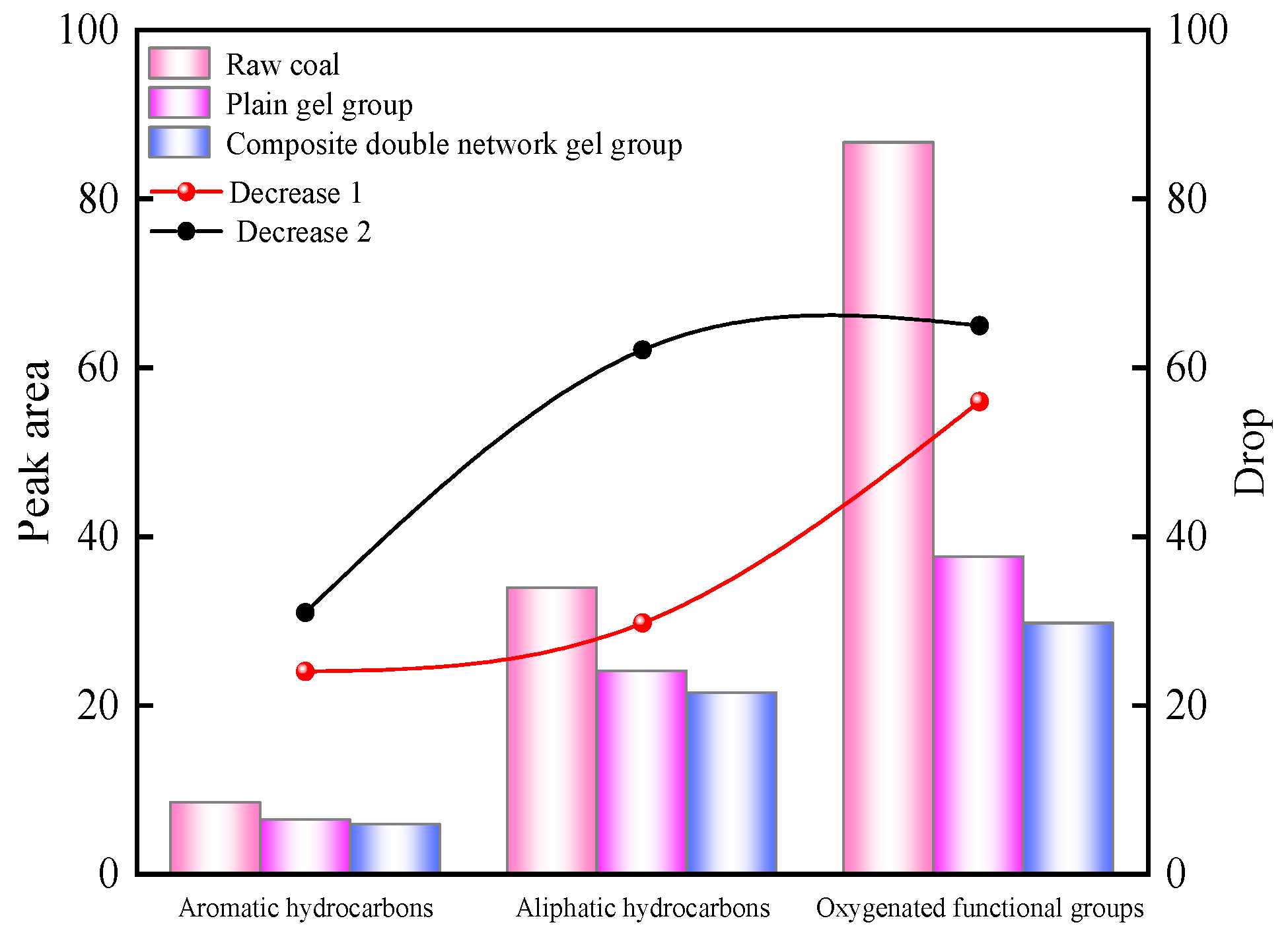
2.5.1. Infrared Spectroscopy Analysis
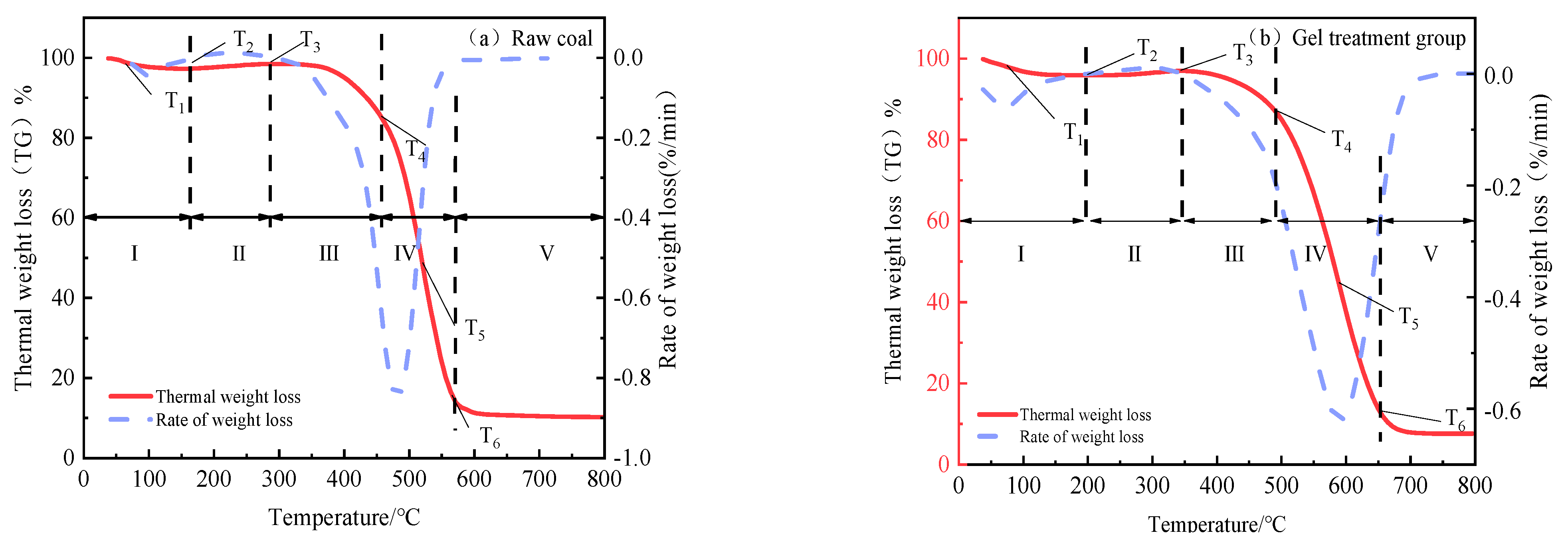
2.5.2. Thermogravimetry
3. Materials and Methods
3.1. Experimental Materials and Equipment
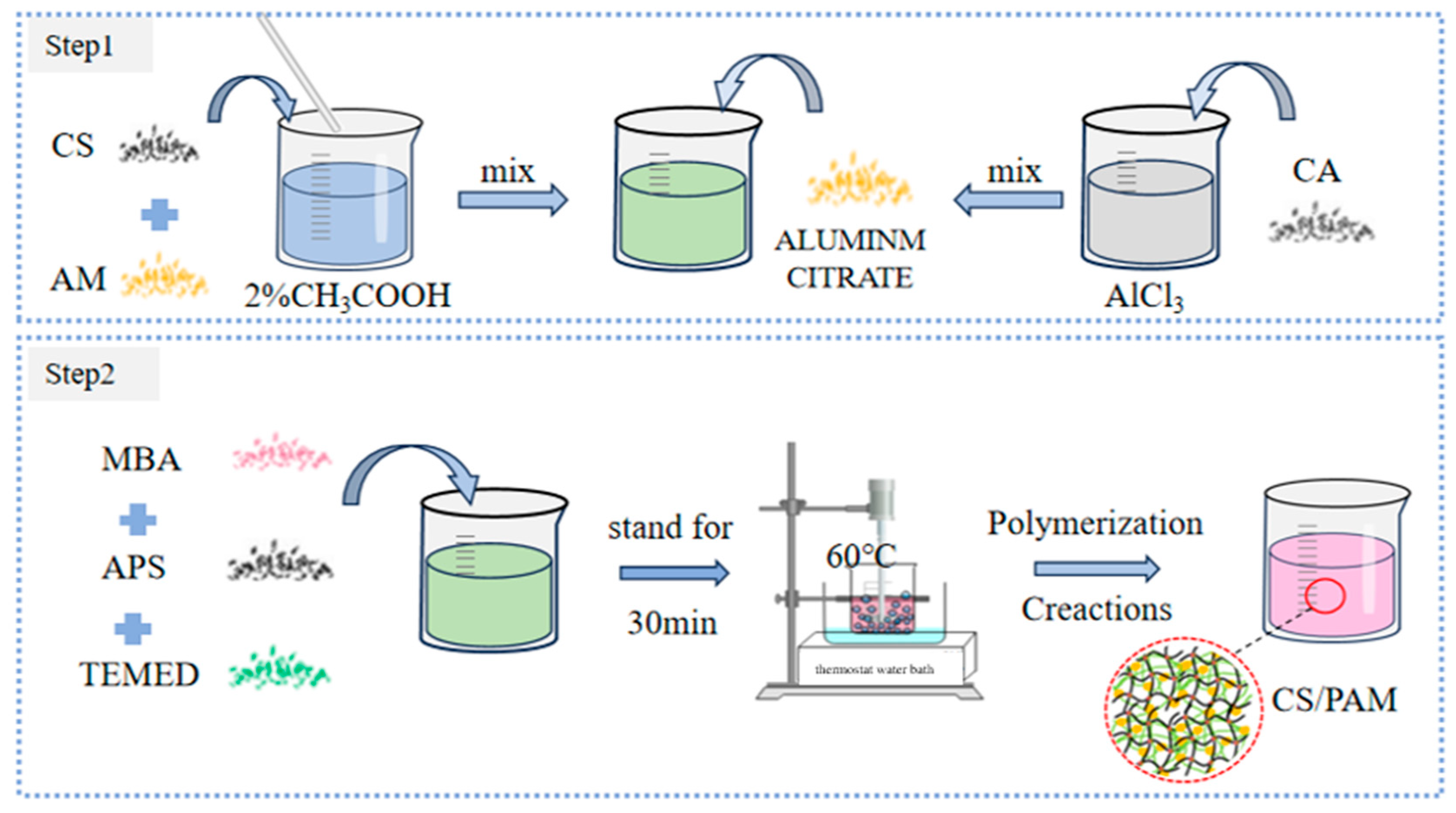
3.2. Sample Preparation Figures
3.3. Study of Basic Properties of Double-Network Gel
3.3.1. Microstructure Characterization of Double-Network Gel
3.3.2. Water Retention Test of Double-Network Gel
- (1)
- Water retention test under constant temperature conditions
- (2)
- Water retention test under heating conditions
3.3.3. Mechanical Property Testing
3.4. Characteristics of Suppressing Low-Temperature Oxidation of Coal
3.4.1. Infrared Spectroscopy Test
3.4.2. TGA
4. Conclusions
- (1)
- The ratio of the composite double-network gel was determined. The final gel formula was selected as 2.5 wt% CS, 11 wt% AM, 0.6‰ MBA, and 2 wt% acetic acid solution, with the initiator APS and the catalyst TEMED participating in the polymerization reaction.
- (2)
- The chitosan/polyacrylamide water/metal ion composite double-network gel has good water retention and coal spontaneous combustion inhibition properties. The gel forms a smooth and dense film on the surface of the coal body, which can encapsulate the coal body and lock in water, reducing the water loss rate by 10%. Compared with raw coal, the composite double-network gel treatment group exhibited an increase in structures representing polysaccharides, as well as a shift in hydrogen bonds, demonstrating the formation of the double-network structure. In addition, the content of -OH and aliphatic hydrocarbon active functional groups in the coal molecules decreased by 24.9% and 31%, respectively, compared to raw coal. As the temperature increased, the characteristic temperature points for coal spontaneous combustion were delayed.
- (3)
- The chitosan/polyacrylamide water/metal ion composite double-network gel has good anti-destruction and compression properties. The deformation memory of the composite double-network gel decreased by 6.5% compared to the plain gel, and the compressive strength of the composite double-network gel increased by 59.96% compared to the plain gel.
- (4)
- In this work, only the fire prevention and extinguishing of a single coal type were studied. In order to improve the universality of the gel in the prevention and control of coal spontaneous combustion, different types of coal will be studied in future research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Xu, K.; Reniers, G.; You, G. Statistical analysis the characteristics of extraordinarily severe coal mine accidents (ESCMAs) in China from 1950 to 2018. Process Saf. Environ. Prot. 2020, 133, 332–340. [Google Scholar] [CrossRef]
- Zhu, W.; Yao, N.; Yan, J. Exploring the determinants of disaster coverage: Evidence from coal mine disasters coverage in China. Int. J. Disaster Risk Reduct. 2018, 31, 856–861. [Google Scholar] [CrossRef]
- Qin, B.; Jia, Y.; Lu, Y.; Li, Y.; Wang, D.; Chen, C. Micro fly-ash particles stabilized Pickering foams and its combustion-retardant characteristics. Fuel 2015, 154, 174–180. [Google Scholar] [CrossRef]
- Qin, B.; Dou, G.; Wang, Y.; Xin, H.; Ma, L.; Wang, D. A superabsorbent hydrogel–ascorbic acid composite inhibitor for the suppression of coal oxidation. Fuel 2017, 190, 129–135. [Google Scholar] [CrossRef]
- Wang, G.; Yan, G.; Zhang, X.; Du, W.; Huang, Q.; Sun, L.; Zhang, X. Research and development of foamed gel for controlling the spontaneous combustion of coal in coal mine. J. Loss Prev. Process Ind. 2016, 44, 474–486. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Engineering of tough double network hydrogels. Macromol. Chem. Phys. 2016, 217, 1022–1036. [Google Scholar] [CrossRef]
- Guo, Q.; Ren, W.; Zhu, J.; Shi, J. Study on the composition and structure of foamed gel for fire prevention and extinguishing in coal mines. Process Saf. Environ. Prot. 2019, 128, 176–183. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, B. Film-forming property and oxygen barrier characteristic of gel-stabilized foam used for controlling spontaneous combustion of coal. Energy Fuels 2021, 35, 12083–12090. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, J.; Liu, X.; He, Y.; Jin, S.; Lou, F.; Guo, R. Preparation and characterization of DOPO-MMT co-stabilized gel foam for inhibiting coal spontaneous combustion. Fuel 2024, 373, 132042. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Ren, T.; Qiao, M.; Yan, J. Influence of methane on the prediction index gases of coal spontaneous combustion: A case study in Xishan coalfield, China. Fuel 2021, 289, 119852. [Google Scholar] [CrossRef]
- Tang, Z.; Xu, G.; Yang, S.; Deng, J.; Xu, Q.; Chang, P. Fire-retardant foam designed to control the spontaneous combustion and the fire of coal: Flame retardant and extinguishing properties. Powder Technol. 2021, 384, 258–266. [Google Scholar] [CrossRef]
- Dong, S.; Lu, X.; Wang, D.; Wang, H.; Zheng, K.; Shi, Q.; Chen, M. Experimental investigation of the fire-fighting characteristics of aqueous foam in underground goaf. Process Saf. Environ. Prot. 2017, 106, 239–245. [Google Scholar] [CrossRef]
- Ren, X.; Hu, X.; Xue, D.; Li, Y.; Shao, Z.; Dong, H.; Lu, W. Novel sodium silicate/polymer composite gels for the prevention of spontaneous combustion of coal. J. Hazard. Mater. 2019, 371, 643–654. [Google Scholar] [CrossRef]
- Fan, Y.J.; Zhao, Y.Y.; Hu, X.M.; Wu, M.Y.; Xue, D. A novel fire prevention and control plastogel to inhibit spontaneous combustion of coal: Its characteristics and engineering applications. Fuel 2020, 263, 116693. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, T.; Xiao, B.; Xian, X.; Lai, N. Preparation of Polyacrylamide Konjac Gum Double Network Gel Liquid Bridge Plug. Chem. Technol. Fuels Oils 2024, 60, 113–118. [Google Scholar] [CrossRef]
- Chen, C.; Li, D.; Abe, K.; Yano, H. Formation of high strength double-network gels from cellulose nanofiber/polyacrylamide via NaOH gelation treatment. Cellulose 2018, 25, 5089–5097. [Google Scholar] [CrossRef]
- Chen, J.; Ao, Y.; Lin, T.; Yang, X.; Peng, J.; Huang, W.; Zhai, M. High-toughness polyacrylamide gel containing hydrophobic crosslinking and its double network gel. Polymer 2016, 87, 73–80. [Google Scholar] [CrossRef]
- Sun, B.; Wang, Z.; He, Q.; Fan, W.; Cai, S. Porous double network gels with high toughness, high stretchability and fast solvent-absorption. Soft Matter 2017, 13, 6852–6857. [Google Scholar] [CrossRef]
- Matsuda, T.; Nakajima, T.; Fukuda, Y.; Hong, W.; Sakai, T.; Kurokawa, T.; Gong, J.P. Yielding criteria of double network hydrogels. Macromolecules 2016, 49, 1865–1872. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, G.; Ding, H.; Wang, H.; Li, J.; Yu, R.; Wang, P. Study on the inhibition performance of double network physicochemical nanocomposite gel inhibitor on coal spontaneous combustion. Fuel 2023, 350, 128697. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, W.; Guo, Z.; Su, X.; Liu, Y.; Meng, H.; Yang, J. Tough, highly adaptable and self-healing integrated supercapacitor based on double network gel polymer electrolyte. Energy 2023, 264, 126244. [Google Scholar] [CrossRef]
- Drozdov, A.D.; Christiansen, J.D. Double-network gels with dynamic bonds under multi-cycle deformation. J. Mech. Behav. Biomed. Mater. 2018, 88, 58–68. [Google Scholar] [CrossRef]
- Drozdov, A.D.; Christiansen, J.D.; Dusunceli, N.; Sanporean, C.G. Self-recovery and fatigue of double-network gels with permanent and reversible bonds. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 438–453. [Google Scholar] [CrossRef]
- Nakajima, T.; Kurokawa, T.; Ahmed, S.; Wu, W.L.; Gong, J.P. Characterization of internal fracture process of double network hydrogels under uniaxial elongation. Soft Matter 2013, 9, 1955–1966. [Google Scholar] [CrossRef]
- King, D.R.; Okumura, T.; Takahashi, R.; Kurokawa, T.; Gong, J.P. Macroscale double networks: Design criteria for optimizing strength and toughness. ACS Appl. Mater. Interfaces 2019, 11, 35343–35353. [Google Scholar] [CrossRef]
- Ali, M.; Al-Suhaibani, Z.; Almuzaiqer, R.; Al-Salem, K.; Nuhait, A.; Algubllan, F.; Alqahtani, I. Sunflower and Watermelon Seeds and Their Hybrids with Pineapple Leaf Fibers as New Novel Thermal Insulation and Sound-Absorbing Materials. Polymers 2023, 15, 4422. [Google Scholar] [CrossRef]
- Na, Y.H.; Kurokawa, T.; Katsuyama, Y.; Tsukeshiba, H.; Gong, J.P.; Osada, Y.; Shibayama, M. Structural characteristics of double network gels with extremely high mechanical strength. Macromolecules 2004, 37, 5370–5374. [Google Scholar] [CrossRef]
- Onifade, M.; Lawal, A.I.; Abdulsalam, J.; Genc, B.; Bada, S.; Said, K.O.; Gbadamosi, A.R. Development of multiple soft computing models for estimating organic and inorganic constituents in coal. Int. J. Min. Sci. Technol. 2021, 31, 483–494. [Google Scholar] [CrossRef]
- Ali, M.; Al-Suhaibani, Z.; Almuzaiqer, R.; Albahbooh, A.; Al-Salem, K.; Nuhait, A. New Composites Derived from the Natural Fiber Polymers of Discarded Date Palm Surface and Pineapple Leaf Fibers for Thermal Insulation and Sound Absorption. Polymers 2024, 16, 1002. [Google Scholar] [CrossRef]


| Level | Factor | |||
|---|---|---|---|---|
| A | B | C | D | |
| CS Content/% | AM Content/% | MBA Content/‰ | Blank Group | |
| 1 | 2 | 10 | 0.4 | 1 |
| 2 | 2.5 | 11 | 0.6 | 2 |
| 3 | 3 | 12 | 0.8 | 3 |
| Experiment Number | CS Content (%) | AM Content (%) | MBA Content (%) | Gelatinization Time (min) | Viscosity (mPa·s) | Strength (N/m2) |
|---|---|---|---|---|---|---|
| 1 | 2 | 10 | 0.4 | 15 | 3329 | 3423 |
| 2 | 2 | 11 | 0.8 | 3.75 | 3511 | 5225 |
| 3 | 2 | 12 | 0.6 | 10 | 3853 | 7846 |
| 4 | 2.5 | 10 | 0.8 | 2.5 | 6440 | 2320 |
| 5 | 2.5 | 11 | 0.6 | 8 | 6541 | 6726 |
| 6 | 2.5 | 12 | 0.4 | 20 | 6673 | 2968 |
| 7 | 3 | 10 | 0.6 | 6.6 | 9037 | 2105 |
| 8 | 3 | 11 | 0.4 | 17.5 | 9385 | 4346 |
| 9 | 3 | 12 | 0.8 | 5 | 9825 | 3326 |
| Source of Differences | Sum of Squares of Dispersion | Degree of Freedom | Mean Squared | F | p |
|---|---|---|---|---|---|
| CS | 0.572 | 2 | 0.286 | 0.533 | 0.652 |
| AM | 19.822 | 2 | 9.911 | 18.496 | 0.051 |
| MBA | 295.355 | 2 | 147.677 | 275.603 | 0.004 |
| Source of Differences | Sum of Squares of Dispersion | Degree of Freedom | Mean Squared | F | p |
|---|---|---|---|---|---|
| CS | 513.646 | 2 | 256.823 | 106.5 | 0.001 |
| AM | 40.228 | 2 | 20.114 | 8.346 | 0.107 |
| MBA | 3.025 | 2 | 1.512 | 0.628 | 0.614 |
| Source of Differences | Sum of Squares of Dispersion | Degree of Freedom | Mean Squared | F | p |
|---|---|---|---|---|---|
| CS | 77.991 | 2 | 98.995 | 2.427 | 0.292 |
| AM | 128.475 | 2 | 64.237 | 3.997 | 0.200 |
| MBA | 76.679 | 2 | 38.339 | 2.386 | 0.295 |
| Coal Samples | T1 (°C) | T2 (°C) | T3 (°C) | T4 (°C) | T5 (°C) | T6 (°C) |
|---|---|---|---|---|---|---|
| Raw coal | 62.04 | 160.77 | 287.89 | 454.08 | 525.16 | 564.67 |
| Composite double-network gel treatment group | 69.05 | 200.39 | 343.47 | 485.47 | 588.34 | 650.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, Z.; Fu, W.; Zhao, Y. Preparation and Properties of Composite Double-Network Gel for Inhibiting Coal Spontaneous Combustion. Molecules 2024, 29, 3365. https://doi.org/10.3390/molecules29143365
Wang J, Zhang Z, Fu W, Zhao Y. Preparation and Properties of Composite Double-Network Gel for Inhibiting Coal Spontaneous Combustion. Molecules. 2024; 29(14):3365. https://doi.org/10.3390/molecules29143365
Chicago/Turabian StyleWang, Jianguo, Zhenzhen Zhang, Wen Fu, and Yifan Zhao. 2024. "Preparation and Properties of Composite Double-Network Gel for Inhibiting Coal Spontaneous Combustion" Molecules 29, no. 14: 3365. https://doi.org/10.3390/molecules29143365
APA StyleWang, J., Zhang, Z., Fu, W., & Zhao, Y. (2024). Preparation and Properties of Composite Double-Network Gel for Inhibiting Coal Spontaneous Combustion. Molecules, 29(14), 3365. https://doi.org/10.3390/molecules29143365





